Open Access Article
Open Access ArticleAdsorption of heavy metal onto biomass-derived activated carbon: review
Baoying Wanga,
Jingming Lana,
Chunmiao Bo a,
Bolin Gong
a,
Bolin Gong *a and
Junjie Ou
*a and
Junjie Ou *abc
*abc
aSchool of Chemistry and Chemical Engineering, Key Laboratory for Chemical Engineering and Technology, State Ethnic Affairs Commission, Ningxia Key Laboratory of Solar Chemical Conversion Technology, North Minzu University, Yinchuan 750021, PR China. E-mail: gongbl@nxu.edu.cn
bCAS Key Laboratory of Separation Science for Analytical Chemistry, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian 116023, China. E-mail: junjieou@dicp.ac.cn
cUniversity of Chinese Academy of Sciences, Beijing 100049, China
First published on 31st January 2023
Abstract
Due to the rapid development of the social economy and the massive increase in population, human beings continue to undertake processing, and commercial manufacturing activities of heavy metals, which has caused serious damage to the environment and human health. Heavy metals lead to serious environmental problems such as soil contamination and water pollution. Human health and the living environment are closely affected by the handling of heavy metals. Researchers must find several simple, economical and practical methods to adsorb heavy metals. Adsorption technology has been recognized as an efficient and economic strategy, exhibiting the advantages of recovering and reusing adsorbents. Biomass-derived activated carbon adsorbents offer large adjustable specific surface area, hierarchically porous structure, strong adsorption capacity, and excellent high economic applicability. This paper focuses on reviewing the preparation methods of biomass-derived activated carbon in the past five years. The application of representative biomass-derived activated carbon in the adsorption of heavy metals preferentially was described to optimize the critical parameters of the activation type of samples and process conditions. The key factors of the adsorbent, the physicochemical properties of the heavy metals, and the adsorption conditions affecting the adsorption of heavy metals are highlighted. In addition, the challenges faced by biomass-derived activated carbon are also discussed.
1. Introduction
Heavy metals can cause serious harm to the environment and human health because most of them are toxic, mutagenic and carcinogenic.1 Heavy metal pollution is one of the environmental pollution caused by heavy metals or heavy metal-derived compounds such as lead, cadmium, cobalt, and copper, which are produced by mining, waste gas discharge, sewage irrigation and industrial wastewater discharge.2 These metals are also considered harmful at concentrations greater than or equal to 5 mg L−1.3 These metal ions enter the atmosphere, water and soil, resulting in the concentration of heavy metals in the natural environment seriously exceeding the standard.4 Moreover, heavy metals in various chemical forms will accumulate, remain, and even migrate through the biological chain of the ecosystem after entering the environment, further leading to irreversible harm to the environment and the human body. The entry of heavy metals into human living environments is shown in Fig. 1.5 Great efforts have been made to develop efficient techniques for heavy metal removal in recent years, such as ion exchange,6 chemical precipitation,7 electrochemistry,8 membrane filtration,9 and adsorption.10 The carbon-based adsorption technique is widely applied in environmental protection, and biomass-derived activated carbon is recognized as one of the most economical and promising adsorbents for heavy metal removal. Fig. 2 shows recent publications obtained searching for “Heavy metal pollution” and “Removal of heavy metal” in Topic via Web of Science. As can be seen, there were over 3500 publications related to heavy metal pollution and over 2500 publications associated with the removal of heavy metals in the period of 2018–2022.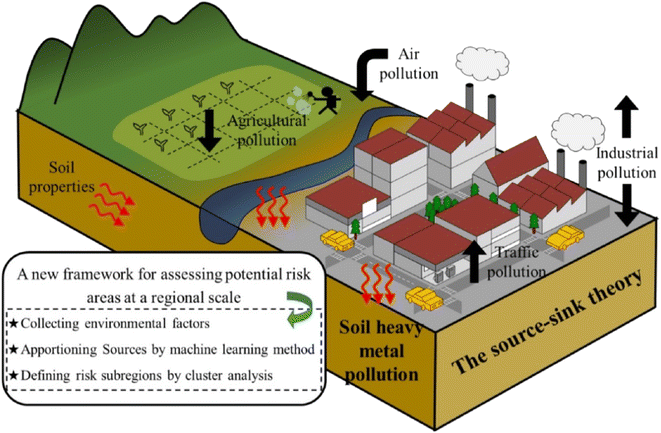 | ||
| Fig. 1 Environmental pollution map of heavy metals. This figure has been adapted/reproduced from ref. 11 with permission from Journal of Hazardous Materials, copyright 2023. | ||
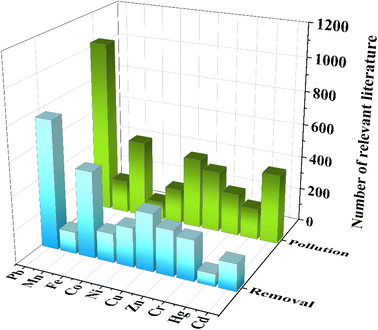 | ||
| Fig. 2 Number of scientific publications related to the removal of various heavy metals with different techniques. | ||
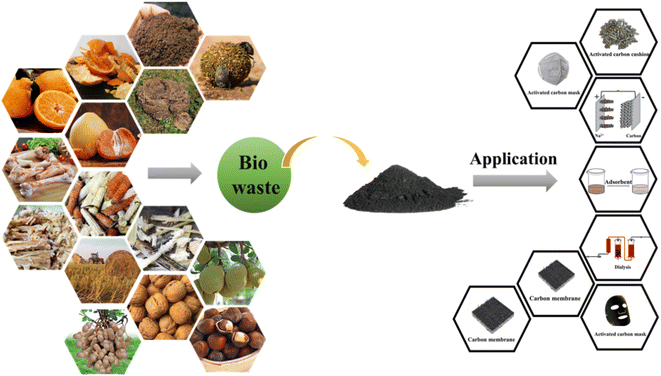
The world produces a large amount of agricultural waste discarded indiscriminately every year, resulting in a huge waste of resources. The rational application of agricultural waste can not only reduce environmental pressure, but also create economic value, so it is necessary to develop new ways of recycling agricultural waste. Agricultural waste refers to the waste generated from agricultural production, livestock breeding, agricultural and sideline products processing, and life activities of rural residents, mainly including residues in farmland and orchard, livestock manure, and residues in agricultural and sideline products and domestic waste. These wastes not only contain 65% to 90% of carbon, hydrogen, and oxygen, but also are rich in nitrogen, phosphorus, potassium, calcium, magnesium, sulfur, as well as trace elements. It is essential to pay attention to the extensive use of agricultural waste for sustainable development. Agricultural waste is usually the first choice to prepare biomass-derived activated carbon, attributing to its high specific surface area, high stability, excellent adsorption properties, simple production process and low price.12
This paper focuses on the advanced preparation progress of biomass-derived activated carbon and its application in the adsorption of heavy metals from wastewater in the past five years. By collecting relevant information about heavy metals adsorbed by biomass and using appropriate language, the carbonization, activation and modification of biomass-derived activated carbon are reviewed. Furthermore, key factors controlling the adsorption process are summarized. The challenges of biomass-activated carbon facing in the adsorption of heavy metals are also discussed. The summary scheme of whole article is shown in Fig. 3.
2. Research status and control methods of heavy metals
2.1 Sources of heavy metals
Heavy metals are a large family with about 45 members in chemistry. Heavy metals have the commonness of irritation, target organ toxicity, carcinogenicity and immunotoxicity, and excessive discharge of them in nature will seriously endanger the health of water, soil and organism.13–17 Heavy metals in the atmosphere mainly come from gases and dust produced by energy, transportation, metallurgy and building materials. The wastewater from industrial and mining enterprises is discharged into the sewer and mixed with domestic wastewater without diversion treatment, increasing the content of heavy metal in the soil of sewage irrigation area year by year.18 During the stacking or treatment of both mining and industrial solid wastes, heavy metals are easy to move due to sunlight, rain and water washing and diffuse into the surrounding soil and water body in a radial and funnel shape. Pesticides, chemical fertilizers, and plastic film are necessary agricultural materials to promote the development of agricultural production. However, the unreasonable long-term application can also lead to heavy metal pollution of soil.Table 1 shows the sources and health effects of some harmful heavy metals such as lead, cadmium, mercury, chromium, and zinc ordinary in living. Any of these heavy metals can cause headache, dizziness, insomnia, forgetfulness, nervous disorder, joint pain, stones, cancer, etc19. For example, lead intake of human body is mainly through meat, fruits, fish, shrimp, eggs, etc, while lead in grains and vegetables comes from the soil, water. There are two primary sources of lead in the atmosphere, man-made emissions and natural sources. Natural source emissions are about one-tenth of man-made emissions. It is reported that the average proportion of zinc measured in the soil near the smelter in Korea is 45.8–83.3%. Zinc concentration ranges higher than normal land. The average concentrations of lead, zinc and cadmium near the plant in northern Iran (302, 311 and 9.83 mg kg−1, respectively) are also significantly higher than those in the control soil.20,21 Lead has harmful effects on multiple organ systems through different mechanisms. Some heavy metals in water can be transformed into more toxic metal compounds under the action of microorganisms. Japan's pain is caused by cadmium entering the water body, painful disease is a strange disease that first occurred in the watershed of Toyama Prefecture, Japan, causing a large loss of calcium in human bones, osteoporosis, bone atrophy and joint pain. There was a patient who sneezed and broke many parts of his body. The other patient finally had 73 fractures in his whole body, and his length was shortened by 30 cm. His illness was very miserable. Painful disease has been prevalent in the local area for more than 20 years, causing more than 200 deaths.22 After ingesting mercury, the human body will directly enter the liver and seriously damage the brain's optic nerve. Chromium poisoning can cause limb numbness and mental abnormalities. And the clinical manifestations of zinc poisoning are abdominal pain, vomiting, diarrhea, gastrointestinal bleeding, anorexia, fatigue and lethargy. Copper is an essential material for the development of industrialization and urbanization, and needed by more than 90% of modern industrial enterprises, copper smelters have caused severe pollution. For example, more than 130 hectares of farmland near the largest non-ferrous metal smelters in China have been polluted.23 Heavy metal pollution is common and especially harmful. It is urgent to remove heavy metal pollution.
| Heavy metal | Main sources | Harm to human body | Ref. |
|---|---|---|---|
| Lead | Cosmetics, puffed food, water pipes, cast type, radioactive radiation, X-ray protective equipment, etc. | Anemia, neurological disorders, renal injury | 24,25 |
| Cadmium | Industrial wastewater, waste batteries, pigments, plastic stabilizers, coating corrosion prevention of steel parts, etc. | Severe bone softening and visceral dysfunction | 26,27 |
| Mercury | Precious metal smelting, cosmetics, lighting lamps, aquatic organisms, dental materials, etc. | Damage brain cells and kidneys, causing systemic poisoning | 28 |
| Chromium | Leather preparation, metal chromium plating, inferior cosmetic raw materials, etc. | Pharyngitis, bronchitis, rhinitis, dermatitis, tuberculosis | 29–31 |
| Zinc | Machinery manufacturing, and emissions from industries such as galvanizing, instrumentation, organic synthesis and papermaking | Metabolic disorder, anemia, zinc fever, etc. | 32,33 |
2.2 Removal methods of heavy metals
There are many treatment methods for heavy metal wastewater, including methods of ion exchange, chemical precipitation, electrochemical, membrane filtration, adsorption, etc. Table 2 shows a few methods commonly utilized to remove heavy metals and summarizes their advantages and disadvantages.| Methods | Advantages | Disadvantages | Ref. |
|---|---|---|---|
| Ion exchange | High removal rate, high concentration of useful substances, high recovery rate, simple equipment and convenient operation and control | High pre-treatment requirements, excessive regeneration waste, long experimental cycles, high salt consumption and poor general applicability | 34 |
| Chemical precipitation | Simple process, low equipment investment, convenient and safe operation | More chemical precipitation and high treatment cost | 35 |
| Electrochemical | No requirement of oxidant, flocculant and other chemicals, no secondary pollution, equipment with small volume, small floor area and convenient and flexible operation, low operation cost and remarkable pollutant removal effect | Low treatment efficiency of trace samples, large power consumption, and requirements of conductive salt for wastewater quality | 36 |
| Membrane filtration | Low energy consumption, high efficiency, simple process and low investment | Membrane fouling, frequent membrane replacement, high cost | 37 |
| Adsorption | Good selectivity, high stability, good treatment effect, convenient management, high adsorption efficiency, simple process, convenient installation, good reusability and simple material replacement | High usage and high cost | 38 |
| Adsorbent | BET surface area (m2 g−1) | Aperture (nm) | Characteristic | Ref. | |
|---|---|---|---|---|---|
| Polymer adsorbent | Polyacrylamide resin | ≤1000 | 0.3–4 | High adsorption rate, large adsorption capacity, good performance, stable to alkali, unstable to inorganic acids, good adsorption performance for organic wastewater. Poor antistatic property, poor selectivity after regeneration and heat resistance | 55 |
| Inorganic adsorbent | Aluminium oxide | 200–500 | 2–50 | Strong affinity, good purification performance of acid waste gas. Low service life | 56 |
| Zeolite | 500–1000 | 0.4–1 | Regular microporous structure, good thermal stability, hydrothermal stability, strong selectivity, good adsorption performance. Poor anion adsorption, hole blockage | 57 | |
| Carbon based adsorbent | Activated carbon | 500–1000 | 2–50 | Rich microporous and mesoporous structures, adsorption of organic compounds, heavy metals, heavy hydrocarbons and other organic substances | 58 |
| Biomass adsorption material | Microalgae | — | — | Fast growth and reproduction, strong tolerance, high removal rate. Poor system stability, prone to pollution and death, high requirements for training equipment, not conducive to practical application | 59 |
| Agricultural waste | — | — | Wide source, low cost, adsorption of dyes and heavy metals. Not recyclable, easy to cause secondary pollution | 60 | |
Common polymer adsorption materials include polystyrene, polyacrylate, polyacrylamide resins with good plasticity. Their functional groups with specific properties can be easily introduced through chemical modification. Additionally, polymer materials can also be swelled, which will expand their three-dimensional structure and is conducive to the mass transfer rate of adsorption. Researcher studied the adsorption performance of lead and copper ions using macroporous polystyrenic chelating resin with a thiourea functional group. The ion-exchange recovery of Cu2+ exceeded 95% with resin dosage of 0.070 g mL−1 in the pH range of 2.5–4.5.61 The resins have the advantages of fast adsorption speed, high adsorption capacity and wide application range. Furthermore, they can be utilized well at low temperature and normal pressure conditions and regenerated after simple treatment. However, the disadvantage of ion exchange resin is relatively low selectivity.62 The ions of the resin itself are generally low valence ions, so when the resin contacts with water, the high valence ions in water will be adsorbed and the low valence ions will be released. For example, divalent ions are more easily adsorbed than monovalent ions.63
Common inorganic adsorbents include alumina and zeolite, which have the characteristics of rich reserves and low price. Alumina is made by heating and dehydration of aluminum hydrate, whose properties depend on the structural state of initial hydroxide.64 It is divided into alkaline alumina, acidic alumina, and neutral alumina. Alkaline alumina is suitable for separating alkaline substances (such as amines and alkaloids) and acid-sensitive samples (such as acetals and glycosides) and neutral substances such as hydrocarbons and steroids. Acidic alumina is appropriate for separating acidic substances such as organic acids, amino acids, pigments and aldehydes. Neutral alumina is proper for separating aldehydes, ketones, quinones, glycosides, nitro compounds and unstable substances in an alkaline medium, such as esters and lactones.65 The activated carbon was prepared using rice husk and reacted with it using caustic soda and alumina at 500 °C. The adsorption efficiency of uranium was 96.35% at an equilibrium time of 1 h, and an initial uranium concentration of 121 mg L−1.66 Activated alumina also shows good adsorption properties due to its high pore surface activity and affinity for water.67 Zeolite also showed excellent adsorption properties. It has a uniform pore size. And it has been widely used in gas adsorption and separation, gas and liquid drying and separation of n-isoalkanes. Compared with other adsorbents, zeolite also has special adsorption performance. Under the condition of low partial pressure (or low concentration) and high temperature, the molecular sieve is significantly superior over other adsorbents. The pores of zeolite are easy to be blocked.68
Carbon-based adsorbent refers to the porous carbonaceous material with high specific surface made of coal or organic matter. Activated carbon is one of the most widely used carbonaceous materials. A new type of activated carbon was prepared, it takes straw as precursor because the carbon element content in the straw was about 40%. The removal efficiencies of heavy metals (Cu2+, Cr4+ and Fe3+) aren't less than 90%. And the adsorption–desorption experiments indicated that the adsorbent was sufficiently reusable.69 Activated carbon contains many pores and has an excellent adsorption capacity it is applied in a wide range of applications such as waste water treatment, decolorization, catalysis and medical applications.70
Bio-adsorbent refers to biomaterials that can adsorb heavy metals and other pollutants. At present, fungi, bacteria, algae and agricultural wastes are widely studied and applied as biosorption materials. This material generally contains a large number of active groups such as hydroxyl, carboxyl and amino groups, which can have electrostatic adsorption, chelation, coordination and inorganic micro precipitation with heavy metal ions. In northern Chile, Chlorella and Echinococcus scenarios were tested for metal mine tail water removal. The removal rates of copper and molybdenum by microalgae were 64.7% and 99.9%.71 Although biomass materials are good, biomass such as microalgae is not easy to survive, and the adsorption capacity is not high. The adsorption capacity of pomelo peel to methylene blue and chromium ions in wastewater was explored. The maximum adsorption capacities for methylene blue and chromium ions were 219 mg g −1 and 11.3 mg g −1, respectively. The main adsorption mechanism for methylene blue is electrostatic attraction and hydrogen bond formation, while the adsorption mechanism for chromium ion is electrostatic attraction.72 Although biomass adsorbent has the advantages of low price, its adsorption capacity is lower than other adsorption materials.
