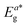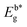DOI:
10.1039/D3RA00214D
(Paper)
RSC Adv., 2023,
13, 9033-9045
Optical, electrochemical and photophysical analyses of heteroleptic luminescent Ln(III) complexes for lighting applications†
Received
11th January 2023
, Accepted 10th March 2023
First published on 20th March 2023
Abstract
A series of lanthanide complexes have been synthesized with fluorinated 1,3-diketones and heteroaromatic ancillary moieties. Spectroscopic studies reveal the attachment of the respective lanthanide ion to the oxygen site of β-diketone and nitrogen site of auxiliary moieties. The conducting behavior of the complexes is proposed by their optical energy gaps which lie in the range of semiconductors. The emission profiles of the lanthanide complexes demonstrate red and green luminescence owing to the distinctive transitions of Sm3+ and Tb3+ ions, respectively. Energy transfer via antenna effect clearly reveals the effective transfer of energy from the chromophoric moiety to the Ln3+ ion. The prepared conducting and luminescent Ln(III) complexes might be employed as the emitting component in designing OLEDs.
1 Introduction
The coordination compounds of lanthanide (Ln) ions having a +3 oxidation state exhibit exciting photo-luminescent features which are promising for their vast range of applicability in diverse fields comprising optical amplifiers,1 sensors,2 lasers,3 fluorescent probes,4,5 single molecule magnets (SMM)6,7 and OLEDs.8,9 These Ln complexes have narrow monochromatic emission peaks due to intraconfigurational transitions which belong to the 4f subshell, large Stokes displacement, long emissive state life-time, and good quantum yield.10,11 However, the solitary metal ions cannot be utilized as luminescent materials due to parity prohibited transition and low molar absorptivity in the ultraviolet-visible (UV-vis) region. The above outcomes demonstrate the weak and low luminescent efficiency of lanthanides.12,13 Thus, trivalent ions typically form coordinated complexes with organic ligands. These organic moieties possess strong absorption in the UV-vis region and upon coordination with the metal ion transfer the absorbed energy to it. Therefore the organic moiety proficiently sensitizes and improves the photoluminescence efficiency of 4f ions. This mechanism is titled as “antenna effect”.14–16 Existence of solvent units in lanthanide complexes are greatly unsuitable on account of highly energized stretching vibrators of hydroxyl and amino modes which leads to luminescence quenching.17,18 It can prominently restrict the practicability of ternary complexes as luminescent material. The high energetic oscillators in chromophoric moieties are also reasonable to quench the luminescence phenomenon in lanthanide ions.19,20 Thus, the substitution of C–H by less energetic C–F bond minimizes the luminescence quenching. The additional assistance of fluorination is the heavy atom effect, which can increase the efficiency of intersystem crossing (ISC) from singlet to triplet level of ligand.21–23 Neutral ligands can substitute the solvent molecules and form coordinatively saturated lanthanide complexes via hard donor sites i.e. nitrogen and oxygen. These ligands have provided rigidity and thermal stability to the complexes. Asymmetric coordinating environment around central ion has also enhanced the luminescence characteristics.24
Lanthanide ions such as europium, samarium and terbium have exhibit bright red, orange and green emission, respectively in visible-range of the electro-magnetic spectrum (EMS). Now we have taken Tb3+ and Sm3+ ions for synthesis of ternary complexes. Tb3+ ion exhibits green emission which is the component of red-green-blue system accredited to transition of 5D4 → 7F5 and situated about emissive wavelength of 545 nm.25,26 The ternary samarium complexes show red luminescence due to 4G5/2 → 6H9/2 (648 nm) transition.27,28
Here, we have reported eight Ln(III) complexes based on fluorinated di-ketone 2,2-dimethyl-6,6,7,7,8,8,8-heptafluoro-3,5-octanedione (Hfodo) and N-donor ancillary units which are 2,2′-bipyridine [Bpy], 5,5′-dibromo-2,2′-bipyridine [DBr], 5-bromo-5′-(3,4-(ethylenedioxy)thien-2-yl)-2,2′-bipyridine [MD] and 5,5′-bis(3,4-(ethylenedioxy)thien-2-yl)-2,2′-bipyridine [DD]. The main aim of our work is to assess the electronic impact of substituents on the optoelectronic and photophysical characteristics of synthesized complexes. The complexes Tb(Hfodo)3Bpy (T1), Tb(Hfodo)3DBr (T2), Tb(Hfodo)3MD (T3), Tb(Hfodo)3DD (T4), Sm(Hfodo)3Bpy (S1), Sm(Hfodo)3DBr (S2), Sm(Hfodo)3MD (S3) and Sm(Hfodo)3DD (S4) have been reported. These lanthanide complexes were examined by elemental investigation, spectroscopically, thermal gravimetric and electro-chemical technique. Complexes were colorimetrically analyzed by using the results of photoluminescence emission.
2 Experimental
2.1 Chemicals and instrumentation
The chemicals comprising Bpy, Hfodo, TbCl3·6H2O and SmCl3·6H2O were bought from Sigma Aldrich (SA). The reagent such as 25% NH4OH solution and solvents viz., methyl carbinol and hexyl hydride has been utilized directly. The ligands namely DBr, MD and DD have been synthesized in lab.29 The composition of CHN in lanthanide complexes was inspected via 2400-CHN Analyzer. The FTIR and proton NMR spectral data was measured on a PerkinElmer 400 FTIR spectrophotometer and FT NMR spectrometer respectively. The proton NMR signals were obtained in CDCl3 and [(CH3)4Si] as reference. Absorption and electrochemical analyses were performed on as respective instrument such as Shimadzu UV VIS 2450 and Potentiostat-4000. PL spectral information was measured on a Horiba Fluorolog 3. Thermal-gravimetric [TG] and differential-thermal-gravimetric [DTG] patterns were obtained under nitrogen atmosphere on a Hitachi Simultaneous Thermogravimetric Analyzer-7300.
2.2 Synthesis
Fig. 1 represents the schematic way for synthesizing lanthanide complexes.30–33 Ln(III) complexes were made by adding 6.48 mmol of 25% NH4OH to 6.48 mmol of Hfodo in 5 mL of methyl carbinol. Beaker was retained undisturbed and closed till the smell of ammonia evaporates. Then, 2.16 mmol of alcoholic solution of lanthanide (respective samarium and terbium) chloride hexahydrate and substituted 2,2′-bipyridine ligands were dispensed to ammoniated solution of Hfodo. The resulting mixture (pH = 6–7) was agitated for 12 hours. After that, leaves the beaker for slow disappearance of solvent. Methyl carbinol and hexyl hydride (2–3 times) were employed to wash the solid residue.
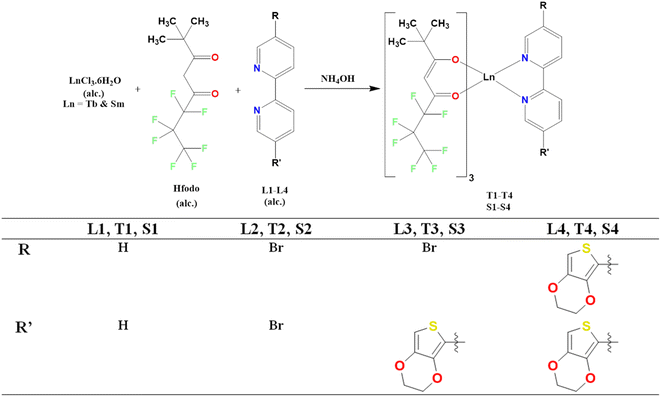 |
| | Fig. 1 Schematic representation of ternary lanthanide complexes. | |
3 Results and discussion
3.1 Primary interpretation
The basic (CHN) compositional data as well as preliminary findings such as appearance and quantity of the complexes is listed in Table 1. The preliminary findings confirm that the estimated outcomes are accordant with the expected data. Furthermore, the findings illustrate that lanthanide compounds are formed in precise stoichiometric proportions. The synthesized lanthanide complexes are solvable in dichloromethane and dimethylsulfoxide.
