Open Access Article
Open Access ArticleA review on the synthesis of metal oxide nanomaterials by microwave induced solution combustion
Ziyang Yin,
Si Li,
Xiang Li,
Wuyang Shi,
Wei Liu,
Zhengxia Gao,
Mengya Tao,
Chengliang Ma * and
Yuan Liu
* and
Yuan Liu
Henan Key Laboratory of High Temperature Functional Ceramics, School of Material Science and Engineering, Zhengzhou University, 75 Daxue Road, Zhengzhou 450052, China. E-mail: machengliang@zzu.edu.cn
First published on 20th January 2023
Abstract
With the increasing awareness of environmental protection and health, the preparation of metal oxide nanomaterials by environmentally friendly methods is favored by more and more researchers both at home and abroad. The preparation of metal oxide nanomaterials by a microwave-induced solution combustion synthesis method can significantly reduce the reaction time, energy consumption, and the use of toxic chemicals, which is an energy-saving, environmentally friendly method for nanomaterials synthesis. In addition, the microwave-induced solution combustion synthesis (MISCS) method has many advantages such as fast reaction speed, high selectivity, small product size, and homogeneous composition. This paper briefly describes the mechanism of the MISCS and its advantages in the synthesis of metal oxide nanomaterials, the related studies on microwave-induced solution combustion synthesis of metal oxide nanomaterials, and the effects of process parameters on the microstructure and properties of metal oxide nanomaterials synthesized by MISCS. Furthermore, some technical difficulties facing the synthesis of metal oxide nanomaterials by MISCS are summarized, and the future direction has also been prospected.
1 Introduction
The concept of nanomaterials first originated in the early 1980s and was proposed by German scientist H. Gleiter.1 In the late 1980s, this concept gained rapid development and attracted the attention of scientists worldwide. On the basis of this, a lot of research work related to nanomaterials was carried out. Materials that exist in at least one dimension in three-dimensional space with nanoscale dimensions (1–100 nm) or are composed of them as basic units can be called nanomaterials.2 Compared to ordinary materials, the particle size of nanomaterials is on the order of nanometers. The fine particle size gives rise to corresponding changes in the crystal structure and surface electronic structure of nanomaterials. Therefore, nanomaterials possess many special effects: small size effect,3 quantum size effect,4 surface effect,5 and macroscopic quantum tunneling effect.6 Due to these special effects, nanomaterials have optical, magnetic, electrical, and thermal properties that are different from those of ordinary materials.7 These differences enable nanomaterials to have a wide range of applications in catalysts,8,9 adsorbents,10,11 antimicrobial agents,12,13 functional ceramics,14,15 and flame retardants.16The liquid phase combustion synthesis, also named solution combustion synthesis (SCS) which start to be implemented in 1981 with the Jain method.17 SCS is a simple, fast, efficient and versatile method. The method can be used to prepare oxide materials with controlled properties (homogeneity and high purity) for a wide number of applications, such as catalysts, solid fuel cells and electronics.18–22 SCS is based on propellant chemistry and the stoichiometric ratio of oxidant to fuel. It involves an exothermic reaction between metal nitrate (oxidant) and organic fuel. The solution combustion synthesis method consists of three main steps: (1) formation of combustion mixture, (2) formation of gel and (3) combustion of gel. Most metal oxides can be obtained by reacting metal nitrates with fuel. Nitrates are the preferred metal precursors because they provide water/alcohol soluble metal ions and ensure homogeneity.
According to the different ignition modes, the solution combustion synthesis method can be divided into the traditional solution combustion synthesis method and the microwave-induced solution combustion synthesis method. The traditional solution combustion synthesis method transfers heat from the outside to the inside of the material by means of heat conduction. This heat transfer mechanism leads to a certain temperature gradient in the material, which leads to the formation of different crystalline phases and the growth of particles. Microwave induced solution combustion synthesis is a new method of nanomaterials preparation based on traditional solution combustion synthesis. The synthesis method has a different heating mechanism than the conventional solution combustion synthesis method. Microwave-induced solution combustion is mainly due to the rapid oscillation of molecular dipoles in the presence of microwaves. This oscillation leads to a higher molecular collision rate, which generates more heat.23 Due to its unique heating mechanism, the microwave heating allows for volumetric heating of the material, resulting in a more uniform heat distribution within the material.24 In addition, the microwave-induced solution combustion synthesis method can rapidly prepare a wide variety of nanomaterials with excellent physicochemical properties. Fig. 1 shows the schematic of the two different heating modes.
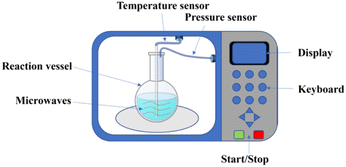
Nowadays, a wide variety of equipment for microwave-assisted reaction synthesis exists in chemical synthesis laboratories. In the early days of microwave synthesis, the main chemical synthesis reactions were carried out in home microwave ovens. The equipment is cheap and convenient, but the parameters of the reaction process were precisely unknown, such as the reaction temperature, the pressure inside the reaction vessel and the power of the microwave.25 Due to these uncertainties, the control of the safety and reproducibility of the synthesis process has become particularly important. Therefore, it is necessary to use an advanced microwave reaction system. This system needs to be equipped with various probes and sensors to monitor the temperature and pressure inside the reaction vessel. The devices can avoid the hazards of high temperature and pressure caused by the violent reaction of solvents in the closed reaction vessel. A schematic diagram of the microwave-assisted reactor system is shown in Fig. 2. The microwave device will provide uniform heating of the entire reactant system. In addition, a temperature sensor and a pressure sensor are inserted in the reaction vessel to monitor the temperature and pressure inside the reaction vessel, respectively.23
2 Microwave-induced solution combustion synthesis method
In microwave chemistry, the microwave heating mechanism is based on the “microwave dielectric heating” effect for efficient heating of materials. This phenomenon depends on the ability of a particular raw material (oxidant or organic fuel) to absorb microwave energy and convert it into heat.26 Microwave-induced solution combustion is induced by two main mechanisms of heating: dipole polarization and ionic conductivity.27 When the MW field is applied, the dipole and polar units of the reactants in the reaction mixture try to align with the microwave electric field frequency, and the dipoles constantly try to realign the oscillating electric field.28 This type of process leads to molecular friction and dielectric loss, which results in energy loss in the form of heat. The heat generated by this process is directly related to the ability of the substrate itself to align with the microwave field frequency. If the dipole does not have enough time to rearrange under the action of the microwave electric field, or cannot reorient quickly with the microwave field, the phenomenon of heating will not occur. The electric field oscillations of 2.45 GHz lead to the rotation of many polar molecules in the liquid, resulting in resistive heating in the medium (dielectric loss).29When microwave electric field is applied, the charged particles dissolved in the reaction mixture oscillate back and forth in response to the applied MW electric field and collide with neighboring molecules, thus generating heat. The heat generated under the ion conduction mechanism is much higher than that generated under the dipole polarization mechanism. The reaction mixtures consisting of free ions exhibit transient heating, which is important for the preparation of nanostructures in ionic liquids.30 The heating characteristics of the reacting material under microwave radiation conditions depend on its dielectric properties. The dielectric properties of a material are mainly determined by two parameters: (1) the dielectric constant ε′′ (the ability of the material to be polarized by the applied electric field) and (2) the dielectric loss ε′ (the ability of the material to dissipate thermal energy by converting the absorbed MW energy into heat).31 The ability of a given substance to convert electromagnetic energy into thermal energy at a given frequency and temperature is determined by the so-called loss factor tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ = ε′′/ε′. In general, solvents can be classified according to their loss factor as high absorbing (tan