In a word, the adsorption method is considered as one of the most favorable methods to deal with pollutant due to its advantages of good treatment effect, wide application range, easy recovery, comprehensive source of adsorbent, reusability and the treatment effect of adsorption method is less affected by the concentration of heavy metal ions.73 The carbonaceous materials are considered as the efficient adsorbent because of high surface area and a variety of active surface sites.74 However, the regeneration of industrial activated carbon is a high cost and complex process, which makes the application of this material economically infeasible. In addition, other types of carbon materials are not suitable to be adsorbents because of their high cost or poor adsorption performance. For example, the adsorbents were prepared by oxidation of multiwall carbon nanotube, then derivatizing the oxidized product with hydroxyl amine, hydrazine and amino acid. The adsorption capacity of the adsorbent for lead ion is 24.34 mg g−1.25 In order to reduce the overall cost and increase adsorption capacity in this adsorption process, using low-cost and due to the rich structure of biomass as the precursor of activated carbon has become a promising strategy.
3. Biomass-derived activated carbon
As one of the most miraculous materials in nature, carbon materials have excellent properties such as resistance to acids and bases, corrosion, high and low temperatures, and biocompatibility. Additionally, through its sp electron orbital hybridization, it can mutate into different forms and properties, including excellent electrical conductor, semiconductor material and insulator, opaque black material or explicit transparent material and so on. Fig. 4 shows the manifestations of carbon with different dimensions.75 Carbon materials have played a significant role throughout history as human civilization has progressed. At each stage of history, carbon had a unique expression. Since the invention of fire, porous charcoal has been used consciously or unconsciously. For example, the use of charcoal to stop bleeding, the use of soot to heal wounds, the use of carbon sterile sheets. From the deodorization and disinfection of clothes, food and housing in daily life to the profound evolution of living places. From protective escape materials in personal space to environmental purification in public places.76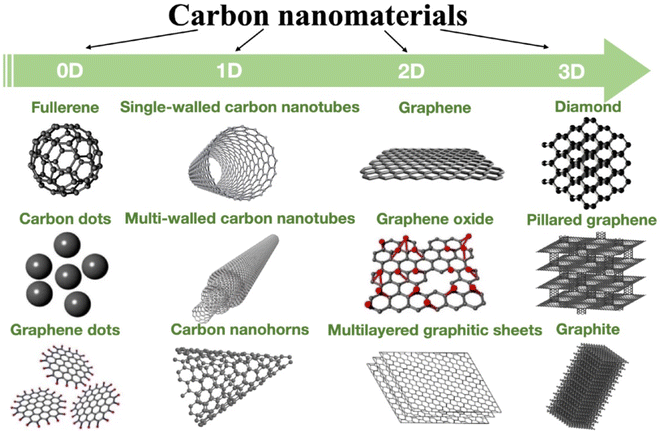 | ||
| Fig. 4 Carbon allotropes in different dimensions. Environmental pollution map of heavy metals. This figure has been adapted/reproduced from ref. 75 with permission from Carbon, copyright 2023. | ||
Different types of activated carbon have emerged following the advent of coal and oil and the rapid development of modern industry. Activated carbon is widely used, and the raw materials for manufacturing activated carbon are also various. There are many sources of raw materials for making activated carbon, including animals, plants, coal, petroleum by-products, and other raw materials such as synthetic resin and organic substances. In general, carbon materials can be divided into three categories, as summarized in Table 4. Mineral activated carbon can be widely used in many fields of liquid phase application and gas-phase application, it is difficult to make high-performance activated carbon in high-demand fields due to containing high impurities. Furthermore, with the increase of raw material cost in recent years, its advantages gradually receded. Activated carbon prepared from waste car tires has also been widely used. The waste car tires need high-temperature pyrolysis, which consumes a lot of energy and costs a lot. Among many raw materials, the advantages of biomass to prepare activated carbon are particularly prominent. Modern agriculture uses a large amount of chemical fertilizer to replace farmyard organic fertilizer and artificial feed to replace agricultural waste feed. In addition, the intensive and large-scale development of modern agriculture has broken the waste recycling in traditional agriculture. As a result, much agricultural waste has been accumulated, resulting in serious environmental problems and resource waste. Therefore, the rational utilization of agricultural waste resources has become a great challenge faced by most countries. The preparation of activated carbon from biomass realizes the concept of waste utilization and resource utilization. The harmless treatment of agricultural waste is an effective way to control agricultural, environmental pollution, improve the rural environment and develop a circular and sustainable economy. As a result, activated carbon fabricated from biomass has made great contributions to energy conservation and environmental protection through waste reuse.
| Activated carbon | Raw materials | Shape | Characteristics | Ref. |
|---|---|---|---|---|
| Biomass-activated carbon | Coconut husk, wood flour, coffee bean stalks, oil palm husk, sugarcane bagasse, manure, animal bones, etc. | Powdered, honeycomb, columnar, granular and powdered activated carbon, etc. | Wide aperture distribution, and most micropores. Adsorb substances of different sizes | 77–79 |
| Mineral raw materials activated carbon | Peat, bituminous coal, anthracite, bitumen and petroleum and their processing products, etc. | Columnar activated carbon, honeycomb activated carbon, spherical activated carbon, raw coal crushed carbon, etc. | Flat mesopores and micropores. Adsorb small molecules and macromolecules with large molecular diameter | 80,81 |
| Other raw materials activated carbon | Waste rubber, waste plastic, etc. | Columnar activated carbon, etc. | Adsorb organic matter and heavy metals | 82,83 |
According to the definition of the International Energy Agency (IEA), “biomass” refers to various organisms formed through photosynthesis.84 Its definition can be divided into broad and narrow sense.85 The former includes all plants, microorganisms, and waste generated by them, while the latter refers to inedible crop by-products, agricultural and forestry wastes such as wheat straw, fruit shell, wood, and other lignocellulose, and livestock manure (Fig. 5).86 Biomass materials are renewable, widely available, inexpensive, environmentally compatible, porous and environmentally friendly, and the adsorbents prepared after modification have the advantages of high selectivity and removal rates.87,88
Biomass activated carbon and activated carbon are both carbonaceous pyrolysis materials and important products of environmental technology. The main raw materials of activated carbon are divided into four categories, including coal activated carbon, wood activated carbon, synthetic activated carbon and other types of activated carbon. The precursor material of biomass activated carbon is only biomass.
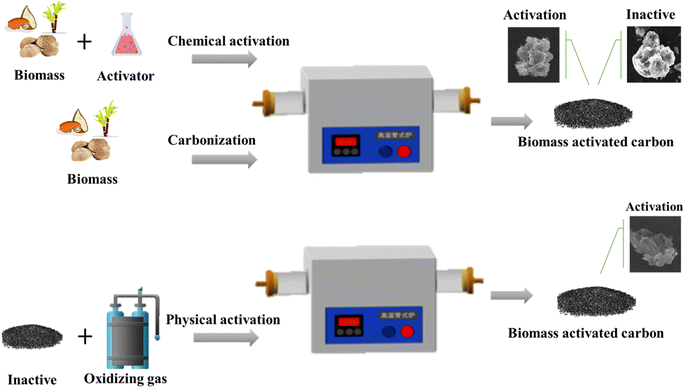
3.1 Preparation of biomass derived activated carbon
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, and the carbonization temperature was 500 °C. Under these conditions, the removal rate of chromium was more than 98%, and the maximum adsorption capacity was 9.99 mg g−1. The concentration of residual chromium in water was less than 0.05 mg L−1. In another method, the carbonized carbon is activated with an activator in a certain ratio at high temperature.
2, and the carbonization temperature was 500 °C. Under these conditions, the removal rate of chromium was more than 98%, and the maximum adsorption capacity was 9.99 mg g−1. The concentration of residual chromium in water was less than 0.05 mg L−1. In another method, the carbonized carbon is activated with an activator in a certain ratio at high temperature.Chemical activation is known to induce specific surface features of porosity and functionality which play a definite role in enhancing the adsorptive potential of the developed activated carbons. The physical and chemical properties of activated carbon are affected by the type of biological reagent and the impregnation time. The effect of zinc chloride and potassium hydroxide on the activation of lignocellulose activated carbon were investigated, respectively.98 The carbon impregnated with zinc chloride produces micropores, while the carbon impregnated with potassium hydroxide forms a wide pore size distribution. The activated carbon obtained by both activation methods exhibited large specific surface area, and the pore size is mainly composed of mesopores and micropores. The chemical activation method possesses the advantages of good treatment efficiency of activated carbon, wide application range and easy recovery. In the process of chemical activation, many chemical reagents such as potassium hydroxide, phosphoric acid, zinc chloride, and potassium carbonate are the most commonly used as chemical activators (Fig. 7).
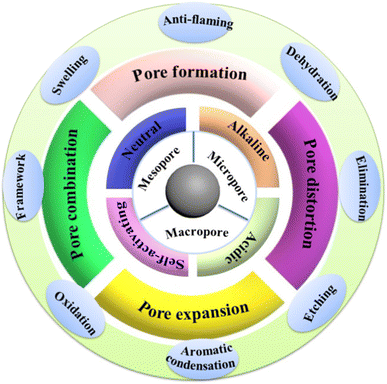 | ||
| Fig. 7 Schematic diagram of activation mechanism by alkaline and acidic activating agent. Carbon allotropes in different dimensions. Environmental pollution map of heavy metals. This figure has been adapted/reproduced from ref. 99 with permission from Science of the Total Environment, copyright 2023. | ||
Table 5 shows in detail the effect of different raw materials and different proportions of activators on the specific surface area of activated carbon. Activators can be divided into alkaline, acidic, neutral, self-activator so on. Carbon materials with different raw materials and activators have different specific surface area. Phosphoric acid is a common acidic activator. When phosphoric acid is used, the activation temperature is lower, and the activation time is shorter. Potassium and sodium hydroxide are the most effective activators for preparing biomass-derived activated carbon with a high specific surface area. The specific surface area of biomass-derived activated carbon prepared from Chinese fir bark and longan shell with potassium hydroxide is higher than 1000 m2 g−1. The specific surface area of activated carbon derived from guava seed and honeycomb biomass activated by sodium hydroxide is higher than 2000 m2 g−1. It is generally believed that potassium hydroxide and sodium hydroxide can inhibit the formation of tar, reduce the activation reaction temperature, accelerate the removal of non-carbon components and improve the carbonize reaction rate. Sodium amino and magnesium chloride are neutral activators. The specific surface area of biomass-derived activated carbon prepared by them is higher than 1000 m2 g−1, the activator for a neutral activator is used at higher temperature. The biomass-derived activated carbon activated by potassium phosphate as a self-activator has slightly lower specific surface area, it can simplify the procedure, reduce the cost and the risk of environmental consequences, and better adapt to scalable production.
| Biomass source | Activator | Activating agent to biomass ratio | Activation conditions | BET surface, area (m2 g−1) | Ref. |
|---|---|---|---|---|---|
| Coconut shell | Phosphoric acid | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
800 °C, 2 h | 2648 | 100 |
| Orange peel | Phosphoric acid | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
700 °C, 1 h | 2210 | 101 |
| Coffee grounds | Phosphoric acid | 1.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
450 °C, 1 h | 925 | 102 |
| Chinese fir bark | Potassium hydroxide | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
700 °C, 2 h | 1242 | 103 |
| Longan shell | Potassium hydroxide | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
800 °C, 2 h | 3260 | 104 |
| Guava seed | Sodium hydroxide | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
800 °C, 1 h | 2574 | 105 |
| Honeycomb | Sodium hydroxide | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
650 °C, 2 h | 3291 | 106 |
| Chestnut shell | Sodium amino | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
450 °C, 1 h | 2615 | 107 |
| Textile | Magnesium chloride | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
900 °C, 1.5 h | 1307 | 108 |
| Lignin | Potassium phosphate | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
900 °C, 2 h | 477 | 109 |
Physical activation is also known as gas activation. The feedstock is carbonized in the absence of oxygen or inert gas at a certain temperature.110–113 The commonly used activation gases include oxidizing gases such as steam, air, CO2 and O2.114–118 In the process of activation, carbon reacts endothermically with H2O and CO2, and the reaction rate is moderate. The reaction between carbon and O2 is exothermic, and the reaction rate is fast.119–124 Therefore, the reaction time is shortened when O2 is added to the mixture to burn the intermediate products H2 and CO and release heat. And the reaction of carbon with CO2 and water vapor makes the porous structure highly developed.125 Physical activation also has little environmental pollution.126 The physical activation method of CO2 and steam agent to activate were investigated.127 The activation under the condition of CO2 and steam saturation correspondingly increases the specific surface area from 89 to 653 m2 g−1 and from 89 to 1015 m2 g−1, respectively. Steam also promotes the generation of mesoporous structures of carbon products, thus expanding their potential applications. Jiang et al.128 also investigated the pore properties and structural characteristics of activated carbon prepared from coconut shells. According to the thermochemical properties of coconut shell, the process was characterized by carbonization at 500 °C with a constant heating rate of 10 °C min−1 under nitrogen flow and then switching to gasification with CO2. The gas temperature in the same reactor was set to 700–900 °C for activation. The pores of coconut shell-activated carbon increased with the increase of activation temperature and holding time. These findings are attributed to the severe reaction of lignocellulose-based carbon with CO2. The surface area reached 1100 m2 g−1, and the mesoporous rate exceeded 40%. Physical activation method shows excellent advantages. More different biomass precursors are activated by the different activation method, whose experimental conditions and biomass-derived activated carbon physical and chemical properties are presented in Table 6. It is easy to find that the type of activator, the time and temperature of carbonization, the ratio of raw materials to activator, and the raw materials have severe impacts on the specific surface area of activated carbon.
