Open Access Article
Open Access ArticleEfficient and recyclable Nd3+-doped CoFe2O4 for boosted visible light-driven photocatalytic degradation of Rhodamine B dye†
Loan T. T. Nguyen a,
Hang T. T. Nguyenb,
Lan T. H. Nguyena,
Anh T. T. Duonga,
Hai Q. Nguyena,
Viet T. M. Ngoa,
Nhuong V. Vu
a,
Hang T. T. Nguyenb,
Lan T. H. Nguyena,
Anh T. T. Duonga,
Hai Q. Nguyena,
Viet T. M. Ngoa,
Nhuong V. Vu a,
Duyen Thi Cam Nguyencd and
Thuan Van Tran
a,
Duyen Thi Cam Nguyencd and
Thuan Van Tran *cd
*cd
aFaculty of Chemistry, Thai Nguyen University of Education, Thai Nguyen 240000, Vietnam
bFaculty of Automotive and Power Machinery Engineering, Thai Nguyen University of Technology, Thai Nguyen, 24000, Vietnam
cInstitute of Applied Technology and Sustainable Development, Nguyen Tat Thanh University, 298-300A Nguyen Tat Thanh, District 4, Ho Chi Minh City, 755414, Vietnam. E-mail: tranvt@ntt.edu.vn; ttran@gradcenter.cuny.edu; tranuv@gmail.com; Fax: (+84)-028-39-404-759; Tel: (+84)-028-3941-1211
dFaculty of Environmental and Food Engineering, Nguyen Tat Thanh University, 298-300A Nguyen Tat Thanh, District 4, Ho Chi Minh City 755414, Vietnam
First published on 11th April 2023
Abstract
Rare earth metal doping spinel ferrites offer excellent electronic, magnetic, and photocatalytic properties, but they have not been well explored for environmental mitigation. Herein, we report the facile fabrication of novel CoNdxFe2−xO4 (x = 0–0.05) photocatalysts based on Nd3+ incorporated into CoFe2O4 for the degradation of Rhodamine B under visible light irradiation. The Nd3+ dopant considerably increased the specific surface area (35 m2 g−1) and enhanced the degradation performance (94.7%) of CoNdxFe2−xO4 catalysts. Nd3+-doped CoFe2O4 played a role in the formation of radicals, including ˙OH, h+, and ˙O2−. With high recyclability and performance, CoNd0.05Fe1.95O4 nanoparticles can be efficient and reusable photocatalysts for degrading organic dyes, including Rhodamine B from wastewaters.
1. Introduction
Discharging the effluent containing organic dyes into water sources has been recently posing enormous impacts on aquatic ecosystems.1 The nature of dyes is water-soluble and low biodegradable, accumulating in durable wastewaters.2 Consequently, they increase chemical and biochemical oxygen demands, which depletes water-dissolved oxygen, thereby causing the biological disruption of the photosynthetic activities of aquatic microorganisms.3 The conventional methods of wastewater management, such as membrane filtration and coagulation processes, are mismatched with cost-effectiveness and performance.4 Therefore, an increasing demand for the development of effective treatment techniques has been addressed. The visible light-driven photocatalytic dye degradation process is proposed as a feasible method with the main advantages of treatment efficiency and technical simplicity.5Over the past years, nanostructured photocatalysts, including but not limited to TiO2, ZnO, and WO3, have observed significant growth and have been utilized for various applications in wastewater treatment, electrochemical, energy storage, and sensing.6,7 Ferrite-based semiconductor photocatalysts are of great interest owing to their unique electronic structure and magnetism.8 Among common ferrites, CoFe2O4 has a narrow band gap energy of 1.6–2.4 eV, making it capable of harvesting photons from visible light.9 Despite such potential, CoFe2O4 nanoparticles still show limited photocatalytic activity because rapid recombination between electrons and holes can significantly lower the catalytic performance of CoFe2O4.10 To address this issue, researchers have explored the use of CoFe2O4 with various rare earth dopants, such as Dy and Ce, to intercept this recombination and improve photocatalytic efficiency. Chen et al. reported the partial substitution of Fe3+ with Dy3+ to create a new lattice of CoFe2O4.11 These authors observed that Dy3+-doped CoFe2O4 exhibited good photocatalytic performance against methyl orange (78.7%). Zhu et al. synthesized Ce3+-doped CoFe2O4 using the hydrothermal method and found an orange II degradation percentage of 98.5% for 60 min.12 However, only few studies have investigated the introduction of rare earth metals into CoFe2O4 and their application in the photocatalytic removal of dyes.
