Open Access Article
Open Access ArticleA Review: Pharmacological Activity and Phytochemical Profile of Abelmoschus esculentus (2010–2022)
Marwa A. M. Abdel-Razek a,
Miada F. Abdelwahab
a,
Miada F. Abdelwahab a,
Usama Ramadan Abdelmohsen
a,
Usama Ramadan Abdelmohsen *ab and
Ashraf N. E. Hamed
*ab and
Ashraf N. E. Hamed a
a
aDepartment of Pharmacognosy, Faculty of Pharmacy, Minia University, 61519 Minia, Egypt. E-mail: usama.ramadan@mu.edu.eg
bDepartment of Pharmacognosy, Faculty of Pharmacy, Deraya University, Universities Zone, 61111 New Minia City, Egypt
First published on 19th May 2023
Abstract
Abelmoschus esculentus L. Moench (okra) which belongs to the family Malvaceae is a commonly consumed vegetable that consists of the seed component which is rich in polyphenolic compounds. The aim of this study is to highlight the chemical and biological diversity of A. esculentus. This plant contains many vitamins, minerals, proteins and carbohydrates in addition to flavonoids, terpenes, phenolic compounds and sterols. These variations in the chemical composition resulted in different therapeutic activities including antidiabetic, hypolipidemic, antioxidant, antimicrobial, anticancer, wound healing, hepatoprotective, immunomodulator, neuroprotective, and gastroprotective activities in addition to cardioprotective activity.
1. Introduction
Abelmoschus esculentus, also known as Hibiscus esculentus L. (family: Malvaceae), is a valuable vegetable that is widely cultivated in Africa, Asia, Southern Europe and America. It is sometimes referred to as Ladies finger, okra, or gumbo.1 The dried seeds of the plant are the most nutrient-dense component. The seed oil can be consumed and the waste meal from oil extraction is an excellent source of protein.2 A. esculentus promotes relief from emotional and mental illnesses like depression and laziness. It has a powerful cure for ulcers and promotes joint health. It is used for sore throats, gastrointestinal irritations and pulmonary inflammations. By modulating how quickly sugar is absorbed from the digestive tract, the fibers in A. esculentus contribute to blood sugar stabilization. The A. esculentus polysaccharide was found to have hepatoprotective properties, according to earlier investigations.3A. esculentus is very rich in flavonoids. It has been known that flavonoids are a very important bioactive substance existing in the plant world. Additionally, it is very rich in terpenes, vitamins and steroidal derivatives. A. esculentus can not only be used as a kind of medicine for preventing cardiovascular and cerebrovascular diseases, but can also have physiological activities like antihyperlipidemia, antioxidant, antidiabetic, anticancer and cancer prevention, immune regulation, etc.4
2. Methodology
From 2010 to 2022, systematic research was carried out for the previous literature (58 peer-reviewed articles) in reputable scientific search engines with a broad coverage were employed including Pubmed, Google Scholar, El Sevier, SciFinder, Dictionary of Natural Products and Science Direct for reported compounds and biological activities of A. esculentus (in vitro, in vivo and medical). Botanical studies were excluded.3. Pharmacological activities
3.1. Antidiabetic and antihyperlipidemic activity
The antidiabetic and antihyperlipidemic activities of A. esculentus peel and seed powder (AEPP and AESP) were examined in streptozotocin-induced diabetic rats. AEPP and AESP did not exhibit any toxicity or fatality in an acute toxicity investigation up to a dose of 2 g kg−1. Therefore, one by fifth and one by tenth doses of both powders were chosen in order to evaluate the antidiabetic action. Glibenclamide (5 mg kg−1) was orally administered as the standard drug. When AEPP and AESP were orally given to diabetic rats at doses of 100 and 200 mg kg−1, there was a significant decrease in blood glucose levels (89.50–109.67 mg kg−1) compared to diabetic control rats (306 mg kg−1). In addition, a significant increase in the level of hemoglobin and total protein, with decreased level of glycosylated hemoglobin and serum glutamate-pyruvate transferase were also observed following the treatment. Interestingly, elevated lipid profile levels in diabetic rats have reverted to normal after the administration of AEPP and AESP.4The ethanolic and aqueous extracts of the dried fruit powder of A. esculentus were tested for their antidiabetic action on alloxan induced diabetic rats. Alloxan monohydrate was given intraperitoneally at a dose of 150 mg kg−1 after 18 h fasting. The animals received the extracts orally at a dose of 300 mg kg−1, whereas glibenclamide was orally administered (0.5 mg kg−1) as a standard. The results demonstrated that the aqueous extract exhibited hypoglycemic activity better than the ethanolic extract, it has significantly decreased blood glucose levels (194.4 mg kg−1) when compared to control group (341.83 mg kg−1).5
The in vitro antidiabetic activity of A. esculentus was evaluated through determination of α-glucosidase and α-amylase inhibitory activity of the aqueous extracts of A. esculentus peel (AAPP) and seed (AASP). The AAPP and AASP exhibited a notable concentration-dependent inhibition of α-glucosidase (IC50 = 142.69 ± 0.32 g mL−1 and 150.47 ± 0.28) g mL−1 and α-amylase (IC50 = 132.63 ± 0.16 g mL−1 and 147.23 ± 0.21) g mL−1, supporting the hypoglycemic impact of A. esculentus aqueous extracts.6
The hypoglycemic activity of A. esculentus whole plant was tested via oral glucose tolerance test. Following 30 min of the oral administration of the crude methanolic extract, n-hexane and chloroform fractions (200 mg kg−1 body weight), standard glibenclamide (10 mg kg−1 body weight) and control (0.15 mL/10 g body weight). Albino mice were given a glucose load (2 g kg−1 body weight). The blood glucose levels of the animals were determined using a glucometer and glucose oxidase-peroxidase reactive strips at intervals of 30, 90 and 150 min. The study demonstrated the prominent hypoglycemic activity of the crude methanolic extract which has significantly lowered blood sugar levels after the first, second and third hours by 41.25, 43.15 and 46.50%, respectively.7
The hypolipidemic and hypoglycemic activities of A. esculentus pods have been reported by Fan et al. The in vivo experiment involved 6 female mice that were fed on high fat diet for 6 weeks. Serum triglycerides, total cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL) as well as blood glucose levels were measured. The results indicated that the blood glucose and triglycerides were reduced to normal levels, while the total cholesterol level has remained unchanged.8
Tian and coworkers assessed the therapeutic effect of okra extract on gestational diabetes mellitus. The study was performed on twenty-seven pregnant rats and diabetes was induced by streptozotocin. The intervention group was administered okra extract in a daily oral dose of 200 mg kg−1. After 19 days, all rats were anaesthetized to collect blood samples. The blood glucose and serum lipid levels after the treatment were significantly improved, indicating that okra extract had stabilized the blood glucose level and obviously enhanced insulin sensitivity in gestational diabetes mellitus rats.9
In 2015, the antihyperglycemic activity of A. esculentus pods was investigated in alloxan-induced diabetic rats with 100 mg kg−1 aqueous extracts of A. esculentus fruits. After, treatment for 14 days, blood samples were collected and the fasting blood glucose levels was measured at days 3, 7 and 14. The aqueous and dried powdered form of A. esculentus significantly decreased the glucose level in blood to normal level, showing an anti-hyperglycemic activity comparable to that of glibenclamide.10
The in vitro antidiabetic property of A. esculentus was identified through investigating the dried fruit methanolic, ethanolic and aqueous extracts at concentrations of 50, 100, 150, 200 and 250 μg mL−1. The study revealed that the antidiabetic action of the studied plant could be attributed to its efficient concentration-dependent inhibitory activity on α-amylase and α-glucosidase enzymes. The methanolic extract exhibited the highest α-amylase as well as α-glucosidase inhibitory potential compared to ethanol and aqueous extracts. The methanolic extract demonstrated 92.3% α-glucosidase inhibition at the highest concentration (250 μg mL−1) whereas the aqueous and ethanolic extracts indicated 84.4% and 88.6% inhibition, respectively. The maximum α-amylase inhibitory activity of the methanolic extract was 91.6% (at concentration of 250 μg mL−1) while the ethanolic and aqueous extracts exhibited 86.9% and 82% inhibition, respectively.11
Another study utilized alloxan-induced diabetic rats as test subjects to determine the antidiabetic effects of three different parts from the fruits of one variety of A. esculentus (Ex-Maradi Okra); Whole Okra (WO), Okra Peel (OP) and Okra Seed (OS). Diabetes was brought on by giving a single dose of alloxan (120 mg kg−1) intraperitoneally. The main groups of rats were randomly created; WO, OP, OS in addition to three control groups; metformin, normal and diabetic. The administered doses were 100, 200 and 300 mg kg−1, whereas metformin was given at a dose of 500 mg kg−1. The effect of the treatment with the plant different parts was determined via lipid profiles, glycated hemoglobin and blood sugar levels. Glycated hemoglobin was measured using colorimetric method and glucose was determined using the glucose oxidase/peroxidase technique, while the lipid profile parameters were estimated by enzymatic method. The results indicated that all biochemical changes including hyperglycemia, hyperlipidemia and increased glycated hemoglobin were markedly restored to near normal levels following the treatment by the various parts of Ex-Maradi Okra fruit variety. The study demonstrated that Okra-based antidiabetic nutraceuticals could be developed which may be effective in the management of diabetes mellitus and its complications.12