δ = ε′′/ε′. In general, solvents can be classified according to their loss factor as high absorbing (tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ > 0.5), medium absorbing (tan
δ > 0.5), medium absorbing (tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ = 0.1–0.5) and low absorbing (tan
δ = 0.1–0.5) and low absorbing (tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ < 0.1).26
δ < 0.1).26
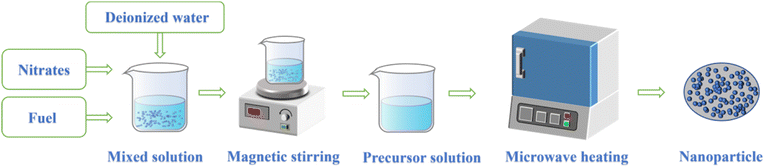
The microwave-induced solution combustion synthesis method is also called microwave-assisted solution combustion synthesis method. Fig. 3 shows the synthetic route of microwave-induced solution combustion method. Firstly, inorganic metal salts, deionized water, and fuel are prepared into a mixed solution. Then the mixed solution is dissolved on a magnetic stirrer until it is well mixed to obtain the precursor solution. Finally, the precursor solution was placed in a microwave oven for microwave heating to form the nanomaterials. In the process of solution combustion, the whole combustion phase only lasts for a few minutes. Besides, the inorganic metal salts in the reactants are mainly used as oxidants, and the fuel and its decomposition products are mainly used as reducing agents, while microwaves play the role of inducing redox reactions. In the process of microwave-induced combustion synthesis, nitrates of metals are often used as oxidants while urea (CO(NH2)2), citric acid (C6H8O7), glycine (C2H5NO2), and plant extracts commonly are often used as fuels.24
3 Microwave-induced combustion synthesis of nanomaterials
3.1 Single metal oxide nanomaterials
The microwave-induced solution combustion synthesis has the advantages of short heating time, uniform heating, and high purity. Therefore, this approach can be used to rapidly prepare high purity single metal oxide nanomaterials. At present, researchers have synthesized various kinds of single metal oxide nanomaterials by microwave-induced solution combustion, and the prepared nanomaterials have been applied in various fields. Table 1 lists some relevant reports on the synthesis of single metal oxide nanomaterials by microwave induced solution combustion. Patra et al. group used Mn(NO3)2 as oxidant and C2H5NO2 as fuel to prepare Mn3O4 with high specific capacitance and small particle size using microwave induced combustion synthesis method. These Mn3O4 nanoparticles can operate over a wide range of potentials and are inherently environmentally friendly. Therefore, The mixed Mn3O4 NPs can be used as an effective electrode material for electrochemical supercapacitors. The average crystallite size of MW synthesized Mn3O4 nanoparticles is calculated to be 28.08 nm by using the Debye Scherer equation. Fig. 4 shows that the microstructure is in the form of sponge like aggregates.32 The single metal oxide nanomaterials prepared by microwave-induced combustion synthesis have small particle sizes and high specific surface area, which can be used as antibacterial agents and adsorbents with efficient antibacterial properties and adsorption capacity.| Synthetic materials | Raw materials | Material characteristics | Particle/crystallite size (nm) | Applications |
|---|---|---|---|---|
| Mn3O4 | Mn(NO3)2·4H2O | High crystallinity | 28.08 | Electrode material32 |
| C2H5NO2 | Excellent capacitance | |||
| High purity | ||||
| CuO | Cu(NO3)2 Moringa oleifera and pomegranate leaves | Small particle size | 14.82/15.82 | Antibacterial agent33 |
| High crystallinity | ||||
| MgO | Mg(NO3)2·6H2O | Small particle size | 18 | Adsorbent34 |
| C2H5NO2 | High porosity | |||
| Nb2O5 | NH4 [NbO(C2O4)2-(H2O)2](H2O) | High crystallinity | 5–110 | Photocatalysis35 |
| NH4NO3 | Small particle size | |||
| CO(NH2)2/C6H8O7 | High purity | |||
| ZnO | Zn(NO3)2 fruit, seed, and pulp extracts of C. colocynthis | Desirable antioxidant | 27–85 | Antibacterial agent36 |
| High antibacterial activity | ||||
| Fe3O4 | Fe(NO3)3·9H2O | Small particle size | 22–30 | Magnetic resonance imaging37 |
| C6H8O7 | High saturation magnetization strength | |||
| Al2O3 | Al(NO3)3·9H2O | Small particle size | 30–40 | Catalytic support38 |
| CO(NH2)2 | High specific surface area | |||
| CeO2 | Ce(NO3)4 | High crystallinity | 18.23–23.83 | Catalyst39 |
| CO(NH2)2 | High purity |
 | ||
| Fig. 4 The SEM image of Mn3O4 nanoparticles [reprinted with permission from ref. 32. Copyright 2021 Elsevier]. | ||
Anand et al. group successfully prepared CuO nanoparticles by microwave-induced combustion synthesis using copper nitrate as oxidant and Moringa oleifera or Punica granatum extracts as fuel. The crystallization properties of CuO nanoparticles were studied by X-ray diffraction. It was found that the average grain size of CuO nanoparticles prepared with Moringa oleifera extract as fuel was 18.42 nm and the average grain size of CuO nanoparticles prepared using Punica granatum extract as fuel was 15.82 nm. The SEM images showed that the morphology of CuO NPs synthesized using Moringa oleifera and Punica granatum was spherical with an average grain size between 12–18 nm, which is in agreement with the powder XRD results. Fig. 5 shows that the spherical shape of nano-CuO. In addition, the antibacterial activity of the copper oxide nanoparticles prepared by the method was studied. The results showed that the copper oxide nanoparticles prepared by this method can be used to cure urinary tract infections.33 Priyadarshini et al. group synthesized cubic MgO nanoparticles with a grain size of 18 nm by microwave induced combustion synthesis, which can be used as an adsorbent to efficiently remove organic dyes from wastewater. The FESEM analysis showed the aggregation of small rod-like structured MgO particles with grain size in the range of 60–85 nm.34
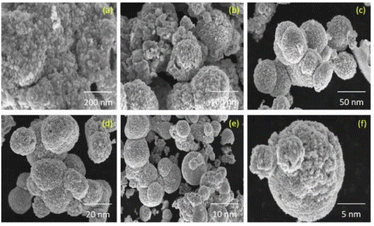 | ||
| Fig. 5 The SEM images of CuO NPs synthesized: (a–c) Moringa oleifera, (d–f) Punica granatum [reprinted with permission from ref. 33. Copyright 2021 Elsevier]. | ||
In the microwave induced solution combustion process, a series of process parameters such as microwave power, oxidant-to-fuel dosage ratio, type of fuel, and pH value of the precursor solution play a decisive role in the structure and properties of the products. Luiz et al. group successfully prepared Nb2O5 powder. The effect of the amount of ammonium niobium oxalate and urea on the particle size of the product was studied. The results showed that when the oxidant-to-fuel ratio is stoichiometric, Nb2O5 with larger particle size is produced, while Nb2O5 with smaller particle size is obtained with either excess fuel or excess oxidant. This is mainly because the amount of oxidant and fuel can change the reaction synthesis temperature, which affects the size of the resulting particles. The TEM and XRD analyses were consistent. In Fig. 6, the TEM results confirmed that the diameters of the obtained niobium pentoxide (Nb2O5) nanoparticles were in the range of 5–110 nm. The HRTEM images of C1 samples showed the presence of orthogonal crystal structures with planar spacing of 0.52 nm and 0.24 nm, corresponding to (130) and (181) planes, respectively. Besides, the (002) and (001) planar spacing of C2 and C3 samples were 0.19 and 0.39 nm, respectively.35
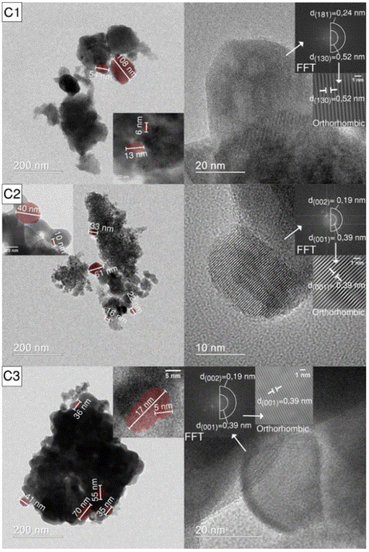 | ||
| Fig. 6 TEM (left) and HRTEM (right) images of the C1, C2 and C3 samples: (C1) stoichiometric, (C2) excess oxidant, (C3) excess fuel [reprinted with permission from ref. 35. Copyright 2021 doiSerbia]. | ||
Azizi et al. group synthesized ZnO nanoparticles with mean particle size between 27 and 85 nm by different bio-fuels. The microscopic morphology of the ZnO particles synthesized using fruits, seeds and pulp as fuels were flower-like, hexagonal and block nanostructures, respectively.36 Radpour et al. group synthesized Fe3O4 powders by conventional ignition and microwave ignition methods. When φ = 1, the sample prepared by conventional ignition contains only the Fe3O4 phase, while the sample made by microwave ignition contains the Fe3O4 phase and some impurity phases such as α-Fe2O3 and FeO.37 Maziviero et al. group successfully synthesized Al2O3 powders with high specific surface area (>200 m2 g−1) at microwave power of 450 W and lower fuel content. In addition, Fig. 7 shows the morphologies of the samples. The micrographs revealed powders with plate-like aggregates with irregular sizes.38 Tamizhdurai et al. group successfully synthesized CeO2 powders at microwave power of 750 W.39 Microwave induced solution combustion method can rapidly and efficiently prepare different types of nano single metal oxides. The comprehensive properties of single metal oxide nanomaterials can be improved by process control.
