Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Solubility of ammonium metal fluorides in aqueous ethanol mixtures – implications for scandium recovery by antisolvent crystallization†
Edward Michael Peters‡
 *a,
Michael Svärd
*a,
Michael Svärd b and
Kerstin Forsberg
b and
Kerstin Forsberg b
b
aMEAB Chemie Technik GmbH, Dennewartstraße 25, 52068, Aachen, Germany
bKTH Royal Institute of Technology, Department of Chemical Engineering, SE-100 44, Stockholm, Sweden. E-mail: kerstino@kth.se
First published on 5th January 2023
Abstract
The recovery of scandium from waste streams of other mining and metallurgical processing industries is gaining research interest due to the scarcity of scandium-containing ores. Hydrometallurgical techniques such as leaching, solvent extraction and crystallization amongst others have been successfully applied to recover scandium salts from such waste streams. Scandium can be recovered as (NH4)3ScF6 by antisolvent crystallization from NH4F strip liquors obtained after solvent extraction. The coextraction of metal impurities such as Fe, Al, Zr and Ti causes contamination of the final solid product. The extent of coprecipitation of ammonium metal fluorides depends on their initial concentration in the strip liquor and their solubility in the NH4F–antisolvent mixtures. Here, the solubility of ammonium metal fluorides of Sc, Zr, Fe, Al and Ti is reported separately in 3 mol L−1 NH4F–ethanol mixtures at 25 °C as well as in a system containing all five solid phases. The solubility of (NH4)3ZrF7 is slightly higher than that of (NH4)3ScF6, while the solubilities of (NH4)3FeF6 and (NH4)3AlF6 are significantly lower in comparison to (NH4)3ScF6. The solubility of (NH4)2TiF6 is 1–2 orders of magnitude higher than those of other ammonium metal fluorides. When a mixture of ammonium metal fluoride salts is dissolved in the same 3 mol L−1 NH4F–ethanol mixture as for the individual salts, the resultant solubility of the ammonium metal fluorides of Sc, Zr and Fe decreases significantly, while the resultant solubility of ammonium aluminum hexafluoride increases. This is likely due to changes in solution speciation with increased NH4F concentration and ionic strength.
Introduction
The recovery of scandium from waste streams of other mining and metallurgical industries has gained enormous interest and is essential to meet the increasing global scandium demand. Scandium is usually present in these streams in quantities of economic value and examples of such streams are the bauxite residue from the alumina industry, titanium dioxide acidic waste, slags from iron smelting, as well as uranium and tin processing waste streams.1–4 Several techniques have been explored to recover scandium from such streams and these include hydrometallurgical techniques such as leaching, solvent extraction and crystallization.1,2,5–7 In recent research, it was reported that antisolvent crystallization is a viable method for recovering scandium as ammonium scandium hexafluoride, (NH4)3ScF6, from NH4F strip liquors obtained after acidic leaching and solvent extraction. The percentage recovery of (NH4)3ScF6 from the strip liquors was reported to be ca. 99% at an ethanol to strip liquor volumetric ratio of ca. 0.8, and the purity of the solid product approached 99%.8,9During the leaching and solvent extraction stages, some metals such as Fe, Al, Ti, Zr, V, U and Th are usually coextracted and end up in the NH4F strip liquor, although a high degree of selectivity can be achieved in the solvent extraction stage. These metals contaminate the solid product obtained during crystallization and it was observed that they are usually present in the solid product in proportions that reflect their relative abundances in the strip liquor.10 The commercial use of scandium products in specialized applications such as solid oxide fuel cells, solid oxide electrolyzer cells and 3D printing often requires very high purities >99% Sc2O3 and/or ScF3. Increasing the final product purity to such levels requires knowledge of the solubility of species likely to contaminate the product during antisolvent crystallization, which could facilitate the development of an antisolvent crystallization strategy, such as fractional crystallization, to further purify the product. Therefore, it is necessary