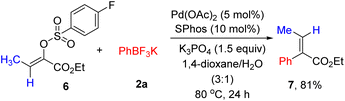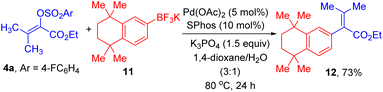Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
α-Arylsulfonyloxyacrylates: attractive O-centered electrophiles for synthesis of α-substituted acrylates via Pd-catalysed Suzuki reactions†‡
Zhongya Zhanga,
Li Zhang*d,
Linge Huaie,
Zhentao Wang*e and
Yewen Fang *abc
*abc
aKey Laboratory of Degraded and Unused Land Consolidation Engineering, The Ministry of Land and Resources of China, College of Environmental Science and Engineering, Chang'an University, No. 126 Yanta Road, Xi'an 710054, China
bSchool of Materials and Chemical Engineering, Ningbo University of Technology, No. 201 Fenghua Road, Ningbo 315211, China. E-mail: fang@nbut.edu.cn
cZhejiang Institute of Tianjin University, No. 201 Fenghua Road, Ningbo 315211, China
dSchool of Fundamental Science, Zhejiang Pharmaceutical University, No. 666 Siming Road, Ningbo 315500, China. E-mail: zhangl@zjpu.edu.cn
eCollege of Chemistry and Material Science, Shandong Agricultural University, No. 61 Daizong Road, Tai'an 271018, China. E-mail: wzht423@mail.ustc.edu.cn
First published on 20th March 2023
Abstract
We herein report α-arylsulfonyloxyacrylates as a kind of useful and attractive O-centered electrophiles for Suzuki cross-coupling reactions. A range of α-(hetero)aryl substituted acrylates has been prepared via the palladium-catalysed C–C cross-coupling reactions between potassium (hetero)aryltrifluoroborates and α-arylsulfonyloxyacrylates. Moreover, α-arylsulfonyloxyacrylate could also react with B-alkyl-9-BBN to produce α-alkyl substituted acrylates. The synthetic application of this new method was demonstrated by the preparation of the intermediate for synthesis of retinoid X receptors-selective retinoids. These Suzuki reaction-based protocols feature broad substrate scope, generality, and mild reaction conditions.
Introduction
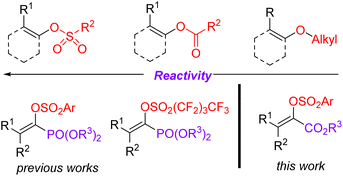
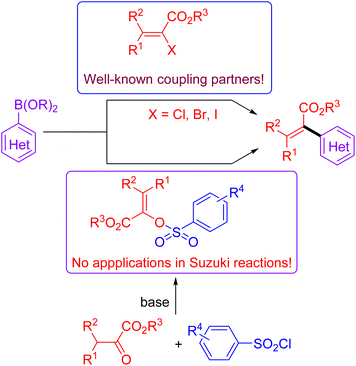
The Suzuki cross-coupling reaction is considered to be one of the most robust methods in modern organic synthesis, providing a rapid and straightforward strategy for constructing C–C bond formation.1 Taking advantage of the easy availability of coupling partners as well as their stabilities towards air and moisture, the synthetic community would like to choose the Suzuki reaction as their choice for C–C bond formation.2 Due to the marvelous progress on the supporting ligands3 and the preparation of organoboron derivatives,4 protocols based on Suzuki reactions become more reliable and practical. Moreover, the applications of non-noble metal catalysts5 and the use of continuous-flow reactors for Suzuki reactions meet the requirement of sustainable development of chemistry.6 Of note, the development of reliable electrophiles has also been the subject of Suzuki cross-coupling reactions.7 As for the available electrophilic Suzuki coupling partners, the utilization of C–O electrophiles as surrogates for organic halides is especially attractive due to their flexibility and generality as well as practicality. Compared to the many well-documented methods for synthesis of aromatic halides, the site-specific preparation of alkenyl halides with expected configuration is still a big challenge. Consequently, there has been increasing interest on the Suzuki cross-coupling reactions using enol-based compounds as the coupling electrophiles. Among the many enol-derived electrophiles, alkenyl sulfonates are especially attractive due to their good stability and high reactivity (Fig. 1).8Acrylates and their derivatives are a kind of fundamental monomers and structural motifs. In addition to the wide applications in polymer chemistry, α-substituted acrylates are useful acceptors for both nucleophiles and nucleophilic radicals.9 As a result, many efforts have been devoted to their preparation and the further transformations. Among the available strategies, transition-metal-catalysed cross-coupling reactions are undoubtedly indispensable tools. Interestingly, an access to α,β-unsaturated esters has been realized via palladium-catalysed reactions using diazo compounds as the coupling partners.10 Not surprisingly, the Suzuki reaction-based protocols for the preparation of α-substituted acrylates have been extensively investigated.11 In contrast to the applications of α-halo acrylates in Suzuki reactions, there is still no available reports dealing with the preparation of α-substituted