Herein, we incorporated neodymium (Nd) into CoFe2O4 to enhance the photocatalytic efficiency of CoNdxFe2−xO4. Nd with an electronic configure ([Xe]4f46s2) is one of the most reactive lanthanides. It is hypothesized that Nd3+ substitution into the lattice of CoFe2O4 leads possibly to the formation of oxygen vacancies and surface defects, enhancing the electron transfer and hindering the recombination capability of electrons and holes.13 Although the synthesis and magnetic properties of CoNdxFe2−xO4 have been reported previously, the photocatalytic performance of this nanomaterial has not yet been investigated. Therefore, this study aims to synthesize and investigate photocatalytic CoNdxFe2−xO4 with different Nd3+ doping ratios from 0 to 5% by molar. The structure of the CoNdxFe2−xO4 nanocomposites was analyzed, and their photocatalytic activity was examined under visible light. In addition, the plausible mechanism of Rhodamine B degradation in the presence of CoNdxFe2−xO4/H2O2/visible light catalytic system was suggested.
2. Experimental
2.1. Synthesis of Nd-doped CoFe2O4 nanocomposites
Nd3+-doped CoFe2O4, other names CoNdxFe2−xO4 (x = 0, 0.01, 0.03, 0.05), could be facilely synthesized as follows. The process involved dissolving 500 mg of urea, 1 mmol of cobalt nitrate, (2−x) mmol of iron(III) nitrate and x mmol of neodymium nitrate in 30 mL of pure H2O. The liquid was heated to 100 °C under stirring, maintained at this temperature for 6 h, and dried in an oven overnight. The solid was calcined at 500 °C for 4 h to obtain the final CoNdxFe2−xO4 products.2.2. Photocatalytic experiments
To assess the efficiency of Nd3+-doped CoFe2O4 nanocomposites in photodegradation reaction, a visible-light source of 30 W provided by LED lamp emitting light within the wavelength range of 400–700 nm filtered by a UV filter, with a rate luminous flux of 2300 lm, and a color rendering index of 80 was used. The light source was fixed 12 cm higher than the RhB solution surface. To begin the experiment, a fixed amount (0.75 g L−1) of the CoNdxFe2−xO4 was added to 0.1 L of 10 mg L−1 Rhodamine B solution. Subsequently, a 30% hydroperoxide solution was slowly dropwised to reach a concentration of 0.1 M. After sorption in the dark was equilibrated for 60 min, the photocatalytic system was exposed to a visible light source for 0–180 min. The concentration of RhB was calibrated using a UV-Vis spectrophotometer at 554 nm. The effect of CoNdxFe2−xO4 dose on RhB degradation efficiency was examined at 0.5, 0.75, and 1 g L−1, while H2O2 concentration was tested at 0.05, 0.1, and 0.15 M.2.3. Recyclability study
After the catalytic experiment was completed, the CoNd0.05Fe1.95O4 catalyst was separated from the reaction and washed with pure water and ethanol to clean the Rhodamine B dye. Afterward, the reused CoNd0.05Fe1.95O4 was dried overnight in an oven and used as a catalyst for further experiments. In detail, 0.75 g L−1 of recycled CoNdxFe2−xO4 and 30% H2O2 with a concentration of 0.1 M were added to 0.1 L of 10 mg L−1 Rhodamine B solution. After 60 min of stirring in the dark, the photocatalytic system was exposed to a visible light source for 180 min. This process was repeated until the degradation efficiency against Rhodamine B dye was significantly reduced.3. Results and discussion