to investigate the solubility of ammonium metal fluorides of Sc and major impurity metals such as Fe, Al, Zr and Ti in solvent mixtures typical of the antisolvent crystallization process. In a recent study, the solubility of (NH4)3ScF6 has been investigated at 25 °C in NH4F solutions of concentration 2–5 mol L−1 dosed with alcohols namely methanol, ethanol, 2-propanol, and 1,3-propane-diol as well as in pure NH4F solutions of concentration 0.1–12.2 mol L−1.9,11 The solubility of (NH4)3ScF6 in HF solutions of concentrations up to 24 mol L−1 at 30 and 50 °C is also reported in literature, from which the interpolated solubility value at 3 mol L−1 HF and 25 °C is about 3.6 g Sc per L.12
The phases of the impurity metals that are likely to co-crystallize include (NH4)3FeF6, (NH4)3AlF6, (NH4)3ZrF7 and (NH4)2TiF6 or (NH4)3TiF7. Such ammonium metal fluorides including the Sc phase have been previously reported to exhibit almost similar XRD patterns when crystallized as a mixture, even from solutions that contained higher quantities of Zr, making their identification and quantification challenging.10 The heptafluoride phases of Zr13,14 and Ti15,16 were reported to be the stable phases under ambient conditions and thermal decomposition of these phases to the hexafluorides was observed to occur at 297 °C and 107 °C, respectively. The solubility of (NH4)2TiF6 in water at 25 °C is reported to be 260 g L−1 and that of (NH4)3ZrF7 is reported over a temperature range of 0–104 °C to be in the range ca. 0.4–1.2 mol L−1 (ca. 111–334 g L−1).17 The solubility of (NH4)3FeF6 was observed to increase with increasing HF concentration (≤20 g L−1) and temperature (0–60 °C) at constant NH4HF2 concentration and decrease upon increasing the NH4HF2 concentration (50–150 g L−1).18 The increase in solubility with increased HF concentration could be due to increased complex formation between Fe3+ and F− anions, while the increase in NH4+ concentration introduces a common ion which promotes crystallization of (NH4)3FeF6. The solubility of (NH4)3AlF6 and NH4AlF4 in water at 25 °C are reported as 8 and 0.5 g L−1, respectively.19 (NH4)3FeF6 and (NH4)3AlF6 also undergo thermal decomposition to their respective tetrafluoride phases at ca. 140 and 170 °C, respectively.20
It has been reported that (NH4)3ScF6 is the stable phase and can transform into other phases such as (NH4)5Sc3F14 and NH4ScF4 at different fluoride concentrations (0.1–7 mol L−1) and temperatures (18 and 90 °C), with the tetrafluoride phase being the stable phase in pure water.21,22 Similar phase transformations were also observed in NH4F media at fluoride concentrations below 0.8 mol L−1 and beyond this concentration, no phase transformation was observed.22 Thermodynamic modelling of a Sc–F system revealed that higher order ScF complexes become more stable with increased fluoride concentration, and are also stable at alkaline pH values compared to lower order ScF complexes, while that of higher order ScOH complexes increase with increasing pH.8 The stability of complexes is expressed by their stability or formation constants.
Solution speciation plays a significant role in determining which phases are likely to crystallize under specific conditions. In the presence of other metal ions and other solvents such as ethanol, the solution speciation can be significantly altered since there is competition for ligands amongst the metal ions and there is high possibility of ethanol–cation, ethanol–ligand and ethanol–water interactions which adds to the complexity of the system speciation. This can significantly alter the solubility of ammonium metal fluorides. As far as we know, there is no data in literature on the solubility of ammonium metal fluorides in NH4F–ethanol mixtures except the data published for (NH4)3ScF6 in such systems.11 For this reason, the solubility is determined herein for ammonium metal fluorides of Zr, Fe, and Al separately in 3 mol L−1 NH4F–ethanol mixtures at 25 °C and compared with the published data for (NH4)3ScF6.11 The solubility is also determined in a system containing a mixture of all five salts including the Ti phase in 3 mol L−1 NH4F–ethanol mixtures at 25 °C to investigate the extent to which the solubility of these compounds is influenced by the presence of other metal salts in solution.