Table 1 CHN data of lanthanide complexes
| Complex |
Color |
Yield (%) |
C% obs.(calcd.) |
H% obs.(calcd.) |
N% obs.(calcd.) |
| T1 |
White |
40 |
39.85 (39.91) |
3.41 (3.43) |
2.40 (2.33) |
| T2 |
White |
33 |
35.37 (35.29) |
2.91 (2.89) |
1.99 (2.06) |
| T3 |
Yellow |
48 |
38.76 (38.83) |
3.17 (3.12) |
1.95 (1.97) |
| T4 |
Yellow |
37 |
42.05 (42.09) |
3.39 (3.33) |
1.92 (1.89) |
| S1 |
White |
35 |
40.38 (40.30) |
3.17 (3.21) |
2.29 (2.35) |
| S2 |
White |
45 |
35.63 (35.59) |
2.71 (2.69) |
2.01 (2.08) |
| S3 |
Yellow |
50 |
39.05 (39.15) |
2.99 (2.93) |
2.03 (1.99) |
| S4 |
Yellow |
39 |
42.39 (42.42) |
3.19 (3.15) |
1.89 (1.90) |
3.2 IR and 1H-NMR spectra evaluation
FTIR spectral information of Ln(III) complexes are mentioned in Table 2. No band at 3300 cm−1 indicates that water molecules do not exist in the complexes. Stretching vibrations of ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H unit are liable for the peak at 3060 cm−1. The FTIR spectrum of diketone ligand reveal a prominent peak due to C
C–H unit are liable for the peak at 3060 cm−1. The FTIR spectrum of diketone ligand reveal a prominent peak due to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group which is moved to the lesser frequency after complexation. This suggests the attachment of the diketone to metal ion via oxygen.34,35 The finding of peaks at about 1070–1100 cm−1 correspondent to C–Br unit existed in T2, T3, S2 and S3. In FTIR profiles of some complexes (T3, T4, S3 and S4) two peaks in scope of 688–722 cm−1 are present due to C–S–C bond.29 The peaks around 455–475 cm−1 (Ln–O) and 531–538 cm−1 (Ln–N), confirms the involvement of Hfodo and ancillary moieties with the Ln3+ ion.36–38
O group which is moved to the lesser frequency after complexation. This suggests the attachment of the diketone to metal ion via oxygen.34,35 The finding of peaks at about 1070–1100 cm−1 correspondent to C–Br unit existed in T2, T3, S2 and S3. In FTIR profiles of some complexes (T3, T4, S3 and S4) two peaks in scope of 688–722 cm−1 are present due to C–S–C bond.29 The peaks around 455–475 cm−1 (Ln–O) and 531–538 cm−1 (Ln–N), confirms the involvement of Hfodo and ancillary moieties with the Ln3+ ion.36–38
Table 2 FTIR spectral information (in cm −1) of lanthanide complexes
| Complex |
ν(Ln–O) |
ν(Ln–N) |
ν(C–S–C) |
ν(C–Br) |
ν(C–N) |
ν(C–F) |
ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) C) |
ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) N) |
ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) O) |
ν(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H, –C–H) C–H, –C–H) |
| T1 |
466 |
530 |
— |
— |
1125 |
1347 |
1464 |
1542 |
1605 |
3072, 2970 |
| T2 |
458 |
535 |
— |
1086 |
1123 |
1356 |
1456 |
1539 |
1611 |
3077, 2967 |
| T3 |
478 |
538 |
722, 690 |
1073 |
1122 |
1346 |
1474 |
1538 |
1620 |
3075, 2970 |
| T4 |
472 |
534 |
718, 688 |
— |
1120 |
1350 |
1468 |
1547 |
1616 |
3075, 2972 |
| S1 |
470 |
532 |
— |
— |
1122 |
1349 |
1460 |
1540 |
1615 |
3051, 2977 |
| S2 |
457 |
538 |
— |
1100 |
1123 |
1356 |
1456 |
1543 |
1620 |
3049, 2974 |
| S3 |
477 |
537 |
722, 689 |
1102 |
1121 |
1346 |
1473 |
1539 |
1619 |
3055, 2972 |
| S4 |
472 |
535 |
719, 692 |
— |
1124 |
1354 |
1466 |
1542 |
1618 |
3052, 2975 |
Table 3 lists1H-NMR data of prepared complexes and 1,3-diketone. In their lanthanide complexes, the methine proton (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H) signal of Hfodo is displaced towards the lower chemical shift. The paramagnetic property of terbium and samarium ions causes noticeable alterations in the 1H-NMR spectrum data of ligands.39–41 The paramagnetic nature of Tb3+ greatly affects the position of signals of auxiliary units. Fig. S1–S16† represents IR and NMR spectral profiles of ternary terbium and samarium complexes respectively.
C–H) signal of Hfodo is displaced towards the lower chemical shift. The paramagnetic property of terbium and samarium ions causes noticeable alterations in the 1H-NMR spectrum data of ligands.39–41 The paramagnetic nature of Tb3+ greatly affects the position of signals of auxiliary units. Fig. S1–S16† represents IR and NMR spectral profiles of ternary terbium and samarium complexes respectively.
Table 3 1H-NMR data (ppm) of prepared complexes
| Complex |
Peaks due to Hfodo |
Peaks due to neutral ligands |
| Hfodo |
14.82 (–OH), 5.62 (methine), 3.56 (–CH2), 1.29 (–C(CH3)3) |
— |
| T1 |
116.08 (3H, methine –CH), 1.24–0.93 (27H, –CH3) |
−9.45 (2H), −21.11 (2H), −30.05 (2H), −45.60 (2H) |
| T2 |
121.19 (3H, methine –CH), 1.40–0.69 (27H, –CH3) |
−2.07 (2H), −15.85 (2H), −25.01 (2H) |
| T3 |
118.48 (3H, methine –CH), 1.28–0.84 (27H, –CH3) |
−3.40 (2H), −4.72 (2H), −9.58 (2H), −12.16 (2H), −13.08 (1H), −16.83 (1H), −26.15 (1H) |
| T4 |
118.39 (3H, methine –CH), 1.33–0.84 (27H, –CH3) |
−3.38 (2H), −4.74 (4H), −5.68 (2H), −6.67 (2H), −9.59 (1H), −117.1–13.19 (2H), −17.01 (1H), −18.70 (1H), −26.24 (1H) |
| S1 |
6.89 (3H, methine –CH), 1.06 (27H, –CH3) |
7.66 (2H), 7.59 (2H), 7.46 (2H), 7.05 (2H) |
| S2 |
6.86 (3H, methine –CH), 1.08 (27H, –CH3) |
8.71 (2H), 8.29 (2H), 7.93 (2H) |
| S3 |
6.94 (3H, methine –CH), 1.04 (27H, –CH3) |
8.81 (1H), 8.37 (1H), 8.08 (2H), 7.55 (1H), 7.39 (1H), 6.50 (1H), 4.96 (4H) |
| S4 |
6.96 (3H, methine –CH), 1.06 (27H, –CH3) |
7.95 (2H), 7.59 (2H), 6.75 (2H), 6.32 (2H), 4.32 (4H), 4.13 (8H) |
3.3 Absorption spectral study
At room temperature, UV–visible absorption spectra of Ln(III) complexes were recorded in dichloromethane (DCM) solvent (10−5 M). Fig. 2 exhibit the absorption patterns of Hfodo and Ln(III) complexes. The spectral profiles of synthesized complexes specify a band in 280–380 nm associated with the π–π* transitions of chelated moieties. Bathochromic shift is observed with change in ancillary moieties from Bpy to DD. Bands due to free units get displaced after complexation with metal ion.42,43 With the assistance of spectral profiles, optical band-gap (Eg) was designed (eqn (1)).
