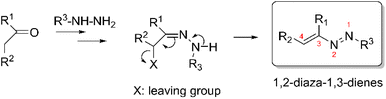Open Access Article
Open Access ArticleNovel synthesis of 1,2-diaza-1,3-dienes with potential biological activity from cinnamic acids and diazonium salts of anilines†
Veronica Vida*a,
Martina Minisinib,
Mario Mardirossianc,
Claudio Brancolinib,
Marco Scocchic,
Cristina Forzatoa and
Federico Berti*a
aDipartimento di Scienze Chimiche e Farmaceutiche, Università di Trieste, Via Giorgieri 1, 34127, Trieste, Italy. E-mail: veronica.vida@phd.units.it; fberti@units.it
bDipartimento di Area Medica, Università di Udine, Piazzale Kolbe 4, 33100, Udine, Italy
cDipartimento di Scienze della Vita, Università di Trieste, Via Giorgieri 8, 34127, Trieste, Italy
First published on 21st December 2022
Abstract
Cinnamic acids are an important class of phenolic compounds, which have many beneficial effects on human health but are also interesting synthetic intermediates thanks to the presence of several reactive sites. While studying the reactivity of cinnamic acids with diazonium salts from aromatic amines, an unexpected reactivity has been discovered, leading to the formation of 1,2-diaza-1,3-dienes instead of traditional diazo-coupling products. The new compounds have been fully characterized by mono and bidimensional NMR spectroscopy and mass spectrometry. Preliminary studies on the biological activity of the compounds have been carried out testing both their antibacterial and antitumor activity, leading to promising results.
1 Introduction
According to the literature, 1,2-diaza-1,3-dienes (DDs) can be considered as hydrazine derivatives of carbonyl compounds, since they are usually prepared by 1,4-elimination of a good leaving group from a hydrazone moiety (Scheme 1).1 The leaving group can be either present in the starting carbonyl compound or it can be introduced on the hydrazone intermediate,1,2 but other methods of synthesis can be found in the literature, although they are related to specific reactions and have limited applications.2 As an example, the treatment of α,β-epoxyketones with hydrazines, initially producing the corresponding α,β-epoxyhydrazones, then gives α-hydroxyazoalkenes by opening of the epoxy ring.2The chemical properties of DDs are related to the conjugated heterodiene system and to the type of substituents present.3 The characteristic of this heterodiene system is represented by the electrophilic property of C-4, promoted by the electron-withdrawing effect of the azo group, leading the 1,2-diaza-1,3-dienes to be good Michael acceptors through a regioselective nucleophilic attack at the terminal carbon atom in the 4-position.1 Depending on the nucleophile used, which can be either a carbon atom or a hetero atom such as oxygen, nitrogen, sulfur, selenium and phosphorus, highly functionalized hydrazones can be formed, which represent the starting point for many reactions involved in the formation of heterocycle systems.1 The presence of substituents on both carbon and nitrogen atoms is another factor that influences both the reactivity and stability of the system.3 In fact, the presence of electron withdrawing groups on one or both of the terminal carbon and nitrogen atoms, improves the stability and enhances the electrophilic character of the diazadienes, favoring the regioselective nucleophilic attack in 4-position but also making them ideal substrates for the “inverse-electron demand” Diels–Alder reaction.1,3 In general, aryl groups favor the stability but not the reactivity, due to the absence of functionalities which are useful for further reactions, whereas alkyl groups or terminal C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond do not favor stability, leading to collateral reactions.3
C bond do not favor stability, leading to collateral reactions.3
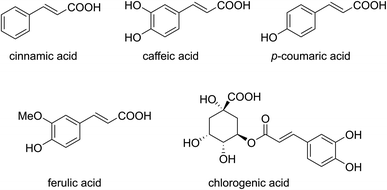
Cinnamic acids are a class of compounds with a phenylpropanoid skeleton (C6–C3), which are largely present in the plant kingdom as free compounds or in a conjugated form.4 Coffee beans, cocoa, tea, apples, brassica vegetables, grapes, citrus, pears are particularly rich of cinnamic acids, which are also present as ester conjugates with quinic acid, to give the class of compounds known as chlorogenic acids,4–9 or esters with other acids, sugars or lipids, or forming amides with amino acids (Fig. 1).4,10 They are also precursors of many natural products such as coumarins, lignans, isoflavonoids, flavonoids, stilbenes, aurones, anthocyanins, spermidines, and tannins,4 but they can also be synthesized artificially through the Perkin reaction or a Knoevenagel condensation reaction from aromatic aldehydes.11,12 In the last years, cinnamic acids received a lot of interest due to their biological activities, which include anticancer, antioxidant, antimicrobial, as well as applications in diabetes, tuberculosis, malaria and cardiovascular diseases.4,13 They are also very interesting from the synthetic point of view thanks to the presence of multiple reactive sites, such as the polarized alkenyl moiety and the carboxylic group, being applied in total synthesis of some natural products.13 Several reactions for these compounds have been reported in the literature, such as electrophilic addition, Michael addition and reactivity of the carboxylic acid moiety, but the most important one is probably the decarboxylative formation of C–C and C–heteroatom bonds.13
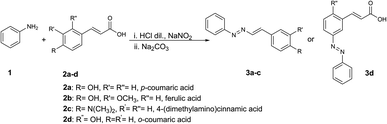
Due to our interest on different aspects of the chemistry of cinnamic acids, in the attempt of studying the reactivity of diazonium salts from aromatic amines with cinnamic acids, we have observed the unexpected formation of DDs (Scheme 2), thus unveiling a new reaction that could be exploited to develop novel synthetic pathways to these important compounds. The reaction described in the present work for formation of DDs is very advantageous since it takes place under mild reaction conditions, in an aqueous environment, without the need for any catalyst, and with very simple work-up procedures. We report here several examples of reactivity between diazonium salts and different substituted cinnamic acids and a reaction mechanism is proposed. Moreover, as the synthesized DDs have, in our opinion, potential biological activities due to the presence of the activated system which makes them reactive towards nucleophilic groups inside biomolecules, we have also carried out preliminary tests on the activities of the novel compounds on cancer cells and on bacteria.