Methodology
Ammonium scandium fluoride was synthesized by reacting near-saturated solutions of Sc2(SO4)3 (prepared using >99.9 wt% Sc2(SO4)3) and NH4F (prepared using >98 wt% NH4F) and details of the procedure are published in literature.11 Fe(NO3)3·9H2O of purity >98 wt%, ZrF4 of purity >98 wt%, aluminum isopropoxide (Al[OCH(CH3)2]3) of purity >98 wt% and TiF4 of purity >98 wt% were used to prepare near-saturated solutions of Fe(NO)3, ZrF4, Al[OCH(CH3)2]3 and ca. 30 wt% TiF4 in 6 mol L−1 hydrofluoric (HF) acid. These solutions were reacted with saturated solutions of NH4F to synthesize the corresponding ammonium metal fluorides, and the solids obtained were washed with ca. 10 mL of ethanol, dried under ambient conditions for at least 48 hours, and analyzed by powder X-ray diffraction (XRD) (SIEMENS™ D5000) to determine the phase of the solid and by inductively coupled plasma optical emission spectroscopy (ICP-OES) (ThermoScientific iCAP™ 7400) to determine the purity. For powder XRD measurements, the solid sample was pulverized in a mortar and carefully spread over the sample holder and compacted to maintain a flat surface in order to minimize noise generation. The XRD analysis was conducted using an X-ray source of Cu Kα radiation (λ = 1.5406 Å). For ICP-OES analysis, a known weight of the solid sample was first dissolved in distilled water and further diluted in a 3.45% v/v HNO3 acid matrix. Six calibration standards ranging between 0.01 and 50 mg L−1 were prepared from commercial standards containing 1000 mg L−1 of the metal in a HNO3 acid matrix. The solubility was determined in quaternary systems of H2O–NH4F–ethanol–(NH4)xMey+F(x+y), where Me is the metal of concern and also in octonary systems of H2O–NH4F–ethanol and all 5 ammonium metal fluorides.Quaternary system
A 3 mol L−1 NH4F solution was prepared, and ethanol was added to 20 mL of this solution to attain ethanol concentrations in the range 0.5–9 mol L−1. About 1 g of the synthesized solid phase (except the Ti phase) was added to the NH4F–ethanol mixtures and the suspension was maintained at 25 °C for 24 hours under agitation at 250 rpm using magnetic stirrers. Temperature control was achieved by means of a thermal water bath equipped with a PT100 temperature sensor with an accuracy of ±0.01 °C. A Traceable® temperature sensor was used to verify the suspension temperatures in the experimental containers with temperature variations amongst the containers not exceeding ±0.04 °C. At the end of the experiments, supernatant samples were collected by means of a syringe fitted with a 0.22 μm PVDF membrane filter. The suspensions were then vacuum filtered, and the solids were dried under ambient conditions and analyzed by powder XRD to check if any phase transformation had occurred. The solubility of each phase for each experimental condition in terms of total metal concentration was determined by analyzing the supernatant samples using ICP-OES after diluting the sample in a 3.45% v/v HNO3 acid matrix. The densities of the supernatant samples were also determined by measuring their volumes and weights during dilution. Experiments were conducted in duplicate to assess the reproducibility.Octonary system
Four experiments were conducted for each experimental condition, and these were grouped into two sets of duplicates. The first set of duplicate experiments was conducted using 5 g of 3 mol L−1 NH4F solution and the second set was conducted using 10 g of 3 mol L−1 NH4F solution. Ethanol was added to the NH4F solutions to attain concentrations in the range 0.5–2 mol L−1. The procedure was similar to the one described for the quaternary system except that 1–2 g of each of the phases of Sc, Fe, Al and Zr and about 4–6 g of the Ti phase were added to each experimental container to assess the effect of other metal salts on the