Open Access Article
Open Access ArticleHighly sensitive ultra-performance liquid chromatography coupled with triple quadrupole mass spectrometry detection method for levoglucosan based on Na+ enhancing its ionization efficiency†
Xiunan Yao ab,
Ninglian Wang*abc,
Xingwang Zhengd,
Quanlian Lie,
Ewerton Santosf,
Linda Maharjane,
Junjie Wangab,
Zhihui Guod,
Jiahua Guoab,
Huan Zhangab,
Kui Zhengab,
Jingquan Wue and
Yao Lie
ab,
Ninglian Wang*abc,
Xingwang Zhengd,
Quanlian Lie,
Ewerton Santosf,
Linda Maharjane,
Junjie Wangab,
Zhihui Guod,
Jiahua Guoab,
Huan Zhangab,
Kui Zhengab,
Jingquan Wue and
Yao Lie
aShaanxi Key Laboratory of Earth Surface System and Environmental Carrying Capacity, Xi'an, 710127, China. E-mail: nlwang@nwu.edu.cn
bCollege of Urban and Environmental Sciences, Northwest University, Xi'an, 710127, China
cCAS Center for Excellence in Tibetan Plateau Earth Sciences, Beijing, 100101, China
dSchool of Chemistry & Chemical Engineering, Shaanxi Normal University, Xi'an 710062, China
eState Key Laboratory of Cryospheric Science, Northwest Institute of Eco-Environment and Resources, CAS, Lanzhou 730000, China
fDepartment of Earth, Environmental and Planetary Sciences, Brown University, Providence, RI 02912, USA
First published on 1st March 2023
Abstract
The sensitive determination of levoglucosan in aqueous samples has great significance for the study of biomass burning. Although some sensitive high-performance liquid chromatography/mass spectrometry (HPLC/MS) detection methods have been developed for levoglucosan, there are still plenty of shortcomings, such as complicated sample pre-treatment procedures, large-amount sample requirements, and poor reproducibility. Herein, a new method for the determination of levoglucosan in the aqueous sample was developed using ultra-performance liquid chromatography with triple quadrupole mass spectrometry (UPLC-MS/MS). In this method, we firstly found that compared with H+, Na+ could effectively enhance the ionization efficiency of levoglucosan, even though the content of H+ is higher in the environment. Moreover, the precursor ion m/z 185.1 [M + Na]+ could be used as a quantitative ion to sensitively detect levoglucosan in aqueous samples. Only 2 μL of un-pretreated sample is required for one injection in this method, and great linearity was obtained (R2 = 0.9992) using the external standard method when the concentration of levoglucosan was 0.5–50 ng mL−1. The limit of detection (LOD) and quantification (LOQ) were 0.1 ng mL−1 (0.2 pg absolute mass injected) and 0.3 ng mL−1, respectively. Acceptable repeatability, reproducibility, and recovery were achieved. This method has the advantages of high sensitivity, good stability, good reproducibility, and simple operation, which could be widely used for the detection of different concentrations of levoglucosan in various water samples, especially for the detection of samples with low content such as ice core or snow samples.