3.1. Characterization
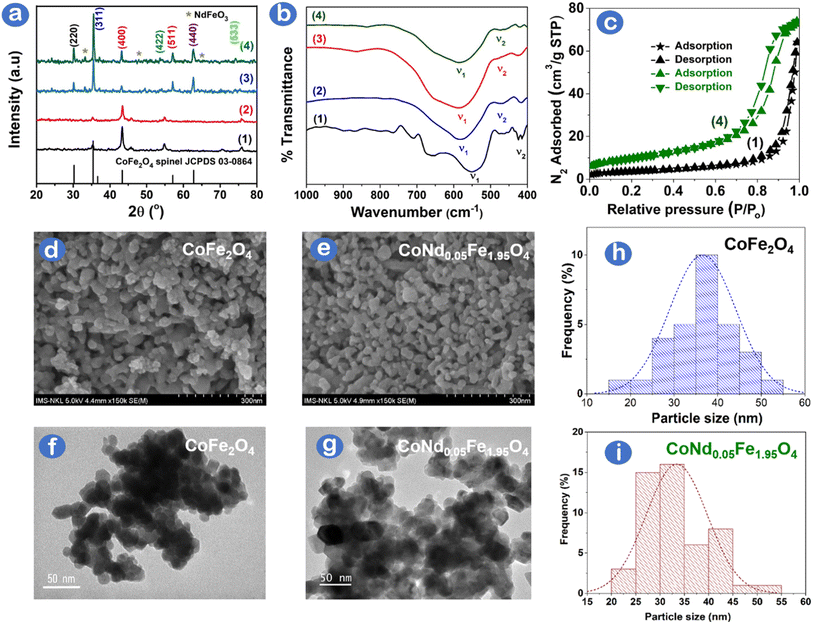
The X-ray diffraction patterns of CoNdxFe2−xO4 (x = 0, 0.01, 0.03, 0.05) nanoparticles are characterized, as shown in Fig. 1a. Diffraction patterns indicated the presence of well-defined peaks at 2θ degrees for the as-synthesized CoFe2O4, with lattice planes at 30.2° (220), 35.3° (331), 43.2° (400), 54.8° (422), 57.1° (511), and 62.7° (440) matching the standard pattern of spinel cubic CoFe2O4 structure as per JCPDS card no. 03-0864. The presence of ortho-ferrite NdFeO3 single phase became more prominent with increased Nd3+ dopants caused by the substitution of Fe3+ by Nd3+. Because Nd3+ ions have a larger radius than those of Fe3+, the atomic substitution in the CoFe2O4 lattice is restrained, resulting in the aggregation of Nd3+ on the grain boundary to form NdFeO3.14 As Fe3+ substituted by Nd3+ increased, the peak intensity at the (311) plane increased, and a slight shift in the (311) angle as a result of unstable d-spacing was observed in Table S1,† confirming the transfer of Nd3+ ions into octahedral and tetrahedral sites to replace Co2+/Fe3+ ions. CoNdxFe2−xO4 crystallite sizes were calculated based on the Scherrer equation for full width at half maximum of the (311) plane. Table S1† shows an upward crystallite size trend (14.35–29.14 nm) with increasing Nd3+ dopant, which suggests that Nd3+ substitution might extend unit cells, leading to increased lattice constants.The surface chemistry of Nd-doped CoFe2O4 can be examined using the FT-IR spectra, as illustrated in Fig. 1b. The two transmittance bands ν1 and ν2 shown in Table S1† reflect tetrahedral and octahedral metal (Fe, Nd)-oxygen bonds, respectively. Absorption bands shifting increasingly to the high-frequency band were attributable to altering lattice parameters and ionic redistribution as a result of the incorporation of rare earth-like Nd3+ into the spinel CoFe2O4 structure.15 The optical characteristics and Tauc plots of (αhν)2 versus photon energy (hν) of CoNdxFe2−xO4 can be evaluated using UV-Vis DRS spectra, as depicted in Fig. S1.† It is noticeable that the bandgap energy (Eg) decreased (1.57–1.35 eV) as the Nd3+ doping (0–5%) increased, confirming the change in the electric structure of CoNdxFe2−xO4 a result of the bandgap renormalization effect.16 Specifically, a dynamic screening of Coulomb repulsion may decrease the electronic bandgap of the CoNdxFe2−xO4 semiconductor as it partially cancels the Moss–Burstein shift.17 The result contradicted an enhanced bandgap caused by the quantum confinement effect as reported previously.18 Moreover, magnetic properties of CoNdxFe2−xO4 via vibrating sample magnetometer showed that magnetic hysteresis loops were smaller and saturation magnetization values decreased from 47 to 29 emu g−1 with higher Nd3+ doping in CoFe2O4 (Fig. S2†). The magnetic results herein agree with the previous report,19 which can be normally due to the lower paramagnetic moment of substituted Nd3+ ions than ferromagnetic Fe3+ ions.
