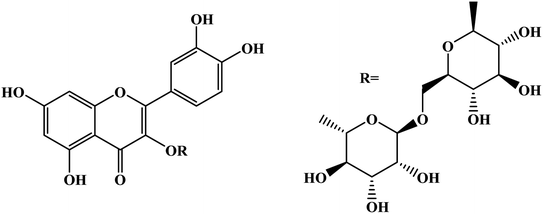
Open Access Article
Open Access ArticleExploiting poly(safranine) and poly(luminol) for sensing applications. A mini review
Salsabeel Al-Sodies
 ab,
Abdullah M. Asiri
*ac,
Khalid A. Alamry
a and
Mahmoud A. Hussein
ab,
Abdullah M. Asiri
*ac,
Khalid A. Alamry
a and
Mahmoud A. Hussein
 *ad
*ad
aChemistry Department, Faculty of Science, King Abdulaziz University, P. O. Box 80203, Jeddah, 21589, Saudi Arabia. E-mail: mahussein74@yahoo.com; maabdo@kau.edu.sa; aasiri@kau.edu.sa
bDepartment of Chemistry, Faculty of Science, Taibah University, Al-Madinah Al-Munawarah 30002, Saudi Arabia
cCenter of Excellence for Advanced Materials Research (CEAMR), King Abdulaziz University, Jeddah 21589, Saudi Arabia
dChemistry Department, Faculty of Science, Assiut University, Assiut, 71516, Egypt
First published on 24th March 2023
Abstract
Sensor applications have captivated numerous scientists in the electroactivity field lately. Between toxic target analytes and biomolecules, many articles investigated the function of the obtained products in sensing utilization and the ability of applying the gained sensor in real sample tests. Safranine and luminol have a unique polymeric constructor combined with different nanomaterials and have been explored as sensors for different analytes through electrochemical and chemical techniques. This work presents the first review of poly(safranine) and poly(luminol) in sensor applications toward assorted analytes. An illustration for the two main types of oxidative polymerization synthetic methods for our targeted compounds has been displayed including chemical and electrochemical techniques. Furthermore, a comprehensive summary for their impressive impact as electrochemical sensors in the last few decades has been additionally introduced.
Salsabeel Al-Sodies is a PhD student at Chemistry department, King Abdulaziz University. She is also working as a Lecturer of Organic Chemistry at Chemistry Department, Faculty of Science, Taibah University, Almadina Almonawara Saudi Arabia. She obtained her master's degree in organic chemistry of ionic liquid compounds for different applications. Her work includes the synthesis and characterization of variable types of polymers, poly(ionic liquid)s and their related nanocomposites for different applications. |
1. Introduction
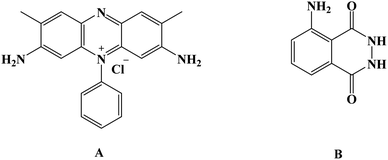
Aromatic amines and diamines such as benzidine, phenylenediamines, naphthidine, diaminopyridines, and their derivatives represent a motivating class that lured attention in the research field of oxidative polymerization of conducting polymers.1 Luminol, safranine dyes, phenosafranine, and safranine (Safranine T, Basic Red 2) are renowned derivatives with numerous uses in sundry electro-applications.2 Due to their enormous conjugated π-system, its investigated polymers are structurally expected to have substantial conductivities and high thermal and mechanical stability.3,4 These captivating characteristics make organic redox-active substances excellent applicants for variable utilization, from biomedical engineering, sensors, organic light-emitting diodes, and energy storage devices to electrochromic devices.5Bearing in mind that polymers have a prominent place in every industrial corner of our everyday lives due to their elastic properties, exploring the polymer activity of the organic structure of many compounds in several fields has drawn scientists' attention. By virtue of the need for an inclusive overview of such polymers, both safranine and luminol polymeric moieties systems have infrequently been explored.6,7 Over and above, the distinguishing chemical characteristics of their polymers are essential to understand since these characteristics of the designated polymers have multiple applications and potentials, from electro and biosensors to biomedicine.
Given that its primary application was in crime scenes, luminol [Fig. 1] and its derivatives were confronted with various applications in analytical chemistry and biotechnology fields as efficient electrochemiluminescence conducting polymers.8,9 Inevitably, new designs of polymers backbone regarding the amendments of luminol structure have appeared in recent years in order to attune the electrochemiluminescence efficiency, sensitivity, and quantum yield.10 Recently, the uses of luminol and its small molecule derivatives have been widely employed to develop sensing properties toward materials as both electrochemical and biosensors.7,47–61
Over the past few years, water-soluble dyes, such as carmine, methylene dye derivatives, and other dyes, have been broadly employed in catalyzing oxidation or reducing organic molecules as mediators.11–13 Safranine [Fig. 1] is a red-colored water-soluble phenazine-type dye with an extensive heterocyclic conjugated system in addition to an electron donor-NH2. It has been applied extensively in several researches comprising pharmaceutical industries applications as well as textiles, cosmetics, sensors, and many others. However, safranine polymer has yet to be discovered in electrochemical studies.3,4
While we establish no allegation that the list of reported examples in this review is complete, the selection articles within this review were chosen to display how infrequent the utilization of both poly(safranine and luminol) and their composites in electrochemical/biosensors scope in the current research domain in spite of the low detection limits resulted of these moieties in the published articles which expand the opportunities window to explore more target materials with different form of composites. At the outset, we will address the main reported oxidative polymerization methods, embracing electrochemical and chemical approaches in a polymeric backbone. Following this, we will explore the published studies of the mentioned units exploited in electro and biosensors. At long last, perspective on future research towards different applications and possibilities studies will be provided of luminol and safranine-based polymer derivatives.
2. Oxidative polymerization techniques
2.1. Electrochemical method
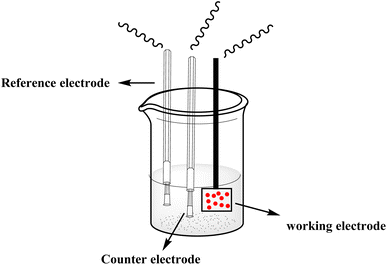
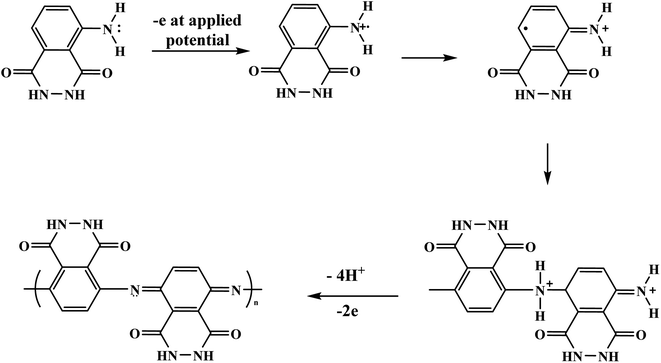
With the continuous demands to evolve susceptible sensors to sustain the current progression in electrochemical technology for electrocatalysis and detection, many researchers have explored this matter. One of the possible methods for attending to these desires is using electroactive conducting polymer films to manufacture the accompanying devices.14–16 Because of its reproducibility and simplicity, electrochemical polymerization is a conventional technique generally utilized for synthesizing electroactive conducting polymer films.17 In the usual procedure, potentiostatic, potential step, galvanostatic, or potential sweep employ for monomer electro-polymerization.18–21 Consequently, various factors determine the characteristics and the applications of the polymer's films formed, for instance doping effect, applied potential, potential sweep direction, electrolyte solution, monomer nature, solvent effect, pH, temperature, and the potential volumetric window.22–24 Fundamentally, in this technique, an electrochemical cell [Fig. 2] comprising a monomer, immersed working electrode, doping agent, and the electrolyte solution under applying potential. Thus, the monomer will be deposited on the working electrode surface, forming a conducting polymer film via an electrochemical oxidization process to generate free radicals that initiate the polymerization progression.25This polymerization approach occurs through a widely recognized mechanism that entails monomer oxidation on the electrode surface by forming cationic radicals. Electrochemical polymerization of luminol illustrated in Scheme 1, the first step informs the formation of a cation radical via electron transfer from sp3 orbital to the electrode surface when oxidation ensues.
