Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Strebluses E–H, four new stilbene-like derivatives from the stems of Streblus ilicifolius†
Tho Huu Leabc,
Phu Hoang Dang abc,
Hai Xuan Nguyenabc,
Truong Nhat Van Doabc,
Nhan Trung Nguyen*abc and
Mai Thanh Thi Nguyen*abc
abc,
Hai Xuan Nguyenabc,
Truong Nhat Van Doabc,
Nhan Trung Nguyen*abc and
Mai Thanh Thi Nguyen*abc
aFaculty of Chemistry, University of Science, Ho Chi Minh City, 72711, Vietnam
bVietnam National University, Ho Chi Minh City, 71300, Vietnam. E-mail: nttmai@hcmus.edu.vn; ntnhan@hcmus.edu.vn
cResearch Lab for Drug Discovery and Development, University of Science, Ho Chi Minh City, 72711, Vietnam
First published on 22nd December 2022
Abstract
Following bioactivity-guided isolation, four new stilbene-like derivatives, named Strebluses E–H, were isolated from the EtOAc-soluble fraction of the stems of Streblus ilicifolius (Moraceae). Their chemical structures were elucidated based on NMR spectroscopic data interpretation and optical rotation calculation. Streblus E possesses potent tyrosinase inhibitory activity with an IC50 value of 0.1 μM. Oxy-tyrosinase has two bound Cu2+ ions and a peroxide group in the binding site, which has a role in the catalytic oxidation. Thus, a docking study of Streblus E with oxy-tyrosinase was performed to analyze the ligand–protein interactions. With in silico modelling, the S value and the ligand–protein interactions suggested that Streblus E showed lower binding affinity for oxy-tyrosinase than that of Streblus C.
Introduction
Streblus ilicifolius (S. Vidal) Corn. (Moraceae) is widely distributed in India, China, and South Asia.1 Several phytochemical studies of S. ilicifolius have been carried out, leading to the identification of amide glycosides, coumarins, stilbenes, lignans, and polyphenols. In addition, the anti-tyrosinase, antimicrobial, and anti-inflammatory activities of these chemical constituents has been reported.2–4Our continued studies on the bioactivity-guided phytochemical investigation of medicinal plants for tyrosinase inhibitory activity5–9 has led to the identification of two coumarins (Strebluses A and B) and two stilbene-like derivatives (Strebluses C and D) from the stems of Streblus ilicifolius, among which Streblus C exhibited a remarkable inhibitory effect with an IC50 value of 0.01 μM.10,11 Thus, this phytochemical study was continuously performed, leading to the isolation of four new stilbene-like derivatives, Strebluses E–H (1–4). Their structures were determined by NMR spectroscopic interpretation. In addition, their absolute configurations were identified based on the optical rotation calculation. Herein, the tyrosinase inhibitory activity assays and the molecular docking studies with the oxy-tyrosinase were performed.