Fig. 1c shows N2 adsorption/desorption isotherm plots of CoFe2O4 and CoNd0.05Fe1.95O4 with the characteristics of Type II (nonporous or macroporous) based on IUPAC classification. CoFe2O4. However, a minor hysteresis loop (H2) of CoNd0.05Fe1.95O4 rather than CoFe2O4 was observed, indicating a disordered, well-undefined and defected interface of CoNd0.05Fe1.95O4.20 Incorporating Nd3+ ions into CoFe2O4 lattice caused the redistribution of Co2+/Fe3+ cations and defected crystal lattice.21 The specific surface area of CoNd0.05Fe1.95O4 was 35.0 m2 g−1 compared with 12.7 m2 g−1 for CoFe2O4 (Table S2†). The total pore volume of CoNd0.05Fe1.95O4 (0.114 cm3 g−1) was also higher than that of CoFe2O4 (0.099 cm3 g−1), suggesting that Nd3+ substitution improved the porosity of the origin spinel ferrite. It was interpreted that doping of neodymium elements might optimize the surface structure of cobalt ferrite, leading to a decrease in grain size with an enhanced porous structure and surface area. The substitution of other rare earth metals (e.g., Ce3+, La3+, and Y3+) into CoFe2O4 was also found to significantly increase the surface area (4.39–6.95 m2 g−1) of rare earth-modified CoFe2O4 compared with bare CoFe2O4 (2.13 m2 g−1) as reported by Gao et al.22 With higher area and defected surface, CoNd0.05Fe1.95O4 was expected to have more active sites for photocatalytic performance.
The morphology and inherent structure of CoFe2O4 and CoNd0.05Fe1.95O4 are examined by SEM/TEM images, as illustrated in Fig. 1d–g. CoFe2O4 and CoNd0.05Fe1.95O4 exhibit nanospherical nanoparticles, with a slight degree of clustering, which was highly commensurate with the morphology of Nd3+ doped CoFe2O4 as published previously.21,23 A minor decrease in average particle size from 35 nm (CoFe2O4) to 30 nm (CoNd0.05Fe1.95O4) is shown in Fig. 1h and g. This trend can be explained that Nd3+ incorporation might intercept the crystal growth of CoFe2O4 during combustion synthesis. Fig. S3† illustrates the chemical composition of nanocomposites with three major elements (11.44% Co, 26.94% Fe, and 61.62% O) for CoFe2O4 and four major elements (13.41% Co, 21.34% Fe, 64.59% O, and 0.66% Nd) for CoNd0.05Fe1.95O4. Otherwise, no unusual peaks in the EDX spectra suggest that the samples are highly pure.
3.2. Photocatalytic study
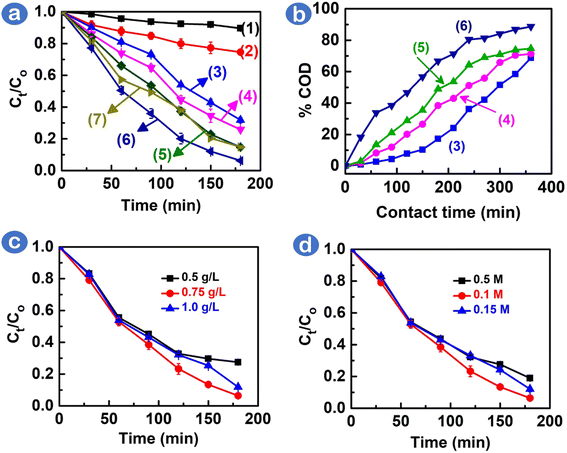
The catalytic photodegradation of Rhodamine B dye as a test substance in the presence of CoNdxFe2−xO4 (x = 0, 0.01, 0.03, and 0.05) nanoparticles was conducted, as depicted in Fig. 2a. As depicted in Table S3,† the Rhodamine B removal percentage was almost insufficient (12.9–29.4%) in the absence of either the CoFe2O4 catalyst or H2O2 oxidant under visible light. The reaction rates were also slow (0.7 × 10−3–1.4 × 10−3 min−1) in these cases, indicating that Rhodamine B dye could be removed owing to adsorption onto CoFe2O4. When both 0.1 M H2O2 and CoFe2O4 catalysts were added under visible light conditions, 71.7% of Rhodamine B dye was removed by CoFe2O4. It is therefore believed that the catalyst, oxidant, and visible light source played a necessary role in the photocatalytic degradation of Rhodamine B dye. With increasing ratios of Nd3+ doping (0–0.05), the catalysts