Open Access Article
Open Access ArticleTiO2/porous carbon as a new nanocomposite and catalyst for the preparation of 4H-pyrimido[2,1-b]benzimidazoles†
Zahra Jaliliana,
Ahmad Reza Moosavi-Zare *b,
Mohammad Ghadermazi
*b,
Mohammad Ghadermazi *a and
Hamid Goudarziafsharb
*a and
Hamid Goudarziafsharb
aDepartment of Chemistry, University of Kurdistan, P.O. Box 66135-416, Sanandaj, Iran. E-mail: mghadermazi@yahoo.com
bDepartment of Chemical Engineering, Hamedan University of Technology, Hamedan, 65155, Iran. E-mail: moosavizare@yahoo.com
First published on 4th April 2023
Abstract
A nano TiO2/porous carbon nanocomposite (TiO2/PCN) was designed by the pyrolysis of peanut shells as bio waste with nano titanium dioxide. In the presented nanocomposite, titanium dioxide is properly placed in the positions and pores of the porous carbon, so that it acts as an optimal catalyst in the nanocomposite structure. The structure of TiO2/PCN was studied by various analyses such as Fourier transform infrared spectroscopy (FT-IR), energy-dispersive X-ray Spectroscopy (EDX), scanning electron microscopy (SEM), SEM coupled EDX (SEM mapping), transmission electron microscopy (TEM), X-ray fluorescence (XRF) and BET. TiO2/PCN was successfully tested as a nano catalyst for the preparation of some 4H-pyrimido[2,1-b]benzimidazoles in high yields (90–97%) and short reaction times (45–80 min).
1. Introduction
4H-pyrimido[2,1-b]benzazoles including 4H-pyrimido[2,1-b]benzimidazole derivatives are important heterocyclic compound derivatives and are used in the structure of various drugs due to some significant biological activities such as anti-inflammatory,1 anti-tumor,2 anti-bacterial3 and anti-fungal properties.4One of the important strategies for the preparation of 4H-pyrimido[2,1-b]benzimidazoles is the multi-component condensation reaction of A β-keto ester with various aldehydes and 2-aminobenzimidazole.5 The multi-component reaction is an important strategy for the synthesis of organic compounds due to preparing the main product in one step, without the formation of side products. Preventing the loss of energy, materials and solvent are some other advantages of this protocol.6–13
Multi-component synthesis of 4H-pyrimido[2,1-b]benzazoles was reported using various catalysis such as, nano-[Co–4CSP]Cl2,5 iron fluoride,14 Fe3O4@nano-cellulose/TiCl,15 chitosan,16 nano-Fe3O4@SiO2–TiCl3,17 H3PO4–Al2O3,18 nano-TiCl2/cellulose,19 acetic acid,20 TMGT,21 1,3-disulfonic acid imidazolium trifluoroacetate,22 [bmim]BF4,23 GO@PSA-Cu24 and poly (vinylpyrrolidonium) perchlorate.25 Due to importance of 4H-pyrimido[2,1-b]benzimidazoles, the find of the more efficient method for the preparation of these significant compounds are still needed.
Millions of tons of biomass waste annually are produced as renewable materials in the world. These wastes can be created from the remains of plants or animals. Low price, low density, ability to return to the environment, good electrical conductivity, easy pyrolysis and degradable structure are important advantages of this category of waste materials.26–30 One of the biggest renewable sources of carbon is biomass, which is cheap and widely accessible.31,32 Peanut shell is one of the important agricultural wastes, which due to its easy pyrolysis, low density and high fiber content, is a suitable template and precursor for the production of porous carbon with a high effective surface. Placing metal oxides and other functional groups in the porous carbon substrate causes chemical activation and its high catalytic ability in promoting chemical reactions.33
Peanut shell as bio-waste material was successfully used for the design of various catalysts which applied in different chemical process such as cycloaddition of epoxides with CO2,33 degradation of organic pollutant,34 production aromatic rich monomer compounds,35 synthesis of 5-hydroxymethylfurfural from glucose, fructose, cellulose and agricultural wastes,36 esterification of cyclohexene with formic acid37 and synthesis of 1,2,3-triazoles.38
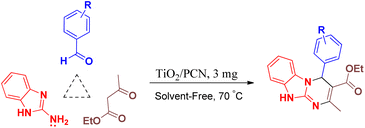
Herein, we have used peanut shell as bio-waste for the preparation of porous carbon as a template for the placement of nano titanium dioxide on it to give TiO2/porous carbon nanocomposite (TiO2/PCN). TiO2/PCN as a novel and efficient heterogeneous catalyst was successfully used for the preparation of 4H-pyrimido[2,1-b]benzimidazoles (Scheme 1).
