DOI:
10.1039/D3RA00892D
(Paper)
RSC Adv., 2023,
13, 11441-11449
Reactions of noble-metal oxides in ionic liquids near room temperature†
Received
9th February 2023
, Accepted 14th March 2023
First published on 11th April 2023
Abstract
The reaction of Ag2O, Au2O3, and HgO with CuCl, CuI, AgCl, AgI, AuCl, and AuI in ionic liquids ([EMIm]Cl, [BMIm]Cl) near room temperature (20–80 °C) is evaluated and results in the new compounds (C8H14N2)CuCl, (C8H14N2)AgI, (C6H10N2)AuCl, [(C8H14N2)2Hg][CuCl3], [(C8H14N2)2Hg][AgCl3], and [EMIm][Ag2I2Cl]. Thereof, (C8H14N2)CuCl, (C8H14N2)AgI, (C6H10N2)AuCl, [(C8H14N2)2Hg][CuCl3], and [(C8H14N2)2Hg][AgCl3] are NHC complexes (NHC: N-heterocyclic carbene) with M–C bonds (M: Cu, Ag, Au, Hg). Whereas (C8H14N2)CuCl and (C8H14N2)AgI crystallize as single molecules, (C6H10N2)AuCl is dimerized via aurophilic interactions. [(C8H14N2)2Hg][CuCl3] and [(C8H14N2)2Hg][AgCl3] exhibit Hg atoms with two Hg–C bonds. Moreover, (C8H14N2)AgI shows intense green fluorescence at room temperature with a quantum yield of 44%, whereas all other compounds do not show any emission at room temperature. Finally, [EMIm][Ag2I2Cl] is not an NHC compound but contains ∞1[AgI1/2I2/4Cl1/2]− chains with infinite d10–d10 interaction of the silver atoms. The title compounds are characterized by single-crystal structure analysis, infrared spectroscopy, thermogravimetry, and fluorescence spectroscopy.
Introduction
Ionic liquids have significantly advanced the synthesis of inorganic compounds and, in the meantime, resulted in several unique compounds that most probably would have not been obtainable in conventional solvents.1 The polychloride [Et4N]2[(Cl3)2·Cl2], the intermetalloid [CuBi8]3+ cluster cation in [CuBi8][AlCl4]2[Al2Cl7], the heavy-metal porphyrin-analogue [Hg4Te8(Te2)4]8− in [DMIm]8[Hg4Te8(Te2)4] ([DMIm]+: 1-decyl-3-methylimidazolium), the ligand-stabilized [Ga5]5+ pentagon, the linear uranyl-type [N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) U
U![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N] complex and supertetrahedral selenido germanate clusters are some exemplary compounds.2 We could contribute the synthesis of the three-dimensional polybromide [C4MPyr]2[(Br)2·(Br2)9], the highly coordinated Sn(II)I8 subunit in the carbonyl [SnI8{Fe(CO)4}4][Al2Cl7]2, or crown-ether coordination compounds like Mn2I4(18-crown-6) with unique fluorescence features such as 100% quantum yield, strong second-harmonic generation (SHG) and intense orange emission via SHG-driven excitation in the infrared spectral range.3 Often, ionic-liquid-based synthesis can be performed near room temperature (≤100 °C), which is ideal to realize new metastable compounds with kinetic control (i.e. at low activation energy).4 Key features of ionic liquids, in this regard, relate to the high thermal and chemical stability and the weakly coordinating properties.1,5
N] complex and supertetrahedral selenido germanate clusters are some exemplary compounds.2 We could contribute the synthesis of the three-dimensional polybromide [C4MPyr]2[(Br)2·(Br2)9], the highly coordinated Sn(II)I8 subunit in the carbonyl [SnI8{Fe(CO)4}4][Al2Cl7]2, or crown-ether coordination compounds like Mn2I4(18-crown-6) with unique fluorescence features such as 100% quantum yield, strong second-harmonic generation (SHG) and intense orange emission via SHG-driven excitation in the infrared spectral range.3 Often, ionic-liquid-based synthesis can be performed near room temperature (≤100 °C), which is ideal to realize new metastable compounds with kinetic control (i.e. at low activation energy).4 Key features of ionic liquids, in this regard, relate to the high thermal and chemical stability and the weakly coordinating properties.1,5
Until now, the synthesis of metastable compounds in ionic liquids was mainly focused on metal halides, metal selenides/tellurides or metal clusters.1–5 The synthesis of new metal oxides and compounds using metal oxides as starting materials, in contrast, was barely addressed, which can be attributed to the high lattice energy of many metal oxides and their low solubility in ionic liquids. Several studies, however, have successfully shown options to dissolve metal oxides in ionic liquids.6 In the course of our studies on ionic-liquid-based syntheses and the optional use of metal oxides as starting materials, we have reacted Ag2O, Au2O3, and HgO with CuCl, CuI, AgCl, AgI, AuCl, and AuI in every combination of the respective metal oxides and metal halides, whereof many lead to new compounds. As results, the novel NHC complexes (NHC: N-heterocyclic carbene) (C8H14N2)CuCl (1), (C8H14N2)AgI (2), (C6H10N2)AuCl (3), [(C8H14N2)2Hg][CuCl3] (4), [(C8H14N2)2Hg][AgCl3] (5), as well as [EMIm][Ag2I2Cl] (6) with an infinite ∞1[AgI1/2I2/4Cl1/2]− chain showing d10–d10 interaction of the silver atoms.
Results and discussion
Ionic-liquid-based synthesis
All reactions were performed using the metal oxides Ag2O, Au2O3, and HgO as well as the metal halides CuCl, CuI, AgCl, AgI, AuCl, and AuI as the starting materials. [EMIm]Cl and [BMIm]Cl ([EMIm]: 1-ethyl-3-methylimidazolium, [BMIm]: 1-butyl-3-methylimidazolium) are introduced to form ionic liquids after reaction with the aforementioned metal halides. All compounds and sample handling needs to be performed with inert conditions in order to avoid hydrolysis. Interestingly, the reactions already start at room temperature (20 °C). For 1 and 4, leaving the ampoule at room temperature for 4 days is sufficient to grow single crystals with a yield of 40–60%. For 2, 3, 5, and 6, the reactions also start at room temperature but were accelerated and completed by sleight heating to 40–80 °C. After 4 days, moisture-sensitive, colourless crystals were formed with good yield (40–60%) (Fig. 1). The synthesis of the title compounds 1–6 can be rationalized based on the following reactions:
| 2C8H15N2Cl + Ag2O + 2CuCl → 2(C8H14N2)CuCl (1) + 2AgCl + H2O |
| 2C6H11N2Cl + Ag2O + 2AuI → 2(C6H10N2)AgI (2) + 2AuCl + H2O |
| 3C6H11N2Cl + 0.5Au2O3 + 3AuCl → 3(C6H10N2)AuCl (3) + AuCl3 + 1.5H2O |
| 2C8H15N2Cl + HgO + CuCl → [(C8H14N2)2Hg][CuCl3] (4) + H2O |
| 2C8H15N2Cl + HgO + AgCl → [(C8H14N2)2Hg][AgCl3] (5) + H2O |
| [EMIm]Cl + 2 AgI* → [EMIm][Ag2I2Cl] (6) (*Note that Ag2O was also present in this reaction) |
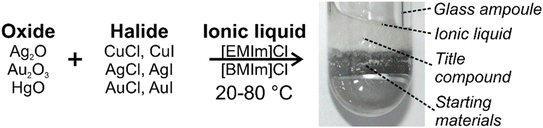 |
| | Fig. 1 Scheme to illustrate the reactions of noble-metal oxides and halides in ionic liquids near room temperature to obtain the title compounds 1–6. | |