| Biomass source | Experimental condition | BET surface, area (m2 g−1) | Ref. | |
|---|---|---|---|---|
| Carbonization | Activation | |||
| Bean dregs | Nitrogen, 800 °C, 0.5 h | 30 mL steam, 1000 °C, 0.5 h | 1004 | 129 |
| Bamboo | Nitrogen, 10 °C min−1 950 °C | 80 mL min−1 ammonia, 950 °C, 2 h | 2032 | 130 |
| Eucalyptus original wood chip | Oxygen, 2.5 °C min−1 700 °C | 700 °C, 2 h | 870 | 131 |
| Rice husk | 200 mL min−1 nitrogen, 900 °C | 0.4 mL min−1 steam, 900 °C, 0.5 h | 1343 | 132 |
| Mushroom substrate | 200 cm3 min−1 nitrogen, 900 °C | 2.0 mL min−1 steam, 900 °C, 1 h | 332 | 133 |
| Jute | Nitrogen, 500 °C, 1 h | Carbon dioxide, 800 °C, 1.5 h | 1120 | 134 |
| Amazon Peshawar fiber | Oxygen, 100 °C h−1 550 °C, 1 h | 150 mL min−1 carbon dioxide, 800 °C, 2 h | 804 | 135 |
| Hops | 170 mL min−1 nitrogen, 10 °C min−1 550 °C, 1 h | 250 mL min−1 carbon dioxide, 800 °C, 1 h | 417 | 136 |
| Apple branch | Oxygen, 500 °C | Nitrogen, carbon dioxide, 500 °C, 2 h | 526 | 137 |
| Large | 100 cm3 min−1 nitrogen, 5 °C min−1 300 °C, 2 h | 165 cm3 min−1 carbon dioxide, 750 °C, 1 h | 740 | 138 |
3.2 Modification of biomass-derived activated carbon
The adsorption performance of biomass-derived activated carbon is determined by its physical and chemical properties. The physical property of biomass-derived activated carbon refer to its specific surface area and pore structure, and the adsorption capacity of carbon is affected by these factors. The chemical property of biomass-derived activated carbon depends on the type and number of surface functional groups, which impact the interaction between the activated carbon and pollutants.139–145 The existing biomass-derived activated carbon possesses certain limitations in the actual wastewater treatment due to large variety of pollutants and high standard requirements for wastewater discharge.146–148 Modification techniques for biomass-derived activated carbon are very important. And the physicochemical properties of biomass-derived activated carbon are closely related to the raw materials, carbonization method, activation and modification techniques. The modification techniques of activated carbon include physical, chemical or microbiological methods by changing the physical structural properties or surface chemical functional groups of biomass-derived activated carbon.149,150Acid modification technique is the oxidation treatment of activated carbon with oxidizing agents such as nitric acid, sulfuric acid, citric acid, and hypochlorous acid under appropriate conditions.151–154 The functional groups on the surface of activated carbon are oxygen-containing functional groups, such as hydroxyl, carboxyl, carbonyl and lactone groups, which are introduced through the oxidation pathway.155–157 The introduction of carboxyl groups on the surface of biomass-derived activated carbon will play an important role in the adsorption of metal ions. The hydrogen in the carboxyl group will be ion-exchanged by metal ions, and the carboxyl group will chelate with metal ions.158,159 Li et al.160 employed in situ modification of the activated carbon with phosphoric acid and disodium EDTA salts to increase the adsorption of nickel ions. The adsorption capacity was increased from 15.4 to 27.9 mg g−1. Nickel ions were adsorbed by cation exchange, electrostatic interaction and surface complexation mechanism, and the adsorption amount was proportional to its surface functional groups under appropriate environments. A similar experiment is that the spent coffee grounds were used as a precursor to obtain activated carbons. The raw material was modified with phosphoric acid. The specific surface area of the carbon increased to 720 m2 g−1, and the pore volume increased to 0.334 cm3 g−1. And all the obtained carbons possessed acidic (mainly carboxyl) groups and exhibited an amorphous structure.161 These results indicated that activated carbon-containing carboxyl groups have very promising adsorption prospects. The pyrolysis-driven eucalyptus carbon was treated with phosphoric acid.162, whose surface area was increased almost fivefold from 253 to 1265 m2 g−1. The modified biomass-derived activated carbon was able to remove 99.76% of chromium, which was higher than the unmodified activated carbon (25.24%). Such high adsorption capacity was attributed to not only excellent specific surface area, but also the phosphoric acid modification which increases the oxygen-containing functional groups on the surface. Li et al.163 investigated the metal removal efficiency of carbonized sewage sludge obtained at 500 °C, and nitric acid modification. The prepared biomass-derived activated carbons removed 98.9%, 42.6% and 34.6% of copper, zinc and aluminum ions, respectively, in less than 5 min. The complexation of functional groups to metal ions not only shortened the adsorption time, but also exhibited higher removal efficiency.
Alkali modification of activated carbon refers to the use of alkaline reagents such as sodium hydroxide, potassium hydroxide and ammonia to modify the activated carbon.164,165 For example, the ZnO-activated carbon was successfully prepared from sugarcane bagasse with sodium hydroxide impregnation, followed by carbonization, and the ZnO-activated carbon nanocomposite was finally synthesized by the hydrothermal method.166 The removal efficiency of chromium reached 97% at pH = 2. Additionally, the adsorption capacity of activated carbon for chromium ions in wastewater was increased after the modification in a high-temperature environment. Biomass-derived activated carbon is modified in the temperature range of 950 °C, producing a surface area >2000 m2 g−1.118 The microporous structure, specific surface area, iodine adsorption value, and nitrogen-containing groups on the surface of modified activated carbon increased. Similarly, researchers synthesized biomass-derived activated carbon with Schiff base structure by ammonia-assisted hydrothermal treatment of industrial hemicellulose and applied to adsorb toxic hexavalent chromium, possessing a high adsorption capacity of 349.6 mg g−1.167 There were electrostatic gravitational, redox, and chelation interactions between the amino groups, Schiff base structure, heterocyclic nitrogen of biomass-derived activated carbon and chromium ions. Although alkali modification has great advantage of improving the adsorption capacity, the adsorption process is often complex.168–170
Loading modification of activated carbon means that activated carbon is treated by soaking in the solution of the loaded material, and surfactants such as polyethylene polyamine and polyethyleneimine are combined to the surface of activated carbon. Such activators do not significantly change the acidity and alkalinity of the activated carbon surface, can improve the adsorption capacity of the activated carbon for pollutants.171,172 Jiang et al.173 investigated the removal of lead ions from wastewater by polyethyleneimine loaded activated carbon, and the removal rate of lead ions increased from 32.6 to 214 mg g−1. Through the study of polyethyleneimine modified activated carbon, it is not difficult to find that the adsorption capacity of modified activated carbon for formaldehyde also increased significantly.174 The adsorption capacity of unmodified activated carbon was 190 mg g−1, and the adsorption capacity of activated carbon after loading 2.50 g of polyethyleneimine was up to 650 mg g−1, which was about 3.42 times that of unmodified activated carbon.
Plasma modification is an efficient, easy-to-operate, and environmentally friendly surface modification technique. In many recent researches, it plays an essential role as a new technology for the modification of activated carbon. The pore structure of activated carbon is changed, and the surface functional groups such as acidic hydroxyl, carbonyl and carboxyl are generated.175–177 Activated carbon particles from peat soils were modified by a flying jet plasma torch. The samples were dried at 70 °C for two nights, carbonized at 800 °C for 4 h, and then sieved on a 2 mm sieve. The surface morphology and chemical composition changed significantly. The increase in mass oxygen percentage indicated an increase in the carbonyl C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group, and higher polarity and solubility in polar liquids, an effective surface area increased from 1069 to 1270 m2 g−1, which more conducive to adsorption.178
O group, and higher polarity and solubility in polar liquids, an effective surface area increased from 1069 to 1270 m2 g−1, which more conducive to adsorption.178
In summary, acid modification is one of the most applied and technically mature methods so far, which is also one of the most beneficial methods to improve the adsorption capacity of activated carbon for metal ions in aqueous solution.194–198 The adsorption capacity of metal ions after alkali modification is weaker than that after acid modification. The reason is that alkali modification reduces the amount of acidic oxygen-containing functional groups on the surface of activated carbon, resulting in the binding sites reduction of metal ions on the surface of activated carbon. Moreover, the increase of nitrogen-containing functional groups enhances the alkalinity, and the competition mechanism between OH− and metal ions under an alkaline environment leads to the decrease of adsorption performance of activated carbon for metal ions. Heating modification and alkali modification are beneficial to improve the adsorption of organic matter in aqueous solutions by activated carbon.199–204 Loading modification allows for targeted loading of chemical substances on the surface of the activated carbon to enhance its adsorption capacity for the target substance.205 Microbial modification, which uses microorganisms adsorbed on the surface of activated carbon, can pre-degrade organic matter in an aqueous solution for the purpose of extending the service life of activated carbon.206 The special properties of activated carbon have led to its wide application as an adsorbent in wastewater treatment.207 With the development of activated carbon modification technology, special modification treatment can be applied to activated carbon according to the characteristics of pollutants in the water environment so as to realize the improvement of selective adsorption capacity of activated carbon in water treatment. Although many modification technologies have been proposed in recent years, many of them are not suitable for practical application because of their low efficiency, high energy consumption or serious toxic by-products. The research on the modification of activated carbon by combining existing modification methods is not yet in-depth. And the modification technology to improve the removal ability of two or more pollutants (such as metal ions and organic matter) has not yet been developed.
4. Key factors to control the adsorption of heavy metals by biomass derived activated carbon
4.1 Characteristics of biomass derived activated carbon
Three important factors including specific surface area, pore structure, and surface chemical functional group would affect the adsorption capacity of biomass-derived activated carbon.208,209The specific surface area of carbon materials can be increased by increasing the proper temperature and concentration of acid or alkali treatment, too high or too low temperature and pH will lead to the collapse of existing pores, thus reducing the specific surface area. Although most of the research has been conducted on activated carbons with large specific surface areas.215,216 However, a high surface area of activated carbon does not necessarily mean that it has the best adsorption capacity for organic compounds. Kharrazi et al.217 investigated the effect of pretreatment of elm sawdust with alkali metal on the adsorption of lead and chromium ions. The adsorption capacity of activated carbon for lead ions increased significantly from 233 to 1430 mg g−1. However, the specific surface area of the magnesium chloride leached pretreated carbon (585 m2 g−1) was found to be significantly reduced compared to the pristine carbon from the nitrogen adsorption/desorption isotherms and pore size distribution. In the preparation of oxidized mesoporous carbon with fluffy structure from petroleum asphalt, it can be found that the specific surface area decreased from 479 to 334 m2 g−1. However, the prepared carbon exhibited excellent absorption performance for the removal of malachite green and led ions with maximum adsorption capacities of 963 and 198 mg g−1, respectively.218
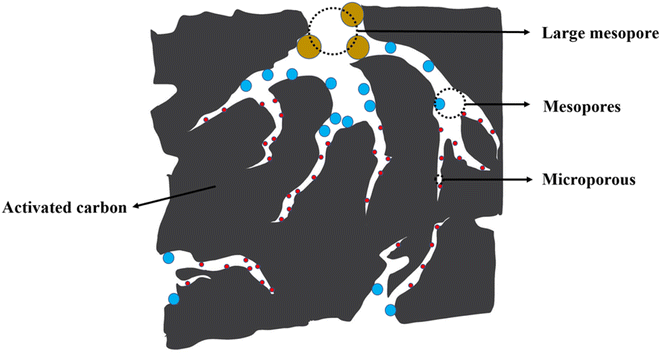
Macropores and mesopores can be utilized as diffusion channels for adsorbate molecules to enter the interior of activated carbon particles quickly. Micropores can adsorb pollutants through pore size interception. At the same time, mesopores can produce capillary condensation and adsorb macromolecules that cannot enter micropores. The pore structure is one of the main factors determining activated carbon's adsorption properties.229 Physical properties such as specific surface area and pore size heavily influence the adsorption capacity of activated carbon.
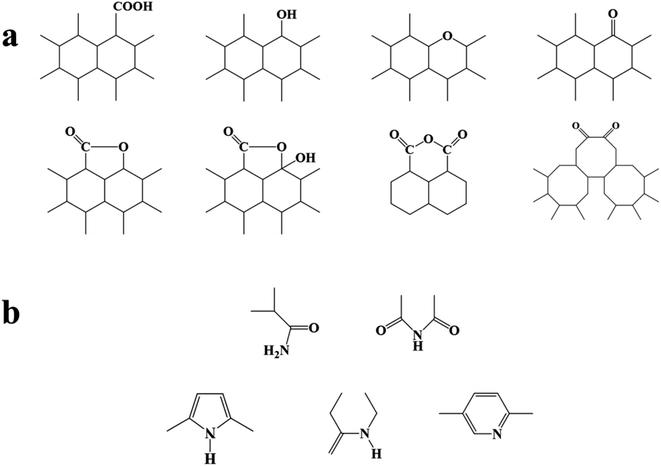 | ||
| Fig. 9 (a) Common oxygen-containing groups on the surface of activated carbon, (b) common nitrogen-containing groups on the surface of activated carbon. | ||
At activation temperature up to 100 °C, carbon adsorbs oxygen. The adsorbed oxygen molecules are decomposed into oxygen atoms, which are bonded to unsaturated bonds on the carbon surface, producing oxygen complexes. Due to their unstable nature, hydroxyl groups or other basic groups eventually form when they meet water. It also forms basic oxygen groups at 800 °C to 1000 °C in the presence of a little oxygen. The same phenomenon occurs when the activated carbon is cooled at low temperature in contact with oxygen. However, if the starting temperature of the activated carbon is 300 °C and the maximum temperature does not exceed 450 °C, some acidic oxygen groups will appear. Oxygen groups show three types of acidity, basicity, and neutrality, which are related to the oxidation phase.236–238 In general, liquid oxidation favors the formation of carboxylic acids, while gas oxidation favors the formation of hydroxyl and carbonyl groups.
In addition to oxygen-containing functional groups, the surface of carbon may also contain nitrogen-containing functional groups. Its main source is the introduction of ammonium, nitric acid and nitrogen-containing compounds in the preparation of activated carbon. Nitrogen atoms take different forms due to different conditions in the preparation of activated carbon. The nitrogen group on the surface of carbon is very stable. When heated at 900 to 1200 °C, most of the nitrogen changes to the free state, and a small portion locates at the surface of carbon in the formation of hydrogen cyanide compound, etc.239
Sulfur containing groups are also common functional groups in carbon materials. And fixing an appropriate amount of sulfur-containing functional groups on the surface of activated carbon will significantly increase the adsorption capacity of metal ions such as mercury and cadmium. Carbon raw materials are treated with sulfur, hydrogen sulfide, sulfur dioxide, carbon disulfide, or sulfides such as sodium thiosulfate at high temperature to produce more surface sulfur-containing groups.240 The sulfur group on the carbon surface is very stable in strong alkaline solutions and stabilizes after losing a few in strong acid solutions. The presence of surface sulfur groups tends to narrow the pore distribution of activated carbon.241,242
4.2 Adsorption conditions
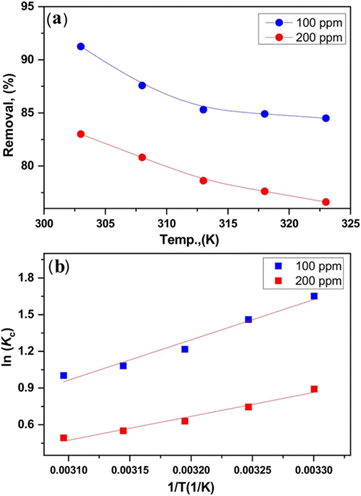 | ||
| Fig. 10 (a) Effect of temperature on removal of chromium ion by ACPH800, (b) Van't Hoff plot for chromium ion adsorption on ACPH800. This figure has been adapted from ref. 250 with permission from Colloids and Surfaces A: Physicochemical and Engineering Aspects, copyright 2023. | ||
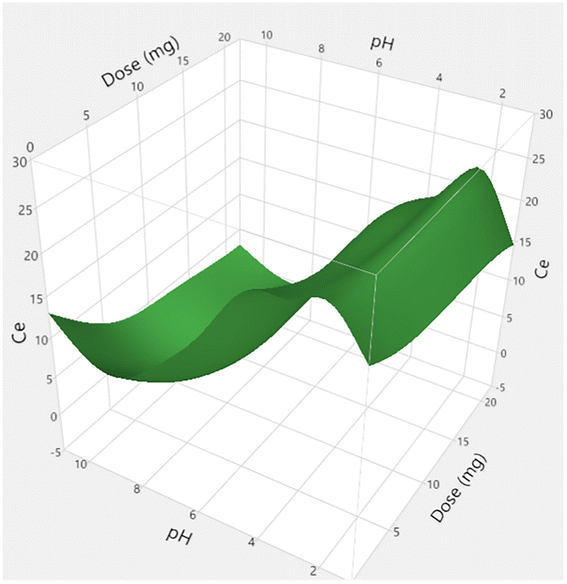 | ||
| Fig. 11 Surface plot of predicted adsorption capacity. This figure has been adapted from ref. 255 with permission from Journal of Molecular Liquids, copyright 2023. | ||
The amount of activated carbon is one of the conditions affecting the adequacy of the adsorption process. If the adsorption sites per unit mass of activated carbon are certain, a large amount of activated carbon will increase the adsorption capacity, it will also increase the cost of wastewater treatment, so choosing the amount of activated carbon with high adsorption capacity and relatively low cost is one of important factors to remove pollutants and reduce the cost of activated carbon adsorption treatment.