2. Results and discussion
In this work, we have used peanut shell to prepare the porous carbon according the previous literature.31 In this regard, peanut shell as bio-waste material was crushed, burned and then, mixed with nano TiO2 in the mass ratio 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. This presented mixture was heated in 600 °C for 6 hours to prepare TiO2/porous carbon nanocomposite (TiO2/PCN). TiO2/PCN was prepared by pyrolysis of peanut shell with nano TiO2 in rutile form (Fig. 1). This composite was structured in nano size and dispersed in the surface of porous carbon with high and effective surface and large volume which could be applied as an efficient catalyst in organic synthesis.
1. This presented mixture was heated in 600 °C for 6 hours to prepare TiO2/porous carbon nanocomposite (TiO2/PCN). TiO2/PCN was prepared by pyrolysis of peanut shell with nano TiO2 in rutile form (Fig. 1). This composite was structured in nano size and dispersed in the surface of porous carbon with high and effective surface and large volume which could be applied as an efficient catalyst in organic synthesis.
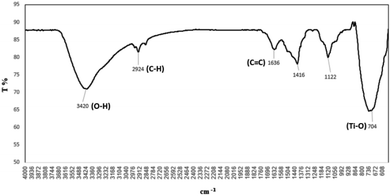
FT-IR spectrum of TiO2/PCN was studied and a broad peak appeared at 3500–3600 cm−1 which could be related to vibration of the O–H bonds from hydroxyl groups.37 Another peak at 1636 cm−1 is belonged to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond vibrations of the carbon skeleton39,40 (Fig. 2). FT-IR spectrum of TiO2 was compared with TiO2/PCN. The images were given in ESI† (Fig. S1).
C bond vibrations of the carbon skeleton39,40 (Fig. 2). FT-IR spectrum of TiO2 was compared with TiO2/PCN. The images were given in ESI† (Fig. S1).
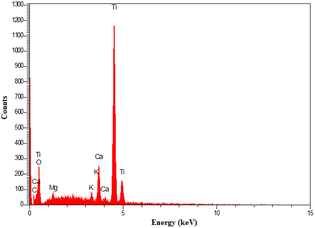
The presented elements in TiO2/PCN were investigated by energy-dispersive X-ray spectroscopy (EDX) (Fig. 3). From the examination of Fig. 3, it is clear that the desired elements such as carbon, oxygen, titanium and other elements in the peanut shell structure namely calcium, magnesium and potassium were also observed in the prepared nanocomposite.
In the next investigation, the distribution of the elements in the prepared nanocomposite structure was investigated and studied by SEM mapping analysis (Fig. 4). As it is found from the Fig. 4, indicates that, the elements of carbon, titanium, oxygen and to a lesser amount potassium, calcium and magnesium are distributed well in the nanocomposite structure.
Types of elemental oxides in the nanocomposite structure determined by X-ray fluorescence (XRF) analysis. The results were obtained in Fig. S2 in ESI.†
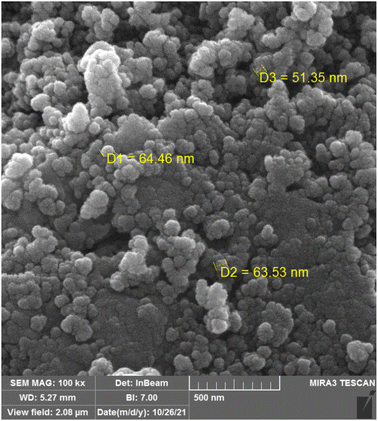
The morphology and the particle size of TiO2/PCN were investigated by scanning electron microscopy (SEM) (Fig. 5). As it is shown in Fig. 5, indicates that the presented particles from TiO2/PCN, were prepared in size less than 100 nanometers (Fig. 5).
The size of the prepared nanocomposite was also investigated by transmission electron microscopy (TEM). According that, as it is displayed from Fig. 6, demonstrated that the particles of TiO2/PCN were obtained in nano size.
