Open Access Article
Open Access ArticleThe key role of pretreatment for the one-step and multi-step conversions of European lignocellulosic materials into furan compounds
Maroua Kammouna,
Antigoni Margellou b,
Vesislava B. Totevac,
Anna Aladjadjiyand,
Andreai F. Sousa
b,
Vesislava B. Totevac,
Anna Aladjadjiyand,
Andreai F. Sousa ef,
Santiago V. Luisg,
Eduardo Garcia-Verdugo
ef,
Santiago V. Luisg,
Eduardo Garcia-Verdugo g,
Konstantinos S. Triantafyllidis
g,
Konstantinos S. Triantafyllidis b and
Aurore Richel
b and
Aurore Richel *a
*a
aLaboratory of Biomass and Green Technologies, University of Liege, Belgium. E-mail: a.richel@uliege.be
bDepartment of Chemistry, Aristotle University of Thessaloniki, 54124 Thessaloniki, Greece
cDepartment of Textile, Leather and Fuels, University of Chemical Technology and Metallurgy, Sofia, Bulgaria
dNational Biomass Association, Plovdiv, Bulgaria
eCICECO—Aveiro Institute of Materials, Department of Chemistry, University of Aveiro, 3810-193 Aveiro, Portugal
fCentre for Mechanical Engineering, Materials and Processes, Department of Chemical Engineering, University of Coimbra Rua Sílvio Lima—Polo II, 3030-790 Coimbra, Portugal
gDpt. of Inorganic and Organic Chemistry, Supramolecular and Sustainable Chemistry Group, University Jaume I, Avda Sos Baynat s/n, E-12071-Castellon, Spain
First published on 18th July 2023
Abstract
Nowadays, an increased interest from the chemical industry towards the furanic compounds production, renewable molecules alternatives to fossil molecules, which can be transformed into a wide range of chemicals and biopolymers. These molecules are produced following hexose and pentose dehydration. In this context, lignocellulosic biomass, owing to its richness in carbohydrates, notably cellulose and hemicellulose, can be the starting material for monosaccharide supply to be converted into bio-based products. Nevertheless, processing biomass is essential to overcome the recalcitrance of biomass, cellulose crystallinity, and lignin crosslinked structure. The previous reports describe only the furanic compound production from monosaccharides, without considering the starting raw material from which they would be extracted, and without paying attention to raw material pretreatment for the furan production pathway, nor the mass balance of the whole process. Taking account of these shortcomings, this review focuses, firstly, on the conversion potential of different European abundant lignocellulosic matrices into 5-hydroxymethyl furfural and 2-furfural based on their chemical composition. The second line of discussion is focused on the many technological approaches reported so far for the conversion of feedstocks into furan intermediates for polymer technology but highlighting those adopting the minimum possible steps and with the lowest possible environmental impact. The focus of this review is to providing an updated discussion of the important issues relevant to bringing chemically furan derivatives into a market context within a green European context.
1. Introduction
The transition from petroleum-based refineries to novel biorefineries is a key to maintaining sustainable global development and preserving the future of the planet. To adapt this context in an action plan, natural resources other than petrol should be involved as input to generate different value-added products, such as chemicals, materials, and fuels. Featured by guaranteed supply, large availability, constant production, and reasonable cost, renewable sources constitute a key element in facing challenges reduce and fossil resource use. Vegetal biomass implication represents an advanced conception of the emerged existing model of food and paper biorefineries while producing a wide range of different platform molecules fulfilling the different societal requirements. Moreover, renewable biomass introduction has a beneficial impact on the balance of complex trade-offs among the socioeconomic and environmental points of view by promoting value-added product generation, creating new opportunities for employment, and reducing fossil resource use.1 The furan compound-driven biorefinery approach, based on lignocellulosic biomass as a renewable resource, has lately