Nguekouo et al. investigated the effect of two cooking methods (boiling and roasting) on the antidiabetic potential of fruits and seeds of A. esculentus in type 2 diabetic rats. The crude fiber, total phenolic content and in vitro free radical scavenging ability of the various fruit and seed preparations were evaluated. Wistar rats that had developed type 2 diabetes as a result of streptozotocin and a high-fat diet were divided into six groups of ten at random and they were given either metformin (300 mg kg−1) or suspensions (200 mg kg−1) of one of the following for 28 days: untreated fruits (UTF), boiled fruits, untreated seeds and roasted seeds. Untreated nondiabetic and diabetic animals made up the controls. Every week, fasting blood glucose levels were assessed. At the end of the experiment, the lipid profile and indicators of liver and kidney damage were determined. The A. esculentus fruits and seeds' fiber content was unaffected by boiling or roasting, but their total phenolic content and ability to scavenge free radicals were significantly reduced. Rats' blood glucose levels were considerably lowered after receiving processed, UTF and seed suspensions daily. Along with a significant increase in the serum level of total protein and it was also found that the serum levels of triglycerides, total cholesterol, LDL cholesterol, transaminases (alanine aminotransferase and aspartate aminotransferase), creatinine and urea had all significantly decreased. The antidiabetic activity of A. esculentus fruits and seeds was not influenced by boiling or roasting.13
Feeding high-fat diet (60% fat) for thirty days combined with streptozotocin injection (35 mg kg−1) was used by Majd et al. to develop diabetes in rats. Following diabetes induction, okra powder (200 mg kg−1) was ingested for 30 days. Serum levels of insulin, HDL, LDL, total cholesterol, fasting blood sugar (FBS) and triglycerides were assessed. The number of β-cells in pancreatic islets was also determined. In addition, PPAR-γ and PPAR-α mRNAs expression were measured in pancreas using real time polymerase chain reaction (PCR) analysis. Okra supplementation had significantly reduced the elevated levels of FBS, total cholesterol and TG and decreased homeostasis model assessment of basal insulin resistance index in diabetic rats. The elevated expression levels of PPAR-γ and PPAR-α genes in diabetic rats was down-regulated in okra-treated rats. Interestingly, okra had improved the pancreatic tissue damage including vacuolization and decreased β-cells mass in diabetic rats. The results confirmed the anti-hyperglycemic and hypolipidemic effects of okra.14
The main polysaccharide fraction of the pods of A. esculentus aqueous extract was purified. The hypoglycemic impact of the polysaccharide rhamnogalacturonan (50 mg kg−1 by intragastric administration, 200 mg kg−1 intragastrical administration) was reported in vivo. Diabetes was induced by an intraperitoneal injection with 1% streptozotocin (45 mg kg−1 body weight). The results revealed that the high-dose rhamnogalacturonan group, compared with model group, demonstrated decreased blood glucose level and glucose tolerance.15
A novel polysaccharide isolated from okra, OP, was assessed to determine its impact on mice fed with a high-fat diet combined with an intraperitoneal injection of 100 mg kg−1 streptozotocin twice, to induce diabetes mellitus. The study demonstrated that an eight-week administration of OP at 200 and 400 mg kg−1 body weight had prominently alleviated the symptoms related to elevated blood glucose, triglycerides, total cholesterol and low-density lipoprotein cholesterol (LDL-C), as well as reduced high-density lipoprotein cholesterol (HDL-C). The OP treatment increased the hepatic glycogen and decreased the mussy hepatic cords and liver fibrosis in diabetic mice. The results suggested that OP exerts its antidiabetic effect through modulating oxidative stress by PI3K/AKT/GSK3β pathway-medicated Nrf2 transport.16
Abscisic acid was isolated and purified from A. esculentus. Its content was reported to be (6.0 μg mL−1 distilled water). An in vitro investigation had been performed to indicate the hypoglycemic activity of abscisic acid. It was suggested that abscisic acid at a concentration of 4.4 μg g−1 present in 500 mg of sample is able to lower blood glucose level.17
Elkhalifa et al. demonstrated the antidiabetic potential of a newly developed okra mucilage biopolymer using enzyme inhibitory assay of α-amylase and α-glucosidase. The investigation revealed that okra mucilage biopolymer at different concentrations (1, 2, 3, 4 and 5 mg mL−1) had concentration dependent inhibitory activities. It found 9.9, 16.5, 24.5, 28.2 and 49.8 α-amylase inhibitory activity as well as 30, 41.5, 50.5, 62.2 and 69.7 α-glucosidase inhibitory effect. These results suggested the possible application of okra mucilage biopolymer for the development of antidiabetic agent.18
The in vivo antihyperglycemic activity of the aqueous fruits extract of A. esculentus (100 and 200 mg kg−1) was determined using high-fat diet-streptozotocin rat model for 14 days. The standardized extract had significantly decreased the fasting plasma glucose level in a dose dependent manner, when compared to diabetic control, with an EC50 value of 141.4 mg kg−1.19
The considerable amount of soluble dietary fibers found in A. esculentus are assumed to be the reason for the delay of glucose absorption from the gut. Nevertheless, its function in concurrent administration with frequently prescribed drugs, such as metformin and acarbose for diabetes, is unknown. Therefore, using a mouse model of glucose-induced hyperglycemia, this study evaluated the impact of A. esculentus pod methanolic extract given concurrently with metformin and acarbose. Each male Swiss Webster mouse was given a gastric lavage dose of 2.5 g per kg per body weight of glucose to induce hyperglycemia. The experimental animals were divided into five sets as follows: the first group as the negative control, the second group as the positive control, the third group received metformin only, the fourth group were given metformin and acarbose and the fifth group received metformin, acarbose and A. esculentus pod extract. In comparison with the positive control, metformin alone or when combined with acarbose significantly lowered glucose levels. On the other hand, the synergistic antihyperglycemic impact of metformin and acarbose was diminished when the medication was given along with the extract. Blood glucose levels were 4.50 mmol L−1 (fourth group) and 6.58 mmol L−1 after 150 min (fifth group). According to this study, taking A. esculentus pod extract along with metformin and acarbose might decrease the efficiency of antidiabetic medications; as a result, concurrent consumption A. esculentus pod extract with conventional medications is not advised.20
Siddique et al. evaluated the in vitro antidiabetic potential of A. esculentus methanolic extract, employing an inhibitory model for the enzymes α-amylase and α-glucosidase. At doses of 50–200 μg mL−1, the percentage inhibition of α-glucosidase and α-amylase ranged from 14.36 ± 0.099 to 19.23 ± 0.172% and 15.89 ± 1.877 to 37.19 ± 7.430%, respectively. Additionally, the yeast glucose uptake assay revealed that glucose absorption was directly proportional to extract concentration and inversely proportional to glucose molar concentration. The maximum glucose uptake percentage by A. esculentus methanolic extract was 68.420 1.752% at a concentration of 3 mg mL−1 extract and 5 mM glucose concentration.21
Tunisian okra pods showed a relevant inhibitory potential on α-amylase (IC50 = 125 μg mL−1) and α-glucosidase (IC50 = 110 μg mL−1). The high inhibitory potential of Tunisian A. esculentus could be explained by the synergic effect of polysaccharides and flavonoids present in okra fruit, and is not only due to the polysaccharides group from okra.42
3.2. Antioxidant activity
The antioxidant activity of A. esculentus seeds and pulp was evaluated by DPPH (2, 2-diphenyl-1-picrylhydrazyl) free radical scavenging and ABTS (2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid)) radical cation decolorization assays. Extraction of seeds and pulp with methanol yielded 1.97 and 3.53%, respectively. The extracts exerted a powerful antioxidant activity with a scavenging activity more than 50% in both methods. The IC50 values was calculated in DPPH method as 44.1 and 55.73 mg mL−1 for seeds and pulp, respectively. Meanwhile, the IC50 values in the ABTS assay was measured as 74.33 and 24.91 mg mL−1 for seeds and pulp, respectively.1The in vitro antioxidant activity of the aqueous and methanolic seed extracts of A. esculentus were evaluated using DPPH radical scavenging, ferric reducing antioxidant power, antioxidant power against β-carotene–linoleic acid assay and chelating effect on ferrous ions, compared with the reference BHT (butylated hydroxytoluene). The antioxidant power of all extracts increased with the increase in concentrations. At 0.125 to 2.0 mg mL−1, the scavenging activities of aqueous extract and methanolic extract on DPPH assay ranged from 9.05 to 75.35% and 9.11 to 82.42%, respectively. While, the reference BHT (0.12 to 2.0 mg mL−1) gave significant free radicals scavenging activity in a dose dependent manner ranging from 21.99 to 87.66%. The maximum ferric reducing power of the extracts was observed at 1 mg mL−1. On the other hand, the strongest chelating effect on ferrous ions (77.60%) was obtained from methanolic extract at 1.0 mg mL−1, while BHT exhibited 84.20% chelating effect. Furthermore, the extracts exhibited antioxidant activity against β-carotene–linoleic acid at 0.25 to 10.0 mg mL−1 ranging from 35.84 to 92.76% for aqueous extract, 40.68 to 97.76% for methanolic extract and 55 to 99.21% for BHT.2
Free radicals scavenging capacity of the okra extract was determined using the stable free radical containing DPPH. It can accept an electron or hydrogen radical, the odd electron in it makes the solution to appear deep violet in color. The absorption vanishes when DPPH accepts an electron resulting in decolorization. An antioxidant's DPPH radical scavenging ability is supposed to be due to hydrogen donating property. Free radical scavenging activity of the extracts on DPPH radicals increased with increase in concentration. At 0.125 to 2.0 mg mL−1, the scavenging activities of aqueous, ethanol and methanol on DPPH radical ranged from 11.3 to 79.3%, 11.5 to 90.5% and 11.7 to 94.5% respectively. Similarly, the reference BHT (0.12 to 2.0 mg mL−1) showed significant free radicals scavenging activities dose dependently ranging from 22.7 to 95.4%. Scavenging capacity of methanol extract was found to be higher than ethanol and aqueous extracts of Abelmoschus esculentus.11