Introduction
Biomass burning is an important source of greenhouse gases and aerosols that significantly influence the atmospheric environment and climate.1–3 To study the impact of biomass burning on climate change or ancient fire records, extensive research has been conducted on the important chemical composition of biomass burning products, monosaccharide anhydrides (MAs), including levoglucosan and its isomers mannosan and galactosan.4,5 MAs only could be generated by the thermal decomposition reaction of cellulose or hemicellulose when the temperature is higher than 300 °C, and cannot be produced in normal human activities.4,6 In addition, previous studies have shown that MAs can normally remain stable for over ten days under most atmospheric conditions during transportation.7,8 Because of these properties, MAs are often used as indicators of biomass burning. Among the three isomers of MAs, levoglucosan is an absolutely dominant constituent, accounting for more than 90% of MAs and is usually the focus of MA detection.6,9In past decades, a range of analytical methods available for levoglucosan have been developed10–15 and mass spectrometry (MS) detection has been widely used due to its excellent selectivity and high sensitivity. Among them, methods based on anion exchange and gas chromatography/mass spectrometry (GC/MS) were commonly used to detect levoglucosan in aerosol samples but not in aqueous samples. Because levoglucosan is a strong hydrophilic compound,10,16 it is very arduous to concentrate or extract low concentrations of levoglucosan from aqueous samples such as ice or snow samples with other solvents. Therefore, high-performance liquid chromatography/mass spectrometry (HPLC/MS) detection methods have been developed to detect levoglucosan in aqueous samples.10,11,17
HPLC/MS detection does not require complex sample pretreatment. By adjusting the flow phase gradient and selecting a suitable chromatographic column, the detection efficiency of HPLC/MS can be improved for complex water sample analysis. Most of the available levoglucosan HPLC/MS detection methods use [M + H]+ or [M–H]− as the precursor ions. The sensitivity, chromatographic conditions, and mass spectrometric conditions of each detection method are very different.10,11,17 The method developed by Yao et al.11 only could achieve a sensitivity of 10 ng mL−1, which is not appealing for the detection of some snow and ice samples that have a low content of levoglucosan, moreover, a relatively large volume of sample is required for injections, and single-needle analysis takes about 60 min. Even though great sensitivity, recovery, repeatability, and reproducibility were obtained, in the method developed by You et al.17 there is a need to add acetonitrile to the filtered sample at a ratio of 50/50 (v/v), which would introduce significant error caused by the operator and the pretreatment process. In the work of Andrea et al.,10 the detection method was only applicable to the concentration ranges between 4 and 30 pg mL−1, and this linear range was too small for many ice and snow samples.
Moreover, during actual detections, the results are easily affected by factors such as instrument performance, operators, and seasonal changes, resulting in poor reproducibility.
Under normal circumstances, the sample volume of an aqueous sample is generally small and the content of levoglucosan is relatively low, especially for snow and ice samples. The sensitivity of a detection method must be higher enough to meet the detection requirements. Moreover, a complex pretreatment process may have an impact on the repeatability and accuracy of the detection. Therefore, the objectives of this study were to develop a new method with characteristics of simplicity, sensitivity, and stability to detect low-concentration levoglucosan in aqueous samples.
The ionization efficiency is the key factor to determine the sensitivity of mass spectrometry methods. This study found that the ionization efficiency of levoglucosan combined with Na+ was much higher than that combined with H+. Good stability and high sensitivity could be achieved in the positive mode if [M + Na]+ was adopted as the precursor ion and quantitative ion when detecting levoglucosan. Based on this study, a new analytical method for detecting levoglucosan in aqueous samples performed on UPLC-MS/MS has been developed. This method requires lower sample volume and no pretreatment while achieving sufficient sensitivity, accuracy, and reproducibility in low-content levoglucosan detection, which is appropriate for the detection of the analytes in samples with limited volume and low content, such as the ice core or snow samples.
Experimental section
Instrument and chemicals
All experiments in this study were performed using the Agilent 1290 UPLC coupled with Agilent 6470 triple quadrupole mass spectrometry system (USA) which was equipped with a Jet stream electrospray ionization (AJS-ESI) source, all data were collected and analyzed using mass-hunter software developed by Agilent company.HPLC grade methanol, formic acid (10%), and sodium formate used in the experiment were obtained from Fisher Scientific (USA). Ultrapure water was obtained from Milli-Q IQ7000D ultrapure water system (USA). Standard levoglucosan (1,6-anhydro-β-D-glucopyranose, 99%, CAS-498-07-7) was obtained from Aladdin (Shanghai, China). Mannosan (1,6-anhydro-β-D-mannopyranose, 98%, CAS-9036-88-8) was obtained from Sigma-Aldrich (St. Louis, USA). Galactosan (1,6-anhydro-β-D-galactopyranose, 97%, CAS-39300-87-3) was obtained from TRC (Toronto, Canada).
Levoglucosan, mannosan, and galactosan standard stock solutions were prepared with ultrapure water at a concentration of 1000 μg mL−1 and stored in the dark at a temperature of 4 °C, and diluted stepwise using ultrapure water before use.
