Open Access Article
Open Access ArticleSilver nanoparticles deposited on a cotton fabric surface via an in situ method using reactive hyperbranched polymers and their antibacterial properties
Hongxia Chena,
Guangyu Zhang b,
Wei Zhang
b,
Wei Zhang *b and
Weidong Gao*a
*b and
Weidong Gao*a
aCollege of Textile Science and Engineering, Jiangnan University, Wuxi 214122, P. R. China. E-mail: gaowd3@163.com
bSchool of Textile and Clothing, Nantong University, Nantong 226019, P. R. China. E-mail: zhangwei@ntu.edu.cn
First published on 12th April 2023
Abstract
This study introduces a new method for the synthesis of silver nanoparticles on a cotton fabric surface by an in situ method. Reactive hyperbranched polymer (EPDA-HBP) was synthesized using epoxy chloropropane dimethylamine and amino hyperbranched polymer. Then, the fabric was modified with reactive hyperbranched polymer to obtain the amino-grafted fabric. The prepared fiber can complex Ag+ and convert Ag+ to Ag0 through the reducibility of amino acids. EPDA-HBP-grafted cotton fibers and silver nanoparticle-coated fibers were then characterized by FTIR, antibacterial, FE-SEM, EDS, and XPS methods. FE-SEM, EDS, and XPS indicated that Ag NPs were uniformly coated on the cotton fabric. FTIR results confirmed that EPDA-HBP was grafted onto the surface of cotton fiber. When the Ag content was more than 180 mg kg−1, the treated cotton fabric showed above 99.9% bacterial reduction against Escherichia coli and Staphylococcus aureus.
1. Introduction
Cotton fabric has been widely used to manufacture a large quantity of apparels, home furnishings, and various textile-based industrial products owing to attractive advantages such as softness, comfort, high strength and flexibility, good moisture absorption and low cost.1,2 As a natural and abundant biomaterial, cotton is mainly composed of cellulose with polymerization degrees ranging from 1 × 104 to 1 × 105.3 However, the hydrophilic and porous structure of cotton surfaces creates a very suitable environment for the growth of microorganisms upon contact with water and cotton fabrics.4 Hence, cotton fibers and fabrics easily act as carriers for microorganisms, such as pathogenic and odor-generating bacteria, causing damage to their mechanical properties and structural stability, which severely restricts their practical applications.5 Therefore, it is urgent to render cotton fabrics with effective antimicrobial performance.Owing to a growing demand for the production of antibacterial textile fabric, an urgent need for different antibacterial agents suitable for textile application on the market has arisen. Good antibacterial agents must be easily applied at the textile mill, economical and safe for the consumer to wear, which vary with respect to their chemical structures, functions, and effects on humans and the environment.6–9 According to the different mechanisms of antibacterial agents, scientists have focused extensive research on exposing fabrics or fibers to different chemical or physical processes to endow cotton fabrics with antibacterial properties. The application of different metal nanoparticles to antibacterial agents has attracted considerable interest due to their novel physicochemical properties and their potential applications.10 For example, ZnO nanoparticles (NPs) have self-cleaning properties, UV-blocking, and applications in cotton fabric.11,12
Meanwhile, biocidal silver, which is characterized in various forms, such as metallic, salt, and oxidation states (Ag0, Ag+, Ag2+, and Ag3+), is suitable for a wide range of industrial applications and research.13,14 Therefore, with the development of nanoscience and nanotechnology, an increased risk of bacterial resistance to existing organic antimicrobial agents, the application of silver nanoparticles to potential antimicrobial agents has attracted considerable interest because of its robustness and broad-spectrum antimicrobial activity, low bacterial resistance, and high biosafety.15–17 Traditional production of silver NPs mainly involves different methodologies, such as physical, chemical and biological methods. Among these methodologies, chemical reduction methodology has been regarded as the most common methodology for the production of silver and other metallic NPs.18 In recent research, researchers have tended to believe that the antimicrobial mechanism of Ag NPs is mainly derived from the inhibition of microbial cells through the accumulation of silver in the form of silver NPs in cell membranes, affecting metabolic processes such as the inhibition of key enzymes that play roles in respiratory processes and other pathways.19,20 Moreover, Ag NPs benefiting from their excellent antimicrobial activity and unique antimicrobial mechanism have been widely used in food preservation, water disinfection, and medical devices.21