Results and discussion
Structural elucidation of four new isolated compounds from S. ilicifolius
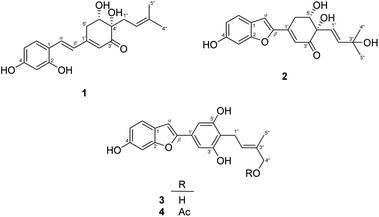
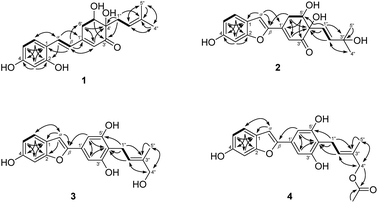
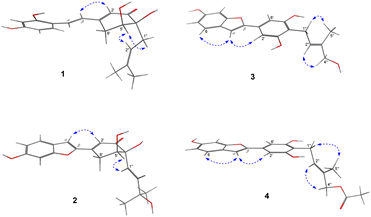
The EtOAc-soluble fraction of S. ilicifolius stems was chromatographed to obtain four undescribed stilbene-like derivatives, Strebluses E–H (1–4) (Fig. 1).Compound 1, Streblus E, showed a molecular formula to be C19H22O5 based on the quasi-molecular ion at m/z 331.1545 [M + H]+ (calcd for C19H23O5+, 331.1540) in the HRESIMS spectrum. The 1H and 13C NMR spectra showed signals of the prenylated stilbene-like feature having the 5,6-dihydroxycyclohex-2-en-1-one moiety (Tables 1 and 2), which resembled those of Streblus C except for the absence of the acetonide group.10 The observed HMBC correlations (Fig. 2) indicated the presence of the 2,4-dihydroxyphenyl group in 1. The 5,6-dihydroxycyclohex-2-en-1-one substructure was established based on the HMBC correlations from H-2′ to C-4′ and C-6′, from H-5′ to C-1′ and C-3′, from 5′-OH to C-5′ and C-6′, and from 4′-OH to C-3′ and C-4′. The HMBC correlations from H-α to C-2, C-6, and C-1′, from H-β to C-1, C-1′, C-2′, and C-6′ were supportive of the Cα–C1 and Cβ–C1′ bonds. In addition, the C-4′ prenyl group was assigned based on the HMBC correlations. The NOESY correlation between H-5′/H2-1′′/H-2′′ deduced the presence of the cis-diol (Fig. 3). In addition, the 3J coupling constants between H-5′ and H2-6′ were 5.6 and 3.2 Hz, to suggest the equatorial configuration of H-5′.12 The preferred conformers of cis-(R,R)-diol 1 were established by molecular mechanics calculation using MMFF94 force field.13 The obtained conformers were reoptimized using B3LYP density functional theory (DFT) method with the 6-31G* basis set, to obtain the most preferred conformer with 95.5% Boltzmann distribution. The optical rotation calculation at the sodium D line (λ = 589.3 nm) was carried out using DFT-B3LYP/6-311++G(2d,2p) function with the polarizable continuum model (PCM) for methanol.14,15 The calculated [α]D value of cis-(R,R)-diol 1 was obtained as −694.12, while the experimental value of [α]D +630.0 (c 0.01, MeOH) was the opposite in sign. Thus, an (S,S) absolute configuration was concluded for Streblus E (1), which was deacetonide-ent-Streblus C.
| Position | δH (J, Hz) | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| 3 | 6.46, d (2.4) | 7.00, d (2.1) | 6.96, d (2.2) | 6.95, d (2.0) |
| 5 | 6.42, dd (8.5, 2.4) | 6.87, dd (8.5, 2.1) | 6.80, dd (8.4, 2.2) | 6.80, dd (8.4, 2.0) |
| 6 | 7.47, d (8.5) | 7.52, d (8.5) | 7.38, d (8.4) | 7.38, d (8.4) |
| 2′ | 5.97, brs | 6.55, d (2.3) | 6.92, s | 6.92, s |
| 5′ | 4.23, dd (5.6, 3.2) | 4.15, dd (2.9, 2.5) | ||