Selection of the experimental conditions
The performance of the instrument was optimized using an aqueous solution of 1 μg mL−1 levoglucosan standard substance. The mass spectrometric conditions were optimized by infusing a single standard of three isomers in both positive and negative ionization, respectively. Ultrapure water and methanol were used as mobile phases. The optimized mass spectrometric parameters were applied following parameters: gas temperature 300 °C, gas flow 5 L min−1, sheath gas temperature 250 °C, sheath gas flow 11 L min−1, capillary 3.5 kV, and nozzle voltage 500 V.The BEH Amide column (2.1 × 100 mm, 1.7 μm, Waters, USA) was chosen for the separation after comparison, and the optimized column temperature was 40 °C. The mobile phase comprised ultrapure water containing 0.1% formic acid (mobile A) and methanol (mobile B), the mobile phase ratio was A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B = 80
B = 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, and the flow rate of isocratic elution was 0.1 mL min−1. The analytical process of one injection lasted 5.00 min, while the post-running time was 2.00 min. All details of the selection of the experimental conditions can be found in the ESI.†
20, and the flow rate of isocratic elution was 0.1 mL min−1. The analytical process of one injection lasted 5.00 min, while the post-running time was 2.00 min. All details of the selection of the experimental conditions can be found in the ESI.†
Sample preparation
Ice-core samples used in this experiment were processed using a reported pre-treatment method.10 Snow and other aqueous samples were not pretreated. All sample vials were repeatedly ultrasonically cleaned with ultrapure water and methanol and were dried in a hundred-grade ultra-clean workbench before use. In order to verify whether the filtration would introduce sample contamination, both the polyether sulfone (PES) filter and online filter were tested, with results shown in the ESI (Fig. S1†). To avoid potential loss and contamination of samples caused by complex preprocessing, no pretreatment was performed on these samples. The sample injection volume was reduced to minimize matrix effects, solvent effects, and damage to the chromatographic column. Through comparative experiments, the single injection volume of each sample was determined at 2 μL, and about 300 μL samples were transferred into the cleaned sample vial with a pipette before analyzing.Results and discussion
Detection of levoglucosan and its isomers
The ion transitions of these three isomers were selected by direct infusion of a single standard solution with a concentration of 1 μg mL−1 into the ion source of the mass spectrometer (185.1/185.1 and 185.1/125 for levoglucosan, 185.1/185.1 and 185.1/122.9 for galactosan, 185.1/185.1 and 185.1/106.8 for mannosan). As shown in Fig. S2,† even at such a high concentration, the response intensities of galactosan and mannosan were much lower than that of levoglucosan. Considering the content of MAs in snow and ice samples is generally at a trace level and the content of levoglucosan is dominant among MAs in the environment,9 the response of trace levels of galactosan and mannosan can be ignored. Thus, the follow-up study was focused on the detection of levoglucosan.The investigation of levoglucosan ionization efficiency with Na+
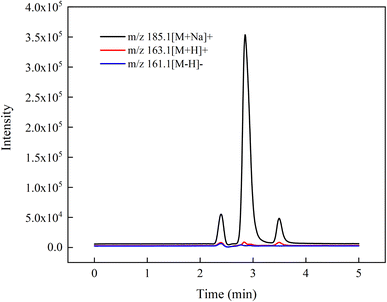
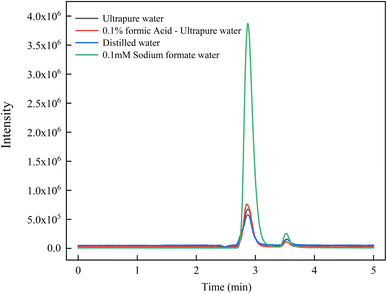
By comparing the response of the levoglucosan standard solution in different ionization modes, it was found that the response of the levoglucosan standard substance in the positive ionization mode was better than that in the negative ionization mode. In addition, the [M + H]+ response was much lower than [M + Na]+ in the positive mode (Fig. 1).In order to investigate the enhancement effect of Na+ on the ionization efficiency of levoglucosan, distilled water, ultra-pure water, 0.1% formic acid–ultrapure water, and 0.1 mM sodium formate solution were used as mobile phase A, respectively. According to the ICP-MS detection data provided by Merck Millipore, the Na+ content of ultrapure water produced by the Milli-Q IQ system is between 0.68–68 nanograms per liter, and distilled water usually has a relatively low content of Na+. Corresponding to the content of Na+ in different aqueous phases, the mass spectrometric response of levoglucosan using ultra-pure water as the aqueous phases, was higher than that using distilled water. The mass spectrometric response of levoglucosan was higher than that of other aqueous phases when 0.1 mM sodium formate solution was used as the aqueous phase (Fig. 2). These results demonstrated that Na+ could significantly enhance the ionization efficiency of levoglucosan.