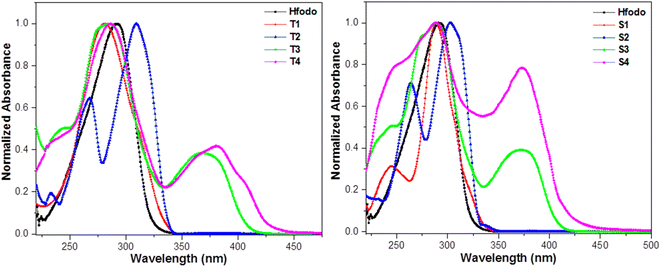 |
| | Fig. 2 Absorption plots of synthesized Hfodo and Ln(III) complexes. | |
The above relation includes absorption co-efficient (α); energy of photon (ℏν) and optical parameter (n).44,45 Eg was determined by extrapolating a line to (αhυ)2 = 0 as given in Fig. 3. Eg declines in a particular series i.e. from T1 to T4 and S1–S4 suggesting the increase in conjugation. The band gap value lies in the range of semiconducting materials.
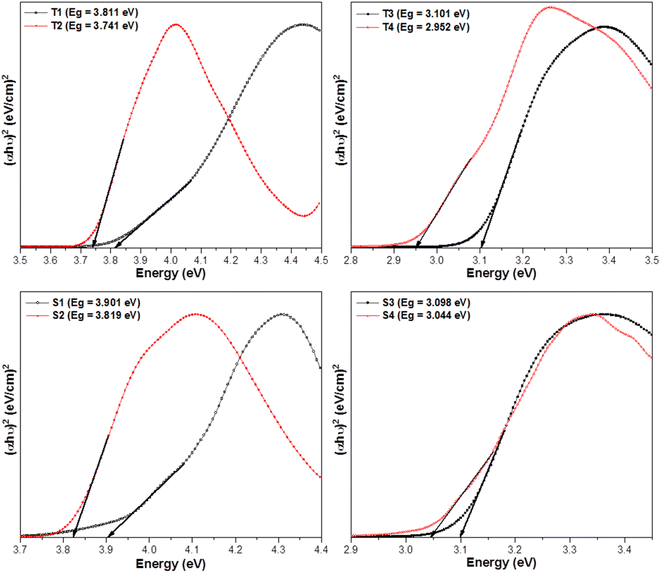 |
| | Fig. 3 Tauc's profiles of prepared complexes. | |
3.4 Thermal gravimetric study
The thermograms of Ln(III) complexes were collected in order to measure their thermal strength. The thermograms of T1, T4, S1 and S4 are thoroughly examined here because the disintegration tendencies of all the prepared complexes are quite comparable. Fig. 4 displays the thermogram of above mentioned complexes which demonstrates the single step disintegration. The unavailability of spectral variation up to 120 °C suggests the anhydrous nature of prepared compounds. T1 shows 85.23% (calcd: 86.79%) mass loss in temperature range of 213–330 °C while T4 exhibit 85.74% (calcd: 89.30%) mass loss in 127–368 °C due to the removal of ligands attached to Tb(III) ion. In case of S1 and S4, huge mass loss of 89.67% (calcd: 87.42%) and 90.15% (calcd: 89.82%) in 225–343 °C and 246–366 °C is attributed to removal of three Hfodo and single neutral unit respectively. The residual product formed after decomposition was due to oxides of terbium and samarium.46–48 The peaks in DTG curves at 267 °C (T1), 275 °C (T4), 308 °C (S1) and 358 °C (S4) support their decomposition pattern.
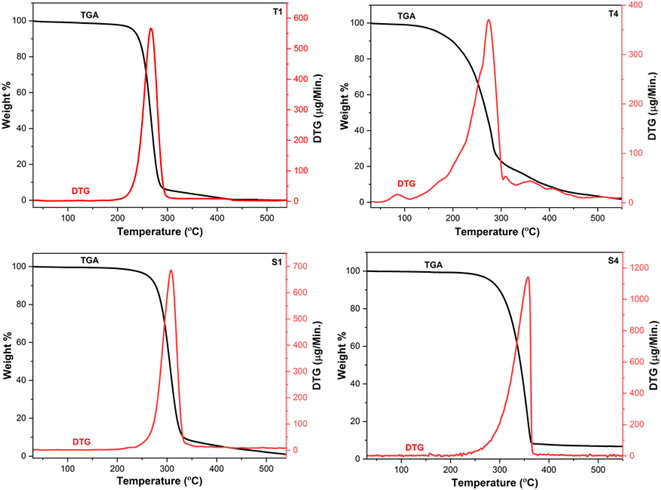 |
| | Fig. 4 Thermograms of T1, T4, S1 and S4. | |
3.5 Electrochemical study
A potential range of −2 V to +4 V and a scanning frequency of 0.1 V s−1 are often used to record cyclovoltammograms (CV). 0.1 M tetrabutylammonium perchlorate in DCM was used as supporting electrolyte and silver wire as a reference electrode. The concentration of ferrocene and the complexes were 10−3 M. CV of synthesized complexes (T1, T4, S1 and S4) with ferrocene in inset is shown in Fig. 5. As the working electrode and counter electrode, respectively, we have chosen glassy carbon (C) and platinum wire. According to eqn (2) and (3), the energy was measure for HOMO and LUMO.49,50| | |
EHOMO = −[(Eox) + 3.77] eV
| (2) |
| | |
ELUMO = −[(Ered) + 3.77] eV
| (3) |
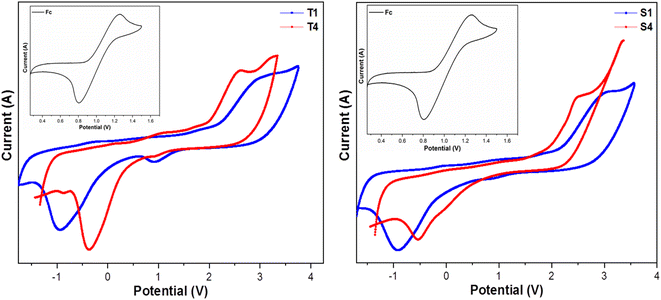 |
| | Fig. 5 Cyclovoltammograms of lanthanide complexes. | |
Ferrocene (Fc) has a half potential of 1.03 V.51 Table 4 lists the electro-chemical parameters of prepared complexes. The electronic band gap of complexes is in the category of semiconductors, implying their conductive nature. Values of electronic band gap determined from electrochemical data corroborate with the optical band gap values obtained from absorption spectral data. The change in values of redox potentials of synthesized complexes from that of heteroaromatic auxiliary units suggests the formation of ternary lanthanide complexes.29,52
Table 4 Electro-chemical data of complexes (T1, T4, S1 & S4)a
| Complex |
Eox (V) |
Ered (V) |
EHOMO (eV) |
ELUMO (eV) |
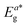
(eV) |
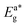 : electronic band gap. : electronic band gap. |
| T1 |
2.924 |
−0.928 |
−6.694 |
−2.842 |
3.852 |
| T4 |
2.606 |
−0.357 |
−6.376 |
−3.413 |
2.963 |
| S1 |
3.013 |
−0.918 |
−6.783 |
−2.852 |
3.931 |
| S4 |
2.476 |
−0.527 |
−6.246 |
−3.243 |
3.003 |
3.6 Powder XRD analysis
To get an idea about crystalline nature of synthesized ternary complexes, their powder X-ray diffraction (XRD) patterns were recorded. Fig. 6(a and b) demonstrates the diffractogramms of lanthanide complexes recorded at Bragg's angle of 2θ in range of 10°–50°. The sharp peaks in XRD profiles suggest that the crystalline nature of synthesized complexes. From powder XRD patterns, it can be found that the prepared complexes hold different degree of crystallinity.53 High crystallinity degree in ternary Ln(III) complexes is evidenced by better defined peaks in their diffractogramms.