exhibited improved catalytic activity for the degradation of Rhodamine B dye (68.2–94.7%). The highest degradation efficiency and kinetic rate were observed under a CoNd0.05Fe1.95O4/H2O2/visible light system at 94.7% for 180 min and 5.3 × 10−3 min−1, respectively. With a higher doping of Nd3+ (x = 0.01), however, the CoNd0.1Fe1.9O4 showed lower catalytic activity, with 85.1% of RhB degradation efficiency. This finding could, therefore, be because of the contribution of 5% Nd3+ substituted in CoFe2O4 lattice that is enough for accelerating electron transfer and prevention of electron–hole recombination.To assess the amount of oxygen necessary to oxidize the organic matter in aquatic wastewaters, the chemical oxygen demand (COD) index was measured. The results of the photocatalytic study of CoNdxFe2−xO4 nanocomposites on the removal of Rhodamine B dye are shown in Fig. 2b. The COD percentage obtained after 360 min is as follows: CoFe2O4 (71.7%) < CoNd0.01Fe1.99O4 (76.5%) < CoNd0.03Fe1.97O4 (81.2%) < CoNd0.05Fe1.95O4 (89.5%). The best catalyst, CoNd0.05Fe1.95O4, was able to significantly reduce the COD value of the Rhodamine B wastewater sample from 394.7 mg L−1 to 41.0 mg L−1. This result demonstrates an efficient Rhodamine B mineralization of CoNd0.05Fe1.95O4 into less toxic compounds, such as CO2, H2O, and N2.24,25 Several photocatalytic degradation pathways for the mineralization of Rhodamine B could be deethylation, ring opening, and degradation of aryl chromophore.26 Overall, our results indicated a good degree of RhB dye mineralization catalyzed by CoNd0.05Fe1.95O4, which can make dyes-containing wastewater cleaner and safer.
Several factors (e.g., catalyst loading and H2O2 concentration) can influence the visible light photocatalytic degradation reaction of Rhodamine B. Fig. 2c shows that the optimal amount of CoNd0.05Fe1.95O4 catalyst was 0.75 g L−1, which led to a 93.4% removal efficiency of the Rhodamine B dye. Furthermore, the influence of H2O2 concentration was studied, with oxidant concentrations ranging from 0.05 to 0.15 M. Fig. 2d depicts that 0.1 M H2O2 concentration was found to be optimal, as this oxidant provided a sufficient amount of reactive oxygen radicals for the degradation of dyes.
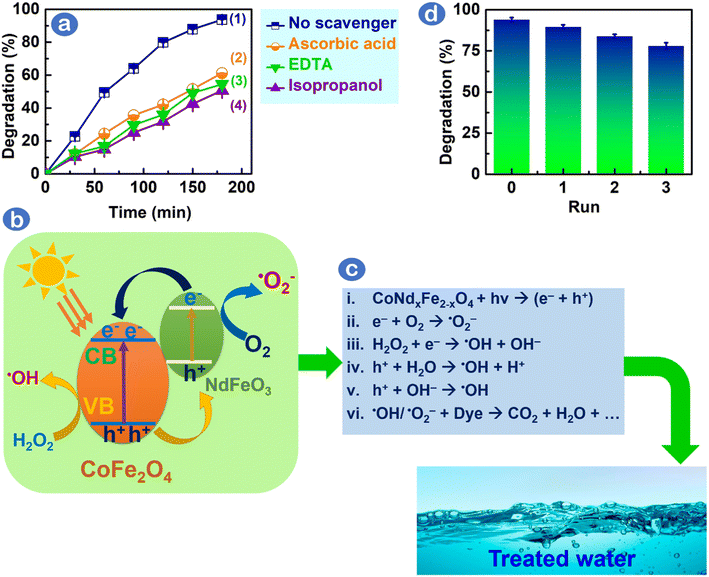
Scavenging experiments can be performed to comprehend the significance of radicals, such as ˙OH and ˙O2−, under CoNd0.05Fe1.95O4/H2O2/visible-light system. Fig. 3a illustrates that the Rhodamine B degradation efficiencies of Rhodamine B dye over CoNd0.05Fe1.95O4 catalyst were considerably reduced (49.8–61.6%) as one of the scavengers for trapping radicals, e.g., ascorbic acid (˙O2−), ethylenediaminetetraacetic acid (h+), and isopropyl alcohol (˙OH), was added into the reaction. Table S4† shows a comparison of degradation efficiencies and pseudo-first order kinetic rates (k1) as follows: EDTA (49.8%, 2.7 × 10−3 min−1) < isopropyl alcohol (52.1%, 2.9 × 10−3 min−1) < ascorbic acid (61.6%, 3.4 × 10−3 min−1) < no scavengers (93.7%, 5.3 × 10−3 min−1). Because the degradation efficiency did not differ significantly under various scavengers, radical species, including ˙OH, ˙O2−, and h+, might take main responsibility for the degradation of Rhodamine B.