solubility of each of the phases. The suspensions were agitated at 250 rpm over 24 hours and temperature variations not exceeding ±0.1 °C were detected amongst the containers. It has been shown in previous research that equilibrium is attained after 5 hours for the Sc phase11 and after 3–4 hours for ammonium metal fluorides of Ti and Nb in the Ti–Nb–NH4F–HF aqueous system.17Comparison of published solubility data for (NH4)3ScF6 obtained from the supersaturated state over 72 hours,9 and from the undersaturated state11 over 24 hours shows negligible discrepancies. Sampling and analysis were conducted as described for the quaternary system. Since the system contained a mixture of salts, calculations were conducted after obtaining the solubility data to determine if the quantity of the Ti phase added was in excess, to ascertain that it did not dissolve completely. The solids added were determined to be in excess of the solubility of the Ti phase in these solution mixtures and was ca. 35 ± 5 wt% for the experiments conducted with 0.5 mol L−1 ethanol, which is higher than the reported solubility of (NH4)2TiF6 in water (260 g L−1).17
Results and discussion
Phase determination
The synthesized phases were analyzed by powder XRD and verified to be (NH4)3ScF6 of monoclinic structure, (NH4)3FeF6, (NH4)3AlF6, (NH4)3ZrF7 of cubic structure, and (NH4)2TiF6 of hexagonal structure, respectively. The XRD diffractograms are shown in Fig. 1, and the patterns with overlaid peak positions of reference patterns are given in ESI.† For the Ti Phase, the pattern of (NH4)2TiF6 matched the peak positions almost perfectly, although the peak intensities of the two patterns are not proportionate. It can also be noted that the reference patterns of (NH4)3TiF6 and (NH4)3TiF7 do not match the peaks in the obtained diffractogram, despite the fact that the heptafluoride phase has been reported to transform into the hexafluoride at 107 °C.15,16 For this reason, the solubility of the Ti phase is presented as g (NH4)2TiF6 per kg solution. The purity of the solid phases as determined by ICP-OES were 99.96 wt% for (NH4)3ScF6 and >99.9 wt% for the other ammonium metal fluorides. The accuracy in purity determination was within ±0.03 wt%.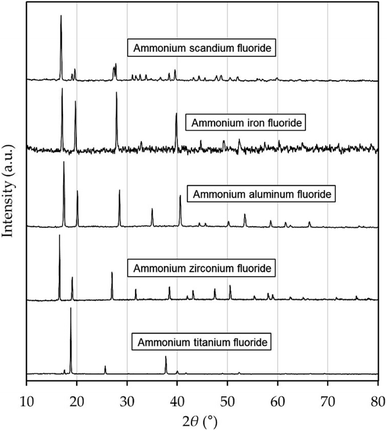 | ||
| Fig. 1 XRD diffractograms of synthesized phases. These were matched with reference patterns obtained from the Powder Diffraction File Database (PDF-2 2021). | ||
Solubility
 | ||
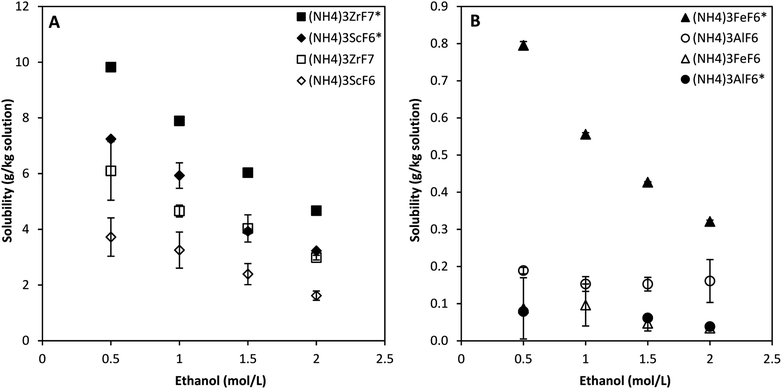
| Fig. 2 Solubility of ammonium metal fluorides in 3 mol L−1 NH4F–ethanol mixtures at 25 °C for the quaternary system. Data for the Sc phase is obtained from literature.11 Error bars denote the standard deviations from the mean of 2 experimental repeats. | ||
The solubility of (NH4)3ScF6 and (NH4)3ZrF7 in 3 mol L−1 NH4F solution containing ethanol at a concentration of 0.5 mol L−1 total solution is about 10 and 7 g kg−1 solution, respectively, while that of (NH4)3FeF6 and (NH4)3AlF6 are ca. 0.8 and 0.1 g kg−1 solution, respectively. The solubility of all phases decreases asymptotically with increased ethanol concentration, which correlates to the reduction in the effective dielectric constant of the solvent mixture as the ethanol concentration increases.11,23 This promotes ion pairing and the crystallization of the respective solid phases of these compounds. Solvents of higher dielectric constant promote complete dissociation in solution. The calculated estimates of the effective dielectric constants of 3 mol L−1 NH4F solutions dosed with ethanol to attain ethanol concentrations in the range 0.5–9 mol L−1 on a total solution basis at 25 °C are published in literature.11 In other terms, the solubility of the phases decreases with increase in the concentration of a solvent (ethanol), in which the phases are almost insoluble.