The antioxidant activity of fresh immature fruits of extracted different varieties from okra was studied. The variety V16 demonstrated the richest source of phenolic compounds among all varieties and the highest DPPH scavenging activity at each concentration, in comparison with the control vitamin C at 250 μg mL−1. The variety V16 revealed an inhibition percentage of 87.17%, compared with vitamin C which had an inhibition percentage of 97.2%.21
Liao et al. explored the free radical scavenging activity for A. esculentus using the DPPH radical scavenging assay and ferric reducing antioxidant power. The tested extracts were 80% methanolic extract of the flower, fruit, leaf and seeds of A. esculentus. The results indicated that all extracts have strong antioxidant activity, with the flower and fruit extract being the most potent due to their higher phenolic and flavonoids content.22
Wang et al. determined the remarkable antioxidant activity of polysaccharides separated from fresh A. esculentus pods employing the DPPH assay. Vitamin C was used as the positive antioxidant reference. The isolated polysaccharides exhibited EC50 value (0.261 mg mL−1) that is significantly higher than that of vitamin C (0.013 mg mL−1).23
The antioxidant effect of the acid-soluble pectin components obtained from A. esculentus were investigated. The results indicated that pectin displayed antioxidant property, including radical scavenging activities on DPPH free radicals, ABTS+ free radicals, O2˙− free radicals and OH˙ free radicals. The scavenging effects increased in a concentration dependent manner. At the concentration of 10 mg mL−1, the IC50 value of pectin components for O2˙− radical was 9.8 mg mL−1, the ABTS+ radical scavenging rate was 58.0% and the OH˙ free radical scavenging rate was 53%.24
β-Carotene bleaching assay is also a common way to evaluate the antioxidant potential of hydroxylated fullerenols. Okra displayed outstanding antioxidant activities on DPPH (EC50 = 1.03 mg mL−1) and β-carotene bleaching (EC50 = 0.47 mg mL−1). The high antioxidant activities may be explained by the high content of phenolic compounds holding hydroxyl groups, which donates protons to free radicals to scavenge them.42
3.3. Antimicrobial activity
Mollick and coworkers studied the antibacterial activity of the gold nanoparticles (Au NPs) of A. esculentus pulp aqueous extract against using the agar diffusion method. The Au NPs solution (0.2 mg mL−1) exhibited an excellent antibacterial activity against the five tested bacterial strains; Bacillus subtilis, Bacillus cereus, Micrococcus luteus, Pseudomonas aeruginosa and Escherichia coli, with inhibition zones of 26, 24, 35, 21 and 15 mm, respectively.25The antibacterial effects of A. esculentus silver nanoparticles (Ag NPs) on the Gram-positive pathogens; Bacillus subtilis, Staphylococcus aureus, Staphylococcus epidermidis and Streptococcus pyogenes as well as the Gram-negative pathogens; Klebsiella pneumoniae, Escherichia coli, Pseudomonas aeruginosa, Proteus vulgaris, Salmonella typhimurium and Shigella sonnei were tested. The antibacterial activity against the selected bacterial strains was carried out using the agar well diffusion method. The A. esculentus Ag NPs solution was prepared at a concentration of 100 μg mL−1 and the positive control antibiotic was ciprofloxacin (1 mg mL−1). The Ag NPs solution exhibited antibacterial properties against all the tested Gram-positive and Gram-negative microbial pathogens. However, Gram-negative bacterial growth was more inhibited than Gram-positive bacterial growth. At a dose of 100 μL, P. vulgaris was the most inhibited bacterium (16 ± 1.0 mm), followed by K. pneumoniae (14 ± 0.5 mm) and S. sonnei (14 ± 0.7 mm).26
The agar-well diffusion process was followed to analyze the bactericidal potential of green synthesized CeO2 NPs counter to S. aureus (Gram-positive) and K. pneumonia (Gram-negative) bacterial strains. First, the nutrient agar plates were prepared using 38 g of agar powder dissolved in 1 L of distilled water; then, the agar solution was autoclaved at 121 °C for 20 min. Then, it was cooled, mixed well, and poured into Petri plates (20 mL per plate); then, the agar plates were swabbed with bacterial strains. At that time, these wells were exposed to 0.05 mL of the solution containing CeO2 nanoparticles at various concentrations. Then, these plates were incubated at 37 °C for 24 h. Then, the antibacterial activities of such nanoparticles were evaluated by measuring the inhibition zone around the wells.30
3.4. Anticancer activity
The golden nanoparticles of okra was used to evaluate 3 cell lines of cancer in vitro. Jurkat (human acute myeloid leukemia), K562 (human chronic myeloid leukemia) and DL (Dalton's lymphoma) cells were exposed to Au NPs at concentrations of 0, 1, 5, 10, 25 and 50 μg mL−1 for 24 h and cytotoxicity was determined using the MTT assay. The results show that Au NPs up to the concentration of 50 μg mL−1 produce a significant reduction in the viability of Jurkat cells. The reduction in viability of Au NP-treated cancer cells occurs in a dose-dependent fashion. The Au NP-exposed Jurkat cell viability significantly decreased by 45.1%, 48.6%, 81.3% and 87.2% at 5, 10, 25 and 50 μg mL−1 doses. In the cases of K562 cells and DL cells, viability was significantly decreased by 38.38% and 50.165% and by 28.51% and 48.165% at 25 and 50 μg mL−1 doses, respectively.25Devanesan and AlSalhi reported the cytotoxic activity of the silver nanoparticles (Ag NPs) synthesized using A. esculentus flowers extract. The results confirmed the tumor-inhibiting effect of the synthesized nanoparticles which reduced the cell viability of the tested cancer cell lines A-549 (lung cancer) and TERT-4 (mesenchymal cancer) in a concentration dependent manner. Concentrations of 25 and 50 μg mL−1 of Ag NPs significantly decreased the cell viability and cell death was very significant at a dose of 100 μg mL−1 compared with that of the control drug 5-fluorouracil. The IC50 values of the synthesized Ag NPs against A-549 and TERT-4 was 1.74 and 1.65 μg mL−1, respectively.26
The anticancer activity of A. esculentus flower ethanolic extract was investigated against the human liver cancer HePG2 cell line using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. The sample concentrations of 1000, 500, 250, 125 and 62.5 μg mL−1 demonstrated a significant anticancer activity with CTC50 values 68.37, 57.28, 48.91, 34.86, 29.49 μg mL−1 against HepG2 cell line, respectively.27
The anticancer potential of the raw polysaccharide aqueous extract of A. esculentus was tested against human liver cancer cells (Huh7it) using 10 μg doxorubicin® as a positive control. The cell proliferation was measured by MTT assay, whereas the cell apoptosis, necrosis and cell cycle analysis were performed using Annexin V FITC-PI antibody test and flow cytometry. The polysaccharide extract significantly inhibited Huh7it cell growth and brought on apoptosis. The used dose of the extract (600 μg mL−1) resulted in 5.82% late cell apoptosis and 5.62% early apoptosis, promoting cell cycle arrest during the G0/G1 phase.28
The potential anti-proliferative effect of lectin, purified from A. esculentus, on human U87 glioma cells was proved using MTT assay. Lectin revealed a significant concentration dependent cytotoxic activity with an IC50 value of 21 μg mL−1. The cytotoxic results were comparable with temozolomide (1%) employed as positive control. In addition, the experiment reported that lectin increased the expression of apoptotic caspases with downregulation of clock and Bmal1 circadian genes. It was also demonstrated that lectin induced G0/G1 cell cycle arrest and increased the intracellular reactive oxygen species generation.29
The greenly synthesized cerium oxide (CeO2) nanoparticles from A. esculentus extract were assessed for their in vitro cytotoxicity against HeLa (cervical cancer cells) using MTT assay. The CeO2 nanoparticles induced loss in cellular viability in a dose-dependent manner. They reduced the cellular viability of HeLa cells 93%, 75%, 66%, 55%, 43% and 33% at increasing concentrations (10, 25, 50, 100 and 125 μg mL−1). The IC50 value was calculated as 85.74 μg mL−1 for the HeLa cells viability.30
In 2022, the anticancer activity of the ethanolic extract of red A. esculentus pods against breast cancer was investigated in rats induced by N-methyl-N-nitrosourea (MNU). The findings demonstrated that A. esculentus pods significantly downregulated interleukin IL-6, IL-1β, tumour necrosis factor TNF-α, IL-17, IL-10 and tumour growth factor at doses of 100 and 200 mg kg−1 body weight using 10 μg methotrexate as a standard. Additionally, doses of 200 mg kg−1 produced a considerably thinner mammary gland epithelium, enhanced CD4+ and CD8+ T cell activity and inhibited the growth of mammary gland epithelial cells.31
The cytotoxic potential of Tunisian okra pods against human non-small cell lung cancer (NCL-H460), human breast adenocarcinoma cell line (MCF-7), human cervical cancer cells (HELA), and human liver hepatocellular cells (HEPG-2) were investigated. The results showed that okra pods induced cytotoxicity in a dose-dependent manner and inhibited the development of NCL-H460, MCF-7, HELA and HEPG-2 cells to 50% at concentrations of 49.62, 56.40, 67.27 and 167.95 mg mL−1, respectively.42
3.5. Wound healing activity