At present, studies on coating strategies of NPs on biological fibers have been widely dependent on solution-based assembly technology, including pad dry cure finishing, spraying, in situ deposition, and solgel coating.22,23 However, biological fibers have difficulty efficiently taking up Ag NPs due to their lack of sufficient compatibility with biological materials. An alternative green approach for generating metal NP coatings is molecule-induced NP self-assembly on the biomolecule surface, which is described as the spontaneous spatial arrangement of NPs functionalized by functional polymers that can recognize biomolecular targets.24 Xu et al. designed an environmentally friendly, energy-efficient, bottom-up nanocoating strategy for cotton fibers through the cooperative self-assembly of heterogeneous Ag NPs functionalized by amino-terminated hyperbranched poly(amidoamine) (HBPAA) and hydroxyl-terminated hyperbranched poly(amine-ester) (HBPAE).25 In addition, another strategy to coat nanomaterials is that silver NPs were prepared by a polyol process with microwave heating and incorporated on cotton fabric surfaces. It was found that an antibacterial fabric with 758 mg kg−1 silver nanoparticles on the surface of cotton was highly effective in killing test bacteria.26
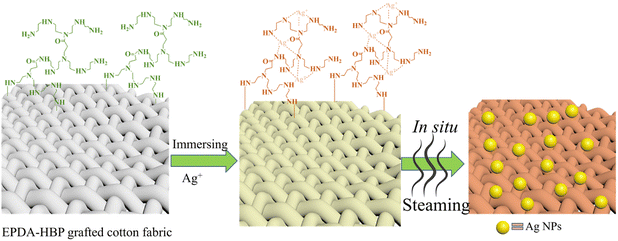
In our previous research, an amino hyperbranched polymer (HBP) containing amino groups, a spherical three-dimensional structure and inner nanocavities27 was synthesized. The amino group can easily produce chemical adsorption with metallic ions, and its nanocavities were applied to the controlled synthesis of Ag and ZnO NPs. In the coating process, metallic NPS coated on cellulose fiber mainly through intermolecular interactions between hydroxy and amino groups, the lack of chemical bonds to link with cotton fibers which will bring about unsatisfied laundering durability. By introducing cationic group into the cotton by chemical bond fibers is one of the most popular research subjects.28,29 In this work, EPDA-HBP was synthesized, and EPDA-HBP was grafted onto cotton fibers to prepare amino modified cotton fibers. Then, EPDA-HBP was used as an admittable agent to complex Ag+. Under thermal steam conditions, Ag+ was restored to Ag0 by the amino group. Compared with the reported methods, the synthetic process of EPDA-HBP is simple and inexpensive. During the coating process, EPDA-HBP was used as a reducing agent and adhesive to fix Ag NPs on the surface of cotton fabric to provide antimicrobial properties, and no other additives were used.
2. Experimental
2.1. Materials
Cotton fabric with composition of cellulose (plain weave and basic weight of 130 g m−2) was obtained from Suzhou Weiyuan Corporation of China. Amino HBP (Mw = 7800, Mn = 2900) was prepared as described in our paper.27 Dimethylamine (33% in water), epoxy chloropropane, sodium hydroxide and AgNO3 (analytically pure) were purchased from Guoyao Chemical Reagent, China. Staphylococcus aureus (S. aureus) (ATCC 6538) and Escherichia coli (E. coli) (ATCC 8099) were obtained from Shanghai Luwei Technology Co., Ltd (China).2.2. Synthesis of EPDA-HBP
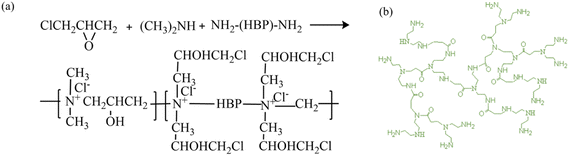
The mass ratio of epoxy chloropropane, dimethylamine and amino HBP was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.4
0.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2, and the reaction mechanism of synthesis of EPDA-HBP is shown in Scheme 1.30 Epoxy chloropropane was added into the four-port bottle with a condensed tube, dimethylamine was put in a dropping funnel and dripped slowly into the four-port bottle, and the reaction system was maintained at 30 °C for 3 h. After the reaction was complete, HBP was added to the reaction system, and the temperature was increased to 70 °C. The reaction was continued for 5 hours, the unreacted material was removed under low pressure using automatic rotary vacuum evaporator, and then EPDA-HBP was obtained.