| 6′ | 2.94–2.96, m | 3.15, ddd (18.3, 2.9, 2.3) | 6.92, s | 6.92, s |
| 3.08, dd (18.3, 2.5) | ||||
| α | 7.36, d (16.3) | 7.33, s | 6.91, brs | 6.91, brs |
| β | 7.00, d (16.3) | |||
| 1′′ | 2.42, dd (14.7, 7.6) | 5.91, d (15.5) | 3.43, d (7.3) | 3.45, d (7.3) |
| 2.34, dd (14.7, 6.9) | ||||
| 2′′ | 5.19, dd (7.6, 6.9) | 6.10, d (15.5) | 5.57, tq (7.3, 1.3) | 5.66, tq (7.3, 1.3) |
| 4′′ | 1.67, s | 1.22, s | 3.90, d (6.2) | 4.41, s |
| 5′′ | 1.57, s | 1.19, s | 1.80, d (1.3) | 1.82, d (1.3) |
| 2-OH | 8.91, s | |||
| 4-OH | 8.68, s | 8.93, s | 8.46, s | 8.46, s |
| 4′-OH | 4.10, s | 4.37, s | ||
| 5′-OH | 3.65, s | 4.00, s | 8.33, s | 8.42, s |
| 3′′-OH | 3.62, s | |||
| 3′-OH | 8.33, s | 8.42, s | ||
| 4′′-OH | 3.58, t (6.2) | |||
| 4′′-OAc | 1.98, s | |||
| Position | δC, type C | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| 1 | 116.3 | 122.0 | 122.7 | 122.7 |
| 2 | 158.2 | 158.1 | 156.7 | 156.7 |
| 3 | 103.7 | 98.3 | 98.4 | 98.4 |
| 4 | 160.8 | 158.9 | 156.6 | 156.6 |
| 5 | 109.0 | 114.3 | 113.2 | 113.2 |
| 6 | 129.5 | 123.4 | 121.8 | 121.9 |
| 1′ | 154.9 | 144.2 | 130.0 | 130.2 |
| 2′ | 123.0 | 119.0 | 103.8 | 103.9 |
| 3′ | 201.1 | 199.1 | 157.3 | 157.3 |
| 4′ | 79.7 | 80.3 | 116.1 | 115.3 |
| 5′ | 72.9 | 74.5 | 157.3 | 157.3 |
| 6′ | 31.9 | 32.1 | 103.9 | 103.9 |
| α | 132.1 | 111.0 | 101.5 | 101.6 |
| β | 126.4 | 153.2 | 155.8 | 155.7 |
| 1′′ | 35.5 | 124.6 | 22.7 | 22.8 |
| 2′′ | 119.0 | 142.4 | 123.9 | 128.5 |
| 3′′ | 134.8 | 70.3 | 135.7 | 130.6 |
| 4′′ | 26.1 | 30.4 | 68.6 | 70.5 |
| 5′′ | 18.1 | 30.2 | 13.9 | 14.1 |
4′′-OCO![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) H3 H3 |
20.8 | |||
4′′-O![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) OCH3 OCH3 |
170.8 | |||
Compound 2, Streblus F, showed the sodium adduct molecular ion at m/z 367.1169 [M + Na]+ (calcd for C19H20O6Na+, 367.1152) in the HRESIMS spectrum. The 1H and 13C NMR spectra of 2 closely resembled those of Streblus D, except for the presence of the 3-hydroxyisopent-1(E)-enyl group [δH 5.91 (d, J = 15.5 Hz, H-1′′), 6.10 (d, J = 15.5 Hz, H-2′′), 1.22 and 1.19 (s, 2 × 3′′-CH3)] instead of the prenyl group in Streblus D (Tables 1 and 2). The observed NOESY correlation between H-5′ and H-1′′ confirmed the cis-orientation of the C-4′ and C-5′ hydroxy groups (Fig. 3). In addition, the equatorial configuration of H-5′ was suggested based on its 3J coupling constants of 2.9 and 2.5 Hz.12 The conformational analysis for (R,R)-2 was obtained seven conformers with a total Boltzmann weight >99%. The calculated [α]D value of (R,R)-2 was +127.95, opposite in sign to its experimental value [α]D −134.0 (c 0.01, MeOH). Thus, the structure of Streblus F (2) was concluded as 4′S,5′S.