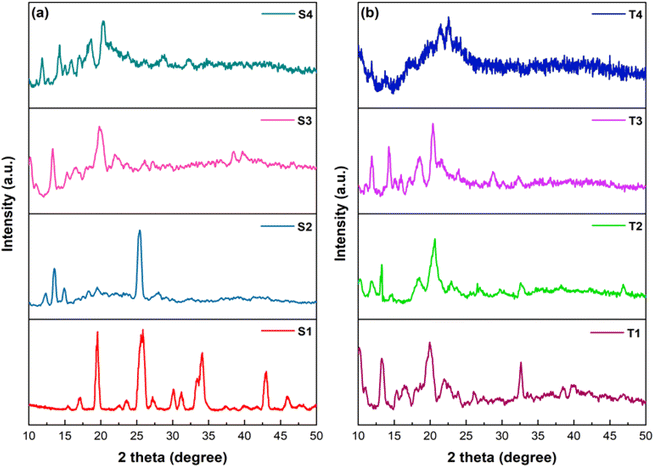 |
| | Fig. 6 Powder X-ray diffraction (XRD) patterns of S1–S4 (a) and T1–T4 (b) complexes. | |
3.7 PL study
The photoluminescence profiles of terbium (T1–T4) and samarium (S1–S4) complexes monitored in solid form are demonstrated in Fig. 7 and 8 respectively. Excitation profiles of Tb(III) complexes evince the broad band of ligand as well as few weak intense peaks owing to f–f transitions of metal ion. Emission spectral profile of ternary terbium complexes were obtained at their respective excitation wavelength (λex) in solid state. Emission spectra display peaks in 480–620 nm, characteristic of Tb3+ ion. The peaks in photoluminescence emission spectra are positioned at 490, 546, 590 and 617 nm attributed to transitions from excited 5D4 state to lower situated 7F6, 5, 4, 3 states of Tb3+ ion separately.54,55 Along with these peaks, the least intense peaks were also seen corresponding to 5D4 → 7F2–0. Dominant peak present at around 546 nm is answerable for green emission of T1–T4 complexes.56
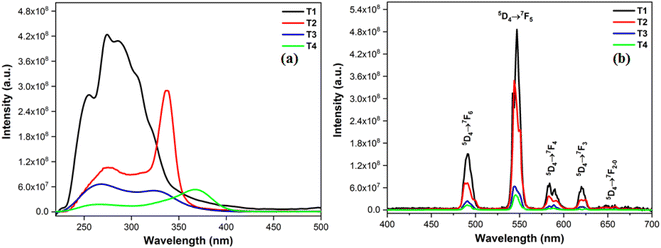 |
| | Fig. 7 (a) Excitation (at λem = 549) and (b) emission spectral profiles T1–T4. | |
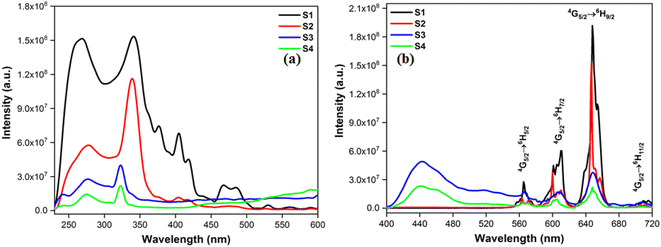 |
| | Fig. 8 (a) Excitation (at λem = 648) and (b) emission spectral profiles S1–S4. | |
Excitation spectra of S1–S4 evince broad band of ligand as well as few weak intense pinnacles relative to f–f transitions of Sm3+ ion. Emission profiles show characteristic peaks of at 564 nm (04G5/2 → 06H5/2), 601 nm (4G 5/2 → 6H7/2), 648 nm (4G5/2 → 6H9/2) and 707 nm (4G5/2 → 6H11/2) transition.57,58 Ligand dependent bands were appeared in emission spectra of S3 and S4. The emission spectra of S1–S4 evident most pronounced peak at 648 nm depends on the coordinating surrounding of Sm3+ ion.59 The PL emission intensity decreases in a particular series however it is larger than their binary complex. Hence, the prepared complexes showed enhanced luminescence behavior. The order of emission intensity in synthesized complexes is also supported by energy transfer phenomenon as shown in Fig. 9. Triplet (T) state energy of Hfodo was determined from the phosphorescence spectral profile (Fig. S17†) of its binary complex with Gd(III) i.e. [Gd(Hfodo)3(H2O)2]. The shortest wavelength in the phosphorescence spectrum provides the energy of triplet state of Hfodo. The T level energy of Bpy and its derivatives has already been reported in literature.60 Energy transfer parameters of diketone and neutral moieties are listed in Table S1.† eqn (4) was used to estimate the branching ratio (β) of synthesized complexes.61
| |
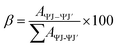 | (4) |
Here,
AψJ–ψ’J denotes the integrated area of PL emission peaks. The value of
β for hyper-sensitive peak is ∼60% as listed in Tables S2
† (
T1–T4) &
S3 (
S1–S4), which supports the efficacy of these substances in lasers.
62 The intensity ratio of
5D
4 →
7F
6/
5D
4 →
7F
5 for Tb(
III) and
4G
5/2 →
6H
9/2/
4G
5/2 →
6H
5/2 for Sm(
III) demonstrates the asymmetric environment around metal ion.
63 The quantum yield for synthesized Ln complexes is measured against the reference
i.e., quinine bisulphate in dilute sulphuric acid. It is calculated in order to investigate the influence of different neutral moieties on the PL intensity of complexes.
Eqn (5) is used for the measurement of relative quantum yield (
Φs).
64| |
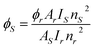 | (5) |
Here,
Φr,
A,
I,
s,
r and
n represent quantum yield reference, absorbance at the excitation wavelength, integrated emission intensity, sample, reference and refractive index of solvent, respectively.
65 The value of
Φr is 54.6%. Quantum yield is found to be in the declining manner and support the outcomes obtained from emission spectral profiles. The numerous absorption and photo-physical data are compiled in
Table 5.