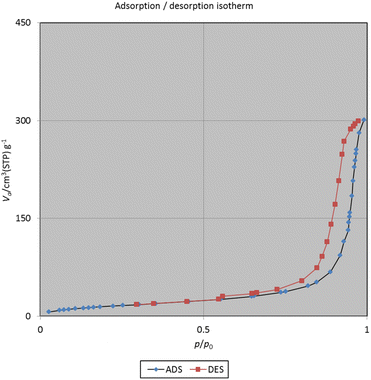
Because of the porous structure of catalyst particles, to show the effective surface of complex to catalyze the reactions, the volume and size of the cavities included in TiO2/PCN nanocomposite were studied. The specific surface area, mean pore diameter and total pore volume of TiO2/PCN nanocomposite are 60.151 m2 g−1, 30.848 nm and 0.4639 cm3 g−1 respectively (Table 1 and Fig. 7).
| Sample | Specific surface area (m2 g−1) | Mean pore diameter (nm) | Total pore volume (cm3 g−1) |
|---|---|---|---|
| TiO2/PCN | 60.151 | 30.848 | 0.4639 |
To find the best reaction condition, at first the reaction of 2-aminobenzimidazole, 4-chlorobenzaldehyde and ethyl acetoacetate was selected as a model reaction and various conditions such as amount of catalyst, temperature, kinds of solvent vs. solvent-free condition were examined on this reaction. After this investigation, the best yield and short reaction time were obtained in the presence of 3 mg of the catalyst at 70 °C under solvent-free condition. The model reaction was also tested in the absence of catalyst which the yield of the obtained product was not suitable (Table 2). To show the efficiency of the prepared nanocomposite as a catalyst in comparison with the moieties in its structure, nano titanium dioxide and porous carbon were separately tested on the model reaction which the obtained results were not better than the main catalyst. The obtained results from these reactions were given in Table 2. Moreover, various allotropes of titanium dioxide namely rutile and anatase used in the structure of nanocomposite and tested on the model reaction which the prepared composite with nano titanium dioxide in rutile form was more effective than anatase form to catalyze the reaction (Table 2).
| Entry | Catalyst (mg) | Solvent | Temperature (°C) | Time (min) | Yielda (%) |
|---|---|---|---|---|---|
| a Isolated yields. | |||||
| 1 | TiO2/PCN (1) | — | 90 | 60 | 74 |
| 2 | TiO2/PCN (3) | — | 90 | 60 | 95 |
| 3 | TiO2/PCN (6) | — | 90 | 60 | 95 |
| 4 | (−) | — | 90 | 60 | 19 |
| 5 | TiO2/PCN (3) | — | 25 | 60 | — |
| 6 | TiO2/PCN (3) | — | 50 | 60 | 43 |
| 7 | TiO2/PCN (3) | — | 70 | 60 | 95 |
| 8 | TiO2/PCN (3) | — | 100 | 60 | 95 |
| 9 | TiO2/PCN (3) | n-Hexane | Reflux | 60 | 16 |
| 10 | TiO2/PCN (3) | Dichloromethane | Reflux | 60 | 54 |
| 11 | TiO2/PCN (3) | Ethyl acetate | Reflux | 60 | 71 |
| 12 | TiO2/PCN (3) | Ethanol | Reflux | 60 | 95 |
| 13 | TiO2 (3) | — | 70 | 60 | 25 |
| 14 | PCN (3) | — | 70 | 60 | 5 |
After the optimization of the reaction condition, to investigate the generality and the efficiency of the catalyst, various aldehydes containing electron-releasing groups, electron-withdrawing groups and halogens on the aromatic ring were reacted with ethyl acetoacetate and 2-aminobenzothiazole to give 4H-pyrimido[2,1-b]benzimidazoles in high yields and short reaction times. The obtained results were given in Table 3.