Streblus G (3) showed the molecular formula C19H18O5, as deduced from the negative HRESIMS spectrum at m/z 325.1087 [M − H]− (calcd for C19H17O5−, 325.1081). The 1H spectrum showed signals for a 1,3,4-trisubstituted [δH 7.38 (d, J = 8.4 Hz, H-6), 6.96 (d, J = 2.2 Hz, H-3), 6.80 (dd, J = 8.4, 2.2 Hz, H-5)] and a 1,3,4,5-tetrasubstituted [δH 6.92 (s, H-2′ and H-6′)] aromatic rings, an olefinic proton [δH 6.91 (brs, H-α)], a hydroxylated prenyl group [δH 3.43 (d, J = 7.3 Hz, H2-1′′), 5.57 (tq, J = 7.3, 1.3 Hz, H-2′′), 3.90 (d, J = 6.2 Hz, H2-4′′), 1.80 (d, J = 1.3 Hz, H3-5′′), 3.58 (t, J = 6.2 Hz, 4′′-OH)], and two hydroxy groups [δH 8.46 (s, 6-OH), 8.33 (s, 3′-OH and 5′-OH)]. The 13C NMR data exhibited resonances for 14 aromatic carbons [δC 98.3–157.2] and a hydroxylated prenyl group [δC 135.5 (C-3′′), 123.7 (C-2′′), 68.5 (C-4′′), 22.5 (C-1′′), 13.7 (C-5′′)] (Table 2). These data resembled closely those of moracin M, except for the presence of the hydroxylated prenyl group at C-4′.16 The HMBC correlations from H-6 to C-α, C-4, and C-2, from H-5 to C-1 and C-3, from H-3 to C-1 and C-5, from H-α to C-β, C-1, and C-2, from H-2′/H-6′ to C-β permitted the structural assignment of 3 as shown (Fig. 2). The C-3′ and C-5′ hydroxy groups were identified by the HMBC correlations with two corresponding oxygenated aromatic carbons. In addition, the HMBC correlations from H2-1′′ and H-2′′ to C-4′, from H-2′′ and H3-5′′ to C-4′′ indicated the location of the 4′′-hydroxyprenyl group at C-4′. The NOESY correlations between H-6/H-α/H-2′(6′), H2-1′′/H3-5′′, and H-2′′/H2-4′′ confirmed the presence of the 2-phenylbenzofuran moiety and the (2′′E)-4′′-hydroxyprenyl group (Fig. 3). Thus, the structure of Streblus G (3) was concluded as 4′-(4′′-hydroxyprenyl)moracin M.
Streblus H (4) showed a sodiated molecular ion peak at m/z 391.1151 [M + Na]+ (calcd for C21H20O6Na, 391.1152) in the HRESIMS spectrum. The 1H and 13C NMR spectra of 4 resembled closely those of 3, except for the presence of signals for an acetyl group [δH 2.08; δC 170.8, 20.8] (Table 2). The HMBC correlation between the H2-4′′ oxymethylene protons and the acetoxy carbonyl at δC 170.8 suggested that the acetylation happened at C-4′′ (Fig. 2). In addition, the NOESY correlations between H2-1′′/H3-5′′ and H-2′′/H2-4′′ supported the E configuration of the double bond in 4′′-acetoxyprenyl group (Fig. 3). Thus, the structure of Streblus H (4) was assigned as 4′′-acetylstreblus G.
Tyrosinase inhibitory activity and docking studies
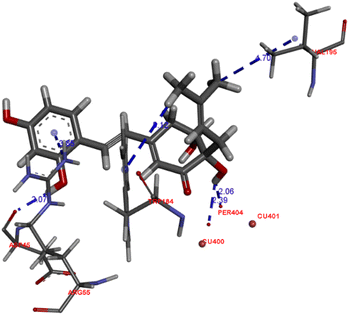
All isolated compounds were tested for their tyrosinase inhibitory activities.17 Kojic acid was used as a positive control. Streblus E (1) showed potent inhibitory effect with an IC50 value of 0.1 μM as compared to that of kojic acid (IC50, 44.6 μM). All remaining compounds were inactive (IC50 > 100 μM). The docking study of 1 was performed with MOE following our previous procedure.9 In the binding pocket of the top-rank pose, 1 showed the H-donor interaction from the C-4′ hydroxy group to peroxide bridge PER404, and from the C-2 hydroxy group to ASP45 residue (Fig. 4). The aromatic ring had the π-cation interaction with ARG55 residue. In addition, the prenyl group showed the σ–σ and π–σ interactions with VAL195 and TRP184, respectively.The genus Streblus is a small deciduous shrub, which includes 20 species and mainly distributed in South China and South Asia. The phytochemical studies of Streblus ilicifolius were carried out to obtain various class of compounds. In our previous studies, we reported the structure and the anti-tyrosinase evaluation of four new compounds, in which Streblus C showed noteworthy inhibitory activity.10,11 With these interesting results, we continued to carry out the bioactivity-guided isolation, leading to the identification of four new stilbene-like derivatives were isolated as well as their in vitro and in silico tyrosinase inhibitory activity were reported. In this study, Streblus E (1) showed potent inhibitory effect with an IC50 value of 0.1 μM. The 2,4-resorcinol subunit highly contributed to inhibitory activity.18 In addition, the formation of the five-membered ring gave rise to the loss of inhibitory effect.19 With in silico modelling, the S value and the ligand–protein interactions suggested that 1 showed lower binding affinity for oxy-tyrosinase than that of Streblus C.10 This result was used to clarify the remarkable difference in IC50 values of Strebluses C (0.01 μM) and E (0.1 μM).
