Open Access Article
Open Access ArticleA facile approach to obtain super-hydrophobicity for cotton fiber fabrics†
Zhengrong Lia,
Junxin Wua,
Yidi Wangb,
Yuxin Lia,
Gang Huang a,
Bin Fei*b,
Zhixiong Xuc,
Yong Zhangc and
Yangling Li
a,
Bin Fei*b,
Zhixiong Xuc,
Yong Zhangc and
Yangling Li *ac
*ac
aSchool of Textile Materials and Engineering, Wuyi University, Jiangmen, 529020, China
bNano Center, Institute of Textiles & Clothing, Hong Kong Polytechnic University, Hong Kong, China. E-mail: bin.fei@polyu.edu.hk
cCCOBATO (Dongguan) Technology, Ltd, Dongguan, 523000, China
First published on 21st March 2023
Abstract
It is a challenging task to directly apply emulsified silicone oil to the surface of cotton fabric to obtain superhydrophobic properties. In this work, a temperature-responsive microgel was first synthesized and the particle size and distribution of the microgel, thermo-responsiveness, and hydrophobicity of the microgel membrane were investigated. Then, through an emulsifying PMHS/water system with microgels as a Pickering emulsifier, a series of Pickering emulsions were obtained. The results showed that the emulsion had the best stability when the microgel content was 2.14 wt% and the mass ratio of PMHS/water was 3/7. The optical microscopy showed that the oil phase could be uniformly dispersed in aqueous solution, and the liquid phase particle size was about 10–22 μm. And stratification of the Pickering emulsion did not occur when placed at room temperature for over one month. Finally, when the addition of Pickering emulsion is 50 g L−1 and the rolling rate is 80%, through a simple two-dip-two-padding treatment, a cotton fabric can obtain the superhydrophobic effect with a static contact angle of 149.6° at 25 °C and 156.4° at 45 °C. The development of this work provides a simple method to make cotton fabric obtain superhydrophobic effects.
Introduction
Cotton fabrics are currently one of the most popular textiles because of their wide availability, low price, easy availability, and excellent properties such as biodegradability, breathability, softness, comfort, and warmth.1–5 However, the polyhydroxy structure at the molecular level makes it easy to absorb water or moisture, which easily causes bacteria and microorganisms to multiply on the surface and accumulate over time, resulting in poor durability and hindering use in certain areas. Therefore, it is important to offer hydrophobicity for cotton fabrics to improve their durability by means of physical or chemical modification.Currently, the hydrophobic finishing agents for cotton fabrics mainly include fluorine-containing perfluorooctane sulfonyl derivatives (PFOS),6,7 silicones,8 aluminum soap/paraffin/metal salt finishing agents,9,10 pyridine quaternary ammonium salts,11 long-chain fatty amides compounds,12 aliphatic hydrocarbon melamine derivatives13 etc. Kasra Khorsand Kazemi et al.14 used silica nanoparticles and low surface energy fluorosilanes to prepare superhydrophobic fabrics with static contact angles up to 164°. Ebenezer Kobina Sam et al.15 prepared a modified fabric by dip-coating polydimethylsiloxane (PDMS) and fluoroalkylsilane (FAS) on linen, and the static contact angle of the hydrophobically modified fabric could reach 151°. Esfandiar Pakdel et al.16 applied a fluorine-free coating formulation composed of TiO2 or nitrogen-doped TiO2 and PDMS polymer to cotton fabric by a simple dip coating method, and the static contact angle of the final prepared superhydrophobic fabric could reach 156.7°. However, the usage of fluorine-containing hydrophobic agents is restricted because of the PFOS, which are extremely harmful to humans and environment.17 Silicone-based poly(methylhydrosiloxane) (PMHS) is often used as a high-efficiency hydrophobic agent because of its low surface energy, good hydrophobicity and skin feeling.18–20 The current methods for achieving super-hydrophobicity with silicone on the surface of fabrics are mainly achieved in the following two ways: (1) fluorosilicon modification, that is, introducing fluorine on the molecular structural unit of silicone to further reduce the surface tension of the fluorosilicon coating and improve the water resistance.21,22 The contact angle after coating realizes the superhydrophobic property on the material surface. For example, the contact angle of the fluorosilicon coating is greater than 140°. (2) Designing on the assembly form of the surface structure of the lotus leaf, a structure similar to the surface morphology of the lotus leaf is assembled.23–25 Lin et al.26 prepared a variety of superhydrophobic surfaces by combining PMHS with SiO2 nanoparticles to build a rough layered structure on the substrate surface. These surfaces have been shown to exhibit excellent self-cleaning, oil/water separation properties. However, the introduction of fluorine element, which can bind to calcium ions in human bones and the complexity of surface structure of lotus – like leaves make those two processing methods mentioned above rarely applied in practice. Therefore, it is of great significance to find a simple, practical and effective way to improve the water repellency of cotton fabrics.