V. Ferreira et al. reported an electrochemical study that incorporated synthesis and characteristics of luminol with aniline undergo electrochemical polymerization. The process performed in acidic media potentiodynamically using various concentration ration of monomers, the authors discuss the ratio effect from (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to (1
1) to (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60). The inclusion of aniline block in the polymer construction reflected on current density and potential profile under acidic media of both characterization and polymerization. At lower potential than aniline where luminol oxidized reasons inhibition aniline oxidation and impact polyaniline integration in the films. The combination of poly(luminol–aniline) films demonstrates electroactive and steady behavior and exhibits similar performance in basic conditions similar to the diversity of polyaniline films.26
60). The inclusion of aniline block in the polymer construction reflected on current density and potential profile under acidic media of both characterization and polymerization. At lower potential than aniline where luminol oxidized reasons inhibition aniline oxidation and impact polyaniline integration in the films. The combination of poly(luminol–aniline) films demonstrates electroactive and steady behavior and exhibits similar performance in basic conditions similar to the diversity of polyaniline films.26
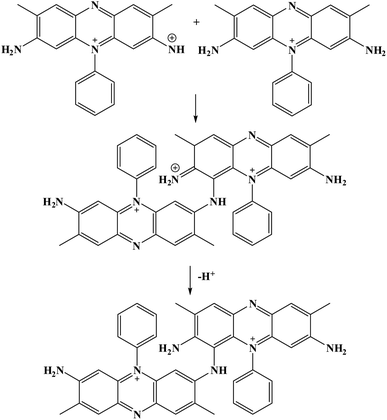
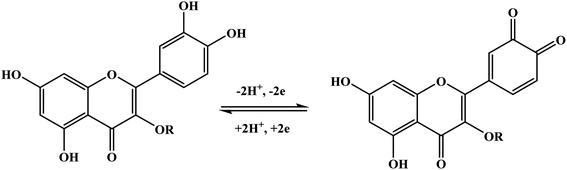
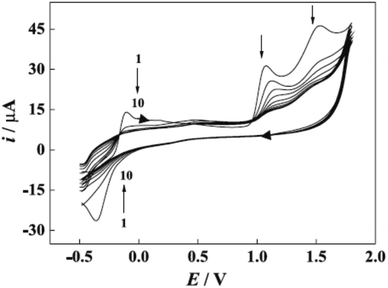
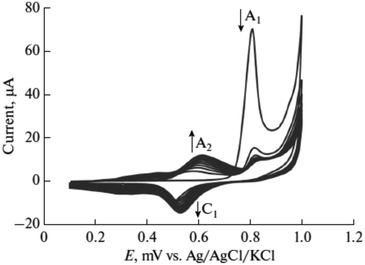
Furthermore, potential cycling electro-polymerization of phenazine safranine at carbon electrodes in nitrate solutions and chloride solution (pH 5.5) into several pH (2.0, 5.5, and 7.0) was reported.27 The investigation of obtained polymer electroactive properties has been examined by cyclic voltammetry (CV) utilizing various electrolyte solutions under −0.8 to 1.2 V in AgCl/Ag referenced electrode for 18 cycles. Chloride solution, pH 5.5, resulted the highest surface coverage for the film formed with 8.7 nmol cm−2 due to the deprotonation being fast at this pH of dimers and oligomers [Scheme 2]. Authors explored other film coverages from 5.3 to 7.8 nmol cm−2, and similar to polymers gained from other phenazine dyes their coverages scored lower. While the polymerization process appeared to be the fastest at pH 5.5, the best electrochemical characteristics resulted at pH 2.0. In most cases, and according to the assessment of cyclic voltammograms, electrochemical processes coated electrode polymer is restricted to surface reactions. An example of a voltammogram to the electropolymerization process of poly(safranine) illustrates in Fig. 3.
 | ||
| Fig. 3 CV recorded of poly(safranine) electro-polymerization in 0.1 M of sulfuric acid and scan rate: 50 mV s−1. This figure has been adapted/reproduced from ref. 6 with permission from PubliSBQ, copyright (2023). | ||
2.2. Chemical method
Chemical oxidative polymerization (COP) is used for polymer synthesis based on amine or diamine aromatic compounds. The method involves monomers that have a high oxidation tendency with distinct electron donor characteristics. In the chemical approach, monomer oxidation is accomplished through an inorganic (or organic) oxidizing agent. The initial polymer growth begins with cation or cation radical sites created in the monomer (polymer) core. Ammonium persulfate is one of the most widespread chemical redox initiators reported in this experiment; among other oxidizing agents, various aqueous media applicable to the oxidation process and a diversity of oxidants can also be exploited.5,28,29Additionally, determined by the formulation conditions, the polymers might be soluble in several organic solvents or insoluble whatsoever;30 likewise, the variability of properties, numerous supramolecular morphologies, and polymer yield controlled by the reaction conditions, whether in acidic or basic media. Myriad parameters are considered necessary in charge of the resulted characteristics and morphology;31,32 this embraces especially the nature of chemical oxidants, acid protonated, solvent, the intermediates formed within the oxidation, molar proportion, and reactants concentrations, temperature, the presence of the additives (e.g., surfactants, colloidal stabilizers) and others. It may be partially evident for the experimenter which parameters can guide the synthesis toward the desired polymers.
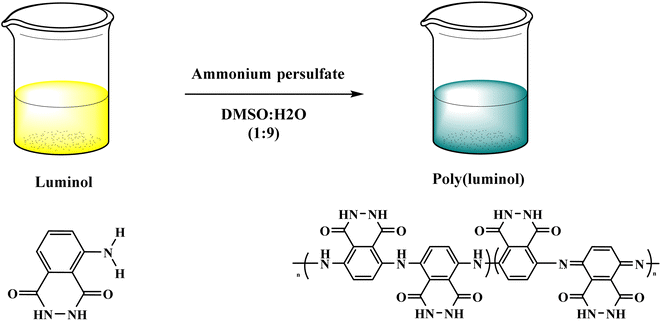
In this context, poly luminol with electroactive behavior has been synthesized through oxidative chemical reaction by J. N'Diaye et al.33 The polymerization reaction of luminol was performed in DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (9
H2O (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and ammonium persulfate [Fig. 4], the process shows fast kinetics with reversible oxidation/reduction reactions. The desired polymer was examined by FT-IR, and data confirmed the transformation of primary amine to secondary amine groups. Glass transition appeared at 99 °C while melting temperature at 152 °C when polymers were investigated by thermal analysis and uncovered the semi-crystalline nature of poly luminol. The writer mentions the possibility of utilizing the corresponding polymer in energy storage applications.
1) and ammonium persulfate [Fig. 4], the process shows fast kinetics with reversible oxidation/reduction reactions. The desired polymer was examined by FT-IR, and data confirmed the transformation of primary amine to secondary amine groups. Glass transition appeared at 99 °C while melting temperature at 152 °C when polymers were investigated by thermal analysis and uncovered the semi-crystalline nature of poly luminol. The writer mentions the possibility of utilizing the corresponding polymer in energy storage applications.
3. Poly(safranine) in sensor applications
Electrochemical applications of poly(safranine) in the mold of sensors were reported in a handful of articles in the literature, mainly through the electrochemical polymerization films method. An innovative sensor for 4-nitrophenol in a water sample was studied using poly(safranine) modified on glassy carbon electrodes.3 Cyclic voltammetry (CV) was used to analyze the electrochemical performance of the polymer film toward 4-NP in phosphate-buffered saline (PBS) at (pH = 6.0) as well as adsorptive linear stripping voltammetry (LSV). Poly(safranine) film was molded on the GCE surface at a scan rate of 0.1 V s−1 for 15 cycles in sweeps among −1.6 and 2.0 V, and the cyclic voltammogram shows a definite reduction peak at −1.05 V, confirming the sensing of the corresponding polymer toward the target with detection limit 3.0 × 10−8 M [Fig. 5]. An excellent linear relationship was observed with a concentration range between 8.0 × 10−8 to 4.0 × 10−5 M of 4-NP reduction current at poly(safranine) film. The impact of pH was covered, and the study displayed a negative response of the reduction peak with increasing pH, whereas, at pH 6.0, the maximum value was presented. The research suggested that the equality of protons and electrons transfer explains this result. The sensor was applied in fruit and natural water sample to examine the response toward the traces of 4-NP, and the data appears with promising results.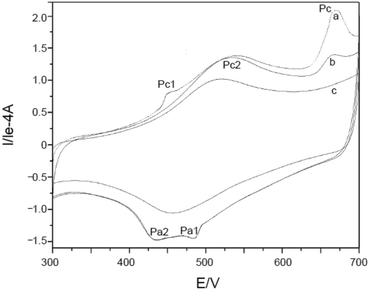 | ||
| Fig. 5 Cyclic voltammetry of first and second cycle of poly(safranine) in the presence of 4-NP (curve a and b) and without (curve c). This figure has been adapted/reproduced from ref. 3 with permission from WILEY – VCH via CCC marketplace, copyright (2023). | ||
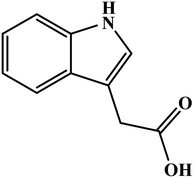
Poly(safranine) has been employed in the biosensor field in addition to the chemical target substances. T. Gan and the research team34 investigate a biosensor to a sort of auxin hormone called indole-3-acetic acid (IAA) [Fig. 6] based on poly(safranine) and nanocomposites of reduced graphene oxide (rGO). The team claimed the novelty of this sensor which combines selectivity with sensitivity, unlike others determining (IAA), reported techniques. Electrodepositing dipping–drying method used to prepare the desired polymer on a pre-anodized graphite electrode (AGE) coated by rGO film layer. Scanning electron microscopic (SEM) revealed a rougher surface of electropolymerized safranine comparable to the morphology of rGO/AGE while forming a smooth surface mirror-like with AGE. In the electrochemical study of the sensor response, CV demonstrated two oxidation peaks located at 0.56 and 0.64 V, respectively, in the first cycle. In contrast, in the reverse sweep, at −0.05, a weak reduction peak appears. A study of pH influence was executed as well, giving the maximum result when it reads 8.0, and this attributed to the anionic behavior of IAA from pH 4.75 and higher, which make electrostatic interaction grow stronger with the cationic positive-charged poly(safranine) film and enhances the response toward IAA in the alkalescent atmosphere. Meanwhile, the detection limit was calculated at 5.0 × 10−8 M. A good linear relationship of the voltammetric current was with IAA concentration in the range 1.0 × 10−7 to 7.0 × 10−6 M. This sensor has been tested in the real sample examination to the determine the IAA in the extract versatile plant leaves and the recoveries diverse in the range of 97.71–103.43%.
Novel biosensor discovered in the literature survey using polyazine-modified GCE. L. Niu and colleagues6 examined for the first time the ability of electrochemically polymerized safranine to determine uric acid (UA) and epinephrine (EP) in the coexistence of ascorbic acid (AA). The preparation procedure of the electro safranine films involves submerged GCE in 2.0 × 10−3 M of safranine solution embracing in (H2SO4 0.1 M) in −0.5 to 1.8 V potential range with ten cycles of scan rate (50 mV s−1). The article discusses using different acids besides sulfuric acid, and the latter has been chosen for its smooth film formation. The effect of the cycle number of electro-polymer formation revealed an enhancement of surface coverage growth until it reached 120 cycles when the polymer started to desquamate; it was found out that as soon as the cycle number is expanded to 100, maintained relationship observed between the scan rate and peak current. Consequently, no changes are noticed in the polymer redox mechanism by increasing the cycle number since diffusion of electrons dominates the surface process [Fig. 7].