evolved. The carbohydrate part of LCB (free sugars such as hexoses or pentoses, or from oligo- or polysaccharides) can be derived into versatile furan-based molecules, such as 5-HMF (5-hydroxymethylfurfural) or 2-F (2-furfural), which are promising bridges between biomass and a panel of possible chemicals and biopolymers.2 In fact, 5-HMF and 2-F were identified as platform molecules since the early 2000s and both were obtained by the selective dehydration of C5 or C6 monosaccharides (including representative C5 sugars, such as D-xylose or C6 entities, such as D-glucose, D-fructose, or D-mannose).3Macromolecules in LCB have different susceptibilities to pretreatment, and the most common situation is hemicellulose removal as it consists of short side chains, and has a lower degree of polymerization, and inferior crystallinity compared to cellulose. During hydrothermal pretreatment, hemicellulose is easily extracted into oligo or polysaccharides or monomers as free sugars. In contrast, a low yield of cellulose is extracted into cellobiose and glucose.4–6 The acid medium attacks hemicellulose and promotes its removal, and during LHW, autohydrolysis is induced through hydronium catalyzed reactions, leading to hemicellulose extraction.7,8 Ionic liquids (IL) can dissolve both hemicellulose and cellulose by dint of the polarity of N–O bonds, which breaks cellulose hydrogen bond, forming new hydrogen bonds with the solute.9,10 Cellulose has low solubility in deep eutectic solvents (DESs) compared to lignin and hemicellulose. DESs especially remove lignin and hemicellulose, due to their intrinsic ionic nature, which breaks the supramolecular H-bonds stabilizing the hemicellulose–lignin complex, allowing easily the breaking of the covalent bonds and leaving a cellulose rich-solid.11,12 Supercritical fluids reduce cellulose crystallinity, promote its decomposition into α- and β-glucosides and deal with direct carbohydrate hydrolysis.13 The recovered sugars from cellulose and hemicellulose can be directly dehydrated to furanic compounds or undergo some added hydrolysis to be transformed. Their conversion depends on pretreatment parameters and their structural changes.
Many technical papers have been published on the only production routes involving “simple” monosaccharides such as xylose,14 glucose,15 and fructose.16 However, this review pays excessive attention to the whole biorefinery schema starting from raw materials from which carbohydrates would be extracted and the mass balance on the whole value chain. It is an unquestionable fact that from an industrial perspective, the issue of exploiting lignocellulosic resources, which are more strategic as sources of (C6 and C5) carbohydrates that can be converted into furan-based compounds of interest for the polymer materials sector.
Therefore, within a green European context, the present review intends to put forward two particular lines of thought. On the one hand, the choice of the most suitable lignocellulosic biomasses as the raw material to be converted into furan intermediates. Thirty-three European lignocellulosic biomasses emerging from agricultural residues, agri-food byproducts, energy crops, forest matrices, plant grasses, and non-food sectors are discussed.
On the other hand, this review describes in detail all the technological approaches allowing us to convert these matrices into furan compounds of interest. Focusing on the green processes, particularly under hydrothermal conditions and using neoteric solvents, potential carbohydrate extraction and furanic compound formation in the fewest possible steps and with the lowest possible environmental impact are investigated.
2. Furanic compounds' potential towards new bio-polymers
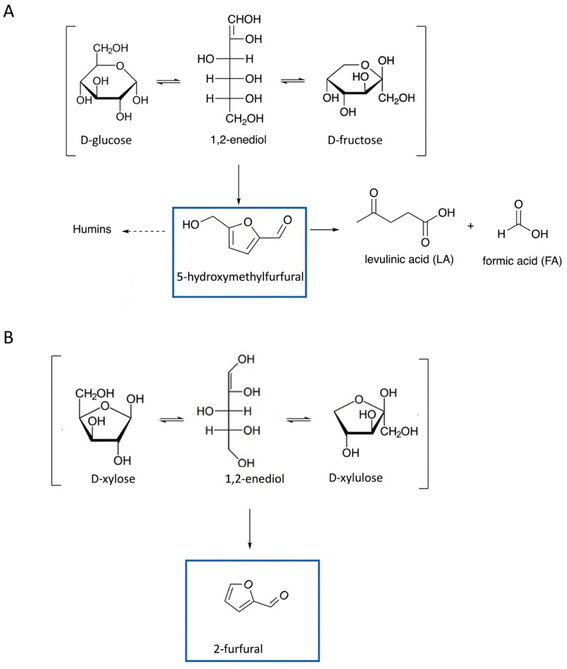