Pickering emulsion are emulsions that are stabilized by solid micro- and nanoparticles locating at oil–water interface, have been extensively used in scientific research and industrial production due to their edge in biocompatibility and stability compared with traditional emulsions.27–29 Substituting solid particles for traditional surfactants, Pickering emulsions are more stable against coalescence and can obtain many useful properties.30–32 Among the present methods to build controllable Pickering emulsions, tuning the amphiphilicity of particles is comparatively effective and has attracted enormous attention.33–35 Here, we synthesis a kind of thermosensitive microgel to be used as Pickering emulsifier, where the PNIPAm acts as thermosensitive and hydrophobic part and the PEI acts as a hydrophilic part. By changing the PMHS/water ratio, a series of Pickering emulsions was achieved. The cotton fabrics could obtain super-hydrophobicity property by simply dipping and padding method, showing a significant improvement in the field of cotton fabric. This simple and effective way can greatly improve the water repellency of cotton fabric and make it obtain superhydrophobic ability. And this work provides a new application approach for Pickering emulsion in textile finishing.
Results and discussion
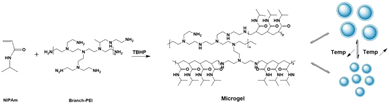
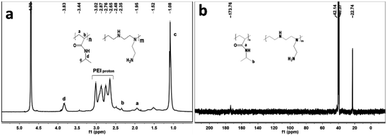
The microgel preparation process is shown in Fig. 1. A temperature-sensitive microgel was prepared by thermal initiation using N-isopropyl acrylamide as monomer, MBA as crosslinking agent, and polyethylene imine and TBHP as redox initiation system. Among them, N-isopropyl acrylamide gave microgel the feature of thermal-sensitivity response. Meanwhile, the active polyethylene imine forms a redox pair with TBHP, which is cracked at high temperature to generate free radicals on the primary amino group, and then the free radicals initiated the polymerization of N-isopropyl acrylamide in solution to form the grafted copolymer PEI-g-PNIPAm microgel, where the main chain is PEI and the side chain is PNIPAm.36 Furthermore, the reaction temperature is much higher than the lower critical solution temperature of N-isopropyl acrylamide (≈30 °C), so the N-isopropyl acrylamide exhibited phase separation phenomenon during the whole reaction process, resulting a milky-white microgel solution, as shown in Fig. S1.†In order to confirm the reaction between PEI and NIPAm monomer, after processed by dialyzed and lyophilized, the PEI-g-PNIPAm microgel's 1H-NMR and 13C-NMR spectra had been shown in Fig. 2. In Fig. 2a, the peaks at 1.08 ppm were attributed to the methyl hydrogen atoms at PNIPAm and the peaks at 3.83 ppm were attributed to the tertiary hydrogen atoms of the PNIPAm, while the peaks between 2.5 and 3.5 ppm were attributed to the PEI.37 In Fig. 2b, the peak at 173.76 ppm was attributed to the carbon atoms of carbonyl group of the PNIPAm parts and the peaks at 22.72 ppm was attributed to the methyl carbon atoms of the PNIPAm parts. The PEI's signal did not appear due to the unique structure of the PEI-g-PNIPAm microgel.36
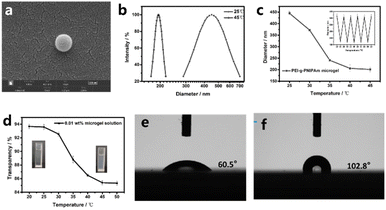