| Entry | Product | Time (min) | Yielda (%) | M.p °C (Lit.)ref |
|---|---|---|---|---|
| a Isolated yield. | ||||
| 1 | 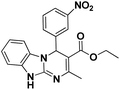 |
65 | 97 | 293–295 (291–292)5 |
| 2 |  |
75 | 92 | 258–260 (261–263)22 |
| 3 |  |
65 | 95 | 290–292 (294–296)23 |
| 4 |  |
70 | 90 | 255–258 |
| 5 |  |
65 | 94 | 304–306 (>300)23 |
| 6 | 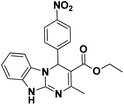 |
45 | 95 | 298–300 (>300)23 |
| 7 | 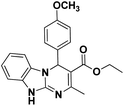 |
80 | 80 | 258–260 (256–258)25 |
| 8 | 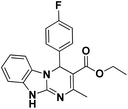 |
75 | 87 | 307–309 (>300)23 |
| 9 | 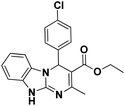 |
60 | 95 | 308–310 (302–303)41 |
| 10 | 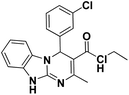 |
65 | 97 | 245–247 (250–252)24 |
| 11 | 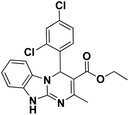 |
70 | 95 | 315–318 (>300)41 |
| 12 | 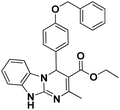 |
75 | 95 | 261–263 |
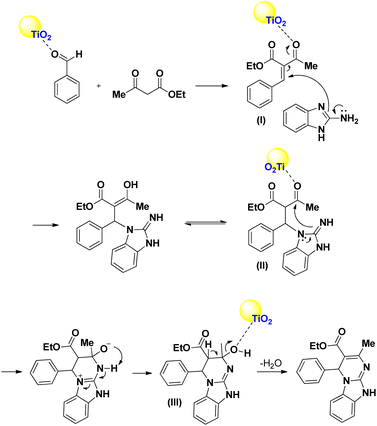
In a suggested mechanism, which is supported by the literature,5–25,41 ethyl acetoacetate reacted with aldehyde which is activated by TiO2/PCN to prepare intermediate (I). Then, 2-aminobenzimidazole is reacted with intermediate (I) to give (II). In the next step, by the intramolecular nucleophilic attack to carbonyl group in intermediate (II) which is activated by TiO2/PCN, intermediate (III) is generated. Finally, by removing of one molecule of H2O from (III), the desired product is obtained (Scheme 2).
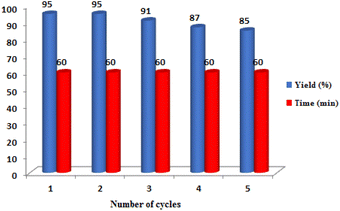
The reusability of TiO2/PCN as a nanocomposite and catalyst was also tested on the reaction of 2-aminobenzimidazole, 4-chlorobenzaldehyde and ethyl acetoacetate. After the completion of the reaction, the reaction mixture was extracted by warm ethanol and separated from the catalyst by simple filtration. The separated catalyst was washed with ethyl acetate, dried and successfully reused for four times (Fig. 8). The FT-IR of reused catalyst in comparison with fresh catalyst was given in Fig. S3 in ESI.†
The catalytic ability of the new nanocomposite is increased due to the distribution of nano titanium dioxide in the porous carbon support and the increase in the surface of the catalyst in comparison with the porous carbon and titanium dioxide separately. Using peanut shell as biological waste is important and economical in designing the structure of this nanocomposite.
3. Conclusions
In summary, TiO2/porous carbon nanocomposite (TiO2/PCN) was prepared by the pyrolysis of peanut shell, as bio-waste and green material, with nano titanium dioxide in rutile form. TiO2/PCN was fully studied by various techniques including FT-IR, EDX, SEM mapping, SEM, TEM, BET and XRF analyses. TiO2/PCN was successfully used as an efficient and reusable heterogeneous catalyst for the preparation of some 4H-pyrimido[2,1-b]benzimidazoles.4. Experimental
4.1. Procedure for the synthesis of TiO2/PCN
Peanut shells were crushed and ground in a mortar and then burned. Then, burnt peanut shell (0.075 g) was mixed with nano titanium dioxide (0.025 g) in rutile form. The presented mixture was placed in the oven and heated for 6 hours at 600 °C to produce TiO2/PCN nanocomposite.4.2. General procedure for the synthesis of 4H-pyrimido[2,1-b]benzimidazoles
A mixture of aromatic aldehyde, (1 mmol), ethyl acetoacetate (1 mmol, 0.13 g), 2-aminobenzimidazole (1 mmol, 0.133 g) and TiO2/PCN nanocomposite (3 mg) as a catalyst was added to 25 mL round-bottomed flask connected to a reflux condenser and stirred at 70 °C under solvent-free conditions. After the completion of the reaction, as monitored by TLC, the reaction mixture was extracted with warm ethanol (10 mL) and separated from the catalyst by simple filtration. Finally, the desired product was purified by the recrystallization in ethanol (90%).4.3. Selected spectral data of compounds
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 701; 1H-NMR: (250 MHz, DMSO-d6): δ (ppm) 1.07 (t, J = 7.50 Hz, 3H), 2.43 (s, 3H), 3.61 (s, 3H), 3.70 (s, 3H), 3.94 (q, J = 7.50 Hz, 2H), 6.58 (s, 1H), 6.72 (d, J = 7.50 Hz, 1H), 6.83 (d, J = 7.50 Hz, 2H), 6.91–7.02 (m, 2H), 7.29 (d, J = 7.50, 1H), 10.74 (s, 1H); 13CNMR: (62.5 MHz, DMSO-d6): δ (ppm); 14.3, 18.9, 51.7, 55.6, 56.4, 59.5, 96.8, 109.7, 112.8, 113.5, 115.5, 117.0, 120.4, 121.9, 130.9, 132.2, 142.6, 146.3, 147.4, 151.1, 153.3, 165.6.