The wound-healing potential of the green synthesized cerium oxide (CeO2) nanoparticles from A. esculentus was investigated. In the experiment, male albino rats (9–10 weeks old, weighed between 220–250 g) were given free access to water and food at room temperature and then divided into three groups (each with 4 rats). Group one received chitosan hydrogel membrane (control group). Group two received chitosan hydrogel membrane loaded with 1% CeO2 nanoparticles and group three received 5% CeO2 nanoparticles. Rats were anesthetized before having 2 cm of their skin completely excised. The prepared nanoparticles were daily applied locally at the site of the wound and the wound diameters were measured every day. The healing process was assessed by tracking the regular changes in wound colour. The experiment results demonstrated that the groups treated with CeO2 nanoparticles incorporated in chitosan hydrogel membrane exhibited better healing after 11 days. Metal oxide nanoparticles have been recently reported to decrease bacterial contamination and wound inflammation, furthermore, they improve collagen formation and tensile strength and affect the regulation of fibrogenic cytokines which greatly ameliorate the healing process.303.6. Hepatoprotective activity
The ethanol extract of the root of A. esculentus was evaluated for its hepatoprotective effect. The experiment was carried out using carbon tetrachloride (CCl4) intoxicated HepG2 cell line and Wistar rats and the hepatoprotective effect was determined through estimation of the levels of hepatic and antioxidant markers. A. esculentus root extract demonstrated IC50 values for DPPH and hydroxy radical scavenging tests of 270.99 and 532.86 μg mL−1, respectively. HepG2 cells were incubated with CCl4, which significantly reduced the viability of the cells and enhanced transaminase leakage. Pre-treatment with the extract (at concentrations of 200 and 400 g mL−1) considerably reduced the rate of cell death by 31.25% and 39.04%, respectively. When compared to cells treated with CCl4 at concentrations of 100, 200 and 400 g mL−1, the treatment reduced ALT leakage by 18.6, 38.5 and 52.1%, respectively. The transaminases, ALP, MDA, total bilirubin and hepatic TNF-α levels were all decreased, on the other hand the antioxidant levels were increased in a dose-dependent manner. Interestingly, the histological observation of the liver sections demonstrated reduced steatosis, necrosis and inflammation.32The preventive action of ethanolic extract of okra against liver injury was evaluated in mice using carbon tetrachloride-induced hepatotoxicity model using silymarin as a positive control. The ethanolic extract at 200 mg Kg−1 exerted significant dose-dependent hepatoprotection by decreasing the CCl4-induced elevation of serum SGOT, SGPT, ALP, GGT, cholesterol, triglycerides and malondialdehyde non-protein sulfhydryls and total protein levels in the liver tissue. A significant reduction was also observed in pentobarbital-induced sleeping time in mice.56
3.7. Immunomodulating activity
The aqueous extract of A. esculentus flowers was subjected to purification to yield a water-soluble polysaccharide. The immunomodulating activities of the purified polysaccharide was evaluated. It significantly reduced HepG2 cells proliferation, enhanced the phagocytic ability and elevated the nitric oxide production, TNF-α and IL-1β secretion. In addition, the polysaccharide strongly elevated NF-κB levels, which is considered as an important transcription factor modulating the expression of NO, iNOS and TNF-α.333.8. Neurological activity
Mairuae and coworkers reported the effect of A. esculentus on various Alzheimer's disease-associated cellular processes in H63D variant HFE cells. Treatment of H63D varient HFE cells with okra or quercetin significantly reduced reactive oxygen species, hydrogen peroxide as well as protein oxidation when compared with untreated cells. The study suggested the beneficial role of okra in minimizing the risk of Alzheimer's disease another neurodegenerative disorders related to oxidative stress.343.9. Gastro protective effect
The gastroprotective mechanism of A. esculentus lectin on gastropathy induced by ethanol was evaluated. Fasted mice treated with ethanol 99.9% (0.2 mL per animal, p.o.) previously receiving (0.01, 0.1, 1.0, 10 and 50 mg kg−1, i.v.), saline (5 mL kg−1, i.v.) or ranitidine (80 mg kg−1 p.o.). Gastric oxidative stress, tissue hemoglobin content and microscopic features were investigated with the purpose of characterizing the okra lectin gastroprotective effect. A. esculentus lectin (1 mg kg−1) was able to prevent mucosal damage from ethanol. However, the lectin effect was reversed by naloxone and yohimbine, demonstrating that opioids and alpha-2 adrenergic receptors were involved in this gastric protective effect. While, nitric oxide or prostaglandins were not implicated. The antioxidant effect of lectin is probably involved in the defensive mechanism of action.35A. esculentus extract was assessed for its gastroprotective effect against ethanol-induced acute gastric mucosal injury in Wistar rats. The animals were treated with okra (500, 250 and 100 mg kg−1), famotidine (20 mg kg−1) and quercetin (75 mg kg−1). Following 60 min, all rats were given 1 mL of 80% ethanol. All groups were sacrificed one hour after ethanol administration. Okra extract at doses of 500, 250 and 100 mg kg−1, famotidine and quercetin inhibited the development of ulcers by 81.0, 67.5, 67.0, 76.3 and 72.4%, respectively. Okra dose of 500 mg kg−1 had a significant reduction in edema, bleeding, inflammation scores and oxidant levels. In addition, serum β carotene and retinol levels were significantly increased.36
The aqueous fresh extract from immature okra fruits was investigated for its antiadhesive properties against Helicobacter pylori. Human gastric epithelial AGS cells and FITC-labeled H. pylori J99, 2 clinical isolates were employed in an in vitro flow cytometric experiment. Bacterial adherence to AGS cells was inhibited by okra fruit extract in a dose-dependent manner, with inhibition ranging from 20% to 70% in the dose range of 0.2 to 2 mg mL−1.37
3.10. Weight reduction potential
One hundred and eight individuals with BMIs (body mass indices) between 25 and 35 kg m−2 participated in a double-blind, randomized, placebo-controlled experiment in which they received either low or high doses of IQP-AE-103 (a combination of dehydrated powder of A. esculentus pods and inulin) or a placebo. Individuals were given two capsules of the IQP-AE-103 after three main meals every day for 12 weeks after a 2 week “run-in” phase. They were told to follow a hypocaloric diet that was balanced nutritionally and corresponded to their own energy needs. At baseline, 2, 4, 8 and 12 weeks, waist and hip circumference, body weight, body fat and other measurements were taken. At the same intervals, subjects also rated their emotions of hunger and fullness using visual analogue scales and their level of yearning for food on a 5-point scale. Before and after the study, blood samples were obtained for safety laboratory parameters. After 12 weeks, the high-dose IQP-AE-103 group lost considerably more weight (5.03 ± 2.50 kg) than both the low-dose group (3.01 ± 2.19 kg) and the placebo (0.98 ± 2.06 kg). IQP-AE-103 at a high dose also reduced appetite in 66% of individuals. Interestingly, high dose IQP-AE-103 group experienced a reduction in total cholesterol, LDL-cholesterol and triglycerides levels at the end of study, compared with the placebo group. There were no negative effects associated with IQP-AE-103 consumption suggesting that it might be a reliable and secure weight loss treatment.383.11. Cardioprotective activity
The impact of okra fruit mucilage (crude water extract and water fraction) on lipid parameters was studied in rats fed on a high-fat diet. Rats with elevated cholesterol were given okra crude water extract (500 and 1000 mg kg−1 body weight) or water fraction (50 and 100 mg kg−1 body weight) along with a high-fat diet for one week. The total cholesterol, triglycerides, LDL and VLDL lipid fractions as well as the atherogenic index were reduced by crude water extract (1000 mg) and the water fraction of okra (50 and 100 mg). The mucilage had the potential to elevate the HDL level in the test the group. These findings imply that okra fruit water fraction and crude water extract could be employed as “heart-friendly” vegetables by modulating blood lipid levels.393.12. Nootropic activity
Doreddula et al. made atrial to examine okra nootropic activity using mouse model. Mouse was gently placed in the illuminated compartment for the acquisition trial and the door between the two compartments was opened 10 s later. When the mouse entered the dark compartment, the door automatically closed and an electrical foot shock (0.5 mA) of 2 s duration was delivered through the stainless steel rods. Twenty-four hours after this acquisition trial, the mouse was again placed in the illuminated compartment for a retention trial. The time taken for a mouse to enter the dark compartment after its door was opened was defined as step-down latency for both acquisition and retention trials. Latency for entering the dark compartment was recorded up to 300 s. If a mouse did not enter the dark compartment within 300 s, the mouse was removed and assigned a latency score of 300 s.The step-down latency of scopolamine (79.4 ± 15 s) treated mice in the passive avoidance task was significantly shorter than that of saline control mice (269.02 ± 23 s). The shorter step-down latency of scopolamine was significantly reversed by pretreatment with reference drug, piracetam (223.2 ± 16.1 s). Similarly, A. esculentus (171.01 ± 18.6 s) significantly reversed the scopolamine-induced memory impairment.2
3.13. Anti-stress activity
Another trial from Doreddula et al. to evaluate the anti-stress activity on mice. All the animals were pretreated with the respective vehicle or extract or standard drug for 7 days before the induction of stress. After 30 min of pretreatment on day 7, mice were exposed to stressful stimuli induced by restraining the animals in polyvinyl chloride (PVC) restrainers of 105 mm length and 32 mm diameter for a period of 4 hours. Following the induction of stress, the mice were evaluated for behavioural changes. A different set of mice treated as above and the blood was withdrawn from the retro orbital plexus and plasma corticosterone, glucose, total protein, cholesterol, and triglycerides were measured.2The A. esculentus showed significant reduction in immobilization stress when compared to the stress group. The acute restraint stress-induced group showed a marked increase in serum glucose [F(4, 25) = 8.56], corticosterone [F(4, 25) = 14.34], cholesterol [F(4, 35) = 9.107], and triglycerides [F(4, 35) = 13.79] in mice. These stress-induced elevated levels of biochemical parameters were significantly reversed by A. esculentus for 7 days once daily and with reference drug, diazepam (2 mg kg−1, i.p).2