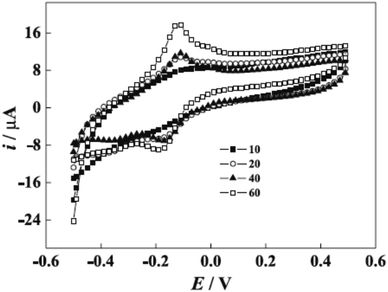 | ||
| Fig. 7 Voltammogram of poly(safranine) generated at different cycles (10, 20, 40, and 60 cycles) in 0.1 M sulfuric acid solution. This figure has been adapted/reproduced from ref. 6 with permission from PubliSBQ, copyright (2023). | ||
However, protonation of UA (pKa1 = 5.40) and EP (pKa = 8.88) when the pH is below 5.40 for UA and 8.88 for EP is the main explained reason for the pH solution has its maximum effect at 4.4 for UA and 7.0 for EP [Fig. 8]. In the electrocatalytic currents, heightened and well-separated potentials were ascertained for EP and UA. Linear anodic peak currents of EP and UA were seen at 6.0 × 10−6 to 1.0 × 10−4 M of the corresponding concentrations with 2.0 × 10−7 M detection limits for EP and 4.3 × 10−6 M for UA [Fig. 9]. Furthermore, the stability and interference studies have been reviewed on the modified electrode and presented good stability along with sensitivity. In the real sample test, acceptable results were accomplished for the sensing of EP and UA in human urine sample injection solutions by EP.
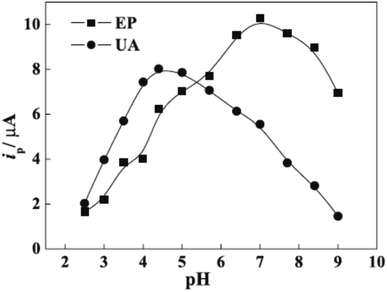 | ||
| Fig. 8 pH effect on epinephrine (EP) and uric acid (UA) oxidation. This figure has been adapted/reproduced from ref. 6 with permission from PubliSBQ, copyright (2023). | ||
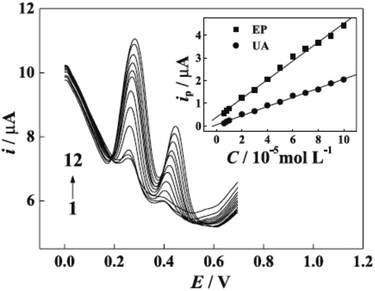 | ||
| Fig. 9 Differential pulse voltammetry (DVP) of UA and EP at different concentrations. Concentrations of EP (10−5 M): (1) 0.6, (2) 0.8, (3) 1.0, (4) 2.0, (5) 3.0, (6) 4.0, (7) 5.0, (8) 6.0, (9) 7.0, (10) 8.0, (11) 9.0, (12) 10; concentrations of UA (10−5 M): (1) 0.6, (2) 0.8, (3) 1.0, (4) 2.0, (5) 3.0, (6) 4.0, (7) 5.0, (8) 6.0, (9) 7.0, (10) 8.0, (11) 9.0, (12) 10; inset graphs are the plots of anodic peak currents to the corresponding concentrations of EP and UA. This figure has been adapted/reproduced from ref. 6 with permission from PubliSBQ, copyright (2023). | ||
Considerable efforts extended to achieve more sensitive and selective sensors utilizing poly(safranine) involving substances closer to daily life, such as determining the caffeine in tea samples.4 Contrary to some analytical techniques that require expensive equipments and complicated processes, the electrochemical approach makes a convenient pathway and more environment-friendly strategy to determine caffeine. With that said, safranine was electropolymerized on a GCE in phosphate buffer solution (pH 5.8) from −0.8 to 1.2 V in 20 cycles. The obtained polymer was then dried and coated with Nafion solution in order to prevent the anions interferences that may happen. The uniform film was examined for caffeine oxidation to exhibit a superior electrocatalytic capability. Cyclic voltammetry was used to study the sensing activity of the polymer under potential within 1.0 to 1.5 V in sulfuric acid. In the presence of caffeine solution (1.0 × 10−5 M), neither bare electrode nor electrode coated with Nafion gave a redox response. Notwithstanding, a distinct anodic peak at 1.38 V was detected when poly(safranine) was introduced; this response raised linearly with caffeine concentration in between 3 × 10−7 to 1.0 × 10−4 M. The detection limit (1 × 10−7 M) was recorded, showing a lower result than other reported analytical researchers. The ability of this method has been proven in the real sample test using green, oolong, and black tea, and the recoveries were 103.3, 106.4, and 96.6, respectively.
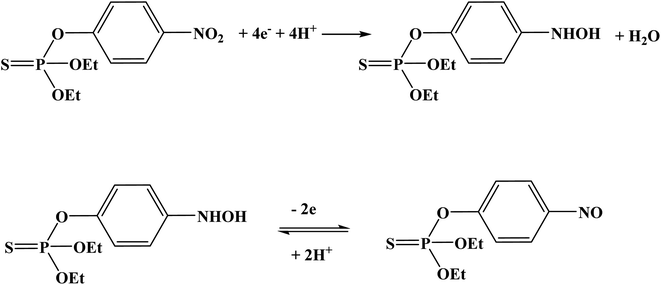
Organophosphate pesticides were aimed to be determined utilizing another designed poly(safranine) on modified GCE.35 Parathion is an OPs that is readily absorbed by the skin through inhibition of the enzyme acetylcholinesterase (AChE). The novel sensor electrode film has been prepared via an electropolymerization process in a solution of PBS (pH 6.0) and sweeps in the range −1.6 to 2.0 V for 15 cycles under 0.1 V s−1 scan rate and the obtained film tested as a sensor for 1.9 × 10−5 M solution of parathion pesticide sample. Voltammogram responded positively with a prominent reduction peak at −0.60 V [Fig. 10] that credited to the transfer of 4 electrons in the reduction mechanism to convert the nitro group in parathion to hydroxylamine derivative [Scheme 3]. Parathion concentrations between 3.43 × 10−8 and 3.43 × 10−5 showed a good linear relationship of the reduction current in linear sweep voltammograms with a detection limit equal to 1.0 × 10−8 M. Scan rate and pH influence were also studied, and the results revealed that the increase in linearity was in scan rate between 0.01 to 0.2 V s−1, and increasing in pH values presented a negative shift with a maximum response at 5.5 to 6.5 [Fig. 11]. Apple sample was used to test the selectivity and the sensitivity of the obtained sensor by determining the trace amounts of parathion; the recoveries of the pesticide spiked recorded between 97.7 to 107.2%.
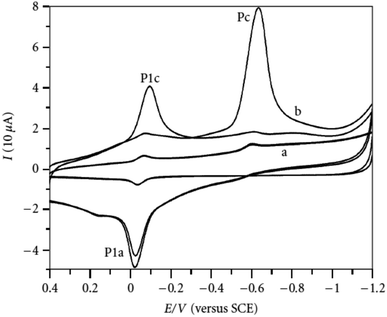 | ||
| Fig. 10 Voltammogram response of parathion (1.9 × 10−5 M) at (a) bare electrode (GCE), (b) poly(safranine) film-modified electrode. This figure has been adapted/reproduced from ref. 35 with permission from Hindawi, copyright (2023). | ||
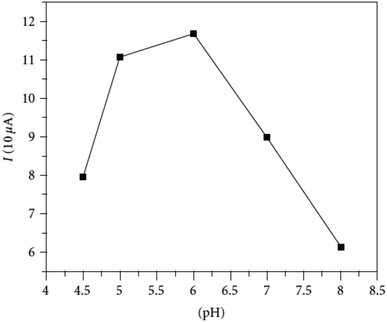 | ||
| Fig. 11 pH effect of parathion (3.43 × 10−5 M) on peak current at fabricated poly(safranine) film under scan rate: 100 mV s−1. This figure has been adapted/reproduced from ref. 35 with permission from Hindawi, copyright (2023). | ||
In this scope of applications, another type of analyte was tested for determination by poly(safranine). Jasmonates and their derivatives are a class of plant hormones that play a primary role in plant growth and response to any tissue wounding. Poly(safranine) based graphene oxide as an electron transfer intermediary has been reported in the form of a sufficient technique to determine methyl jasmonate [Fig. 12] through one step polymerization process.36 Electrochemical activity study of the obtained electrode film revealed a voltammogram with a strong outcome of the designed polymer toward the target material in the form of a noticeable oxidation peak at 1.54 V. In optimum anodic current, the linearity enhanced of the analyte in concentrations between of 1.0 to 10 μM and 30 to 3000 μM. A formed electrocatalytic film was effectively applied to determine the target substance in samples of both jasmine essential oil and rice spikelets, and the study came with satisfactory results.
Similarly, D. Saritha et al.37 reported an electro-polymerization of poly(safranine) intended for a novel electrochemical sensor constructed with nano NiO materials on the surface of modulated carbon paste electrode (CPE). The obtained composite was applied to detect rutin [Fig. 13], which is a natural flavonol glycoside with versatile biological activities-usage cyclic and differential pulse voltammetric methods in 0.01 mM of safranine and supporting electrolytes (PBS 0.1 M) at (pH 5.5).
The procedure was executed in potential between 0.0 to 0.07 V for 20 cycles under 50 mV s−1 scan rate [Fig. 14]. Since nickel oxide nanoparticles hold superparamagnetic characteristics, the modified CPE with poly(safranine/nano NiO) is conjectured to display exceptional electrochemical performance, high stability and sensitivity, and better conductivity. The CV data shows two distinct redox peaks at 0.232 V and 0.188 mV with maximum peak currents at the analyte concentration range of 1.61 × 10−8 M to 2.30 × 10−7 M [Fig. 15]. As stated in the research, the exceeded pH over 5.5 causes the amino group in safranine to be easily protonated, negative ions increase, and electrostatic repulsion between rutin and NiO/safranine contributes to the current reduction [Scheme 4]. Detection and quantification limit values were 0.54 × 10−8 M and 1.61 × 10−8 M, respectively. Buckwheat and green tea samples were utilized for the actual sample study to test the response of the modified electrode film to the rutin company with interfering constituents. The recoveries' performance was recorded in a range of 96.5% to 102.8%. Thus, the electro-polymerization process provided a significant augmentation to the electrochemical response, as in 6 times greater than the bare CPE and 3 times greater than CPE over nano (NiO) materials.