2 Results and discussion
2.1 Structural elucidation
As a first attempt to study the reactivity in a diazo-coupling reaction of differently substituted cinnamic acids with diazonium salts from aromatic amines, the reaction between the diazonium salt of aniline 1, obtained with sodium nitrite and diluted hydrochloric acid, with p-coumaric acid 2a in sodium carbonate, in a stoichiometry of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
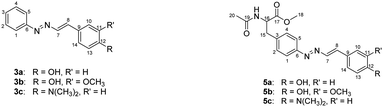
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 respectively, was performed (Scheme 2). Immediately after addition of p-coumaric acid 2a to the diazonium salt solution, the formation of a red precipitate has been observed, which has been isolated and characterized without the need of further purification. Spectroscopic analysis of the compound led to the assignment of the structure of 1,2-diaza-1,3-diene 3a which was unexpectedly formed. In fact, the presence of the electron donating hydroxyl group in para position, with respect to the conjugated double bond of p-coumaric acid, was expected to lead the reaction towards a traditional diazo-coupling reaction with the diazonium salt of aniline.
2 respectively, was performed (Scheme 2). Immediately after addition of p-coumaric acid 2a to the diazonium salt solution, the formation of a red precipitate has been observed, which has been isolated and characterized without the need of further purification. Spectroscopic analysis of the compound led to the assignment of the structure of 1,2-diaza-1,3-diene 3a which was unexpectedly formed. In fact, the presence of the electron donating hydroxyl group in para position, with respect to the conjugated double bond of p-coumaric acid, was expected to lead the reaction towards a traditional diazo-coupling reaction with the diazonium salt of aniline.
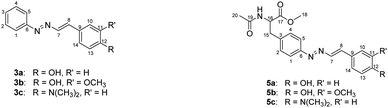
Integration of the 1H-NMR signals (DMSO-d6) of the obtained product resulted in the assignment of 11 protons instead of the 10 expected in case of a diazo-coupling reaction, which is compatible with the formation of a diazadiene as 3a. The assignments of 1H-NMR and 13C-NMR signals are reported in Tables 1 and 2. For compound 3a, the two doublets at 7.64 ppm and 6.85 ppm (H-10/14 and H-11/13 in Table 1), each integrating for 2 protons with a coupling constant of 8.6 Hz, had the same pattern of the corresponding protons in the starting reagent p-coumaric acid 2a, to demonstrate that the formation of a traditional diazo coupling product was not possible, since in that case there should be the loss of one proton replaced by the nitrogen atom of the diazonium salt, and this would completely change the multiplicity and integration of the signals. A completely different chemical shift was observed for the vinyl protons H-7 and H-8 (Table 1), one at 8.02 ppm and the other under the multiplet at 7.73 ppm, with a coupling constant of 13.7 Hz, whereas the coupling constant between these two protons in the starting cinnamic acid is of 16.0 Hz, and the two protons have chemical shifts of 7.48 ppm and 6.28 ppm. Diazadiene 3a was formed as the only product with a sufficient purity so no further purification was needed.
| Position | 3a | 3b | 3c | 5a | 5b | 5c |
|---|---|---|---|---|---|---|
| 1 and 5 | 7.73, m | 7.74, m | 7.70, m | 7.64, m | 7.66, m | 7.63, m |
| 2 and 4 | 7.53, m | 7.53, m | 7.51, m | 7.36, d (8.3) | 7.37, d (8.4) | 7.34, d (8.4) |
| 3 | 7.47, m | 7.47, m | 7.43, m | — | — | — |
| 7 | 8.02, d (13.7) | 8.11, d (13.6) | 8.02, d (13.6) | 8.00, d (13.7) | 8.09, d (13.7) | 8.01, d (13.6) |
| 8 | 7.73, m | 7.70, d (13.6) | 7.67, d (13.6) | 7.64, m | 7.66, m | 7.63, m |
| 10 | 7.64, d (8.6) | 7.40, d (2.0) | 7.61, d (8.9) | 7.64, m | 7.39, d (2.0) | 7.63, m |
| 11 | 6.85, d (8.6) | — | 6.76, d (8.9) | 6.84, d (8.7) | 6.76, d (9.0) | |
| 13 | 6.85, d (8.6) | 6.85, d (8.1) | 6.76, d (8.9) | 6.84, d (8.7) | 6.84, d (8.2) | 6.76, d (9.0) |
| 14 | 7.64, d (8.6) | 7.22, dd (8.1, 2.0) | 7.61, d (8.9) | 7.64, m | 7.20, dd (8.2, 2.0) | 7.63, m |
| OCH3 | — | 3.85, s | — | — | 3.84, s | — |
| N(CH3)2 | — | — | 3.00, s | — | — | 3.00, s |
| 15 | — | — | — | 3.08 and 2.95, dd (13.8, 5.6, 9.3) | 3.09 and 2.95, dd (13.7, 5.6, 9.3) | 3.07 and 2.94, dd (13.8, 5.6, 9.3) |
| 16 | — | — | — | 4.51, ddd (9.3, 7.8, 5.6) | 4.51, ddd (9.3, 7.8, 5.6) | 4.50, ddd (9.3, 7.8, 5.6) |
| 18 | — | — | — | 3.61, s | 3.61, s | 3.61, s |
| 20 | — | — | — | 1.80, s | 1.80, s | 1.80, s |
| NH | — | — | — | 8.38, d (7.8) | 8.38, d (7.8) | 8.37, d (7.8) |
| Position | Compound | |||||
|---|---|---|---|---|---|---|
| 3a | 3b | 3c | 5a | 5b | 5c | |
| 1 and 5 | 122.02 | 121.99 | 121.82 | 121.91 | 121.92 | 121.80 |
| 2 and 4 | 129.39 | 129.35 | 129.28 | 130.04 | 130.07 | 130.05 |
| 3 | 130.49 | 130.43 | 129.90 | 140.05 | 140.05 | 139.50 |
| 6 | 152.46 | 152.48 | 152.64 | 151.23 | 151.26 | 151.54 or 151.48 |
| 7 | 143.94 | 144.29 | 142.59 | 143.94 | 144.30 | 142.66 |
| 8 | 143.49 | 143.71 | 144.01 | 143.06 | 143.33 | 143.71 |