701; 1H-NMR: (250 MHz, DMSO-d6): δ (ppm) 1.07 (t, J = 7.50 Hz, 3H), 2.43 (s, 3H), 3.61 (s, 3H), 3.70 (s, 3H), 3.94 (q, J = 7.50 Hz, 2H), 6.58 (s, 1H), 6.72 (d, J = 7.50 Hz, 1H), 6.83 (d, J = 7.50 Hz, 2H), 6.91–7.02 (m, 2H), 7.29 (d, J = 7.50, 1H), 10.74 (s, 1H); 13CNMR: (62.5 MHz, DMSO-d6): δ (ppm); 14.3, 18.9, 51.7, 55.6, 56.4, 59.5, 96.8, 109.7, 112.8, 113.5, 115.5, 117.0, 120.4, 121.9, 130.9, 132.2, 142.6, 146.3, 147.4, 151.1, 153.3, 165.6.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the Hamedan University of Technology and University of Kurdistan, for financial support to our research group.Notes and references
- S. Nalawade, V. Deshmukh and S. Chaudhari, J. Pharm. Res., 2013, 7, 433 CAS.
- M. T. Gabr, N. S. El-Gohary, E. R. El-Bendary and M. M. El-Kerdawy, Eur. J. Med. Chem., 2014, 85, 576 CrossRef CAS PubMed.
- P. H. Tran, T.-P. T. Bui, X.-Q. B. Lam and X.-T. T. Nguyen, RSC Adv., 2018, 8, 36392 RSC.
- M. S. Chaitanya, G. Nagendrappa and V. P. Vaidya, J. Chem. Pharm. Res., 2010, 2, 206 CAS.
- A. R. Moosavi-Zare, H. Goudarziafshar and P. Fashi, Res. Chem. Intermed., 2020, 46, 5567 CrossRef CAS.
- A. Khazaei, A. R. Moosavi-Zare, H. Afshar-Hezarkhani and V. Khakyzadeh, Eurasian Chem. Commun., 2020, 2, 27 CrossRef CAS.
- A. R. Moosavi-Zare and H. Afshar-Hezarkhani, Eurasian Chem. Commun., 2020, 2, 465 CrossRef CAS.
- A. R. Moosavi-Zare, H. Goudarziafshar, Z. Jalilian and F. Hosseinabadi, Chem. Methodol., 2022, 6, 571 CAS.
- A. Khazaei, F. Gohari-Ghalil, M. Tavasoli, M. Rezaei-Gohar and A. R. Moosavi-Zare, Chem. Methodol., 2020, 4, 543 CAS.
- N. Irannejad-Gheshlaghchaei, A. Zare, A. Banaei, H. Kaveh and N. Varavi, Chem. Methodol., 2020, 4, 400 CrossRef CAS.
- M. A. Zolfigol, S. Baghery, A. R. Moosavi-Zare and S. M. Vahdat, J. Mol. Catal. A: Chem., 2015, 409, 216 CrossRef CAS.
- A. Khazaei, H. A. Alavi Nik and A. R. Moosavi-Zare, J. Chin. Chem. Soc., 2015, 62, 675 CrossRef CAS.
- A. R. Moosavi-Zare, M. A. Zolfigol, E. Noroozizadeh, O. Khaledian and B. Shirmardi Shaghasemi, Res. Chem. Intermed., 2016, 42, 4759 CrossRef CAS.
- A. B. Atar, Y. S. Jeong and Y. T. Jeong, Tetrahedron, 2014, 70, 5207 CrossRef CAS.
- S. Azad and B. B. F. Mirjalili, RSC Adv., 2016, 6, 96928 RSC.