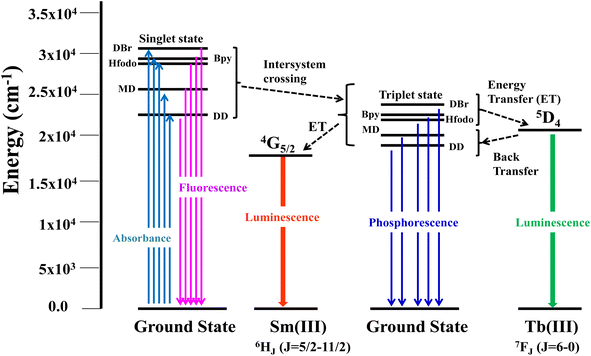 |
| | Fig. 9 Energy transfer (Antenna Effect) in ternary complexes. | |
Table 5 Some photo–physical parameters of lanthanide complexesa
| Complex |
λabs (nm) |
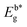
(eV) |
λex (nm) |
λem (nm) |
FWHM (nm) |
Intensity ratio |
Φs (%) |
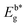 : optical band gap. : optical band gap. |
| T1 |
281 |
3.811 |
274 |
544 |
8.69 |
0.282 |
6.24 |
| T2 |
309 |
3.741 |
337 |
547 |
9.08 |
0.253 |
5.67 |
| T3 |
369 |
3.101 |
268 |
543 |
10.26 |
0.357 |
4.39 |
| T4 |
382 |
2.951 |
369 |
545 |
7.95 |
0.363 |
3.81 |
| S1 |
288 |
3.901 |
268 |
648 |
9.56 |
10.090 |
5.13 |
| S2 |
303 |
3.819 |
339 |
647 |
8.08 |
12.388 |
5.02 |
| S3 |
373 |
3.098 |
323 |
649 |
14.08 |
11.101 |
2.65 |
| S4 |
373 |
3.044 |
324 |
648 |
10.96 |
9.545 |
1.76 |
3.8 Colorimetric study
The color (x, y) co-ordinates was obtained from emission spectral data. These co-ordinates positioned in the greenish and orange red regime of Fig. 10 relative to T1–T4 and S1–S4, separately. The florescent photographs of T1 and S1 are also shown in CIE (x, y) diagram of complexes. From (x, y), the other colorimetric parameters i.e., (u′, v′) and color temperature (CT) were estimated through eqn (6) and (7).66–68 (u′, v′) with CT values are manifested in Fig. 11.| |
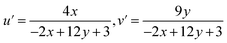 | (6) |
| | |
CT = −437n3 + 3601n2 − 6861n + 5514.31
| (7) |
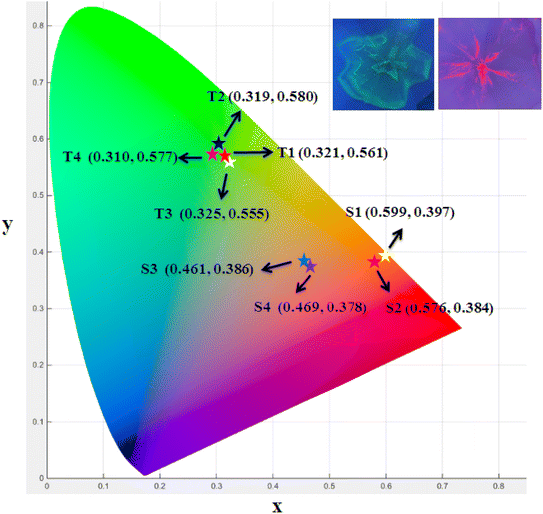 |
| | Fig. 10 CIE (x, y) coordinates of prepared complexes (inset figures are fluorescent photographs of T1 and S1). | |
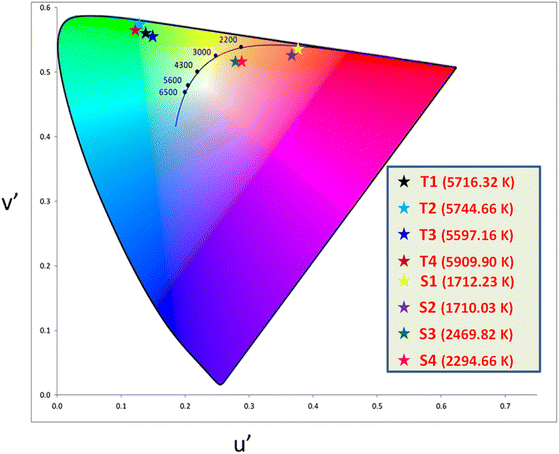 |
| | Fig. 11 CIE (u′, v′) parameters of prepared complexes. | |
In the relation (7), n characterizes the inverse-slope line and was assessed via eqn (8).
| |
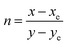 | (8) |
Here,
xe and
ye represents the color epicenter having respective entity
i.e. 0.332 and 0.186. Color purity (CP) of prepared Ln complexes was determined from
eqn (9).
| |
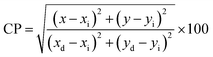 | (9) |
Here, (
xi &
yi) with value 0.333 are the illuminated points. The value of dominant points
xd = 0.290 (green) & 0.688 (red) and
yd = 0.600 (green) & 0.331 (red).
69–71 The relative luminance value for a certain color in terms of RGB parameters is also calculated. The color characteristics for ternary lanthanide complexes are listed in
Table 6.
Table 6 Colorimetric parameters of prepared complexes
| Complex |
(x, y) |
(u′, v′) |
CP (%) |
R |
G |
B |
Luminance (%) |
| T1 |
(0.321, 0.561) |
(0.141, 0.555) |
84.42 |
110 |
254 |
57 |
82 |
| T2 |
(0.319, 0.580) |
(0.137, 0.559) |
91.48 |
96 |
254 |
28 |
81 |
| T3 |
(0.325, 0.555) |
(0.144, 0.554) |
82.14 |
120 |
255 |
61 |
83 |
| T4 |
(0.310, 0.577) |
(0.133, 0.558) |
90.62 |
79 |
255 |
46 |
81 |
| S1 |
(0.599, 0.397) |
(0.365, 0.544) |
77.07 |
255 |
98 |
0 |
60 |
| S2 |
(0.576, 0.384) |
(0.359, 0.535) |
69.94 |
255 |
101 |
0 |
61 |
| S3 |
(0.461, 0.386) |
(0.275, 0.518) |
39.02 |
255 |
157 |
101 |
72 |
| S4 |
(0.469, 0.378) |
(0.284, 0.516) |
40.35 |
255 |
148 |
100 |
70 |
4 Conclusions
A different series of Ln complexes with fluorinated primary and heteroaromatic secondary ligand have been prepared and examined. The outcome of infrared study reveals the coordination of ligands through hard donor atoms (–O and –N). The complexes exhibit band at higher wavelength as compared to Hfodo which illustrates the stability of ligand orbitals on complexation. The high luminescent intensity recommends the superior ligand sensitization for solid sample. Synthesized terbium and samarium complexes emit bright green and orange red emission which is the constituent of tricolor system. The prepared lanthanide complexes are potential applicant in laser diodes due to high β value corresponding to the hyper-sensitive transition and the range of band gap further prove their utility as conducting material in fabricating displays.
Ethical statement
The article does not involve any study performed on animals or human by any of the authors.
Data availability
The authors affirm that the information/data of this research article is available inside the article.
Author contributions
Anjli Hooda = data curation, writing – original draft; Devender Singh = writing – review & editing, supervision; Anuj Dalal = investigation; Kapeesha Nehra = formal analysis; Sumit Kumar = visualization; Rajender Singh Malik = software; Ramesh Kumar = methodology; Parvin Kumar = resources; Brijesh Rathi = validation.
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors (DS and AH) wish to thank SERB-DST, New Delhi [EMR/2016/006135] and CSIR for SRF [Award No.: 09/382(0255)/2020-EMR-I] for financially supporting this work.
References
- L. N. Sun, H. J. Zhang, L. S. Fu, F. Y. Liu, Q. G. Meng, C. Y. Peng and J. B. Yu, A New Sol–Gel Material Doped with an Erbium Complex and Its Potential Optical-Amplification Application, Adv. Funct. Mater., 2005, 15, 1041–1048 CrossRef CAS.
- Y. Jia, J. Wang, L. Zhao and B. Yan, Eu3+-β-diketone functionalized covalent organic framework hybrid material as a sensitive and rapid response fluorescent sensor for glutaraldehyde, Talanta, 2022, 236, 122877 CrossRef CAS PubMed.
- L. N. Sun, H. J. Zhang, Q. G. Meng, F. Y. Liu, L. S. Fu, C. Y. Peng, J. B. Yu, G. L. Zheng and S. B. Wang, Near-infrared luminescent hybrid materials doped with lanthanide (Ln) complexes (Ln= Nd, Yb) and their possible laser application, J. Phys. Chem. B, 2005, 109, 6174–6182 CrossRef CAS PubMed.
- J. Yuan and G. Wang, Lanthanide-based luminescence probes and time-resolved luminescence bioassays, TrAC, Trends Anal. Chem., 2006, 25, 490–500 CrossRef CAS.