Conclusions
Four new stilbene-like derivatives, named Strebluses E–H (1–4), were isolated from the EtOAc-soluble fraction of the stems of Streblus ilicifolius (Moraceae). Their structures were elucidated based on NMR spectroscopic data interpretation with the aid of optical rotation calculation. Streblus E (1) showed potent tyrosinase inhibitory effect with an IC50 value of 0.1 μM.Experimental
General experimental procedures
Optical values were measured on a Shimadzu UV-1800 spectrophotometer (Shimadzu Pte., Ltd., Singapore). NMR spectra were acquired on a Bruker Avance III 500 spectrometer (Bruker BioSpin AG, Bangkok, Thailand). Chemical shifts are expressed as δ values. HRESIMS data were acquired on Bruker micrOTOF-QII mass spectrometer (Bruker Singapore Pte., Ltd., Singapore). Column chromatography (CC) was carried out using silica gel 60, 0.06–0.2 mm (Scharlau, Barcelona, Spain) and LiChroprep RP-18, 40–63 μm (Merck KGaA, Darmstadt, Germany). Kieselgel 60 F254 or RP-18 F254 plates for thin-layer chromatography (TLC) were purchased from Merck (Merck KGaA, Darmstadt, Germany). Mushroom tyrosinase (EC 1.14.18.1; 3933 U mL−1) and L-dihydroxyphenylalanine (L-DOPA) were obtained from Sigma-Aldrich (Sigma-Aldrich Pte Ltd, Singapore). Other chemicals were of the highest grade available.Plant material
The stems of Streblus ilicifolius were collected at Hoai Nhon District, Binh Dinh Province, Vietnam, in October 2017. Its scientific name was identified by Dr Rer. Nat. Anh Tuan Dang-Le, Faculty of Biology and Biotechnology, University of Science, Ho Chi Minh City, Vietnam. A sample (MCE0052) has been deposited at the Department of Medicinal Chemistry, Faculty of Chemistry, University of Science, Ho Chi Minh City, Vietnam.Extraction and isolation
The dried powdered stems of S. ilicifolius (7.0 kg) were exhaustively extracted in a Soxhlet extractor with n-hexane, EtOAc, and MeOH to yield n-hexane – (64.8 g), EtOAc – (117.2 g), and MeOH – (378.0 g) soluble fractions, respectively. The EtOAc-soluble fraction was chromatographed by silica gel CC (15 × 150 cm) and eluted with MeOH–CHCl3 mixtures (v/v, 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 → 100
100 → 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) to afford 18 fractions (Fr.1–Fr.18). Fraction Fr.8 (0.8 g) was subjected to further silica gel CC and was eluted with MeOH–CHCl3 (v/v, 0
0) to afford 18 fractions (Fr.1–Fr.18). Fraction Fr.8 (0.8 g) was subjected to further silica gel CC and was eluted with MeOH–CHCl3 (v/v, 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 → 100
100 → 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) mixtures to yield 2 fraction (Fr.8.1 and Fr.8.2). Fraction Fr.8.1 (445 mg) was chromatographed over a silica gel column with EtOAc–CHCl3 mixtures (v/v, 0
0) mixtures to yield 2 fraction (Fr.8.1 and Fr.8.2). Fraction Fr.8.1 (445 mg) was chromatographed over a silica gel column with EtOAc–CHCl3 mixtures (v/v, 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 → 100
100 → 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) mixtures to obtain five fractions (Fr.8.1.1 and Fr.8.1.5). Fraction Fr.8.1.4 (50.9 mg) was purified by preparative TLC with an EtOAc–CHCl3 mixture (v/v, 30