In regard of a reaction of Ag2O, Au2O3, and HgO, the metal chlorides CuCl, AgCl, and AuCl turned out to be most reactive, which can be ascribed to the more ionic character in comparison to the respective metal iodides. Although all possible combinations of the respective metal oxides and metal halides were evaluated, some did not result in the formation and crystallization of new compounds, which can be also related to the solubility of the respective metal halide in the ionic liquid. The reaction of Ag2O with AgI is particularly different from all other reactions since the presence of Ag2O was necessary to obtain [EMIm][Ag2I2Cl] although it is not part of the aforementioned reaction. Obviously, the argentophilic interaction in 6 leads to a thermodynamically more stable compound than the formation of a NHC complex. Nevertheless, the Ag+ excess originating from Ag2O is crucial and promotes the argentophilic interactions. However, the dominating reaction relates to the formation of NHC complexes 1–5. Generally, NHC ligands serve as strong sigma-donating ligands and result in a rich coordination chemistry with almost all transition metals.7 Some NHC complexes show promising properties and applications, including homogeneous catalysis with second-generation Grubbs catalysts as one of the most prominent examples.8 Cu–NHC complexes have become popular due to their ability to activate C–H bonds.9 Furthermore, Au– and Pt–NHC complexes received specific attention due to their potential suitability as anti-cancer agents.10 Among the NHC complexes, the ligands essentially contain an imidazole or a derivative as central unit, which is usually modified by sterically demanding groups, such as in 1,3-dimesityl-imidazol-4,5-dihydro-2-ylidene (SIMes).7,11 After the discovery of the very first NHC complex by Wanzlick et al. in 1968,12 NHC complexes were prepared by different approaches, most often using transmetallation strategies.7,13 In addition to these conventional approaches, few examples also show the synthesis of NHC complexes in ionic liquids,6b,14 including electrochemical reduction in ionic liquids.15
Structural characterization
(C8H14N2)CuCl (1) crystallizes in the form of colourless rhombuses in the orthorhombic, non-centrosymmetric and chiral space group P212121 (ESI: Table S1 and Fig. S1†). Herein, a deprotonated imidazole is connected to a CuCl unit via Cu–C bonding (Fig. 2a). The C–Cu–Cl arrangement is almost linear (173.7(2)°) and with a Cu–C distance of 186.9(5) pm (Table 1). Both is in good agreement with literature data (186.6–195.3 pm, 174–180°).16 Similarly, the Cu–Cl distance (210.9(2) pm) is well in agreement with comparable compounds (e.g. 208.8 pm in (C41H32N2O2)CuCl).16a In fact, the composition of 1 was already claimed in a patent to improve bleaching agents. However, its structure and composition were not characterized.17 With [Ag(EMIm-ylide)2][X] (X: SCN, CN2, OAc), Binnemans et al. also showed the synthesis of comparable Ag–NHC complexes in ionic liquids for the first time,6b which were obtained by reaction of Ag2O and the ionic liquid [EMIm][X] (X: SCN, CN2, OAc) only. Despite of comparable experimental conditions, the reaction of Ag2O with CuCl in [BMIm]Cl in our case did not result in an Ag–NHC compound but the Cu–NHC compound 1, which can be ascribed to the presence of CuCl as well as of Ag2O, whereas Binnemans et al. examined the reaction of Ag2O only in the ionic liquid [EMIm][X].6b Taking the reaction mechanism proposed by Binnemans et al. into account,6b we suggest a modified two-step reaction for the formation of 1 (Fig. 2d). First of all, Ag2O as a base subtracts H+ from [BMIm]+ and Cl− coordinates to Ag+. Secondly, the intermediate decomposes with cleavage of the C–H bond and coordination of the carbene to Cu+, followed by the formation of (C8H14N2)CuCl, AgCl, and H2O (Fig. 2d).
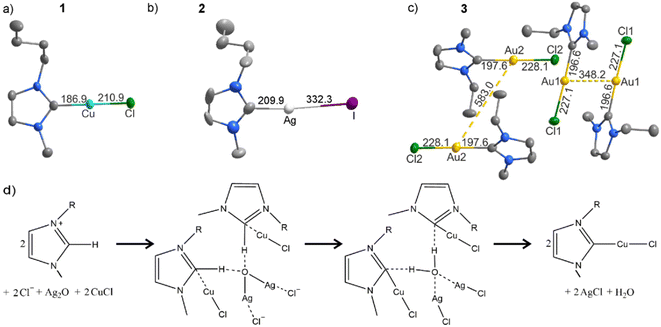 |
| | Fig. 2 Molecular structures of the NHC complexes (C8H14N2)CuCl (1) (a), (C8H14N2)AgI (2) (b) (C6H10N2)AuCl (3) (c), and scheme of the reaction mechanism illustrated for 1 (d). | |
Table 1 Comparison of selected distances in (C8H14N2)CuCl (1), (C6H10N2)AgI (2), (C6H10N2)AuCl] (3), [(C8H14N2)2Hg][CuCl3] (4), and [(C8H14N2)2Hg][AgCl3] (5) with literature data
| Atoms |
Compound |
Distance/pm |
| Cu–Cl |
1 |
210.9(2) |
| |
(C41H32N2O2)CuCl16a |
208.8 |
| |
CuCl27 |
238.2 |
| Cu–C |
1 |
186.9(5) |
| |
(C41H32N2O2)CuCl16a |
186.9 |
| |
(C21H36N2)CuCl16c |
195.3 |
| |
(C27H38N2)CuCl16b |
189.6 |
| Ag–I |
3 |
332.3(2) |
| |
(C21H26N2)AgI18a |
256.6 |
| |
(C27H36N2)AgI18b |
254.7 |
| |
(C27H38N2)AgI18c |
257.4 |
| |
AgI AgI4 |
281.0 |
| Ag–C |
3 |
210.0(2) |
| |
(C21H26N2)AgI18a |
211.3 |
| Au–Cl |
2 |
227.5(3), 228.1(2) |
| |
(C7H12N2)AuCl19a |
228.8 |
| |
(C5H8N2)AuCl20 |
228.8 |
| |
AuCl19b |
230.0 |
| Au–C |
2 |
196.2(11), 197.7(9) |
| |
(C7H12N2)AuCl19a |
198.6 |
| |
(C5H8N2)AuCl20 |
197.9 |
| Hg–Cl |
4 |
299.4(2) |
| |
[S4N3][HgCl3]26 |
234.5, 243.3, 248.5, 302.2, 320.4 |
| |
HgCl2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 32 32 |
228.4, 230.1 |
| Hg–C |
4 |
208.0(5) |
| |
[(C15H12N2)2Hg][ClO4]2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25a 25a |
206.0 |
| |
(C16H18N4)Hg(PF6)2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 34 34 |
206.7 |
| Cu–Cl |
4 |
222.2(3), 227.5(2) |
| |
CuCl27 |
238.2 |
| Hg–Cl |
5 |
304.4(1) |
| |
[S4N3][HgCl3]26 |
234.5, 243.3, 248.5, 302.2, 320.4 |
| |
HgCl2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33 33 |
228.4, 230.1 |
| Hg–C |
5 |
206.8(3) |
| |
[(C15H12N2)2Hg][ClO4]2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25a 25a |
206.0 |
| |
(C16H18N4)Hg(PF6)2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 34 34 |
206.7 |
| Ag–Cl |
5 |
247.0(1), 250.4(1) |
| |
CsAgCl2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28a 28a |
249.2, 266.7, 287.2 |
| |
AgCl28b |
277.3 |