- M. Elbanowski and B. M
![[a with combining cedilla]](https://www.rsc.org/images/entities/char_0061_0327.gif) kowska, The lanthanides as luminescent probes in investigations of biochemical systems, J. Photochem. Photobiol., A, 1996, 99, 85–92 CrossRef CAS.
kowska, The lanthanides as luminescent probes in investigations of biochemical systems, J. Photochem. Photobiol., A, 1996, 99, 85–92 CrossRef CAS. - J. Tang and P. Zhang, Lanthanide single-ion molecular magnets, Lanthanide Single Molecule Magnets, 2015, pp. 41–90 Search PubMed.
- R. Marin, G. Brunet and M. Murugesu, Shining new light on multifunctional lanthanide single-molecule magnets, Angew. Chem., Int. Ed. Engl., 2021, 60, 1728–1746 CrossRef CAS PubMed.
- A. Dalal, K. Nehra, A. Hooda, D. Singh, P. Kumar, S. Kumar, R. S. Malik and B. Rathi, Luminous lanthanide diketonates: Review on synthesis and optoelectronic characterizations, Inorg. Chim. Acta, 2023, 121406 CrossRef CAS.
- D. Singh, S. Bhagwan, R. K. Saini, V. Nishal and I. Singh, Development in Organic Light-Emitting Materials and Their Potential Applications, Adv. Magn. Opt. Mater., 2016, 473–519 Search PubMed.
- A. Dalal, K. Nehra, A. Hooda, D. Singh, S. Kumar and R. S. Malik, Synthesis, photophysical characteristics and geometry optimization of Tris(2-benzoylacetophenonate)europium complexes with 2,2′-Bipyridine derivatives, J. Lumin., 2022, 247, 118873 CrossRef CAS.
- K. Nehra, A. Dalal, A. Hooda, S. Singh and D. Singh, Computational and Spectroscopic Evaluation of 1,10-Phenanthroline based Eu(III) Fluorinated β-Diketonate Complexes for Displays, J. Lumin., 2022, 251, 119111 CrossRef CAS.
- Z. Ahmed, W. A. Dar and K. Iftikhar, Synthesis and luminescence study of a highly volatile Sm (III) complex, Inorg. Chim. Acta, 2012, 392, 446–453 CrossRef CAS.
- K. Nehra, A. Dalal, A. Hooda, D. Singh, S. Kumar, R. S. Malik and P. Kumar, Influence of coordinating environment on photophysical properties of UV excited sharp red emitting material: Judd Ofelt analysis, J. Photochem. Photobiol., A, 2022, 430, 113999 CrossRef CAS.
- X. Guo, H. Guo, L. Fu, L. D. Carlos, R. S. Ferreira, L. Sun, R. Deng and H. Zhang, Novel near-infrared luminescent hybrid materials covalently linking with lanthanide [Nd(III), Er(III), Yb(III), and Sm(III)] complexes via a primary β-Diketone ligand: synthesis and photophysical studies, J. Phys. Chem. C, 2009, 113, 12538–12545 CrossRef CAS.
- D. Singh, V. Tanwar, S. Bhagwan and I. Singh, Recent Advancements in Luminescent Materials and their Potential Applications, Adv. Magn. Opt. Mater., 2016, 317–352 Search PubMed.
- K. Nehra, A. Dalal, A. Hooda, D. Singh and S. Kumar, Exploration of newly synthesized red luminescent material of samarium for display applications, Inorg. Chem. Commun., 2022, 139, 109361 CrossRef CAS.
- K. Nehra, A. Dalal, A. Hooda, P. Kumar, D. Singh, S. Kumar, R. S. Malik and P. Kumar, Luminous terbium and samarium complexes with diacetylmethane and substituted 1,10-phenanthroline derivatives for display applications: Preparation and optoelectronic investigations, J. Lumin., 2022, 249, 119032 CrossRef CAS.
- A. Dalal, K. Nehra, A. Hooda, D. Singh, S. Kumar and R. S. Malik, Red emissive ternary europium complexes: synthesis, optical, and luminescence characteristics, Luminescence, 2022, 37, 1309–1320 CrossRef CAS PubMed.
- A. Bellusci, G. Barberio, A. Crispini, M. Ghedini, M. La Deda and D. Pucci, Synthesis and luminescent properties of novel lanthanide(III) β-diketone complexes with nitrogen p, p ‘-disubstituted aromatic ligands, Inorg. Chem., 2005, 44, 1818–1825 CrossRef CAS PubMed.
- A. Døssing, Luminescence from lanthanide (3+) ions in solution, Eur. J. Inorg. Chem., 2005, 2005, 1425–1434 CrossRef.
- A. Dalal, K. Nehra, A. Hooda, S. Singh, S. Bhagwan, D. Singh and S. Kumar, 2,2′-Bipyridine based fluorinated β-Diketonate Eu(III) complexes as red emitter for display applications, Inorg. Chem. Commun., 2022, 140, 109399 CrossRef CAS.
- J. Shi, Y. Hou, W. Chu, X. Shi, H. Gu, B. Wang and Z. Sun, Crystal structure and highly luminescent properties studies of bis-β-diketonate lanthanide complexes, Inorg. Chem., 2013, 52, 5013–5022 CrossRef CAS PubMed.
- K. Nehra, A. Dalal, A. Hooda, D. Singh, S. Kumar and R. S. Malik, Heteroleptic luminous ternary europium Complexes: Synthesis, electrochemical and photophysical investigation, Chem. Phys. Lett., 2022, 800, 139675 CrossRef CAS.
- K. Nehra, A. Dalal, A. Hooda, S. Bhagwan, R. K. Saini, B. Mari, S. Kumar and D. Singh, Lanthanides β-diketonate complexes as energy-efficient emissive materials: A review, J. Mol. Struct., 2021, 1249, 131531 CrossRef.
- A. Dalal, K. Nehra, A. Hooda, D. Singh, K. Jakhar and S. Kumar, Preparation and photoluminescent characteristics of green Tb(III) complexes with β-diketones and N donor auxiliary ligands, Inorg. Chem. Commun., 2022, 139, 109349 CrossRef CAS.
- A. Dalal, A. Hooda, K. Nehra, D. Singh, S. Kumar, R. S. Malik and P. Kumar, Effect of substituted 2,2′-bipyridine derivatives on luminescence characteristics of green emissive terbium complexes: Spectroscopic and optical analysis, J. Mol. Struct., 2022, 1265, 133343 CrossRef CAS.
- A. Dalal, K. Nehra, A. Hooda, P. Kumar, D. Singh, S. Kumar, R. Kumar and P. Kumar, Red emissive β-diketonate Ln(III) complexes for displays: Preparation, spectroscopic and optical investigations, Optik, 2023, 276, 170648 CrossRef CAS.
- K. Nehra, A. Dalal, A. Hooda, R. K. Saini, D. Singh and S. Kumar, Synthesis and photoluminescence characterization of the complexes of samarium dibenzoylmethanates with 1,10-phenanthroline derivatives, Polyhedron, 2022, 217, 115730 CrossRef CAS.
- A. Dalal, K. Nehra, A. Hooda, D. Singh, R. S. Malik and S. Kumar, Synthesis and optoelectronic features of 5,5’-Bis(3,4-(ethylenedioxy)thien-2-yl)-2,2’-bipyridine, Optik, 2021, 248, 167942 CrossRef CAS.
- W. A. Dar, A. B. Ganaie and K. Iftikhar, Synthesis and photoluminescence study of two new complexes [Sm(hfaa)3(impy)2] and [Eu(hfaa)3(impy)2] and their PMMA based hybrid films, J. Lumin., 2018, 202, 438–449 CrossRef CAS.
- A. Hooda, D. Singh, A. Dalal, K. Nehra, S. Kumar, R. S. Malik, R. Kumar and P. Kumar, Preparation, Spectral and Judd Ofelt Analyses of Luminous Octa-coordinated Europium(III) Complexes, J. Photochem. Photobiol., A, 2023, 440, 114646 CrossRef CAS.