3.14. Anti-inflammatory activity
The anti-inflammatory activity of okra pectin examined using the inflammation model of RAW 264.7 cells. The model established at concentrations (25, 50, 100, 200, 400 and 800 μg mL−1) for one day. The production of NO normally secreted by macrophages was reflected in the blank group and increased significantly in the modeling groups, in which cells were treated with LPS for 24 h in the presence of AOP at different concentrations. Thus, the inflammation model of RAW 264.7 cells was established successfully when the concentration of AOP >400 μg mL−1, the production of NO was significantly reduced closer to that of the blank group, indicating that the cells were protected by okra pectin.243.15. Antinociceptive activity
Romdhane et al. studied the inhibition percentages of writhing for Tunisian A. esculentus. The reference drug inhibited 63.65% of the writhing response induced by acetic acid. The results showed that okra pod extract induces in a dose-dependent manner the antinociceptive potential. Okra pods extract reduced significantly the number of writhing, which is related to the release of endogenous substances including histamines, serotonin, bradykinin and prostaglandin. The antinociceptive activity of the extract of Tunisian okra pods at 100 mg kg−1 (58.11%) were found to be higher than that of the methanolic extract of okra roots at the same concentration (36.99%) and okra water extract (30.76%).42Pritam et al. tested the analgesic activity of A. manihot leaves in vivo by two methods. Swiss albino mice (25–30 g) of either sex were divided into eight groups containing six animals in each. A control group received normal saline solution, while three groups received petroleum ether extract at doses 100, 200 and 400 mg kg−1 p.o, respectively. The remaining three groups received methanol extract at doses 100, 200 and 400 mg kg−1 p.o. Pentazocine (10 mg kg−1) was administered to eighth group. The temperature of hot plate was maintained at 55 ± 0.5 °C. The rats were placed individually on hot plate and time between placement and licking of paws, shaking or jumping off the surface was recorded by using Eddys hot plate apparatus (Spacelab). As a response latency rat with baseline latencies of less than 5 s or more than 15 s were eliminated from the study and cut off latency time was set at 15 s to avoid tissue damage. After determination of base line response latencies, hot plate latencies were re determined at 0, 30, 60, 90 and 180 min after drug administration. The Swiss albino mice (25–30 g) of either sex were divided into eight groups containing six animals in each. The rats were fasted for 12 h prior to induction of analgesia. The control group received normal saline solution, while three groups received petroleum ether extract at doses 100, 200 and 400 mg kg−1 p.o., respectively. The remaining three groups received methanol extract at doses 100, 200 and 400 mg kg−1 p.o. Pentazocine (10 mg kg−1) was administered to eighth group. The lower 5 cm portion of the tail was marked. This part of the tail was immersed in a cup of freshly filled water of exactly 55 °C. Within a few seconds the rat reacted by withdrawing the tail. The reaction time was recorded by a stopwatch. After each determination the tail was carefully dried by a cloth. The reaction time was determined before and periodically after oral administration of the test substance, e.g., after 0, 30, 60, 90 and 180 min. The cut off time of the immersion was kept 15 s. The withdrawal time of untreated animals was between 1 and 5.5 s. A withdrawal time of more than 6 s therefore is regarded as a positive response. The results obtained indicate that the extracts possessed significant analgesic activity, which was found to be dose-dependent. A significant inhibition in pain threshold in hot-plate test was exhibited. However, in flick test, highest analgesic activity was observed only with 400 mg kg−1 dose as compared with the standard drug.58
4. Natural products from A. esculentus
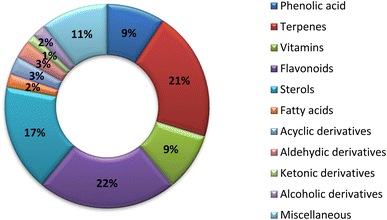
Nwachukwu et al. studied the percentage of flavonoids in okra leaves and fruits. The total highest phenolic content was found in young leaves (0.99 mg g−1) and the total flavonoids were highest in mature leaves (0.79 mgQE g−1). The antioxidant activity of A. esculentus is mainly attributed to the flavonoidal percentage.40Thirteen phenolic acids were reported from A. esculentus that had diverse biological activities. Rosmarinic acid and chlorogenic acid may be responsible for the anti-inflammatory properties of A. esculentus.41 Terpenoids are widely distributed in A. esculentus, with 29 terpenoidal compounds have been reported. β-Carotene and lycopene tetraterpenoidal compounds in addition to β-caryophyllene a sesquiterpenoidal compound presented powerful antioxidant and anticancer activities.42 Vitamins are widely distributed in A. esculentus in both types water and fat soluble.43 In the current survey 31 flavonoidal derivatives were reported as 5,7,3′,4′-tetrahydroxy flavonol-3-O-[β-D-glucopyranosyl-(1→6)]-β-D-glucopyranoside had antioxidant activity.46 A. esculentus also had several steroidal compounds including β-sitosterol and daucosterol.47 Finally, there are uncategorized or miscellaneous group including 15 compounds. Eugenol had anticancer activity against different cell lines,42 while 3-hydroxy-2,3-dihydroimidazo [1,5-a] pyridin-8(5H)-one-5-β-glucopyranoside that exhibited positive inotropic effect on the heart (increase in amplitude of the heart), while it also possess negative chronotropic effect (decrease in frequency).48
5. Phytochemical profile
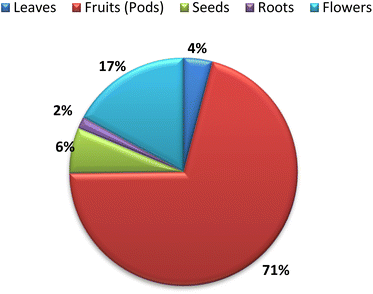
The nutritional value and chemical composition of A. esculentus vary according to the part used. The fruits (pods) that presented in Fig. 1, leaves, seeds and roots contain diverse chemical compounds belonging to different classes. This variation in chemical composition is responsible for the different biological activities. Flavonoids and terpenes are the main secondary metabolites detected in all parts of A. esculentus. Table 1 and Fig. 2 illustrate the isolated compounds from 2010 to 2022.| No. | Compound name | Molecular weight | Molecular formula | Part used | Ref. |
|---|---|---|---|---|---|
| (1) Phenolic acids | |||||
| 1 | Oxalic acid | 90.03 | C2H2O4 | Pods | 42 |
| 2 | Fumaric acid | 116.07 | C4H4O4 | Pods | 42 |
| 3 | Malic acid | 134.08 | C4H6O5 | Pods | 42 |
| 4 | p-Methoxy benzoic acid | 152.15 | C8H8O3 | Flowers and fruits | 50 |
| 5 | Coumaric acid (Syn.: p-hydroxycinnamic acid) | 164.15 | C9H8O3 | Leaves | 49 |
| 6 | Vanillic acid | 168.14 | C8H8O4 | Pods | 42 |
| 7 | Gallic acid (Syn.: 3,4,5-trihydroxybenzoic acid) | 170.11 | C7H6O5 | Roots | 41 |
| 8 | Shikimic acid (Syn.: shikimate) | 174.15 | C7H10O5 | Pods | 42 |
| 9 | Caffeic acid (Syn.: 3,4-dihydroxycinnamic acid) | 180.15 | C9H8O4 | Leaves | 49 |
| 10 | Citric acid (Syn.: aciletten) | 192.12 | C6H8O7 | Pods | 42 |
| 11 | Ferulic acid (Syn.: 4-hydroxy-3-methoxycinnamic acid) | 194.18 | C10H10O4 | Leaves | 49 |
| 12 | Chlorogenic acid (Syn.: 3-O-caffeoylquinic acid) | 354.30 | C16H18O9 | Leaves | 49 |
| 13 | Rosmarinic acid (Syn.: rosemary acid) | 360.31 | C18H16O8 | Roots | 41 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| (2) Terpenes | |||||
| (2i) Monoterpenoidal compounds | |||||
| 14 | Limonene (Syn.: cajeputene or cinene) | 136.23 | C10H16 | Pods | 42 |
| 15 | α-Pinene (Syn.: acintene A) | 136.23 | C10H16 | Pods | 42 |
| 16 | β-Pinene (Syn.: nopinene) | 136.23 | C10H16 | Pods | 42 |
| 17 | Carvone (Syn.: karvon) | 150.21 | C10H14O | Pods | 42 |
| 18 | β-Cyclocitral | 152.23 | C10H16O | Pods | 42 |
| 19 | Camphor (Syn.: 2-camphanone) | 152.23 | C10H16O | Pods | 42 |
| 20 | Dihydrocarvone | 152.23 | C10H16O | Pods | 42 |
| 21 | Terpineol (Syn.:p-menth-1-en-8-ol) | 154.24 | C10H18O | Pods | 42 |
| 22 | Linalool (Syn.: linalyl alcohol) | 154.24 | C10H18O | Pods | 42 |
| 23 | Menth-2-en-1-ol | 154.24 | C10H18O | Pods | 42 |
| 24 | Menthol | 156.26 | C10H20O | Pods | 42 |
| 25 | Δ8,9-Dehydro-4-hydroxythymol dimethyl ether | 192.27 | C12H16O2 | Pods | 42 |
| 26 | Dihydro-β-ionone | 194.31 | C13H22O | Pods | 42 |
| 27 | Exo fenchyl acetate | 196.29 | C12H20O2 | Pods | 42 |
| 28 | α-Terpenyl acetate | 196.29 | C12H20O2 | Pods | 42 |
| (2ii) Sesquiterpenoidal compounds | |||||
| 29 | α-Cubebene | 204.35 | C15H24 | Pods | 42 |
| 30 | β-Caryophyllene | 204.35 | C15H24 | Pods | 42 |
| 31 | Alloaromadendrene (Syn.: aromadendrene) | 204.35 | C15H24 | Pods | 42 |
| 32 | ε-Muurolene | 204.35 | C15H24 | Pods | 42 |
| 33 | Abscisic acid | 264.31 | C15H20O4 | Fruits | 17 |
| (2iii) Triterpenoidal compounds | |||||
| 34 | Lupeol (Syn.: clerodol or monogynol B) | 426.71 | C30H50O | — | 57 |
| 35 | Ursolic acid (Syn.: malol and urson) | 456.70 | C30H48O3 | Fruits | 48 |
| 36 | (3β,21β)-19,21-Epoxylup-20(29)-en-3-yl acetate | 482.73 | C32H52O3 | Fruits | 51 |
| 37 | (3β)-9,18-Dihydroxyolean-12-en-3-yl acetate | 484.75 | C32H52O3 | Fruits | 51 |
| 38 | Ursene-3-O-β-D-glucopyranoside | 588.44 | C36H60O6 | Fruits | 48 |
| 39 | Olean-12-en-3-O-β-D-glucopyranoside | 616.47 | C38H64O6 | Fruits | 45 |
| (2iv) Tetraterpenoidal compounds | |||||
| 40 | β-Carotene | 536.87 | C40H56 | Fruit | 43 |
| 41 | Lycopene | 536.90 | C40H56 | Seeds | 42 |
| 42 | Zeaxanthin | 568.88 | C40H56O2 | Fruits | 43 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| (3) Vitamins | |||||
| 43 | Niacin (Syn.: Vit. B3) | 123.10 | C6H5NO2 | Fruits | 43 |
| 44 | Pyridoxine (Syn.: Vit. B6) | 169.17 | C8H11NO3 | Fruits | 43 |
| 45 | Menadione (Syn.: Vit. K3) | 172.18 | C11H8O2 | Fruits | 43 |
| 46 | Ascorbic acid (Syn.: Vit. C) | 176.12 | C6H8O6 | Fruits | 43 |