The length of adsorption time is a key factor affecting the adequacy of the removal effect. By increasing the adsorption time appropriately, the amount of activated carbon adsorption will effectively increase. However, the increase in adsorption time increases the stagnation time of wastewater, reduces the overall wastewater treatment efficiency, and increases the wastewater treatment cost. Therefore, selecting the appropriate adsorption time is beneficial to improve the adsorption efficiency of activated carbon.
5. Conclusions and prospects
To date, human activities have led to the accumulation of various heavy metals at a rate that exceeds the capacity to treat or manage them. In the last five years, public awareness of environmental protection and the selection of suitable sorbents are among the most challenging things in removing heavy metal pollution.Although many methods for removing heavy metals have been proposed in recent years, many of these techniques are not suitable for commercial application due to low efficiency, high energy consumption, or poor selectivity. Biomass-derived activated carbon has shown prospective potential due to its large specific surface area, rich pore structure, high stability and relatively low cost.
In this paper, the sources of heavy metals, removal methods and preparation methods of biomass activated carbon were reviewed. The key factors of adsorbents, physical and chemical properties of heavy metals and adsorption conditions affecting heavy metal adsorption were emphatically introduced. In addition, most of heavy metals adsorbed on biomass-derived activated carbon materials can be recovered by desorption processes under various conditions. Biomass is widely utilized as a precursor for the formation of activated carbon. The choice of biomass, type of carbonization, and activation can also affect the porosity and surface area of the carbon. Under suitable conditions or appropriate modifications, almost all of biomass-derived activated carbons exhibit high adsorption capacity of heavy metal—both the physical form and the chemical functional groups of the adsorbent influence the adsorption of heavy metals. In general, larger specific surface areas and smaller pore sizes favor adsorption, while the influence of functional groups is related to the pH of heavy metal-containing wastewater. Adsorption conditions such as temperature and pH content seriously affect the adsorption of heavy metals. Too high a temperature is not favorable for heavy metal adsorption. Too high or too low a pH level can also hurt adsorption.
Currently, “green” is the trend of today's society, there is no doubt that the application of biomass-activated carbon in wastewater is becoming more promising. However, some knowledge gaps still need to be filled to improve further the adsorption capacity of biomass-derived activated carbon for heavy metals in actual complex samples. Reducing the production cost of carbon adsorbents, the large-scale production of biomass-derived carbon is complex due to the limitation of harsh reaction conditions, high equipment requirements, and high dosage of modifier agents. Therefore, researchers must seek simple synthetic methods to meet the mass production of adsorbents from biomass-derived activated carbon. In addition, several challenges need to be overcome. For example, the aim is to improve the selectivity of carbon adsorbents for heavy metals. Furthermore, most of the current co-modifications of biomass-derived activated carbons are still based on laboratory studies, so the reproducibility of commercial biomass-derived activated carbons is an issue that we must consider. Shortly, the preparation of biomass-derived activated carbon will certainly be commercialized in a simple, fast and easy way.
Conflicts of interest
All authors disclosed no relevant relationships.Acknowledgements
Financial support is gratefully acknowledged from the National Natural Science Foundation of China (No. 21974137), the CAS-Weigao Research & Development Program ([2017]-009), the Innovation Program of Science and Research from the Dalian Institute of Chemical Physics (DICPI202005) and the National Natural Science Foundation of Ningxia (No. 2021AAC02017) to J. Ou, as well as the Introduction Flexible Team of Ningxia (No. 2019RXTD0004), the National Natural Science Foundation of China (No. 22164001) and the Key research and development program of Ningxia (No. 2022BFE02002) and the Key materials and Key technology innovation team for industrial biological processes (KJT2019004) to B. Gong.References
- X. Zhang, B. Gao, A. E. Creamer, C. Cao and Y. Li, Adsorption of VOCs onto engineered carbon materials: a review, J. Hazard. Mater., 2017, 338, 102–123 CrossRef CAS PubMed.
- S. Yu, B. Du, A. Baheiduola, C. Geng and J. Liu, HCB dechlorination combined with heavy metals immobilization in MSWI fly ash by using n-Al/CaO dispersion mixture, J. Hazard. Mater., 2020, 392, 122510 CrossRef CAS PubMed.
- R. D. F. Rios, P. J. B. Bueno, J. C. S. Terra and F. C. C. Moura, Influence of the surface modification of granular-activated carbon synthesized from macauba on heavy metal sorption, Environ. Sci. Pollut. Res., 2022, 02, 42 Search PubMed.
- H. Qin, T. Hu, Y. Zhai, N. Lu and J. Aliyeva, The improved methods of heavy metals removal by biosorbents: a review, Environ. Pollut., 2020, 258, 113777 CrossRef CAS PubMed.
- X. Jia, T. Fu, B. Hu, Z. Shi, L. Zhou and Y. Zhu, Identification of the potential risk areas for soil heavy metal pollution based on the source-sink theory, J. Hazard. Mater., 2020, 393, 122424 CrossRef CAS PubMed.
- P. R. Yaashikaa, P. S. Kumar, S. Jeevanantham and R. Saravanan, A review on bioremediation approach for heavy metal detoxification and accumulation in plants, Environ. Pollut., 2022, 301, 119035 CrossRef CAS PubMed.
- W. Zeng, W. Guo, B. Li, Z. Wei, D. D. Dionysiou and R. Xiao, Kinetics and mechanistic aspects of removal of heavy metal through gas-liquid sulfide precipitation: a computational and experimental study, J. Hazard. Mater., 2021, 408, 124868 CrossRef CAS PubMed.
- Z. Wang, Z. Tan, H. Li, S. Yuan, Y. Zhang and Y. Dong, Direct current electrochemical method for removal and recovery of heavy metals from water using straw biochar electrode, J. Cleaner Prod., 2022, 339, 130746 CrossRef CAS.
- F. Zhu, Y. M. Zheng, B. G. Zhang and Y. R. Dai, A critical review on the electrospun nanofibrous membranes for the adsorption of heavy metals in water treatment, J. Hazard. Mater., 2021, 401, 123608 CrossRef CAS PubMed.
- W. S. Chai, J. Y. Cheun, P. S. Kumar, M. Mubashir, Z. Majeed, F. Banat, S.-H. Ho and P. L. Show, A review on conventional and novel materials towards heavy metal adsorption in wastewater treatment application, J. Cleaner Prod., 2021, 296, 126589 CrossRef CAS.
- X. L. Jia, T. T. Fu, B. F. Hu, Z. Shi, L. Q. Zhou and Y. W. Zhu, Identification of the potential risk areas for soil heavy metal pollution based on the source-sink theory, J. Hazard. Mater., 2020, 393, 122424 CrossRef CAS PubMed.
- H. Li, M. J. Li, F. Zheng, J. Wang, L. Chen, P. F. Hu, Q. Zhen, S. Bashir and J. L. Liu, Efficient removal of water pollutants by hierarchical porous zeolite-activated carbon prepared from coal gangue and bamboo, J. Cleaner Prod., 2021, 325, 129322 CrossRef CAS.
- N. Naqash, S. Prakash, D. Kapoor and R. Singh, Interaction of freshwater microplastics with biota and heavy metals: a review, Environ. Chem. Lett., 2020, 18, 1813–1824 CrossRef CAS.
- X. Zhang, J. Zhou, Z. Xu, P. Zhu and J. Liu, Characterization of heavy metals in textile sludge with hydrothermal carbonization treatment, J. Hazard. Mater., 2021, 402, 123635 CrossRef CAS PubMed.
- A. T. Ubando, A. D. M. Africa, M. C. Maniquiz-Redillas, A. B. Culaba, W. H. Chen and J. S. Chang, Microalgal biosorption of heavy metals: A comprehensive bibliometric review, J. Hazard. Mater., 2021, 402, 123431 CrossRef CAS PubMed.
- L. S. Miranda, B. Wijesiri, G. A. Ayoko, P. Egodawatta and A. Goonetilleke, Water-sediment interactions and mobility of heavy metals in aquatic environments, Water Res., 2021, 202, 117386 CrossRef CAS PubMed.
- C. Tang, Z. Liu, C. Peng, L.-Y. Chai, K. Kuroda, M. Okido and Y.-X. Song, New insights into the interaction between heavy metals and struvite: struvite as platform for heterogeneous nucleation of heavy metal hydroxide, Chem. Eng. J., 2019, 365, 60–69 CrossRef CAS.
- P. Sherugar, M. Padaki, N. S. Naik, S. D. George and D. H. K. Murthy, Biomass-derived versatile activated carbon removes both heavy metals and dye molecules from wastewater with near-unity efficiency: mechanism and kinetics, Chemosphere, 2022, 287, 132085 CrossRef CAS PubMed.
- Q. Zhong, C. Cruz-Paredes, S. Zhang and J. Rousk, Can heavy metal pollution induce bacterial resistance to heavy metals and antibiotics in soils from an ancient land-mine?, J. Hazard. Mater., 2021, 411, 124962 CrossRef CAS PubMed.
- M. J. Kang, Y. K. Kwon, S. Yu, P. K. Lee, H. S. Park and N. Song, Assessment of Zn pollution sources and apportionment in agricultural soils impacted by a Zn smelter in South Korea, J. Hazard. Mater., 2019, 364, 475–487 CrossRef CAS PubMed.
- M. Ghayoraneh and A. Qishlaqi, Concentration, distribution and speciation of toxic metals in soils along a transect around a Zn/Pb smelter in the northwest of Iran, J. Geochem. Explor., 2017, 180, 1–14 CrossRef CAS.
- H. Liu, C. Cheng and H. M. Wu, Sustainable utilization of wetland biomass for activated carbon production: a review on recent advances in modification and activation methods, Sci. Total Environ., 2021, 790, 148214 CrossRef CAS PubMed.
- J. Zhang, X. Sun, J. Deng, G. Li, Z. Li, J. Jiang, Q. Wu and L. Duan, Emission characteristics of heavy metals from a typical copper smelting plant, J. Hazard. Mater., 2022, 424, 127311 CrossRef CAS PubMed.
- M. Cartro-Sabate, P. Mayor, M. Orta-Martinez and A. Rosell-Mele, Anthropogenic lead in Amazonian wildlife, Nat. Sustain., 2019, 2, 702–709 CrossRef.
- M. P. Taylor, C. F. Isley and J. Glover, Prevalence of childhood lead poisoning and respiratory disease associated with lead smelter emissions, Environ. Int., 2019, 127, 340–352 CrossRef CAS PubMed.
- H. T. Liu, K. Liu, H. Y. Fu, R. Ji and X. L. Qu, Sunlight mediated cadmium release from colored microplastics containing cadmium pigment in aqueous phase, Environ. Pollut., 2020, 263, 114484 CrossRef CAS PubMed.
- N. Ma, R. N. Cai and C. M. Sun, Threonine dehydratase enhances bacterial cadmium resistance via driving cysteine desulfuration and biomineralization of cadmium sulfide nanocrystals, J. Hazard. Mater., 2021, 417, 126102 CrossRef CAS PubMed.
- Y. L. Zhao, X. G. Li, X. B. Jia and S. Y. Gao, Why and how to tailor the vertical coordinate of pore size distribution to construct ORR-active carbon materials?, Nano Energy, 2019, 58, 384–391 CrossRef CAS.
- D. Mamais, C. Noutsopoulos, I. Kavallari, E. Nyktari, A. Kaldis, E. Panousi, G. Nikitopoulos, K. Antoniou and M. Nasioka, Biological groundwater treatment for chromium removal at low hexavalent chromium concentrations, Chemosphere, 2016, 152, 238–244 CrossRef CAS PubMed.
- J. B. Vincent and H. C. Lukaski, Chromium, Adv. Nutr., 2018, 9, 505–506 CrossRef PubMed.
- E. M. Hamilton, S. D. Young, E. H. Bailey and M. J. Watts, Chromium speciation in foodstuffs: A review, Food Chem., 2018, 250, 105–112 CrossRef CAS PubMed.
- Y. X. An, Q. Fu, D. H. Zhang, Y. Y. Wang and Z. L. Tang, Performance evaluation of activated carbon with different pore sizes and functional groups for VOC adsorption by molecular simulation, Chemosphere, 2019, 227, 9–16 CrossRef CAS PubMed.
- H. Sturikova, O. Krystofova, D. Huska and V. Adam, Zinc, zinc nanoparticles and plants, J. Hazard. Mater., 2018, 349, 101–110 CrossRef CAS PubMed.
- G. Cho, Y. Park, Y. K. Hong and D. H. Ha, Ion exchange: an advanced synthetic method for complex nanoparticles, Nano Convergence, 2019, 6, 39 CrossRef PubMed.
- L. Shi, B. R. Duan, Z. G. Zhu, C. F. Sun, J. Zhou and A. Walsh, Preparing copper catalyst by ultrasound-assisted chemical precipitation method, Ultrason. Sonochem., 2020, 64, 105013 CrossRef PubMed.
- M. Z. Momcilovic, M. S. Randelovic, M. M. Purenovic, J. S. Dordevic, A. Onjia and B. Matovic, Morpho-structural, adsorption and electrochemical characteristics of serpentinite, Sep. Purif. Technol., 2016, 163, 72–78 CrossRef CAS.
- X. Yin, Z. J. Zhang, H. Y. Ma, S. Venkateswaran and B. S. Hsiao, Ultra-fine electrospun nanofibrous membranes for multicomponent wastewater treatment: Filtration and adsorption, Sep. Purif. Technol., 2020, 242, 116794 CrossRef CAS.
- A. Mojiri, J. L. Zhou, B. Robinson, A. Ohashi, N. Ozaki, T. Kindaichi, H. Farraji and M. Vakili, Pesticides in aquatic environments and their removal by adsorption methods, Chemosphere, 2020, 253, 126646 CrossRef CAS PubMed.
- I. A. Aguayo-Villarreal, A. Bonilla-Petriciolet and R. Muñiz-Valencia, Preparation of activated carbons from pecan nutshell and their application in the antagonistic adsorption of heavy metal ions, J. Mol. Liq., 2017, 230, 686–695 CrossRef CAS.
- T. Xiong, X. Yuan, X. Cao, H. Wang, L. Jiang, Z. Wu and Y. Liu, Mechanistic insights into heavy metals affinity in magnetic MnO2@Fe3O4/poly(m-phenylenediamine) core-shell adsorbent, Ecotoxicol. Environ. Saf., 2020, 192, 110326 CrossRef CAS PubMed.
- S. Singh, D. Kapoor, S. Khasnabis, J. Singh and P. C. Ramamurthy, Mechanism and kinetics of adsorption and removal of heavy metals from wastewater using nanomaterials, Environ. Chem. Lett., 2021, 19, 2351–2381 CrossRef CAS.
- Q. Chen, Y. Yao, X. Li, J. Lu, J. Zhou and Z. Huang, Comparison of heavy metal removals from aqueous solutions by chemical precipitation and characteristics of precipitates, J. Water Process. Eng., 2018, 26, 289–300 CrossRef.