- P. K. Sahu, P. K. Sahu, S. K. Gupta and D. D. Agarwal, Ind. Eng. Chem. Res., 2014, 53, 2085 CrossRef CAS.
- S. A. Fazeli-Attar and B. B. Fatameh Mirjalili, Res. Chem. Intermed., 2018, 44, 6419 CrossRef CAS.
- H. R. Shaterian, N. Fahimi and K. Azizi, Res. Chem. Intermed., 2014, 40, 1879 CrossRef CAS.
- S. Azad and B. B. Fatameh Mirjalili, Res. Chem. Intermed., 2017, 43, 1723 CrossRef CAS.
- P. K. Sahu, P. K. Sahu, Y. Sharma and D. D. Agarwal, J. Heterocycl. Chem., 2014, 51, 1193 CrossRef CAS.
- A. Shaabani, A. Rahmati and S. Naderi, Bioorg. Med. Chem. Lett., 2005, 15, 5553 CrossRef CAS PubMed.
- N. Basirat, S. S. Sajadikhah and A. Zare, Res. Chem. Intermed., 2020, 46, 3263 CrossRef CAS.
- C. Yao, S. Lei, C. Wang, T. Li, C. Yu, X. Wang and S. Tu, J. Heterocycl. Chem., 2010, 47, 26 CAS.
- N. Shekarlab, R. Ghorbani-Vaghei and S. Alavinia, Appl. Organomet. Chem., 2020, e5918 CAS.
- M. Abedini, F. Shirini, M. Mousapour and O. Goli-Jolodar, Res. Chem. Intermed., 2016, 42, 6221 CrossRef CAS.
- X. B. Lu and D. J. Darensbourg, Chem. Soc. Rev., 2012, 41, 1462 RSC.
- C. Wang, J. Kim, J. Tang, J. Na, Y.-M. Kang, M. Kim, H. Lim, Y. Bando, J. Li and Y. Yamauchi, Angew. Chem., Int. Ed., 2020, 59, 2066 CrossRef CAS PubMed.
- G. Singh, K. S. Lakhi, S. Sil, S. V. Bhosale, I. Kim, K. Albahily and A. Vinu, Carbon, 2019, 148, 164 CrossRef CAS.
- Y. Chen, X. Zhang, W. Chen, H. Yang and H. Chen, Bioresour. Technol., 2017, 246, 101 CrossRef CAS PubMed.
- Y. Li, G. Wang, T. Wei, Z. Fan and P. Yan, Nano Energy, 2016, 19, 165 CrossRef CAS.
- X. Chen, S. Song, H. Li, G. Gözaydın and N. Yan, Acc. Chem. Res., 2021, 54, 1711 CrossRef CAS PubMed.
- K. Lee, Y. Jing, Y. Wang and N. Yan, Nat. Rev. Chem., 2022, 6, 635 CrossRef PubMed.
- M. Ding, X. Liu and J. Yao, New J. Chem., 2021, 45, 4147 RSC.
- Q. An, C. Liu, S. Deng, Y. Jiao, M. Tang, M. Yang, Z. Ye and B. Zhao, Chemosphere, 2022, 304, 135308 CrossRef CAS PubMed.
- M. Cao, C. Long, S. Sun, Y. Zhao, J. Luo and D. Wu, J. Energy Inst., 2021, 96, 90 CrossRef CAS.
- K.-L. Chang, S. C. Muega, B. I. G. Ofrasio, W.-H. Chen, E. G. Barte, R. R. M. Abarca and M. D. G. de Luna, Chemosphere, 2022, 291, 132829 CrossRef CAS PubMed.
- W. Xue, H. Zhao, J. Yao, F. Li and Y. Wang, Chin. J. Catal., 2016, 37, 769 CrossRef CAS.
- Z. Dolatkhah, A. Mohammadkhani, S. Javanshir and A. Bazgir, BMC Chem., 2019, 13, 97 CrossRef PubMed.
- M. Ding, S. Chen, X. Q. Liu, L. B. Sun, J. Lu and H. L. Jiang, ChemSusChem, 2017, 10, 1898 CrossRef CAS PubMed.
- C. Liu, C. Zhang, H. Song, X. Nan, H. Fu and G. Cao, J. Mater. Chem. A, 2016, 4, 3362 RSC.
- S. Tu, Q. Shao, D. Zhou, L. Cao, F. Shi and C. Li, J. Heterocycl. Chem., 2007, 44, 1401 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra00367a |
| This journal is © The Royal Society of Chemistry 2023 |