acrylates using O-centered coupling electrophiles (Scheme 1). Inspired by our recent works on the Suzuki cross-coupling reactions using α-phosphonovinyl arylsulfonates as the electrophilic coupling partners (Fig. 1),12 we wonder that α-arylsulfonyloxyacrylates could serve as the O-centered electrophile candidate. According to the available reports, α-arylsulfonyloxyacrylates could be easily prepared from inexpensive aryl sulfonyl chloride with pyruvate derivatives in presence of base. Moreover, in addition to environmentally benign character, the α-arylsulfonyloxyacrylates would be more stable than α-haloacrylates coupling partners. We herein report a new protocol for synthesis of α-(hetero)aryl acrylates via the Suzuki reactions between arylsulfonyloxyacrylates and potassium (hetero)aryltrifluoroborates enabled by palladium catalysis.13 Moreover, α-alkyl acrylates were also prepared via the palladium catalysed C–C cross-coupling reactions of α-arylsulfonyloxyacrylates with B-alkyl-9-BBN.14
Results and discussion
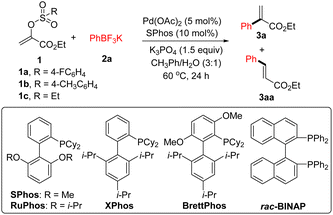
We began our studies by investigating the Suzuki coupling reaction of potassium phenyltrifluoroborate 2a with 1a derived from ethyl pyruvate and 4-fluorobenzenesulfonyl chloride. As outlined in Table 1, a simple survey of experimental parameters led us to identify the optimal reaction conditions (5 mol% Pd(OAc)2, 10 mol% SPhos, 1.5 equiv. of K3PO4, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixed CH3Ph/H2O, 60 °C, 24 h) (entry 1).15 Other Buchwald-type ligands were screened and no better result was observed (entries 2–4). The combination of Pd(OAc)2 and rac-BINAP did not lead to the expected product formation (entry 5). With tricyclohexylphosphine as the supporting ligand, a mixture of 3a and 3aa was observed (entry 6). Possibly, the side product 3aa would be formed when the β-hydride elimination pathway took place ahead of transmetallation process.16 Again, we found that a mixture of 3a and 3aa was achieved using Pd(OAc)2, t-Bu3PH·BF4, and K3PO4 (entry 7). Other inorganic bases such as Cs2CO3, K2CO3, and t-BuOK gave inferior results (entries 8–10). Moreover, no better results were observed using other palladium catalysts (entries 11 and 12). As for the electrophiles, α-tosyloxyacrylate 1b could serve as the alternative, albeit in low yield (entry 13). However, no desired product could be observed using α-ethylsulfonyloxyacrylate 1c as the coupling partner (entry 14). With phenylboronic acid instead of 2a as the nucleophilic coupling partner, the desired product 3a was contaminated by the formation of 3aa (entry 15). Similar result was achieved using phenylboronic acid pinacol ester as the coupling partner (entry 16). In the absence of base, treatment of 1a with 2a under the palladium catalysed reaction conditions gave 3a in 49% yield (entry 17). Control experiments confirmed that both ligand and catalyst were required for this transformation (entries 18 and 19).
1 mixed CH3Ph/H2O, 60 °C, 24 h) (entry 1).15 Other Buchwald-type ligands were screened and no better result was observed (entries 2–4). The combination of Pd(OAc)2 and rac-BINAP did not lead to the expected product formation (entry 5). With tricyclohexylphosphine as the supporting ligand, a mixture of 3a and 3aa was observed (entry 6). Possibly, the side product 3aa would be formed when the β-hydride elimination pathway took place ahead of transmetallation process.16 Again, we found that a mixture of 3a and 3aa was achieved using Pd(OAc)2, t-Bu3PH·BF4, and K3PO4 (entry 7). Other inorganic bases such as Cs2CO3, K2CO3, and t-BuOK gave inferior results (entries 8–10). Moreover, no better results were observed using other palladium catalysts (entries 11 and 12). As for the electrophiles, α-tosyloxyacrylate 1b could serve as the alternative, albeit in low yield (entry 13). However, no desired product could be observed using α-ethylsulfonyloxyacrylate 1c as the coupling partner (entry 14). With phenylboronic acid instead of 2a as the nucleophilic coupling partner, the desired product 3a was contaminated by the formation of 3aa (entry 15). Similar result was achieved using phenylboronic acid pinacol ester as the coupling partner (entry 16). In the absence of base, treatment of 1a with 2a under the palladium catalysed reaction conditions gave 3a in 49% yield (entry 17). Control experiments confirmed that both ligand and catalyst were required for this transformation (entries 18 and 19).