- K. Nehra, A. Dalal, A. Hooda, S. Singh, D. Singh, S. Kumar, R. S. Malik, R. Kumar and P. Kumar, Red luminous Eu(III) complexes: Preparation, spectral, optical and theoretical evaluation, Inorg. Chim. Acta, 2022, 539, 121007 CrossRef CAS.
- R. Ilmi and K. Iftikhar, Photophysical properties of Lanthanide(III) 1,1,1-trifluoro-2,4-pentanedione complexes with 2,2’-Bipyridyl: an experimental and theoretical investigation, J. Photochem. Photobiol., A, 2017, 333, 142–155 CrossRef CAS.
- X. Liu, S. Gao, L. Wang, L. Shen and J. Jiang, Synthesis, luminescent properties and theoretical study of novel ternary samarium (III) and dysprosium (III) complexes, Rare Met., 2011, 30, 284–288 CrossRef CAS.
- A. Hooda, K. Nehra, A. Dalal, S. Singh, R. K. Saini, S. Kumar and D. Singh, Deep red emissive octacoordinated heteroleptic Sm(III) complexes: preparation and spectroscopic investigation, J. Mol. Struct., 2022, 1260, 132848 CrossRef CAS.
- A. Hooda, D. Singh, K. Nehra, A. Dalal, S. Kumar, R. S. Malik, B. Rathi and P. Kumar, Luminescent Tb(III) Complexes with Lewis Bases for Displays: Synthesis and Spectral Investigation, Inorg. Chem. Commun., 2023, 151, 110583 CrossRef CAS.
- A. Dalal, K. Nehra, A. Hooda, R. K. Saini, D. Singh, S. Kumar and R. S. Malik, Preparation and optoelectronic enhancement of trivalent terbium complexes with fluorinated β-diketone and bidentate ancillary ligands, J. Mater. Sci.: Mater. Electron., 2022, 33, 12984–12996 CrossRef CAS.
- J. A. Costa, R. A. de Jesus, D. D. Dorst, I. M. Pinatti, L. M. Oliveira, M. E. de Mesquita and C. M. Paranhos, Photoluminescent properties of the europium and terbium complexes covalently bonded to functionalized mesoporous material PABA-MCM-41, J. Lumin., 2017, 192, 1149–1156 CrossRef CAS.
- T. Cantat, F. Jaroschik, F. Nief, L. Ricard, N. Mezailles and Le P. Floch, New mono-and bis-carbene samarium complexes: synthesis, X-ray crystal structures and reactivity, Chem. Commun., 2005, 41, 5178–5180 RSC.
- K. Nehra, A. Dalal, A. Hooda, S. Bhagwan, K. Jakhar, D. Singh, R. S. Malik, S. Kumar and B. Rathi, Synthesis, thermal and photoluminescence investigation of Tb(III) β-diketonates with 1,10-phenanthroline derivatives, J. Lumin., 2022, 119233 CrossRef CAS.
- A. Dalal, K. Nehra, A. Hooda, D. Singh, J. Dhankhar and S. Kumar, Fluorinated β-diketone-based Sm(III) complexes: spectroscopic and optoelectronic characteristics, Luminescence, 2022, 37, 1328–1334 CrossRef CAS PubMed.
- A. Hooda, A. Dalal, K. Nehra, S. Singh, S. Kumar and D. Singh, Red-emitting β-diketonate Eu(III) Complexes With Substituted 1,10-phenanthroline Derivatives: Optoelectronic and Spectroscopic Analysis, J. Fluoresc., 2022, 32, 1413–1424 CrossRef CAS PubMed.
- A. Dalal, K. Nehra, A. Hooda, S. Bhagwan, R. K. Saini, D. Singh and S. Kumar, Luminescent heteroleptic samarium (III) complexes: Synthesis, optical and photophysical investigation, Inorg. Chem. Commun., 2022, 141, 109620 CrossRef CAS.
- K. Nehra, A. Dalal, A. Hooda, K. Jakhar, D. Singh and S. Kumar, Preparation, optoelectronic and spectroscopic analysis of fluorinated heteroleptic samarium complexes for display applications, Inorg. Chim. Acta, 2022, 537, 120958 CrossRef CAS.
- V. Kesarwani and V. K. Rai, Optical thermometry and broad infrared luminescence in highly sensitized TBO glass, Opt. Laser Technol., 2022, 146, 107535 CrossRef CAS.
- H. Wang, P. He, H. Yan and M. Gong, Synthesis, characteristics and luminescent properties of a new europium (III) organic complex applied in near UV LED, Sens. Actuators, B, 2011, 156, 6 CrossRef CAS.
- X. Liu, S. Gao, L. Wang, J. Jiang and L. Shen, Synthesis, luminescent properties and theoretical study of a new β-diketone and its rare earth ternary complexes, Rare Met., 2011, 30, 289–293 CrossRef.
- A. Chauhan and R. Langyan, Preparation and optical features of samarium(III) complexes introducing bidentate fluorinate and secondary ligands, J. Mater. Sci.: Mater. Electron., 2020, 31, 22085–22097 CrossRef CAS.
- A. Dalal, K. Nehra, A. Hooda, S. Singh, D. Singh and S. Kumar, Synthesis, Optoelectronic and Photoluminescent Characterizations of Green Luminous Heteroleptic Ternary Terbium Complexes, J. Fluoresc., 2022, 32, 1019–1029 CrossRef CAS PubMed.
- K. Nehra, A. Dalal, A. Hooda, D. Singh, R. S. Malik and S. Kumar, Spectroscopic and optoelectronic investigations of 3,8-Bis(3,4-(ethylenedioxy)thien-2-yl)-1,10-phenanthroline, J. Mater. Sci.: Mater. Electron., 2022, 33, 115–125 CrossRef CAS.
- N. G. Tsierkezos, Cyclic voltammetric studies of ferrocene in nonaqueous solvents in the temperature range from 248.15 to 298.15 K, J. Solution Chem., 2007, 36, 289–302 CrossRef CAS.
- R. Boddula and S. Vaidyanathan, White light emissive bipolar ligand and their EuIII complex for white/red light emitting diodes, J. Photochem. Photobiol., A, 2017, 347, 26–40 CrossRef CAS.
- M. Sengar and A. K. Narula, Luminescence sensitization of Eu(III) complexes with aromatic Schiff base and N, N’-donor heterocyclic ligands: synthesis, luminescent properties and energy transfer, J. Fluoresc., 2019, 29, 111–120 CrossRef CAS PubMed.
- A. Hooda, A. Dalal, K. Nehra, D. Singh, S. Kumar, R. S. Malik and P. Kumar, Preparation and optical investigation of green luminescent ternary terbium complexes with aromatic β-diketone, Chem. Phys. Lett., 2022, 794, 139495 CrossRef CAS.
- J. Manzur, C. Poblete, J. Morales, R. C. De Santana, L. J. Queiroz Maia, A. Vega, P. Fuentealba and E. Spodine, Enhancement of terbium(III)-centered luminescence by tuning the triplet energy level of substituted pyridylamino-4-R-phenoxo tripodal ligands, Inorg. Chem., 2020, 59, 5447–5455 CrossRef CAS PubMed.
- K. Nehra, A. Dalal, A. Hooda, S. Singh, D. Singh and S. Kumar, Spectroscopic and Optical Investigation of 1,10-Phenanthroline based Tb(III) β-Diketonate Complexes, Inorg. Chim. Acta, 2022, 536, 120860 CrossRef CAS.
- A. B. Ganaie and K. Iftikhar, Theoretical Modeling (Sparkle RM1 and PM7) and Crystal Structures of the Luminescent Dinuclear Sm(III) and Eu(III) Complexes of 6,6,7,7,8,8,8-Heptafluoro-2,2-dimethyl-3,5-octanedione and 2,3-Bis(2-pyridyl) pyrazine: Determination of Individual Spectroscopic Parameters for Two Unique Eu3+ Sites, ACS Omega, 2021, 6, 21207–21226 CrossRef CAS PubMed.