Since we did not observe any crystalline Ag–NHC compound upon reacting of Ag2O with AgCl and AgI, we have performed syntheses with Ag2O, Au2O3, AuCl, and AuI. Here, the compounds (C8H14N2)AgI (2) and (C6H10N2)AuCl (3) were obtained and crystallize in the space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) and Cc (ESI: Table S1, Fig. S2 and S3†). The structure of 2 compares to 1 with a deprotonated imidazole connected to a AgI unit via Ag–C bonding (Fig. 2b). The Ag–C distance is 210.0(2) pm, the Ag–I distance is (332.3(2) pm) (Table 1). Both are well in agreement with literature data (Ag–C: 206–211 pm, Ag–I: 256–257 pm).18 The C–Ag–I arrangement is again almost linear (175.2(5)°). The Au–NHC compound 3 also exhibits structural features similar to 1 and 2, however, with molecular units dimerized via aurophilic interactions (Fig. 2c). Here, Au–C bonding is observed with distances of 196.6(11) and 197.6(9) pm (Table 1) and almost linear C–Au–Cl arrangement (176.8(3)–179.2(3)°). Distance and angle compare to literature data (Au–C: 198 pm, 174–180°).19 Similarly, the Au–Cl distances (227.1(3), 228.1(2) pm) are well in agreement with comparable compounds, such as (C7H12N2)AuCl (228.8 pm) or AuCl (230.0 pm).19a,20 Finally, short intermolecular Au⋯Au distances (348.2(1) pm in 3) are observed that are only slightly larger than the doubled van der Waals radius (332 pm) (Fig. 2c).21 Such aurophilic interactions are characteristic for Au(I) and have been frequently observed.22 A direct single-step formation of Au–NHC compounds such as for 3, to the best of our knowledge, has not been shown in literature before and has yet performed by transmetallation of Ag–NHC compounds in a two-step synthesis.23
and Cc (ESI: Table S1, Fig. S2 and S3†). The structure of 2 compares to 1 with a deprotonated imidazole connected to a AgI unit via Ag–C bonding (Fig. 2b). The Ag–C distance is 210.0(2) pm, the Ag–I distance is (332.3(2) pm) (Table 1). Both are well in agreement with literature data (Ag–C: 206–211 pm, Ag–I: 256–257 pm).18 The C–Ag–I arrangement is again almost linear (175.2(5)°). The Au–NHC compound 3 also exhibits structural features similar to 1 and 2, however, with molecular units dimerized via aurophilic interactions (Fig. 2c). Here, Au–C bonding is observed with distances of 196.6(11) and 197.6(9) pm (Table 1) and almost linear C–Au–Cl arrangement (176.8(3)–179.2(3)°). Distance and angle compare to literature data (Au–C: 198 pm, 174–180°).19 Similarly, the Au–Cl distances (227.1(3), 228.1(2) pm) are well in agreement with comparable compounds, such as (C7H12N2)AuCl (228.8 pm) or AuCl (230.0 pm).19a,20 Finally, short intermolecular Au⋯Au distances (348.2(1) pm in 3) are observed that are only slightly larger than the doubled van der Waals radius (332 pm) (Fig. 2c).21 Such aurophilic interactions are characteristic for Au(I) and have been frequently observed.22 A direct single-step formation of Au–NHC compounds such as for 3, to the best of our knowledge, has not been shown in literature before and has yet performed by transmetallation of Ag–NHC compounds in a two-step synthesis.23
Beside using Ag2O and Au2O3 as a base to obtain NHC compounds, we also have evaluated the reaction of CuCl and AgCl with HgO as a base. In this context, Pelz et al. already pointed to the feasibility of acid–base reactions of imidazolium cations and HgO.24 We have reacted HgO with CuCl or AgCl in [BMIm]Cl as the ionic liquid, which resulted in colourless crystals of [(C8H14N2)2Hg][CuCl3] (4) and [(C8H14N2)2Hg][AgCl3] (5). Both crystallize in the monoclinic space group C2/c (ESI: Table S2, Fig. S4 and S5†). As the most relevant building unit, a central Hg2+ cation bridges two BMIm-ylide units. Furthermore, trigonal planar [(Cu,Ag)Cl3]2− units coordinate with two Cl atoms to the central Hg2+. The third Cl atom of each [(Cu,Ag)Cl3]2− unit is bridging to another Hg2+, so that infinite –[Cl2(Cu,Ag)Cl]–⋯[(C8H14N2)2Hg2+]–[Cl2(Cu,Ag)Cl]−⋯ strands are formed (Fig. 3).
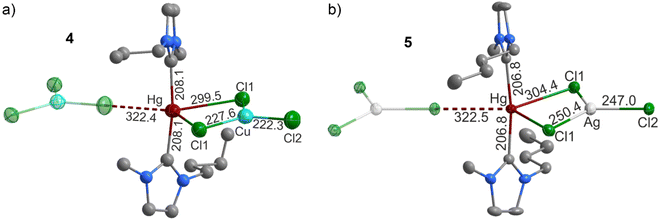 |
| | Fig. 3 Molecular structures of the NHC complexes [(C8H14N2)2Hg][CuCl3] (4) (a) and [(C8H14N2)2Hg][AgCl3] (5) (b). | |
In regard of the distances, Hg–C distances of 208.0(5) pm (4) and 206.8(3) pm (5) fit well with known Hg–NHC compounds (204.6(5)–209.2(5) pm).25 The Hg–Cl distances are 227.6(1) and 299.5(2) pm in 4 as well as 250.4(1) pm and 304.4(1) in 5 for the μ2-binding [(Cu,Ag)Cl3]2− units, which compares to [S4N3][HgCl3] (234.5–320.4 pm).26 μ1-Binding is accompanied with longer Hg⋯Cl distances of 322.4(1) pm in 4 and 322.5(1) pm in 5 (Table 1). The Cu–Cl distances in the [CuCl3]2− unit of 4 with 222.2(3)–227.5(2) pm and are slightly shorter than in CuCl (238.2 pm),27 which reflects the threefold coordination in the title compound as compared to a tetrahedral coordination of Cu+ cation in CuCl. For similar reasons, the Ag–Cl distances of the [AgCl3]2− unit in 5 with 247.0(1)–250.4(1) pm are slightly shorter than in CsAgCl2 (249.2–287.2 pm) or AgCl (277.3 pm).28 Interestingly, for 5 a comparably short Hg⋯Ag distance of 334.5(1) pm is observed, which is slightly above the van der Waals distance (327 pm) but still points to a weak interaction.21 Finally, it needs to be noticed that the butyl chain of the BMIm-ylide in 4 is disordered, which was tackled by split positions for all carbon atoms with a probability of 33 and 67%. Although Hg–NHC complexes are amongst the first described for this class of compounds,7,13,25 the here observed structure and coordination, to the best of our knowledge, are described for the first time. Thus, a coordination of trigonal planar [CuCl3]2−/[AgCl3]2− units in a Hg–NHC complex is new. Trigonal planar [CuCl3]2−/[AgCl3]2− units are rarely observed in general and typically require sterical stabilization by sterically demanding organic ligands,29 or hydrogen bonds.30 In the case of Ag+, such trigonal planar arrangement is known from [PPh4]2[Ag2Cl4], but only with a strongly distorted [Ag2Cl4]2− unit,31 or from the rigid network in the Zintl phase Li17Ag3Sn6.32
In difference to the formation of NHC complexes by the reaction of Ag2O, Au2O3, HgO with CuCl, AgCl, AuCl, the reaction of Ag2O and AgI in [EMIm]Cl did not result in an NHC compound but in the formation of [EMIm][Ag2I2Cl]. 6 crystallizes with colourless needles in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (ESI: Table S2 and Fig. S6†). The compound is composed of infinite anionic ∞1[AgI2/4I1/2Cl1/2]− chains, which are separated by [EMIm]+ cations (Fig. 4). In these chains, the Ag atoms are coordinated by three I and one Cl atom to form distorted Ag(1)I3Cl and Ag(2)I3Cl tetrahedra with two crystallographically independent Ag sites. All AgI3Cl tetrahedra are connected via edge-sharing over all edges to four adjacent AgI3Cl tetrahedra (Fig. 4a). Such edge-sharing is rare and in difference, for instance, to the corner-sharing AgI4 tetrahedra in the binary sphalerite-type AgI.35 Most remarkably, two I atoms in 6 belong to four different AgI3Cl tetrahedra. The Ag–I distances in 6 range from 277.9(1) to 291.9(1) pm, which is comparable to AgI (281.4 pm). Due to the edge-sharing of tetrahedra in 6, the longer Ag–I distances in comparison to AgI with corner-sharing tetrahedra are to be expected. In contrast, the Ag–Cl distances (261.0(2), 261.1(2) pm) in 6 are slightly shorter than in AgCl (277.2 pm),35 which can be attributed to the lower coordination in comparison to NaCl-type AgCl.