The solubility of the phases decreases in the order Zr4+, Sc3+, Fe3+ and Al3+ which correlates to the increase in charge density of the metal ion for the trivalent metals. The calculated charge densities are presented in the ESI.† However, Zr4+ has a higher charge density than Sc3+, yet the solubility of the Zr phase is higher than that of the Sc phase and this is because the solubility of a compound depends mainly on the energy required to break bonds and is therefore not always correlated to the charge density of the metal ion. The solubility data is presented in Table 1 as averages of two experimental repeats together with their standard deviations. There was no phase transformation detected by powder XRD.
| [Ethanol] (mol L−1) | Solubility g (NH4)3ZrF7 per kg solution | Solubility g (NH4)3FeF6 per kg solution | Solubility g (NH4)3AlF6 per kg solution |
|---|---|---|---|
| 0.5 | 9.8 ± 0.115 | 0.80 ± 0.010 | 0.079 ± 0.003 |
| 1.0 | 7.890 ± 0.003 | 0.556 ± 0.005 | — |
| 1.5 | 6.03 ± 0.059 | 0.427 ± 0.002 | 0.062 ± 0.003 |
| 2.0 | 4.67 ± 0.048 | 0.321 ± 0.004 | 0.038 ± 0.003 |
| 3.0 | 2.788 ± 0.001 | 0.189 ± 0.000 | 0.028 ± 0.004 |
| 4.0 | 1.72 ± 0.035 | 0.112 ± 0.004 | — |
| 6.0 | 0.80 ± 0.050 | 0.047 ± 0.006 | — |
| 8.0 | 0.303 ± 0.004 | 0.025 ± 0.003 | — |
 | ||
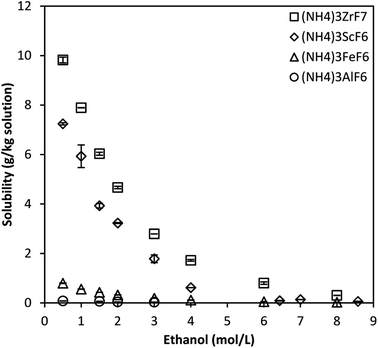
| Fig. 3 Solubility of ammonium metal fluorides in 3 mol L−1 NH4F–ethanol mixtures at 25 °C for the octonary system. Error bars denote the standard deviations from the mean of 3–4 experimental repeats. | ||
A similar trend in which the solubility of the phases decreases with increased ethanol concentration is observed. The solubility of (NH4)2TiF6 is much higher, about 1–2 orders of magnitude higher than that of other ammonium metal fluorides. This is attributed to the fact that titanium rarely exists as Ti4+ in solution and tends to form the stable titanyl complex, TiO2+, in solution.24
Table 2 shows the solubility data of these phases in the octonary system containing all five salts in a NH4F–H2O–ethanol system at 25 °C. The data is presented as averages of 3–4 values with their associated standard deviations.