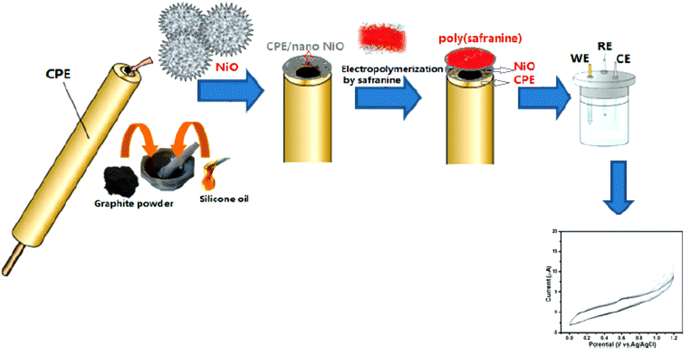 | ||
| Fig. 14 Schematic representation of electropolymerizing process of safranine-modified CPE with NiO. This figure has been adapted/reproduced from ref. 37 with permission from ESG, copyright (2023). | ||
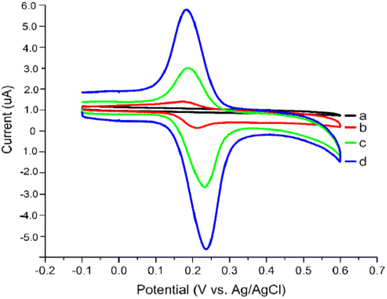 | ||
| Fig. 15 Voltammogram response for (a) bare CPE, (b) bare electrode in the presence of rutin, (c) rutin at NiO modified CPE, (d) rutin at poly(safranine)/nano NiO CPE. This figure has been adapted/reproduced from ref. 37 with permission from ESG, copyright (2023). | ||
Quercetin is one of the antioxidants in food and has a similar structure to rutin was studied as a target analyte for poly(safranine) on GCE as well.38 Modified electrode film illustrates a high electrocatalytic activity for quercetin electrochemical reaction [Fig. 16]. The same result as in rutin, quercetin showed reversible performance throughout the adsorption-controlled procedure with a transfer of two electrons. Linear concentration is exhibited in square wave voltammetry (SWV) data between 0.01 μM to 8 μM with reasonable detection and quantification limits [Fig. 17]. Additionally, stability, repeatability, reproducibility, and sensitivity have been studied for the obtained sensor, and the values displayed good selectivity for quercetin among different materials. In the real sample test, wine and fruit juice were used, and their recorded recovery of 90.7 to 105.5% was compared with those performed by high-performance liquid chromatography (HPLC), resulting in a steady value with the gained from HPLC.
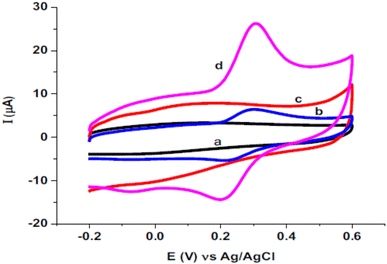 | ||
| Fig. 16 Voltammogram of (a) bare electrode, (b) bare electrode in presence of quercetin, (c) poly(safranine)/GCE in absence of quercetin, (d) poly(safranine)/GCE in presence of quercetin. This figure has been adapted/reproduced from ref. 38 with permission from ELSEVIER via CCC marketplace, copyright (2023). | ||
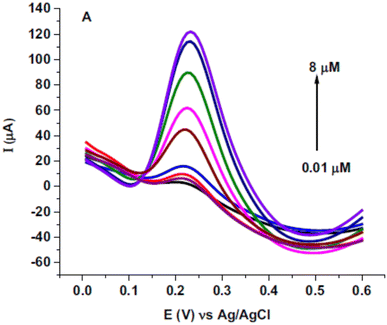 | ||
| Fig. 17 Square wave voltammetry (SWV) for diverse concentrations of quercetin (0.01, 0.05, 0.25, 0.75, 2.75, 4.25, 6, 7.5 and 8 μM) in pH 5.6 at poly(safranine) on GCE. This figure has been adapted/reproduced from ref. 38 with permission from ELSEVIER via CCC marketplace, copyright (2023). | ||
Amalgamation between multi-walled carbon nanotubes (MWCNT), electropolymerized safranine, and copper oxide nanoparticles (CuO) was reviewed as a novel sensor for catechol and hydroquinone substances.39 The method was performed on pencil graphite electrodes (PGE) in deep eutectic solvent (acid-doped ethaline) to formulate the film electrode alongside three diverse nanostructured designs. Among using different acids media, HCl, HNO3, HClO4, and H2SO4, the growth of polymer film gave excellent outcomes when poly(safranine) was electrodeposited on the above combination in HCl conditions. Obtained film-coated electrodes were examined using CV and electrochemical impedance spectroscopy to test their sensor performance toward the objective materials. The modified film electrode delivered both sensitivity and selectivity detection of catechol and hydroquinone with pronounced oxidation peak of catechol at around 0.22 V and 0.12 V for hydroquinone due to the united impact of MWCNT/CuO nanocomposite. The determination of the analytes was studied regarding milk and tap water samples, and results approved the novel sensor applicability, making it an encouraging method to sense the target materials.
As can be deduced, significant efforts have mainly focused on preparing poly(safranine) via electrochemical methods, and it is worth mention chemical polymerization of safranine has not been reported, to the best of our acknowledge, as an approach method for preparing safranine in sensor applications. In this regard, new opportunities may open the research window for developing methods and addressing poly(safranine) in electrochemical applications through versatile chemical oxidizing agents and chemically green techniques. Summary of the above results utilizing poly(safranine) in sensor applications in Table 1.
| Sensor | Target | Detection limit | Electrode | Ref. |
|---|---|---|---|---|
| Poly(safranine) | 4-Nitrophenol (4-NP) | 3.0 × 10−8 M | Glassy carbon electrode (GCE) | 3 |
| Poly(safranine)/rGO | Indole-3-acetic acid (IAA) | 5.0 × 10−8 M | Pre-anodized graphite electrode (AGE) | 34 |
| Poly(safranine) | Epinephrine (EP) | 2.0 × 10−7 M (EP) | GCE | 6 |
| Uric acid (UA) | 4.3 × 10−6 M (UA) | |||
| Poly(safranine) | Caffeine content in tea | 1 × 10−7 M | GCE | 4 |
| Poly(safranine) | Parathion | 1.0 × 10−8 M | GCE | 35 |
| Poly(safranine)/rGO | Methyl jasmonate | 5.0 × 10−7 M | GCE | 36 |
| Poly(safranine)/nano NiO | Rutin in buckwheat and green tea samples | 0.54 × 10−8 M | Carbon paste electrode (CPE) | 37 |
| Poly(safranine) | Quercetin in wine and fruit juice samples | 5.0 × 10−9 M | GCE | 38 |
| Poly(safranine)/MWCNTs/CuO nanocomposite | Hydroquinone (HQ) | 3.0 × 10−7 M (HQ) | Pencil graphite electrodes (PGE) | 39 |
| Catechol (CAT) | 7.0 × 10−7 M (CAT) |
4. Poly(luminol) in sensor applications
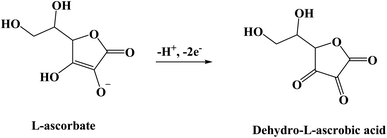
Luminol (5-amino-2,3-dihydro-phthalazine-1,4-dione) [Fig. 1] has broadly exploited in several fields, including chemistry, biochemistry, and thoroughly in electrochemiluminescence and chemiluminescence detection. The presence of an amino group in luminol improves the intensity of chemiluminescence as an electron-donating group to the system. It reflects positively on the electrochemical properties of poly(luminol) and its utilization in sensor applications.40–46S. Ashok Kumar47 and colleagues reviewed a novel composite electrode film of electropolymerized luminol under acidic media (0.1 M sulfuric acid) conducted with zinc oxide (ZnO) nanoparticle on modified (GCE). The coated electrode film was then studied for electrochemical analysis as a sensor for ascorbic acid (AA), two electrons and one proton transfer were the essence of the oxidation of L-ascorbate to dehydro-L-ascorbic acid [Scheme 5]. It was projected that the comparable kinetic mechanism is involve in the ascorbate oxidation on the poly(aniline)–poly(vinylsulfonate) composite modified film where a complex is designed between the polymer and the ascorbate followed by bound ascorbate oxidation. Versatile instruments were applied to demonstrate the surface of the construction of the obtained hybrid comprising scanning electron microscope (SEM) and atomic force microscope (AFM). Particle sizes of the hybrid film were recorded <100 nm alongside with observable blue colored on the electrode surface. PLu/ZnO-NPs modified electrode film electroactivity was studied in a pH range (1 to 11). Data displays an increasing oxidation peak with pH values until 7.0, and a negative response started with higher pH values. Compared with the bare electrode, the modified one exhibits excellent electroactivity toward ascorbic acid in the shape of a significant rise of anodic current [Fig. 18]. The sensing response for the analyte had a broad linear in AA concentrations between 1 × 10−6 to 3.6 × 10−4 M with 3 seconds response time and low detection limit. Upon the above results, commercial vitamin C samples were analyzed to examine the efficiency of the polymer-modified electrode in a real sample study, and the results came with an arrangement with the described values.