| 9 | 125.63 | 126.19 | 122.00 | 125.64 | 126.21 | 122.09 |
| 10 | 130.31 | 111.09 | 129.97 | 130.22 | 111.04 | 130.00 |
| 11 | 116.05 | 148.05 | 111.98 | 116.01 | 148.07 | 112.07 |
| 12 | 159.70 | 149.18 | 151.49 | 159.63 | 149.17 | 151.54 or 151.48 |
| 13 | 116.05 | 115.79 | 111.98 | 116.01 | 115.80 | 112.07 |
| 14 | 130.31 | 123.21 | 129.97 | 130.22 | 123.19 | 130.00 |
| OCH3 | — | 55.72 | — | — | 55.73 | — |
| N(CH3)2 | — | — | 39.5 | — | — | 39.52 |
| 15 | — | — | — | 36.53 | 36.55 | 36.58 |
| 16 | — | — | — | 53.37 | 53.39 | 53.49 |
| 17 | — | — | — | 172.05 | 172.07 | 172.14 |
| 18 | — | — | — | 51.89 | 51.91 | 51.96 |
| 19 | — | — | — | 169.37 | 169.40 | 169.55 |
| 20 | — | — | — | 22.24 | 22.26 | 22.30 |
13C-NMR (Table 2) and bidimensional HSQC and HMBC spectra (see ESI†) were registered to confirm the formation of the DD and fully characterize the new product. In particular, looking at the 13C-NMR spectrum, it is important to highlight the absence of the carboxylic group of the starting reagent p-coumaric acid, and the presence of 10 carbon signals, three of which quaternary. All these data are in line with the hypothesized structure of a DD considering that in case of a diazo-coupling reaction, due to the loss of one proton on the aromatic ring portion of the cinnamic acid, three more carbons should be observed, and there would be two more quaternary carbons. In this way it was possible to confirm that we were in front of a new reaction, never reported in literature before, leading to the formation of DD.
Structure of compound 3a was also confirmed by accurate mass analysis, showing a peak at m/z 225.1023 [M + H]+ (theorical 225.1022) and 247.0843 [M + Na]+ (theorical 247.0842) (see ESI†).
A second reaction was performed using ferulic acid 2b, which differs from 2a by the presence of a methoxy group in ortho position with respect to the OH. In this case the reaction has been tried using two different stoichiometric ratios, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 as in the case of p-coumaric acid, and 1
2 as in the case of p-coumaric acid, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. In all the tested conditions, the formation of the corresponding DD 3b has been observed, even if the presence of variable amounts of byproducts has been observed in some cases depending on the experimental conditions, which has made us think that the presence of one further electron donating group might increase the reactivity of the product in side reactions. Different work-up strategies have been evaluated, since it has been observed that in some cases precipitation of the final product did not occur, and extraction with organic solvent was necessary to recover all the product. The best condition resulted to be the 1
1. In all the tested conditions, the formation of the corresponding DD 3b has been observed, even if the presence of variable amounts of byproducts has been observed in some cases depending on the experimental conditions, which has made us think that the presence of one further electron donating group might increase the reactivity of the product in side reactions. Different work-up strategies have been evaluated, since it has been observed that in some cases precipitation of the final product did not occur, and extraction with organic solvent was necessary to recover all the product. The best condition resulted to be the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 stoichiometry, extracting the aqueous solution with ethyl acetate, leading to a yield of 98% of the diazadiene 3b, that resulted pure enough to be used without further purification. Also in this case, the formation of diazadiene 3b has been proved through 1H-NMR and 13C-NMR analysis which confirmed retention of the pattern of the original ferulic acid, without the loss of any aromatic proton of the starting cinnamic acid part. Structural assignment was also confirmed by mass spectrometry analysis since two peaks at 255.1120 and 277.0947 m/z were observed corresponding to [M + H]+ and [M + Na]+ ions respectively.
2 stoichiometry, extracting the aqueous solution with ethyl acetate, leading to a yield of 98% of the diazadiene 3b, that resulted pure enough to be used without further purification. Also in this case, the formation of diazadiene 3b has been proved through 1H-NMR and 13C-NMR analysis which confirmed retention of the pattern of the original ferulic acid, without the loss of any aromatic proton of the starting cinnamic acid part. Structural assignment was also confirmed by mass spectrometry analysis since two peaks at 255.1120 and 277.0947 m/z were observed corresponding to [M + H]+ and [M + Na]+ ions respectively.
The reaction between aniline 1 with 4-(dimethylamino)cinnamic acid 2c using a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry confirmed this type of reactivity giving compound 3c as the only product with an 81% yield. 1H-NMR, 13C-NMR spectroscopy and mass spectrometry analyses led to the assignment of the diazadiene structure to compound 3c.