The SEM showed that the microgel had a regular spherical morphology, with a particle size of about 200 nm, and a slightly rough as shown in Fig. 3a. The microgel's roughness may be directly related to the PNIPAm's hydrophobic chain.38 The temperature-sensitive PNIPAm segments in PEI-g-PNIPAm microgels shrink irregularly, and the internal stress generated during this process caused irregular folding on the surface of the microgel, resulting in a rough morphology on the surface of the microgel. Next, the microgel's temperature sensitivity was investigated. The result from the dynamic light scattering showed that the average particle size of PEI-g-PNIPAm microgels at 25 °C was about 469 nm with polymer dispersity index (PDI) as 0.01 (Fig. 2b and S2†), while when the temperature heated to 45 °C, the PEI-g-PNIPAm's average particle size shrank to 193 nm with PDI still as 0.01 (Fig. 2b and S3†). That demonstrated the PEI-g-PNIPAm microgel owned homogeneous particle size distribution and good temperature sensitivity. It should be noted that the hydraulic diameter of the microgel obtained by the dynamic light scattering is about 2.5 times higher than the actual microgel's diameter due to the strong hydrophilic ability of PEI part in PEI-g-PNIPAm microgel, showing that the microgel has better ampholytic characteristics. In order to describe in details the PEI-g-PNIPAm microgel size's kinetic changing in the actual heating process, the relationship between microgel size and temperature was investigated. The results (Fig. 3c) showed the PEI-g-PNIPAm microgel size gradually decreased from 446 nm at 25 °C to 190 nm at 40 °C with the gradual increase of temperature. When the temperature over 40 °C, there was no significant change in particle size. This process is similar to the temperature change behaviors of pure poly N-isopropyl acrylamide microgel,39 where the hydrophobic ability of PNIPAm fragments in PEI-g-PNIPAm microgel is gradually enhanced during the gradual heating process, which promotes the discharge of water molecules from the inside of the PEI-g-PNIPAm microgel, resulting in microgel's size gradual reduction. When the temperature rises to about 45 °C, the water molecules inside the microgel have basically been completely discharged, so the particle size no longer changes significantly. Furthermore, the PEI-g-PNIPAm microgel's heat reversibility was investigated, as shown in Fig. 3c. PEI-g-PNIPAm microgel's size could change easily accompany with temperature changing between 25 °C and 45 °C. During all the five cycle process, the PEI-g-PNIPAm microgels' size, either at 25 °C or at 45 °C, still keep almost same value. It demonstrated that this PEI-g-PNIPAm microgel possessed better heat reversibility. Next, the light transmittance of PEI-g-PNIPAm microgel solution with a mass fraction of 0.1 wt% was investigated, as shown in Fig. 3d, as the solution temperature gradually increased, the light transmittance of PEI-g-PNIPAm microgel solution gradually decreased from 93.7% at 25 °C to 85.6% at 45 °C, after which this transparent state was maintained. Finally, the membrane properties of PEI-g-PNIPAm microgel after drying was investigated. After uniformly coated the PEI-g-PNIPAm microgel solution on the glass sheet by spin coating, a PEI-g-PNIPAm microgel film with a uniform and flat surface was obtained by carefully controlling the volatilization rate of water. As shown in Fig. 3e and f, the contact angle of the PEI-g-PNIPAm microgel membrane is about 62.5° at low temperature conditions (25 °C), showing a certain hydrophilic capacity. Correspondingly, at high temperature conditions (45 °C), the contact angle of PEI-g-PNIPAm membrane is about 102.8°, showing a certain hydrophobic capacity.