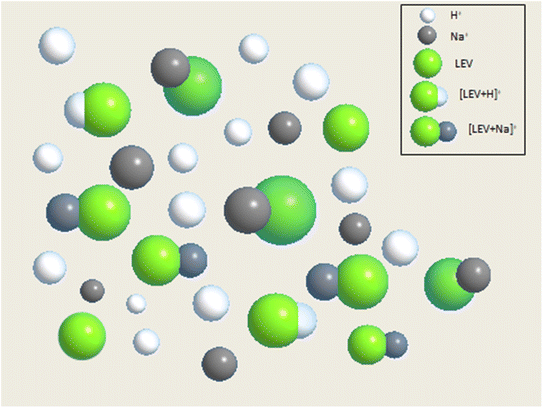
With the results achieved from the above experiment, we consider that the ionization efficiency of Na+ with levoglucosan was much higher than H+ with levoglucosan in UPLC-MS detection, even though the content of H+ was higher in the environment (Scheme 1).
While a large amount of free H+ in formic acid does not combine with the LEV to form the precursor ions, the ionization environment provided by H+ could effectively improve the ionization efficiency of Na+ and levoglucosan. The mass spectrometric response of levoglucosan, as shown in Fig. 2, using 0.1% formic acid–ultrapure water as the aqueous phases was higher than that observed using ultra-pure water and proves its influence.
Nonetheless, subsequent experiment results showed that extra Na+ could significantly inhibit the formation of production ions, which seriously affects the accuracy of qualitative analysis, even though it could enhance the response strength of quantitative ion mass spectrometry. Furthermore, sodium formate could not completely evaporate in the mobile phase, which might cause mass spectrometry contamination or capillary clogging. Since the content of levoglucosan in water samples is generally at the nanogram level, the content of Na+ in the mobile phase and environment can fully meet the demand for levoglucosan ionization. After comprehensive consideration, 0.1% formic water was finally selected as the aqueous phase, [M + Na]+ m/z 185.1/185.1 was used as the precursor ion for the selection of qualitative and quantitative ions. After repeated comparison and screening, it was found that the ion ratio between the product ions was unstable, which has a certain influence on accurate quantification. Finally, the precursor ion m/z 185.1/185.1 was used as a quantitative ion, and the production ion m/z 185.1/125.1 was used as a qualitative ion. All subsequent experiments were performed in the positive mode through multiple reactions monitoring (MRM).
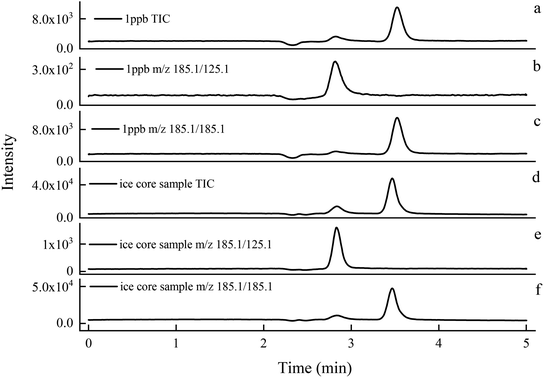
Under the optimal experimental conditions, the chromatograms of 1 ng mL−1 standard solution and ice core sample were compared. As can be seen in Fig. 3 that the retention time of levoglucosan was 2.89 min, and both the ice core sample and the standard solution had obvious responses even at very low concentrations. The chromatographic peak of qualitative ion was not interfered with by the impurity peak, and thus, the chromatographic peak of quantitative ion could be accurately located and quantified.