| [Ethanol] (mol L−1) | Equivalent equilibrium [NH4F] (mol L−1) | Solubility g (NH4)3ZrF7 per kg solution | Solubility g (NH4)3ScF6 per kg solution | Solubility g (NH4)3FeF6 per kg solution | Solubility g (NH4)3AlF6 per kg solution | Solubility g (NH4)2TiF6 per kg solution |
|---|---|---|---|---|---|---|
| 0.5 | 4.38 | 6 ± 1.052 | 3.7 ± 0.689 | 0.09 ± 0.082 | 0.189 ± 0.009 | 125 ± 8.1 |
| 1.0 | 4.24 | 4.7 ± 0.213 | 3.3 ± 0.647 | 0.10 ± 0.056 | 0.15 ± 0.020 | 113 ± 6.1 |
| 1.5 | 4.06 | 4.0 ± 0.489 | 2.4 ± 0.379 | 0.05 ± 0.021 | 0.15 ± 0.018 | 97 ± 1.7 |
| 2.0 | 3.91 | 2.98 ± 0.084 | 1.6 ± 0.167 | 0.034 ± 0.003 | 0.16 ± 0.058 | 84 ± 2.6 |
The molar quantity of NH4F ions increased due to solubilization of both NH4+ and F− ions contained in the salts. The NH4F concentration values presented in Table 2 are the sum of the initial 3 mol L−1 NH4F and the equivalent molar quantity of NH4+ ions that dissolved from all 5 salts and equilibrated with the solid mixture. The term ‘equivalent equilibrium’ is used since the molar quantity of NH4F that dissolved was computed as the sum of the molar quantities of NH4+ ions released by each salt. However, an excess molar quantity of F− ions were released by each salt since the molar ratio of fluoride to ammonium ion concentration is 2.33 for (NH4)3ZrF7, 2 for (NH4)3ScF6, (NH4)3FeF6, (NH4)3AlF6 and 3 for (NH4)2TiF6.
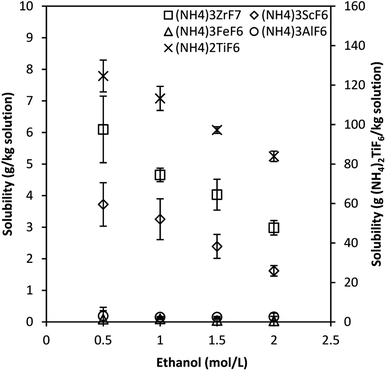
Fig. 4 compares the solubility of ammonium metal fluorides of Sc, Zr, Fe and Al determined in the quaternary and octonary systems at 25 °C. The initial NH4F concentration was 3 mol L−1 in all experiments.
It is observed that the solubility of (NH4)3ZrF7, (NH4)3ScF6 and (NH4)3FeF6 is significantly reduced, by about 37%, 46% and 88%, respectively, for the octonary system compared with the single-salt systems. Furthermore, an increase in the solubility of (NH4)3AlF6 by about 65% was observed in the octonary system. The total equivalent concentration of NH4F is higher in the octonary system compared to the single-salt systems (see Table 2), which likely reduces the solubility of species due to the common ion effect, but this does not explain the increase in the solubility of the Al phase.
The changes in solubility are also partly due to changes in solution speciation since the ionic strength increases, and the presence of five metal ions implies that there is competition amongst the ions for ligands in solution. The stability of metal–ligand complexes is expressed in terms of stability or formation constants and the presence of ethanol complicates the system speciation. The stability constants of relevant complexes in the presence of ethanol could not be found in literature.
The XRD diffractogram of the solid mixture after the solubility experiments in the octonary system is presented in the ESI.† The XRD pattern contains multiple peaks on specific 2θ angles which are almost matched with the reference patterns of the five ammonium metal fluorides. It can therefore be reasonably assumed that no phase transformations occurred for any of the solid phases under the experimental conditions in this study.
The remarkable changes in the solubility of the ammonium metal fluorides observed in the octonary system compared with the single-metal systems emphasize that the supersaturation experienced by each phase in a system containing a mixture of salts should not be evaluated using solubility data reported in terms of total metal dissolved in pure systems. It illustrates the importance of expressing the supersaturation in terms of the true driving force considering the chemical speciation, which unfortunately is challenging in mixed solvent systems. For instance, a strip liquor that contains 0.02 g (NH4)3FeF6 could be supersaturated with respect to the Fe phase in the octonary system at ethanol concentration ≥3 mol L−1 (by extrapolation of the solubility data in Table 2), while it remains undersaturated in the quaternary system at ethanol concentration of 8 mol L−1 (see Table 1). The supersaturation experienced by each solid phase can be expressed in terms of the initial total concentration of the metal of that phase and the solubility in terms of total metal concentration of the phase in the system considered. In the typical strip liquors with Sc concentration of ca. 2.5 g kg−1 solution as presented in published studies,9,10 the Sc phase attains supersaturation at an ethanol concentration of about 1.5 mol L−1 and the impurity phases become supersaturated at different stages as the ethanol concentration is increased. This means that the purity of the solid phase can be improved by operating at low ethanol concentration, but at the expense of the yield and by conducting stage-wise crystallization. Considering process economy, it would be preferable to maximize the yield given that the purity of the solid is within desired specifications.