0) mixtures to obtain five fractions (Fr.8.1.1 and Fr.8.1.5). Fraction Fr.8.1.4 (50.9 mg) was purified by preparative TLC with an EtOAc–CHCl3 mixture (v/v, 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70) to afford compound 2 (3.0 mg). Fraction Fr.11 (4.7 g) was subjected to silica gel CC with MeOH–CHCl3 mixtures (v/v, 0
70) to afford compound 2 (3.0 mg). Fraction Fr.11 (4.7 g) was subjected to silica gel CC with MeOH–CHCl3 mixtures (v/v, 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 → 100
100 → 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) to obtain six fractions (Fr.11.1–Fr.11.6). Fraction Fr.11.5 (849 mg) was separated by silica gel CC with EtOAc–CHCl3 mixtures (v/v, 0
0) to obtain six fractions (Fr.11.1–Fr.11.6). Fraction Fr.11.5 (849 mg) was separated by silica gel CC with EtOAc–CHCl3 mixtures (v/v, 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 → 100
100 → 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) to obtain four fractions (Fr.11.5.1–Fr.11.5.4). Fraction Fr.11.5.2 (118 mg) was purified by CC with Me2CO–n-hexane mixtures (v/v, 0
0) to obtain four fractions (Fr.11.5.1–Fr.11.5.4). Fraction Fr.11.5.2 (118 mg) was purified by CC with Me2CO–n-hexane mixtures (v/v, 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 → 100
100 → 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0), then the resulting fraction was purified by preparative TLC with an EtOAc–n-hexane mixture (v/v, 50
0), then the resulting fraction was purified by preparative TLC with an EtOAc–n-hexane mixture (v/v, 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) to afford compound 4 (4.0 mg). Fraction Fr.14 (19.6 g) was separated by silica gel CC with MeOH–CHCl3 mixtures (v/v, 0
50) to afford compound 4 (4.0 mg). Fraction Fr.14 (19.6 g) was separated by silica gel CC with MeOH–CHCl3 mixtures (v/v, 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 → 100
100 → 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) to obtain 11 fractions (Fr.14.1–Fr.14.11). Fraction Fr.14.9 (8.8 g) was loaded onto a silica gel column and eluted with Me2CO–CHCl3 mixtures (v/v, 0
0) to obtain 11 fractions (Fr.14.1–Fr.14.11). Fraction Fr.14.9 (8.8 g) was loaded onto a silica gel column and eluted with Me2CO–CHCl3 mixtures (v/v, 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 → 70
100 → 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30) to give 20 fractions (Fr.14.9.1–Fr.14.9.20). Fraction Fr.14.9.5 (41.4 mg) was chromatographed over a silica gel column with a MeOH–CHCl3 mixture (v/v, 10
30) to give 20 fractions (Fr.14.9.1–Fr.14.9.20). Fraction Fr.14.9.5 (41.4 mg) was chromatographed over a silica gel column with a MeOH–CHCl3 mixture (v/v, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90) to afford compound 3 (4.0 mg). Fraction Fr.14.9.8 (46.8 mg) was separated by CC with Me2CO–CHCl3 mixtures (v/v, 0
90) to afford compound 3 (4.0 mg). Fraction Fr.14.9.8 (46.8 mg) was separated by CC with Me2CO–CHCl3 mixtures (v/v, 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 → 70
100 → 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30), then the resulting fractions were purified by preparative reversed-phase TLC with a H2O–MeOH mixture (v/v, 30
30), then the resulting fractions were purified by preparative reversed-phase TLC with a H2O–MeOH mixture (v/v, 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70) to afford compound 1 (3.0 mg).