Analytical performances
According to ICH's recommendations,18 this method was validated on the basis of the optimized conditions in terms of repeatability and reproducibility, linearity, recovery, the limit of detection (LOD), and the limit of quantitation (LOQ).Detection limit and quantitative limit
The LOD was quantified three times using procedural blanks, and the mean value was 0.1 ng mL−1. The limit of quantification (LOQ) was estimated to be three times the LOD, which was met at 0.3 ng mL−1.Reproducibility and repeatability
The method reproducibility was evaluated by detecting a snow sample with a concentration of 3.88 ng mL−1 taken at three different times, the relative standard deviation (RSD) value was calculated to be 10.5%; the repeatability of the method was evaluated by 10 consecutive measurements using a 5 ng mL−1 levoglucosan standard solution (Tables S1 and S2†), the RSD was calculated to be 5.0%. The results indicate that this method has relatively good reproducibility and repeatability.Calibration
A part of mass spectrometry methods was calibrated by the internal standard method. However, the internal standard methods cannot eliminate the environmental, sample handling, and container contamination that may exist in actual testing. Therefore, this method used an external standard method for quantitative analysis to increase the reliability of the test results. The levoglucosan concentration of the calibration curve varied from 0.5 to 50 ng mL−1 at 6 concentration levels (0.50, 1.00, 5.00, 10.00, 20.00, and 50.00 ng mL−1). A good linearity was obtained (Fig. 4), and the coefficient of determination (R2) value was 0.9992.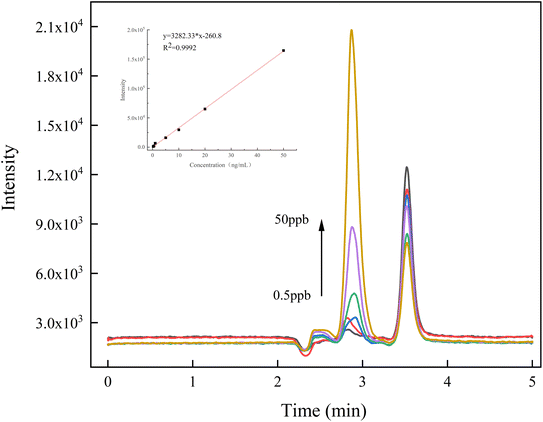 | ||
| Fig. 4 Calibration curve and chromatogram for standard solutions of levoglucosan at different concentrations. | ||
Recovery and matrix effect
In order to verify the influence of the matrix effect of different samples, three different ice core samples, snow samples, and river water samples with different concentrations of levoglucosan were selected for the recovery experiment. The ice core sample was collected from the Tanggula Mountains on the Qinghai-Tibet Plateau (longitude: 92.07, latitude: 33.10), the snow sample was collected from the Heihe fixed monitoring station in Heilongjiang Province (longitude: 100.24, latitude: 38.01), and the river sample was sampled from the tributaries of the Han River in Shaanxi Province (longitude: 107.20, latitude: 33.38).As shown in Table 1, the results of three consecutive measurements showed that the recovery rate was 92% for the ice core sample with a concentration of 1.5 ng mL−1, 97.8%, and 97.2% for the snow sample with a concentration of 7.52 ng mL−1, and 99.7% and 101.6% for river water sample with a concentration of 13.63 ng mL−1, respectively. The results demonstrate that the matrix effect has a limited influence on the detection of levoglucosan in practical samples.
| Sample | Sample concentrationa (ng mL−1) | Adding concentrationb (ng mL−1) | Final concentrationc (ng mL−1) | Recovery (%) |
|---|---|---|---|---|
| a Note: a is the original concentration of the sample (average value of three detections); b is the added amount of levoglucosan standard solution; c is the detected concentration of the sample after adding a standard solution. The ± values in the sample (a) and final (c) concentrations column means standard deviations (±SD). | ||||
| Ice-core sample | 1.50 ± 0.08 | 1 | 2.42 ± 0.09 | 92 |
| Snow sample | 7.52 ± 0.11 | 5 | 12.41 ± 0.25 | 97.8 |
| 10 | 17.24 ± 0.20 | 97.2 | ||
| River water sample | 13.63 ± 0.07 | 5 | 18.62 ± 0.10 | 99.7 |
| 10 | 23.79 ± 0.14 | 101.6 | ||
Sample analysis
To verify the reliability of this method, the results of snow samples were compared with those of similar and adjacent areas reported in other literature,19,20 with the results shown in Table 2. The comparison results show that the detection concentration of snow samples collected in similar areas between the current and studies in the literature are very close, the results of samples from the adjacent areas are basically at the same concentration level as in this study, indicating that this method is reliable.| Region | Latitude (N) | Longitude (E) | Sampling time | Number of samples | Concentration (ng mL−1) | References |