| Entry | Deviation from standard conditions | Yield of 3a (%) |
|---|---|---|
| a Standard reaction conditions: a reaction mixture of 1a (0.2 mmol), 2a (0.26 mmol), Pd(OAc)2 (5 mol%), SPhos (10 mol%), K3PO4 (0.3 mmol), and CH3Ph/H2O (3.0 mL/1.0 mL) was stirred at 60 °C for 24 h.b Yield of the isolated product 3a.c NMR yield (500 MHz) was reported by use of p-nitroacetophenone as an internal standard.d NMR yields of the 3aa were reported in the brackets. | ||
| 1 | None | 73 |
| 2 | RuPhos instead of SPhos | 49 |
| 3 | XPhos instead of SPhos | 67 |
| 4 | BrettPhos instead of SPhos | 27 |
| 5c | rac-BINAP instead of SPhos | 0 |
| 6c,d | Cy3PH·BF4 instead of SPhos | 36 (12) |
| 7c,d | t-Bu3PH·BF4 instead of SPhos | 37 (28) |
| 8c,d | Cs2CO3 instead of K3PO4 | 52 (7) |
| 9c,d | K2CO3 instead of K3PO4 | 52 (9) |
| 10 | t-BuOK instead of K3PO4 | 52 |
| 11 | PdCl2 instead of Pd(OAc)2 | 62 |
| 12 | Pd2(dba)3 instead of Pd(OAc)2 | 45 |
| 13 | 1b instead of 1a | 37 |
| 14c | 1c instead of 1a | 0 |
| 15c,d | PhB(OH)2 instead of 2a | 40 (20) |
| 16c,d | PhBpin instead of 2a | 31(16) |
| 17 | Without base | 49 |
| 18c | Without ligand | 0 |
| 19c | Without catalyst | 0 |
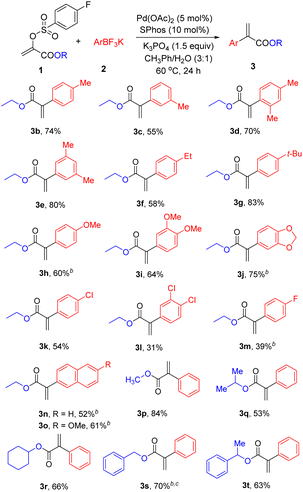
After the optimal reaction conditions were established for this Pd-catalysed Suzuki reactions, the scope of aryl trifluoroborates and α-arylsulfonyloxyacrylates was initially tested (Table 2). A range of potassium aryltrifluoroborate was firstly investigated. Generally, both electron-rich and electron-deficient aryl trifluoroborates proved viable coupling partners, giving 3b–3o in 31–83% yields. Notably, steric variance on the phenyl ring did not lead to an obvious influence on the reaction efficiency. The aryl trifluoroborates having para- (3b), meta- (3c, 3e), or ortho- (3d) substituents were all eligible to forge the desired ethyl α-aryl acrylates. Pleasingly, the cross-coupling reactions of substituted aryl trifluoroborates (-ethyl, -tert-butyl, and -methoxyl) worked well to give the desired products 3f–3j in 58–83% yields. Noticeably, chloro substituent is well tolerated in this reaction conditions (3k and 3l), which is advantageous for the further decoration. Likewise, potassium 4-fluorophenyltrifluoroborate could be coupled with 1a using this new protocol, obtaining 3m in moderate yield. Moreover, aryl trifluoroborates bearing a naphthalene ring can be transformed to the corresponding products 3n and 3o in 52% and 61% yields, respectively. After the evaluation of scope of aryl trifluoroborates, various alkyl α-phenylacrylates were prepared under the standard cross-coupling reaction conditions. In addition to efficient access to methyl and iso-propyl α-phenylacrylates (3p, 3q), cyclohexyl α-phenylacrylate (3r) could be nicely prepared in 66% yield. Additionally, benzyl α-phenylacrylates (3s, 3t) were also successfully prepared in efficient manner.