- Y. Hasegawa, S. I. Tsuruoka, T. Yoshida, H. Kawai and T. Kawai, Enhanced deep-red luminescence of tris (hexafluoroacetylacetonato) samarium (III) complex with phenanthroline in solution by control of ligand coordination, J. Phys. Chem. A, 2008, 112, 803–807 CrossRef CAS PubMed.
- A. Hooda, K. Nehra, A. Dalal, S. Singh, S. Bhagwan, K. Jakhar and D. Singh, Preparation and photoluminescent analysis of Sm3+ complexes based on unsymmetrical conjugated chromophoric ligand, J. Mater. Sci.: Mater. Electron., 2022, 33, 11132–11142 CrossRef CAS.
- A. Hooda, A. Dalal, K. Nehra, P. Kumar, D. Singh, S. Kumar, R. S. Malik, R. Kumar and P. Kumar, Mononuclear luminous β-diketonate Ln(III) complexes with heteroaromatic auxiliary ligands: Synthesis and luminescent characteristics, Luminescence, 2022, 37, 1921–1931 CrossRef CAS PubMed.
- G. C. Righini and M. Ferrari, Photoluminescence of rare-earth-doped glasses, Riv.Nuovo Cimento, 2005, 28, 1–53 CAS.
- A. Zhang, J. Zhang, Q. Pan, S. Wang, H. Jia and B. Xu, Synthesis, photoluminescence and intramolecular energy transfer model of a dysprosium complex, J. Lumin., 2012, 132, 965–971 CrossRef CAS.
- A. B. Ganaie, A. Ali and K. Iftikhar, Synthesis, structure, phase controlled colour tuning of dinuclear Pr(III) and Tb(III) complexes with fluorinated β-diketone and heterocyclic Lewis base as UV light converters, Polyhedron, 2021, 212, 115592 CrossRef.
- A. J. Kanimozhi and V. Alexander, Synthesis and photophysical and magnetic studies of ternary lanthanide (III) complexes of naphthyl chromophore functionalized imidazo [4,5-f][1,10]phenanthroline and dibenzoylmethane, Dalton Trans., 2017, 46, 8562–8571, 10.1039/C7DT01133D.
- C. Yang, J. Luo, J. Ma, M. Lu, L. Liang and B. Tong, Synthesis and photoluminescent properties of four novel trinuclear europium complexes based on two tris-β-diketones ligands, Dyes Pigm., 2011, 92, 696–704 CrossRef.
- A. Hooda, A. Dalal, K. Nehra, S. Singh, D. Singh, S. Kumar and R. S. Malik, Red luminous ternary europium complexes: Optoelectronic and photophysical analysis, J. Lumin., 2022, 248, 118989 CrossRef CAS.
- K. Nehra, A. Dalal, A. Hooda, R. K. Saini, D. Singh, S. Kumar, R. S. Malik, R. Kumar and P. Kumar, Synthesis of green emissive terbium(III) complexes for displays: Optical, electrochemical and photoluminescent analysis, Luminescence, 2023, 38, 56–63 CrossRef CAS PubMed.
- A. Dalal, K. Nehra, A. Hooda, S. Singh, D. Singh, S. Kumar, R. S. Malik and P. Kumar, Preparation, spectroscopic and thermal investigation of fluorinated Sm(III) β-diketonates with bidentate N donor ligands, Chem. Phys. Lett., 2022, 800, 139672 CrossRef CAS.
- A. Hooda, K. Nehra, A. Dalal, S. Singh, R. K. Saini, S. Kumar and D. Singh, Terbium Complexes of an Asymmetric β-diketone: Preparation, Photophysical and Thermal Investigation, Inorg. Chim. Acta, 2022, 536, 120881 CrossRef CAS.
- A. Hooda, K. Nehra, A. Dalal, S. Bhagwan, I. Gupta, D. Singh and S. Kumar, Luminescent Features of Ternary Europium Complexes: Photophysical and Optoelectronic Evaluation, J. Fluoresc., 2022, 32, 1529–1541 CrossRef CAS PubMed.
- A. Hooda, A. Dalal, K. Nehra, P. Kumar, D. Singh, R. S. Malik and S. Kumar, Heteroleptic Eu(III) emissive complexes: Luminescent, optoelectronic and theoretical investigation, J. Lumin., 2022, 252, 119272 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry 2023 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article *a,
Anuj Dalala,
Kapeesha Nehraa,
Sumit Kumarb,
Rajender Singh Malikb,
Ramesh Kumarc and
Parvin Kumarc
*a,
Anuj Dalala,
Kapeesha Nehraa,
Sumit Kumarb,
Rajender Singh Malikb,
Ramesh Kumarc and
Parvin Kumarc
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H unit are liable for the peak at 3060 cm−1. The FTIR spectrum of diketone ligand reveal a prominent peak due to C
C–H unit are liable for the peak at 3060 cm−1. The FTIR spectrum of diketone ligand reveal a prominent peak due to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group which is moved to the lesser frequency after complexation. This suggests the attachment of the diketone to metal ion via oxygen.34,35 The finding of peaks at about 1070–1100 cm−1 correspondent to C–Br unit existed in T2, T3, S2 and S3. In FTIR profiles of some complexes (T3, T4, S3 and S4) two peaks in scope of 688–722 cm−1 are present due to C–S–C bond.29 The peaks around 455–475 cm−1 (Ln–O) and 531–538 cm−1 (Ln–N), confirms the involvement of Hfodo and ancillary moieties with the Ln3+ ion.36–38
O group which is moved to the lesser frequency after complexation. This suggests the attachment of the diketone to metal ion via oxygen.34,35 The finding of peaks at about 1070–1100 cm−1 correspondent to C–Br unit existed in T2, T3, S2 and S3. In FTIR profiles of some complexes (T3, T4, S3 and S4) two peaks in scope of 688–722 cm−1 are present due to C–S–C bond.29 The peaks around 455–475 cm−1 (Ln–O) and 531–538 cm−1 (Ln–N), confirms the involvement of Hfodo and ancillary moieties with the Ln3+ ion.36–38
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C)
C)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N)
N)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)
O)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H, –C–H)
C–H, –C–H)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H) signal of Hfodo is displaced towards the lower chemical shift. The paramagnetic property of terbium and samarium ions causes noticeable alterations in the 1H-NMR spectrum data of ligands.39–41 The paramagnetic nature of Tb3+ greatly affects the position of signals of auxiliary units. Fig. S1–S16† represents IR and NMR spectral profiles of ternary terbium and samarium complexes respectively.
C–H) signal of Hfodo is displaced towards the lower chemical shift. The paramagnetic property of terbium and samarium ions causes noticeable alterations in the 1H-NMR spectrum data of ligands.39–41 The paramagnetic nature of Tb3+ greatly affects the position of signals of auxiliary units. Fig. S1–S16† represents IR and NMR spectral profiles of ternary terbium and samarium complexes respectively.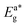 : electronic band gap.
: electronic band gap.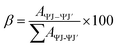
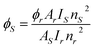
 : optical band gap.
: optical band gap.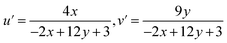

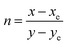
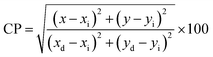
![[a with combining cedilla]](https://www.rsc.org/images/entities/char_0061_0327.gif) kowska, The lanthanides as luminescent probes in investigations of biochemical systems, J. Photochem. Photobiol., A, 1996, 99, 85–92 CrossRef CAS.
kowska, The lanthanides as luminescent probes in investigations of biochemical systems, J. Photochem. Photobiol., A, 1996, 99, 85–92 CrossRef CAS.