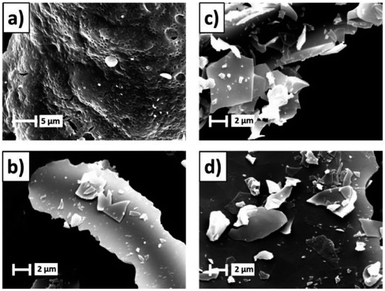 | ||
| Fig. 7 The SEM images of the aluminas: (a) non-calcined; (b–d) calcined at 550, 750, 950 °C [reprinted with permission from ref. 38. Copyright 2021 Elsevier]. | ||
3.2 Composite metal oxide nanomaterials
Composite metal oxides nanomaterials have a wide range of applications. Many methods have been derived for the preparation of composite metal oxide nanomaterials. The methods mainly include the sol–gel method,40 microemulsion method,41 co-precipitation method,42 hydrothermal method,43 and microwave-induced solution combustion method, etc. Among them, the microwave-induced solution combustion synthesis method is a green and efficient method and its simple operation, uniform heating, short heating time, and small particle size of products.Therefore, the method is widely used for the preparation of the composite metal oxide nanomaterials. Table 2 lists some relevant reports on the synthesis of composite metal oxide nanomaterials by microwave-induced solution combustion.
| Synthetic materials | Raw materials | Material characteristics | Particle/crystallite size (nm) | Applications |
|---|---|---|---|---|
| MnFe2O4 | Mn(NO3)2·4H2O | Small particle size | 7–40 | Catalyst44 |
| Fe(NO3)3·9H2O | High crystallinity | |||
| C6H8O7 | ||||
| CoFe2O4 | Co(NO3)2·6H2O | — | — | Photocatalysis45 |
| Fe(NO3)3·9H2O | ||||
| CO(NH2)2/C2H5NO2/C6H8O7 | ||||
| NiFe2O4 | Ni(NO3)2·6H2O | Small particle size | 50–70 | Catalyst51 |
| Fe(NO3)3·9H2O | High crystallinity | |||
| Plant extract | High purity | |||
| BiFeO3 | Bi(NO3)3·5H2O | Good electrochemical properties | 50–90 | Catalyst52 |
| Fe(NO3)3·9H2O | High crystallinity | |||
| C6H8O7 | ||||
| Y2O3–MgO | Mg(NO3)2·6H2O | Small particle size | 19 | Transparent ceramic53 |
| Y(NO3)3·6H2O | High thermal stability | |||
| C6H8O7 | ||||
| ZnAl2O4 | Zn(NO3)2·4H2O | High surface area | — | Catalyst carrier54 |
| Al(NO3)3·9H2O glycine/ethylene glycol/acetic acid | ||||
| ZnFe2O4 | Zn(NO3)2·4H2O | — | 37/74 | Solar cells55 |
| Fe(NO3)3·9H2O | ||||
| Plant extract solution | ||||
| Li4SiO4 | Si(OC2H5)4 | Small particle size | 25 | Ceramic tritium breeders56 |
| LiNO3 | High purity | |||
| C6H8O7 | Favourable sinterability |
The composite metal oxide nanomaterials prepared by microwave-induced solution combustion method have nanoscale microscopic size as well as high crystallinity. de Andrade et al. group successfully synthesized MnFe2O4 nanoparticles. The results of X-ray diffraction spectra showed that the synthesized material was the cubic phase of MnFe2O4 (JCPDS 73-1964). Fig. 8 shows that the formed MnFe2O4 nanoparticles were irregularly agglomerated in shape. The catalytic degradation of the malachite green (MG) cationic dye was investigated using a mixture of H2O2 and MnFe2O4 nanoparticles. The results suggested that the dye was completely decomposed after 60 min forming colourless products. In Fig. 9, Fig. 9(a) and (b) shows HRTEM images of MnFe2O4 nanoparticles. High-resolution transmission electron microscopy was used to better estimate the size of the formed particles. Their high magnification TEM images in Fig. 9(c) and (d) shows that the material consists of nanoparticles in the range of 7–40 nm.44
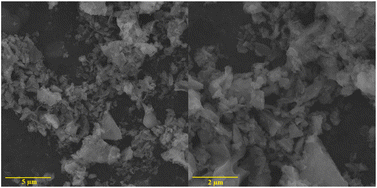 | ||
| Fig. 8 The SEM image of MnFe2O4 nanoparticles [reprinted with permission from ref. 44. Copyright 2021 Elsevier]. | ||
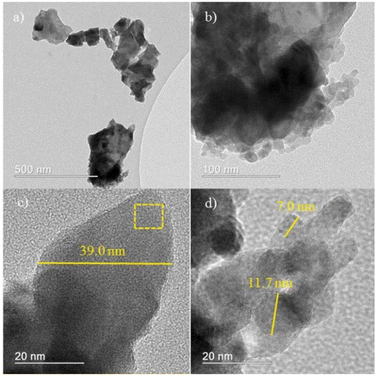 | ||
| Fig. 9 High-resolution transmission electron microscopy (HRTEM) images (a–d) [reprinted with permission from ref. 44. Copyright 2021 Elsevier]. | ||
Microwave-induced solution combustion synthesis can be applied as a simple and effective synthesis method to improve the catalytic properties of spinel ferrite structured nanoparticles. Karakas et al. group successfully synthesized CoFe2O4 nanoparticles with thermally and chemically stable structures. By further investigating the effects of fuel type on the photocatalytic properties of the synthesized nanoparticles. The results showed that the dye was almost completely removed from the solution when the samples were prepared using citric acid. In addition, the removal efficiencies of the samples prepared with urea and glycine were 99% and 96%, respectively. Therefore, the improvement of the catalytic activity of cobalt ferrite nanoparticles can be achieved by adjusting the type of fuel.45
Compared with the conventional combustion method, the composite metal oxide nanomaterials synthesized by microwave-induced combustion have better microstructure and properties. Javadi et al. group successfully synthesized Cobalt ferrite (CoFe2O4) nanoparticles by conventional and microwave heating techniques. The results showed that the spherical CoFe2O4 nanoparticles synthesized by microwave heating are larger and have a higher saturation magnetization intensity compared to conventional heating.46 Haghi et al. group successfully prepared LiFePO4 powders using CTAB as fuel by conventional and microwave-assisted solution combustion methods. Compared with the conventional heating method, the LiFePO4 powder synthesized by microwave heating has higher crystallinity, larger particle size and smaller specific surface area. In addition, the LiFePO4 powders prepared by microwave heating method exhibited higher electrical conductivity due to lower electrode resistance.47 Ragupathi et al. group successfully prepared nickel aluminate (NiAl2O4) using Opuntia dilenii haw as plant extract by conventional and microwave-assisted solution combustion methods. They found that the NiAl2O4 prepared by microwave combustion method is uniform in size and well-defined shape with crystallinity. The NiAl2O4 prepared by microwave combustion method was found to possess a higher specific surface area and smaller grain size than the NiAl2O4 synthesized by conventional combustion method.48 Ajamein et al. group successfully synthesized Cobalt CuO–ZnO–Al2O3 nanocatalyst using ethylene glycol as fuel in the microwave oven and the conventional furnace. The results showed that the CuO prepared by the MCM method exhibited smaller particles, higher surface area and better crystallinity.49 Kombaiah et al. group successfully prepared nanocrystalline ZnFe2O4 samples using Hibiscus rosa-sinensis plant extract as fuel by conventional and microwave-assisted solution combustion methods. The results showed that the ZnFe2O4 nanoparticles prepared by microwave-assisted combustion exhibited higher specific surface area and smaller grain size than the ZnFe2O4 nanoparticles prepared by conventional combustion.50 In addition, Kombaiah et al. group successfully synthesized NiFe2O4 using plant extract as a fuel. Nickel nitrate and ferric nitrate was mixed in a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 in a mortar pestle. Then the samples and plant extracts were dissolved double distilled water and stirred into a homogeneous clear solution. Finally, the solution will be ignited in a muffle and microwave oven to form NiFe2O4 powder. The results of XRD showed the synthesis of NiFe2O4 in a single crystalline phase with an average crystallite size of 50–70 nm. The particle sizes of the synthesized NiFe2O4 nanoparticles was observed using scanning electron microscopy (SEM). The NiFe2O4 nanoparticles with particle sizes in the range of 100–200 nm were synthesized by conventional combustion. However, the size of NiFe2O4 nanoparticles synthesized by microwave combustion was between 50–100 nm. Fig. 10 shows that the SEM image of NiFe2O4 synthesized by conventional solution combustion and microwave induced solution combustion. In addition, the catalytic reduction ability of NiFe2O4 nanoparticles for different dyes such as Rhodamine-B (RhB), Methylene blue (MB), Rose Bengal (RB), and Congo red (CR) was investigated. The results showed that the NiFe2O4 nanoparticles can be used as high potential catalyst for the reduction of organic dyes in wastewater treatment applications.51