The role of radicals (˙OH, ˙O2−, and h+) on Rhodamine B degradation catalyzed by CoNd0.05Fe1.95O4 under visible light is elucidated herein, as demonstrated in Fig. 3b. Initially, incorporating Nd3+ into CoFe2O4 changes the electronic density and metal–oxygen bonding energy in CoFe2O4 crystal lattice, thereby exerting the formation of oxygen vacancies and defected surface on CoNd0.05Fe1.95O4 photocatalyst.23 Partially filled f-orbitals of Nd3+ ions (Nd3+/Nd2+, E0 = −0.40 V vs. NHE) enable visible light-photoexcited electrons to transfer easily from the valence band of CoFe2O4 to the Nd3+ doping energy level in NdFeO3.27 Consequently, Nd3+ f-orbitals might hinder the photogenerated e–/h+ pair recombination, thereby improving degradation performance. Fig. 3c illustrates the interaction between e– and O2 to create ˙O2− and between e– and H2O2 to create ˙OH. Holes (h+) interact with H2O/OH− to form ˙OH. It is suggested that ˙OH and ˙O2− species reacted with Rhodamine B to degrade into mediators, fragments and final products, such as H2O and CO2. To assess the stability of CoNd0.05Fe1.95O4, we examined Rhodamine B degradation efficiency after the photocatalytic cycle. As shown in Fig. 3d, CoNd0.05Fe1.95O4 can be reused several times, and the final cycle showed a degradation efficiency of 78%, suggesting that this catalyst had high stability. To check the crystalline structure of the CoNd0.05Fe1.95O4 catalyst after use, XRD patterns were examined, as depicted in Fig. S4.† The primary peaks at (220), (311), (400), (422), (511), (440), and (533) were still maintained, suggesting that the CoNd0.05Fe1.95O4 structure was stable after the recycling process.
The photocatalytic efficiency of CoNd0.05Fe1.95O4 can be compared with other catalysts. As depicted in Table 1, CoNd0.05Fe1.95O4 in this study demonstrated higher dye degradation performance (94.7%) than other photocatalysts. Moreover, this reaction was conducted under mild conditions, i.e., a visible light source instead of a UV light condition, as reported in previous studies. This comparison suggests that the CoNd0.05Fe1.95O4 could be a competitive photocatalyst for degrading hazardous dyes, such as Rhodamine B, in wastewater under visible light condition.
| No. | Nanocomposite | Light source | H (%) | Ref. |
|---|---|---|---|---|
| 1 | CoNd0.05Fe1.95O4 | Visible light | 94.7 | This work |
| 2 | ZnFe2O4-50%@ZnO | Visible light | 79 | 28 |
| 3 | NiFe2O4@HAp-Sn2+ | Visible light | 84.4 | 29 |
| 4 | NiFe2O4 | Visible light | 90 | 30 |
| 5 | ZnFe@CuS | Visible light | 93 | 31 |
| 6 | ZnFe2O4 | Visible light | 94 | 30 |
| 7 | MIL-101(Cr)/RGO/ZnFe2O4 | Visible light | 94 | 24 |
| 8 | ZnFe2O4/graphene oxide | Visible light | 94 | 32 |
| 9 | Ni0.5Zn0.5Fe2O4 | Visible light | 98 | 30 |
| 10 | Zn-doped Fe3O4 | UV light | 97 | 33 |
| 11 | NiFe2O4 | UV light | 84 | 34 |
| 12 | AuSe QDs@Cs2Fe2O4 | UV light | 99.2 | 35 |
| 13 | Mg0.4Zn(0.6−xx)CaxFe2O4 | UV light | 99.5 | 36 |
| 14 | Chitin biochar-based ZnFe2O4 | Solar light | 100 | 37 |
4. Conclusion
Novel CoNdxFe2−xO4 catalysts with various amounts of Nd3+ dopant were synthesized and characterized. Nd3+ substitution into CoFe2O4 lattice changed its electronic and magnetic structure. Bandgaps and saturation magnetization decreased as the Nd3+ doping increased. Moreover, CoNd0.05Fe1.95O4 obtained small particle sizes and a large surface area (35 m2 g−1). The highest degradation efficiency was obtained (94.7%) at 0.75 g L−1 of CoNd0.05Fe1.95O4 and 0.1 M H2O2 under visible light irradiation. The main radical species, such as ˙OH, h+, and ˙O2−, were found to influence the mechanism of Rhodamine B dye degradation. Additionally, the catalyst demonstrated good stability and recyclability. These results suggest that CoNd0.05Fe1.95O4 nanoparticles can be an efficient and reusable photocatalyst for degrading organic dyes in water.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This research is funded by Thai Nguyen University of Education under grant number TNUE-2022-05.References
- R. Al-Tohamy, S. S. Ali, F. Li, K. M. Okasha, Y. A. G. Mahmoud, T. Elsamahy, H. Jiao, Y. Fu and J. Sun, Ecotoxicol. Environ. Saf., 2022, 231, 113160 CrossRef CAS PubMed.