(ESI: Table S2 and Fig. S6†). The compound is composed of infinite anionic ∞1[AgI2/4I1/2Cl1/2]− chains, which are separated by [EMIm]+ cations (Fig. 4). In these chains, the Ag atoms are coordinated by three I and one Cl atom to form distorted Ag(1)I3Cl and Ag(2)I3Cl tetrahedra with two crystallographically independent Ag sites. All AgI3Cl tetrahedra are connected via edge-sharing over all edges to four adjacent AgI3Cl tetrahedra (Fig. 4a). Such edge-sharing is rare and in difference, for instance, to the corner-sharing AgI4 tetrahedra in the binary sphalerite-type AgI.35 Most remarkably, two I atoms in 6 belong to four different AgI3Cl tetrahedra. The Ag–I distances in 6 range from 277.9(1) to 291.9(1) pm, which is comparable to AgI (281.4 pm). Due to the edge-sharing of tetrahedra in 6, the longer Ag–I distances in comparison to AgI with corner-sharing tetrahedra are to be expected. In contrast, the Ag–Cl distances (261.0(2), 261.1(2) pm) in 6 are slightly shorter than in AgCl (277.2 pm),35 which can be attributed to the lower coordination in comparison to NaCl-type AgCl.
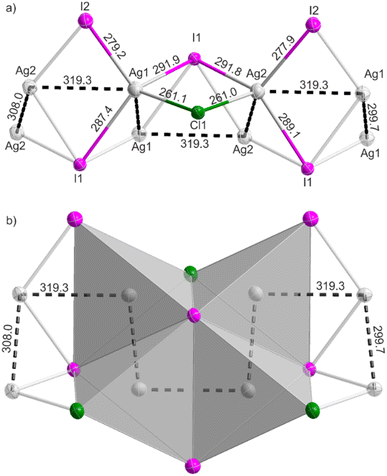 |
| | Fig. 4 ∞1[AgI2/4I1/2Cl1/2]− chain with d10–d10 interaction in [EMIm][Ag2I2Cl] (6). | |
Due to the edge-sharing AgI3Cl tetrahedra, moreover, short Ag–Ag distances are observed in the ∞1[AgI2/4I1/2Cl1/2]− chains of 6 (Fig. 4b). Thus, the Ag–Ag distances are at 299.7(1), 308.0(1), and 319.3(1) pm. All of them are below 330 pm, which was considered as attractive d10–d10 interaction by Jansen.36 Hence, an infinite zig-zag chain of attractive Ag–Ag distances is present in 6. These Ag–Ag distances are of course longer than in elemental silver (288.9 pm), which reflects the positive charge of the Ag+ cations. In principle, compounds with chain-like [Ag2I3]− anions were already described in the literature.37 However, a formation of infinite Ag+ chains with attractive d10–d10 interaction was only discussed for [C8H9N2][Ag2I3] by Zhang.38 This compound has a comparable connectivity of AgI4 tetrahedra as in 6 but only contains iodine and exhibits significantly longer Ag–Ag distances (322.9–334.9 pm) than in 6. Moreover, this compound requires a synthesis in concentrated HI.38 A combination of chlorine and iodine in chain-like halido argentates (I) is generally rare and does not result in Ag chains with attractive d10–d10 interactions.39 In sum, a compound like 6 with infinite ∞1[AgI2/4I1/2Cl1/2]− chains and zig-zag-shaped Ag–Ag chains was obtained for the first time and prepared by ionic-liquid-based synthesis.
Material properties
Beside the structural properties, chemical composition, thermal stability and fluorescence properties of the title compounds were examined by Fourier-transformed infrared (FT-IR) spectroscopy, thermogravimetry (TG), and photoluminescence spectroscopy (PL). FT-IR spectra of the NHC compounds 1, 3–5 are dominated by the imidazolium cations with characteristic vibrations of ν(C–H): 3300–2800 cm−1, ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N): 1650–1600 cm−1, as well as the fingerprint area at 1500–500 cm−1 (ESI: Fig. S7†). Moreover, the Hg–C stretching vibration at 720–680 cm−1 is observed in the spectra of 4 and 5. Similarly, the characteristic vibrations of the [EMIm]+ cation are observed for 6 (ESI: Fig. S8†). The absence of O–H-related vibrations at 3600–3000 cm−1 confirms the absence of moisture.
N): 1650–1600 cm−1, as well as the fingerprint area at 1500–500 cm−1 (ESI: Fig. S7†). Moreover, the Hg–C stretching vibration at 720–680 cm−1 is observed in the spectra of 4 and 5. Similarly, the characteristic vibrations of the [EMIm]+ cation are observed for 6 (ESI: Fig. S8†). The absence of O–H-related vibrations at 3600–3000 cm−1 confirms the absence of moisture.
TG analysis was performed, on the one hand, to examine the thermal stability of the title compounds, and on the other hand, to confirm the chemical composition via total organic combustion analysis (Fig. 5). All title compounds show thermal decomposition with 1 to 3 decompositions steps, which are summarized in Table 2. Except for 4, which starts to decompose already slightly above room temperature, the inset of thermal decomposition is at 150 to 200 °C and is initiated by the decomposition of the EMIm/BMIm-ylides of 1 and 3–5 as well as the [EMIm]+ cation in 6. At higher temperature, HCl, I2, CuCl, AgCl, or HgCl2 evaporate (Table 2). The decomposition ends with the metals (Cu, Ag, Au) or metal halides (CuCl, AgCl) as solid residues. The observed mass loss fits well with the calculated values, and thus, also confirms the composition and purity of the title compounds.