2 in a mortar pestle. Then the samples and plant extracts were dissolved double distilled water and stirred into a homogeneous clear solution. Finally, the solution will be ignited in a muffle and microwave oven to form NiFe2O4 powder. The results of XRD showed the synthesis of NiFe2O4 in a single crystalline phase with an average crystallite size of 50–70 nm. The particle sizes of the synthesized NiFe2O4 nanoparticles was observed using scanning electron microscopy (SEM). The NiFe2O4 nanoparticles with particle sizes in the range of 100–200 nm were synthesized by conventional combustion. However, the size of NiFe2O4 nanoparticles synthesized by microwave combustion was between 50–100 nm. Fig. 10 shows that the SEM image of NiFe2O4 synthesized by conventional solution combustion and microwave induced solution combustion. In addition, the catalytic reduction ability of NiFe2O4 nanoparticles for different dyes such as Rhodamine-B (RhB), Methylene blue (MB), Rose Bengal (RB), and Congo red (CR) was investigated. The results showed that the NiFe2O4 nanoparticles can be used as high potential catalyst for the reduction of organic dyes in wastewater treatment applications.51
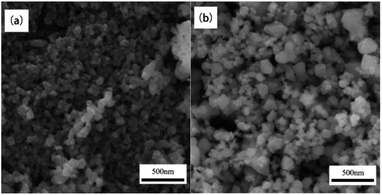 | ||
| Fig. 10 The SEM image of NiFe2O4 nanoparticles: (a) conventional solution combustion synthesis, (b) microwave induced solution combustion synthesis [reprinted with permission from ref. 51. Copyright 2019 Elsevier]. | ||
Moradi et al. group successfully prepared BiFeO3 and BiFeO3 (CTAB/PVA/PEG) nanocomposites by a one-step green combustion synthesis. Hexadecyl trimethyl ammonium bromide (CTAB), polyvinyl alcohol (PVA), and polyethylene glycol (PEG-6000) are used as surfactants. The effect of surfactants on the catalytic performance of bismuth ferrate was investigated. The results showed that BiFeO3-CTAB nano composites exhibited the highest degradation rate of methyl orange dye contamination, and the degradation rate could be increased up to 97% in aqueous solution.52 Mathew et al. group prepared Y2O3–MgO nanocomposites by one-step microwave sintering. The XRD analysis results showed the presence of ultrafine nanostructured Y2O3 and MgO cubic phases with an average microcrystal size of about 19 nm. Fig. 11(a) shows the TEM image of Y2O3–MgO nanocomposite. TEM images exhibited that the crystal size ranges from 12 nm to 24 nm. Fig. 11(b) shows the different crystal planes of Y2O3–MgO nanocomposites. From the clearly visible grains, the grain size is calculated to be 15.6 nm, corresponding to the (222) plane of cubic crystal structure of Yttrium.53
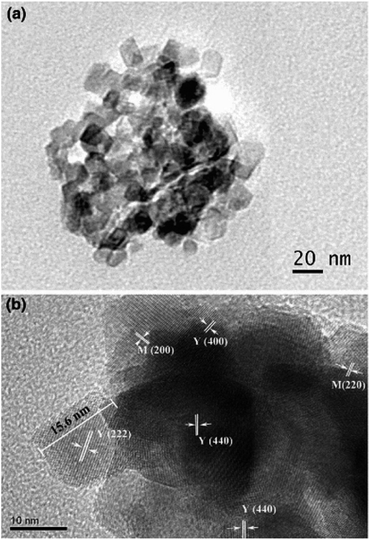 | ||
| Fig. 11 (a) TEM image of as-prepared nano Y2O3–MgO nanocomposite. (b) HRTEM image of different crystallographic planes [reprinted with permission from ref. 53. Copyright 2017 Indian Academy of Science]. | ||
Talati et al. group successfully synthesized ZnAl2O4 nanoparticles using Zn(NO3)2·4H2O and Al(NO3)3·9H2O as oxidants and glycine (Gly), ethylene glycol (EG), and acetic acid (AA) as reducing agents by the one-step microwave combustion (MW) method and muffle-furnace combustion (MF). In the process of synthesis, Zn(NO3)2·4H2O and Al(NO3)3·9H2O in Zn/Al molar ratio of 1/2 were dissolved into deionized water. Then the fuel was added into the solution at the stoichiometric ratio and vigorously stirred. In the next stage, the mixture temperature was raised to 60 °C and continue stirring until a transparent gel was formed. Finally, the generated transparent gel was put into a microwave oven at 950 W for 2 min to synthesize ZnAl2O4 nanoparticles. As shown in Fig. 12(a), the surface morphology of the ZnO nanophotocatalyst synthesized with glycine consists of spherical nanoparticles with large pores. In Fig. 12(b) and (c), the surface morphology of the ZnAl2O4 nanophotocatalyst synthesized by ethylene glycol and acetic acid consists of continuous spherical agglomerates with low porosity. In contrast, the synthesized Zn-spinel samples with glycine (Fig. 12(d) and (e)) have porous structures with big combustion pores.54 Compared with the conventional solution combustion method, the microwave-induced solution combustion method is a more promising simple, efficient and energy saving material preparation method, which produces composite metal oxide nanomaterials with better stability and higher purity. Due to its unique heating mechanism, the process of microwave-induced solution combustion can reduce the production of impurity phases.
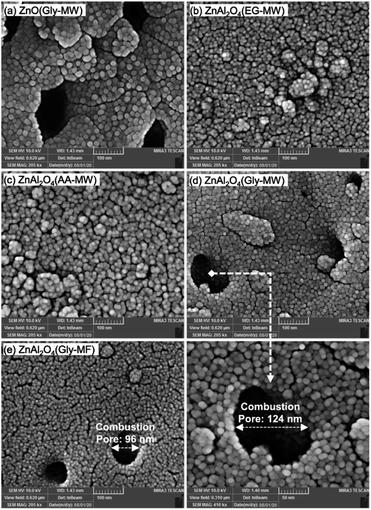 | ||
| Fig. 12 The FESEM image of Zn-spinel nanophotocatalysts: (a) ZnO(Gly-MW), (b) ZnAl2O4(EG-MW), (c) ZnAl2O4(AA-MW), (d) ZnAl2O4(Gly-MW), (e) ZnAl2O4(Gly-MF) [reprinted with permission from ref. 54. Copyright 2022 Elsevier]. | ||
Kombaiah et al. group successfully prepared ZnFe2O4 nanomaterials using iron nitrate and zinc nitrate as oxidants and plant extracts as fuel (reducing agent) by conventional solution combustion and microwave-induced solution combustion methods. In addition, they studied the effect of two different heating methods on the performance of the synthesized materials. The results showed that the maximum conversion rate of solar cells composed of ZnFe2O4 prepared by microwave-induced combustion method could reach 0.22%, which is higher than the conversion efficiency of samples prepared by conventional combustion method. The particle sizes of the synthesized NiFe2O4 nanoparticles was observed using scanning electron microscopy (SEM). The ZnFe2O4 nanoparticles with particle sizes in the range of 5–15 nm were synthesized by conventional combustion. However, the size of ZnFe2O4 nanoparticles synthesized by microwave combustion was between 30-50 nm.55 Zhou et al. group synthesized lithium orthosilicate (Li4SiO4) by microwave induced solution combustion synthesis using tetraethyl orthosilicate (Si(OC2H5)4) and lithium nitrate (LiNO3) as oxidants and citric acid as fuel. The research found that the content of Li2SiO3 in the product could be effectively reduced by regulating the microwave power and the ratio of fuel to oxide. When the microwave power output is 1100 W and the φ value is 0.75, the Li2SiO3 content can be minimized. In addition, the thermal analysis revealed that the relative density of the ceramic embryos after calcination at the sintering temperature of 1373 K was as high as 96%, which is an ideal material for tritium-augmented ceramics.56 Sundararajan et al. group synthesized NiFe2O4 and ZnFe2O4 nanoparticles with average grain size ranging from 16 to 20 nm using microwave induced solution combustion method.57
Mahmoud et al. group successfully prepared NiFe2O4 using metal nitrates and urea as starting materials by a microwave-induced combustion method and investigated the effect of fuel-to-oxidizer ratio on their particle size during the combustion process. The results showed that the particle size can be systematically controlled by simply changing the urea/metal nitrate ratio. When the urea is relatively low, the sample is less crystalline.58
3.3 Ion doping metal oxide nanomaterials
The microwave-induced solution combustion synthesis method has also been mostly used to prepare ion-doped metal oxide nanomaterials. This method is not only simple, low-cost, and efficient, but also can improve the microstructure and properties of ion-doped nano-metal oxides. Zhao et al. group successfully prepared neodymium aluminum lutetium-doped garnet (LuAG: Nd) phosphor materials with particle sizes ranging from 100 to 130 nm using microwave induced solution combustion synthesis. Fig. 13 shows the microscopic morphology of the synthesized LuAG: Nd at different calcination temperatures. The results showed that the microwave induced solution combustion synthesized LuAG: Nd grains as regular spherical grains when the calcination temperature was 900 °C.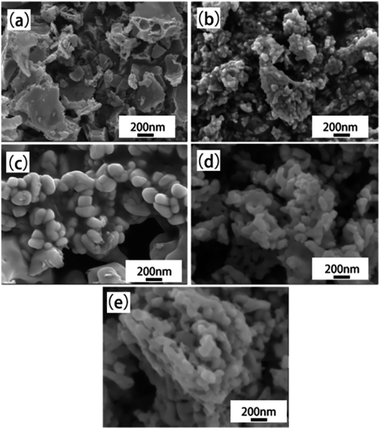 | ||
| Fig. 13 The SEM images of Lu2.85Nd0.15Al5O12 powders prepared with different temperatures: (a) 800 °C, (b) 850 °C, (c) 900 °C, (d) 950 °C, (e) 1000 °C [reprinted with permission from ref. 59. Copyright 2020 Elsevier]. | ||
In addition, the fluorescence emission spectra of LuAG: Nd powders with different Nd3+ doping concentrations were detected. The results showed that the highest emission peak intensity of fluorescence spectrum can be observed at 1064 nm for LuAG: Nd powders when the Nd3+ doping concentration is 5 mol%. The results indicated that the LuAG: Nd phosphor synthesized by microwave-induced solution combustion is a promising optical material.59