- T. V. Tran, D. T. C. Nguyen, P. S. Kumar, A. T. M. Din, A. S. Qazaq and D.-V. N. Vo, Environ. Res., 2022, 214, 113925 CrossRef CAS PubMed.
- M. Shabir, M. Yasin, M. Hussain, I. Shafiq, P. Akhter, A.-S. Nizami, B.-H. Jeon and Y.-K. Park, J. Ind. Eng. Chem., 2022, 112, 1–19 CrossRef CAS.
- R. Yadav, T. S. Chundawat, P. K. Surolia and D. Vaya, J. Phys. Chem. Solids, 2022, 165, 110691 CrossRef CAS.
- T. Wang, J. Zheng, J. Cai, Q. Liu and X. Zhang, Sci. Total Environ., 2022, 839, 155955 CrossRef CAS PubMed.
- J. Lincho, A. Zaleska-Medynska, R. C. Martins and J. Gomes, Sci. Total Environ., 2022, 837, 155776 CrossRef CAS PubMed.
- F. Siddique, S. Gonzalez-Cortes, A. Mirzaei, T. Xiao, M. A. Rafiq and X. Zhang, Nanoscale, 2022, 14, 11806–11868 RSC.
- H. S. Jarusheh, A. Yusuf, F. Banat, M. A. Haija and G. Palmisano, J. Environ. Chem. Eng., 2022, 10, 108204 CrossRef CAS.
- J. Chen, Y. Wang and Y. Deng, J. Alloys Compd., 2013, 552, 65–69 CrossRef CAS.
- B. Aslibeiki, N. Eskandarzadeh, H. Jalili, A. Ghotbi Varzaneh, P. Kameli, I. Orue, V. Chernenko, A. Hajalilou, L. P. Ferreira and M. M. Cruz, Ceram. Int., 2022, 48, 27995–28005 CrossRef CAS.
- S. Chen, D. Jiang, G. Zeng, H. Chi, L. Li, Y. He, F. Ke, J. D. Xiao and S. Ye, Mater. Lett., 2021, 284, 128966 CrossRef CAS.
- F. Zhu, Q. Ji, Y. Lei, J. Ma, Q. Xiao, Y. Yang and S. Komarneni, Chemosphere, 2022, 291, 132765 CrossRef CAS PubMed.
- F. Sharifianjazi, M. Moradi, N. Parvin, A. Nemati, A. Jafari Rad, N. Sheysi, A. Abouchenari, A. Mohammadi, S. Karbasi, Z. Ahmadi, A. Esmaeilkhanian, M. Irani, A. Pakseresht, S. Sahmani and M. Shahedi Asl, Ceram. Int., 2020, 46, 18391–18412 CrossRef CAS.
- R. S. Yadav, J. Havlica, J. Masilko, L. Kalina, J. Wasserbauer, M. Hajdúchová, V. Enev, I. Kuřitka and Z. Kožáková, J. Magn. Magn. Mater., 2016, 399, 109–117 CrossRef CAS.
- Z. A. Gilani, M. F. Warsi, M. N. Anjum, I. Shakir, S. Naseem, S. Riaz and M. A. Khan, J. Alloys Compd., 2015, 639, 268–273 CrossRef CAS.
- M. M. Ugeda, A. J. Bradley, S.-F. Shi, F. H. da Jornada, Y. Zhang, D. Y. Qiu, W. Ruan, S.-K. Mo, Z. Hussain, Z.-X. Shen, F. Wang, S. G. Louie and M. F. Crommie, Nat. Mater., 2014, 13, 1091–1095 CrossRef CAS PubMed.
