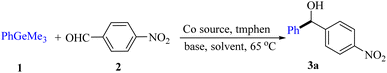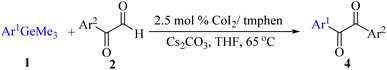Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Co-catalyzed arylation of aldehydes and aryltrimethylgermanes†
Qiang Zhang *,
Xiao Zou,
Ningqi Zhang and
Bo Liu
*,
Xiao Zou,
Ningqi Zhang and
Bo Liu
Shaanxi Key Laboratory of Catalysis, School of Chemistry and Environmental Science, Shaanxi University of Technology, Han zhong, 723001, P. R. China. E-mail: zhangqiang22@126.com
First published on 10th March 2023
Abstract
A novel cobalt-catalyzed protocol for the synthesis of carbinol derivatives and benzil derivatives has been developed. In the presence of CoI2 as the catalyst and tmphen (3,4,7,8-tetramethyl-1,10-phenanthroline) as the ligand, the corresponding arylated products were obtained from the addition of aryltrimethylgermanes to aromatic aldehydes and arylglyoxals in moderate to excellent yields under air atmosphere.
1. Introduction
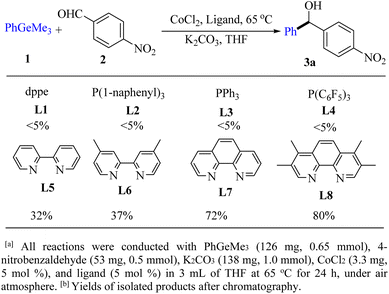
In the past decade, transition metal catalysis has been recognized as a powerful synthetic tool for diarylmethanols through the addition of organometallic reagents.1,2 Organogermanes3 have received much less attention so far, compared with their congeners such as organosilanes4 and organostannanes due to their lower reactivity, the higher cost of germanium relative to silicon5 and the less reported synthetic methodology of organogermanes.5–9 To the best of our knowledge, organogermanes are more susceptible to breaking the C–Ge bond than arylsilane analogues,9 and have lower carbon–metal bond energy and a larger covalent radius than their silicon counterparts in group IVA. However, examples of employing aryltrimethylgermanes in addition reactions have been never reported before. Our previous work10 prompted us to explore the possibility of employing low-cost catalysts in addition reactions. Herein, we report our preliminary results on the first example of cobalt-catalyzed addition of aromatic aldehydes and arylglyoxals with ArGeMe3 using a CoI2/tmphen catalytic system.The reaction of PhGeMe3 (1a) and 4-nitrobenzaldehyde (2a) was firstly chosen as the model reaction for this cobalt-catalyst system (Fig. 1).
Ligands were firstly screened since it often plays an important role in transition-metal-catalyzed chemistry.9 The effects of phosphine ligand with different electron-donating, electron-withdrawing and steric hindrance groups were examined (Fig. 1, L1–L4), but no target product was detected. However, the yield of 3a could be improved to 80% when the combination of CoCl2 and tmphen (L8) was employed (Fig. 1, L8). Subsequently, various reaction conditions concerning the types and amount of cobalt sources, the effects of time and temperature, bases, solvents, were examined to increase the yield of product (Table 1). After extensive screening, the optimized reaction condition was established as follows: CoI2 (2.5 mol%), tmphen (L8, 2.5 mol%), K2CO3 (1.0 mmol), THF (3.0 mL), ArGeMe3 (0.65 mmol) and aldehydes (0.5 mmol), 65 °C, 12 h. Among the bases we used, K2CO3 was superior to other bases such as NaHCO3, Na2CO3, NaOAc, KF, Li2CO3, and Cs2CO3. 14% yield of benzophenone was detected when using Cs2CO3 as the base under model reaction condition (Table 1, entry 7). The choice of solvents was also crucial to the reaction. THF was proved to be the best one of all the solvents we chosed.