70) to afford compound 1 (3.0 mg).
Streblus E (1): yellow, amorphous powder; 1H and 13C NMR (500 MHz, acetone-d6, see Tables 1 and 2); HRESIMS m/z 331.1545 [M + H]+ (calcd for C19H23O5+, 331.1540).
Streblus F (2): yellow, amorphous powder; 1H and 13C NMR (500 MHz, acetone-d6, see Tables 1 and 2); HRESIMS m/z 367.1169 [M + Na]+ (calcd for C19H20O6Na+, 367.1152).
Streblus G (3): yellow, amorphous powder; 1H and 13C NMR (500 MHz, acetone-d6, see Tables 1 and 2); HRESIMS m/z 325.1087 [M − H]− (calcd for C19H17O5−, 325.1081).
Streblus H (4): yellow, amorphous powder; 1H and 13C NMR (500 MHz, acetone-d6, see Tables 1 and 2); HRESIMS m/z 391.1151 [M + Na]+ (calcd for C21H20O6Na+, 391.1152).
Tyrosinase inhibitory assay
All pure compounds were dissolved in DMSO and tested at concentrations of 0.01–100 μM. Assay mixtures in 0.1 M phosphate buffer pH 6.8 were prepared immediately before use, consisting of 100 μL of tyrosinase solution (15 U mL−1) and 1900 μL of test solution. These mixtures were preincubated at 32 °C for 30 min, followed by 1000 μL of L-DOPA 1.5 mM in pH 6.8 phosphate buffer, and incubated at 32 °C for 7 min. The absorbance (A) at 475 nm was acquired on Shimadzu UV-1800 spectrophotometer. The inhibitory percentage (I%) was calculated according to the formula: I% = [(Acontrol − Asample)/Acontrol] × 100%. All experiments were performed in triplicate and data were represented as the mean of three samples with standard deviation.Optical rotation calculation
The conformational searches were performed on Spartan'18 (Wave function, Inc., Irvine, USA) using Merck molecular force field (MMFF). All conformers with Boltzmann weight > 1% were optimized using DFT method at the B3LYP/6-31G* level in the gas phase. The optical rotation calculations at 589.3 nm were carried out using the DFT-B3LYP with 6-311++G(2d,2p) basis set in IEFPCM solvation model for methanol. These calculations were performed on Gaussian 16 Rev. C.01 (Gaussian, Inc., Wallingford, USA). The calculated optical rotation values were expressed as the Boltzmann-weighted average of all output data.Molecular docking
Docking studies were performed with Molecular Operating Environment 2019 (MOE 2019.0102) suite (Chemical Computing Group ULC, Montreal, Canada). All structures were minimized up to 0.0001 gradients using the Amber12:EHT force field. The oxy-tyrosinase structure (1WX2) was prepared following our previous procedure. The molecular docking procedure was carried out with Triangle Matcher placement, Induced Fit refinement, and two scoring methods (London dG and GBVI/WSA dG). Five top results with the negative binding free energy value (S value) were selected to show up. The ligand interactions were carried out using BIOVIA Discovery Studio Visualizer 2016 (Dassault Systèmes Americas Corp., Waltham, USA).Author contributions
MTTN and NTN designed the work. THL, HXN, and TNVD directed the experiments. THL and TNVD performed tyrosinase inhibitory assay. PHD performed molecular docking and optical rotation calculations. The manuscript was written by THL, PHD, MTTN, and NTN.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research is funded by Vietnam National University Ho Chi Minh City (VNU-HCM) under grant number NCM2020-18-01.Notes and references
- B. Singh, A. Chettri, D. Adhikari and S. K. Barik, J. Bot. Res. Inst. Tex., 2012, 23, 611–614 Search PubMed.