- J. Q. Tang, J. B. Xi, J. X. Yu, R. A. Chi and J. D. Chen, Novel combined method of biosorption and chemical precipitation for recovery of Pb(2+) from wastewater, Environ. Sci. Pollut. Res. Int., 2018, 25, 28705–28712 CrossRef CAS PubMed.
- M. A. Ganzoury, C. Chidiac, J. Kurtz and C. F. de Lannoy, CNT-sorbents for heavy metals: electrochemical regeneration and closed-loop recycling, J. Hazard. Mater., 2020, 393, 122432 CrossRef CAS PubMed.
- X. Yang, L. Liu, W. Tan, C. Liu, Z. Dang and G. Qiu, Remediation of heavy metal contaminated soils by organic acid extraction and electrochemical adsorption, Environ. Pollut., 2020, 264, 114745 CrossRef CAS PubMed.
- R. F. T. Tagne, R. C. T. Temgoua, D. R. T. Tchuifon, T. Vintila, A. S. Ngueabouo and S. G. Anagho, Development of an electroanalytical method using activated rice husk-derived carbon for the detection of a paraquat herbicide, Carbon Trends, 2021, 4, 100060 CrossRef.
- X. Pei, L. Gan, Z. Tong, H. Gao, S. Meng, W. Zhang, P. Wang and Y. Chen, Robust cellulose-based composite adsorption membrane for heavy metal removal, J. Hazard. Mater., 2021, 406, 124746 CrossRef CAS PubMed.
- J. E. Efome, D. Rana, T. Matsuura and C. Q. Lan, Experiment and modeling for flux and permeate concentration of heavy metal ion in adsorptive membrane filtration using a metal-organic framework incorporated nanofibrous membrane, Chem. Eng. J., 2018, 352, 737–744 CrossRef CAS.
- A. Almasian, M. Giahi, G. Chizari Fard, S. A. Dehdast and L. Maleknia, Removal of heavy metal ions by modified PAN/PANI-nylon core-shell nanofibers membrane: filtration performance, antifouling and regeneration behavior, Chem. Eng. J., 2018, 351, 1166–1178 CrossRef CAS.
- S. Deng, X. H. Liu, J. B. Liao, H. Lin and F. Liu, PEI modified multiwalled carbon nanotube as a novel additive in PAN nanofiber membrane for enhanced removal of heavy metal ions, Chem. Eng. J., 2019, 375, 122086 CrossRef CAS.
- R. Wang, L. Deng, X. Fan, K. Li, H. Lu and W. Li, Removal of heavy metal ion cobalt (II) from wastewater via adsorption method using microcrystalline cellulose-magnesium hydroxide, Int. J. Biol. Macromol., 2021, 189, 607–617 CrossRef CAS PubMed.
- D. Jiang, Y. Yang, C. Huang, M. Huang, J. Chen, T. Rao and X. Ran, Removal of the heavy metal ion nickel (II) via an adsorption method using flower globular magnesium hydroxide, J. Hazard. Mater., 2019, 373, 131–140 CrossRef CAS PubMed.
- S. Sun, Q. Yu, M. Li, H. Zhao and C. Wu, Preparation of coffee-shell activated carbon and its application for water vapor adsorption, Renewable Energy, 2019, 142, 11–19 CrossRef CAS.
- P. Saket, M. Kashyap, K. Bala and A. Joshi, Microalgae and bio-polymeric adsorbents: an integrative approach giving new directions to wastewater treatment, Int. J. Phytorem., 2022, 24, 536–556 CrossRef PubMed.
- K. X. Wang, L. P. Yang, H. X. Li and F. Zhang, Surfactant Pyrolysis-Guided in Situ Fabrication of Primary Amine-Rich Ordered Mesoporous Phenolic Resin Displaying Efficient Heavy Metal Removal, ACS Appl. Mater. Interfaces, 2019, 11, 21815–21821 CrossRef CAS PubMed.
- H. Y. Ren, H. Y. Shen and Y. Z. Liu, Adsorption of CO2 with tetraethylammonium glycine ionic liquid modified alumina in the Rotating Adsorption Bed, J. CO2 Util., 2022, 58, 101925 CrossRef CAS.
- Z. Y. Yang, D. C. Wang, Z. Y. Meng and Y. Y. Li, Adsorption separation of CH4/N-2 on modified coal-based carbon molecular sieve, Sep. Purif. Technol., 2019, 218, 130–137 CrossRef CAS.
- J. Shahrivar and M. Gharabaghi, Separation of AuCN2- by activated carbon and functionalized graphene/activated carbon composite, Adv. Powder Technol., 2020, 31, 4648–4656 CrossRef CAS.
- A. B. Saralegui, V. Willson, N. Caracciolo, M. N. Piol and S. P. Boeykens, Macrophyte biomass productivity for heavy metal adsorption, J. Environ. Manage., 2021, 289, 112398 CrossRef CAS PubMed.
- M. N. Piol, C. Dickerman, M. P. Ardanza, A. Saralegui and S. P. Boeykens, Simultaneous removal of chromate and phosphate using different operational combinations for their adsorption on dolomite and banana peel, J. Environ. Manage., 2021, 288, 112378 CrossRef PubMed.
- S. Elfeghe, Q. Sheng and Y. Zhang, Separation of Lead and Copper Ions in Acidic Media Using an Ion-Exchange Resin with a Thiourea Functional Group, ACS Omega, 2022, 7, 13042–13049 CrossRef CAS PubMed.
- A. Freni, L. Calabrese, A. Malara, P. Frontera and L. Bonaccorsi, Silica gel microfibres by electrospinning for adsorption chillers, Energy, 2019, 187, 113607 CrossRef.
- S. Batra, A. Awasthi, M. Iqbal and D. Datta, Solvent impregnated resins for the treatment of aqueous solutions containing different compounds: a review, Rev. Chem. Eng., 2022, 38, 209–242 CrossRef CAS.
- A. Boretskaya, I. Il'yasov, S. Egorova, A. Popov and A. Lamberov, Modification of a phase-inhomogeneous alumina support of a palladium catalyst. Part I: effect of the amorphous phase on the textural and acidic characteristics of alumina and methods for controlling its phase homogeneity, Mater. Today Chem., 2020, 18, 121102 Search PubMed.
- S. I. Alhassan, L. Huang, Y. He, L. Yan, B. Wu and H. Wang, Fluoride removal from water using alumina and aluminum-based composites: a comprehensive review of progress, Crit. Rev. Environ. Sci. Technol., 2020, 51, 2051–2085 CrossRef.
- W. M. Youssef, M. S. Hagag and A. H. Ali, Synthesis, characterization and application of composite derived from rice husk ash with aluminium oxide for sorption of uranium, Adsorpt. Sci. Technol., 2018, 36, 1274–1293 CrossRef CAS.
- M. Aazza, H. Ahlafi, H. Moussout and H. Maghat, Adsorption of meta-nitrophenol onto alumina and HDTMA modified alumina: kinetic, isotherm and mechanism investigations, J. Mol. Liq., 2018, 268, 587–597 CrossRef CAS.
- N. Jiang, R. Shang, S. G. J. Heijman and L. C. Rietveld, High-silica zeolites for adsorption of organic micro-pollutants in water treatment: a review, Water Res., 2018, 144, 145–161 CrossRef CAS PubMed.
- H. Wang, J. Xu, X. Liu and L. Sheng, Preparation of straw activated carbon and its application in wastewater treatment: a review, J. Cleaner Prod., 2021, 283, 124671 CrossRef CAS.
- B. Gorska, E. Frackowiak and F. Beguin, Redox active electrolytes in carbon/carbon electrochemical capacitors, Curr. Opin. Electrochem., 2018, 9, 95–105 CrossRef CAS.
- C. Urrutia, E. Yañez-Mansilla and D. Jeison, Bioremoval of heavy metals from metal mine tailings water using microalgae biomass, Algal Res., 2019, 43, 101659 CrossRef.
- V.-P. Dinh, T.-D.-T. Huynh, H. M. Le, V.-D. Nguyen, V.-A. Dao, N. Q. Hung, L. A. Tuyen, S. Lee, J. Yi, T. D. Nguyen and L. V. Tan, Insight into the adsorption mechanisms of methylene blue and chromium(iii) from aqueous solution onto pomelo fruit peel, RSC Adv., 2019, 9, 25847–25860 RSC.
- S. S. Fiyadh, M. A. AlSaadi, W. Z. Jaafar, M. K. AlOmar, S. S. Fayaed, N. S. Mohd, L. S. Hin and A. El-Shafie, Review on heavy metal adsorption processes by carbon nanotubes, J. Cleaner Prod., 2019, 230, 783–793 CrossRef CAS.
- K. Du, S. Li, L. Zhao, L. Qiao, H. Ai and X. Liu, One-Step Growth of Porous Cellulose Beads Directly on Bamboo Fibers via Oxidation-Derived Method in Aqueous Phase and Their Potential for Heavy Metal Ions Adsorption, ACS Sustainable Chem. Eng., 2018, 6, 17068–17075 CrossRef CAS.
- K. Cheng, S. Wallaert, H. Ardebili and A. Karim, Advanced triboelectric nanogenerators based on low-dimension carbon materials: a review, Carbon, 2022, 194, 81–103 CrossRef CAS.
- N. Abu Bakar, N. Othman, Z. M. Yunus, W. A. H. Altowayti, M. Tahir, N. Fitriani and S. N. A. Mohd-Salleh, An insight review of lignocellulosic materials as activated carbon precursor for textile wastewater treatment, Environ. Technol. Innovation, 2021, 22, 101445 CrossRef.
- K. J. Guan, L. L. Zhang, F. Y. Zhu, H. J. Li, H. C. Sheng and Y. Guo, Multi-layer SiC-graphene oxide-hydroxyapatite bioactive coating for carbon/carbon composites, J. Alloys Compd., 2020, 821, 101132 CrossRef.
- F. Zhao, L. L. Zhang, Y. Guo, H. C. Sheng, P. X. Zhang, Y. X. Zhang, Q. Li, H. Y. Yang and S. V. Mikhalovsky, Mechanically strong and bioactive carbon fiber-SiC nanowire-hydroxyapatite-pyrolytic carbon composites for bone implant application, Ceram. Int., 2021, 47, 3389–3400 CrossRef CAS.
- L. L. Zhang, S. X. Li, H. J. Li and L. N. Pei, Bioactive surface modification of carbon/carbon composites with multilayer SiC-SiC nanowire-Si doped hydroxyapatite coating, J. Alloys Compd., 2018, 740, 109–117 CrossRef CAS.
- L. C. Zhong, Y. S. Zhang, T. Wang, Y. Ji, P. Norris and W. P. Pan, Optimized methods for preparing activated carbon from rock asphalt using orthogonal experimental design, J. Therm. Anal. Calorim., 2019, 136, 1989–1999 CrossRef CAS.
- J. Wang, T. L. Liu, Q. X. Huang, Z. Y. Ma, Y. Chi and J. H. Yan, Production and characterization of high quality activated carbon from oily sludge, Fuel Process. Technol., 2017, 162, 13–19 CrossRef CAS.
- C. Guclu, K. Alper, M. Erdem, K. Tekin and S. Karagoz, Activated carbons from co-carbonization of waste truck tires and spent tea leaves, Sustainable Chem. Pharm., 2021, 21, 100410 CrossRef CAS.
- R. B. Gonzalez-Gonzalez, L. T. Gonzalez, S. Iglesias-Gonzalez, E. Gonzalez-Gonzalez, S. O. Martinez-Chapa, M. Madou, M. M. Alvarez and A. Mendoza, Characterization of Chemically Activated Pyrolytic Carbon Black Derived from Waste Tires as a Candidate for Nanomaterial Precursor, Nanomaterials, 2020, 10, 2213 CrossRef CAS PubMed.
- D. Meier, B. van de Beld, A. V. Bridgwater, D. C. Elliott, A. Oasmaa and F. Preto, State-of-the-art of fast pyrolysis in IEA bioenergy member countries, Renewable Sustainable Energy Rev., 2013, 20, 619–641 CrossRef.
- L. Gao, C. Li, S. Li, W. Zhang, X. Du, L. Huang, Y. Zhu, Y. Zhai and G. Zeng, Superior performance and resistance to SO2 and H2O over CoOx-modified MnOx/biomass activated carbons for simultaneous Hg0 and NO removal, Chem. Eng. J., 2019, 371, 781–795 CrossRef CAS.
- L. Gao, C. Li, J. Zhang, X. Du, S. Li, J. Zeng, Y. Yi and G. Zeng, Simultaneous removal of NO and Hg0 from simulated flue gas over CoOx -CeO2 loaded biomass activated carbon derived from maize straw at low temperatures, Chem. Eng. J., 2018, 342, 339–349 CrossRef CAS.
- M. Antar, D. Lyu, M. Nazari, A. Shah, X. Zhou and D. L. Smith, Biomass for a sustainable bioeconomy: an overview of world biomass production and utilization, Renewable Sustainable Energy Rev., 2021, 139, 110691 CrossRef CAS.
- A. A. Salema, R. M. W. Ting and Y. K. Shang, Pyrolysis of blend (oil palm biomass and sawdust) biomass using TG-MS, Bioresour. Technol., 2019, 274, 439–446 CrossRef CAS PubMed.
- M. Tymoszuk, K. Mroczek, S. Kalisz and H. Kubiczek, An investigation of biomass grindability, Energy, 2019, 183, 116–126 CrossRef.
- S. Tong, Y. Sun, X. Li, Z. Hu, N. Worasuwannarak, H. Liu, H. Hu, G. Luo and H. Yao, Gas-pressurized torrefaction of biomass wastes: co-gasification of gas-pressurized torrefied biomass with coal, Bioresour. Technol., 2021, 321, 124505 CrossRef CAS PubMed.
- A. C. Eloka-Eboka and F. L. Inambao, Effects of CO2 sequestration on lipid and biomass productivity in microalgal biomass production, Appl. Energy, 2017, 195, 1100–1111 CrossRef CAS.
- P. Bober, J. Kovářová, J. Pfleger, J. Stejskal, M. Trchová, I. Novák and D. Berek, Twin carbons: the carbonization of cellulose or carbonized cellulose coated with a conducting polymer, polyaniline, Carbon, 2016, 109, 836–842 CrossRef CAS.
- J. González-Arias, M. E. Sánchez, J. Cara-Jiménez, F. M. Baena-Moreno and Z. Zhang, Hydrothermal carbonization of biomass and waste: a review, Environ. Chem. Lett., 2021, 20, 211–221 CrossRef.
- J. Xu, C. Li, L. Dai, C. Xu, Y. Zhong, F. Yu and C. Si, Biomass Fractionation and Lignin Fractionation towards Lignin Valorization, Chemsuschem, 2020, 13, 4284–4295 CrossRef CAS PubMed.
- Z. Heidarinejad, M. H. Dehghani, M. Heidari, G. Javedan, I. Ali and M. Sillanpää, Methods for preparation and activation of activated carbon: a review, Environ. Chem. Lett., 2020, 18, 393–415 CrossRef CAS.
- A. Volperts, A. Plavniece, G. Dobele, A. Zhurinsh, I. Kruusenberg, K. Kaare, J. Locs, L. Tamasauskaite-Tamasiunaite and E. Norkus, Biomass based activated carbons for fuel cells, Renewable Energy, 2019, 141, 40–45 CrossRef CAS.
- H. Li, P. Gao, J. Cui, F. Zhang, F. Wang and J. Cheng, Preparation and Cr(VI) removal performance of corncob activated carbon, Environ. Sci. Pollut. Res. Int., 2018, 25, 20743–20755 CrossRef CAS PubMed.
- A. Nayak, B. Bhushan, V. Gupta and P. Sharma, Chemically activated carbon from lignocellulosic wastes for heavy metal wastewater remediation: effect of activation conditions, J. Colloid Interface Sci., 2017, 493, 228–240 CrossRef CAS PubMed.