|---|---|---|---|---|---|---|
| a Note: the ± values in the concentration column means standard deviations (±SD). | ||||||
| Southern TP | 28.23 | 85.61 | 2012.5 | 4 | 26.66 ± 4.77 | You et al.19 |
| 30.11 | 90.27 | 2014.6 | 7 | 11.72 ± 15.61 | Q. Li et al.20 | |
| 28.64 | 86.11 | 2021.1 | 3 | 19.74 ± 5.96 | This method | |
| Southeastern TP | 29.20 | 96.90 | 2012.11 | 19 | 1.41 ± 1.69 | You et al.19 |
| 31.09 | 96.49 | 2021.1 | 3 | 1.85 ± 1.38 | This method | |
| Northeastern TP | 39.25 | 97.75 | 2012.9 | 3 | 2.56 ± 0.53 | You et al.19 |
| 39.14 | 97.46 | 2014.6 | 8 | 0.50 ± 0.06 | Q. Li et al.20 | |
| 39.26 | 96.33 | 2014.6 | 6 | 0.44 ± 0.32 | Q. Li et al.20 | |
| 39.26 | 97.75 | 2020.8 | 7 | 4.25 ± 1.50 | This method | |
Conclusion
In this work, we found that levoglucosan combined with Na+ could achieve higher ionization efficiency in a positive mode than that combined with H+ in mass spectrometry detection. On the basis of this phenomenon, a new, simple, efficient, and sensitive UPLC-MS/MS method has been proposed for the determination of levoglucosan in aqueous samples based on Na+-promoted ionization efficiency. Consequently, precursor ion m/z 185.1 [M + Na]+ served as the precursor ion and quantitative ion, and the product ion was used as the qualitative ion. Due to this, the influence of environmental factors on quantitative ions could be minimized and obtained better stability and reproducibility. This method has been verified to have excellent sensitivity, accuracy, reliable recovery, and good linearity in concentration ranges between 0.5 to 50 ng mL−1, which could meet the requirement of multiple types of aqueous samples with different concentrations at the same time. In addition, the excellent sensitivity makes this method only require a sample volume of 2 μL in one injection and 5 min for analysis, without any pretreatment, which is very suitable for the detection of trace concentrations of levoglucosan in limited-volume samples such as ice cores or snow.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is supported by the National Natural Science Foundation of China (Grant No. 41971088), and the Strategic Priority Research Program of the Chinese Academy of Sciences (Grant No. XDA20060201).References
- M. O. Andreae and P. Merlet, Emission of trace gases and aerosols from biomass burning, Global Biogeochem. Cycles, 2001, 15(4), 955–966 CrossRef CAS.
- O. Gustafsson, M. Krusa, Z. Zencak, R. J. Sheesley, L. Granat, E. Engstrom, P. S. Praveen, P. S. P. Rao, C. Leck and H. Rodhe, Brown Clouds over South Asia: Biomass or Fossil Fuel Combustion?, Science, 2009, 323(5913), 495–498 CrossRef CAS PubMed.
- G. R. Van der Werf, J. T. Randerson, L. Giglio, G. J. Collatz, M. Mu, P. S. Kasibhatla, D. C. Morton, R. S. DeFries, Y. Jin and T. T. van Leeuwen, Global fire emissions and the contribution of deforestation, savanna, forest, agricultural, and peat fires (1997–2009), Atmos. Chem. Phys., 2010, 10(23), 11707–11735, DOI:10.5194/acp-10-11707-2010.
- B. R. T. Simoneit, Biomass burning — a review of organic tracers for smoke from incomplete combustion, Appl. Geochem., 2002, 17, 129–162 CrossRef CAS.
- M. Legrand, J. McConnell, H. Fischer, E. W. Wolff, S. Preunkert, M. Arienzo, N. Chellman, D. Leuenberger, O. Maselli, P. Place, M. Sigl, S. Schüpbach and M. Flannigan, Boreal fire records in Northern Hemisphere ice cores: a review, Clim. Past, 2016, 12(10), 2033–2059, DOI:10.5194/cp-12-2033-2016.
- B. R. T. Simoneit, J. J. Schauer, C. G. Nolte, D. R. Oros, V. O. Elias, M. P. Fraser, W. F. Rogge and G. R. Cass, Levoglucosan, a tracer for cellulose in biomass burning and atmospheric particles, Atmos. Environ., 1999, 33, 173–182 CrossRef CAS.