- P. D. Cunningham, A. T. Hanbicki, K. M. McCreary and B. T. Jonker, ACS Nano, 2017, 11, 12601–12608 CrossRef CAS PubMed.
- A. M. S. Arulanantham, K. V. Gunavathy, M. Antony, N. Sundaramurthy, M. M. Stephy, P. Mohanraj and V. Ganesh, Chem. Pap., 2022, 76, 6349–6358 CrossRef CAS.
- M. A. Ahmed, N. Okasha, A. A. Mohamed and I. Mmdouh, J. Magn. Magn. Mater., 2014, 358–359, 32–37 CrossRef CAS.
- A. Svidrytski, D. Hlushkou, M. Thommes, P. A. Monson and U. Tallarek, J. Phys. Chem. C, 2020, 124, 21646–21655 CrossRef CAS.
- M. A. Almessiere, Y. Slimani, S. Güner, M. Nawaz, A. Baykal, F. Aldakheel, S. Akhtar, I. Ercan, I. Belenli and B. Ozçelik, Ceram. Int., 2019, 45, 8222–8232 CrossRef CAS.
- J. Gao, G. Pu, C. Yuan, M. Gao, X. Lu and S. Jia, Fuel, 2022, 326, 124933 CrossRef CAS.
- K. L. Routray, S. Saha, D. Sanyal and D. Behera, Mater. Res. Express, 2019, 6, 026107 CrossRef.
- L. Nirumand, S. Farhadi, A. Zabardasti and A. Khataee, Ultrason. Sonochem., 2018, 42, 647–658 CrossRef CAS PubMed.
- A. H. Mady, M. L. Baynosa, D. Tuma and J. J. Shim, Appl. Catal., B, 2017, 203, 416–427 CrossRef CAS.
- L. T. T. Nguyen, H. T. T. Nguyen, L. T. H. Nguyen, A. T. T. Duong, H. Q. Nguyen, N. D. Bui, V. T. M. Ngo, D. T. C. Nguyen and T. V. Tran, Environ. Res., 2022, 214, 114130 CrossRef CAS PubMed.
- G. L. Colpani, R. C. F. Zeferino, M. Zanetti, J. M. M. Mello, L. L. Silva and M. A. Fiori, in Photocatalytic Systems by Design: Materials, Mechanisms and Applications, Elsevier, 2021, pp. 23–53 Search PubMed.
- L. T. T. Nguyen, D.-V. N. Vo, L. T. H. Nguyen, A. T. T. Duong, H. Q. Nguyen, N. M. Chu, D. T. C. Nguyen and T. V. Tran, Environ. Technol. Innovation, 2022, 25, 102130 CrossRef CAS.
- K. C. Das, S. S. Dhar, D. G. Thakurata and J. Das, J. Cleaner Prod., 2021, 290, 125172 CrossRef CAS.
- S. A. Jadhav, M. V. Khedkar, D. D. Andhare, S. B. Gopale and K. M. Jadhav, Ceram. Int., 2021, 47, 13980–13993 CrossRef CAS.
- M. Shakil, U. Inayat, M. Ashraf, M. Tanveer, S. S. A. Gillani and A. Dahshan, Optik, 2023, 272, 170353 CrossRef CAS.
- N. Nadeem, M. Zahid, A. Tabasum, A. Mansha, A. Jilani, I. A. Bhatti and H. N. Bhatti, Mater. Res. Express, 2020, 7, 15519 CrossRef CAS.
- A. Manohar, K. Chintagumpala and K. H. Kim, J. Mater. Sci.: Mater. Electron., 2021, 32, 8778–8787 CrossRef CAS.
- K. R. Sanadi, K. C. Rathod, M. L. Gaur, R. R. Powar, V. G. Parale, R. S. Patil, S. H. Burungale and A. V. Mali, Bull. Mater. Sci., 2021, 44, 265 CrossRef CAS.
- F. T. Alshorifi, A. A. Alswat and R. S. Salama, Heliyon, 2022, 8, e09652 CrossRef CAS PubMed.
- S. Kumari, N. Dhanda, A. Thakur, V. Gupta, S. Singh, R. Kumar, S. Hameed and P. Thakur, Ceram. Int., 2023, 49(8), 12469–12480 CrossRef CAS.
- N. Welter, J. Leichtweis, S. Silvestri, P. I. Z. Sánchez, A. C. C. Mejía and E. Carissimi, J. Alloys Compd., 2022, 901, 163758 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra00971h |
| This journal is © The Royal Society of Chemistry 2023 |