| Entry | Catalyst | Base | Solvent | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: 1 (126 mg, 0.65 mmol), 2 (76 mg, 0.5 mmol), cobalt source (5 mol%), tmphen (L8, 5.9 mg, 5 mol%); base (1.0 mmol), solvent (3 mL), 65 °C for 12 h, under air in reaction tubes.b Yields of isolated products after chromatography.c CoI2 (3.9 mg, 2.5 mol%), tmphen (L8, 3.0 mg, 2.5 mol%).d CoI2 (15.6 mg, 10 mol%), tmphen (L8, 11.8 mg, 10 mol%). | ||||
| 1 | — | — | THF | N.R |
| 2 | CoCl2 | — | THF | N.R |
| 3 | CoCl2 | NaHCO3 | THF | 21 |
| 4 | CoCl2 | Na2CO3 | THF | 51 |
| 5 | CoCl2 | NaOAc | THF | 28 |
| 6 | CoCl2 | KF | THF | 53 |
| 7 | CoCl2 | Cs2CO3 | THF | 71 |
| 8 | CoCl2 | K2CO3 | THF | 80 |
| 9 | CoCl2 | K2CO3 | DME | 62 |
| 10 | CoCl2 | K2CO3 | CH3CN | <5 |
| 11 | CoCl2 | K2CO3 | DMF | <5 |
| 12 | CoCl2 | K2CO3 | Dioxane | 37 |
| 13c | CoI2 | K2CO3 | THF | 92 |
| 14d | CoI2 | K2CO3 | THF | 90 |
| 15 | CoI2 | K2CO3 | THF | 87 |
| 16 | CoBr2 | K2CO3 | THF | 67 |
| 17 | Co(OAc)2 | K2CO3 | THF | 58 |
| 18 | Co(C5H5)2 | K2CO3 | THF | 14 |
| 19 | Co3O4 | K2CO3 | THF | <5 |
| 20 | PdCl2 | K2CO3 | THF | <5 |
| 21 | RhCl3·3H2O | K2CO3 | THF | <5 |
Subsequently, various reaction conditions concerning the types and amounts of cobalt sources, the effects of time and temperature, bases, solvents, were examined to increase the yield (Table 1). After extensive screening, the optimized reaction condition was established as follows: CoI2 (2.5 mol%), tmphen (L8, 2.5 mol%), K2CO3 (1.0 mmol), THF (3.0 mL), ArGeMe3 (0.65 mmol) and aldehydes (0.5 mmol), 65 °C, 12 h. Among the bases we used, K2CO3 was superior to other bases such as NaHCO3, Na2CO3, NaOAc, KF, Li2CO3, and Cs2CO3. 14% yield of benzophenone was detected when using Cs2CO3 as the base under model reaction condition (Table 1, entry 7). The choice of solvents was also crucial to the reaction. THF was proved to be the best one of all the solvents we chosed.
With the optimized conditions in hand, a variety of aldehydes with electron-rich, electron-deficient and steric hindrance was examined to broaden the extent of the reaction. Typical functional groups such as methyl, methoxyl, fiuoro, chloro were well tolerated under the reaction conditions. Electron-deficient analogues of aldehyde reacted with ArGeMe3 easily and gave biarylmethanols in good yields (Table 2, entries 1–10). Particularly, 4-formylbenzaldehyde could react with PhGeMe3 and the product of 3n and keep one formyl group untouched (Table 2, entry 14). The chloro and bromo groups untouched in this catalytic system (Table 2, entries 9 and 10). Unfortunately, the reaction was stopped by using aldehydes with neutral and electron-rich groups or aliphatic aldehydes due to its low activity to aryltrimethylgermane under this reaction condition. However, butyraldehyde or 4-methoxybenzaldehyde as substrate react with phenyltrimethylgermane did not give the responding products. Similarly, tetramethylgermane as substrate react with 4-nitro-phenyladehyde also did not give the responding products.