- Y. Gao, Q. Y. Yue, B. Y. Gao and A. M. Li, Insight into activated carbon from different kinds of chemical activating agents: a review, Sci. Total Environ., 2020, 746, 141094 CrossRef CAS PubMed.
- Z. B. Zhang, Y. Q. Lei, D. W. Li, J. W. Zhao, Y. K. Wang, G. Y. Zhou, C. W. Yan and Q. X. He, Sudden heating of H3PO4-loaded coconut shell in CO2 flow to produce super activated carbon and its application for benzene adsorption, Renewable Energy, 2020, 153, 1091–1099 CrossRef CAS.
- Q. L. Wei, Z. M. Chen, Y. Y. Cheng, X. F. Wang, X. M. Yang and Z. C. Wang, Preparation and electrochemical performance of orange peel based-activated carbons activated by different activators, Colloids Surf., A, 2019, 574, 221–227 CrossRef CAS.
- A. Reffas, V. Bernardet, B. David, L. Reinert, M. B. Lehocine, M. Dubois, N. Batisse and L. Duclaux, Carbons prepared from coffee grounds by H3PO4 activation: characterization and adsorption of methylene blue and Nylosan Red N-2RBL, J. Hazard. Mater., 2010, 175, 779–788 CrossRef CAS PubMed.
- L. Luo, T. Chen, Z. Li, Z. Zhang, W. Zhao and M. Fan, Heteroatom self-doped activated biocarbons from fir bark and their excellent performance for carbon dioxide adsorption, J. CO2 Util., 2018, 25, 89–98 CrossRef CAS.
- H. M. Wei, H. J. Chen, N. Fu, J. Chen, G. X. Lan, W. Qian, Y. P. Liu, H. L. Lin and S. Han, Excellent electrochemical properties and large CO2 capture of nitrogen-doped activated porous carbon synthesised from waste longan shells, Electrochim. Acta, 2017, 231, 403–411 CrossRef CAS.
- O. Pezoti, A. L. Cazetta, K. C. Bedin, L. S. Souza, A. C. Martins, T. L. Silva, O. O. Santos, J. V. Visentainer and V. C. Almeida, NaOH-activated carbon of high surface area produced from guava seeds as a high-efficiency adsorbent for amoxicillin removal: kinetic, isotherm and thermodynamic studies, Chem. Eng. J., 2016, 288, 778–788 CrossRef CAS.
- Y. Yu, N. Qiao, D. J. Wang, Q. Z. Zhu, F. Fu, R. Q. Cao, R. Wang, W. Liu and B. Xu, Fluffy honeycomb-like activated carbon from popcorn with high surface area and well-developed porosity for ultra-high efficiency adsorption of organic dyes, Bioresour. Technol., 2019, 285, 121340 CrossRef CAS PubMed.
- L. L. Rao, S. F. Liu, L. L. Wang, C. D. Ma, J. Y. Wu, L. Y. An and X. Hu, N-doped porous carbons from low-temperature and single-step sodium amide activation of carbonized water chestnut shell with excellent CO2 capture performance, Chem. Eng. J., 2019, 359, 428–435 CrossRef CAS.
- Z. H. Xu, Z. H. Yuan, D. F. Zhang, W. F. Chen, Y. X. Huang, T. Q. Zhang, D. Q. Tian, H. X. Deng, Y. W. Zhou and Z. H. Sun, Highly mesoporous activated carbon synthesized by pyrolysis of waste polyester textiles and MgCl2: physiochemical characteristics and pore-forming mechanism, J. Cleaner Prod., 2018, 192, 453–461 CrossRef CAS.
- Y. B. Xi, D. J. Yang, X. Q. Qiu, H. Wang, J. H. Huang and Q. Li, Renewable lignin-based carbon with a remarkable electrochemical performance from potassium compound activation, Ind. Crops Prod., 2018, 124, 747–754 CrossRef CAS.
- I. Yang, M. Jung, M.-S. Kim, D. Choi and J. C. Jung, Physical and chemical activation mechanisms of carbon materials based on the microdomain model, J. Mater. Chem. A, 2021, 9, 9815–9825 RSC.
- W. Jiang, X. Xing, S. Li, X. Zhang and W. Wang, Synthesis, characterization and machine learning based performance prediction of straw activated carbon, J. Cleaner Prod., 2019, 212, 1210–1223 CrossRef CAS.
- C. Nieto-Delgado, D. Partida-Gutierrez and J. R. Rangel-Mendez, Preparation of activated carbon cloths from renewable natural fabrics and their performance during the adsorption of model organic and inorganic pollutants in water, J. Cleaner Prod., 2019, 213, 650–658 CrossRef CAS.
- Y. Ding, Y. Li, Y. Dai, X. Han, B. Xing, L. Zhu, K. Qiu and S. Wang, A novel approach for preparing in situ nitrogen doped carbon via pyrolysis of bean pulp for supercapacitors, Energy, 2021, 216, 114231 CrossRef.
- M. Wang, L. Y. Chu, Z. Y. Li, A. M. Messinis, Y. Q. Ding, L. Hu and J. B. Ma, Dinitrogen and Carbon Dioxide Activation to Form C-N Bonds at Room Temperature: A New Mechanism Revealed by Experimental and Theoretical Studies, J. Phys. Chem. Lett., 2021, 12, 3490–3496 CrossRef CAS PubMed.
- M. Arif, G. Liu, M. Zia ur Rehman, B. Yousaf, R. Ahmed, M. M. Mian, A. Ashraf, M. A. Mujtaba Munir, M. S. Rashid and A. Naeem, Carbon dioxide activated biochar-clay mineral composite efficiently removes ciprofloxacin from contaminated water-Reveals an incubation study, J. Cleaner Prod., 2022, 332, 130079 CrossRef CAS.
- A. Trubetskaya, J. Kling, O. Ershag, T. M. Attard and E. Schroder, Removal of phenol and chlorine from wastewater using steam activated biomass soot and tire carbon black, J. Hazard. Mater., 2019, 365, 846–856 CrossRef CAS PubMed.
- J. H. Kwak, M. S. Islam, S. Wang, S. A. Messele, M. A. Naeth, M. G. El-Din and S. X. Chang, Biochar properties and lead(II) adsorption capacity depend on feedstock type, pyrolysis temperature, and steam activation, Chemosphere, 2019, 231, 393–404 CrossRef CAS PubMed.
- X. Liu, S. Zuo, N. Cui and S. Wang, Investigation of ammonia/steam activation for the scalable production of high-surface area nitrogen-containing activated carbons, Carbon, 2022, 191, 581–592 CrossRef CAS.
- P. N. Y. Yek, R. K. Liew, M. S. Osman, C. L. Lee, J. H. Chuah, Y. K. Park and S. S. Lam, Microwave steam activation, an innovative pyrolysis approach to convert waste palm shell into highly microporous activated carbon, J. Environ. Manage., 2019, 236, 245–253 CrossRef CAS PubMed.
- W. Yang, H. Chen, X. Han, S. Ding, Y. Shan and Y. Liu, Preparation of magnetic Co-Fe modified porous carbon from agricultural wastes by microwave and steam activation for mercury removal, J. Hazard. Mater., 2020, 381, 120981 CrossRef CAS PubMed.
- S. S. Lam, P. N. Y. Yek, Y. S. Ok, C. C. Chong, R. K. Liew, D. C. W. Tsang, Y. K. Park, Z. Liu, C. S. Wong and W. Peng, Engineering pyrolysis biochar via single-step microwave steam activation for hazardous landfill leachate treatment, J. Hazard. Mater., 2020, 390, 121649 CrossRef CAS PubMed.
- A. D. Igalavithana, S. W. Choi, J. Shang, A. Hanif, P. D. Dissanayake, D. C. W. Tsang, J. H. Kwon, K. B. Lee and Y. S. Ok, Carbon dioxide capture in biochar produced from pine sawdust and paper mill sludge: effect of porous structure and surface chemistry, Sci. Total Environ., 2020, 739, 139845 CrossRef CAS PubMed.
- J. Yan and R. Li, Simple and low-cost production of magnetite/graphene nanocomposites for heavy metal ions adsorption, Sci. Total Environ., 2022, 813, 152604 CrossRef CAS PubMed.
- M. Manoj, C. Muhamed Ashraf, M. Jasna, K. M. Anilkumar, B. Jinisha, V. S. Pradeep and S. Jayalekshmi, Biomass-derived, activated carbon-sulfur composite cathode with a bifunctional interlayer of functionalized carbon nanotubes for lithium-sulfur cells, J. Colloid Interface Sci., 2019, 535, 287–299 CrossRef CAS PubMed.
- S. Satyro, H. Li, A. M. Dehkhoda, R. McMillan, N. Ellis and S. A. Baldwin, Application of Fe-biochar composites for selenium (Se(+6)) removal from aqueous solution and effect of the presence of competing anions under environmentally relevant conditions, J. Environ. Manage., 2021, 277, 111472 CrossRef CAS PubMed.
- P. N. Y. Yek, C. Li, W. Peng, C. S. Wong, R. K. Liew, W. A. Wan Mahari, C. Sonne and S. S. Lam, Production of modified biochar to treat landfill leachate using integrated microwave pyrolytic CO2 activation, Chem. Eng. J., 2021, 425, 131886 CrossRef CAS.
- N. T. Mai, M. N. Nguyen, T. Tsubota, P. L. T. Nguyen and N. H. Nguyen, Evolution of physico-chemical properties of Dicranopteris linearis-derived activated carbon under various physical activation atmospheres, Sci. Rep., 2021, 11, 14430 CrossRef CAS PubMed.
- W.-T. Tsai and T.-J. Jiang, Mesoporous activated carbon produced from coconut shell using a single-step physical activation process, Biomass Convers. Biorefin., 2018, 8, 711–718 CrossRef CAS.
- B. Chu, Y. Amano and M. Machida, Preparation of bean dreg derived N-doped activated carbon with high adsorption for Cr(VI), Colloids Surf., A, 2020, 586, 110121 CrossRef.
- S. Mopoung and N. Dejang, Activated carbon preparation from eucalyptus wood chips using continuous carbonization-steam activation process in a batch intermittent rotary kiln, Sci. Rep., 2021, 11, 139487 Search PubMed.
- P. N. Y. Yek, R. K. Liew, M. S. Osma, C. L. Lee, J. H. Chuah, Y. K. Park and S. S. Lam, Microwave steam activation, an innovative pyrolysis approach to convert waste palm shell into highly microporous activated carbon, J. Environ. Manage., 2019, 236, 245–253 CrossRef CAS PubMed.
- J. H. Lee, Y. J. Heo and S. J. Park, Effect of silica removal and steam activation on extra-porous activated carbons from rice husks for methane storage, Int. J. Hydrogen Energy, 2018, 43, 22377–22384 CrossRef CAS.
- D. D. Sewu, H. Jung, S. S. Kim, D. S. Lee and S. H. Woo, Decolorization of cationic and anionic dye-laden wastewater by steam-activated biochar produced at an industrial-scale from spent mushroom substrate, Bioresour. Technol., 2019, 277, 77–86 CrossRef CAS PubMed.
- W. F. Chen, F. F. He, S. J. Zhang, H. Xv and Z. H. Xv, Development of porosity and surface chemistry of textile waste jute-based activated carbon by physical activation, Environ. Sci. Pollut. Res., 2018, 25, 9840–9848 CrossRef CAS PubMed.
- J. P. Castro, J. R. C. Nobre, M. L. Bianchi, P. F. Trugilho, A. Napoli, B. S. Chiou, T. G. Williams, D. F. Wood, R. J. Avena-Bustillos, W. J. Orts and G. H. D. Tonoli, Activated carbons prepared by physical activation from different pretreatments of amazon piassava fibers, J. Nat. Fibers, 2019, 16, 961–976 CrossRef CAS.
- A. Bazan-Wozniak, P. Nowicki and R. Pietrzak, Removal of NO2 from gas stream by activated bio-carbons from physical activation of residue of supercritical extraction of hops, Chem. Eng. Res. Des., 2021, 166, 67–73 CrossRef CAS.
- T. T. Wang, D. Zhang, K. K. Fang, W. Zhu, Q. Peng and Z. G. Xie, Enhanced nitrate removal by physical activation and Mg/Al layered double hydroxide modified biochar derived from wood waste: adsorption characteristics and mechanisms, J. Environ. Chem. Eng., 2021, 9, 105184 CrossRef CAS.
- H. G. Zanella, L. Spessato, G. K. P. Lopes, J. T. C. Yokoyama, M. C. Silva, P. S. C. Souza, A. Ronix, A. L. Cazetta and V. C. Almeida, Caffeine adsorption on activated biochar derived from macrophytes (Eichornia crassipes), J. Mol. Liq., 2021, 340, 117206 CrossRef CAS.
- R. Chen, X. Li, Q. Huang, H. Ling, Y. Yang and X. Wang, Self-assembled porous biomass carbon/RGO/nanocellulose hybrid aerogels for self-supporting supercapacitor electrodes, Chem. Eng. J., 2021, 412, 128755 CrossRef CAS.
- C. Wang, L. Cao, J. Huang, J. Li and K. Kajiyoshi, Divergent thinking and its application in biomass carbon electrode preparation, Renewable Sustainable Energy Rev., 2021, 138, 110564 CrossRef CAS.
- Z. Tang, Y. Wang, Z. Zheng and X. Luo, In situ dual growth of graphitic structures in biomass carbon to yield a potassium-ion battery anode with high initial coulombic efficiency, J. Mater. Chem. A, 2021, 9, 9191–9202 RSC.
- W. Tian, L. Wang, K. Huo and X. He, Red phosphorus filled biomass carbon as high-capacity and long-life anode for sodium-ion batteries, J. Power Sources, 2019, 430, 60–66 CrossRef CAS.
- B. Zhang, C. Wang, D. Liu, Y. Liu, X. Yu and L. Wang, Boosting ORR Electrocatalytic Performance of Metal-Free Mesoporous Biomass Carbon by Synergism of Huge Specific Surface Area and Ultrahigh Pyridinic Nitrogen Doping, ACS Sustainable Chem. Eng., 2018, 6, 13807–13812 CrossRef CAS.
- W.-B. Jiao, Y.-Q. Zhang, K. Yu, J.-R. Zhou, H.-L. Cao and J. Lü, Porous Graphitic Biomass Carbons as Sustainable Adsorption and Controlled Release Carriers for Atrazine Fixation, ACS Sustainable Chem. Eng., 2019, 7, 20180–20189 CrossRef CAS.
- P. Wang, Z. Gong, K. Ye, Y. Gao, K. Zhu, J. Yan, G. Wang and D. Cao, N-rich biomass carbon derived from hemp as a full carbon-based potassium ion hybrid capacitor anode, Appl. Surf. Sci., 2021, 553, 149569 CrossRef CAS.
- S. Gleizer, R. Ben-Nissan, Y. M. Bar-On, N. Antonovsky, E. Noor, Y. Zohar, G. Jona, E. Krieger, M. Shamshoum, A. Bar-Even and R. Milo, Conversion of Escherichia coli to Generate All Biomass Carbon from CO2, Cell, 2019, 179, 1255–1263 CrossRef CAS PubMed.
- X. Xu, Z. Meng, X. Zhu, S. Zhang and W.-Q. Han, Biomass carbon composited FeS2 as cathode materials for high-rate rechargeable lithium-ion battery, J. Power Sources, 2018, 380, 12–17 CrossRef CAS.
- Q. Yang, Y. Shi, Y. Fang, Y. Dong, Q. Ni, Y. Zhu and Y. Fu, Construction of polyaniline aligned on magnetic functionalized biomass carbon giving excellent microwave absorption properties, Compos. Sci. Technol., 2019, 174, 176–183 CrossRef CAS.
- S. Sun, Z. Yin, B. Cong, W. Hong, X. Zhou, Y. Wang, Y. Wang and G. Chen, Crystalline carbon modified hierarchical porous iron and nitrogen co-doped carbon for efficient electrocatalytic oxygen reduction, J. Colloid Interface Sci., 2021, 594, 864–873 CrossRef CAS PubMed.