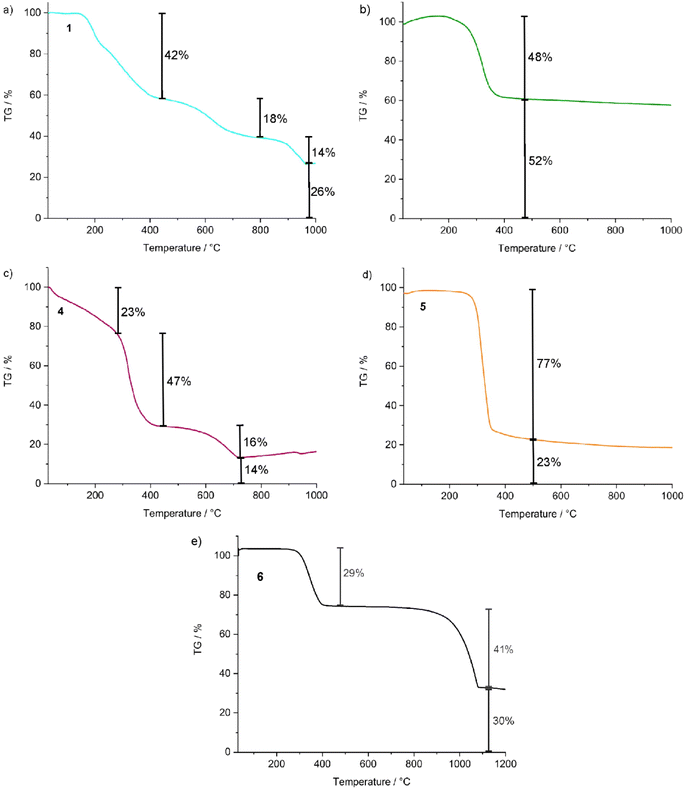 |
| | Fig. 5 TG analysis of the compounds (C8H14N2)CuCl (1), (C6H10N2)AuCl] (3), [(C8H14N2)2Hg][CuCl3] (4), [(C8H14N2)2Hg][AgCl3] (5), and [C6H11N2][Ag2I2Cl] (6). | |
Table 2 TG analysis of the compounds 1, 2, 4, 5 and 6
| Compound |
Temperature range/°C |
Mass loss/% |
Calculated mass loss/% |
| (C8H14N2)CuCl (1) |
140–450 |
42 |
|
| |
450–800 |
18 |
58 (C8H13N2) |
| |
800–960 |
14 |
15 (HCl) |
| |
|
26 (residue) |
27 (Cu) |
| (C6H10N2)AuCl (2) |
200–400 |
42 |
42 (C6H10N2) |
| |
|
58 (residue) |
58 (Au) |
| [(C8H14N2)2Hg][CuCl3] (4) |
20–270 |
23 |
22 (2C5H10) |
| |
270–420 |
47 |
42 (HgCl2) |
| |
500–750 |
16 |
21 (2C3H4N2) |
| |
|
14 (residue) |
15 (CuCl) |
| [(C8H14N2)2Hg][AgCl3] (5) |
250–500 |
77 |
79 (2C8H14N2, HgCl2) |
| |
|
23 (residue) |
21 (AgCl) |
| [C6H11N2][Ag2I2Cl] (6) |
260–420 |
29 |
24 (C6H10N2, HCl) |
| |
800–1100 |
41 |
41 (I2) |
| |
|
30 (residue) |
35 (2Ag) |
Whereas the compounds 1 and 3–6 do not show any PL at room temperature, intense yellow emission is observed for (C8H14N2)AgI (2), which is visible for the naked eye under UV-excitation (Fig. 6). Excitation and emission spectra indicate strong absorption below 400 nm and intense emission at 400–550 nm with a maximum at 572 nm. The efficiency of the photoluminescence processes was quantified with an absolute quantum yield of 44%, following the method given by Friend et al.40 A comparable luminescence was frequently observed for coinage metal complexes but predominately for Cu(I) and Au(I) compounds.41 In contrast, a luminescence of molecular Ag(I) complexes was rarely reported and is usually limited to quantum yields <20% at room temperature.42
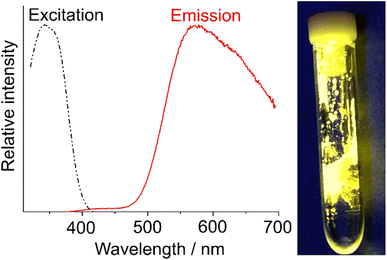 |
| | Fig. 6 PL spectra with excitation and emission of (C8H14N2)AgI (2) as well as a photo of the sample. | |
Conclusions
The noble metal oxides Ag2O, Au2O3, and HgO are reacted with the respective noble metal halides CuCl, CuI, AgCl, AgI, AuCl, and AuI in ionic liquids ([EMIm]Cl, [BMIm]Cl) near room temperature (20–80 °C). Most often, the reactions start already at room temperature. Certain heating accelerates and completes the reaction. As a result, the NHC complexes (C8H14N2)CuCl, (C8H14N2)AgI, (C6H10N2)AuCl, [(C8H14N2)2Hg][CuCl3] and [(C8H14N2)2Hg][AgCl3] are formed as new compounds. They contain EMIm- and BMIm-ylides with Cu–C, Ag–C, Au–C, and Hg–C bonds. (C6H10N2)AuCl is dimerized via aurophilic interactions. [(C8H14N2)2Hg][CuCl3] and [(C8H14N2)2Hg][AgCl3] contain central Hg atoms with two Hg–C bonds of BMIm-ylides. Furthermore, trigonal planar [CuCl3]− and [AgCl3]− connect the [(C8H14N2)2Hg]+ cations via alternating μ1- and μ3-binding to infinite chains. The reaction of Ag2O with AgI in [EMIm]Cl does not result in an NHC compound but yields [EMIm][Ag2I2Cl], which exhibits ∞1[AgI1/2I2/4Cl1/2]− chains with infinite d10–d10 interaction of silver atoms. Structure, composition, and thermal properties of all compounds were examined by single-crystal structure analysis, infrared spectroscopy, and thermogravimetry. Finally, (C8H14N2)AgI shows intense green fluorescence at room temperature with a quantum yield of 44%. Metal oxides, in general, were rarely used as starting materials in ionic-liquid-based synthesis until now, which can be related to their low solubility and reactivity. The here presented reactions and compounds, however, show that metal oxides nevertheless can be used for reactions already at room temperature, which offers various options for chemical synthesis and the formation of new compounds.
Experimental
Synthesis
General. All reactions and sample handling were carried out under dried argon atmosphere using standard Schlenk techniques or glove boxes. Reactions were performed in Schlenk flasks and glass ampoules. The starting materials AgCl (99.9%, Alfa Aesar), Ag2O (≥99%, Alfa Aesar), AuCl (99.9%, Sigma Aldrich), Au2O3 (99%, abcr), CuCl (≥99%, Alfa Aesar), and HgO (99%, Alfa Aesar) were used as received. The ionic liquid [BMIm]Cl (99.9%, IoLiTec) was dried at 130 °C at reduced pressure (1 × 10−3 mbar) for 3 days. All compounds were handled and stored in argon-filled glove boxes (MBraun Unilab, c(O2, H2O) < 0.1 ppm).
(C8H14N2)CuCl (1). 100.0 mg (0.573 mmol) of [BMIm]Cl, 57.0 mg (0.573 mmol) of CuCl, and 50.0 mg (0.216 mmol) of Ag2O were mixed and filled in a glass ampoule. After 4 days at room temperature, colourless rod-like crystals were obtained with a yield of about 40%. The compound is sensitive to moisture and must be handled under inert gas conditions.
(C6H10N2)AgI (2). 100 mg (0.374 mmol) of [BMIm]Cl, 121 mg AuI (0.376 mmol), and 50 mg of Ag2O (0.216 mmol) were mixed and filled in a glass ampoule. This mixture was heated to 80 °C, and the temperature was kept for 96 h. After cooling to room temperature with a rate of 1 K per h, colourless crystals were obtained with a yield of about 40%. These crystals are stable in air.
(C6H10N2)AuCl (3). 100 mg (0.682 mmol) of [EMIm]Cl, 158 mg (0.682 mmol) of AuCl, and 80 mg (0.181 mmol) of Au2O3 were mixed and filled in a glass ampoule. This mixture was heated to 60 °C, and the temperature was kept for 96 h. After cooling to room temperature with a rate of 1 K per h, colourless, moisture-sensitive crystals were obtained with a yield of about 40%.
[(C8H14N2)2Hg][CuCl3] (4). 100.0 mg (0.573 mmol) of [BMIm]Cl, 57.0 mg (0.576 mmol) of CuCl, and 50.0 mg of (0.231 mmol) of HgO were mixed and filled into a glass ampoule. Again, the reaction occurred already at room temperature. After 4 days, rhombic, colourless crystals were obtained with a yield of about 60%. The crystals are highly moisture sensitive and must be handled under inert gas atmosphere.
[(C8H14N2)2Hg][AgCl3] (5). 100.0 mg (0.573 mmol) of [BMIm]Cl, 82.0 mg (0.573 mmol) of AgCl, and 50 mg (0.231 mmol) of HgO were mixed and filled in a glass ampoule. The reaction again started at room temperature. The growth of suitable single crystals was promoted by heating to 80 °C. The ampoule was kept at 80 °C for 96 h and then cooled to room temperature with a rate of 1 K per h. Colourless, moisture-sensitive, rhombic crystals were obtained with a yield of about 50%.
[EMIm][Ag2I2Cl] (6). 100.0 mg (0.682 mmol) of [EMIm]Cl, 160.0 mg (0.682 mmol) of AgCl and 50 mg (0.32 mmol) of Ag2O were mixed in a glass ampoule. The ampoule was heated to 45 °C and kept at this temperature for 96 h. Subsequently, the ampoule was cooled to room temperature with a rate of 1 K per h. Crystals of 5 were obtained as colourless needles with a yield of about 40%. The compound is sensitive to moisture and needs to be handled under inert gas conditions.
Analytical equipment
Detailed information on the analytical equipment can be obtained from the ESI.† Further details of the crystal structure investigation may be obtained from the joint CCDC/FIZ Karlsruhe deposition service on quoting the depository number CSD-No. 2104796–2104800 and 2220642.