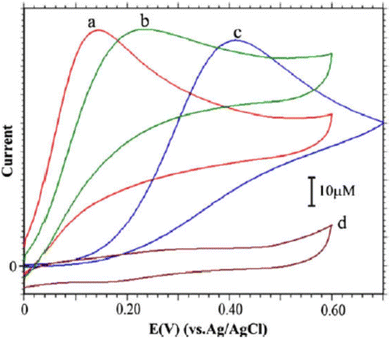 | ||
| Fig. 18 CVs of 5 mM of (AA) with: PLu/ZnO-NPs/GCE (curve a), PLu/GCE (curve b) and bare GCE (curve c) in pH 7.0 PBS + PLu/ZnO-NPs/GCE in the absences of AA in PBS (pH 7.0) solution (curve d). Scan rate = 10 mV s−1. This figure has been adapted/reproduced from ref. 47 with permission from ELSEVIER via CCC marketplace, copyright (2023). | ||
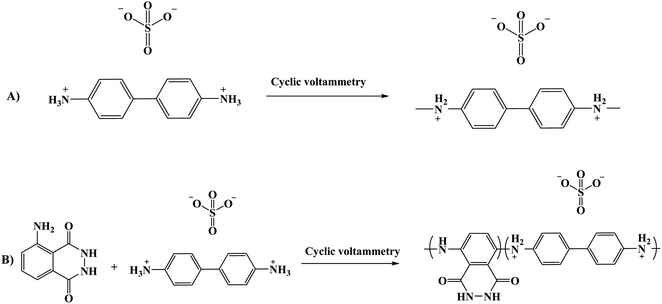
Another composite film through an electro-polymerization technique based on poly(luminol–benzidine) has been investigated on the surface of graphite electrode.48 The process was carried out on a three-electrodes system under a potential of −0.2 to 1.0 V for 30 sweep cycles and 5.0 × 10−4 M solutions of luminol, same as for benzidine concentration. The desired modified electrode film was examined for its electrochemiluminescence (ECL) operative as a sensor toward hydrogen peroxide (H2O2). The (ELC) mechanism of the composite films consist of electrochemically oxidized of poly(luminol) at first to polymeric azasemiquinone radical on the electrode surface when applied an appropriate electro-potential to the electrode. Concurrently, super oxygen anion radicals (O2˙−) could appear coming from the reduction of the dissolved oxygen by the construction of poly(luminol), followed by the reaction of (O2˙−) with the polymeric azasemiquinone radical to generate ECL signal. Furthermore, in the sight of H2O2, polymeric azasemiquinone radical will react with the electrooxidized H2O2 to form polymeric 3-aminophthalate in the exited state, which resulted in the enhanced ECL signal when returning to the ground state [Fig. 19]. Scientific data presented a higher fluorescence quantum yield of polymeric 3-aminophthalate compared with poly(luminol), and the article suggested that the dissimilar performance of electrochemiluminescence of poly(luminol–benzidine) hybrid films could be driven by the photophysical property modulation of polymeric benzidine to the poly(luminol) in the composite films.
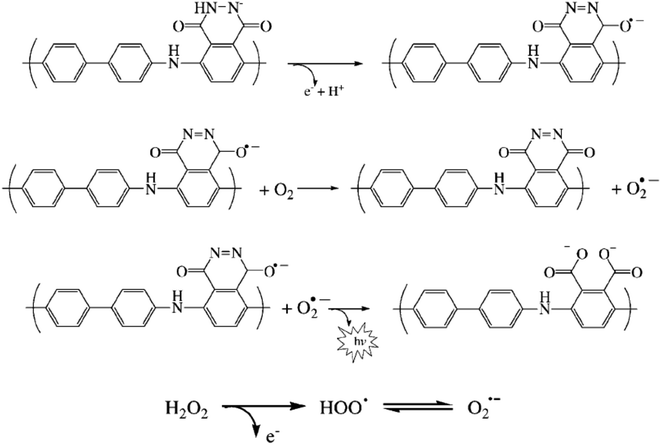 | ||
| Fig. 19 Proposed ECL mechanism for polymeric luminol in alkaline solution. This figure has been adapted/reproduced from ref. 48 with permission from WILEY – VCH via CCC marketplace, copyright (2023). | ||
Additional sensor for hydrogen peroxide was explored through galvanostatic polymerization as a new class of method to prepare poly(luminol) films onto screen-printed electrodes (SPEs).49 Similar data of the new technique was observed for the gained film in respect of responses to H2O2 compared with the ones found through potentiostatic mode. Cyclic voltammetry was used to test the film performance by utilizing choline oxidase as an enzyme model, immobilized in silica gel designed on both luminol before and after being polymerization. The results confirmed the ability of luminol to be polymerized inside a silica gel. In either event, concentrations of micromolar choline could provide sensitive detection of the target substance, and it opens the window for many other oxidase-substrate compounds.
A biosensor for glucose has been proposed by electro oxidizing approach of poly(luminol) with polymeric aniline and nanowires to form a composite film on the surface of graphite electrode in an acidic environment.50 The prepared film was carried out in CV under potential between −0.2 to 1.0 V and 0.2 V s−1 scan rate for 50 cycles using 0.001 M of luminol and 0.025 M of aniline. Synthesized electrode film impacted ECL response in the form of distinct oxidation at 0.2 V and 0.5 V, and a new one was revealed from the second scan at the potential of −0.55 V, which can be explained as polymeric luminol electrogenerated species oxidation area might react with modified polymer reduced species at the electrode surface. The study of (ELC) performance shows that the electropolymerized composite forms an exemplary biosensor for glucose by providing a significant surface addition to the presence of nanoparticles interface on the electrode surface, improving the response toward the glucose enzyme loading. The developed biosensor demonstrated the response of relative standard deviation to 1.0 × 10−6 M glucose was 4.3% for eleven consecutive conclusions.
One more attempt to design an electrochemical sensor for H2O2 designed through electropolymerized luminol synthesized on screen-printed gold electrode has been reported via J. Ballesta-Claver et al.51 A copolymerization study of luminol-bearing manifold monomers was performed. The applied potential interval was between −0.2 to 1.0 V to formulate the polymeric films in H2SO4 (0.2 M). Poly(luminol–3,3′,5,5′-tetramethylbenzidine) shows growth at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 ratio with great efficiently and ECL emission with similar mechanism to the one mentioned previously.48 In linearization analysis, detection limits were calculated at 2.6 × 10−9 M, illustrating an obvious 4-decade width concentration range. The designated screen-printed electrode sensors will be suitable for oxidase substrate determination in ECL single-use biosensors.
5 ratio with great efficiently and ECL emission with similar mechanism to the one mentioned previously.48 In linearization analysis, detection limits were calculated at 2.6 × 10−9 M, illustrating an obvious 4-decade width concentration range. The designated screen-printed electrode sensors will be suitable for oxidase substrate determination in ECL single-use biosensors.
Determining biomolecules such as dopamine (DA) and uric acid (UA) were also reviewed based on poly(luminol) modified film on GCE. A composite of multi-walled carbon nanotubes and gold nanoparticles combined with β-cyclodextrin was efficiently developed and tested as a biosensor.52 The electrochemical study results show the vital role of the gold nanoparticles in the catalytic activity of the obtained film toward dopamine addition to MWCNTs, which dramatically boosts electron transfer. While there is no noticeable response to the bare electrode, the desired composite displays unmistakable redox peaks between 0.20 and 0.33 V. Multiples of proton exchange process with a reversible electron transfer progression is occurred during the proposed mechanism. The linearity of the current response presents a low detection limit of 1.9 × 10−7 M with rises in analyte's concentration from 1.0 × 10−6 to 5.6 × 10−5 M. Besides, the interferences study of uric acid and ascorbic acid are successfully reduced, and the modified electrode's suitability was established through dopamine measurement in dopamine hydrochloride injection.
An additional biosensor was reported toward cholesterol via the electrochemiluminescence process through layers formation on gold screen-printed cells.53 Electro-construction of novel luminol copolymers used as a detection layer with diverse click-chemistry-based biotinylated pyrroles, introducing an innovative electroluminescent property of biosensor with an original transduction layer. Cyclic voltammetry applies to study the electroactivity with 5 cycles in a 10−3 M solution of the obtained polymer at pH 7.0. Incubation with biotin vinyl sulfone was used to prepare the oxidase cholesterol, then immobilized it on the gained polymer usage the interaction of avidin–biotin. The results show the linearity of cholesterol was maximum between 1.5 × 10−5 M and 8.0 × 10−4 M and 7.9% accuracy in the case of 9.0 × 10−5 M while exhibits 14.1% for 2.0 × 10−4 M. In compared to a reference procedure, acceptable outcomes have been presented in real sample test of cholesterol determination utilizing serum samples.
Poly(luminol) was used by the same means to detect health hazard materials such as free chlorine content in drinking water. An optical sensor based on distinguishing the chemiluminescence signal generated by thin immobilized polymeric luminol film was disposed by electro-polymerization method on the planar indium-tin-oxide (ITO) electrode surface.54 The method was executed by several techniques to prepare poly(luminol), including cyclic voltammetry and pulsed amperometry. The data shows that the films prepared using the CV approach came with the highest luminescence signal. At the same time, pulsed amperometry revealed 80% less luminescence and closed to no signal accomplished in case of constant potential electrolysis. In phosphate buffer, reducing in signal intensity was detected at concentrations above 0.5 mM by a quenching mechanism produced by the analyte or the high amount of free chlorine. Hypochlorite detection limit was below 5 × 10−7 M at pH 8.0, and the signal of chemiluminescence exhibits a drop over 1 mM of analyte concentration. Tap water samples were tested to confirm the applicability of the prepared polymer.
Along the same line, the copolymerization of aniline with luminol was also studied and applied in sensor applications. X. Wei and team55 examined the electrochemical deposition of poly(aniline–luminol) onto a nanohybrid of AuAg/TiO2 indium tin oxide coated glass. The modified electrode was employed for flow-injection-analysis (FIA) detection and characterized in cyclic voltammetry. Results show more robust ECL emission performance for target analytes alongside outstanding stability and sensitive response to reactive oxygen species. Subsequently, the determination of FIA mode hydrogen peroxide manifests suitable results upon residue detection in the specimen.