Conclusions
The solubility of ammonium metal fluorides has been determined at 25 °C in quaternary systems containing a single solid phase, water, NH4F and ethanol, as well as in octonary systems containing all five salts, water, NH4F and ethanol. The data shows that the solubility of ammonium metal fluorides in the quaternary systems decreases asymptotically with increase in ethanol concentration and occurs in the cationic order Zr4+, Sc3+, Fe3+ and Al3+. This corresponds to increase in charge density for the trivalent cations while the tetravalent cation (Zr4+) deviates from this trend. The presence of other metal salts in the octonary system has a significant effect on the amount of ammonium metal fluorides dissolved. Compared with the respective single-metal systems, the solubility of the ammonium metal fluorides of zirconium, scandium and iron decrease remarkably in the octonary system, while that of (NH4)3AlF6 increases significantly. The changes in solubility are attributed partly to changes in solution speciation as the solution ionic strength increases, and partly to the common ion effect caused by the increase in NH4F concentration in the octonary system. In the mixed salt system, there is increase in competition for ligands amongst the metal ions, which is further complicated by the presence of ethanol in the system. The solubility of (NH4)2TiF6 in the octonary system was determined to be 1–2 orders of magnitude higher than that of other phases, most likely due to the formation of the stable TiO2+ ion.Author contributions
E. M. Peters: conceptualization, methodology, validation, formal analysis, investigation, writing original draft and editing. M. Svärd: conceptualization, writing – review and editing, supervision. K. Forsberg: conceptualization, resources, writing – review and editing, supervision, project administration, funding acquisition.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research has emanated within the SCALE project financed by the European Union Horizon 2020 research and innovation programme under grant agreement no. 730105. Financial support from Scale-up under EIT RawMaterials project agreement 21013 is also gratefully acknowledged.Notes and references
- N. Krishnamurthy and C. K. Gupta, Extractive Metallurgy of Rare Earths, CRC Press Taylor and Francis Group, New York, 2nd edn, 2016 Search PubMed.
- F. Habashi, Extractive Metallurgy of Rare Earths, Can. Metall. Q., 2013, 52(3), 224–233 CrossRef CAS.
- U.S. Geological Survey, Mineral Commodity Summaries 2021, U.S. Geological Survey, Reston Virginia, 2021 Search PubMed.
- H. T. Mnculwane, Scandium Quantification in Selected Inorganic and Organometallic Compounds, University of Free State, Bloemfontein, 2016 Search PubMed.
- W. Wang, Y. Pranolo and C. Y. Cheng, Metallurgical Processes for Scandium Recovery from Various Resources: A Review, Hydrometallurgy, 2011, 108(1–2), 100–108 CrossRef CAS.
- D. Li and C. Wang, Solvent Extraction of Scandium(III) by Cyanex 923 and Cyanex 925, Hydrometallurgy, 1998, 48(3), 301–312 CrossRef CAS.
- D. I. Smirnov and T. V. Molchanova, The Investigation of Sulphuric Acid Sorption Recovery of Scandium and Uranium from the Red Mud of Alumina Production, Hydrometallurgy, 1997, 45(3), 249–259 CrossRef CAS.
- Ş. Kaya, E. M. Peters and K. Forsberg, et al., Scandium Recovery from an Ammonium Fluoride Strip Liquor by Anti-Solvent Crystallization, Metals, 2018, 8(10), 767 CrossRef.
- E. M. Peters, Ş. Kaya, C. Dittrich and K. Forsberg, Recovery of Scandium by Crystallization Techniques, J. Sustain. Metall., 2019, 5(1), 48–56 CrossRef.
- E. M. Peters, C. Dittrich, B. Yagmurlu and K. Forsberg, Co-precipitation of Impurity (TiU, Th) Phases during the Recovery of (NH4)3ScF6 from Strip Liquors by Anti-solvent Crystallization, in Rare Metal Technology, ed. G. Azimi, K. Forsberg, T. Ouchi, H. Kim, S. Alam and A. A. Baba, Springer, Cham, 2020, pp. 177–189 Search PubMed.