| 47 | Pantothenic acid (Syn.: Vit. B5) | 219.23 | C9H17NO5 | Fruits | 43 |
| 48 | Biotin (Syn.: Vit. B7) | 244.31 | C10H16N2O3S | Fruits | 43 |
| 49 | Thiamine (Syn.: Vit. B2) | 267.35 | C12H17ClN4OS | Fruits | 43 |
| 50 | Retinol (Syn.: Vit. A) | 286.45 | C20H30O | Fruits | 43 |
| 51 | Cholecalciferol (Syn.: Vit. D3) | 384.63 | C27H44O | Fruits | 43 |
| 52 | γ-Tocopherol (Syn.: 7,8-dimethyltocol) | 416.70 | C28H48O2 | Fruits | 43 |
| 53 | α-Tocopherol (Syn.: Vit. E) | 430.70 | C29H50O2 | Fruits | 43 |
| 54 | Folic acid (Syn.: Vit. B9 or Vit. M) | 441.40 | C19H19N7O6 | Fruits | 43 |
| 55 | Cyanocoblamin (Syn.: Vit. B12) | 1355.4 | C63H88CoN14O14P | Fruits | 43 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| (4) Flavonoids | |||||
| (4i) Flavone derivatives | |||||
| 56 | Myricetin (Syn.: myricetol or cannabiscetin) | 318.23 | C15H10O8 | Flowers | 52 |
| 57 | Gossypetin (Syn.: articulatidin and equisporol) | 318.23 | C15H10O8 | Seeds | 44 |
| 58 | Hibiscetin | 334.50 | C15H10O9 | Seeds | 44 |
| 59 | Quercetin-8-(2′′-pyrrolidinon-5′-yl)-3′′-O-D-glucopyranoside | 385.32 | C19H15NO8 | Flowers | 52 |
| 60 | Kaempferol-3-O-glucoside (Syn.: astragalin) | 448.37 | C21H19O11 | Flowers | 52 |
| 61 | Quercetin 3-O-glucoside (Syn.: isoquercitrin or quercetin glycoside) | 464.37 | C21H20O12 | Flowers | 52 |
| 62 | Hyperin (Syn.: hyperoside or quercetin 3-galactoside) | 464.38 | C21H20O12 | Flowers and fruits | 50 and 52 |
| 63 | Quercetin3-O-(6′′-O-acetyl)-β-D-galactranoside | 506.41 | C23H22O13 | Pods | 42 |
| 64 | 5,7,3′,4′-Tetrahydroxy-4′′-O-methylflavonol-3-O-β-D-glucopyranoside | 478.11 | C22H22O12 | Seeds | 46 |
| 65 | Cannabiscitrin (Syn.: myricetin 5′-glucoside or isomyricitrin) | 480.40 | C21H20O13 | Flowers | 52 |
| 66 | Quercetin-O-acetylhexoside | 506.4 | C23H28O16 | Pods | 42 |
| 67 | 3-O-Kaempferol-3-O-acetyl-6-O-(p-coumaroyl)-α-D-glucopyranoside | 594.51 | C30H26O13 | Flowers and fruits | 50 and 52 |
| 68 | Kaempferol 3-O-glucoside | 596.49 | C26H28O16 | Pods | 42 |
| 69 | Quercetin-O-pentosyl-hexoside | 596.5 | C26H28O16 | Pods | 42 |
| 70 | Isorhamnetin 3-O-glucoside-7-O-xyloside | 610.50 | C27H30O16 | Pods | 53 |
| 71 | Rutin (Syn.: quercetin 3-rutinoside) | 610.51 | C27H30O16 | Flowers | 52 |
| 72 | Floramanoside D | 624.41 | C28H32O16 | Flowers | 52 |
| 73 | Quercetin diglucoside (Syn.: quercetin-3,4′-O-diglucoside) | 626.50 | C27H30O17 | Flowers | 52 |
| 74 | Quercetin 3-O-gentibioside | 626.51 | C27H30O17 | Flowers | 52 |
| 75 | 5,7,3′,4′-Tetrahydroxyflavonol-3-O-[β-D-glucopyranosyl-(1→6)]-β-D-glucopyranoside | 626.51 | C27H30O17 | Seeds | 46 |
| 76 | Quercetin 3-O-sophoroside | 626.53 | C27H30O17 | Flowers | 52 |
| (4ii) Flavan derivatives | |||||
| (4ii-a) Flavanol derivatives | |||||
| 77 | Catechin | 290.26 | C15H14O6 | Leaves | 49 |
| 78 | Epicatechin | 290.26 | C15H14O6 | Fruits | 1 |
| 79 | Gallocatechin | 306.26 | C15H14O7 | Fruits | 1 |
| 80 | Procyanidin B1 (Syn.: endotelon) | 578.52 | C30H26O12 | Fruits | 1 |
| 81 | Procyanidin B2 (Syn.: procyanidol B2) | 594.50 | C30H26O13 | Fruits | 1 |
| (4ii-b) Flavanonol derivatives | |||||
| 82 | Dihydromyricetin (Syn.: ampelopsin or ampeloptin) | 320.25 | C15H12O8 | Flowers | 52 |
| (4ii-c) Anthocyanin | |||||
| 83 | Cyanidin 3-glucoside (Syn.: kuromanin or idein) | 450.39 | C21H21O11 | Pods | 53 |
| 84 | Cyanidin 4′-glucoside | 450.39 | C21H21O11 | Pods | 53 |
| 85 | Cyanidin 3-sambubioside (Syn.: sambicyanin) | 581.50 | C26H30O15 | Pods | 53 |
| 86 | Delphinidin 3-O-sambubioside | 598.15 | C26H30O16 | Pods | 53 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| (5) Sterols | |||||
| 87 | Ergosta-7, 22-dien-3-ol | 398.66 | C28H46O | — | 57 |
| 88 | Stigmasta-4, 22-dien-3-one | 410.67 | C29H46O | — | 57 |
| 89 | Stigmasterol | 412.69 | C29H48O | — | 57 |
| 90 | Stigmast-4-ene-3-one | 412.70 | C29H48O | — | 47 |
| 91 | β-Sitosterol (Syn.: cupreol or azuprostat) | 414.70 | C29H50O | — | 47 |
| 92 | Stigmast-4,22-dien-3,6-dione | 424.65 | C29H44O2 | — | 57 |
| 93 | 6-Hydroxy-stigmasta-4,22-dien-3-one | 426.32 | C29H46O2 | — | 57 |
| 94 | Stigmast-4-ene-3,6-dione | 426.34 | C29H46O2 | — | 47 |
| 95 | Stigmasta-5,22-dien-3-ol-7-one | 426.67 | C29H46O2 | — | 57 |
| 96 | Stigmasta-5-en-3-ol-7-one | 428.36 | C29H48O2 | — | 57 |
| 97 | 6-Hydroxy-stigmasta-4-en-3-one | 428.69 | C29H48O2 | — | 57 |
| 98 | 5α,6α-Epoxyergost-8(14),22-dien-3β,7α-diol | 428.71 | C28H44O3 | — | 47 |
| 99 | Stigmast-5, 22-diene-3β,7α-diol | 428.71 | C29H48O2 | — | 47 |
| 100 | Stigmast-4,22-diene-3 β,6β-diol | 428.71 | C29H48O2 | — | 47 |
| 101 | Stigmast-5,22-dien-3,7-diol | 428.70 | C29H48O2 | — | 57 |
| 102 | Stigmast-5-en-3,7-diol | 430.70 | C29H50O2 | — | 57 |
| 103 | Ergost-7,22-diene-3β,5α,6β-triol | 430.71 | C28H46O3 | — | 47 |
| 104 | 5α,8α-Epidioxyergost-22-en-3β-ol | 430.70 | C28H46O3 | — | 47 |
| 105 | Stigmast-5-ene-3β,7α-diol | 430.70 | C29H50O2 | — | 47 |
| 106 | Stigmast-4-en-3β,6β-diol | 430.70 | C29H50O2 | — | 47 |
| 107 | Cycloart-23-ene-3β,25-diol | 442.39 | C30H50O2 | — | 47 |
| 108 | Cycloart-25-en-3,24-diol | 442.40 | C30H50O2 | — | 57 |
| 109 | Daucosterol (Syn.: eleutheroside a and β-sitosterol β-D-glucoside) | 576.84 | C35H60O6 | — | 47 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| (6) Fatty acids | |||||
| 110 | Palmitic acid (Syn.: cetylic acid or hexadecanoic acid) | 256.42 | C16H32O2 | Seeds | 54 and 57 |
| 111 | Linoleic acid (Syn.: telfairic acid) | 280.42 | C18H32O2 | Seeds | 54 |
| 112 | Oleic acid (Syn.: octadec-9-enoic acid) | 282.5 | C18H34O2 | Seeds | 54 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| (7) Acyclic derivatives | |||||
| 113 | Undecane | 156.30 | C11H24 | Pods | 42 |
| 114 | Dodecane | 170.33 | C12H26 | Pods | 42 |
| 115 | Tridecane | 184.36 | C13H28 | Pods | 42 |
| 116 | Tetradecane | 198.38 | C14H30 | Pods | 42 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| (8) Aldehydic derivatives | |||||
| 117 | 2-Heptenal | 112.16 | C7H12O | Pods | 42 |
| 118 | Nonanal | 142.23 | C9H18O | Pods | 42 |
| 119 | Decanal (Syn.: decyl aldehyde or caprinaldehyde) | 156.26 | C10H20O | Pods | 42 |
| 120 | Isododecanal | 184.53 | C12H24O | Pods | 42 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| (9) Ketonic derivatives | |||||
| 121 | 4-Hydroxy-4-methyl-2-pentanone (Syn.: diacetone alcohol) | 116.15 | C6H12O2 | Pods | 42 |
| 122 | 6-Methyl-5-hepten-2-on (Syn.: sulcatone) | 126.19 | C8H16O | Pods | 42 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| (10) Alcoholic derivatives | |||||
| 123 | Cyclohexanol | 100.15 | C6H12O | Pods | 42 |
| 124 | 2-Octen-1-ol | 128.21 | C8H16O | Pods | 42 |
| 125 | 3-Octen-1-ol | 128.21 | C8H16O | Pods | 42 |
| 126 | 1-Octanol (Syn.: N-octanol) | 130.22 | C8H18O | Pods | 42 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| (11) Miscellaneous | |||||
| (11i) Cyclic aldehydic derivatives | |||||
| 127 | Benzaldehyde | 106.12 | C7H6O | Pods | 42 |
| 128 | p-Tolualdehyde | 120.15 | C8H8O | Pods | 42 |
| 129 | 1H-Indole-3-carboxaldehyde (Syn.: indole-3-carbaldehyde and 3-formylindole) | 145.16 | C9H7NO | Flowers and fruits | 50 |
| (11ii) Phenyl propanoids | |||||
| 130 | Anethol | 148.20 | C10H12O | Pods | 42 |
| 131 | Eugenol | 164.20 | C10H12O2 | Pods | 42 |
| (11iii) Esters | |||||
| 132 | 2-Hydroxyphenylacetic acid methyl ester | 152.14 | C8H8O3 | Flowers and fruits | 50 |
| 133 | Methyl 2-(2-hydroxyphenyl) acetate | 166.17 | C9H10O3 | Flowers and fruits | 50 |
| (11iv) Amide | |||||
| 134 | N-E-Coumaroyl tyramine (Syn.: paprazine) | 283.32 | C17H17NO3 | Flowers and fruits | 50 |
| 135 | N-E-Feruloyltyramine (Syn.: moupinamide) | 313.30 | C18H19NO4 | Flowers and fruits | 50 |
| (11v) Imidazole alkaloid | |||||
| 136 | 3-Hydroxy-2,3-dihydroimidazo [1,5-a]pyridin-8(5H)-one-5-β-glucopyranoside (Syn.: esculentoside) | 330.29 | C13H18N2O8 | Fruits | 48 |
| (11vi) Neolignan | |||||
| 137 | 7R,8S-Dihydrodehydro-coniferyl alcohol | 360.15 | C20H24O6 | Flowers and fruits | 50 |
| (11vii) Amphetamine | |||||
| 138 | Aurantiamide acetate | 180.23 | C6H12O6 | — | 57 |
| (11viii) Others | |||||
| 139 | β-Glucose | 180.23 | C6H12O6 | Pods | 42 |
| 140 | Uridine | 244.20 | C9H12N2O6 | — | 55 |
| 141 | Perlolyrine (Syn.: 2-dehydrocarbonylflazin) | 264.28 | C16H12N2O2 | Flowers and fruits | 50 |
6. Conclusion
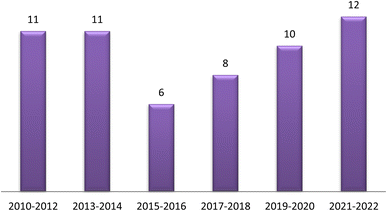
The traditional use of A. esculentus has been related to a variety of pharmacological effects. Over the past 12 years, a significant improvement has been seen in the rate of A. esculentus publications from (2015–2016) to (2021–2022) (Fig. 3). The leaves, fruits (pods), seeds, roots and flowers of A. esculentus have been the subject of research (Fig. 4). Research on A. esculentus reported a variety of intriguing bioactivities, including antidiabetic, hypolipidemic, antioxidant, antimicrobial and anticancer properties in addition to wound healing, hepatoprotective, immunomodulative, neurological, gastroprotective, weight reduction potential, cardioprotective and properties. Also other pharmacological activities were detected as nootropic, antistress, anti-inflammatory and antinociceptive. Undoubtedly, the varied phytochemical composition is responsible for these biological actions. Phenolic acids, terpenes, vitamins and flavonoids constitute the most frequently isolated phytochemical classes from A. esculentus in addition to sterols and fatty acids (Fig. 5). A. esculentus is regarded as a valuable source for natural compounds with potential use in medicine.Even though A. esculentus is used in supplementary medicine, nothing is known about its approved drugs derived from it. To completely comprehend the relationship between the active ingredients and the proven biological actions, in-depth investigations are needed. Last but not least, the anticancer and cardioprotective properties of A. esculentus constitute crucial future research subjects, encompassing the investigation of pure substances, their structure–activity relationship and their mechanisms of action. Finally, due to its significant nutritional content, A. esculentus is a promising option for the development of natural product drugs in addition to its beneficial use as functional food ingredient.