The preparation of biosensors bearing poly(luminol) continued to develop through more hybridization of poly(luminol) with nanoparticles. K. C. Lin et al.56 studied the electroactivity of polymerized luminol combined with poly(neutral red), graphene oxide, and multi-walled carbon nanotubes towards β-nicotinamide adenine dinucleotide [NADH]. The analyte represents one of the coenzymes that plays a significant part in cell energy production and consumption. High sensitivity has been obtained using amperometry of 288.9 μA mM−1 cm−2 to NADH with a concentration between 1.33 × 10−8 to 1.95 × 10−4 M and the limit of detection recorded to be 1.33 × 10−8 M.
In the mold of biosensor detections, research has developed another fabricated poly(luminol) composite sensor for dopamine sensing.57 Electrochemical preparation of poly(luminol–benzidine sulfate) electrode with a constant ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 and diverse concentrations of luminol and benzidine sulfate constructed by cyclic voltammetry was studied. To produce H2O2 for ECL and oxidizing dopamine, the article used tyramine oxidase and immobilized it onto the surface of the modified film, a brief description of copolymer formation mechanism in [Scheme 6]. The results came out with a linear increase of the signal between 1 to 20 nM of dopamine concentration and a detection limit of 0.5 nM. To examined the selectivity, interference study has been applied and it is found that not far from no response to other interferents such as ascorbic acid and uric acid with a 100-fold dopamine concentration.
3 and diverse concentrations of luminol and benzidine sulfate constructed by cyclic voltammetry was studied. To produce H2O2 for ECL and oxidizing dopamine, the article used tyramine oxidase and immobilized it onto the surface of the modified film, a brief description of copolymer formation mechanism in [Scheme 6]. The results came out with a linear increase of the signal between 1 to 20 nM of dopamine concentration and a detection limit of 0.5 nM. To examined the selectivity, interference study has been applied and it is found that not far from no response to other interferents such as ascorbic acid and uric acid with a 100-fold dopamine concentration.
 | ||
| Scheme 6 (A) Electro-polymerization of benzidine sulfate, (B) suggested reaction of composite film formation. | ||
The ongoing formulation of biosensors using the ultrasensitive electrochemiluminescence (ECL) process makes biomolecule analytes a perfect target of poly(luminol) and its composites. Poly(aniline–luminol) constructed with graphene oxide nanocomposite was reviewed to detect alpha-fetoprotein (AFP). For the first time in this part of the review, the chemical approach was used to synthesize poly(luminol) rather than the electrochemical method.58 A straightforward and fast chemical oxidation strategy using (NH4)2S2O8 as an oxidizing agent exhibited exceptional ECL achievement [Fig. 20]. The addition of graphene oxide (GO) surface to the composite brought excellent electron transfer characteristics to the formed poly(aniline–luminol). Through the glutaraldehyde crosslinking method, anti-AFP was immobilized on the surface of a hybrid electrode, and the analytes captured onto the surface by immunoreaction of an antigen–antibody appeared as decreasing ECL intensity. The linear range of 1.7 × 10−12 to 1.7 × 10−8 mg mL−1 displays in ECL immunosensor with low detection limit suggesting a promising biosensor for AFP after human serum sample has been successfully tested and came back with reasonable results [Fig. 21].
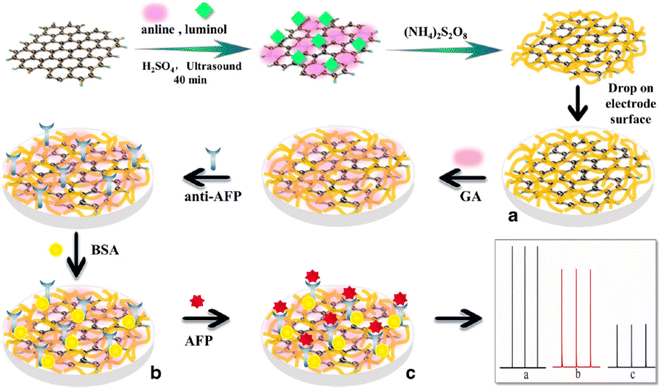 | ||
| Fig. 20 Diagram of AFP immunosensor construction based on the poly(aniline–luminol)/graphene oxide nanocomposite. This figure has been adapted/reproduced from ref. 58 with permission from Springer Nature via CCC marketplace, copyright (2023). | ||
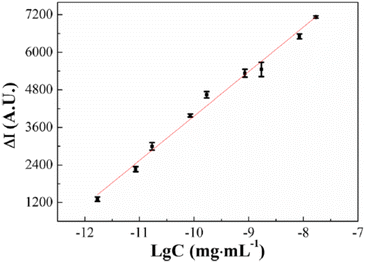 | ||
| Fig. 21 Linear range of 1.7 × 10−12 to 1.7 × 10−8 mg mL−1 AFP immunosensor concentration of ECL response. This figure has been adapted/reproduced from ref. 58 with permission from Springer Nature via CCC marketplace, copyright (2023). | ||
In pharmaceutical formulations, paracetamol was one of the target materials for poly(luminol) fabricated with MWCNTs sensor as well.59 The electropolymerized poly(luminol) was introduced to the GCE surface alongside casting the functionalized multi-walled carbon [Fig. 22]. The sensor efficiency was tested by square wave anodic stripping voltammetry (SWASV) in 40 μM of paracetamol solution at pH 7.0, displaying enhancement of the peak current attributable to the exquisite electronic transformation presented by MWCNTs [Fig. 23 and 24]. The designed electrode has been examined in urine and serum samples to detect the analyte with successfully obtained results. In optimization condition, the interference study results display no interferences were detected in the existence of 200-fold of several materials.
 | ||
| Fig. 22 Electropolymerizing process of luminol voltammogram in 0.5 M sulfuric acid under 50 mV s−1 and for 10 cycles. This figure has been adapted/reproduced from ref. 59 with permission from Springer Nature via CCC marketplace, copyright (2023). | ||
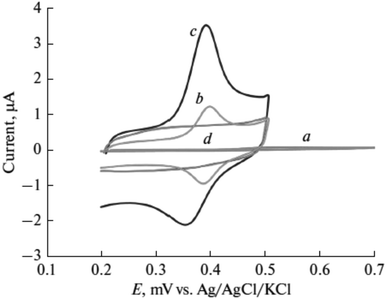 | ||
| Fig. 23 Cyclic voltammetry response of paracetamol (40 μM) at (a) bare electrode, (b) f-MWCNTs on the electrode, (c) poly(luminol/f-MWCNTs), (d) modified electrode in the absence of paracetamol. This figure has been adapted/reproduced from ref. 59 with permission from Springer Nature via CCC marketplace, copyright (2023). | ||
In the same manner, another biosensor has been discussed by Y. Yang and the team using a similar electro pathway to prepare a nanocomposite constructed of poly(luminol–aniline–hemin) to detect one of the vitamin B complexes, choline.60 An enzyme-catalyzed choline oxidase (CHOD) reaction was used to test the sensor performance. In this research, luminol and aniline were co-electropolymerized on the surface of the electrode to construct nanoparticles, and this composite might be employed as nucleation sites in the obtained electropolymerizing procedures. The article considered the catalytic effect of hemin and its ability to merge into the desired hybrid through facile electro-polymerization technique over and above the exhibition of higher intensity response compared with nanocomposites poly(aniline–luminol) alone. The broader linear range for the choline sensing between the concentration of 1 × 10−11 to 1 × 10−7 M in association with applied it to detect choline in milk sample confirms the fabricated film's effectiveness toward the analyte.
One of the latest research developments in this field, using poly(luminol–aniline)/MWCNTs as an inventive biosensor for the hepatitis B virus (HBV) DNA assay, has been published.61 On the electrode surface, the desired coralloid poly(aniline–luminol)-has been fabricated and accompanied by the capture DNA attachment through amide bond construction. A sandwich structure was formed on the electrode surface attributable to ferrocene-labelled DNA hybridized and HBV-DNA. ECL shows an efficient raising of ECL signal output sensor credited to the ferrocene catalysis effect of luminol. The constructed sensor to detect HBV-DNA was tested into both systems with and without H2O2, and the data displays equivalently in characteristics under either scheme, demonstrating the ability to use the presented DNA biosensor excluding H2O2 addition, which make the obtained sensor a has an excellent capability for other DNA sequence detection. In Table 2, sum up of the previous outcomes.