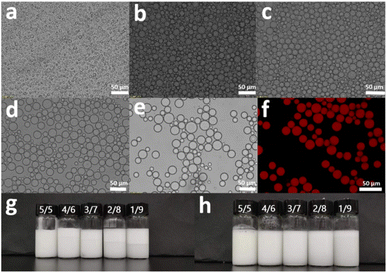
We applied PEI-g-PNIPAm microgel as Pickering emulsifier to prepare an oil/water Pickering emulsion by emulsified PMHS. A series of Pickering emulsions were prepared by adjusting the mass ratio between PMHS and water by fixing the PEI-g-PNIPAm microgel addition amount of 2.14 wt%, without the addition of any other emulsifiers or dispersant. The results are shown in Fig. 3, when the PMHS/water mass ratio is 5/5, the obtained emulsion shows an irregular shape under the optical microscope, and the size of the oil droplets is about 3–6 μm. When the PMHS/water mass ratio is adjusted to 4/6, the appearance of the emulsion droplets begins to gradually transition from irregular shape to spherical regular shape, and during the whole changing process, the size of the emulsion droplets gradually becomes larger, and the particle size range is about 8–20 μm. When the PMHS/water mass ratio is further adjusted to 3/7, the emulsion droplets show a regular spherical shape, the size becomes more uniform, and the droplet size range is about 10–22 μm. By further increasing the PMHS/water mass ratio to 2/8, the emulsion droplets become uniform and complete, and the emulsion droplet size becomes further larger, and the droplet size range is approximately 12–25 μm. When the oil/water mass ratio is 1/9, the morphology and size of the emulsion droplets basically do not change, showing a regular morphology. The stability of the emulsion was further investigated. After the emulsification of the emulsion with different PMHS/water mass ratios, it showed that almost no foam was generated on the surface of the sample with an oil/water mass ratio of 5/5. As the water content in the system gradually increases, the foam layer on the surface of the emulsion gradually get thicker. Especially when the oil/water mass ratio is 1/9, the foam volume in the emulsion occupies almost half of it (Fig. 3g). Nevertheless, the foam disappeared after those series of lotions is left overnight. To study the stability of the emulsions, the emulsions were kept at room temperature for one month. The results showed no significant stratification occurred in all emulsion samples after one month (Fig. 3h). However, there are a small amount of floating PMHS oil on the surface of emulsion with oil/water mass ratio as 2/8 and 1/9, showing the emulsion had good storage stability. Next, we take the sample with an oil/water mass ratio of 3/7 as the study object and labeled as Emulsion-37. The fluorescent dye Nile red was used to stain the PMHS, and the laser confocal microscope observation showed that the hydrophobic PMHS in the Pickering emulsion system was uniformly dispersed in the aqueous phase system to form an oil/water stable system (Fig. 3f).
In addition, the stability of the Pickering emulsion was investigated by adjusting the additive amount of the microgel where the PMHS/water mass ratio kept constant as 3/7. By changing the microgels' addition amount as 1.28 wt%, 1.71 wt%, 2.14 wt% and 2.57 wt%, a series Pickering emulsion had obtained. Those emulsion were treated via a centrifugal manner. After 15 minutes of centrifugation at the speed of 3000 rpm, the emulsions were still not stratified, as shown in Fig. S4.† This shows that when the PMHS/water mass ratio was controlled at 3/7, the picking emulsion with better stability can be obtained by changing the amount of microgel (Fig. 4).