Compared with the conventional combustion method, the ion-doped metal oxide nanomaterials synthesized by microwave-induced combustion have better microstructure and properties. Chen et al. group successfully synthesized pure and well-crystallized BaMgAl10O17: Eu2+ (BAM) nanoscale blue phosphors by adopting a feasible microwave-induced solution combustion synthesis (MISCS) method. By studying the BAM synthesized by traditional solution combustion and microwave solution combustion, the results showed that the BAM phosphor synthesized by microwave solution combustion exhibited good spherical morphology, good crystallinity, and strong blue light emission ability under vacuum ultraviolet (VUV) light excitation. In Fig. 14, The TEM and high-resolution TEM (HRTEM) images of BAM shows the lattice fringes with a lattice spacing of 0.486 nm, corresponding to the (0 0 1) plane of β-alumina-structured BAM. It is known that the dipole molecules (H2O) are constantly rotating in the presence of microwave radiation, which generates heat in the microwave oven. Therefore, the reactions are uniformly heated in the microwave oven compared to the reactions in conventional heating systems,60 which helps to increase the reaction rate. The main advantage of the introduction of microwave into the reaction system is the extremely rapid crystallization kinetics,61 so that smaller particle sizes and well crystallized particles can be imagined.62
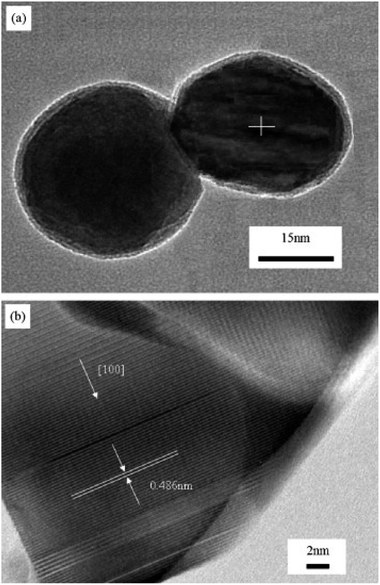 | ||
| Fig. 14 (a) TEM image of BAM nanoparticle; (b) high-resolution TEM image of the BAM in Fig. 2(a) [reprinted with permission from ref. 62. Copyright 2009 Elsevier]. | ||
The ion-doped metal oxide nanomaterials prepared by microwave-induced solution combustion synthesis are characterized by small particle size, large specific surface area, and the preparation process is simple. Medeiros et al. group prepared nickel based catalysts with alumina (Al2O3) as the carrier by microwave-induced solution combustion synthesis using aluminum nitrate nonahydrate and nickel nitrate nonahydrate as oxidants and urea as reducing agent. In addition, the effect of the fuel-to-oxidizer ratio on the catalytic performance of the prepared products was investigated. The results showed that the nickel-based catalysts prepared with alumina (Al2O3) as the carrier at low fuel ratio (fuel-to-oxidizer ratio less than 50% of the stoichiometric ratio) have a high reduction, high specific surface area (265 m2 g−1) and small size (∼15 nm). These properties make the catalytic performance of nickel-based catalysts improved.63 Baskar et al. group synthesized strontium ion-doped cubic crystalline magnesium ferrite using arginine as a fuel after reacting at 700 W microwave power for 15 min. The average particle size was calculated from 24.11 to 33.10 nm by the Debay–Scherrer formula. The presence of Sr, Mg, Fe and O was confirmed by EDX studies.64
Palan et al. group prepared BaLaB9O16 doped and co-doped with Eu3+ and Tb3+ by microwave induced solution combustion. The research found that microwave assisted heating resulted in better sintering properties of the products.65 Ansari et al. group dissolved zinc nitrate, dopant nitrate (Fe, Al, Cr, and Li), and glucose fuel in distilled water in accordance with the stoichiometric ratio, heated in a microwave oven for 2–3 min, and then placed in a preheated furnace at 500 °C. After heating in the furnace for 10 min, Zn0.94M0.06−xLixO (M: Fe3+, Al3+, Gr3+) nanoparticles were synthesized. In addition, the effect of different ion doping on the photocatalytic efficiency of ZnO catalysts in the degradation of rhodamine B dye was investigated. The results showed that the photodegradation efficiencies of CZO (6 mol% Gr3+-doped ZnO), AZO (6 mol% Al3+-doped ZnO), and L3AZO (1.5 mol% Li+-ion co-doped AZO) catalysts were higher compared with the photodegradation efficiencies of ZnO catalysts (89%).66 Kanithan et al. group used nickel nitrate, iron nitrate, and magnesium nitrate as oxidants and arginine as fuel to synthesize undoped nickel ferrite spinel (NiFe2O4) and magnesium doped nickel ferrite spinel (Ni1−xMgxFe2O4). The undoped NiFe2O4 has a single-phase cubic spinel structure with an average crystallite size between 20 and 35 nm, while the magnesium-doped NiFe2O4 also has a cubic spinel structure. In addition, doping of magnesium ions will induce secondary crystallization of the α-Fe2O3 phase. Fig. 15 shows the SEM images of undoped Mg2+ (x = 0) and doped Mg2+ (x = 0.1).67 Dhiwahar et al. group successfully prepared Cu1−xCoxFe2O4 with different cobalt contents by microwave-induced solution combustion method. In addition, the effect of doping with or without cobalt ions on the degradation of rhodamine B by copper ferrites under visible light (k > 420 nm) irradiation was investigated. The results showed that the degradation effect increased with the content of doped cobalt ions.68
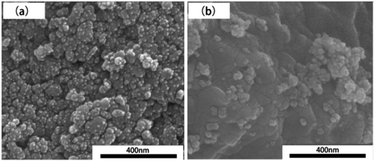 | ||
| Fig. 15 The SEM image of Ni1−xMgxFe2O4 nanoparticles: (a) undoped magnesium ion (x = 0), (b) doped magnesium ion (x = 0.1) [reprinted with permission from ref. 67. Copyright 2022, Elsevier. | ||
Sertkol et al. group successfully synthesized ZnxNi1−xFe2O4 using metal nitrates and urea as starting materials by microwave-assisted combustion method. In Fig. 16, the TEM analysis results shows the presence of necked near-spherical particles with an average size of ∼20 nm.69 Freitas et al. group successfully synthesized nanocrystalline NixCo1−xFe2O4 from nitrate precursors, using urea as fuel. The TEM analysis results showed the presence of Near-spherical and necked particles with an average crystallite size of 30 to 50 nm.70 Chamani et al. group successfully prepared CoxNi1−xferrite nanoparticles with varying compositions by microwave auto-combustion method. In Fig. 17, the high-resolution TEM (HRTEM) images of Co0.9Ni0.1ferrite shows the lattice fringes with a lattice spacing of 0.496 nm, corresponding to the (111) of CoFerrite.71
 | ||
| Fig. 16 TEM micrographs of ZnxNi1−xFe2O4 nanoparticles where x is (a) 0.0, (b) 0.2, and (c) 0.7, synthesized by microwave assisted combustion method [reprinted with permission from ref. 69. Copyright 2009 Elsevier]. | ||
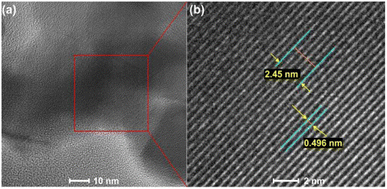 | ||
| Fig. 17 (a, b) HRTEM images of Co0.9Ni0.1Ferrite [reprinted with permission from ref. 71. Copyright 2021 American Chemical Society]. | ||
4 Influence of process parameters related to microwave-induced solution combustion synthesis of nanomaterials
In the process of microwave-induced solution combustion synthesis of nanomaterials, a series of parameters such as microwave power, the dosage ratio of oxidant to fuel, the type of fuel, and the pH value of the precursor solution will have certain effects on the microstructure and properties of the products.4.1 Influence of fuel type
The presence of fuel plays a crucial role in the synthesis of nanomaterials by microwave-induced solution combustion. During the combustion reaction, the fuel not only acts as a reducing agent but also has good complexation. In addition, the fuel can form a gel network with metal ions to prevent early precipitation of metal ions, maintain the compositional homogeneity between all components, and contribute to the formation of strong coordination bonds.72 Therefore, the fuel type has a great influence on the microstructure of the final oxidized material.Azizi et al. group investigated the microstructural changes of the ZnO nanoparticles using zinc nitrate as an oxidant and the fruits, seeds, and pulp of citrus as fuel. The results revealed that the average particle size of the synthesized ZnO nanoparticles ranged from 27 to 85 nm. The shapes of the ZnO particles synthesized with fruits, seeds, and pulp were a flower-like, hexagonal, and blocky nanostructures, respectively.36 Mahu et al. group used urea, citric acid, and maleic acid as fuels and successfully prepared CoFe2O4 and NiFe2O4 nanoparticles. Among the three different fuels, the CoFe2O4 and NiFe2O4 nanoparticle samples prepared with urea as fuel had the smallest grain size and the highest specific surface area.73 Karakas et al. group synthesized CoFe2O4 powders by combustion using Co(NO3)2·6H2O and Fe(NO3)3·9H2O as oxidants and urea, glycine, and citric acid as fuels. The effect of the type of fuel added on the structure and morphology of the synthesized CoFe2O4 nanoparticles was investigated. The results showed that the samples synthesized with urea had the smallest particle size. The samples prepared with glycine had more uniform morphology and proper shape, but their average particle size was observed to be larger than the other samples.45