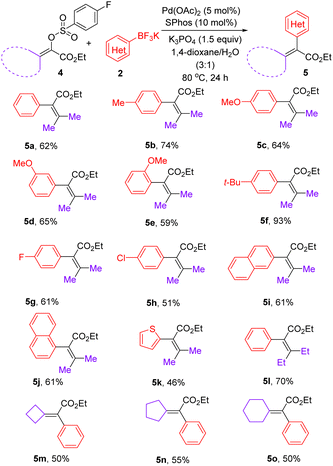
After realizing the synthesis of α-aryl acrylates, we set out to prepare tetrasubstituted α,β-unsaturated esters under the above optimized catalyst system. As shown in Table 3, a range of β,β-disubstituted-α-aryl acrylates could be synthesized via the reactions between β,β-disubstituted-α-arylsulfonyloxyacrylates and aryl trifluoroborates enabled by palladium catalysis. Due to the possible steric effect, increasing the reaction temperature to 80 °C was required for preparation of tetrasubstituted olefins using mixed 1,4-dioxane/H2O as the solvent. Notably, aryl trifluoroborates bearing a methoxyl group at ortho-, meta-, or para-positions on the phenyl ring, were well-tolerated, giving the corresponding products 5c–5e in 59–65% yields. In addition to aryl trifluoroborates bearing electron-neutral and electron-donating substituents, fluoro and chloro substituents were well both accommodated, furnishing the expected products 5g and 5h in 61% and 51% yields, respectively. In addition to the smooth reaction of potassium (2-naphthalene)trifluoroborate with 4a, a sterically demanding potassium (1-naphthalene)trifluoroborate was compatible in this reaction, furnishing 5j in 61% yield. Gratifyingly, potassium 2-thienyltrifluoroborate could undergo coupling reaction with 4a to afford 5k in 46% yield. After the successful synthesis of various ethyl 3-methyl-2-aryl-2-butenoates 5a–5k, β,β-diethyl substituted α-phenyl acrylate 5l could be expectedly prepared in 70% yield. To our delight, a range of exocyclic olefins 5m–5o could be obtained in 50–55% yields.
| a Reaction conditions: a reaction mixture of 4 (0.2 mmol), 2 (0.3 mmol), Pd(OAc)2 (5 mol%), SPhos (10 mol%), K3PO4 (0.3 mmol), and 1,4-dioxane/H2O (3.0 mL/1.0 mL) was stirred at 80 °C for 24 h. |
|---|
 |
Next, the stereoselective cross-coupling of α-arylsulfonyloxyacrylate 6 with potassium phenyltrifluoroborate 2a was briefly investigated. As shown in Scheme 2, full conversion could be achieved and no trace of the alkene geometry erosion was observed, producing (E)-ethyl 2-phenyl-2-butenoate 7 in 81% yield. The results listed in Tables 2, 3 and Scheme 2 nicely demonstrated the generality of (hetero)arylation of arylsulfonyloxyacrylates.
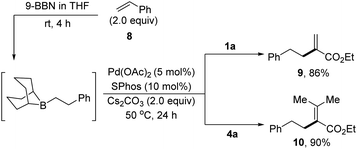
As an extension, the B-alkyl Suzuki cross coupling reactions have been demonstrated with α-arylsulfonyloxyacrylates as the electrophilic coupling partners. As shown in Scheme 3,15e the hydroboration of styrene 8 with 9-BBN (THF, rt) afforded the corresponding B-phenylethyl-9-BBN, which was in situ treated with Cs2CO3 and 1a in the presence of Pd(OAc)2 and SPhos in THF at 50 °C for 24 hours, producing the desired cross-coupled product 9 in 86% yield. Likewise, tetrasubstituted alkene 10 could be prepared in 90% yield via the reactions of B-phenylethyl-9-BBN with 4a.
Lastly, a demonstration of the synthetic value of this method is given by the preparation of 12, which is an important intermediate for synthesis of retinoid X receptors-selective retinoids.17 As displayed in Scheme 4, efficient C–C cross-coupling reaction for formation of 12 could take place between 4a and aryl trifluoroborate 11 under the standard reaction conditions.
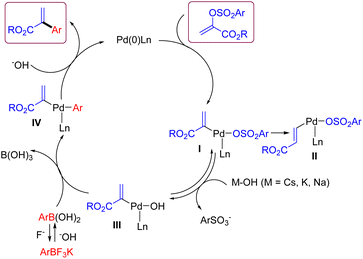
According to the literature reports and our results,18 a plausible reaction mechanism for this Suzuki cross-coupling reaction between α-arylsulfonyloxyacrylate and aryl trifluoroborate is depicted in Scheme 5. The oxidative addition of α-arylsulfonyloxyacrylate onto a ligated Pd(0) species generates arylpalladium(II) complex I. At this stage, a side intermediate II for the formation of alkyl cinnamate would be formed via a competitive pathway involving β–H elimination of I followed by reinsertion of Pd–H species.16 As for the expected pathway, the more reactive organopalladium hydroxide III is formed from the I via the displacement of arylsulfonate ion with HO− anion. Then transmetallation with arylboronic acid takes place to give intermediate IV. Finally, reductive elimination of IV affords the coupling product and regenerates the Pd(0) species with the aid of base.