0.2, and the reaction mechanism of synthesis of EPDA-HBP is shown in Scheme 1.30 Epoxy chloropropane was added into the four-port bottle with a condensed tube, dimethylamine was put in a dropping funnel and dripped slowly into the four-port bottle, and the reaction system was maintained at 30 °C for 3 h. After the reaction was complete, HBP was added to the reaction system, and the temperature was increased to 70 °C. The reaction was continued for 5 hours, the unreacted material was removed under low pressure using automatic rotary vacuum evaporator, and then EPDA-HBP was obtained.
2.3. Preparation of EPDA-HBP grafted cotton fabric
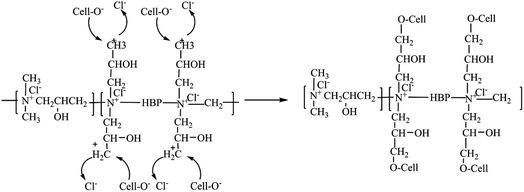
One gram of cotton fabric was added to the EPDA-HBP solution, the amount of PEG-HBP was 8 o.w.f., and the concentration of NaOH was 10 g L−1. The mixtures were placed in a beaker at 70 °C for 90 min. After cooling, the cotton fabric was washed with water and ethyl alcohol separately. The fabric was then dried at 80 °C for 60 min to obtain the EPDA-HBP grafted cotton fabric. The reaction mechanism of the graft-cotton fabric shown in Scheme 2.2.4. Preparation of Ag NPs-coated cotton fabric
EPDA-HBP-grafted cotton fabric was placed in 0.1, 0.2 and 0.3 mM AgNO3 aqueous solutions for 60 min with a liquor ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30. Subsequently, the cotton fabric was steamed (100 °C) for 30 min using a steam engine (BTZS10A, China). Then, the fibers were washed with deionized water and dried at 60 °C to produce the Ag NP-coated cotton fibers.
30. Subsequently, the cotton fabric was steamed (100 °C) for 30 min using a steam engine (BTZS10A, China). Then, the fibers were washed with deionized water and dried at 60 °C to produce the Ag NP-coated cotton fibers.
2.5. Characterization of Ag NPs coated cotton fabric
Fourier transform infrared (FTIR) analysis spectra were obtained on a Nicolet 5700 FTIR spectrophotometer (Thermo Electron Corporation, USA). The surface morphology of the fibers was characterized by field emission scanning electron microscopy (FE-SEM) (Scios DualBeam, Czechia) and energy dispersive spectroscopy (EDS) (Carl Zeiss, EVO 15, Oberkochen, Germany). X-ray photoelectron spectroscopy (XPS) analyses were carried out on an XSAM 800 electron spectrometer (Kratos, UK). The crystalline phase of cotton fabric were analyzed by XRD, (Philips, Amsterdam, The Netherlands). The silver content in the cotton fibers was measured using a Vista MPX inductively coupled plasma atomic emission spectrometer (ICP–AES) (Varian, USA). The silver content was calculated using eqn (1).