Conflicts of interest
There are no conflicts to declare.
Acknowledgements
The authors acknowledge the Deutsche Forschungsgemeinschaft (DFG). Furthermore, the authors thank Prof. Dr Dieter Fenske, Prof. Dr Peter Roesky, and Dr Michael Garmer for data collection on the gallium-jet Stoe StadiVari diffractometer as well as on Stoe Stadi Vari diffractometer.
References
-
(a) T. Zhang, T. Doert, H. Wang, S. Zhang and M. Ruck, Angew. Chem., Int. Ed., 2021, 60, 22148–22165 CrossRef CAS PubMed;
(b) D. Freudenmann, S. Wolf, M. Wolff and C. Feldmann, Angew. Chem., Int. Ed., 2011, 50, 11050–11060 CrossRef CAS PubMed.
-
(a) R. Brueckner, H. Haller, S. Steinhauer, C. Mueller and S. Riedel, Angew. Chem., Int. Ed., 2015, 54, 15579–15583 CrossRef CAS PubMed;
(b) M. Knies, M. Kaiser, A. Isaeva, U. Mueller, T. Doert and M. Ruck, Chem. - Eur. J., 2018, 24, 127–132 CrossRef CAS PubMed;
(c) C. Donsbach, K. Reiter, D. Sundholm, F. Weigend and S. Dehnen, Angew. Chem., Int. Ed., 2018, 57, 8770–8774 CrossRef CAS PubMed;
(d) K. Glootz, D. Himmel, D. Kratzert, B. Butschke, H. Scherer and I. Krossing, Angew. Chem., Int. Ed., 2019, 58, 14162–14166 CrossRef CAS PubMed;
(e) S. S. Rudel, H. L. Deubner, M. Mueller, A. J. Karttunen and F. Kraus, Nat. Chem., 2020, 12, 962–967 CrossRef CAS PubMed;
(f) Z. Wu, I. Nussbruch, S. Nier and S. Dehnen, J. Am. Chem. Soc., 2022, 2, 204–213 CAS.
-
(a) M. Wolff, J. Meyer and C. Feldmann, Angew. Chem., Int. Ed., 2011, 50, 4970–4973 CrossRef CAS PubMed;
(b) S. Wolf, R. Köppe, T. Block, R. Pöttgen, P. W. Roesky and C. Feldmann, Angew. Chem., Int. Ed., 2020, 59, 5510–5514 CrossRef CAS PubMed;
(c) E. Merzlyakova, S. Wolf, S. Lebedkin, L. Bayarjargal, B. L. Neumeier, D. Bartenbach, C. Holzer, W. Klopper, B. Winkler, M. Kappes and C. Feldmann, J. Am. Chem. Soc., 2021, 143, 798–804 CrossRef CAS PubMed.
- C. Feldmann, Angew. Chem., Int. Ed., 2013, 52, 7610–7611 CrossRef CAS PubMed.
-
(a) P. Wasserscheid and T. Welton, Ionic Liquids in Synthesis, Wiley-VCH, Weinheim, 2008 Search PubMed;
(b) B. Kirchner, J. Blasius, V. Alizadeh, A. Gansaeuer and O. Holloczki, J. Phys. Chem. B, 2022, 126, 766–777 CrossRef CAS PubMed.
-
(a) P. Nockemann, B. Thijs, T. N. Parac-Vogt, K. Van Hecke, L. Van Meervelt, B. Tinant, I. Hartenbach, T. Schleid, V. T. Ngan, M. T. Nguyen and K. Binnemans, Inorg. Chem., 2008, 47, 9987–9999 CrossRef CAS PubMed;
(b) S. Wellens, N. R. Brooks, B. Thijs, L. Van Meervelt and K. Binnemans, Dalton Trans., 2014, 43, 3443–3452 RSC;
(c) J. Richter and M. Ruck, RSC Adv., 2019, 9, 29699–29710 RSC;
(d) S. Wolf, S. Seidel, J. Treptow, R. Köppe, P. Roesky and C. Feldmann, Inorg. Chem., 2022, 61, 4018–4023 CrossRef CAS PubMed.
-
(a) P. P. Nair, A. Jayaraj and C. A. Swamy, ChemistrySelect, 2022, 7, e202103517 CrossRef CAS;
(b) Q. Zhao, G. Meng, M. Szostak and S. P. Nolan, Chem. Rev., 2020, 120, 1981–2048 CrossRef CAS PubMed;
(c) W. A. Herrmann and C. Kocher, Angew. Chem., Int. Ed., 1997, 36, 2162–2187 CrossRef CAS.
-
(a) S. S. Bera and M. Szostak, ACS Catal., 2022, 12, 3111–3137 CrossRef CAS PubMed;
(b) P. Schwab, R. H. Grubbs and J. W. Ziller, J. Am. Chem. Soc., 1996, 118, 100–110 CrossRef CAS.
-
(a) Y.-H. Wen, Z.-J. Zhang, S. Li, J. Song and L.-Z. Gong, Nat. Commun., 2022, 13, 1344 CrossRef CAS;
(b) W. Xie and S. Chang, Angew. Chem., Int. Ed., 2016, 55, 1876–1880 CrossRef CAS.
-
(a) S. K. Goetzfried, P. Kapitza, C. M. Gallati, A. Nindl, M. Cziferszky, M. Hermann, K. Wurst, B. Kircher and R. Gust, Dalton Trans., 2022, 51, 1395–1406 RSC;
(b) M. Bian, R. Fan, Z. Yang, Y. Chen, Z. Xu, Y. Lu and W. Liu, J. Med. Chem., 2022, 65, 1848–1866 CrossRef CAS PubMed.
- M.-M. Gan, J.-Q. Liu, L. Zhang, Y. Y. Wang, F. E. Hahn and Y.-F. Han, Chem. Rev., 2018, 118, 9587–9641 CrossRef CAS.
- H.-W. Wanzlick and H.-J. Schönherr, Angew. Chem., Int. Ed., 1968, 7, 141–142 CrossRef CAS.
- F. E. Hahn and M. C. Jahnke, Angew. Chem., Int. Ed., 2008, 47, 3122–3172 CrossRef CAS PubMed.
- C. Donsbach and S. Dehnen, Eur. J. Inorg. Chem., 2018, 4429–4433 CrossRef CAS.
-
(a) I. Chiarotto, M. Feroci and A. Inesi, New J. Chem., 2017, 41, 7840–7843 RSC;
(b) I. Chiarotto, M. Feroci, G. Sotgiu and A. Inesi, Eur. J. Org. Chem., 2013, 326–331 CrossRef CAS.
-
(a) C. Liu, H.-Q. Shen, M.-W. Chen and Y.-G. Zhou, Organomet, 2018, 37, 3756–3769 CrossRef CAS;
(b) S. Díez-González, E. D. Stevens and S. P. A. Nolan, Chem. Commun., 2008, 4747–4749 RSC;
(c) H. Kaur, F. K. Zinn, E. D. Stevens and S. P. Nolan, Organomet, 2004, 23, 1157–1160 CrossRef CAS.
- M. B. Abrams and X. Zhang, Metallocarbene Complex Peroxideactivators, US pat. 8414793B, 2013.
-
(a) M. Iglesias, D. J. Beetstra, J. C. Knight, L.-L. Ooi, A. Stasch, S. Coles, L. Male, M. B. Hursthouse, K. J. Cavell, A. Dervisi and I. A. Fallis, Organomet, 2008, 27, 3279–3289 CrossRef CAS;
(b) D. V. Partyka and N. Deligonul, Inorg. Chem., 2009, 48, 9463–9475 CrossRef CAS PubMed;
(c) V. H. L. Wong, A. J. P. White, T. S. A. Hor and K. K. M. Hii, Chem. Commun., 2015, 51, 17755 Search PubMed;
(d) R. Bloch and H. Möller, Z. Phys. Chem. A, 1930, 152, 245–248 Search PubMed.