| Entry | Product | Yieldb (%) |
|---|---|---|
| a Reaction conditions: ArGeMe3 1 (0.65 mmol), aldehyde 2 (0.5 mmol), CoI2 (3.9 mg, 2.5 mol%), tmphen (L8, 3.0 mg, 2.5 mol%), K2CO3 (138 mg, 1.0 mmol), THF (3 mL), 65 °C for 12 h, under air in pressure tubes.b Yields of isolated products after chromatography. | ||
| 1 | 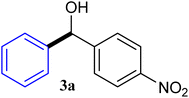 |
92 |
| 2 | 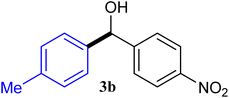 |
94 |
| 3 | 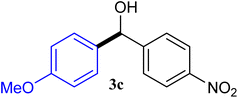 |
93 |
| 4 | 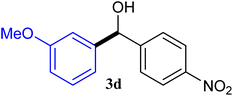 |
93 |
| 5 | 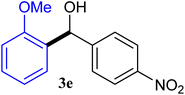 |
91 |
| 6 | 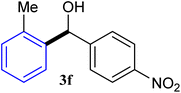 |
92 |
| 7 | 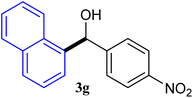 |
93 |
| 8 | 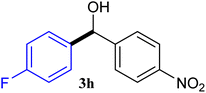 |
95 |
| 9 | 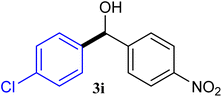 |
92 |
| 10 | 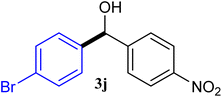 |
91 |
| 11 | 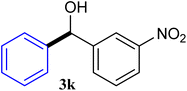 |
85 |
| 12 | 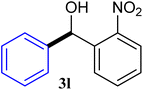 |
76 |
| 13 | 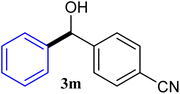 |
87 |
| 14 | 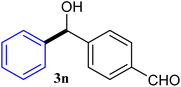 |
93 |
| 15 | 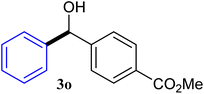 |
55 |
| 16 | 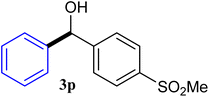 |
91 |
During broadening the extent of the reaction, the phenylglyoxal hydrate was examined to broaden the scope of the reaction, which could be seen as the electron-deficient analogue instead of 4-nitrobenzaldehyde. Only a trace of benzoin formed, the benzil was instead the major final product. Obviously, benzil was resulted from catalytic oxidation of in situ generated benzoin in the presence of K2CO3 in air. It was noteworthy that the over oxidation product could not be detected under argon atmosphere with degassed THF. A more efficient catalytic system with the dual ability to facilitate the addition of ArGeMe3 to phenylglyoxal hydrate was obtained when K2CO3 was exchange by Cs2CO3 as the base. Then, the optimized reaction conditions were then extended to conversions of PhGeMe3 to phenylglyoxal hydrate as follows: CoI2 (3.9 mg, 2.5 mol%), tmphen (L8, 3.0 mg, 2.5 mol%), Cs2CO3 (326 mg, 1.0 mmol), THF (3.0 mL), PhGeMe3(0.65 mmol) and phenylglyoxal hydrate (0.5 mmol), 65 °C, 12 h (Table 3).