Rasouli et al. group successfully prepared Europium oxide doped with zinc oxide using microwave-assisted combustion method. In addition, the effect of different types of fuels on the structural morphology of the synthesized nanoparticles was investigated. The results showed that the morphology of the samples shifted from nanosphere to sponge-like when the fuel mixture composition changed from citric acid to glycine.74 Khort et al. group produced metallic cobalt nanoparticles by microwave-assisted combustion and investigated the effects of different types of fuels (urea, citric acid, glycine and hexamethylenetetramine) on the combustion process and solid product properties. When hexamethylenetetramine was used as a fuel, the results showed that metallic Co nanoparticles with a wide size distribution (∼5–40 nm) could be synthesized by one-step solution combustion under normal air atmosphere.75
4.2 Influence of the dosage ratio of oxidant to fuel
During the reaction of microwave-induced solution combustion synthesis, the different dosage ratios of oxidant to fuel can have a great influence on the microstructure and properties of the products. Luiz et al. group prepared niobium pentoxide (Nb2O5) powder by this method and investigated the effect of the dosage of ammonium niobium oxalate and urea on the microstructure of the products. When the dosage ratio of oxidant to fuel was the stoichiometric ratio, the resulting Nb2O5 had the largest particle size, while excess of fuel or excess of oxidant will give smaller particle size of Nb2O5. This is mainly because the amount of oxidant and fuel can change the temperature of the reaction process, which affects the size of the resulting particles. Excessive amounts of both oxidant and fuel lowered the reaction synthesis temperature and slowed down the growth of the particles.35 Nayebzadeh et al. group used the microwave combustion method to prepare zirconia-alumina nanocatalysts and investigated the effect of fuel-to-oxidizer ratio (F/O) on their structural and chemical properties during the combustion process. The results showed that when the fuel-to-oxidizer ratio increased from 0.5 to 1.0, the combustion reaction temperature and time increased, leading to the formation of ZrO and α-Al2O3. The crystal size of zirconia-alumina nanocatalysts increased when the fuel-to-oxidizer ratio was increased from 1 to 1.5.76Derakhshani et al. group prepared hierarchical porous Ni0.5Zn0.5Fe2O4 ferrite powder using metal nitrate as oxidant and glycine as fuel. By investigating the effect of fuel content on the composition of the synthesized material, the results showed that the pure NiZn ferrite powder was directly crystallized at lower fuel content, and about 2 wt% intermetallic FeNi3 phase appeared at fuel to total metal nitrates molar ratio of 2.77 Frikha et al. group prepared three binary metal oxide catalysts containing Ni, Cu, and Co oxides using microwave-assisted solution combustion method. The results showed that the performance of the catalysts prepared by microwave-assisted solution combustion can be improved by controlling the reactant stoichiometric ratio (RV/OV). Catalysts with the best structural properties and the best catalytic activity can be prepared at the stoichiometric balance (RO/RV = 1). On the contrary, when the RV/OV ratio of the catalysts was non-stoichiometric balance, the combustion reactions were not controlled, thus resulting to poor structural properties of the catalysts.78
4.3 The pH of the precursor solution
The pH of the precursor solution also plays a crucial role in the microwave-induced solution combustion synthesis of nanomaterials, mainly affecting the intensity of the combustion process and the structural morphology of the final oxidized material.72 Radpour et al. group formulated precursor solutions using Al(NO3)3·9H2O as an oxidant and C6H8O7 as fuel. Then ammonia was added to adjust the pH value of the solution to 2, 7, and 9. The iron oxide nanopowders were synthesized at different pH conditions. The average particle sizes of the synthesized Fe3O4 were 10 nm, 25 nm, and 27 nm when the pH values were 2, 7, and 9, respectively. In addition, when the pH = 2, the iron oxide (Fe3O4) was generated, and when the pH = 7 or the pH = 9, in addition to Fe3O4, the FeO phase was also generated.79 Araichimani et al. group prepared Fe3O4-modified SiO2 nanostructures by microwave-induced combustion method using rice husk as the silicon source. The effect of pH of the precursor solution on the ability of the synthesized samples to adsorb Hg2+ was also investigated. The results showed that increasing the pH of the precursor solution could increase the removal rate of Hg2+. The removal rate of Hg2+ reached the maximum when pH = 6. This is mainly due to the fact that only a small amount of mercury ions (Hg2+) are adsorbed on the surface of the prepared samples at lower pH values. Besides, the amount of hydrogen ions (H+) decreased significantly with increasing pH, resulting in enhanced adsorption of Hg2+ on the prepared samples at pH = 6.804.4 Microwave power
In the process of microwave-induced solution combustion synthesis, different microwave powers affect the phase composition and microstructure of the products. Zhou et al. group prepared Li4SiO4 nanoparticles by microwave-induced solution combustion synthesis and investigated the effect of different microwave powers on the composition of the product phases by adjusting the microwave power to 700 W, 900 W, 1100 W, and 1300 W. The results showed that the Li4SiO4 powders produced by microwave-induced solution combustion synthesis at four different microwave powers contained a small amount of Li2SiO3. When the microwave output power was 1100 W, the content of Li2SiO3 decreased to 5%, which improved the purity of the products.56 Yathisha et al. group synthesized prismatic zinc oxide (ZnO) nanostructures by microwave combustion method using zinc nitrate as precursor and ethylene glycol as solvent at different microwave powers (160, 320, 480, 640 and 800 W). XRD and FE-SEM results confirmed the dependence of grain size and growth of ZnO nanostructures on microwave heating power.81Hashemzehi et al. group successfully synthesized zinc-aluminum spinel as well as copper-aluminum spinel by microwave induced solution combustion method. The effect of microwave power on the microstructure of the synthesized zinc-aluminum spinel as well as copper aluminum spinel was also investigated to obtain zinc/copper-aluminum spinel with small grain size and less agglomerated particles. The experimental results showed that the higher microwave power is beneficial to the improvement of the morphology and structure of zinc/copper aluminum spinel nanoparticles. The growth of pore diameter was resulted by increasing microwave power.82
5 Conclusions and prospect
Currently, the microwave-induced solution combustion method is widely used in the synthesis and preparation of nanomaterials. Compared with the traditional heating method, the advantages of the microwave-induced solution combustion method are reflected in the uniform heating of the reaction mixture, high selectivity, clean products, good reproducibility, and energy saving.In addition, the method can significantly shorten the reaction time of the reactants and effectively reduce the generation of by-products during the reaction process, thus contributing to energy conservation and rapid preparation of the target products. Microwave synthesis technology has contributed greatly to the preparation of nanomaterials of various sizes and well-defined structures with the premise of using liquid substances as the reaction medium. Although the microwave-induced solution combustion synthesis method has a series of advantages such as high efficiency and energy saving, there are still some difficulties to be solved:
(1) Regarding the mechanism of microwave heating, some people think that the heat generated by microwave heating is due to depolarization, while others think that the heat generated by microwave heating is caused by Ionic conductance. The mechanism of microwave heating can not be clearly explained at present. Therefore, it is necessary to further understand the nature of the reaction mechanism in the future.