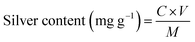 | (1) |
3. Results and discussion
3.1. Characterization of EPDA-HBP-grafted cotton fabric
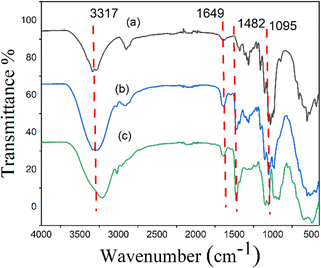
Cellulose molecules contain a certain amount of hydroxy groups, and corona modifiers generally have a salt compound with active groups. Under alkaline conditions, the active base of the cationic modified agent can occur with these hydroxy groups. The grafted cotton fabric was characterized by the FTIR method to further verify the group changes in the reaction. Compared with the FTIR spectrum of pure cotton fiber (Fig. 1a), many new characteristic absorption peaks appeared in the FTIR spectrum of EPDA-HBP grafted cotton fabric (Fig. 1b). For instance, it was found that the absorption at 1649.3 cm−1 corresponded to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch in amide bonds (–CONH–) of the HBP. The absorption at 1482.3 cm−1 and 1095.6 cm−1 in the FTIR spectra of the EPDA-HBP are characteristic of the C–H bend of trimethylammonium groups and the C–OH stretch in the side chain of the quaternary ammonium salt groups. At the same time, the peak type at 3317 cm−1 becomes wider, which can be attributed to the syndrome and vibration of the O–H and N–H.31–33 All the results show that EPDA-HBP has been successfully grafted onto the surface of cotton fabric.
O stretch in amide bonds (–CONH–) of the HBP. The absorption at 1482.3 cm−1 and 1095.6 cm−1 in the FTIR spectra of the EPDA-HBP are characteristic of the C–H bend of trimethylammonium groups and the C–OH stretch in the side chain of the quaternary ammonium salt groups. At the same time, the peak type at 3317 cm−1 becomes wider, which can be attributed to the syndrome and vibration of the O–H and N–H.31–33 All the results show that EPDA-HBP has been successfully grafted onto the surface of cotton fabric.
3.2. Antibacterial properties of Ag NPs-coated cotton fiber
EPDA-HBP-grafted cotton fabric samples were immersed in 0.1, 0.2, and 0.3 mM AgNO3 solutions and marked with a, b and c, respectively, to provide cotton fabric with antibacterial properties. After treatment under hot steaming conditions (100 °C) for 30 min, Ag NPs were coated on the fiber. The reaction mechanism of Ag NPs coated on cotton fabric is shown in Scheme 3. Due to the large number of amino groups and terminal primary amino groups, amino HBP has a strong complexation ability of metal ions in water. When the EDPA-HBP-grafted cotton fabric was immersed in the silver nitrate solution, the grafted HBP on cotton fabric could complex with Ag+. Under hot steam conditions, the amino group reduces Ag+ to form Ag0 and prevents Ag NPs from agglomerating. Ag NPs are limited to the interior of the HBP, and their growth will be subject to physical restrictions on the grid. Therefore, the size of Ag NPs can be effectively controlled. After the reaction, the white fabric gradually changed into gold.Table 1 shows the silver content and antibacterial properties against E. coli and S. aureus of the cotton fabric. The cotton fabric did not show antibacterial activity against E. coli or S. aureus, indicating that cotton fiber alone is not enough to inhibit the growth of bacteria. Attributed to the cationic characteristics of the amino group, the EPDA-HBP grafted fabric shows certain antibacterial activity.35 These results indicate that EPDA-HBP can potentially enhance the antibacterial properties of cotton fiber. In contrast, the Ag NP-coated cotton fabric exhibits excellent antibacterial activity even with Ag contents of 117 mg kg−1. When the concentration of silver reached 180 mg kg−1, bacteria hardly survived on the cotton fabric. The fabric treated with 0.2 mM AgNO3 was further characterized by FE-SEM, EDS and XPS.
| Sample | Sliver content (mg kg−1) | S. aureus | E. coli | ||
|---|---|---|---|---|---|
| Surviving cells (CFU mL−1) | Reduction (%) | Surviving cells (CFU mL−1) | Reduction (%) | ||
| Cotton | — | 6.8 × 105 | — | 2.6 × 105 | — |
| EPDA-HBP | — | 4.7 × 105 | 30.88 | 1.79 × 105 | 31.15 |
| a | 117 | 1.05 × 104 | 98.46 | 4.9 × 103 | 98.11 |
| b | 180 | <10 | 99.99 | <10 | 99.99 |
| c | 250 | <10 | 99.99 | <10 | 99.99 |
The washing durability of functional cotton fabric is a very important factor to consider. After washing 10 times and 30 times, the silver content and antibacterial activity of Ag NP-coated cotton were measured. The results are shown in Table 2. As the washing cycle increased, the silver content and antibacterial activity of the Ag-coated cotton decreased. After 30 washing cycles, the fabric still showed bacterial reductions of 99.08% and 98.92% for S. aureus and E. coli, respectively. The excellent durability of Ag NPs on cotton fibers is attributed to the unique chemical and physical properties of EPDA-HBP, which can trap silver ions in the narrow internal cavity and prevent them from further gathering through steric hindrance effects.