-
(a) P. de Frémont, N. M. Scott, E. D. Stevens and S. P. Nolan, Organomet, 2005, 24, 2411–2418 CrossRef;
(b) J. Strähle and K.-P. Lörcher, Naturforsch. B, 1974, 29, 266–267 CrossRef.
- H. M. J. Wang, C. S. Vasam, T. Y. R. Tsai, S.-H. Chen, A. H. H. Chang and I. J. B. Lin, Organomet, 2005, 24, 486–493 CrossRef CAS.
- A. Bondi, J. Phys. Chem., 1964, 68, 441–451 CrossRef CAS.
- H. Schmidbaur and A. Hubert, Chem. Soc. Rev., 2012, 41, 370–412 RSC.
- K. U. N. Sheikh, H. Amin, R. A. Haque, A. S. Abdul Majid, M. Yaseen and M. A. Iqbal, J. Coord. Chem., 2021, 74, 467–542 CrossRef CAS.
- S. Pelz and F. Mohr, Organomet., 2011, 30, 383–385 CrossRef CAS.
-
(a) A. J. Arduengo, R. L. Harlow, W. J. Marshall and T. K. Prakasha, Heteroat. Chem., 1996, 7, 421–426 CrossRef CAS;
(b) A. J. Arduengo, D. Tapu and W. J. Marshall, Angew. Chem., Int. Ed., 2005, 44, 7240–7244 CrossRef CAS PubMed;
(c) S.-C. Chen, H.-H. Hsueh, C.-H. Chen, C. S. Lee, F.-C. Liu, I. J. B. Lin, G.-H. Lee and S.-M. Peng, Inorg. Chim. Acta, 2009, 362, 3343–3350 CrossRef CAS.
- K. Weidenhammer and M. L. Ziegler, Z. Anorg. Allg. Chem., 1977, 434, 152–156 CrossRef CAS.
- R. W. G. Wyckoff and E. Posnjak, J. Am. Chem. Soc., 1922, 44, 30–36 CrossRef CAS.
-
(a) H.-C. Gaebell, G. Meyer and R. Hoppe, Z. Anorg. Allg. Chem., 1983, 497, 199–205 CrossRef CAS;
(b) S. Hull and D. A. Keen, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 59, 750–761 CrossRef CAS.
-
(a) A. Beheshti, W. Clegg, V. Nobakht, M. Panahi Mehra and L. Russo, Dalton Trans., 2008, 6641–6646 RSC;
(b) R. Balamurugan, M. Palaniandavar and R. S. Gopalan, Inorg. Chem., 2001, 40, 2246–2255 CrossRef CAS PubMed;
(c) M. R. Churchill and F. J. Rotella, Inorg. Chem., 1979, 18, 166–171 CrossRef CAS;
(d) O. Crespo, M. C. Gimeno, P. G. Jones and A. Laguna, J. Chem. Soc., Dalton Trans., 1996, 4583–4588 RSC;
(e) S. Y. Lee, S. Parka and S. S. Lee, Inorg. Chem., 2009, 48, 11335–11341 CrossRef CAS;
(f) C.-Y. Su, Y.-P. Cai, C.-L. Chen, M. D. Smith, W. Kaim and H.-C. zur Loye, J. Am. Chem. Soc., 2003, 125, 8595–8613 CrossRef CAS PubMed.
-
(a) L. Xian, T.-B. Wei and Y.-M. Zhang, J. Coord. Chem., 2004, 57, 453–457 CrossRef CAS;
(b) F. A. Devillanova, A. Diaz, F. Isaia, G. Verani, L. P. Battaglia and A. B. Corradi, J. Coord. Chem., 1986, 15, 161–172 CrossRef CAS.
- G. Helgesson and S. Jagner, J. Chem. Soc., Dalton Trans., 1988, 2117–2120 RSC.
- C. Lupu, C. Downie, A. M. Guloy, T. A. Albright and J.-G. Mao, J. Am. Chem. Soc., 2004, 126, 4386–4397 CrossRef CAS PubMed.
- V. Subramanian and K. Seff, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1980, 36, 2132–2135 CrossRef.
- R. A. Haque, A. W. Salman, T. S. Guan and H. H. Abdallah, J. Organomet. Chem., 2011, 696, 3507–3512 CrossRef CAS.
- S. Hull and D. A. Keen, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 59, 750–761 CrossRef CAS.
- M. Jansen, Angew. Chem., 1987, 26, 1098–1110 CrossRef.
-
(a) T. Sörgel and M. Jansen, Z. Kristallogr. NCS, 2007, 222, 20–22 Search PubMed;
(b) Y.-K. Wang, L.-M. Zhao, Y.-Q. Fu, Z. Chen, X.-Y. Lin, D.-H. Wang, Y. Li, H.-H. Li and Z.-R. Chen, Cryst. Growth Des., 2018, 18, 3827–3840 CrossRef CAS;
(c) B. Xu, R. Wang and X. Wang, Nanoscale, 2012, 4, 2713–2719 RSC.
- C. Zhang, J. Shen, Q. Guan, T. Yu and Y. Fu, Solid State Sci., 2015, 46, 14–18 CrossRef.
-
(a) G. A. Bowmaker, Y. Effend, J. D. Kildea, B. W. Skelton and A. H. White, Aust. J. Chem., 1990, 43, 2113 CrossRef CAS;
(b) R. Frydrych, T. Muschter, I. Brüdgam and H. Hartl, Z. Naturforsch., 1990, 45b, 679–688 CrossRef;
(c) K. Rosenstengel, A. Schulz, O. Niehaus, O. Janka, R. Pöttgen and A. Villinger, Eur. J. Inorg. Chem., 2018, 778–790 CrossRef CAS.
- J. C. de Mello, H. F. Wittmann and R. H. Friend, Adv. Mater., 1997, 9, 230–232 CrossRef CAS.
-
(a) J. M. Lopez-de-Luzuriaga, M. Monge and M. E. Olmos, Dalton Trans., 2017, 46, 2046–2067 RSC;
(b) R. C. Evans, P. Douglas and C. J. Winscom, J. Coord. Chem. Rev., 2006, 250, 2093–2126 CrossRef CAS.