1 stoichiometry confirmed this type of reactivity giving compound 3c as the only product with an 81% yield. 1H-NMR, 13C-NMR spectroscopy and mass spectrometry analyses led to the assignment of the diazadiene structure to compound 3c.
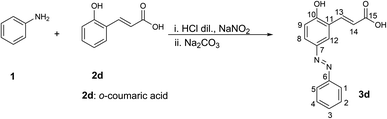
Changing the hydroxycinnamic acid from p-coumaric acid 2a to o-coumaric acid 2d, which has the same electronic effect, the reaction moved towards the formation of the diazo-coupling product 3d. In this case, no precipitate formed, and the reaction mixture was extracted with ethyl acetate, thus obtaining an orange solid, which did not necessitate any further purification. From 1H-NMR analysis, it was possible to identify 10 protons from integration, instead of the 11 in case of formation of the diazadiene. The aromatic part of the cinnamic acid portion presents three signals, each integrating for 1H and the coupling constants observed are in accordance with a substitution at the para position with respect to the OH group of the o-coumaric acid (Scheme 3). The two protons of the double bond do not show relevant changes in the chemical shifts with respect to the starting reagent (as it happens in case of diazadienes) and the coupling constant remains of 16.1 Hz. The formation of diazo-coupling product is also confirmed by the presence of the carboxylic acid carbon at 167.85 ppm in the 13C-NMR spectrum. 2D-NMR as well as mass spectrometry confirm the assigned structure to 3d. From mass spectrometry analysis is possible to find the signal at 269.0 m/z, corresponding to the adduct [M + H]+, and the signal at 291.0 m/z, corresponding to the adduct [M + Na]+. In this reaction, diazo-coupling reaction was likely possible due to a less steric hindrance of the aromatic ring in the activated position.
2.2 Influence of the aromatic amine on reactivity
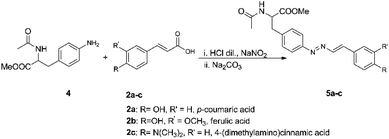
The cinnamic acids 2a–c were further used in the reaction with acetyl-p-amino-phenylalanine methyl ester 4 in order to obtain new diazadiene products (Scheme 4). We have chosen to test the reaction on this amino acid in the view of obtaining biomolecule-related DDs. Acetyl-p-amino-phenylalanine methyl ester has been used for the reaction thus having both the amino and the carboxy terminals of the amino acid protected. In all the three cases it was possible to obtain the corresponding diazadiene products 5a–c in good yields, ranging between 71% and 96%, in a pure form and without the need for any purification. The structures have been confirmed through 1D and 2D-NMR spectra, as well as accurate mass spectrometry.2.3 The mechanism
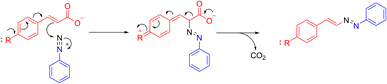
The effect of an electron-donating group at the para position of the cinnamic acids seems to suggest a bielectronic mechanism for the reaction. The hypothesis is that such group promotes the nucleophilic attack of the α vinyl carbon towards the electrophilic diazonium salt. Then, decarboxylation of the intermediate leads to the reestablishing of aromaticity, thus obtaining the completely conjugated DD. The hypothesized mechanism is reported in Scheme 5.This option is very different from that of other reactions involving diazonium salts and unsaturated compounds. One previously documented reaction, involving also cinnamic acid, is the Meerwein arylation reaction, originally discovered in 1939 as the copper-catalyzed addition of an aryl-diazonium salt with unsaturated compound, even if an intramolecular version of the reaction, the Pschorr reaction, was firstly reported in 1896.14 The proposed mechanism is radical, and the reaction takes place better when the olephinic double bond is activated by an electron-withdrawing group, such as carbonyl, cyano or aryl.14,15 The result is the addition of the aryl group from the diazonium salt to the carbon atom in β position with respect to the activating group.15 Anyway, this reaction gave low yields, high catalyst loading and formation of side products.14 Nevertheless, other options alternative to reduction of diazonium salts by copper(I) are now available, leading to aryl radicals, thanks to photoredox catalysis, for example, using of [Ru(bpy)3]2+.14 One particular application of the Meerwein reaction involves cinnamic acid: in this case, the reaction leads to the loss of a carbon dioxide molecule and to the formation of the corresponding stilbene.14,15
In the present work we observed a different reactivity with respect to the Meerwein arylation reaction, although the reaction conditions are not the same, and formation of DDs, which has never been reported in the literature, was the only reaction to take place. We could only find a somehow-correlate reaction reported by Xu and colleagues, in which 4,4-dialkylthio-1,2-diaza-1,3-butadienes are synthesized based on the azo-coupling decarboxylation of α-carboxy ketene dithioacetals with aryldiazonium salts.16
2.4 Biological activity
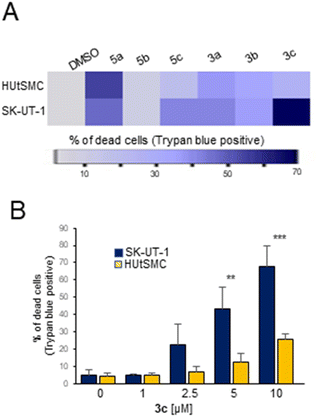
In a first screening, cells were incubated with the different compounds at a concentration of 10 μM for 48 hours. 3c was the compound that caused the highest percentage of cell death in SK-UT-1 cells compared with normal HUtSMC (Fig. 2A). The number of Trypan blue-positive cells was approximately 70% in cancer cells and 25% in normal cells. The other compounds were either ineffective (5b) or showed no differential effect between normal and cancer cells (3a, 3b, 5a). 5c, similar to 3c, elicited a differential cell death response in cancer and normal cells, but was less effective than 3c. Therefore, we investigated the death-promoting effect of 3c in more detail. Dose-dependent studies confirmed the specific cell-killing effect of 3c against LMS cells (Fig. 2B).