Conflicts of interest
There are no conflicts to declare.References
- P. Khomsug, W. Thongjaroenbuangam, N. Pakdeenarong, M. Suttajit and P. Chantiratikul, Antioxidative activities and phenolic content of extracts from okra (Abelmoschus esculentus L.), Res. J. Biol. Sci., 2010, 5, 310–313 CrossRef.
- S. K. Doreddula, S. R. Bonam, D. P. Gaddam, B. S. R. Desu, N. Ramarao and V. Pandy, Phytochemical analysis, antioxidant, antistress, and nootropic activities of aqueous and methanolic seed extracts of ladies finger (Abelmoschus esculentus L.) in mice, Sci. World J., 2014, 2014, 519848, DOI:10.1155/2014/519848.
- L. Hu, W. Yu, Y. Li, N. Prasad and Z. Tang, Antioxidant activity of extract and its major constituents from okra seed on rat hepatocytes injured by carbon tetrachloride, BioMed Res. Int., 2014, 2014, 341291, DOI:10.1155/2014/341291.
- V. Sabitha, S. Ramachandran, K. R. Naveen and K. Panneerselvam, Antidiabetic and antihyperlipidemic potential of Abelmoschus esculentus (L.) Moench. in streptozotocin-induced diabetic rats, J. Pharm. Bioallied Sci., 2011, 3, 397–402, DOI:10.4103/0975-7406.84447.
- D. Saha, B. Jain and V. K. Jain, Phytochemical evaluation and characterization of hypoglycemic activity of various extracts of Abelmoschus esculentus Linn. fruit, Int. J. Pharm. Sci., 2011, 3, 183–185 Search PubMed.
- V. Sabitha, K. Panneerselvam and S. Ramachandran, In vitro α-glucosidase and α-amylase enzyme inhibitory effects in aqueous extracts of Abelmoscus esculentus (L.) Moench, Asian Pac. J. Trop. Biomed., 2012, 2, S162–S164 CrossRef.
- H. Mda and A. M. Sarkarb, Evaluation of biological activities of Abelmoschus esculentus (Malvaceae), Int. J. Curr. Sci, 2014, 10, 43–49 Search PubMed.
- S. Fan, Y. Zhang, Q. Sun, L. Yu, M. Li, B. Zheng, X. Wu, B. Yang, Y. Li and C. Huang, Extract of okra lowers blood glucose and serum lipids in high-fat diet-induced obese C57BL/6 mice, J. Nutr. Biochem., 2014, 25, 702–709 CrossRef CAS.
- Z. H. Tian, F. T. Miao, X. Zhang, Q. H. Wang, N. Lei and L. C. Guo, Therapeutic effect of okra extract on gestational diabetes mellitus rats induced by streptozotocin, Asian Pac. J. Trop. Biomed., 2015, 8, 1038–1042 CrossRef CAS PubMed.
- A. E. Ben-Chioma, D. G. Tamuno-Emine and D. B. Dan, The effect of Abelmoschus esculentus in Alloxan-induced diabetic wistar rat, Int. J. Sci. Res., 2015, 4, 540–543 Search PubMed.
- B. T. Ahmed and S. A. Kumar, Antioxidant and Antidiabetic properties of Abelmoschus esculentus extract–an in vitro assay, Res. J. Biotechnol., 2016, 11, 34–41 CAS.
- I. Yaradua, M. Ibrahim, K. I. Matazu, N. U. Nasir, A. S. Matazu, M. B. Zainab, L. Abdul Rahman, L. Bilbis and A. Y. Abbas, Antidiabetic activity of Abelmoschus esculentus (Ex-Maradi Okra) fruit in alloxan-induced diabetic rats. Niger, J. Biochem. Mol. Biol., 2017, 32, 44–52 Search PubMed.
- Ph. T. Nguekouo, D. Kuate, A. P. Kengne, C. Y. Woumbo, F. A. Tekou and J. E. Oben, Effect of boiling and roasting on the antidiabetic activity of Abelmoschus esculentus (Okra) fruits and seeds in type 2 diabetic rats, J. Food Biochem., 2018, 42, 1266–1269 CrossRef.
- N. E. Majd, M. R. Tabandeh, A. Shahriari and Z. Soleimani, Okra (Abelmoscus esculentus) improved islets structure, and down-regulated PPARs gene expression in pancreas of high-fat diet and streptozotocin-induced diabetic rats, Cell J., 2018, 20, 31–40 Search PubMed.
- J. Liu, Y. Zhao, Q. Wu, A. John, Y. Jiang, J. Yang, H. Liu and B. Yang, Structure characterisation of polysaccharides in vegetable “okra” and evaluation of hypoglycemic activity, Food Chem., 2018, 242, 211–216 CrossRef CAS PubMed.
- Z. Liao, J. Zhang, B. Liu, T. Yan, F. Xu, F. Xiao, B. Wu, K. Bi and Y. Jia, Polysaccharide from okra (Abelmoschus esculentus (L.) Moench) improves antioxidant capacity via PI3K/AKT pathways and Nrf2 translocation in a type 2 diabetes model, Molecules, 2019, 24, 1906 CrossRef CAS PubMed.
- P. Daliu, G. Annunziata, G. C. Tenore and A. Santini, Abscisic acid identification in Okra, Abelmoschus esculentus L. (Moench): Perspective nutraceutical use for the treatment of diabetes, Nat. Prod. Res., 2020, 34, 3–9 CrossRef CAS PubMed.
- A. O. Elkhalifa, E. Al-Shammari, M. Adnan, J. C. Alcantara, A. M. Mehmood, N. E. Eltoum, K. Awadelkareem, B. P. Khan and S. A. Ashraf, Development and Characterization of Novel Biopolymer Derived from Abelmoschus esculentus L. Extract and Its Antidiabetic Potential, Molecules, 2021, 26, 3609 CrossRef CAS PubMed.
- E. L. Peter, P. B. Nagendrappa, C. O. Ajayi and C. D. Sesaazi, Total polyphenols and antihyperglycemic activity of aqueous fruits extract of Abelmoschus esculentus: Modeling and optimization of extraction conditions, PLoS One, 2021, 16, e0250405 CrossRef CAS PubMed.
- M. A. Haque, M. S. Hossain, N. M. Sayed, M. T. Islam, M. R. Khan, F. Ahmmed, F. T. Zohora, D. Ağagündüz, L. C. Ming and R. Capasso, Abelmoschus esculentus (L.) Moench Pod Extract Revealed Antagonistic Effect against the Synergistic Antidiabetic Activity of Metformin and Acarbose upon Concomitant Administration in Glucose-Induced Hyperglycemic Mice, Biologics, 2022, 2, 128–138 CrossRef.
- M. H. Siddique, A. Ashraf, S. Hayat, B. Aslam, M. FakhareAlam, S. Muzammil, M. Atif, M. Shahid, S. Shafeeq and M. Afzal, Antidiabetic and antioxidant potentials of Abelmoschus esculentus: In vitro combined with molecular docking approach, J. Saudi Chem. Soc., 2022, 26, 1014–1018 CrossRef.
- H. Liao, W. Dong, X. Shi, H. Liu and K. Yuan, Analysis and comparison of the active components and antioxidant activities of extracts from Abelmoschus esculentus L., Pharmacogn. Mag., 2012, 8, 156–161 CrossRef CAS PubMed.
- C. Wang, Y. B. Yu, T. T. Chen, Z. W. Wang and J. K. Yan, Innovative preparation, physicochemical characteristics and functional properties of bioactive polysaccharides from fresh okra (Abelmoschus esculentus (L.) Moench), Food Chem., 2020, 320, 126–136 Search PubMed.
- B. Xiong, W. Zhang, Z. Wu, R. Liu, C. Yang, A. Hui, X. Huang and Z. Xian, Preparation, characterization, antioxidant and anti-inflammatory activities of acid-soluble pectin from okra (Abelmoschus esculentus L.), Int. J. Biol. Macromol., 2021, 181, 824–834 CrossRef CAS PubMed.