-
(a) H.-M. Pan, Y.-Y. Ma, D.-Y. Li, S. Wu and Z. Jing, J. Solid State Chem., 2022, 307, 122814 CrossRef CAS;
(b) M. Klein, N. Rau, M. Wende, J. Sundermeyer, G. Cheng, C.-M. Che, A. Schinabeck and H. Yersin, Chem. Mater., 2020, 32, 10365–10382 CrossRef CAS;
(c) T. Teng, K. Li, G. Cheng, Y. Wang, J. Wang, J. Li, C. Zhou, H. Liu, T. Zou, J. Xiong, C. Wu, H.-X. Zhang, C.-M. Che and C. Yang, Inorg. Chem., 2020, 59, 12122–12131 CrossRef CAS PubMed;
(d) R. Hamze, S. Shi, S. C. Kapper, D. S. Muthiah Ravinson, L. Estergreen, M.-C. Jung, A. C. Tadle, R. Haiges, P. I. Djurovich, J. L. Peltier, R. Jazzar, G. Bertrand, S. E. Bradforth and M. E. Thompson, J. Am. Chem. Soc., 2019, 141, 8616–8626 CrossRef CAS PubMed;
(e) M. Z. Shafikov, R. Czerwieniec and H. Yersin, Dalton Trans., 2019, 48, 2802–2806 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Additional data related to the analytical techniques, the crystallographic data, and the unit cells of the title compounds 1–6. CCDC 2104796–2104800 and 2220642. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3ra00892d |
|
| This journal is © The Royal Society of Chemistry 2023 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article *
*
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) U
U![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N] complex and supertetrahedral selenido germanate clusters are some exemplary compounds.2 We could contribute the synthesis of the three-dimensional polybromide [C4MPyr]2[(Br)2·(Br2)9], the highly coordinated Sn(II)I8 subunit in the carbonyl [SnI8{Fe(CO)4}4][Al2Cl7]2, or crown-ether coordination compounds like Mn2I4(18-crown-6) with unique fluorescence features such as 100% quantum yield, strong second-harmonic generation (SHG) and intense orange emission via SHG-driven excitation in the infrared spectral range.3 Often, ionic-liquid-based synthesis can be performed near room temperature (≤100 °C), which is ideal to realize new metastable compounds with kinetic control (i.e. at low activation energy).4 Key features of ionic liquids, in this regard, relate to the high thermal and chemical stability and the weakly coordinating properties.1,5
N] complex and supertetrahedral selenido germanate clusters are some exemplary compounds.2 We could contribute the synthesis of the three-dimensional polybromide [C4MPyr]2[(Br)2·(Br2)9], the highly coordinated Sn(II)I8 subunit in the carbonyl [SnI8{Fe(CO)4}4][Al2Cl7]2, or crown-ether coordination compounds like Mn2I4(18-crown-6) with unique fluorescence features such as 100% quantum yield, strong second-harmonic generation (SHG) and intense orange emission via SHG-driven excitation in the infrared spectral range.3 Often, ionic-liquid-based synthesis can be performed near room temperature (≤100 °C), which is ideal to realize new metastable compounds with kinetic control (i.e. at low activation energy).4 Key features of ionic liquids, in this regard, relate to the high thermal and chemical stability and the weakly coordinating properties.1,5


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 32
32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25a
25a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 34
34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33
33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25a
25a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 34
34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28a
28a![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) and Cc (ESI: Table S1, Fig. S2 and S3†). The structure of 2 compares to 1 with a deprotonated imidazole connected to a AgI unit via Ag–C bonding (Fig. 2b). The Ag–C distance is 210.0(2) pm, the Ag–I distance is (332.3(2) pm) (Table 1). Both are well in agreement with literature data (Ag–C: 206–211 pm, Ag–I: 256–257 pm).18 The C–Ag–I arrangement is again almost linear (175.2(5)°). The Au–NHC compound 3 also exhibits structural features similar to 1 and 2, however, with molecular units dimerized via aurophilic interactions (Fig. 2c). Here, Au–C bonding is observed with distances of 196.6(11) and 197.6(9) pm (Table 1) and almost linear C–Au–Cl arrangement (176.8(3)–179.2(3)°). Distance and angle compare to literature data (Au–C: 198 pm, 174–180°).19 Similarly, the Au–Cl distances (227.1(3), 228.1(2) pm) are well in agreement with comparable compounds, such as (C7H12N2)AuCl (228.8 pm) or AuCl (230.0 pm).19a,20 Finally, short intermolecular Au⋯Au distances (348.2(1) pm in 3) are observed that are only slightly larger than the doubled van der Waals radius (332 pm) (Fig. 2c).21 Such aurophilic interactions are characteristic for Au(I) and have been frequently observed.22 A direct single-step formation of Au–NHC compounds such as for 3, to the best of our knowledge, has not been shown in literature before and has yet performed by transmetallation of Ag–NHC compounds in a two-step synthesis.23
and Cc (ESI: Table S1, Fig. S2 and S3†). The structure of 2 compares to 1 with a deprotonated imidazole connected to a AgI unit via Ag–C bonding (Fig. 2b). The Ag–C distance is 210.0(2) pm, the Ag–I distance is (332.3(2) pm) (Table 1). Both are well in agreement with literature data (Ag–C: 206–211 pm, Ag–I: 256–257 pm).18 The C–Ag–I arrangement is again almost linear (175.2(5)°). The Au–NHC compound 3 also exhibits structural features similar to 1 and 2, however, with molecular units dimerized via aurophilic interactions (Fig. 2c). Here, Au–C bonding is observed with distances of 196.6(11) and 197.6(9) pm (Table 1) and almost linear C–Au–Cl arrangement (176.8(3)–179.2(3)°). Distance and angle compare to literature data (Au–C: 198 pm, 174–180°).19 Similarly, the Au–Cl distances (227.1(3), 228.1(2) pm) are well in agreement with comparable compounds, such as (C7H12N2)AuCl (228.8 pm) or AuCl (230.0 pm).19a,20 Finally, short intermolecular Au⋯Au distances (348.2(1) pm in 3) are observed that are only slightly larger than the doubled van der Waals radius (332 pm) (Fig. 2c).21 Such aurophilic interactions are characteristic for Au(I) and have been frequently observed.22 A direct single-step formation of Au–NHC compounds such as for 3, to the best of our knowledge, has not been shown in literature before and has yet performed by transmetallation of Ag–NHC compounds in a two-step synthesis.23
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (ESI: Table S2 and Fig. S6†). The compound is composed of infinite anionic ∞1[AgI2/4I1/2Cl1/2]− chains, which are separated by [EMIm]+ cations (Fig. 4). In these chains, the Ag atoms are coordinated by three I and one Cl atom to form distorted Ag(1)I3Cl and Ag(2)I3Cl tetrahedra with two crystallographically independent Ag sites. All AgI3Cl tetrahedra are connected via edge-sharing over all edges to four adjacent AgI3Cl tetrahedra (Fig. 4a). Such edge-sharing is rare and in difference, for instance, to the corner-sharing AgI4 tetrahedra in the binary sphalerite-type AgI.35 Most remarkably, two I atoms in 6 belong to four different AgI3Cl tetrahedra. The Ag–I distances in 6 range from 277.9(1) to 291.9(1) pm, which is comparable to AgI (281.4 pm). Due to the edge-sharing of tetrahedra in 6, the longer Ag–I distances in comparison to AgI with corner-sharing tetrahedra are to be expected. In contrast, the Ag–Cl distances (261.0(2), 261.1(2) pm) in 6 are slightly shorter than in AgCl (277.2 pm),35 which can be attributed to the lower coordination in comparison to NaCl-type AgCl.
(ESI: Table S2 and Fig. S6†). The compound is composed of infinite anionic ∞1[AgI2/4I1/2Cl1/2]− chains, which are separated by [EMIm]+ cations (Fig. 4). In these chains, the Ag atoms are coordinated by three I and one Cl atom to form distorted Ag(1)I3Cl and Ag(2)I3Cl tetrahedra with two crystallographically independent Ag sites. All AgI3Cl tetrahedra are connected via edge-sharing over all edges to four adjacent AgI3Cl tetrahedra (Fig. 4a). Such edge-sharing is rare and in difference, for instance, to the corner-sharing AgI4 tetrahedra in the binary sphalerite-type AgI.35 Most remarkably, two I atoms in 6 belong to four different AgI3Cl tetrahedra. The Ag–I distances in 6 range from 277.9(1) to 291.9(1) pm, which is comparable to AgI (281.4 pm). Due to the edge-sharing of tetrahedra in 6, the longer Ag–I distances in comparison to AgI with corner-sharing tetrahedra are to be expected. In contrast, the Ag–Cl distances (261.0(2), 261.1(2) pm) in 6 are slightly shorter than in AgCl (277.2 pm),35 which can be attributed to the lower coordination in comparison to NaCl-type AgCl.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N): 1650–1600 cm−1, as well as the fingerprint area at 1500–500 cm−1 (ESI: Fig. S7†). Moreover, the Hg–C stretching vibration at 720–680 cm−1 is observed in the spectra of 4 and 5. Similarly, the characteristic vibrations of the [EMIm]+ cation are observed for 6 (ESI: Fig. S8†). The absence of O–H-related vibrations at 3600–3000 cm−1 confirms the absence of moisture.
N): 1650–1600 cm−1, as well as the fingerprint area at 1500–500 cm−1 (ESI: Fig. S7†). Moreover, the Hg–C stretching vibration at 720–680 cm−1 is observed in the spectra of 4 and 5. Similarly, the characteristic vibrations of the [EMIm]+ cation are observed for 6 (ESI: Fig. S8†). The absence of O–H-related vibrations at 3600–3000 cm−1 confirms the absence of moisture.