Conclusions
In summary, we have reported α-arylsulfonyloxyacrylates as the useful O-centered electrophiles for the Pd-catalysed Suzuki cross-coupling reactions. Using functionalized potassium (hetero)aryltrifluoroborates as the nucleophilic coupling partners, a list of α-(hetero)aryl substituted acrylates could be modularly prepared via the C–C cross-coupling reactions. Moreover, α-alkyl substituted acrylates could be also efficiently accessed via palladium-catalyzed reactions of α-arylsulfonyloxyacrylate with B-alkyl-9-BBN. We anticipate that these new developed α-arylsulfonyloxyacrylates could find more application for the synthesis of functionalized α,β-unsaturated esters via various C–C cross-coupling reactions enabled by transition-metal catalysis and beyond.Author contributions
YF, LZ, and ZW conceived the idea and designed the research. ZZ and LH performed the research. ZZ, LH, and YF analyzed the data. YF and ZZ wrote the original manuscript. YF and LZ reviewed the manuscript and suggested improvements.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 22271168 and 21801190), the Natural Science Foundation of Ningbo (No. 2021J140), the Science and Technology Innovation 2025 Major Project of Ningbo (No. 2019B10112), and the Scientific Research Fund of Zhejiang Provincial Education Department (No. Y202147855).Notes and references
- (a) N. Miyaura, K. Yamada and A. Suzuki, A new stereospecific cross-coupling by the palladium-catalyzed reaction of 1-alkenylboranes with 1-alkenyl or 1-alkynyl halides, Tetrahedron Lett., 1979, 20, 3437–3440 CrossRef; (b) N. Miyaura and A. Suzuki, Stereoselective Synthesis of Arylated (E)-Alkenes by the Reaction of Alk-1-enylboranes with Aryl Halides in the Presence of Palladium Catalyst, J. Chem. Soc., Chem. Commun., 1979, 866–867 RSC; (c) N. Miyaura and A. Suzuki, Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds, Chem. Rev., 1995, 95, 2457–2483 CrossRef CAS.
- (a) C. C. Johansson Seechurn, M. O. Kitching, T. J. Colacot and V. Snieckus, Palladium-Catalyzed Cross-Coupling: A Historical Contextual Perspective to the 2010 Nobel Prize, Angew. Chem., Int. Ed., 2012, 51, 5062–5086 CrossRef CAS PubMed; (b) K. C. Nicolaou, P. G. Bulger and D. Sarlah, Palladium-Catalyzed Cross-Coupling Reactions in Total Synthesis, Angew. Chem., Int. Ed., 2005, 44, 4442–4489 CrossRef CAS PubMed; (c) A. J. J. Lennox and G. C. Lloyd-Jones, Selection of boron reagents for Suzuki–Miyaura coupling, Chem. Soc. Rev., 2014, 43, 412–443 RSC.
- (a) R. Martin and S. L. Buchwald, Palladium-Catalyzed Suzuki–Miyaura Cross-Coupling Reactions Employing Dialkylbiaryl Phosphine Ligands, Acc. Chem. Res., 2008, 41, 1461–1473 CrossRef CAS PubMed; (b) N. Marion and S. P. Nolan, Well-Defined N-Heterocyclic Carbenes-Palladium(II) Precatalysts for Cross-Coupling Reactions, Acc. Chem. Res., 2008, 41, 1440–1449 CrossRef CAS PubMed.
- (a) F. W. Friese and A. Studer, New avenues for C–B bond formation via radical intermediates, Chem. Sci., 2019, 10, 8503–8518 RSC; (b) E. C. Neeve, S. J. Geier, I. A. I. Mkhalid, S. A. Westcott and T. B. Marder, Diboron(4) Compounds: From Structural Curiosity to Synthetic Workhorse, Chem. Rev., 2016, 116, 9091–9161 CrossRef CAS PubMed; (c) J. Li, A. S. Grillo and M. D. Burke, From Synthesis to Function via Iterative Assembly of N-Methyliminodiacetic Acid Boronate Building Blocks, Acc. Chem. Res., 2015, 48, 2297–2307 CrossRef CAS PubMed; (d) I. A. I. Mkhalid, J. H. Barnard, T. B. Marder, J. M. Murphy and J. F. Hartwig, C–H Activation for the Construction of C–B Bonds, Chem. Rev., 2010, 110, 890–931 CrossRef CAS PubMed; (e) D. M. Volochnyuk, A. O. Gorlova and O. O. Grygorenko, Saturated Boronic Acids, Boronates, and Trifluoroborates: An Update on Their Synthetic and Medicinal Chemistry, Chem.–Eur. J., 2021, 27, 15277–15326 CrossRef CAS PubMed; (f) D. G. Hall, Boronic acids. Preparation and applications in organic synthesis, medicine and materials, WILEY-VCH, Weinheim, 2nd edn, 2011 Search PubMed.