| Sample | Sliver content (mg kg−1) | S. aureus | E. coli | ||
|---|---|---|---|---|---|
| Surviving cells (CFU mL−1) | Reduction (%) | Surviving cells (CFU mL−1) | Reduction (%) | ||
| Cotton | — | 6.8 × 105 | — | 2.6 × 105 | — |
| Ag-cotton | 180 | <10 | 99.99 | <10 | 99.99 |
| 10 times washing | 165 | <10 | 99.99 | <10 | 99.99 |
| 30 times washing | 148 | 6.2 × 103 | 99.08 | 2.8 × 102 | 98.92 |
3.3. Characterization of Ag NPs-coated cotton fiber
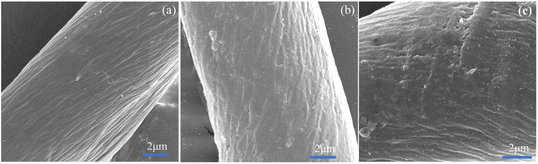
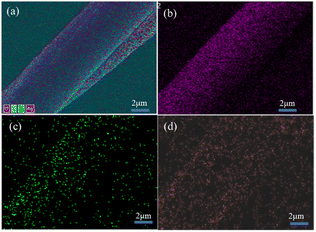
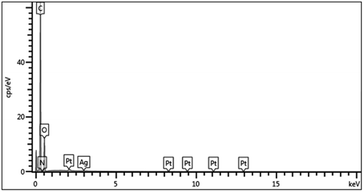
The cotton, EPDA-HBP grafted cotton and Ag-NP-coated cotton fabric were also characterized by FE-SEM. Fig. 2a shows that the surface of the original cotton fiber is smooth, and the structure is dense and uniform.33 The component of cotton mainly cellulose and it can react with sodium hydroxide. After grafting with EPDA-HBP and Ag NPs, the cotton fibers has partial damaged. As shown in Fig. 2b and c, the cotton fiber surface morphology becomes rough, uneven and has a hollow core structure. Many white spots were found on the cotton fiber (Fig. 2c) after treatment with Ag+, and the white spots were uniformly dispersed on the cotton fiber surface.The chemical characteristics of the Ag NP-treated cotton fiber were further examined through EDS analysis of C, O, and Ag to confirm whether the white spots were sliver. Fig. 3 and 4 show that additional N and Ag were found in the cotton fibers, which may be ascribed to the attachment of Ag NPs to the EPDA-HBP grafted fibers.
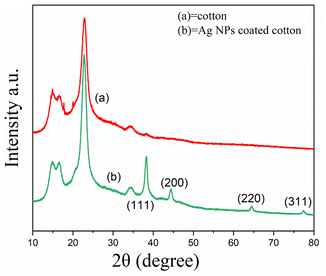
To determine the Ag NPs-coated on cotton fabric, the XRD patterns of the control and treated cotton fabrics were tested. As shown in Fig. 5 the diffraction peaks at 2θ values of at 38.21, 44.31, 64.3, and 77.5 can be indexed to the (111), (200), (220), and (311) diffractions of the standard face-centered-cubic Ag (JCPDS No. 04-0783). No characteristic diffraction peaks were found for AgO, indicating the Ag NPs was coated on the cotton fabric.34
XPS analyses of the Ag NP-coated cotton fibers were conducted to further investigate the Ag NP coating process. Fig. 6a shows that the treated fibers displayed peaks of O 1s, N 1s, and C 1s. Ag 3d peaks at 373 eV were observed after treatment with Ag+, indicating the coating of Ag on the cotton fiber. Ag NPs are easily oxidized when exposed to air without good protection. In Fig. 6b, two peaks at 367.3 and 373.4 eV can be attributed to Ag 3d3/2 and Ag 3d5/2 of metallic Ag NPs, respectively, indicating good protection of Ag NPs by EPDA-HBP.35 The C 1s peaks of cotton fabric could be classified into three categories as follows: C–C (284.6 eV), C–O (286.3 eV), and O–C–O (287.6 eV). These peaks were attributed to cellulose. As shown in Fig. 6c and d, due to the EPDA-HBP and Ag NPs coated on the cotton fabric, the C 1s intensities of the treated cotton fabric decreased.36