| Entry | Product | Yieldb (%) |
|---|---|---|
| a Reaction conditions: ArGeMe3 (0.65 mmol), arylglyoxal (0.5 mmol), CoI2 (3.9 mg, 2.5 mol%), tmphen (L8, 3.0 mg, 2.5 mol%), Cs2CO3 (138 mg, 1.0 mmol), THF (3 mL), 65 °C for 12 h, under air in pressure tubes.b Yields of isolated products after chromatography. | ||
| 1 | 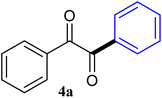 |
95 |
| 2 | 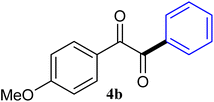 |
94 |
| 3 | 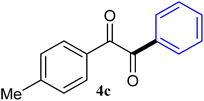 |
93 |
| 4 | 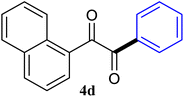 |
92 |
| 5 | 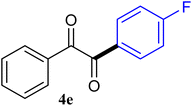 |
91 |
| 6 | 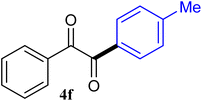 |
93 |
| 7 | 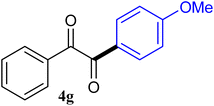 |
92 |
| 8 | 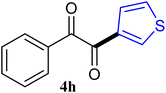 |
67 |
| 9 | 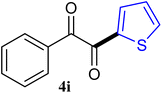 |
59 |
| 10 | 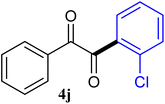 |
78 |
| 11 | 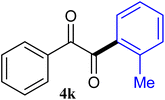 |
87 |
| 12 | 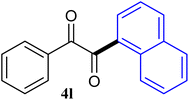 |
91 |
| 13 | 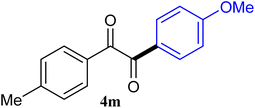 |
92 |
| 14 | 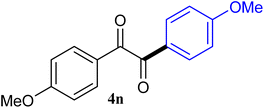 |
94 |
| 15 | 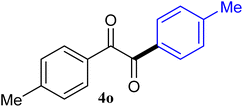 |
93 |
The reactions of different ArGeMe3 with arylglyoxals were examined to broaden the scope of the reaction. All the reactions catalyzed by CoI2/tmphen proceeded well and provided the desired products in good yields. Although the hetero-atoms in the heteroaryltrimethylgermanes might coordinate to transition-metal, trimethyl(thiophen-3-yl)germane and trimethyl(thiophen-2-yl)germane were still good partners for the addition reaction. The corresponding products were isolated in 67% and 59% yields, respectively (Table 3, entries 11 and 12). It seemed that the ortho substituents had little influence on their activities. For instance, (2-chlorophenyl)trimethylgermane, trimethyl(o-tolyl)germane and trimethyl(naphthalen-1-yl)germane could react with phenylglyoxal hydrate to furnish 4j, 4k, and 4l in excellent yields (Table 3, entries 13–15). The comparison of PhGeMe3 and its congener PhSiMe3 was also investigated under the optimised reaction conditions. However, PhSiMe3 was not the proper candidates and recovered the reactants. Similarly, tetramethylgermane as substrate to react phenylglyoxal hydrate did not give the responding products.
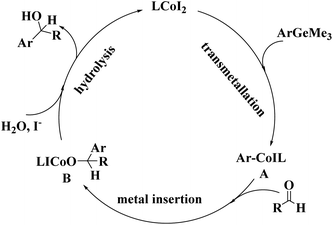
To further understand the mechanism, the model reaction under optimized reaction conditions was studied by gas chromatography-mass spectrometry. The data showed that 1,1′-biphenyl and hexamethyldigermane were the by-products, except for the addition product and the reactants. To account for the present reaction, a plausible mechanism based upon the above experimental results was proposed as follows (Fig. 2).
A plausible mechanism for forming diarylmethanols (Fig. 2): the catalytic cycle may contain three steps: Co(II) undergoes transmetallation to form ArCo(II)IL (A), which exhibits high nucleophilicity toward carbonyl carbon might produce the byproduct with 1,1′-diphenyl and hexamethyldigermane through cross-coupling reaction. Then arylcobalt11 should be transferred to carbonyl carbon through the insertion gives the intermediate (B). Finally, the hydrolysis of intermediate (B) affords the diarylmethanols. Cs2CO3 might facilitate the addition of aryltrimethylgermane to arylglyoxal and prompt the aerobic oxidation of the carbinol; The ICo–OH species reacts with I− to regenerate the active CoI2 for the next cycle.