- T. Mesganaw and N. K. Garg, Ni- and Fe-Catalyzed Cross-Coupling Reactions of Phenol Derivatives, Org. Process Res. Dev., 2013, 17, 29–39 CrossRef CAS.
- (a) T. Noël, S. Kuhn, A. J. Musacchio, K. F. Jensen and S. L. Buchwald, Suzuki–Miyaura Cross-Coupling Reactions in Flow: Multistep Synthesis Enabled by a Microfluidic Extraction, Angew. Chem., Int. Ed., 2011, 50, 5943–5946 CrossRef PubMed; (b) A. Fihri, M. Bouhrara, B. Nekoueishahraki, J.-M. Bassset and V. Polshettiwar, Nanocatalysts for Suzuki cross-coupling reactions, Chem. Soc. Rev., 2011, 40, 5181–5203 RSC.
- (a) D.-G. Yu, B.-J. Li and Z.-J. Shi, Exploration of New C–O Electrophiles in Cross-Coupling Reactions, Acc. Chem. Res., 2010, 43, 1486–1495 CrossRef CAS PubMed; (b) C. M. So and F. Y. Kwong, Palladium-catalyzed cross-coupling reactions of aryl mesylates, Chem. Soc. Rev., 2011, 40, 4963–4972 RSC; (c) J. Cornella, C. Zarate and R. Martin, Metal-catalyzed activation of ethers via C–O bond cleavage: a new strategy for molecular diversity, Chem. Soc. Rev., 2014, 43, 8081–8097 RSC; (d) B. M. Rosen, K. W. Quasdorf, D. A. Wilson, N. Zhang, A.-M. Resmerita, N. K. Garg and V. Percec, Nickel-Catalyzed Cross-Couplings Involving Carbon-Oxygen Bonds, Chem. Rev., 2011, 111, 1346–1416 CrossRef CAS PubMed; (e) J. Yamaguchi, K. Muto and K. Itami, Recent Progress in Nickel-Catalyzed Biaryl Coupling, Eur. J. Org. Chem., 2013, 19–30 CrossRef CAS.
- B.-J. Li, D.-G. Yu, C.-L. Sun and Z.-J. Shi, Activation of “Inert” Alkenyl/Aryl C–O Bond and Its Application in Cross-Coupling Reactions, Chem.–Eur. J., 2011, 17, 1728–1759 CrossRef CAS PubMed.
- (a) T. Guo, L. Zhang, Y. Fang, X. Jin, Y. Li, R. Li, X. Li, W. Cen, X. Liu and Z. Tian, Visible-Light-Promoted Decarboxylative Giese Reactions of α-Aryl Ethenylphosphonates and the Application in the Synthesis of Fosmidomycin Analogue, Adv. Synth. Catal., 2018, 360, 1352–1357 CrossRef CAS; (b) T. Guo, L. Zhang, X. Liu, Y. Fang, X. Jin, Y. Yang, Y. Li, B. Chen and M. Ouyang, Visible-Light-Promoted Redox-Neutral Cyclopropanation Reactions of α-Substituted Vinylphosphonates and Other Michael Acceptors with Chloromethyl Silicate as Methylene Transfer Reagent, Adv. Synth. Catal., 2018, 360, 4459–4463 CrossRef CAS; (c) W. Luo, Y. Yang, Y. Fang, X. Zhang, X. Jin, G. Zhao, L. Zhang, Y. Li, W. Zhou, T. Xia and B. Chen, Photoredox-Catalyzed Cyclopropanation of 1,1-Disubstituted Alkenes via Radical-Polar Crossover Process, Adv. Synth. Catal., 2019, 361, 4215–4221 CrossRef CAS; (d) W. Luo, Y. Fang, L. Zhang, T. Xu, Y. Liu, Y. Li, X. Jin, J. Bao, X. Wu and Z. Zhang, Bromomethyl Silicate: A Robust Methylene Transfer Reagent for Radical-Polar Crossover Cyclopropanation of Alkenes, Eur. J. Org. Chem., 2020, 1778–1781 CrossRef CAS; (e) L. Zhang, Y. Fang, X. Jin, T. Guo, R. Li, Y. Li, X. Li, Y. Yang, M. Yuan and Z. Tian, Efficient synthesis of α-substituted ethylphosphonates via CuH-catalyzed conjugate reduction of terminal alkenylphosphonate, Tetrahedron Lett., 2017, 58, 4538–4541 CrossRef CAS.