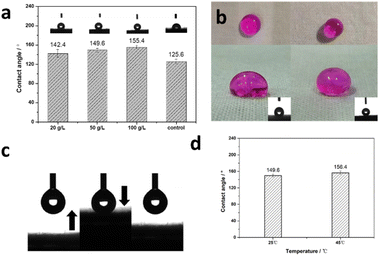
Emulsion-37 emulsion was applied to the post-treatment of cotton fabrics as shown in Fig. 5a. As the amount of Emulsion-37 emulsion is gradually increased, the static contact angle of water droplets on the cotton fabric gradually increase. The static contact angle is 142.4° when the addition is 20 g L−1, and the static contact angle is 149.6° when the addition of Emulsion-37 emulsion is increased to 50 g L−1. Furthermore, when the amount of Emulsion-37 emulsion is increased to 100 g L−1, the static contact angle changed to 155.4°, showing super-hydrophobic ability. Therefore, PEI-g-PNIPAm microgel, as a Pickering emulsifier, has excellence emulsifying ability, and can easily achieve the effect of uniform emulsification of PMHS with strong hydrophobicity. For comparison, the commercial aliphatic hydrocarbon emulsifier 1306, had been investigated its effect on cotton fiber fabrics under the same conditions. As shown in Fig. 5a, the PMHS emulsion emulsified by emulsifier 1306 was added to the working solution with the amount of 50 g L−1 to treated the cotton fiber fabric, and the static water contact angle of the cotton fiber fabric is about 125.6°. In order to illustrate the hydrophobicity vividly, the photography of active-red stained water droplets on emulsifier 1306 and Emulsion-37 coated cotton fiber fabrics was shown in Fig. 5b. So, it proved that PEI-g-PNIPAm microgel had a better emulsification effect than the commercial emulsifier. This excellent hydrophobic property is mainly because during the dipping process, the amino groups (including primary amines and secondary amines) in the PEI components of the PEI-g-PNIPAm microgel can be bonded together with the hydroxyl groups in the fiber through hydrogen bonding, and uniformly attached to the inside or surface of the fiber, which improves the penetration ability of the microgel on the fiber surface. At the same time, during the process of combining the microgel with cellulose, PMHS penetrates into the inside of the cotton fiber fabric, while the PEI-g-PNIPAm microgel, fixed on the surface of the cotton fiber fabric, dried and formed a protective film to prevent the hydrophobic PMHS components from migrating out of the fiber to reduce the water resistance of the cotton fiber fabric in the finishing process.
Further, in order to more intuitively observe the good water-repellent ability brought by Pickering emulsion, water droplets dyed with active red was gradually added to the cotton fiber fabric treated with Pickering emulsion. The lifting process of water droplets on the fabric was shown in Fig. 5c. The water droplets could be easily pulled up from the surface of the treated fabric due to its super-hydrophobic ability. At last the PEI-g-PNIPAm microgel's thermo-responsive ability was investigated. As shown in Fig. 5d, the static water contact angle for Emulsion-37 coated cotton is 149.6° at 25 °C, while it is 156.4° at 45 °C. During those heating increase process, the hydrophobic isopropyl group and PMHS work together to make cotton fabrics exhibit superhydrophobic properties. This result showed that the Emulsion-37 coated cotton could achieve thermo-responsive ability and get more hydrophobic at high temperature. However, it was worth noting that the interaction between the PMHS and the cotton was so weak that the super-hydrophobicity of the cotton surface was unstable after the cotton experienced washing process. The actual static contact angle after one times washing was about 124°, similar to the value of the commercial emulsifier 1306. And the contact angle would decrease quickly accompany with the washing of the PMHS inside of the cotton fiber. Nevertheless, when this cotton fiber is immersed in a non-aqueous solvent containing a Karstedt catalyst, this superhydrophobic property can be preserved for a long time.40 As shown in Fig. S5,† the cotton fibers treated by this method can still maintain super hydrophobic ability after 4 consecutive soaping treatments.
Conclusions
A microgel with temperature sensitivity was prepared with polyethyleneimine as the main chain and poly(N-isopropyl acrylamide) as the side chain. Polyethyleneimine, main chain, gives the microgel hydrophilic properties and the property of improving the adhesion to cellulose. Poly(N-isopropyl acrylamide) is used as the side chain to impart temperature sensitivity to the microgel. Compared with the commercial amphoteric aliphatic hydrocarbon emulsion, the Pickering emulsion prepared by microgel as emulsifier has the following advantages: (1) the water-repellent ability is significantly enhanced at room temperature; (2) the water-repellent ability at high temperature is further improved. The development of this work can give the cotton fiber fabric excellent water-repellent ability by simple dipping and rolling method, which opens up a new direction for the application of Pickering emulsion in the direction of textile finishing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the Guangdong/Hong Kong Joint Foundation of Wuyi University (No. 2019WGALH07) and Wuyi University's special fund (Grant No. 5041700128).Notes and references
- S. Smith, M. Ozturk and M. Frey, Cellulose, 2021, 28, 4485–4494 CrossRef CAS.