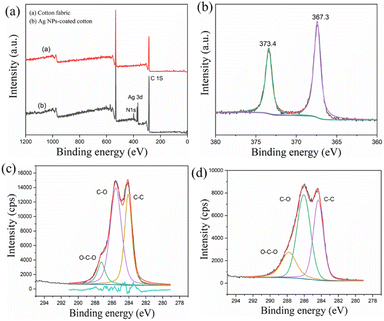 | ||
| Fig. 6 (a) High-resolution XPS spectra, (b) Ag 3d, (c) C 1s of cotton fabric (d) C 1s of Ag NPs coated-cotton fabric. | ||
4. Conclusion
In this research EPDA-HBP grafted cotton fabric was synthesized, amino groups on the cotton fiber could effectively complex Ag+ in an aqueous solution and under a high steaming condition, the Ag+ can convert to Ag0 NPs. During the process, EPDA-HBP plays an important role as a self-reducing agent and a stabilizing agent. FTIR results show that EPDA-HBP has been successfully grafted onto the surface of cotton fabric. SEM, XRD and XPS measurements confirmed the Ag NPs were synthetized and uniformly distributed on the surface of cotton fiber. The Ag contents of the cotton fiber at 180 mg kg−1 show bacterial reduction rates of S. aureus and E. coli above 99.28%. The Ag NPS-coated cotton fabrics showed excellent durable properties, still over 98.77% of bacterial reduction was maintained even after after 30 washing cycles.Author contributions
W. Zang, W. Gao, conceived the project and designed the experiments. H. Chen, and G. Zhang fabricated the samples, conducted the characterizations and performed the supercapacitor tests. H. Chen, W. Gao, and W. Zang revised the manuscript. All authors analyzed the data and contributed to the discussions.Conflicts of interest
The authors declare they have no conflicts interest.Acknowledgements
This work was supported by the Nantong Science and Technology Plan (MS2022077), National Education Department of Jiangsu Province “Qinglan project”.Notes and references
- A. M. Awwad, N. M. Salem and A. O. Abdeen, Int. J. Ind. Chem., 2013, 4, 29 CrossRef.
- L. Bao and X. Li, Adv. Mater., 2012, 24, 3246–3252 CrossRef CAS PubMed.
- M. R. Mohammadi, Int. J. Biol. Macromol., 2018, 109, 476–482 CrossRef PubMed.
- Y. Y. Zhang, Q. B. Xu, F. Y. Fu and X. D. Liu, Cellulose, 2016, 23, 2791–2808 CrossRef CAS.
- G. Dhiman and J. N. Chakraborty, Fash. Text., 2015, 2, 13 CrossRef.
- M. Fouda, E. wdel-Halim and S. Al-Deyab, Carbohydr. Polym., 2013, 92, 943–954 CrossRef CAS PubMed.
- Y. Gao and R. Cranston, Text. Res. J., 2008, 78, 60–72 CrossRef CAS.
- R. Purwar and M. Joshi, AATCC Rev., 2004, 4, 22–26 CAS.
- B. Simoncic and B. Tomsic, Text. Res. J., 2010, 80, 1721–1737 CrossRef CAS.
- S. Eckhardt, P. S. Brunetto, J. Gagnon, M. Priebe, B. Giese and K. M. Fromm, Chem. Rev., 2013, 113, 4708–4754 CrossRef CAS PubMed.
- X. l. Ji, H. Li, Y. Qin and J. Yan, Crystals, 2022, 12, 214 CrossRef CAS.
- A. Chen, Y. Kang, S. Wang, N. Tang and H. Zhao, CIESC J., 2015, 12, 0438–1157 Search PubMed.
- L. Budama, B. A. Çakır, Ö. Topel and N. Hoda, Chem. Eng. J., 2013, 228, 489–495 CrossRef CAS.