(2) The controlled preparation of nanomaterials with different structures, morphologies, and properties can be achieved by regulating the parameter settings of microwave heating methods.
(3) The reaction space of microwave heating equipment is relatively small, which leads to the high cost of mass production. Therefore, further optimization of the microwave heating equipment is required.
(4) The method of microwave-induced solution combustion synthesis does not have the conditions for continuous production. Therefore, it is possible to provide a possibility for continuous production of microwave-induced solution combustion synthesis of nanopowders by improving the synergy between the equipment.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 51472220, No. 51872265), and Collaborative Innovation Major Special Project of Zhengzhou (No. 20XTZX12025).References
- H. Gleiter, Acta. Metall. Sin., 1997, 33, 165–174 CAS
.
- K. Shehzad, Y. Xu, C. Gao and X. Duan, Chem. Soc. Rev., 2016, 45, 5541–5588 RSC
.
- Y. Yao, Y. Wei and S. Chen, Surf. Sci., 2015, 636, 19–24 CrossRef CAS
.
- L. Zhang, L. Yin, C. Wang, N. Lun, Y. Qi and D. Xiang, J. Phys. Chem. C, 2010, 114, 9651–9658 CrossRef CAS
.
- A. Verma and F. Stellacci, Small, 2010, 6, 12–21 CrossRef CAS PubMed
.
- N. V. Ilawe, M. Belen Oviedo and B. M. Wong, J. Mater. Chem. C, 2018, 6, 5857–5864 RSC
.
- Y. R. Parauha, V. Sahu and S. J. Dhoble, Mater. Sci. Eng., B, 2021, 267, 115054 CrossRef CAS
.
- D. Wang and Y. Chang, Nanomaterials, 2022, 12, 572 CrossRef CAS PubMed
.
- D. P. Harris, C. Wan, Y. She, B. R. Beck, D. S. Forbes and B. M. Leonard, Z. Naturforsch., B: J. Chem. Sci., 2021, 76, 803–810 CrossRef CAS
.
- R. Yu, F. Liu, H. Ren, J. Wu and X. Zhang, Environ. Technol., 2018, 39, 2162–2167 CrossRef CAS PubMed
.
- Y. Chang, B. Liu, Z. Huang, Y. Liu, M. Liu and J. Liu, Langmuir, 2020, 36, 1034–1042 CrossRef CAS PubMed
.
- D. Shi, H. Yang and X. Xue, Chin. J. Chem. Eng., 2020, 28, 1474–1482 CrossRef CAS
.
- T. Cai, G. Fang, X. Tian, J.-J. Yin, C. Chen and C. Ge, ACS Nano, 2019, 13, 12694–12702 CrossRef CAS PubMed
.
- L. Katsnelson and B. Kerbel, Non-Ferrous Pyrometall.: Trace Met., Furn. Pract. Energy Effic., Proc. Int. Symp., 2012, 1, 34–37 Search PubMed
.
- L. Zhang, Z. Li, F. Zhen, L. Wang, Q. Zhang, R. Sun, F. A. Selim, C. Wong and H. Chen, J. Mater. Sci., 2017, 52, 8556–8567 CrossRef CAS
.
- W. He, P. Song, B. Yu, Z. Fang and H. Wang, Prog. Mater. Sci., 2020, 114, 100687 CrossRef CAS
.
- S. R. Jain, K. C. Adiga and V. R. Pai Verneker, Combust. Flame, 1981, 40, 71–79 CrossRef CAS
.
- D. Evecan, Ş. S. Kaplan, M. Ş. Sönmez, S. Yıldırım, M. Okutan, H. Deli-gçz and E. Zayim, Microelectron. Eng., 2019, 215, 110989 CrossRef CAS
.
- D. Zheng, G. Wang, W. Huang, B. Wang, W. Ke, J. L. Logsdon, H. Wang, Z. Wang, W. Zhu, J. Yu, M. R. Wasielewski, M. G. Kanatzidis, T. J. Marks and A. Facchetti, Adv. Funct. Mater., 2019, 29, 1900265 CrossRef
.
- S. L. González-Cortés and F. E. Imbert, Appl. Catal., A, 2013, 452, 117–131 CrossRef
.
- K. K. Banger, Y. Yamashita, K. Mori, R. L. Peterson, T. Leedham, J. Rick-ard and H. Sirringhaus, Nat. Mater., 2011, 10, 45–50 CrossRef CAS PubMed
.
- Z. Shao, W. Zhou and Z. Zhu, Prog. Mater. Sci., 2012, 57, 804–874 CrossRef CAS
.
- B. N. Rao and N. Satyanarayana, ECS J. Solid State Sci. Technol., 2021, 10, 103003 CrossRef CAS
.
- G. A. Tompsett, W. C. Conner and K. S. Yngvesson, ChemPhysChem, 2006, 7, 296–319 CrossRef CAS PubMed
.
- Y. J. Zhu and F. Chen, Chem. Rev., 2014, 114, 6462–6555 CrossRef CAS PubMed
.
- C. O. Kappe, Angew. Chem., Int. Ed., 2004, 43, 6250–6284 CrossRef CAS PubMed
.
- D. V. Stass, J. R. Woodward, C. R. Timmel, P. J. Hore and K. A. McLauchlan, Chem. Phys. Lett., 2000, 329, 15–22 CrossRef CAS
.
- M. J. Collins Jr, Future Med. Chem., 2010, 2, 151–155 CrossRef PubMed
.
- B. L. Hayes, Microwave synthesis: Chemistry at the speed of light, Cem Corporation, 2002 Search PubMed
.
- C. O. Kappe, D. Dallinger and S. S. Murphree, Strategies, Instruments, and Protocols, Wiley-VCH, Weinheim, Germany, 2009 Search PubMed
.
- P. Michael and D. áMingos, Chem. Soc. Rev., 1991, 20, 1–47 RSC
.
- T. Patra, J. Panda and T. R. Sahoo, Mater. Today: Proc., 2021, 41, 247–250 CAS
.
- G. T. Anand, S. J. Sundaram, K. Kanimozhi, R. Nithiyavathi and K. Kaviyarasu, Mater. Today: Proc., 2021, 36, 427–434 Search PubMed
.
- B. Priyadarshini, T. Patra and T. R. Sahoo, J. Magnesium Alloys, 2021, 9, 478–488 CrossRef CAS
.
- T. M. Luiz, F. Nakagomi, R. A. Renzetti and G. O. Siqueira, Process. Appl. Ceram., 2021, 15, 128–135 CrossRef CAS
.
- S. Azizi, R. Mohamad and M. M. Shahri, Molecules, 2017, 22, 301 CrossRef PubMed
.
- M. Radpour, S. M. Masoudpanah and S. Alamolhoda, Ceram. Int., 2017, 43, 14756–14762 CrossRef CAS
.