- (a) J. Barluenga, P. Moriel, C. Valdés and F. Aznar, N-Tosylhydrazones as Reagents for Cross-Coupling Reactions: A Route to Polysubstituted Olefins, Angew. Chem., Int. Ed., 2007, 46, 5587–5590 CrossRef CAS PubMed; (b) J. Barluenga, M. Tomás-Gamasa, F. Aznar and C. Valdés, Synthesis of 2-Arylacrylates from Pyruvate by Tosylhydrazide-Promoted Pd-Catalyzed Coupling with Aryl Halides, Chem.–Eur. J., 2010, 16, 12801–12803 CrossRef CAS PubMed; (c) C. Peng, Y. Wang and J. Wang, Palladium-Catalyzed Cross-Coupling of α-Diazocarbonyl Compounds with Arylboronic Acids, J. Am. Chem. Soc., 2008, 130, 1566–1567 CrossRef CAS PubMed; (d) C. Peng, G. Yan, Y. Wang, Y. Jiang, Y. Zhang and J. Wang, Palladium-Catalyzed Coupling Reaction of α-Diazocarbonyl Compounds with Aromatic Boronic Acids or Halides, Synthesis, 2010, 4154–4168 CAS; (e) L. Zhou, Y. Liu, Y. Zhang and J. Wang, Pd-catalyzed coupling of β-hydroxy α-diazocarbonyl compounds with aryl iodides: a migratory insertion/β-hydroxy elimination sequence, Chem. Commun., 2011, 47, 3622–3624 RSC; (f) R. J. Sullivan, G. P. R. Freure and S. G. Newman, Overcoming Scope Limitations in Cross-Coupling of Diazo Nucleophiles by Manipulating Catalyst Speciation and Using Flow Diazo Generation, ACS Catal., 2019, 9, 5623–5630 CrossRef CAS.
- (a) F. Berthiol, H. Doucet and M. Santelli, Suzuki Coupling of 2-Chloroacrylonitrile, Methyl 2-Chloroacrylate, or 2-Chloroprop1-en-3-ol with Arylboronic Acids Catalyzed by a Palladium-Tetraphosphine Complex, Synth. Commun., 2006, 36, 3019–3027 CrossRef CAS; (b) F. Berthiol, H. Doucet and M. Santelli, Synthesis of Polysubstituted Alkenes by Heck Vinylation or Suzuki Cross-Coupling Reactions in the Presence of a Tetraphosphane-Palladium Catalyst, Eur. J. Org. Chem., 2003, 1091–1096 CrossRef CAS.
- (a) Y. Fang, L. Zhang, J. Li, X. Jin, M. Yuan, R. Li, R. Wu and J. Fang, Applications of α-Phosphonovinyl Tosylates in the Synthesis of α-Arylethenylphosphonates via Suzuki-Miyaura Cross-Coupling Reactions, Org. Lett., 2015, 17, 798–801 CrossRef CAS PubMed; (b) M. Yuan, Y. Fang, L. Zhang, X. Jin, M. Tao, Q. Ye, R. Li, J. Li, H. Zheng and J. Gu, Pd-Catalyzed Synthesis of α-Aryl Vinylphosphonates via Suzuki Arylation of α-Phosphonovinyl Nonaflates, Chin. J. Chem., 2015, 33, 1119–1123 CrossRef CAS; (c) Y. Fang, L. Zhang, X. Jin, J. Li, M. Yuan, R. Li, T. Wang, T. Wang, H. Hu and J. Gu, α-Phosphonovinyl Arylsulfonates: An Attractive Partner for the Synthesis of α-Substituted Vinylphosphonates through Palladium-Catalyzed Suzuki Reactions, Eur. J. Org. Chem., 2016, 1577–1587 CrossRef CAS; (d) L. Zhang, Y. Fang, X. Jin, T. Guo, R. Li, Y. Li, X. Li, Q. Ye and X. Luo, Palladium-catalysed coupling of α-halo vinylphosphonate and α-phosphonovinyl sulfonate with alkylzincs: straightforward and versatile synthesis of α-alkyl vinylphosphonates, Org. Chem. Front., 2018, 5, 1457–1461 RSC.