| MIC (μM) | ||
|---|---|---|
| Compound | S. aureus (ATCC 25923) | E. coli (ATCC 25922) |
| 3a | 8 | >64 |
| 3b | 8 | >64 |
| 3c | >64 | >64 |
| 5a | 64 | >64 |
| 5b | 32 | >64 |
| 5c | >64 | >64 |
The compounds 3a, 3b, 5a and 5b inhibited the growth of S. aureus cells when given at 8 μM (3a, 3b), 32 μM (5b) or at 64 μM (5a), whereas E. coli cells were not susceptible to any of the compounds, at least up to 64 μM. It is worth noting that 3c, which caused the highest percentage of cell death in leiomyosarcoma SK-UT-1 cells, did not affect the viability of either bacterial strain, indicating selective cytotoxicity. In contrast, 3b, which showed anti-Staphylococcus activity, did not affect heavily human cells (regardless their tumoral origin or not) and thus may have selective antibacterial effect. On the other hand, the high insensitivity of E. coli to these compounds is not surprising since Gram-negative bacteria, that possess a second membrane barrier, are generally more resistant to small hydrophobic molecules19 such as DDs.
3 Materials and methods
3.1 Chemicals and materials
Acetyl-p-amino-phenylalanine methyl ester was purchased from Bachem, aniline was purchased from BDH.All the other reagents were purchased from Sigma-Aldrich (Milano, Italy).
3.2 Instrumentation
NMR spectra were recorded on Varian 400 or 500 MHz spectrometers (Palo Alto, CA, USA) using DMSO-d6 as the solvent if not otherwise stated. Coupling constants are given in Hertz.Accurate masses were determined on a micrOTOF-Q (Bruker, Billerica, MA, USA). Measurements were registered in positive mode.
Infrared spectra were registered on an ATR-IR IRAffinity-1S Fourier transform infrared spectrophotometer (Shimadzu).
3.3 General procedure for the synthesis of compounds 3a–c and 5a–c
To a solution of aniline (1 mmol, 1 eq.) in 32 mL of 0.1 M hydrochloric acid, cooled in an ice bath, a solution of sodium nitrite (1.2 mmol, 1.2 eq.) in 6.4 mL of distilled water was added dropwise. The reaction mixture was stirred in an ice bath for 15 minutes to enable the formation of the diazonium salt. Subsequently, a cooled solution of cinnamic acid (1 or more eq.) in 32 mL of 0.1 M sodium carbonate was added dropwise to the diazonium salt solution. The reaction mixture suddenly turned colored, and it was left to react 30 minutes at 0 °C and for 30 more minutes at room temperature.Ratio of cinnamic acid/aniline and workup for each product obtained will be described in the specific paragraph.
1H NMR (400 MHz, DMSO-d6) δ 8.02 (1H, d, J = 13.7 Hz, H-7), 7.73 (3H, m, H-1, H-5, H-8), 7.64 (2H, d, J = 8.6 Hz, H-10, H-14), 7.53 (2H, m, H-2, H-4), 7.47 (1H, m, H-3), 6.85 (2H, d, J = 8.6 Hz, H-11, H-13). 13C NMR (101 MHz, DMSO-d6) δ 159.70 (C-12), 152.46 (C-6), 143.94 (C-7), 143.49 (C-8), 130.49 (C-3), 130.31 (C-10, C-14), 129.39 (C-2, C-4), 125.63 (C-9), 122.02 (C-1, C-5), 116.05 (C-11, C-13). MS (ESI+) 225.1023 [M + H]+ (calculated 225.1022); 247.0843 [M + Na]+ (calculated 247.0842). IR (νmax/cm−1) 1602.85 (stretching N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N).
N).
1H NMR (400 MHz, DMSO-d6) δ 8.11 (1H, d, J = 13.6 Hz, H-7), 7.74 (2H, m, H-1, H-5), 7.70 (1H, d, J = 13.6 Hz, H-8), 7.53 (2H, m, H-2, H-4), 7.48 (1H, m, H-3), 7.40 (1H, d, J = 2.0 Hz, H-10), 7.22 (1H, dd, J = 8.1, 2.0 Hz, H-14), 6.85 (1H, d, J = 8.1 Hz, H-13), 3.85 (3H, s, OCH3). 13C NMR (126 MHz, DMSO-d6) δ 152.48 (C-6), 149.18 (C-12), 148.05 (C-11), 144.29 (C-7), 143.71 (C-8), 130.43 (C-3), 129.35 (C-2, C-4), 126.19 (C-9), 123.21 (C-14), 121.99 (C-1, C-5), 115.79 (C-13), 111.09 (C-10), 55.72 (OCH3). MS (ESI+): 255.1120 [M + H]+ (calculated 255.1128), 277.0949 [M + Na]+ (calculated 277.0948). IR (νmax/cm−1) 1587.42 (stretching N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N).
N).