- B. Tomšič, D. Marković, V. Janković, B. Simončič, J. Nikodinovic-Runic, T. Ilic-Tomic and M. Radetić, Cellulose, 2022, 29, 287–302 CrossRef.
- M. Xu, H. Cai, Z. Liu, F. Chen, L. Chen, X. Chen, X. Cheng, F. Dai and Z. Li, Adv. Electron. Mater., 2021, 7, 2100368 CrossRef CAS.
- H. Liu, L. Guo, S. Hu, F. Peng, X. Zhang, H. Yang, X. Sui, Y. Dai, P. Zhou and H. Qi, ACS Appl. Mater. Interfaces, 2022, 14, 34049–34058 CrossRef CAS PubMed.
- K. Sarkar, D. Das, T. K. Chaki and S. Chattopadhyay, Carbon, 2017, 116, 1–14 CrossRef CAS.
- H. Jung, J. Kwon, H. Jung, K. M. Cho, S. J. Yu, S. M. Lee, M. Jeon and S. G. Im, RSC Adv., 2022, 12, 7773–7779 RSC.
- T. Chen, J. Hong, C. Peng, G. Chen, C. Yuan, Y. Xu, B. Zeng and L. Dai, Carbohydr. Polym., 2019, 208, 14–21 CrossRef CAS PubMed.
- M. Przybylak, H. Maciejewski, A. Dutkiewicz, D. Wesołek and M. Władyka-Przybylak, Polym. Degrad. Stab., 2016, 128, 55–64 CrossRef CAS.
- W. Zhang, J. Wu, L. Yu, H. Chen, D. Li, C. Shi, L. Xiao and J. Fan, ACS Appl. Mater. Interfaces, 2021, 13, 52174–52180 CrossRef CAS PubMed.
- M. Wang, M. Peng, Y.-X. Weng, Y.-D. Li and J.-B. Zeng, Cellulose, 2019, 26, 8121–8133 CrossRef CAS.
- Z. Lu, J. Liu, C. Dong, Z. Zhang and D. Wei, Cellulose, 2019, 26, 7483–7494 CrossRef CAS.
- R. Sharif, M. Mohsin, S. Sardar, N. Ramzan and Z. A. Raza, J. Nat. Fibers, 2022, 19, 12473–12485 CrossRef CAS.
- L. Kang, L. Shi, Q. Zeng, B. Liao, B. Wang and X. Guo, Sep. Purif. Technol., 2021, 279, 119737 CrossRef CAS.
- K. K. Kazemi, T. Zarifi, M. Mohseni, R. Narang, K. Golovin and M. H. Zarifi, ACS Appl. Mater. Interfaces, 2021, 13, 34877–34888 CrossRef CAS PubMed.
- E. K. Sam, Y. Ge, J. Liu and X. Lv, Colloids Surf., A, 2021, 625, 126860 CrossRef CAS.
- E. Pakdel, H. Zhao, J. Wang, B. Tang, R. J. Varley and X. Wang, Cellulose, 2021, 28, 8807–8820 CrossRef CAS.
- A. Ali, K. Shaker, Y. Nawab, M. Jabbar, T. Hussain, J. Militky and V. Baheti, J. Ind. Text., 2018, 47, 2153–2183 CrossRef CAS.
- W. Zhu, J. Zhao, X. Wang, X. Liu, J. Yu and B. Ding, J. Colloid Interface Sci., 2019, 556, 541–548 CrossRef CAS PubMed.