- (a) G. A. Molander, Organotrifluoroborates: Another Branch of the Mighty Oak, J. Org. Chem., 2015, 80, 7837–7848 CrossRef CAS PubMed; (b) G. A. Molander and N. Ellis, Organotrifluoroborates: Protected Boronic Acids That Expand the Versatility of the Suzuki Coupling Reaction, Acc. Chem. Res., 2007, 40, 275–286 CrossRef CAS PubMed; (c) S. Darses and J.-P. Genet, Potassium Organotrifluoroborates: New Perspectives in Organic Synthesis, Chem. Rev., 2008, 108, 288–325 CrossRef CAS PubMed.
- (a) G. Seidel and A. Fürstner, Suzuki reactions of extended scope: the ‘9-MeO-9-BBN variant’ as a complementary format for cross-coupling, Chem. Commun., 2012, 48, 2055–2070 RSC; (b) J. El-Maiss, T. M. El Dine, C.-S. Lu, I. Karamé, A. Kanj, K. Polychronopoulou and J. Shaya, Recent Advances in Metal-Catalyzed Alkyl–Boron (C(sp3))–(C(sp2)) Suzuki-Miyaura Cross-Couplings, Catalysts, 2020, 10, 296–320 CrossRef CAS.
- (a) L. Zhang, Y. Fang, X. Jin, H. Xu, R. Li, H. Wu, B. Chen, Y. Zhu, Y. Yang and Z. Tian, Pd-Catalysed Suzuki coupling of α-bromoethenylphosphonates with organotrifluoroborates: a general protocol for the synthesis of terminal α-substituted vinylphosphonates, Org. Biomol. Chem., 2017, 15, 8985–8989 RSC; (b) Y. Fang, M. Yuan, J. Zhang, L. Zhang, X. Jin, R. Li and J. Li, An efficient and straightforward route to terminal vinyl sulfones via palladium-catalyzed Suzuki reactions of alpha-bromo ethenylsulfones, Tetrahedron Lett., 2016, 57, 1460–1463 CrossRef CAS; (c) Y. Fang, L. Zhang, X. Jin, J. Li, M. Yuan, R. Li, H. Gao, J. Fang and Y. Liu, Expedient Synthesis of Terminal Vinylphosphonates by Palladium-Catalyzed C-C Cross-Coupling Reactions of (1-Halovinyl)phosphonates, Synlett, 2015, 26, 980–984 CrossRef CAS; (d) L. Zhang, Modular Synthesis of α-Substituted Alkenyl Acetals by a Palladium-Catalyzed Suzuki Reaction of α-Haloalkenyl Acetals with Organoboranes, Synlett, 2021, 32, 723–727 CrossRef CAS; (e) L. Zhang, J. Shi and Y. Fang, An Alternative to the Arbuzov Reaction: Generation and Transformation of α-Dialkyl-Substituted Methylphosphonate Carbanions via an SET Reduction Process, Synthesis, 2023, 55, 907–918 CrossRef CAS.
- E. M. Woerly, J. E. Miller and M. D. Burke, (1-Bromovinyl)-MIDA boronate: a readily accessible and highlyversatile building block for small molecule synthesis, Tetrahedron, 2013, 69, 7732–7740 CrossRef CAS PubMed.
- M. I. Dawson, L. Jong, P. D. Hobbs, J. F. Cameron, W.-R. Chao, M. Pfahl, M.-O. Lee, B. Shroot and M. Pfahl, Conformational Effects on Retinoid Receptor Selectivity. 2. Effects of Retinoid Bridging Group on Retinoid X ReceptorActivity and Selectivity, J. Med. Chem., 1995, 38, 3368–3383 CrossRef CAS PubMed.
- (a) C. Amatore, G. Le Duc and A. Jutand, Mechanism of Palladium-Catalyzed Suzuki–Miyaura Reactions: Multiple and Antagonistic Roles of Anionic “Bases” and Their Countercations, Chem.–Eur. J., 2013, 19, 10082–10093 CrossRef CAS PubMed; (b) L. Zhang, C. Yang, X. Guo and F. Mo, Research Progress of Suzuki-Miyaura Cross-Coupling Reaction Mechanism, Chin. J. Org. Chem., 2021, 41, 3492–3510 CrossRef.
Footnotes |
| † Dedicated to the 40th Anniversary of Ningbo University of Technology. |
| ‡ Electronic supplementary information (ESI) available: Experimental procedures and compound characterisation data. See DOI: https://doi.org/10.1039/d3ra00401e |
| This journal is © The Royal Society of Chemistry 2023 |