1H NMR (400 MHz, DMSO-d6) δ 8.02 (1H, d, J = 13.6 Hz, H-7), 7.70 (2H, m, H-1, H-5), 7.67 (1H, d, J = 13.6 Hz, H-8) 7.61 (2H, d, J = 8.9 Hz, H-10, H-14), 7.51 (2H, m, H-2, H-4), 7.43 (1H, m, H-3), 6.76 (2H, d, J = 8.9 Hz, H-11, H-13), 3.00 (6H, s, N(CH3)2). 13C NMR (101 MHz, DMSO-d6) δ 152.64 (C-6), 151.49 (C-12), 144.01 (C-8), 142.59 (C-7), 129.97 (C-10, C-14), 129.90 (C-3), 129.28 (C-2, C-4), 122.00 (C-9), 121.82 (C-1, C-5), 111.98 (C-11, C-13), 39.5 (N(CH3)2). MS (ESI+): 252.1492 [M + H]+ (calculated 252.1495), 274.1310 [M + Na]+ (calculated 274.1315). IR (νmax/cm−1) 1600.92 (stretching N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N).
N).
1H NMR (400 MHz, DMSO-d6) δ 8.38 (1H, d, J = 7.8 Hz, NH), 8.00 (1H, d, J = 13.7 Hz, H-7), 7.64 (5H, m, H-1, H-5, H-8, H-10, H-14), 7.36 (2H, d, J = 8.3 Hz, H-2, H-4), 6.84 (2H, d, J = 8.7 Hz, H-11, H-13), 4.51 (1H, ddd, J = 9.3, 7.8, 5.6 Hz, H-16), 3.61 (3H, s, H-18), 3.08 (1H, dd, J = 13.8, 5.6 Hz, H-15), 2.95 (1H, dd, J = 13.8, 9.3 Hz, H-15), 1.80 (3H, s, H-20). 13C NMR (101 MHz, DMSO-d6) δ 172.05 (C-17), 169.37 (C-19), 159.63 (C-12), 151.23 (C-6), 143.94 (C-7), 143.06 (C-8), 140.05 (C-3), 130.22 (C-10, C-14), 130.04 (C-2, C-4), 125.64 (C-9), 121.91 (C-1, C-5), 116.01 (C-11, C-13), 53.37 (C-16), 51.89 (C-18), 36.53 (C-15), 22.24 (C-20). MS (ESI+): 368.1595 [M + H]+ (calculated 368.1605), 390.1417 [M + Na]+ (calculated 390.1424). IR (νmax/cm−1) 1602.85 (stretching N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N).
N).
1H NMR (400 MHz, DMSO-d6) δ 8.38 (1H, d, J = 7.8 Hz, NH), 8.09 (1H, d, J = 13.7 Hz, H-7), 7.66 (3H, m, H-1, H-5, H-8), 7.37 (2H, d, J = 8.4 Hz, H-2, H-4), 7.39 (1H, d, J = 2 Hz, H-10), 7.20 (1H, dd, J = 8.2, 2.0 Hz, H-14), 6.84 (1H, d, J = 8.2 Hz, H-13), 4.51 (1H, ddd, J = 9.3, 7.8, 5.6, H-16), 3.84 (3H, s, OCH3), 3.61 (3H, s, H-18), 3.09 (1H, dd, J = 13.7, 5.6 Hz, H-15), 2.95 (1H, dd, J = 13.7, 9.3 Hz, H-15), 1.80 (3H, s, H-20). 13C NMR (101 MHz, DMSO-d6) δ 172.07 (C-17), 169.40 (C-19), 151.26 (C-6), 149.17 (C-12), 148.07 (C-11), 144.30 (C-7), 143.33 (C-8), 140.05 (C-3), 130.07 (C-2, C-4), 126.21 (C-9), 123.19 (C-14), 121.92 (C-1, C-5), 115.80 (C-13), 111.04 (C-10), 55.73 (OCH3), 53.39 (C-16), 51.91 (C-18), 36.55 (C-15), 22.26 (C-20). MS (ESI+): 398.1704 [M + H]+ (calculated 398.1710), 420.1528 [M + Na]+ (calculated 420.1530). IR (νmax/cm−1) 1598.99 (stretching N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N).
N).
1H NMR (400 MHz, DMSO-d6) δ 8.37 (1H, d, J = 7.8 Hz, NH), 8.01 (1H, d, J = 13.6 Hz, H-7), 7.63 (5H, m, H-1, H-5, H-8, H-10, H-14), 7.34 (2H, d, J = 8.4 Hz, H-2, H-4), 6.76 (2H, d, J = 9.0 Hz, H-11, H-13), 4.50 (1H, ddd, J = 9.3, 7.8, 5.6 Hz, H-16), 3.61 (3H, s, H-18), 3.07 (1H, dd, J = 13.8, 5.6 Hz, H-15), 3.00 (6H, s, N(CH3)2), 2.94 (1H, dd, J = 13.8, 9.3 Hz, H-15), 1.80 (3H, s, H-20). 13C NMR (126 MHz, DMSO-d6) δ 172.14 (C-17), 169.55 (C-19), 151.54 (C-6 or C-12), 151.48 (C-6 or C-12), 143.71 (C-8), 142.66 (C-7), 139.50 (C-3), 130.05 (C-2, C-4), 130.00 (C-10, C-14), 122.09 (C-9), 121.80 (C-1, C-5), 112.07 (C-11, C-13), 53.49 (C-16), 51.96 (C-18), 39.52 (N(CH3)2), 36.58 (C-15), 22.30 (C-20). MS (ESI+): 395.2076 [M + H]+ (calculated 395.2078), 417.1905 [M + Na]+ (calculated 417.1897). IR (νmax/cm−1) 1598.99 (stretching N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N).
N).
1H NMR (400 MHz, DMSO-d6) δ 8.16 (1H, d, J = 2.4 Hz, H-12), 7.85 (4H, m, H-1, H-5, H-8, H-13), 7.58 (2H, m, H-2, H-4), 7.52 (1H, m, H-3), 7.10 (1H, d, J = 8.8 Hz, H-9), 6.66 (1H, d, J = 16.1 Hz, H-14). 13C NMR (101 MHz, DMSO-d6) δ 167.85 (C-15), 159.74 (C-10), 152.00 (C-6), 145.15 (C-7), 138.68 (C-13), 130.80 (C-3), 129.38 (C-2, C-4), 124.84 (C-12), 124.70 (C-8), 122.25 (C-1, C-5), 121.53 (C-11), 119.87 (C-14), 116.94 (C-9). MS (ESI+): 269.0 [M + H]+, 291.0 [M + Na]+.