- M. R. Mollick, B. Bhowmick, D. Mondal, D. Maity, D. Rana, S. Dash, S. Chattopadhyay, S. Roy, J. Sarkar and K. Acharya, Anticancer (in vitro) and antimicrobial effect of gold nanoparticles synthesized using Abelmoschus esculentus (L.) pulp extract via a green route, RSC Adv., 2014, 4, 37838–37848 RSC.
- S. Devanesan and M. S. AlSalhi, Green synthesis of silver nanoparticles using the flower extract of Abelmoschus esculentus for cytotoxicity and antimicrobial studies, Int. J. Nanomed., 2021, 16, 3343–3356 CrossRef PubMed.
- S. Solomon, M. Muruganantham and N. Senthamilselvi, Anticancer activity of Abelmoschus esculentus (flower) against human liver cancer, Int. J. Pharmacol. Biol. Sci., 2016, 6, 154–157 CrossRef.
- S. Hayaza, S. P. Wahyuningsih, R. J. Susilo, A. A. Permanasari, S. A. Husen, D. Winarni, H. Punnapayak and W. Darmanto, Anticancer activity of okra raw polysaccharides extracts against human liver cancer cells, Trop. J. Pharm. Res., 2019, 18, 1667–1672 CAS.
- S. A. Musthafa, K. Muthu, S. J. George, S. Murali, J. Govindaraj and G. Munuswamy-Ramanujam, Lectin isolated from Abelmoschus esculentus induces caspase mediated apoptosis in human U87 glioblastoma cell lines and modulates the expression of circadian clock genes, Toxicon, 2021, 202, 98–109 CrossRef CAS PubMed.
- H. E. Ahmed, Y. Iqbal, M. H. Aziz, M. Atif, Z. Batool, A. Hanif, N. Yaqub, W. A. Farooq, S. Ahmad and A. Fatehmulla, Green synthesis of CeO2 Nanoparticles from the Abelmoschus esculentus extract: evaluation of antioxidant, anticancer, antibacterial, and wound-healing activities, Molecules, 2021, 26, 4659 CrossRef CAS PubMed.
- M. Pramudya, F. R. Dewi, R. W. Wong, D. W. Anggraini, D. Winarni and S. P. Wahyuningsih, Anti-cancer activity of an ethanolic extract of red okra pods (Abelmoschus esculentus L. Moench) in rats induced by N-methyl-N-nitrosourea, Vet. World, 2022, 15, 1177–1184 CAS.
- S. Saravanan, P. Pandikumar, N. Pazhanivel, M. G. Paulraj and S. Ignacimuthu, Hepatoprotective role of Abelmoschus esculentus (Linn.) Moench., on carbon tetrachloride-induced liver injury, Toxicol. Mech. Methods, 2013, 23, 528–536 CrossRef CAS PubMed.
- W. Zheng, T. Zhao, W. Feng, W. Wang, Y. Zou, D. Zheng, M. Takase, Q. Li, H. Wu and L. Yang, Purification, characterization and immunomodulating activity of a polysaccharide from flowers of Abelmoschus esculentus, Carbohydr. Polym., 2014, 106, 335–342 CrossRef CAS PubMed.
- N. Mairuae, J. R. Connor, S. Y. Lee, P. Cheepsunthorn and W. Tongjaroenbuangam, The effects of okra (Abelmoschus esculentus Linn.) on the cellular events associated with Alzheimer's disease in a stably expressed HFE neuroblastoma SH-SY5Y cell line, Neurosci. Lett., 2015, 603, 6–11 CrossRef CAS PubMed.
- K. A. Ribeiro, H. V. Chaves, M. Pereira Filho, I. Ribeiro Pinto, D. Andressa Martins Monteiro, S. Oliveira Matos, T. Santi-Gadelha, C. Alberto de Almeida Gadelha, J. Thalles Jocelino Gomes de Lacerda and L. M. V. Aguiar, Alpha-2 Adrenergic and Opioids Receptors Participation in Mice Gastroprotection of Abelmoschus esculentus Lectin, Curr. Pharm. Des., 2016, 22, 4736–4742 CrossRef CAS.
- D. Ortac, M. Cemek, T. Karaca, S. Büyükokuroğlu, Z. Özdemir, A. Kocaman and S. Göneş, In vivo anti-ulcerogenic effect of okra (Abelmoschus esculentus) on ethanol-induced acute gastric mucosal lesions, Pharm. Biol., 2018, 56, 165–175 CrossRef CAS PubMed.
- J. Messing, C. Thöle, M. Niehues, A. Shevtsova, E. Glocker, T. Borén and A. Hensel, Antiadhesive properties of Abelmoschus esculentus (Okra) immature fruit extract against Helicobacter pylori adhesion, PLoS One, 2014, 9, e84836 CrossRef PubMed.
- R. Uebelhack, U. Bongartz, S. Seibt, G. Bothe, P. Chong, P. De Costa and N. Wszelaki, Double-blind, randomized, three-armed, placebo-controlled, clinical investigation to evaluate the benefit and tolerability of two dosages of IQP-AE-103 in reducing body weight in overweight and moderately obese subjects, J. Obes., 2019, 2019, 3412952, DOI:10.1155/2019/3412952.
- V. S. Kuruwitaarachchige, D. I. Uluwaduge, S. Premakumara and J. Wijayabandara, Cardio protective activity of Abelmoschus esculentus (Okra), Int. J. Food Sci. Nutr., 2018, 3, 39–43 Search PubMed.
- E. C. Nwachukwu, R. Nulit and R. Go, Nutritional and biochemical properties of Malaysian okra variety, Adv. Med. Plant Res., 2014, 2, 16–19 Search PubMed.
- S. Ray, S. Mishra, K. Bisen, S. Singh, B. K. Sarma and H. B. Singh, Modulation in phenolic root exudate profile of Abelmoschus esculentus expressing activation of defense pathway, Microbiol. Res., 2018, 207, 100–107 CrossRef CAS PubMed.
- M. H. Romdhane, H. Chahdoura, L. Barros, M. I. Dias, R. C. Corrêa, P. Morales, M. Ciudad-Mulero, G. Flamini, H. Majdoub and I. C. F. R. Ferreira, Chemical composition, nutritional value, and biological evaluation of Tunisian okra pods (Abelmoschus esculentus L.), Molecules, 2020, 25, 4739 CrossRef CAS PubMed.
- R. Sami, Y. Li, P. Qi, S. Wang, Q. Zhang, F. Han, Y. Ma, J. Jing and L. Jiang, HPLC analysis of water-soluble vitamins (B2, B3, B6, B12, and C) and fat-soluble vitamins (E, K, D, A, and β-carotene) of okra (Abelmoschus esculentus), J. Chem., 2014, 2014, 1–6 CrossRef.
- M. T. Islam, Phytochemical information and pharmacological activities of Okra (Abelmoschus esculentus): A literature-based review, Phytother. Res., 2019, 33, 72–80 CrossRef PubMed.
- B. J. Taiwo, T. D. Popoola, F. R. van Heerden and A. A. Fatokun, Isolation and Characterisation of Two Quercetin Glucosides with Potent Anti-Reactive Oxygen Species (ROS) Activity and an Olean-12-en Triterpene Glucoside from the Fruit of Abelmoschus esculentus (L.), Chem. Biodiversity, 2021, 18, e2000670 CrossRef CAS PubMed.
- H. Liao, H. Liu and K. Yuan, A new flavonol glycoside from the Abelmoschus esculentus Linn, Pharmacogn. Mag., 2012, 8, 12–20 CrossRef CAS PubMed.
- L. Jia, D. Li, L. L. Jing and M. M. Guo, Studies on the chemical constituents from petroleum ether portion of Abelmoschus esculentus, J. Chin. Med. Mater., 2010, 33, 1262–1265 CAS.
- I. O. Kehinde, O. E. Olatunji and A. Adegoke, Electrocardiography effects of Abelmoschus esculentus fruit extract and its isolated compounds using isolated frog heart perfusion, World J. Adv. Res. Rev., 2022, 14, 104–111 CrossRef CAS.
- I. F. Olawuyi, J. J. Park and W. Y. Lee, Effect of extraction conditions on ultrasonic-assisted extraction of polyphenolic compounds from okra (Abelmoschus esculentus L.) leaves, Korean J. Food Preserv., 2020, 27, 476–486 CrossRef.
- S. Mi, R. Guo, C. Xie and X. Huang, Compounds from the flowers and fruits of Abelmoschus esculentus (L.), Asian J. Tradit. Med., 2021, 16, 269–275 Search PubMed.
- Y. Zhou, X. Jia, J. Shi, Y. Xu, L. Jing and L. Jia, Two new pentacyclic triterpenes from Abelmoschus esculentus, Helv. Chim. Acta, 2013, 96, 533–537 CrossRef CAS.
- S. Yang, Y. Zhao, Z. Li, Q. Zuo and S. Qian, Flavonoids from flowers of Abelmoschus manihot, Chem. Nat. Compd., 2018, 54, 257–260 CrossRef CAS.
- Y. Zhang, T. Zhang, Q. Zhao, X. Xie, Y. Li, Q. Chen, F. Cheng, J. Tian, H. Gu and J. Huang, Comparative Transcriptome Analysis of the Accumulation of Anthocyanins Revealed the Underlying Metabolic and Molecular Mechanisms of Purple Pod Coloration in Okra (Abelmoschus esculentus L.), Foods, 2021, 10, 2180 CrossRef CAS PubMed.
- R. L. Jarret, M. L. Wang and I. J. Levy, Seed oil and fatty acid content in okra (Abelmoschus esculentus) and related species, J. Agric. Food Chem., 2011, 59, 4019–4024 CrossRef CAS PubMed.
- N. R. Gaikwad, R. Jadhav and S. Vikhe, Pharmacological Importance of Abelmoschus esculentus, World J. Pharm. Res., 2020, 9, 279–287 CAS.
- S. I. Alqasoumi, Okra Hibiscus esculentus L: A study of its hepatoprotective activity, Saudi Pharm. J., 2012, 20, 135–141 CrossRef CAS PubMed.
- L. Jia, M. Guo, D. Li and L. Jing, Chemical constituents from petroleum ether portion of Abelmoschus esculentus II, China J. Chin. Mater. Med., 2011, 36, 891–895 CAS.
- S. J. Pritam, A. T. Amol, B. B. Sanjay and J. S. Sanjay, Analgesic Activity of Abelmoschus manihot Extracts, Int. J. Pharmacol., 2011, 7, 716–720 CAS.
| This journal is © The Royal Society of Chemistry 2023 |