| Sensor | Target | Detection limit | Electrode | Ref. |
|---|---|---|---|---|
| Poly(luminol)/nano-ZnO | Ascorbic acid (vitamin C) | 1 × 10−6 M | GCE | 47 |
| Poly(luminol–benzidine) | H2O2 | 6 × 10−11 M | Graphite electrode | 48 |
| Poly(luminol) | H2O2 | — | Screen-printed electrodes (SPEs) | 49 |
| Choline | ||||
| Poly(luminol–aniline) nanowires composite (PLANC) | Glucose | 3 × 10−8 M | Graphite electrode | 50 |
| Copolymerization of luminol in the presence of different monomers | H2O2 | 2.6 × 10−9 M | Screen-printed gold electrode (gold SPE) | 51 |
| Gold nanoparticles-poly(luminol) (Plu-AuNPs)/MWCNTs | Dopamine (DA) | 1.9 × 10−7 M (DA) | GCE | 52 |
| Uric acid (UA) | 3.8 × 10−7 M (UA) | |||
| Poly(luminol–biotinylated pyrrole) | Cholesterol | 1.47 × 10−5 M | Gold screen-printed cells | 53 |
| Poly(luminol) | Free chlorine content in drinking water | 5 × 10−7 M | Planar indium-tin-oxide (ITO) electrode | 54 |
| Poly(aniline–luminol) | Flow-injection-analysis (FIA) | 1.0 × 10−12 g | AuAg/TiO2 nanohybrid functionalized indium tin oxide coated glass | 55 |
| Poly(luminol) and poly(neutral red)/GO/(MWCNTs) | β-Nicotinamide adenine dinucleotide [NADH] | 1.33 × 10−8 M | GCE | 56 |
| Poly(luminol–benzidine sulfate) | Dopamine | 5 × 10−10 M | Fluorine-doped tin oxide (FTO) glass | 57 |
| Poly(aniline–luminol)/GO nanocomposite | Alpha-fetoprotein (AFP) | 5 × 10−13 mg mL−1 | Chemical polymerization using (NH4)2S2O8 | 58 |
| Poly(luminol)/(f-MWCNTs) | Paracetamol | 2.5 × 10−8 M | GCE | 59 |
| Poly(aniline–luminol–hemin) nanocomposites | Choline | 1.2 × 10−12 M | Graphite electrode | 60 |
| Poly(aniline–luminol)/MWCNTs | The assay of hepatitis B virus (HBV) DNA | 2.4 × 10−17 M | Graphite electrode | 61 |
5. Conclusion and outlook
Comprehensive researchers promoted polymerized safranine and luminol and their composites in sensor applications and the performance of electroactivity constructions. The above finding reveals the high selectivity and sensitivity among other electrochemical properties of poly(safranine) and poly(luminol) as well as their composites in sensing applications which make these moieties a good choice for future discovering. Almost the entire researches focused on the electrochemical oxidative polymerization approach compared to the chemical method due to the countless benefits of the electrochemical process, including the direct deposition of the films on the surface electrode and the easy way to control the film's thickness with almost zero impurities in the process. The above findings give the chemical technique a wide window to explore; diverse oxidizing agents may apply with eco-friendly methods. The analytes in the literature also revolved around biomolecules, and minimal were discussing toxic heavy metals, which leads to more possibilities of target detections using poly(safranine and luminol) to examine.Conflicts of interest
There are no conflicts to declare.References
- X.-G. Li, M.-R. Huang, W. Duan and Y. L. Yang, Novel Multifunctional Polymers from Aromatic Diamines by Oxidative Polymerizations, Chem. Rev., 2002, 102(9), 2925–3030, DOI:10.1021/cr010423z.
- M. Abdel-Azzem, U. S. Yousef and G. Pierre, A cyclic voltammetric and coulometric study of a modified electrode prepared by electrooxidative polymerization of 1,5-diaminonaphthalene in aqueous acidic medium, Eur. Polym. J., 1998, 34, 819–826, DOI:10.1016/S0014-3057(97)00194-8.
- X.-Y. Liu, A Novel Sensor Based on Electropolymerization Poly(safranine) Film Electrode for Voltammetric Determination of 4-Nitrophenol, Bull. Korean Chem. Soc., 2010, 31(5), 1182–1186, DOI:10.5012/bkcs.2010.31.5.1182.
- S. Guo, Q. Zhu, B. Yang, J. Wang and B. Ye, Determination of caffeine content in tea based on poly(safranine T) electroactive film modified electrode, Food Chem., 2011, 129, 1311–1314, DOI:10.1016/j.foodchem.2011.05.095.
- G. Ć.-Marjanović, N. V. Blinova, M. Trchová and J. Stejskal, Chemical Oxidative Polymerization of Safranines, J. Phys. Chem. B, 2007, 111(9), 2188–2199, DOI:10.1021/jp067407w.
- L. Niu, K. Lian, W. Kang and S. Li, Characterization of Poly(Safranine T)-Modified Electrode and Application for Simultaneous Determination of Epinephrine and Uric Acid Coexisting with Ascorbic Acid, J. Braz. Chem. Soc., 2011, 22(2), 204–210, DOI:10.1590/S0103-50532011000200003.
- C. M. Geiselhart, C. B. Kowollik and H. Mutlu, Untapped toolbox of luminol based polymers, Polym. Chem., 2021, 12, 1732–1748, 10.1039/d1py00034a.
- C. A. Marquette and L. J. Blum, Applications of the luminol chemiluminescent reaction in analytical chemistry, Anal. Bioanal. Chem., 2006, 385, 546–554, DOI:10.1007/s00216-006-0439-9.
- C. Dodeigne, L. Thunus and R. Lejeune, Chemiluminescence as diagnostic tool. A review, Talanta, 2000, 51, 415–439, DOI:10.1016/s0039-9140(99)00294-5.
- P. Khan, D. Idrees, M. A. Moxley, J. A. Corbett, F. Ahmad, G. von Figura, W. S. Sly, A. Waheed and M. I. Hassan, Luminol-based chemiluminescent signals: clinical and non-clinical application and future uses, Appl. Biochem. Biotechnol., 2014, 173, 333–355, DOI:10.1007/s12010-014-0850-1.
- A. A. Ensafi, Determination of Ascorbic Acid by Electrocatalytic Voltammetry with Methylene Blue, Anal. Lett., 2003, 36, 591–604, DOI:10.1081/AL-120018250.
- B. Wang and S. Dong, Sol-gel-derived amperometric biosensor for hydrogen peroxide based on methylene green incorporated in Nafion film, Talanta, 2000, 51, 565–572, DOI:10.1016/s0039-9140(99)00315-x.
- X. Y. Liu, C. Y. Li and S. S. Hu, Voltammetric Sensor for the Determination of Parathion Using an Electropolymerized Poly(Carmine) Film Electrode, Microchim. Acta, 2006, 154, 275–280, DOI:10.1007/s00604-006-0518-9.
- M. A. De Paoli and W. A. Gazotti, Electrochemistry, polymers and opto-electronic devices: A combination with a future, J. Braz. Chem. Soc., 2002, 13, 410–424, DOI:10.1590/S0103-50532002000400003.
- S. Cosnier, Biomolecule immobilization on electrode surfaces by entrapment or attachment to electrochemically polymerized films. A review, Biosens. Bioelectron., 1999, 14, 443–456, DOI:10.1016/s0956-5663(99)00024-x.
- H. T. Santoso, Electrochemical processing of polythiophene films with enhanced structural order, MSc thesis, Georgia Institute of Technology, Atlanta, 2011, p. 117.
- Z. Yong, N. Shuji, W. Kazuya and H. Kazuhito, Hydroxylated and aminated polyaniline nanowire networks for improving anode performance in microbial fuel cells, J. Biosci. Bioeng., 2011, 112, 63–66, DOI:10.1016/j.jbiosc.2011.03.014.
- R. Gupta, M. Singhal, S. K. Nataraj and D. N. Srivastava, A potentiostatic approach of growing polyaniline nanofibers in fractal morphology by interfacial electro-polymerization, RSC Adv., 2016, 6, 110416–110421, 10.1039/C6RA21759A.
- M. J. Bleda-Martínez, C. Peng, S. Zhang, G. Z. Chen, E. Morallón and D. Cazorla Amorós, Electrochemical methods to enhance the capacitance in activated carbon/polyaniline composites, J. Electrochem. Soc., 2008, 155, A672–A678, DOI:10.1149/1.2956969.
- J. Heinze, B. A. Frontana-Uribe and S. Ludwigs, Electrochemistry of conducting polymers persistent models and new concepts, Chem. Rev., 2010, 110, 4724–4771, DOI:10.1021/cr900226k.
- W. Schuhmann, C. Kranz, H. Wohlschliiger and J. Strohmeier, Pulse technique for the electrochemical deposition of polymer films on electrode surfaces, Biosens. Bioelectron., 1997, 12, 1157–1167, DOI:10.1016/s0956-5663(97)00086-9.
- H. Okamoto and T. Kotaka, Structure and properties of polyaniline films prepared via electrochemical polymerization. I: Effect of pH in electrochemical polymerization media on the primary structure and acid dissociation constant of product polyaniline films, Polymer, 1998, 39, 4349–4358, DOI:10.1016/S0032-3861(98)00013-5.
- K. Imanishi, M. Satoh, Y. Yasuda, R. Tsushima and S. Aoki, Solvent effect on electrochemical polymerization of aromatic compounds, J. Electroanal. Chem., 1988, 242, 203–208, DOI:10.1016/0022-0728(88)80251-1.
- L. J. Duic, Z. Mandic and F. Kovacicek, The effect of supporting electrolyte on the electrochemical synthesis, morphology, and conductivity of polyaniline, J. Polym. Sci., Part A: Polym. Chem., 1994, 32, 105–111, DOI:10.1002/pola.1994.080320112.
- G. Fomo, T. Waryo, U. Feleni, P. Baker and E. Iwuoha, Electrochemical Polymerization, in Functional Polymers, Polymers and Polymeric Composites: A Reference Series, ed. M. A. Jafar Mazumder, H. Sheardown and A. Al-Ahmed, Springer Nature Switzerland AG, 2019, pp. 105–131, DOI:10.1007/978-3-319-95987-0_3.
- V. Ferreira, A. C. Cascalheira and L. M. Abrantes, Electrochemical preparation and characterization of Poly(Luminol–Aniline) films, Thin Solid Films, 2008, 516, 3996–4001, DOI:10.1016/j.tsf.2007.08.004.
- R. Pauliukaite, A. Selskiene, A. Malinauskas and C. M. A. Brett, Electrosynthesis and characterisation of poly(safranine T) electroactive polymer films, Thin Solid Films, 2009, 517, 5435–5441, DOI:10.1016/j.tsf.2009.01.092.
- N. Y. Abu-Thabit, Chemical Oxidative Polymerization of Polyaniline: A Practical Approach for Preparation of Smart Conductive Textiles, J. Chem. Educ., 2016, 93(9), 1606–1611, DOI:10.1021/acs.jchemed.6b00060.
- I. Sapurina and J. Stejskal, The mechanism of the oxidative polymerization of aniline and the formation of supramolecular polyaniline structures, Polym. Int., 2008, 57, 1295–1325, DOI:10.1002/pi.2476.
- K. Lee, S. Cho, S. H. Park, A. J. Heeger, C.-W. Lee and S.-H. Lee, Metallic transport in polyaniline, Nature, 2006, 441, 65–68, DOI:10.1038/nature04705.