3.4 Cell culture, drug treatment and cell death evaluation
Human uterine smooth muscle cells (HUtSMC) immortalized with hTERT and SK-UT-1 cells were grown in DMEM supplemented with 10% fetal bovine serum (FBS), L-glutamine (2 mM), penicillin (100 U mL−1), and streptomycin (100 μg mL−1) (Lonza). Cells were tested regularly for mycoplasma contamination through microscope after Hoechst 33342 (Sigma) staining. For cell death evaluation, cells were treated with the indicated compounds for 48 h. Cell death was evaluated through Countess II FL automated cell counter (Invitrogen) after 0.1% Trypan blue (Sigma) staining.3.5 Bacterial strains, antimicrobial activity
The bacterial strains used in this work were E. coli ATCC 25922 and S. aureus ATCC 25923. Bacterial strains were grown overnight at 37 °C in Mueller–Hinton broth (MHB, Difco) with shaking (140 rpm). The day after, the bacterial cultures were diluted 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 in fresh MHB and incubated at 37 °C with shaking (140 rpm) to mid-log phase, until an optical density (OD) at 600 nm ≈ 0.3 was reached. Mid-log bacterial cultures were diluted to 5 × 105 colony-forming units (cfu) per mL in Mueller–Hinton Broth (MHB). To test the antimicrobial activity, the DDs, diluted in MHB to the concentration of 128 μM in the presence of 1.5–2% DMSO, were added to the first wells of a round-bottom 96-well microtiter plate and serially twofold diluted in a final volume of 50 μL of MHB into successive wells. Subsequently, 50 μL of the bacterial suspension 5 × 105 cfu mL−1 (see above) was added to each well. This reduced the final bacterial load to 2.5 × 104 cfu per well and halved the DD concentration in the wells. The same amount of untreated bacteria in MHB + 2% DMSO, without any other added compound, was used as bacterial growth control. MHB only (100 μL) was used to control the medium sterility. The plate was sealed with Parafilm to minimize evaporation and incubated overnight at 37 °C (for approx. 18 h). Data are the median of 3 independent experiments performed as internal duplicates (n = 6).
40 in fresh MHB and incubated at 37 °C with shaking (140 rpm) to mid-log phase, until an optical density (OD) at 600 nm ≈ 0.3 was reached. Mid-log bacterial cultures were diluted to 5 × 105 colony-forming units (cfu) per mL in Mueller–Hinton Broth (MHB). To test the antimicrobial activity, the DDs, diluted in MHB to the concentration of 128 μM in the presence of 1.5–2% DMSO, were added to the first wells of a round-bottom 96-well microtiter plate and serially twofold diluted in a final volume of 50 μL of MHB into successive wells. Subsequently, 50 μL of the bacterial suspension 5 × 105 cfu mL−1 (see above) was added to each well. This reduced the final bacterial load to 2.5 × 104 cfu per well and halved the DD concentration in the wells. The same amount of untreated bacteria in MHB + 2% DMSO, without any other added compound, was used as bacterial growth control. MHB only (100 μL) was used to control the medium sterility. The plate was sealed with Parafilm to minimize evaporation and incubated overnight at 37 °C (for approx. 18 h). Data are the median of 3 independent experiments performed as internal duplicates (n = 6).
4 Conclusions
In this work, the synthesis of six 1,2-diaza-1,3-dienes from substituted cinnamic acids and aromatic amines has been described. The obtained diazadienes have never been reported in literature and their structures have been fully characterized, even if the real novelty is the synthetic procedure adopted to obtain them. In fact, for the first time a new reactivity of diazonium salts from anilines with cinnamic acids has been reported: in particular, the reaction has been carried out with three substituted cinnamic acids, i.e. p-coumaric acid, ferulic acid and 4(dimethylamino)cinnamic acid, and two model anilines, namely aniline and acetyl-p-amino-phenylalanine methyl ester. All the synthesized compounds have also been used for preliminary biological activity evaluation, to establish potential antitumor and antimicrobial activity. Compound 3c is the most effective compound in terms of antitumor activity, since it is the only compound that causes a higher percentage of cell death in LMS cells compared to normal HUtSMC cells, and this was confirmed also through promising dose-dependent studies. Interestingly, compound 3c is completely inactive as an antibacterial against S. aureus, but compound 3b, which do not affect much human cells, shows an anti-Staphylococcus activity with a MIC of 8 μM, so it might have a selective action as antibacterial.Author contributions
Veronica Vida: conceptualization, investigation, methodology, formal analysis, data curation, validation, visualization, writing original draft and writing review and editing. Martina Minisini: investigation, data curation, validation, visualization, writing original draft. Mario Mardirossian: investigation, data curation, validation, writing original draft. Claudio Brancolini: data curation, validation, visualization, writing original draft, writing review and editing, resources and supervision. Marco Scocchi: data curation, validation, visualization, writing original draft, writing review and editing, resources and supervision. Cristina Forzato: conceptualization, validation, visualization, writing original draft, writing review and editing, supervision. Federico Berti: conceptualization, validation, visualization, writing review and editing, supervision, resources, project administration.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Veronica Vida is grateful to Regione Friuli-Venezia Giulia for having granted her PhD fellowship within the European Social Fund 2014–2020.Notes and references
- O. A. Attanasi, L. De Crescentini, G. Favi, P. Filippone, F. Mantellini, F. R. Perrulli and S. Santeusanio, Cultivating the passion to build heterocycles from 1,2-diaza-1,3-dienes: the force of imagination, Eur. J. Org. Chem., 2009, 2009(19), 3109–3127 CrossRef.