- C. J. Hennigan, A. P. Sullivan, J. L. Collett and A. L. Robinson, Levoglucosan stability in biomass burning particles exposed to hydroxyl radicals, Geophys. Res. Lett., 2010, 37(9) DOI:10.1029/2010GL043088.
- M. P. Fraser and K. Lakshmanan, Using levoglucosan as a molecular marker for the long-range transport of biomass combustion aerosols, Environ. Sci. Technol., 2000, 34(21), 4560–4564 CrossRef CAS.
- O. E. Vladimir, R. T. S. Bernd, C. C. RENATO and T. Bruno, Evaluating levoglucosan as an indicator of biomass burning in Carajás, Amazônia: A comparison to the charcoal record, Geochim. Cosmochim. Acta, 2001, 65, 267–272 CrossRef.
- A. Gambaro, R. Zangrando, P. Gabrielli, C. Barbante and P. Cescon, Direct determination of levoglucosan at the picogram per milliliter level in Antarctic ice by high-performance liquid chromatography/electrospray ionization triple quadrupole mass spectrometry, Anal. Chem., 2008, 80(5), 1649–1655 CrossRef CAS PubMed.
- P. Yao, V. F. Schwab, V.-N. Roth, B. Xu, T. Yao and G. Gleixner, Levoglucosan concentrations in ice-core samples from the Tibetan Plateau determined by reverse-phase high-performance liquid chromatography–mass spectrometry, J. Glaciol., 2013, 59(216), 599–612, DOI:10.3189/2013JoG12J157.
- C. You and C. Xu, Review of levoglucosan in glacier snow and ice studies: Recent progress and future perspectives, Sci. Total Environ., 2018, 616–617, 1533–1539, DOI:10.1016/j.scitotenv.2017.10.160.
- G. Schkolnik and Y. Rudich, Detection and quantification of levoglucosan in atmospheric aerosols: a review, Anal. Bioanal. Chem., 2006, 385(1), 26–33, DOI:10.1007/s00216-005-0168-5.
- G. Engling, C. M. Carrico, S. M. Kreidenweis, J. L. Collett Jr, D. E. Day, W. C. Malm, E. Lincoln, W. Min Hao, Y. Iinuma and H. Herrmann, Determination of levoglucosan in biomass combustion aerosol by high-performance anion-exchange chromatography with pulsed amperometric detection, Atmos. Environ., 2006, 40, 299–311, DOI:10.1016/j.atmosenv.2005.12.069.
- C. You, T. Yao, S. Gao, P. Gong and H. Zhao, Simultaneous Determination of Levoglucosan, Mannosan and Galactosan at Trace Levels in Snow Samples by GC/MS, Chromatographia, 2014, 77(13–14), 969–974, DOI:10.1007/s10337-014-2702-0.
- A. A. Ghfar, S. M. Wabaidur, A. Y. Ahmed, Z. A. Alothman, M. R. Khan and N. H. Al-Shaalan, Simultaneous determination of monosaccharides and oligosaccharides in dates using liquid chromatography-electrospray ionization mass spectrometry, Food Chem., 2015, 176, 487–492, DOI:10.1016/j.foodchem.2014.12.035.
- C. You, L. Song, B. Xu and S. Gao, Method for determination of levoglucosan in snow and ice at trace concentration levels using ultra-performance liquid chromatography coupled with triple quadrupole mass spectrometry, Talanta, 2016, 148, 534–538, DOI:10.1016/j.talanta.2015.11.030.
- ICH, Guideline Q2 (R1)—Validation of Analytical Procedures: Text and Methodology, ICH Secretariat, c/o IFPMA, 2005 Search PubMed.
- C. You, C. Xu, B. Xu, H. Zhao and L. Song, Levoglucosan evidence for biomass burning records over Tibetan glaciers, Environ. Pollut., 2016, 216, 173–181, DOI:10.1002/2016JD024904.
- Q. Li, N. Wang, C. Barbante, S. Kang, P. Yao, X. Wan, E. Barbaro, M. del C. V. Hidalgo, A. Gambaro, C. Li, H. Niu, Z. Dong and X. Wu, Levels and spatial distributions of levoglucosan and dissolved organic carbon in snowpits over the Tibetan Plateau glaciers, Sci. Total Environ., 2018, 612, 1340–1347, DOI:10.1016/j.scitotenv.2017.08.267.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ra07419b |
| This journal is © The Royal Society of Chemistry 2023 |