- E. M. Peters, M. Svärd and K. Forsberg, Phase Equilibria of Ammonium Scandium Fluoride Phases in Aqueous Alcohol Mixtures for Metal Recovery by Anti-solvent Crystallization, Sep. Purif. Technol., 2020, 252, 117449 CrossRef CAS.
- S. V. Potapenko, E. G. Rakov and S. V. Khaustov, Solubility of Scandium Trifluoride and Ammonium Hexafluoroscandate in Water and Hydrofluoric Acid, Russ. J. Inorg. Chem., 1995, 40(7), 1073–1076 Search PubMed.
- Fluorine Chemistry, ed. J. H. Simons, Academic Press Inc. Ltd, London, 5th edn, 1964 Search PubMed.
- H. M. Haendler, C. M. Wheeler and D. W. Robinson, The Thermal Decomposition of Ammonium Heptafluorozirconate (IV), J. Am. Chem. Soc., 1952, 74(9), 2352–2353 CrossRef CAS.
- I. N. Flerov, M. S. Molokeev, N. M. Laptash, A. A. Udovenko, E. I. Pogoreltsev, S. V. Mel’nikova and S. V. Misyul, Structural Transformation between Two Cubic Phases of (NH4)3SnF7, J. Fluorine Chem., 2015, 178, 86–92 CrossRef CAS.
- A. V. Kartashev, M. V. Gorev, E. V. Bogdanov, I. N. Flerov and N. M. Laptash, Thermal Properties and Phase Transition in the Fluoride, (NH4)3SnF7, J. Solid State Chem., 2016, 237, 269–273 CrossRef CAS.
- J. Hala, Halides, Oxyhalides and Salts of Halogen Complexes of Titanium, Zirconium, Hafnium, Vanadium, Niobium and Tantalum, in The Solubility Data Series, ed. J. W. Lorimer, International Union of Pure and Applied Chemistry, Oxford, 1989, vol. 40 Search PubMed.
- S. Nishimura, Production of Metallic Oxide and Metal Powders by Crystallization Stripping Applying Solvent Extraction, Miner. Process. Extr. Metall. Rev., 2000, 21(1–5), 143–165 CrossRef CAS.
- M. A. White, H. Shi and J. C. Leiper, Preparation and Characterization of (NH4)3AlF6 and NH4AlF4 including the Perdeutero Salts (ND4)3AlF6 and ND4AlF4, J. Fluorine Chem., 1993, 62(2–3), 211–216 CrossRef CAS.
- D. B. Shinn, D. S. Crocket and H. M. Haendler, The Thermal Decomposition of Ammonium Hexafluoroferrate(III) and Ammonium Hexafluoroaluminate. A New Crystalline Form of Aluminum Fluoride, Inorg. Chem., 1966, 5(11), 1927–1933 CrossRef CAS.
- B. N. Ivanov-Emin, T. N. Susanina and A. I. Ezhov, Investigation of Ammonium Fluoroscandates, Zh. Neorg. Khim., 1967, 12, 23–28 CAS.
- T. A. Sviridova, Yu. V. Sokolova and K. Yu. Pirozhenko, Crystal Structure of (NH4)5Sc3F14, Crystallogr. Rep., 2013, 58(2), 220–225 CrossRef CAS.
- G. Åkerlöf, Dielectric Constants of Some Organic Solvent-Water Mixtures at Various Temperatures, J. Am. Chem. Soc., 1932, 54(11), 4125–4139 CrossRef.
- R. L. Madan, Chemistry for Degree Students, S. Chand Publishing Ltd, New Delhi, 2011 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: XRD patterns of synthesized solids before and after solubility tests and calculated volumetric charge densities of metal cations. See DOI: https://doi.org/10.1039/d2ra07516d |
| ‡ Peters E. M. conducted this research as part of his PhD thesis at KTH Royal Institute of Technology in Stockholm, Sweden. |
| This journal is © The Royal Society of Chemistry 2023 |