- C. Jin, L. Fu, J. Zhu, W. Yang, D. Li and L. Zhou, A hierarchical carbon modified nano-NiS2 cathode with high thermal stability for a high energy thermal battery, J. Mater. Chem. A, 2018, 6, 7123–7132 RSC.
- Y. Ding, Q. Gu, A. Klyushin, X. Huang, S. H. Choudhury, I. Spanos, F. Song, R. Mom, P. Düngen, A. K. Mechler, R. Schlögl and S. Heumann, Dynamic carbon surface chemistry: revealing the role of carbon in electrolytic water oxidation, J. Energy Chem., 2020, 47, 155–159 CrossRef.
- D. Xia, C. Yu, Y. Zhao, Y. Wei, H. Wu, Y. Kang, J. Li, L. Gan and F. Kang, Degradation and regeneration of Fe-N x active sites for the oxygen reduction reaction: the role of surface oxidation, Fe demetallation and local carbon microporosity, Chem. Sci., 2021, 12, 11576–11584 RSC.
- J. Lee and G. H. An, Surface-engineered flexible fibrous supercapacitor electrode for improved electrochemical performance, Appl. Surf. Sci., 2021, 539, 116114 Search PubMed.
- T. Zhao, Y. Yao, D. Li, F. Wu, C. Zhang and B. Gao, Facile low-temperature one-step synthesis of pomelo peel biochar under air atmosphere and its adsorption behaviors for Ag(I) and Pb(II), Sci. Total Environ., 2018, 640–641, 73–79 CrossRef CAS PubMed.
- B. Huo, B. Li, C. Chen, Y. Zhang and D. Wang, Morphological and mineralogical insights into acetic acid modifying and hydraulic process on steel slag for enhanced reactivity, Constr. Build. Mater., 2021, 307, 125004 CrossRef CAS.
- U. S. Rashid and A. N. Bezbaruah, Citric acid modified granular activated carbon for enhanced defluoridation, Chemosphere, 2020, 252, 126639 CrossRef CAS PubMed.
- Z. Huang, J. Zhang, Y. Du, Y. Zhang, X. Wu and G. Jing, Spectroscopic Insights into the Mechanism of Selective Catalytic Reduction of NO by Ammonia on Sulfuric Acid-modified Fe2O3 Surface, ChemCatChem, 2019, 11, 3035–3041 CrossRef CAS.
- S. C. Hu, Y. C. Chen, X. Z. Lin, A. Shiue, P. H. Huang, Y. C. Chen, S. M. Chang, C. H. Tseng and B. Zhou, Characterization and adsorption capacity of potassium permanganate used to modify activated carbon filter media for indoor formaldehyde removal, Environ. Sci. Pollut. Res. Int., 2018, 25, 28525–28545 CrossRef CAS PubMed.
- Z. Juan, Z. Yongke, Z. Yaping, Z. Qingyun and W. Siyu, Influence of the acid groups on activated carbon on the adsorption of bilirubin, Mater. Chem. Phys., 2021, 260, 113107 CrossRef.
- Y. Li, J. Zhang and H. Liu, In situ modification of activated carbon with ethylenediaminetetraacetic acid disodium salt during phosphoric acid activation for enhancement of nickel removal, Powder Technol., 2018, 325, 113–120 CrossRef CAS.
- M. Zięzio, B. Charmas, K. Jedynak, M. Hawryluk and K. Kucio, Preparation and characterization of activated carbons obtained from the waste materials impregnated with phosphoric acid(V), Appl. Nanosci., 2020, 10, 4703–4716 CrossRef.
- H. Zeng, H. Zeng, H. Zhang, A. Shahab, K. Zhang, Y. Lu, I. Nabi, F. Naseem and H. Ullah, Efficient adsorption of Cr (VI) from aqueous environments by phosphoric acid activated eucalyptus biochar, J. Cleaner Prod., 2021, 286, 124964 CrossRef CAS.
- L. Y. Li, X. Gong and O. Abida, Waste-to-resources: exploratory surface modification of sludge-based activated carbon by nitric acid for heavy metal adsorption, Waste Manage., 2019, 87, 375–386 CrossRef CAS PubMed.
- J. O. Ighalo and A. G. Adeniyi, Adsorption of pollutants by plant bark derived adsorbents: an empirical review, J. Water Process. Eng., 2020, 35, 101228 CrossRef.
- H. Han, M. K. Rafiq, T. Zhou, R. Xu, O. Masek and X. Li, A critical review of clay-based composites with enhanced adsorption performance for metal and organic pollutants, J. Hazard. Mater., 2019, 369, 780–796 CrossRef CAS PubMed.
- M. Sharma, M. Joshi, S. Nigam, S. Shree, D. K. Avasthi, R. Adelung, S. K. Srivastava and Y. Kumar Mishra, ZnO tetrapods and activated carbon based hybrid composite: adsorbents for enhanced decontamination of hexavalent chromium from aqueous solution, Chem. Eng. J., 2019, 358, 540–551 CrossRef CAS.
- Y. Wei, H. Wang, X. Zhang and C. Liu, Ammonia-assisted hydrothermal carbon material with schiff base structures synthesized from factory waste hemicelluloses for Cr(VI) adsorption, J. Environ. Chem. Eng., 2021, 9, 106187 CrossRef CAS.
- M. Naushad, A. A. Alqadami, A. A. Al-Kahtani, T. Ahamad, M. R. Awual and T. Tatarchuk, Adsorption of textile dye using para-aminobenzoic acid modified activated carbon: kinetic and equilibrium studies, J. Mol. Liq., 2019, 296, 112075 CrossRef CAS.
- P. Rechnia-Gorący, A. Malaika and M. Kozłowski, Effective conversion of rapeseed oil to biodiesel fuel in the presence of basic activated carbon catalysts, Catal. Today, 2020, 357, 102–112 CrossRef.
- X. Li, X. Wang, L. Wang, P. Ning, Y. Ma, L. Zhong, Y. Wu and L. Yuan, Efficient removal of carbonyl sulfur and hydrogen sulfide from blast furnace gas by one-step catalytic process with modified activated carbon, Appl. Surf. Sci., 2022, 579, 152189 CrossRef CAS.
- F. Morales-Lara, V. K. Abdelkader-Fernández, M. Melguizo, A. Turco, E. Mazzotta, M. Domingo-García, F. J. López-Garzón and M. Pérez-Mendoza, Ultra-small metal nanoparticles supported on carbon nanotubes through surface chelation and hydrogen plasma reduction for methanol electro-oxidation, J. Mater. Chem. A, 2019, 7, 24502–24514 RSC.
- J.-K. Kang, S.-C. Lee, H.-Y. Jang and S.-B. Kim, Synthesis of poly(ethyleneimine)-functionalized mesoporous silica gel with dual loading of host ion and crosslinking for enhanced heavy metal removal in multinary solutions, Microporous Mesoporous Mater., 2021, 311, 110698 CrossRef CAS.
- Q. Jiang, W. Xie, S. Han, Y. Wang and Y. Zhang, Enhanced adsorption of Pb(II) onto modified hydrochar by polyethyleneimine or H3PO4: an analysis of surface property and interface mechanism, Colloids Surf., A, 2019, 583, 113114 CrossRef.
- D. Zhang, M. Zhang, F. Ding, W. Liu, L. Zhang and L. Cui, Efficient removal of formaldehyde by polyethyleneimine modified activated carbon in a fixed bed, Environ. Sci. Pollut. Res. Int., 2020, 27, 18109–18116 CrossRef CAS PubMed.
- E. Haye, N. Job, Y. Wang, S. Penninckx, V. Stergiopoulos, N. Tumanov, M. Cardinal, Y. Busby, J. F. Colomer, B. L. Su, J. J. Pireaux and L. Houssiau, ZnO/Carbon xerogel photocatalysts by low-pressure plasma treatment, the role of the carbon substrate and its plasma functionalization, J. Colloid Interface Sci., 2020, 570, 312–321 CrossRef CAS PubMed.
- X. Shen, Z. Shi, Z. Zhao, X. Wang, C. Li, J. Huang, K. Li and G. Liu, Study of the ablation of a carbon/carbon composite at ∼25 MW/m2 with a nitrogen plasma torch, J. Eur. Ceram. Soc., 2020, 40, 5085–5093 CrossRef CAS.
- A. A. Kava and C. S. Henry, Exploring carbon particle type and plasma treatment to improve electrochemical properties of stencil-printed carbon electrodes, Talanta, 2021, 221, 121553 CrossRef CAS PubMed.
- W. S. Abdul-Majeed and K. O. Al-Riyami, Activation of peat soil carbon and production of carbon nanostructures using a flying jet cold plasma torch, Environ. Chem. Lett., 2019, 17, 1383–1390 CrossRef CAS.
- S. Li, C. Zhang, J. Fu, Y. Zhou, J. Sun, Y. He, F. Nan and Z. Yu, Interfacial modification of carbon fiber by carbon nanotube gas-phase dispersion, Compos. Sci. Technol., 2020, 195, 434242 Search PubMed.
- S. Ali, S. A. U. Rehman, H. Y. Luan, M. U. Farid and H. Huang, Challenges and opportunities in functional carbon nanotubes for membrane-based water treatment and desalination, Sci. Total Environ., 2019, 646, 1126–1139 CrossRef CAS PubMed.
- X. Liu, Y. Han, Y. Cheng and G. Xu, Microwave-assisted ammonia modification of activated carbon for effective removal of phenol from wastewater: DFT and experiment study, Appl. Surf. Sci., 2020, 518, 113221 Search PubMed.
- S. Neha, P. Rajput and N. Remya, Biochar from microwave co-pyrolysis of food waste and polyethylene using different microwave susceptors-Production, modification and application for metformin removal, Environ. Res., 2022, 210, 112922 CrossRef CAS PubMed.
- L. Wang, Z. Chen, H. Wen, Z. Cai, C. He, Z. Wang and W. Yan, Microwave assisted modification of activated carbons by organic acid ammoniums activation for enhanced adsorption of acid red 18, Powder Technol., 2018, 323, 230–237 CrossRef CAS.
- G. Durán-Jiménez, V. Hernández-Montoya, J. Rodríguez Oyarzun, M. Á. Montes-Morán and E. Binner, Pb(II) removal using carbon adsorbents prepared by hybrid heating system: understanding the microwave heating by dielectric characterization and numerical simulation, J. Mol. Liq., 2019, 277, 663–671 CrossRef.
- Y. Zhou, N. Kandel, M. Bartoli, L. F. Serafim, A. E. ElMetwally, S. M. Falkenberg, X. E. Paredes, C. J. Nelson, N. Smith, E. Padovano, W. Zhang, K. J. Mintz, B. C. L. B. Ferreira, E. K. Cilingir, J. Chen, S. K. Shah, R. Prabhakar, A. Tagliaferro, C. Wang and R. M. Leblanc, Structure-activity relationship of carbon nitride dots in inhibiting Tau aggregation, Carbon, 2022, 193, 1–16 CrossRef CAS PubMed.
- X. Ji, E. Abakumov, S. Chigray, S. Saparova, V. Polyakov, W. Wang, D. Wu, C. Li, Y. Huang and X. Xie, Response of carbon and microbial properties to risk elements pollution in arctic soils, J. Hazard. Mater., 2021, 408, 124430 CrossRef CAS PubMed.
- Z. Zhai, M. Luo, Y. Yang, Y. Liu, X. Chen, C. Zhang, J. Huang and J. Chen, Trade-off between microbial carbon use efficiency and microbial phosphorus limitation under salinization in a tidal wetland, Catena, 2022, 209, 105809 CrossRef CAS.
- S. Doetterl, A. A. Berhe, C. Arnold, S. Bodé, P. Fiener, P. Finke, L. Fuchslueger, M. Griepentrog, J. W. Harden, E. Nadeu, J. Schnecker, J. Six, S. Trumbore, K. Van Oost, C. Vogel and P. Boeckx, Links among warming, carbon and microbial dynamics mediated by soil mineral weathering, Nat. Geosci., 2018, 11, 589–593 CrossRef CAS.
- J. Tian, N. Zong, I. P. Hartley, N. He, J. Zhang, D. Powlson, J. Zhou, Y. Kuzyakov, F. Zhang, G. Yu and J. A. J. Dungait, Microbial metabolic response to winter warming stabilizes soil carbon, Global Change Biol., 2021, 27, 2011–2028 CrossRef CAS PubMed.
- J. L. Soong, L. Fuchslueger, S. Maranon-Jimenez, M. S. Torn, I. A. Janssens, J. Penuelas and A. Richter, Microbial carbon limitation: the need for integrating microorganisms into our understanding of ecosystem carbon cycling, Global Change Biol., 2010, 26, 2019–2028 Search PubMed.
- X. Wang, H. Cheng, G. Ye, J. Fan, F. Yao, Y. Wang, Y. Jiao, W. Zhu, H. Huang and D. Ye, Key factors and primary modification methods of activated carbon and their application in adsorption of carbon-based gases: a review, Chemosphere, 2022, 287, 131995 CrossRef CAS PubMed.
- M. S. H. Gohr, A. I. Abd-Elhamid, A. A. El-Shanshory and H. M. A. Soliman, Adsorption of cationic dyes onto chemically modified activated carbon: kinetics and thermodynamic study, J. Mol. Liq., 2022, 346, 118227 CrossRef.
- T. Pang, T. S. Aye Chan, Y. A. C. Jande and J. Shen, Removal of fluoride from water using activated carbon fibres modified with zirconium by a drop-coating method, Chemosphere, 2020, 255, 126950 CrossRef CAS PubMed.
- T. Huang, H. He, P. Zhang, S. Lv, H. Jiang, H. Liu and X. Peng, Laboratory investigation on performance and mechanism of polyphosphoric acid modified bio-asphalt, J. Cleaner Prod., 2022, 333, 130104 CrossRef CAS.
- M. Lu, X. Shi, Q. Feng, X. Li, S. Lian, M. Zhang and R. Guo, Effects of humic acid modified oyster shell addition on lignocellulose degradation and nitrogen transformation during digestate composting, Bioresour. Technol., 2021, 329, 124834 CrossRef CAS PubMed.
- Y. Jin, C. Zeng, Q. F. Lu and Y. Yu, Efficient adsorption of methylene blue and lead ions in aqueous solutions by 5-sulfosalicylic acid modified lignin, Int. J. Biol. Macromol., 2019, 123, 50–58 CrossRef CAS PubMed.
- W. Cheng, Y. Zhu, J. A. Shao, W. Zhang, G. Wu, H. Jiang, J. Hu, Z. Huang, H. Yang and H. Chen, Mitigation of ultrafine particulate matter emission from agricultural biomass pellet combustion by the additive of phosphoric acid modified kaolin, Renewable Energy, 2021, 172, 177–187 CrossRef CAS.
- J. Zhao, Z. Zou, R. Ren, X. Sui, Z. Mao, H. Xu, Y. Zhong, L. Zhang and B. Wang, Chitosan adsorbent reinforced with citric acid modified β-cyclodextrin for highly efficient removal of dyes from reactive dyeing effluents, Eur. Polym. J., 2018, 108, 212–218 CrossRef CAS.
- T. Fujioka, M. Osako, K. Oda, T. Shintani and H. Kodamatani, Impact of heat modification conditions on the removal of N-nitrosodimethylamine by polyamide reverse osmosis membranes, Sep. Purif. Technol., 2020, 247, 116921 CrossRef CAS.
- W.-B. Miao, Y.-Y. Ning, H.-R. Huang, H.-M. Liu, X.-S. Cai and X.-D. Wang, Effect of dry heat modification and the addition of Chinese quince seed gum on the physicochemical properties and structure of tigernut tuber starch, Arabian J. Chem., 2021, 14, 103407 CrossRef CAS.
- W. Zou, X. Zhang and R. Stockmann, Thermally processed lignin reduces the apparent hydrolysis rate of pancreatic α-amylase in starchy foods, Carbohydr. Polym., 2021, 263, 117961 CrossRef CAS PubMed.
- A. Ivanovska, K. Asanovic, M. Jankoska, K. Mihajlovski, L. Pavun and M. Kostic, Multifunctional jute fabrics obtained by different chemical modifications, Cellulose, 2020, 27, 8485–8502 CrossRef CAS.