- O. A. Attanasi and L. Caglioti, Conjugated Azoalkenes: Attractive Products and Versatile Intermediates, Org. Prep. Proced. Int., 1986, 18(5), 299–327 CrossRef CAS.
- O. A. Attanasi, L. D. Crescentini, P. Filippone, F. Mantellini and S. Santeusanio, 1,2-Diaza-1,3-butadienes; just a nice class of compounds, or powerful tools in organic chemistry? reviewing an experience, Arkivoc, 2003, 2002(11), 274–292 Search PubMed.
- J. Guzman, Natural Cinnamic Acids, Synthetic Derivatives and Hybrids with Antimicrobial Activity, Molecules, 2014, 19(12), 19292–19349 Search PubMed.
- V. Sinisi, A. Stevaert, F. Berti, C. Forzato, F. Benedetti, L. Navarini, A. Camps, L. Persoons and K. Vermeire, Chlorogenic Compounds from Coffee Beans Exert Activity against Respiratory Viruses, Planta Med., 2016, 83(07), 615–623 CrossRef PubMed.
- V. Sinisi, K. Boronová, S. Colomban, L. Navarini, F. Berti and C. Forzato, Synthesis of Mono-, Di-, and Tri-3,4-dimethoxycinnamoyl-1,5-γ-quinides, Eur. J. Org. Chem., 2014, 2014(6), 1321–1326 CrossRef CAS.
- A. L. Gutiérrez Ortiz, F. Berti, L. Navarini, P. Crisafulli, S. Colomban and C. Forzato, Aqueous extracts of walnut (Juglans regia L.) leaves: quantitative analyses of hydroxycinnamic and chlorogenic acids, J. Chromatogr. Sci., 2018, 56(8), 753–760 Search PubMed.
- A. L. Gutiérrez Ortiz, F. Berti, W. Solano Sánchez, L. Navarini, S. Colomban, P. Crisafulli and C. Forzato, Distribution of p-coumaroylquinic acids in commercial Coffea spp. of different geographical origin and in other wild coffee species, Food Chem., 2019, 286, 459–466 CrossRef PubMed.
- A. L. Gutiérrez Ortiz, F. Berti, L. Navarini, A. Monteiro, M. Resmini and C. Forzato, Synthesis of p-coumaroylquinic acids and analysis of their interconversion, Tetrahedron: Asymmetry, 2017, 28(3), 419–427 CrossRef.
- F. Berti, L. Navarini, S. Colomban and C. Forzato, Hydroxycinnamoyl Amino Acids Conjugates: A Chiral Pool to Distinguish Commercially Exploited Coffea spp, Molecules, 2020, 25(7), 1704 CrossRef CAS PubMed.
- A. J. Borah and G. Yan, Decarboxylative functionalization of cinnamic acids, Org. Biomol. Chem., 2015, 13(30), 8094–8115 RSC.
- M. Gupta and B. P. Wakhloo, Tetrabutylammoniumbromide mediated Knoevenagel condensation in water: synthesis of cinnamic acids, Arkivoc, 2007, 2007(1), 94–98 Search PubMed.
- L. Chen, L. Zhang, G. Yan and D. Huang, Recent Advances of Cinnamic Acids in Organic Synthesis, Asian J. Org. Chem., 2020, 9(6), 842–862 CrossRef CAS.
- P. Schroll, D. P. Hari and B. König, Photocatalytic Arylation of Alkenes, Alkynes and Enones with Diazonium Salts, ChemistryOpen, 2012, 1(3), 130–133 CrossRef CAS PubMed.
- C. S. Rondestvedt Jr, Arylation of Unsaturated Compounds by Diazonium salts (The Meerwein Arylation Reaction), Org. React., 2004, 11, 189–260 Search PubMed.
- X.-X. Xu, M. Wang, Q. Liu, L. Pan and Y.-L. Zhao, Azo-coupling Decarboxylation Reaction of α-Carboxy Ketene Dithioacetals in Water—a New Route to 1,2-Diaza-1,3-butadienes, Chin. J. Chem., 2006, 24(10), 1431–1434 CrossRef CAS.
- S. George, C. Serrano, M. L. Hensley and I. Ray-Coquard, Soft tissue and uterine leiomyosarcoma, J. Clin. Oncol., 2018, 36(2), 144–150 CrossRef CAS PubMed.
- L. Iuliano, S. Drioli, Y. Pignochino, C. M. Cafiero, M. Minisini, F. D'Este, R. Picco, E. Dalla, G. Giordano, G. Grignani, E. Di Giorgio, F. Benedetti, F. Felluga and C. Brancolini, Enhancing Proteotoxic Stress in Leiomyosarcoma Cells Triggers Mitochondrial Dysfunctions, Cell Death, and Antitumor Activity in vivo, Mol. Cancer Ther., 2021, 20(6), 1039–1051 CrossRef CAS PubMed.
- D. G. Brown, T. L. May-Dracka, M. M. Gagnon and R. Tommasi, Trends and Exceptions of Physical Properties on Antibacterial Activity for Gram-Positive And Gram-Negative Pathogens, J. Med. Chem., 2014, 57(23), 10144–10161 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Mono- and bidimensional NMR spectra and mass spectra of all the newly synthesized compounds. See DOI: https://doi.org/10.1039/d2ra07515f |
| This journal is © The Royal Society of Chemistry 2023 |