- F. V. Maziviero, R. L. B. A. Medeiros, D. M. A. Melo, H. P. Macedo, A. A. S. Oliveira and T. R. Araujo, Mater. Chem. Phys., 2021, 264, 124408 CrossRef CAS
.
- P. Tamizhdurai, S. Sakthinathan, S.-M. Chen, K. Shanthi, S. Sivasanker and P. Sangeetha, Sci. Rep., 2017, 7, 1–13 CrossRef PubMed
.
- S. V. Bhandare, R. Kumar, A. V. Anupama, H. K. Choudhary, V. M. Jali and B. Sahoo, J. Magn. Magn. Mater., 2017, 433, 29–34 CrossRef CAS
.
- A. Karimi, M. A. A. Sadatlu, B. Saberi, H. Shariatmadar and M. Ashjaee, Adv. Powder Technol., 2015, 26, 1529–1536 CrossRef CAS
.
- T. Zhang, D. Gong, S. Lin and J. Yu, Chem. Eng. J., 2022, 449, 137830 CrossRef CAS
.
- M. Srivastava, S. Chaubey and A. K. Ojha, Mater. Chem. Phys., 2009, 118, 174–180 CrossRef CAS
.
- F. V. de Andrade, A. B. de Oliveira, G. O. Siqueira, M. M. Lage, M. R. de Freitas, G. M. de Lima and J. Nuncira, J. Environ. Chem. Eng., 2021, 9, 106232 CrossRef CAS
.
- I. H. Karakas, Ceram. Int., 2021, 47, 5597–5609 CrossRef CAS
.
- S. Javadi, S. M. Masoudpanah and A. Zakeri, J. Sol-Gel Sci. Technol., 2016, 79, 176–183 CrossRef CAS
.
- Z. Haghi and S. M. Masoudpanah, J. Sol-Gel Sci. Technol., 2019, 91, 335–341 CrossRef CAS
.
- C. Ragupathi, J. J. Vijaya and L. J. Kennedy, J. Saudi Chem. Soc., 2017, 21, 231–239 CrossRef
.
- H. Ajamein and M. Haghighi, Energy Convers. Manage., 2016, 118, 231–242 CrossRef CAS
.
- K. Kombaiah, J. J. Vijaya, L. J. Kennedy and M. Bououdina, Ceram. Int., 2016, 42, 2741–2749 CrossRef CAS
.
- K. Kombaiah, J. J. Vijaya, L. J. Kennedy and K. Kaviyarasu, Mater. Chem. Phys., 2019, 221, 11–28 CrossRef CAS
.
- E. Moradi, H. Farajnejad Ghadi, M. Rabbani and R. Rahimi, J. Mater. Sci.: Mater. Electron., 2021, 32, 8237–8248 CrossRef CAS
.
- C. T. Mathew, S. Solomon, J. Koshy and J. K. Thomas, Biomater. Sci., 2017, 40, 1171–1178 CAS
.
- A. Talati and M. Haghighi, Sol. Energy, 2022, 234, 275–293 CrossRef CAS
.
- K. Kombaiah, J. J. Vijaya, L. J. Kennedy and M. Bououdina, Optik, 2017, 129, 57–68 CrossRef CAS
.
- Q. Zhou, J. Zhou, W. Feng, L. Xue, H. Li, L. Guo and Y. Yan, Ceram. Int., 2021, 47, 22006–22015 CrossRef CAS
.
- M. Sundararajan, M. Sukumar, C. S. Dash, A. Sutha, S. Suresh, M. Ubaidullah, A. M. Al-Enizi, M. K. Raza and D. Kumar, Phys. Rev. B: Condens. Matter Mater. Phys., 2022, 644, 414232 CrossRef CAS
.
- M. H. Mahmoud, A. M. Elshahawy, S. A. Makhlouf and H. H. Hamdeh, J. Magn. Magn. Mater., 2013, 343, 21–26 CrossRef CAS
.
- C. Zhao, J. Yu, Y. Zhang, H. Gong, B. Xie, X. Lin, M. Sheng, J. Mao and J. Jing, Ceram. Int., 2020, 46, 17891–17895 CrossRef CAS
.
- R. A. KIMINAMI, M. R. Morelli, D. C. Folz and D. E. Clark, Am. Ceram. Soc. Bull., 2000, 79, 63–67 CAS
.
- W. W. Wang, Mater. Chem. Phys., 2008, 108, 227–231 CrossRef CAS
.
- Z. Chen, Y. Yan, J. Liu, Y. Yin, H. Wen, J. Zhao and S. Li, J. Alloys Compd., 2009, 473, 13–16 CrossRef
.
- R. L. B. A. Medeiros, G. P. Figueredo, H. P. Macedo, A. A. S. Oliveira, R. C. Rabelo-Neto, D. M. A. Melo, R. M. Braga and M. A. F. Melo, Fuel, 2021, 287, 119511 CrossRef CAS
.
- S. Baskar, S. Yuvaraj, P. S. Subudhi, M. Sundararajan and D. Chandra Sekhar, J. Chin. Chem. Soc., 2021, 68, 630–638 CrossRef CAS
.
- C. B. Palan, N. S. Bajaj, S. P. Bhagat and S. K. Omanwar, Luminescence, 2021, 36, 1491–1497 CrossRef CAS PubMed
.
- S. A. Ansari, C. Manjunatha, N. Parveen, B. W. Shivaraj and R. H. Krishna, Dalton Trans., 2021, 50, 14891–14907 RSC
.
- S. Kanithan, N. A. Vignesh, K. M. Katubi, P. S. Subudhi, E. Yanmaz, J. A. Dhanraj, N. S. Alsaiari, k. M. Abualnaja, M. Sukumar, M. Sundararajan, S. Baskar, S. Sahu and C. S. Dash, J. Mol. Struct., 2022, 1265, 133289 CrossRef CAS
.
- A. T. Dhiwahar, S. Maruthamuthu, K. V. Chandekar, M. S. Hamdy, M. Shkir, M. Sundararajan and P. Sakthivel, Adv. Powder Technol., 2021, 32, 4030–4041 CrossRef
.
- M. Sertkol, Y. Köseoğlu, A. Baykal, H. Kavas, A. Bozkurt and M. S. Toprak, J. Alloys Compd., 2009, 486, 325–329 CrossRef CAS
.
- M. R. D. Freitas, G. L. D. Gouveia, L. J. Dalla Costa, A. J. A. D. Oliveira and R. H. G. A. Kiminami, Mater. Res., 2016, 19, 27–32 CrossRef
.
- S. Chamani, M. Khatamian, N. S. Peighambardoust and U. Aydemir, ACS Omega, 2021, 6, 33024–33032 CrossRef CAS PubMed
.
- E. Carlos, R. Martins, E. Fortunato and R. Branquinho, Chem.–Eur. J., 2020, 26, 9099–9125 CrossRef CAS PubMed
.
- E. Mahu, P. Samoila, M. Ignat, C. Cojocaru and V. Harabagiu, Cron. Chim., 2022, 25, 189–202 Search PubMed
.
- S. Rasouli, A. M. Arabi, A. Naeimi and S. M. Hashemi, J. Fluoresc., 2018, 28, 167–172 CrossRef CAS PubMed
.
- A. Khort, K. Podbolotov, R. Serrano-García and Y. Gun’ko, Inorg. Chem., 2018, 57, 1464–1473 CrossRef CAS PubMed
.
- H. Nayebzadeh, N. Saghatoleslami and M. Tabasizadeh, J. Nanostruct. Chem., 2019, 9, 141–152 CrossRef CAS
.
- M. Derakhshani, E. Taheri-Nassaj, M. Jazirehpour and S. M. Masoudpanah, J. Alloys Compd., 2021, 886, 161195 CrossRef CAS
.
- K. Frikha, S. Bennici, J. Bouaziz, K. Chaari and L. Limousy, Energies, 2020, 13, 3126 CrossRef CAS
.
- M. Radpour, S. Alamolhoda and S. M. Masoudpanah, Ceram. Int., 2017, 43, 13729–13734 CrossRef CAS
.
- P. Araichimani, K. Prabu, G. S. Kumar, G. Karunakaran, S. Surendhiran, M. Shkir and H. E. Ali, Ceram. Int., 2022, 48, 10339–10345 CrossRef CAS
.
- R. O. Yathisha and Y. Arthoba Nayaka, Russ. J. Electrochem., 2021, 57, 784–794 CrossRef CAS
.
- M. Hashemzehi, V. Pirouzfar, H. Nayebzadeh and A. Alihosseini, Fuel, 2020, 263, 1–14 CrossRef
.
| This journal is © The Royal Society of Chemistry 2023 |