- X. Li, M. Cao, H. Shan, F. Handan Tezel and B. Li, Chem. Eng. J., 2019, 358, 1101–1113 CrossRef CAS.
- G. Huang, L. Huo, Y. Jin, S. Yuan, R. Zhao, J. Zhao, Z. Li and Y. Li, Prog. Org. Coat., 2022, 163, 106671 CrossRef CAS.
- Q. Li, Z. Fan, C. Chen and Z. Li, J. Text. Inst., 2020, 111, 360–369 CrossRef CAS.
- Y. Zhou, C. Liu, J. Gao, Y. Chen, F. Yu, M. Chen and H. Zhang, Prog. Org. Coat., 2019, 134, 134–144 CrossRef CAS.
- L. Feng, S. Li, Y. Li, H. Li, L. Zhang, J. Zhai, Y. Song, B. Liu, L. Jiang and D. Zhu, Adv. Mater., 2002, 14, 1857–1860 CrossRef CAS.
- B. Liu, Y. He, Y. Fan and X. Wang, Macromol. Rapid Commun., 2006, 27, 1859–1864 CrossRef CAS.
- M. K. Mehrizi and Z. Shahi, in Sustainable Practices in the Textile Industry, 2021, pp. 149–165, DOI:10.1002/9781119818915.ch6.
- W. Lin, M. Cao, K. Olonisakin, R. Li, X. Zhang and W. Yang, Cellulose, 2021, 28, 10425–10439 CrossRef CAS.
- S. Sacanna, W. K. Kegel and A. P. Philipse, Phys. Rev. Lett., 2007, 98, 158301 CrossRef CAS PubMed.
- J. Wu and G.-H. Ma, Small, 2016, 12, 4633–4648 CrossRef CAS PubMed.
- F. Cui, S. Zhao, X. Guan, D. J. McClements, X. Liu, F. Liu and T. Ngai, Food Hydrocolloids, 2021, 119, 106812 CrossRef CAS.
- T. Zhang, H. Jiang, L. Hong and T. Ngai, Chem. Sci., 2022, 13, 10752–10758 RSC.
- X. Zhou, C. Chen, C. Cao, T. Song, H. Yang and W. Song, Chem. Sci., 2018, 9, 2575–2580 RSC.
- Y. Xi, B. Liu, S. Wang, S. Wei, S. Yin, T. Ngai and X. Yang, Chem. Sci., 2022, 13, 2884–2890 RSC.
- B. Wu, C. Yang, Q. Xin, L. Kong, M. Eggersdorfer, J. Ruan, P. Zhao, J. Shan, K. Liu, D. Chen, D. A. Weitz and X. Gao, Adv. Mater., 2021, 33, 2102362 CrossRef CAS PubMed.
- C. Wang, J. Wu, C. Wang, C. Mu, T. Ngai and W. Lin, Food Res. Int., 2022, 157, 111380 CrossRef CAS PubMed.
- T. Zhang, F. Liu, J. Wu and T. Ngai, Particuology, 2022, 64, 110–120 CrossRef CAS.
- P. Li, K. Xu, Y. Tan, C. Lu, Y. Li and P. Wang, Polymer, 2013, 54, 5830–5838 CrossRef CAS.
- M. F. Leung, J. Zhu, F. W. Harris and P. Li, Macromol. Rapid Commun., 2004, 25, 1819–1823 CrossRef CAS.
- M. Destribats, M. Eyharts, V. Lapeyre, E. Sellier, I. Varga, V. Ravaine and V. Schmitt, Langmuir, 2014, 30, 1768–1777 CrossRef CAS PubMed.
- W. Cao, Q. Ding, P. Ding, L. Hu, C. Liu and W. Yu, ACS Appl. Polym. Mater., 2022, 4, 8623–8632 CrossRef CAS.
- H. Lin, Q. Hu, T. Liao, X. Zhang, W. Yang and S. Cai, Polymers, 2020, 12, 833 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ra08189j |
| This journal is © The Royal Society of Chemistry 2023 |