In summary, we describe here the first time a mild cobalt-catalyzed nucleophilic arylation of aromatic aldehydes and arylglyoxals with ArGeMe3 using CoI2/tmphen catalytic system. In the presence of CoI2/tmphen catalytic system, a variety of electron-deficient arylaldehydes and arylglyoxals was found to be suitable substrates for the reaction with ArGeMe3 in moderate to excellent yields. It was noteworthy that our methodology could keep the formyl group chloro and bromo groups untouched for further functionalization. This method might provide potential opportunities for the addition of ArGeMe3 to unsaturated carbon–carbon bonds and unsaturated carbon-hetero bonds. The detailed mechanism of the reaction and further applications of ArGeMe3 are the focus of ongoing efforts in our laboratory.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are grateful to the Shaanxi Province Education Ministry Research Key Foundation (No. 20JS015) and the Foundation of the Introducing Talents Foundation of Shaanxi University of Technology (No. SLGKYQD2-09) for financial support.Notes and references
- K. Fagnou and M. Lautens, Rhodium-catalyzed carbon-carbon bond forming reactions of organometallic compounds, Chem. Rev., 2003, 103, 169 CrossRef CAS PubMed.
- (a) T. C. Wu, J. J. Chen and Y. T. Wu, Nickel-catalyzed tetramerization of alkynes: Synthesis and structure of octatetraenes, Org. Lett., 2011, 13, 4794 CrossRef CAS PubMed; (b) C. Krug and J. F. Hartwig, Direct observation of aldehyde Insertion into Rhodium-aryl and -alkoxide complexes, J. Am. Chem. Soc., 2002, 124, 1674 CrossRef CAS PubMed; (c) M. Pucheault, S. Darses and J. P. Genet, Direct access to ketones from aldehydes via Rhodium-catalyzed cross-coupling reaction with potassium trifluoro(organo)borates, J. Am. Chem. Soc., 2004, 126, 15356 CrossRef CAS PubMed; (d) S. U. Son, S. B. Kim, J. A. Reingold and G. B. Carpenter, et al., An anionic Rhodium η4-quinonoid complex as a multifunctional catalyst for the arylation of aldehydes with arylboronic acids, J. Am. Chem. Soc., 2005, 127, 12238 CrossRef CAS PubMed; (e) H. F. Duan, J. H. Xie and W. J. Shi, et al., Enantioselective rhodium-catalyzed addition of arylboronic acids to aldehydes using chiral spiro monophosphite ligands, Org. Lett., 2006, 8, 1479 CrossRef CAS PubMed; (f) P. M. Gois, A. F. Trindade and L. F. Veiros, et al., Tuning the reactivity of dirhodium(II) complexes with axial N-heterocyclic carbene ligands: the arylation of aldehydes, Angew. Chem., Int. Ed., 2007, 46, 5750 CrossRef CAS PubMed; (g) T. Yamamoto, T. Ohta and Y. Ito, Palladium-catalyzed addition of arylboronic acids to aldehydes, Org. Lett., 2005, 7, 4153 CrossRef CAS PubMed; (h) K. Suzuki, T. Arao and S. Ishii, et al., Use of cheaper metal than Rh, CHCl3-free Pd catalyst, in 1,2-addition of aromatic aldehydes with arylboronic acids, Tetrahedron, 2006, 47, 5789 CrossRef CAS; (i) P. He, Y. Lu and C. G. Dong, et al., Anionic four-electron donor-based palladacycles as catalysts for addition reactions of arylboronic acids with α,β-unsaturated ketones, aldehydes, and α-ketoesters, Org. Lett., 2007, 9, 343 CrossRef CAS PubMed; (j) S. Lin and X. J. Lu, Cationic Pd(II)/bipyridine-catalyzed addition of arylboronic acids to arylaldehydes. One-pot synthesis of unsymmetrical triarylmethanes, Org. Chem., 2007, 72, 9757 CrossRef CAS PubMed; (k) M. Kuriyama, R. Shimazawa and R. J. Shirai, Efficient 1,2-addition of aryl- and alkenylboronic acids to aldehydes catalyzed by the palladium/thioether-imidazolinium chloride system, Org. Chem., 2008, 73, 1597 CrossRef CAS PubMed; (l) Y. Liu, G. Y. Zhang and H. M. Huang, Ni-catalyzed dimerization and arylation of diarylacetylenes with arylboronic acids, Org. Lett., 2017, 19, 6674 CrossRef CAS PubMed; (m) T. Zou, S. S. Pi and J. H. Li, FeCl3-catalyzed 1,2-addition reactions of aryl aldehydes with arylboronic acids, Org. Lett., 2009, 11, 453 CrossRef CAS PubMed.