- S. T. Dubas, P. Kumlangdudsana and P. Potiyaraj, Colloids Surf., A, 2006, 289, 105–109 CrossRef CAS.
- C. Levard, E. M. Hotze, G. V. Lowry and G. E. Brown, Environ. Sci. Technol., 2012, 46, 6900–6914 CrossRef CAS PubMed.
- Y. A. Krutyakov, E. G. Rukhlya, A. V. Artemov, A. Y. Olenin, M. N. lvanov and O. V. Shelyakov, Nanotechnol. Russ., 2008, 3, 756–762 CrossRef.
- H. E. Emam, A. P. Manian, B. Siroká, H. Duelli, B. Redl, A. Pipal and T. Bechtold, J. Cleaner Prod., 2013, 39, 17–23 CrossRef CAS.
- M. T. Yilmaz, H. İspirlis, O. Taylan and E. Dertli, LWT, 2020, 128, 109–497 CrossRef.
- M. Behravan, A. Hossein Panahi, A. Naghizadeh, M. Ziaee, R. Mahdavi and A. Mirzapour, Int. J. Biol. Macromol., 2019, 124, 148–154 CrossRef CAS PubMed.
- M. Carbone, D. T. Donia, G. Sabbatella and R. Antiochia, J. King Saud Univ. Sci., 2016, 28, 273–279 CrossRef.
- R. Kaegi, A. Voegelin, C. Ort, B. Sinnet, B. Thalmann, J. Krismer, H. Hagendorfer, M. Elumelu and E. Mueller, Water Res., 2013, 47, 3866–3877 CrossRef CAS PubMed.
- S. Huda, S. K. Smoukov, H. Nakanishi, B. Kowalczyk, K. J. M. Bishop and B. A. Grzybowski, ACS Appl. Mater. Interfaces, 2010, 2, 1206–1210 CrossRef CAS PubMed.
- A. Mohamed, A. Hassabo, S. Shaarawy and A. Hebeish, Carbohydr. Polym., 2017, 178, 251–259 CrossRef CAS PubMed.
- S. Ravindra, Y. M. Mohan, N. N. Reddy and K. Raju, Colloids Surf., A, 2010, 367, 31–40 CrossRef CAS.
- S. Xu, F. Zhang, L. Yao, C. Zhu, H. Morikawa and Y. Chen, Cellulose, 2017, 24, 1493–1509 CrossRef CAS.
- S. Shahidi, M. Ghoranneviss, B. Moazzenchi, A. Rashidi and M. Mirjalili, Cellulose, 2010, 17, 627–634 CrossRef CAS.
- G. Zhang, Y. Liu, H. Morikawa and Y. Chen, Cellulose, 2013, 20, 1877–1884 CrossRef CAS.
- F. Zhang, X. Wu, Y. Chen and H. Lin, Fibers Polym., 2009, 10, 496–501 CrossRef CAS.
- G. Zhang, Y. Liu, X. Gao and Y. Chen, Nanoscale Res. Lett., 2014, 9, 216 CrossRef PubMed.
- Y. Wang, B. Gao, Q. Yue, X. Zhan, X. Si and C. X. Li, J. Environ. Manage., 2009, 91, 423–431 CrossRef CAS PubMed.
- F. Zhang, Y. Chen, H. Ling and D. Zhang, Fibers Polym., 2009, 10, 141–147 CrossRef CAS.
- T. S. Wu and K. M. Chen, J. Soc. Dyers Colour., 1993, 109, 153–158 CrossRef CAS.
- J. Zhou, D. Cai, Q. Xu, Y. Zhang, F. Fu, H. Diao and X. Liu, RSC Adv., 2018, 8, 24458–24463 RSC.
- A. Patil, S. Jadhav, V. More, K. Sonawane and P. Patil, Colloid J., 2019, 81, 720–727 CrossRef.
- S. Xu, S. Chen, F. Zhang, C. Jiao, J. Song, Y. Chen, H. Lin, Y. Gotoh and H. Morikawa, Mater. Des., 2016, 95, 107–118 CrossRef CAS.
- M. Abid, S. Bouattour and D. Conceicao, RSC Adv., 2016, 6, 58957–58969 RSC.
| This journal is © The Royal Society of Chemistry 2023 |