Furanic compounds, notably 5-HMF and 2-furfural, are promising “starting point” molecules derived from LCB, to form new platform molecules. Fig. 1 provides an overview of the molecular structures of the main furan entities under investigation to date. The economic and environmental stakes related to 5-HMF and 2-furfural are high and supported by multiple research initiatives on the subject, which results in abundant scientific literature.17 The state of the art describes extensively the production of furans from hexoses and pentoses, which have often been considered as the “model case” studies (Fig. 2). A lot of fundamental knowledge has been gained on the role of the catalyst, the solvent (whether it is an aqueous solution, an organic solvent, or an ionic liquid),18 and the reaction mechanism;19 more specifically, D-glucose can be converted via isomerization to D-fructose, which is further dehydrated to 5-HMF, with homogeneous (inorganic acids and bases and metal chlorides) or heterogeneous (metal oxides, metal-doped zeolites, etc.) catalysts exhibiting basicity or Lewis acidity (for D-glucose isomerization) and Brønsted acidity (for D-fructose dehydration to 5-HMF).3,20–23 Different organic solvents, mixtures of water-organic solvents, ionic liquids, and even pure water have been used as reaction media.24,25 Attention should be paid to the selective production of 5-HMF instead of organic acids (levulinic acid and formic acid) and humin by-products, stability of catalysts, and improvement of current separation methods of products, e.g., use of biphasic systems.22,23,26 Similarly, 2-furfural is produced from D-xylose via dehydration. Since the early 20s, the industrial production of 2-F is based on the acidic transformation of biomass derived C5 sugars, but the low yield (50%) obtained, due to undesired side-reactions, led to further research on this process.27 D-Xylose conversion to 2-furfural is catalyzed either by homogeneous organic and inorganic acids (HCl, H2SO4, metal chlorides, etc.) or heterogeneous catalysts, such as metal oxides and zeolites.28,29 Regarding the solvents, water, organic solvents, or mixtures of water with organic solvents are frequently used.28–30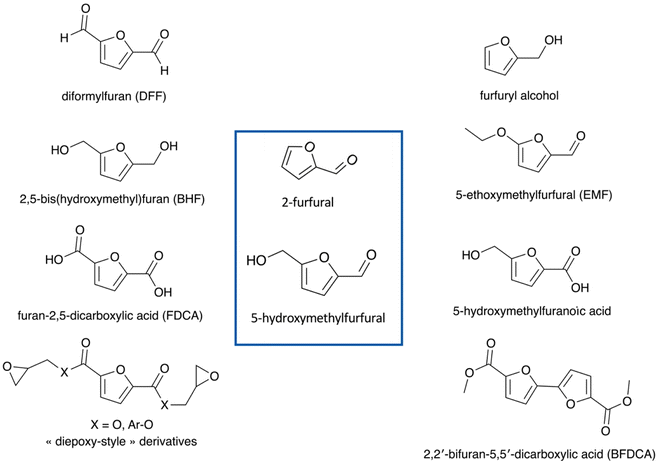 | ||
| Fig. 1 Main molecules derived from 5-HMF or 2-F exploited for the design of new biobased polymers and additives (non-exhaustive list).17 | ||
 | ||
| Fig. 2 Hydrothermal conversion of D-glucose (A) and D-fructose (B) into 5-HMF and derivatives. Reaction intermediates and/or subsequent decomposition (or rearrangement) products are not mentioned.18 | ||
The most reported case study in the scientific literature, and the most studied by many private actors, is undoubtedly the case of 5-HMF-based furan-2,5-dicarboxylic acid (FDCA) allowing obtaining poly(ethylene 2,5-furanoate) (PEF), a possible alternative to the traditional poly(ethylene terephthalate) (PET),31 but also suitable for a myriad of other biopolymers and technical additives.32 Given the chemical reactivity of furan rings and the presence of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–O functional groups on whether 5-HMF or 2-F, numerous studies have been reported on the development of catalytic tools allowing their functionalization into a panel of derived molecules, among which we find 2,2′-bifuran-5,5′-dicarboxylic acid (BFDCA), which offers an alternative to bisphenol A for the design of dimethacrylate resins with thermomechanical properties comparable with those of traditional BisGMA (dimethacrylate derivative of bisphenol A).33,34 The potential of 5-HMF among others is underlined through various works reported in the state of the art, with the alternative synthesis of “symmetrical” 2,5-functional derivatives such as 2,5-bis(hydroxymethyl)furan (BHF), or diformylfuran (DFF), and the “unsymmetrical” monomers such as 5-ethoxymethylfurfural (EMF), furfuryl alcohol (FA), 5-hydroxymethylfuranoic acid, 5-(chloromethyl)furfural (CMF), and diamines and diepoxy analogs.35 Nevertheless, the exploitation of specific chemical tools also allows obtaining other derivatives of interest for the plastics sector, with a loss of the typical furan ring structure, since it is possible to synthesize medium chain diols such as 1,6-hexanediol or even anhydrides (maleic anhydride).36 It is commonly accepted that, in general, all aforementioned furan derivatives offer unique molecular architectures and notable physicochemical properties, opening new prospects in polymer science and technology. However, the great question is not on their value but rather on how to prepare them, starting from lignocellulosic matrices available to explore in the European context.