- (a) P. G. Peter, The chemistry of organic germanium, tin and lead compounds, Rappoport Z. John Wiley & Sons, Ltd., Chichester, 2002, vol. 2, Parts 1–2 Search PubMed; (b) Germanium in organic synthesis in in main group metals in organic synthesis, ed. H. Yamamoto and K. Oshima, JohnWiley & Sons, 2004, pp. 593–619 Search PubMed; (c) A. C. Spivey, C. J. G. Gripton and J. P. C. Hannah, Recent advances in Group 14 cross-coupling: Si and Ge-based alternatives to the Stille reaction, Org. Synth., 2004, 1, 211–226 CrossRef CAS; (d) J. Karthikeyan, K. Parthasarathy and C. H. Cheng, Synthesis of biarylketones and phthalides from organoboronic acids and aldehydes catalyzed by cobalt complexes, Chem. Commun., 2011, 47, 10461 RSC.
- (a) Metal-catalyzed cross-coupling reactions, ed. A. d. Meijere and F. Diederich, Wiley-VCH, Weinheim, Germany, 2004 Search PubMed; (b) R. Lerebours and C. Wolf, Chemoselective nucleophilic arylation and single-step oxidative esterification of aldehydes using siloxanes and a palladium-phosphinous acid as a reaction switch, J. Am. Chem. Soc., 2006, 128, 13052 CrossRef CAS PubMed.
- Z. T. Zhang, J. P. Pitteloud and L. Cabrera, et al., Arylchlorogermanes/TBAF/“moist” toluene: a promising combination for Pd-catalyzed Germyl-Stille cross-coupling, Org. Lett., 2010, 12, 816 CrossRef CAS PubMed.
- (a) M. Kosugi, T. Tanji and Y. Tanaka, et al., Palladium-catalyzed reaction of 1-aza-5-germa-5-organobicyclo[3.3.3]undecane with aryl bromide, J. Organomet. Chem., 1996, 508, 255 CrossRef CAS; (b) J. W. Faller and R. G. Kultyshev, Palladium-catalyzed cross-coupling reactions of allyl, phenyl, alkenyl, and alkynyl germatranes with aryl iodides, Organometallics, 2002, 21, 5911 CrossRef CAS.
- (a) T. Nakamura, H. Kinoshita and H. Shinokubo, et al., Biaryl synthesis from two different aryl halides with tri(2-furyl)germane, Org. Lett., 2002, 4, 3165 CrossRef CAS PubMed; (b) T. Enokido, K. Fugami and M. Endo, et al., Palladium-catalyzed cross-coupling reaction by means of organogermanium trichlorides, Adv. Synth. Catal., 2004, 346, 1685 CrossRef CAS; (c) M. Endo, K. Fugami and H. Enokido T, et al., Palladium-catalyzed cross-coupling reaction using arylgermanium sesquioxide, Adv. Synth. Catal., 2007, 349, 1025 CrossRef CAS; (d) Z. Wang and S. F. Wnuk, Application of vinyl tris(trimethylsilyl)germanes in Pd-catalyzed couplings, J. Org. Chem., 2005, 70, 3281 CrossRef CAS PubMed.