- W. W. Focke, G. E. Wnek and Y. Wei, Influence of oxidation state, pH, and counterion on the conductivity of polyaniline, J. Phys. Chem., 1987, 91, 5813–5818, DOI:10.1021/j100306a059.
- W. Zhong, Y. Wang, Y. Yan, Y. Sun, J. Deng and W. Yang, Fabrication of Shape-Controllable Polyaniline Micro/Nanostructures on Organic Polymer Surfaces: Obtaining Spherical Particles, Wires, and Ribbons, J. Phys. Chem. B, 2007, 111, 3918–3926, DOI:10.1021/jp0678296.
- J. N'Diaye and K. Lian, Investigation of the chemical structure and electrochemical activity of a chemically polymerized luminol, J. Electroanal. Chem., 2019, 839, 90–95, DOI:10.1016/j.jelechem.2019.03.024.
- T. Gan, C. Hua, Z. Chen and S. Hu, A disposable electrochemical sensor for the determination of indole-3-acetic acid based on poly(safranine T)-reduced graphene oxide nanocomposite, Talanta, 2011, 85, 310–316, DOI:10.1016/j.talanta.2011.03.070.
- X. Liu, Electrochemical Sensor for Determination of Parathion Based on Electropolymerization Poly(Safranine) Film Electrode, Int. J. Electrochem., 2011, 1–6, DOI:10.4061/2011/986494.
- J. Sun, Z. Wu, D. Hu, Z. Shi and Z. Lv, Preparation of Reduced Graphene Oxide–Poly(Safranine T) Film via One-Step Polymerization for Electrochemical Determination of Methyl Jasmonate, Fullerenes, Nanotubes, Carbon Nanostruct., 2014, 23, 701–708, DOI:10.1080/1536383X.2014.970685.
- D. Saritha, V. K. Gupta, A. V. B. Reddy, S. Agarwal, M. Moniruzzaman, K. Anitha and G. Madhavi, Development of a Simple, Selective, Stable and Ultrasensitive Poly(safranine/nano NiO) Modified Carbon Paste Electrode for Selective Detection of Rutin in Buckwheat and Green Tea Samples, Int. J. Electrochem. Sci., 2019, 14, 10093–10110, DOI:10.20964/2019.11.48.
- G. Tesfaye, T. Hailu, E. Ele, N. Negash and M. Tessema, Square wave voltammetric determination of quercetin in wine and fruit juice samples at poly (safranine O) modified glassy carbon electrode, Sensing and Bio-Sensing Research, 2021, 34, 100466, DOI:10.1016/j.sbsr.2021.100466.
- B. Dalkiran and C. M. A. Brett, Poly(safranine T)-deep eutectic solvent/copper oxide nanoparticle-carbon nanotube nanocomposite modified electrode and its application to the simultaneous determination of hydroquinone and catechol, Microchem. J., 2022, 179, 107531, DOI:10.1016/j.microc.2022.107531.
- T. Kamidate and H. Watanabe, Peroxidase-catalysed luminol chemiluminescence method for the determination of glutathione, Talanta, 1996, 43, 1733–1738, DOI:10.1016/0039-9140(96)01956-X.
- M. Fisher, S. Harbon and B. R. Rabin, An Amplified Chemiluminescent Assay for the Detection of Alkaline Phosphatase, Anal. Biochem., 1995, 227, 73–79, DOI:10.1006/abio.1995.1254.
- H. Ma, U. Jarzak and W. Thiemann, Synthesis and spectroscopic properties of new luminol-linked calixarene derivatives, Anal. Chim. Acta, 1998, 362, 121–129, DOI:10.1016/S0003-2670(98)00050-6.
- C. E. Taylor IV and S. E. Creager, Electrochemiluminescence-based detection of ferrocene derivatives at monolayer-coated electrodes, J. Electroanal. Chem., 2000, 485, 114–120, DOI:10.1016/S0022-0728(00)00097-8.
- G. Xu and S. Dong, Electrochemiluminescent determination of peroxydisulfate with Cr(bpy)33+ in purely aqueous solution, Anal. Chim. Acta, 2000, 412, 235–240, DOI:10.1016/S0003-2670(00)00723-6.
- O. V. Reshetnyak and E. P. Koval’chuk, A possible scheme of electrochemiluminescence generation on platinum cathodes in aqueous solutions of peroxydisulfates, Electrochim. Acta, 1998, 43, 465–469, DOI:10.1016/S0013-4686(97)00138-2.
- C.-Z. Zhao, N. Egashira, Y. Kurauchi and K. Ohga, Electrochemiluminescence oxalic acid sensor having a platinum electrode coated with chitosan modified with a ruthenium (II) complex, Electrochim. Acta, 1998, 43, 2167–2173, DOI:10.1016/S0013-4686(97)10007-X.
- S. A. Kumar, H.-W. Cheng and S.-M. Chen, Electroanalysis of ascorbic acid (vitamin C) using nano-ZnO/poly(luminol) hybrid film modified electrode, React. Funct. Polym., 2009, 69, 364–370, DOI:10.1016/j.reactfunctpolym.2009.03.001.
- G. Li, X. Zheng and L. Song, Electrochemiluminescence Characterization of Poly(luminol-benzidine) Composite Films and Their Analytical Application, Electroanalysis, 2009, 21(7), 845–852, DOI:10.1002/elan.200804483.
- A. Sassolas, L. J. Blum and B. D. Leca-Bouvier, Polymeric luminol on pre-treated screen-printed electrodes for the design of performant reagentless (bio)sensors, Sens. Actuators, B, 2009, 139, 214–221, DOI:10.1016/j.snb.2009.01.020.
- G. Li, J. Lian, X. Zheng and J. Cao, Electrogenerated chemiluminescence biosensor for glucose based on poly(luminol–aniline) nanowires composite modified electrode, Biosens. Bioelectron., 2010, 26, 643–648, DOI:10.1016/j.bios.2010.07.003.
- J. Ballesta-Claver, M. C. Valencia-Mirón and L. F. Capitán-Vallvey, Copolymerization of luminol on screen-printed cells for single-use electrochemiluminescent sensors, Anal. Bioanal. Chem., 2011, 400, 3041–3051, DOI:10.1007/s00216-011-4965-8.
- D. Jia, J. Daib, H. Yuana, L. Lei and D. Xiaoa, Selective detection of dopamine in the presence of uric acid using a gold nanoparticles-poly(luminol) hybrid film and multi-walled carbon nanotubes with incorporated β-cyclodextrin modified glassy carbon electrode, Talanta, 2011, 85, 2344–2351, DOI:10.1016/j.talanta.2011.07.067.
- J. Ballesta-Clavera, J. Ametis-Cabelloa, J. Morales-Sanfrutosb, A. Megía Fernándezb, M. C. Valencia-Miróna, F. Santoyo-Gonzálezb and L. F. Capitán Vallveya, Electrochemiluminescent disposable cholesterol biosensor based on avidin–biotin assembling with the electroformed luminescent conducting polymer poly(luminol-biotinylated pyrrole), Anal. Chim. Acta, 2012, 754, 91–98, DOI:10.1016/j.aca.2012.10.006.
- M. Szili, I. Kasik, V. Matejec, G. Nagy and B. Kovacs, Poly(luminol) based sensor array for determination of dissolvedchlorine in water, Sens. Actuators, B, 2014, 192, 92–98, DOI:10.1016/j.snb.2013.10.080.
- X. Wei, C. Xiao, C. Liu, K. Wang and Y. Tu, The Sensitized Solid-Phase Electrochemiluminescence of Electrodeposited Poly-Luminol/Aniline on AuAg/TiO2 Nanohybrid Functionalized Electrode for Flow Injection Analysis, Electroanalysis, 2014, 26, 807–814, DOI:10.1002/elan.201300559.
- K. C. Lin, S. Y. Lai and S. M. Chen, A highly sensitive NADH sensor based on a mycelium-like nanocomposite using graphene oxide and multi-walled carbon nanotubes to co-immobilize poly(luminol) and poly(neutral red) hybrid films, Analyst, 2014, 139, 3991–3998, 10.1039/C4AN00536H.
- Y. Wang, S. Hamid, X. Zhang, N. Akhtar, X. Zhang and T. He, An electrochemiluminescent biosensor for dopamine detection using a poly(luminol–benzidine sulfate) electrode modified by tyramine oxidase, New J. Chem., 2017, 41, 1591–1597, 10.1039/C6NJ03338E.
- L. Wei, Y. Zhang, N. Eziz, Y. Yang, G. Li and M. Guan, An ultrasensitive electrochemiluminescence immunosensor for alpha-fetoprotein based on a poly(aniline-luminol)/graphene oxide nanocomposite, Anal. Bioanal. Chem., 2019, 411, 5175–5186, DOI:10.1007/s00216-019-01897-w.
- M. B. Gholivand and E. Ahmadi, Square Wave Anodic Stripping Voltammetric Determination of Paracetamol at Poly Luminol/Functionalized Multi-Walled Carbon Nanotubes Modified Glassy Carbon Electrode, Russ. J. Electrochem., 2019, 55(12), 1151–1161, DOI:10.1134/S102319351912005X.
- Y. Yang, Y. Zhang, L. Wei, G. Li, M. Guan and S. Tian, A Highly Sensitive Electrochemiluminescence Choline Biosensor Based on Poly(aniline-luminol-hemin) Nanocomposites, Electroanalysis, 2019, 31, 624–631, DOI:10.1002/elan.201800582.
- R. Dong, Y. Zhang, J. Huang, M. Habibul and G. Li, Electrochemiluminescence DNA Biosensor for HBV Based on Coralloid Poly(Aniline-Luminol)-MWCNTs and Catalysis of Ferrocene, Electroanalysis, 2022, 34, 1555–1563, DOI:10.1002/elan.202200020.
| This journal is © The Royal Society of Chemistry 2023 |