- J. Shin, Y. G. Lee, S. H. Lee, S. Kim, D. Ochir, Y. Park, J. Kim and K. Chon, Single and competitive adsorptions of micropollutants using pristine and alkali-modified biochars from spent coffee grounds, J. Hazard. Mater., 2020, 400, 123102 CrossRef CAS PubMed.
- B. Yang, S. Ma, R. Cui, S. Sun, J. Wang and S. Li, Simultaneous removal of NOx and SO2 with H2O2 catalyzed by alkali/magnetism-modified fly ash: high efficiency, Low cost and Catalytic mechanism, Chem. Eng. J., 2019, 359, 233–243 CrossRef CAS.
- F. Gao, S. Ahmad, J. Tang, C. Zhang, S. Li, C. Yu, Q. Liu and H. Sun, Enhanced nitrobenzene removal in soil by biochar supported sulfidated nano zerovalent iron: solubilization effect and mechanism, Sci. Total Environ., 2022, 826, 153960 CrossRef CAS PubMed.
- K. Ashwin, A. K. Pattanaik and G. S. Howarth, Polyphenolic bioactives as an emerging group of nutraceuticals for promotion of gut health: a review, Food Biosci., 2021, 44, 101376 CrossRef CAS.
- L. Wu, Y. Cai, S. Wang and Z. Li, Doping of nitrogen into biomass-derived porous carbon with large surface area using N2 non-thermal plasma technique for high-performance supercapacitor, Int. J. Hydrogen Energy, 2021, 46, 2432–2444 CrossRef CAS.
- R. Guillossou, J. Le Roux, R. Mailler, E. Vulliet, C. Morlay, F. Nauleau, J. Gasperi and V. Rocher, Organic micropollutants in a large wastewater treatment plant: what are the benefits of an advanced treatment by activated carbon adsorption in comparison to conventional treatment?, Chemosphere, 2019, 218, 1050–1060 CrossRef CAS PubMed.
- K. K. Kishibayev, J. Serafin, R. R. Tokpayev, T. N. Khavaza, A. A. Atchabarova, D. A. Abduakhytova, Z. T. Ibraimov and J. Sreńscek-Nazzal, Physical and chemical properties of activated carbon synthesized from plant wastes and shungite for CO2 capture, J. Environ. Chem. Eng., 2021, 9, 106798 CrossRef CAS.
- S. Yang, F. Zhao, X. Li, B. Cao, Y. Mo, D. Chen and Y. Chen, Electrode structural changes and their effects on capacitance performance during preparation and charge-discharge processes, J. Energy Storage, 2019, 24, 100799 CrossRef.
- B. Yu, G. Jiang, C. Cao, N. Lei, C. Li, U. Evariste and P. Ma, Preparation and electrochemical properties of ultra-high specific surface area N-doped biomass-porous carbon, J. Energy Storage, 2020, 30, 101537 CrossRef.
- Z. Zhang, T. Wang, H. Zhang, Y. Liu and B. Xing, Adsorption of Pb(II) and Cd(II) by magnetic activated carbon and its mechanism, Sci. Total Environ., 2021, 757, 143910 CrossRef CAS PubMed.
- T. Zhang, S. Wei, G. I. N. Waterhouse, L. Fu, L. Liu, W. Shi, J. Sun and S. Ai, Chromium (VI) adsorption and reduction by humic acid coated nitrogen-doped magnetic porous carbon, Powder Technol., 2020, 360, 55–64 CrossRef CAS.
- Z. Jafari, V. M. Avargani, M. R. Rahimi and S. Mosleh, Magnetic nanoparticles-embedded nitrogen-doped carbon nanotube/porous carbon hybrid derived from a metal-organic framework as a highly efficient adsorbent for selective removal of Pb(II) ions from aqueous solution, J. Mol. Liq., 2020, 318, 113987 CrossRef CAS.
- S. Wu, P. Yan, W. Yang, J. Zhou, H. Wang, L. Che and P. Zhu, ZnCl2 enabled synthesis of activated carbons from ion-exchange resin for efficient removal of Cu(2+) ions from water via capacitive deionization, Chemosphere, 2021, 264, 128557 CrossRef CAS PubMed.
- M. Li, G. Wu, Z. Liu, X. Xi, Y. Xia, J. Ning, D. Yang and A. Dong, Uniformly coating ZnAl layered double oxide nanosheets with ultra-thin carbon by ligand and phase transformation for enhanced adsorption of anionic pollutants, J. Hazard. Mater., 2020, 397, 122766 CrossRef CAS PubMed.
- S. M. Kharrazi, M. Soleimani, M. Jokar, T. Richards, A. Pettersson and N. Mirghaffari, Pretreatment of lignocellulosic waste as a precursor for synthesis of high porous activated carbon and its application for Pb (II) and Cr (VI) adsorption from aqueous solutions, Int. J. Biol. Macromol., 2021, 180, 299–310 CrossRef CAS PubMed.
- X. Zhang, Q. Lin, S. Luo, K. Ruan and K. Peng, Preparation of novel oxidized mesoporous carbon with excellent adsorption performance for removal of malachite green and lead ion, Appl. Surf. Sci., 2018, 442, 322–331 CrossRef CAS.
- X. Zeng, C. Zhao, Y. Yin, T. Nie, N. Xie, R. Yu and G. D. Stucky, Construction of NiCO2O4 nanosheets-covered Ti3C2Tx MXene heterostructure for remarkable electromagnetic microwave absorption, Carbon, 2022, 193, 26–34 CrossRef CAS.
- N. Talreja, S. Jung, L. T. H. Yen and T. Kim, Phenol-formaldehyde-resin-based activated carbons with controlled pore size distribution for high-performance supercapacitors, Chem. Eng. J., 2020, 379, 122332 CrossRef CAS.
- L. Suárez, V. Barranco and T. A. Centeno, Impact of carbon pores size on ionic liquid based-supercapacitor performance, J. Colloid Interface Sci., 2021, 588, 705–712 CrossRef PubMed.
- P. Zhang, J. Wang, W. Fan, Y. Zhong, Y. Zhang, Q. Deng, Z. Zeng and S. Deng, Ultramicroporous carbons with extremely narrow pore size distribution via in situ ionic activation for efficient gas-mixture separation, Chem. Eng. J., 2019, 375, 121931 CrossRef CAS.
- J. Li, B. Michalkiewicz, J. Min, C. Ma, X. Chen, J. Gong, E. Mijowska and T. Tang, Selective preparation of biomass-derived porous carbon with controllable pore sizes toward highly efficient CO2 capture, Chem. Eng. J., 2019, 360, 250–259 CrossRef CAS.
- K. Zou, P. Cai, B. Wang, C. Liu, J. Li, T. Qiu, G. Zou, H. Hou and X. Ji, Insights into Enhanced Capacitive Behavior of Carbon Cathode for Lithium Ion Capacitors: the Coupling of Pore Size and Graphitization Engineering, Nano-Micro Lett., 2020, 12, 121 CrossRef CAS PubMed.
- J. Du, L. Liu, Z. Hu, Y. Yu, Y. Qin and A. Chen, Order Mesoporous Carbon Spheres with Precise Tunable Large Pore Size by Encapsulated Self-Activation Strategy, Adv. Funct. Mater., 2018, 28, 213411 Search PubMed.
- J. Choma, J. Jagiello and M. Jaroniec, Assessing the contribution of micropores and mesopores from nitrogen adsorption on nanoporous carbons: application to pore size analysis, Carbon, 2021, 183, 150–157 CrossRef CAS.
- C. Xue, W. Hao, W. Cheng, J. Ma and R. Li, Effects of pore size distribution of activated carbon (AC) on CuCl dispersion and CO adsorption for CuCl/AC adsorbent, Chem. Eng. J., 2019, 375, 122049 CrossRef CAS.
- X. Bai, B. Quan, C. Kang, X. Zhang, Y. Zheng, J. Song, T. Xia and M. Wang, Activated carbon from tea residue as efficient absorbents for environmental pollutant removal from wastewater, Biomass Convers. Biorefin., 2022, 2, 23–45 Search PubMed.
- S. Dantas, K. C. Struckhoff, M. Thommes and A. V. Neimark, Pore size characterization of micro-mesoporous carbons using CO2 adsorption, Carbon, 2021, 173, 842–848 CrossRef CAS.
- X. Yang, Y. Wan, Y. Zheng, F. He, Z. Yu, J. Huang, H. Wang, Y. S. Ok, Y. Jiang and B. Gao, Surface functional groups of carbon-based adsorbents and their roles in the removal of heavy metals from aqueous solutions: a critical review, Chem. Eng. J., 2019, 366, 608–621 CrossRef CAS PubMed.
- M. Mariana, H. P. S. Abdul Khalil, E. M. Mistar, E. B. Yahya, T. Alfatah, M. Danish and M. Amayreh, Recent advances in activated carbon modification techniques for enhanced heavy metal adsorption, J. Water Process. Eng., 2021, 43, 102221 CrossRef.
- Z. Liu, B. Li, X. Shi, L. Li, Y. Feng, D. Jia and Y. Zhou, Target-oriented synthesis of high synthetic yield carbon dots with tailored surface functional groups for bioimaging of zebrafish, flocculation of heavy metal ions and ethanol detection, Appl. Surf. Sci., 2021, 538, 112314 Search PubMed.
- M. Salahinejad, S. Sadjadi and M. Abdouss, Investigating fluorescence quenching of cysteine-functionalized carbon quantum dots by heavy metal ions: experimental and QSPR studies, J. Mol. Liq., 2021, 334, 116067 CrossRef CAS.
- J. C. Ge and N. J. Choi, Performance of electrospun nanofibrous membranes for trapping of BTX aromatic hydrocarbons and heavy metal ions: mechanisms, isotherms and kinetics, J. Cleaner Prod., 2019, 217, 388–397 CrossRef CAS.
- F. Cao, C. Lian, J. Yu, H. Yang and S. Lin, Study on the adsorption performance and competitive mechanism for heavy metal contaminants removal using novel multi-pore activated carbons derived from recyclable long-root Eichhornia crassipes, Bioresour. Technol., 2019, 276, 211–218 CrossRef CAS PubMed.
- Y. Li, N. Tsend, T. Li, H. Liu, R. Yang, X. Gai, H. Wang and S. Shan, Microwave assisted hydrothermal preparation of rice straw hydrochars for adsorption of organics and heavy metals, Bioresour. Technol., 2019, 273, 136–143 CrossRef CAS PubMed.
- M. S. Islam, J. H. Kwak, C. Nzediegwu, S. Wang, K. Palansuriya, E. E. Kwon, M. A. Naeth, M. G. El-Din, Y. S. Ok and S. X. Chang, Biochar heavy metal removal in aqueous solution depends on feedstock type and pyrolysis purging gas, Environ. Pollut., 2021, 281, 117094 CrossRef CAS PubMed.
- T. Hou, L. Yan, J. Li, Y. Yang, L. Shan, X. Meng, X. Li and Y. Zhao, Adsorption performance and mechanistic study of heavy metals by facile synthesized magnetic layered double oxide/carbon composite from spent adsorbent, Chem. Eng. J., 2020, 384, 123331 CrossRef CAS.
- D.-W. Kim, J.-H. Wee, C.-M. Yang and K. S. Yang, Efficient removals of Hg and Cd in aqueous solution through NaOH-modified activated carbon fiber, Chem. Eng. J., 2020, 392, 123768 CrossRef CAS.
- Q. Yin, L. Si, R. Wang, Z. Zhao, H. Li and Z. Wen, DFT study on the effect of functional groups of carbonaceous surface on ammonium adsorption from water, Chemosphere, 2022, 287, 132294 CrossRef CAS PubMed.
- W. Li and T. J. Bandosz, Analyzing the effect of nitrogen/sulfur groups’ density ratio in porous carbons on the efficiency of CO2 electrochemical reduction, Appl. Surf. Sci., 2021, 569, 151066 CrossRef CAS.
- A. B. Fuertes, G. A. Ferrero, N. Diez and M. Sevilla, A Green Route to High-Surface Area Carbons by Chemical Activation of Biomass-Based Products with Sodium Thiosulfate, ACS Sustainable Chem. Eng., 2018, 6, 16323–16331 CrossRef CAS.
- F. Shen, J. Liu, Z. Zhang, Y. Dong and C. Gu, Density functional study of hydrogen sulfide adsorption mechanism on activated carbon, Fuel Process. Technol., 2018, 171, 258–264 CrossRef CAS.
- D. Li, J. Zhou, Y. Wang, Y. Tian, L. Wei, Z. Zhang, Y. Qiao and J. Li, Effects of activation temperature on densities and volumetric CO2 adsorption performance of alkali-activated carbons, Fuel, 2019, 238, 232–239 CrossRef CAS.
- F. Safdari, A. N. Shamkhali, M. Tafazzoli and G. Parsafar, Adsorption of pollutant cations from their aqueous solutions on graphitic carbon nitride explored by density functional theory, J. Mol. Liq., 2018, 260, 423–435 CrossRef CAS.
- C. Duan, T. Ma, J. Wang and Y. Zhou, Removal of heavy metals from aqueous solution using carbon-based adsorbents: a review, J. Water Process. Eng., 2020, 37, 101339 CrossRef.
- Y. Yuan, Z. An, R. Zhang, X. Wei and B. Lai, Efficiencies and mechanisms of heavy metals adsorption on waste leather-derived high-nitrogen activated carbon, J. Cleaner Prod., 2021, 293, 126215 CrossRef CAS.
- K. M. S. Khalil, W. A. Elhamdy and A. A. Elsamahy, Biomass derived P-doped activated carbon as nanostructured mesoporous adsorbent for chromium(VI) pollutants with pronounced functional efficiency and recyclability, Colloids Surf., A, 2022, 641, 128553 CrossRef CAS.
- N. D. Shooto, Removal of lead(II) and chromium(VI) ions from synthetic wastewater by the roots of harpagophytum procumbens plant, J. Environ. Chem. Eng., 2020, 8, 104541 CrossRef CAS.
- K. M. S. Khalil, W. A. Elhamdy and A. A. Elsamahy, Biomass derived P-doped activated carbon as nanostructured mesoporous adsorbent for chromium(VI) pollutants with pronounced functional efficiency and recyclability, Colloids Surf., A, 2022, 641, 128553 CrossRef CAS.
- D. Yin, Z. Wang, X. Wen, Y. Ding, X. Hou, Y. Geng and Y. Li, Effects of carbon concentration, pH, and bubbling depth on carbon dioxide absorption ratio in microalgae medium, Environ. Sci. Pollut. Res., 2019, 26, 32902–32910 CrossRef CAS PubMed.
- S. Vahedi, M. S. Yazdi, A. R. Arani and M. Azadfalah, A comparative study on the effect of pH values and carbonic additives on the supercapacitive performance of LDH-derived Ni-Co-Al oxide, J. Energy Storage, 2021, 43, 103211 CrossRef.
- B. Misbah Biltayib, M. Bonyani, A. Khan, C.-H. Su and Y.-Y. Yu, Predictive modeling and simulation of wastewater treatment process using nano-based materials: effect of pH and adsorbent dosage, J. Mol. Liq., 2021, 343, 117611 CrossRef CAS.
- T. Kokab, H. S. Ashraf, M. B. Shakoor, A. Jilani, S. R. Ahmad, M. Majid, S. Ali, N. Farid, R. A. Alghamdi, D. A. H. Al-Quwaie and K. R. Hakeem, Effective Removal of Cr(VI) from Wastewater Using Biochar Derived from Walnut Shell, Int. J. Environ. Res. Public Health, 2021, 18, 18 Search PubMed.
- B. M. Biltayib, M. Bonyani, A. Khan, C. H. Su and Y. Y. Yu, Predictive modeling and simulation of wastewater treatment process using nano-based materials: effect of pH and adsorbent dosage, J. Mol. Liq., 2021, 343, 117611 CrossRef.
| This journal is © The Royal Society of Chemistry 2023 |