- S. Dej-adisai, K. Parndaeng and C. Wattanapiromsakul, Trop. J. Pharm. Res., 2016, 15, 497–506 CrossRef CAS.
- G. Zhang, L. Hao, D. Zhou, W. Liu, C. Li, S. Su, X. Xu, X. Huang and J. Li, Biochem. Syst. Ecol., 2019, 87, 103962 CrossRef CAS.
- Y. Huang, X. Huang, G. Tian, W. Zhang, S. Su, X. Xu, J. Li and B. Liu, Nat. Prod. Res., 2022, 36, 1485–1493 CrossRef CAS.
- N. T. Nguyen, M. H. K. Nguyen, H. X. Nguyen, N. K. N. Bui and M. T. T. Nguyen, J. Nat. Prod., 2012, 75, 1951–1955 CrossRef CAS PubMed.
- H. X. Nguyen, N. T. Nguyen, M. H. K. Nguyen, T. H. Le, T. N. Van Do, T. M. Hung and M. T. T. Nguyen, Chem. Cent. J., 2016, 10, 2 CrossRef PubMed.
- P. H. Dang, T. T. Nguyen, T. H. Le, H. X. Nguyen, M. T. T. Nguyen and N. T. Nguyen, Nat. Prod. Res., 2018, 32, 1745–1750 CrossRef CAS PubMed.
- P. H. Dang, L. T. T. Nguyen, H. T. T. Nguyen, T. H. Le, T. N. V. Do, H. X. Nguyen, N. D. Le, M. T. T. Nguyen and N. T. Nguyen, Nat. Prod. Res., 2019, 33, 2883–2889 CrossRef CAS PubMed.
- P. H. Dang, T. H. Le, T. N. V. Do, H. X. Nguyen, M. T. T. Nguyen and N. T. Nguyen, Evidence-Based Complementary Altern. Med., 2021, 2021, 8872920 Search PubMed.
- N. T. Nguyen, P. H. Dang, H. X. Nguyen, T. N. V. Do, T. H. Le, T. Q. H. Le and M. T. T. Nguyen, Evidence-Based Complementary Altern. Med., 2021, 2021, 5561176 Search PubMed.
- N. T. Nguyen, H. X. Nguyen, T. H. Le, D. H. Nguyen, T. N. V. Do, P. H. Dang and M. T. T. Nguyen, Nat. Prod. Res., 2022, 36, 4967–4972 CrossRef CAS.
- Z. Lin, T. Zhu, Y. Fang, Q. Gu and W. Zhu, Phytochemistry, 2008, 69, 1273–1278 CrossRef CAS PubMed.
- T. Lewis-Atwell, P. A. Townsend and M. N. Grayson, Tetrahedron, 2021, 79, 131865 CrossRef CAS.
- P. J. Stephens, F. J. Devlin, J. R. Cheeseman and M. J. Frisch, J. Phys. Chem. A, 2001, 105, 5356–5371 CrossRef CAS.
- T. Aharon and M. Caricato, J. Chem. Theory Comput., 2020, 16, 4408–4415 CrossRef CAS PubMed.
- H. X. Li, J. U. Park, X. D. Su, K. T. Kim, J. S. Kang, Y. R. Kim, Y. H. Kim and S. Y. Yang, Molecules, 2018, 23, 2559 CrossRef PubMed.
- E. T. Arung, I. W. Kusuma, Y. M. Iskandar, S. Yasutake, K. Shimizu and R. Kondo, J. Wood Sci., 2005, 51, 520–525 CrossRef CAS.
- S. Khatib, O. Nerya, R. Musa, M. Shmuel, S. Tamir and J. Vaya, Bioorg. Med. Chem., 2005, 13, 433–441 CrossRef CAS PubMed.
- Z. P. Zheng, K. W. Cheng, Q. Zhu, X. C. Wang, Z. X. Lin and M. Wang, J. Agric. Food Chem., 2010, 58, 5368–5373 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ra07294g |
| This journal is © The Royal Society of Chemistry 2023 |