- (a) A. C. Spivey, C. C. Tseng and J. P. Hannah, et al., Light-fluorous safety-catch arylgermanes-exceptionally robust, photochemically activated precursors for biaryl synthesis by Pd(0) catalyzed cross-coupling, Chem. Commun., 2007, 2926 RSC; (b) A. C. Spivey, C. J. G. Gripton and J. P. Hannah, et al., The development of a ‘safety-catch’ arylgermane for biaryl synthesis by palladium-catalysed Germyl-Stille cross-coupling, Appl. Organomet. Chem., 2007, 21, 572 CrossRef CAS.
- (a) P. Gandeepan, P. Rajamalli and C. H. Cheng, Diastereoselective [3+2] annulation of aromatic/vinylic amides with bicyclic alkenes through cobalt-catalyzed C-H activation and intramolecular nucleophilic addition, Angew. Chem., Int. Ed., 2016, 55, 4308 CrossRef CAS PubMed; (b) S. Prakash, K. Muralirajan and C. H. Cheng, Cobalt-catalyzed oxidative annulation of nitrogen-containing arenes with alkynes: an atom-economical route to heterocyclic quaternary ammonium salts, Angew. Chem., Int. Ed., 2016, 55, 1844 CrossRef CAS PubMed; (c) P. Gandeepan and C. H. Cheng, Cobalt catalysis involving π components in organic synthesis, Acc. Chem. Res., 2015, 48, 1194 CrossRef CAS PubMed; (d) J. Yang, A. Rerat and Y. J. Lim, et al., Cobalt-catalyzed enantio- and diastereoselective intramolecular hydroacylation of trisubstituted alkenes, Angew. Chem., Int. Ed., 2017, 56, 2449 CrossRef CAS PubMed; (e) W. Xu and N. Yoshikai, N-H imine as a powerful directing group for cobalt-catalyzed olefin hydroarylation, Angew. Chem., Int. Ed., 2016, 55, 12731 CrossRef CAS PubMed; (f) J. L. Wu and N. Yoshikai, Cobalt-catalyzed alkenylzincation of unfunctionalized alkynes, Angew. Chem., Int. Ed., 2016, 55, 336 CrossRef CAS PubMed; (g) H. L. Jiang, K. Lang and H. J. Lu, et al., Intramolecular radical aziridination of allylic sulfamoyl azides by cobalt(II)-based metalloradical catalysis: effective construction of strained heterobicyclic structures, Angew. Chem., Int. Ed., 2016, 55, 11604 CrossRef CAS PubMed; (h) Y. H. Chen, S. Grassl and P. Knochel, Cobalt-catalyzed electrophilic amination of aryl- and heteroarylzinc pivalates with N-hydroxylamine benzoates, Angew. Chem., Int. Ed., 2018, 57, 1108 CrossRef CAS PubMed; (i) J. M. Hammann, L. Thomas and Y. H. Chen, et al., Cobalt-catalyzed cross-couplings of bench-stable alkynylzinc pivalates with (hetero)aryl and alkenyl halides, Org. Lett., 2017, 19, 38470 CrossRef PubMed; (j) H. Wang, M. M. Lorion and L. Ackermann, Overcoming the limitations of C-H activation with strongly coordinating N-heterocycles by cobalt catalysis, Angew. Chem., Int. Ed., 2016, 55, 10386 CrossRef CAS PubMed.
- Q. Zhang, C. F. Liu and J. Shi, et al., Palladium-catalyzed N-arylation of amines and amides with aryltrimethylgermanes, Synlett, 2016, 27, 1945 CrossRef CAS.
- T. Mita, S. Hanagata and K. Michigami, et al., Co-catalyzed direct addition of allylic C(sp3)-H bonds to ketones, Org. Lett., 2017, 19, 5876 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra00836c |
| This journal is © The Royal Society of Chemistry 2023 |