O and C–O functional groups on whether 5-HMF or 2-F, numerous studies have been reported on the development of catalytic tools allowing their functionalization into a panel of derived molecules, among which we find 2,2′-bifuran-5,5′-dicarboxylic acid (BFDCA), which offers an alternative to bisphenol A for the design of dimethacrylate resins with thermomechanical properties comparable with those of traditional BisGMA (dimethacrylate derivative of bisphenol A).33,34 The potential of 5-HMF among others is underlined through various works reported in the state of the art, with the alternative synthesis of “symmetrical” 2,5-functional derivatives such as 2,5-bis(hydroxymethyl)furan (BHF), or diformylfuran (DFF), and the “unsymmetrical” monomers such as 5-ethoxymethylfurfural (EMF), furfuryl alcohol (FA), 5-hydroxymethylfuranoic acid, 5-(chloromethyl)furfural (CMF), and diamines and diepoxy analogs.35 Nevertheless, the exploitation of specific chemical tools also allows obtaining other derivatives of interest for the plastics sector, with a loss of the typical furan ring structure, since it is possible to synthesize medium chain diols such as 1,6-hexanediol or even anhydrides (maleic anhydride).36 It is commonly accepted that, in general, all aforementioned furan derivatives offer unique molecular architectures and notable physicochemical properties, opening new prospects in polymer science and technology. However, the great question is not on their value but rather on how to prepare them, starting from lignocellulosic matrices available to explore in the European context.
3. Lignocellulosic matrices as potential inflows for the production of furanic entities
Considering the natural composition of lignocellulosic biomass, highly enriched in carbohydrate polymers namely cellulose (source of D-glucose via hydrolysis) and hemicellulose (mostly rich in C5 and C6 carbohydrates),37 and the attractive perspectives for 5-HMF or 2-F production therefrom, if suitable pretreatment steps are set up for its isolation, several authors' assessments for 2020–2030 have identified four strategic primary sources of lignocellulosic biomass that could support the development of biobased industrial activities, at the European level. These are (1) forest biomass, (2) agricultural residues, (3) non-food (or energy) crops, and (4) food or non-food side-products.38However, one should have in consideration the fact that these four primary sources of lignocellulosic materials are heterogeneously distributed within the EU, both in terms of quantities and territorial repartition. This is due, among other things, to differences in climate (and soil type) between the EU member states, differences in “production tradition”, or differences in the management of the end of life of the by-products. Although it is rather complex to quantify rigorously the available biomass flows in Europe, it can be said that agricultural residues represent the most important sector with a (dry) mass of available lignocellulosic materials of 342 million tons (on an annual basis), followed by the forestry sector with 325 million tons and the forestry by-product sector accounting for 185 million tons. The food waste sector could contribute nearly 89 million tons. However, as already stated before these raw material flows are unevenly distributed in the European zone. Forestry production is massively concentrated in the Nordic countries such as Finland and Sweden, whereas France, Spain, and Slovenia are also producers but to a lesser extent. Agricultural production (mainly cereals) is strongly concentrated in France and Spain. The other European countries contribute in an equivalent way, with specificities of production. This is particularly true for sugar beet (which is produced mainly in Belgium, Germany and the Netherlands) in addition to northern France, and for olive (produced mainly in the southern countries such as Greece, Spain and Portugal).
Table 1 presents the four primary sectors of raw lignocellulosic materials as well as representative examples of interest for Europe. Table 1 also proposes the chemical composition of these lignocellulosic matrices, besides cellulose and hemicellulose previously mentioned, also the aromatic biopolymer lignin together with minor amounts of other organic components (proteins, secondary metabolites, etc.) and minerals.37 As can be seen, this chemical composition is found specific, with cellulose contents fluctuating between 10 and 75% of the dry weight of the starting material, and hemicellulose contents oscillating between 5 and 35%. If we estimate (by simple calculation based on C6 contents) the theoretical yields of 5-hydroxymethylfurfural and 2-furfural that can be obtained, we deduce that all these raw materials are not equivalent in their strict potentiality to offer important yields of 5-HMF. The same conclusion can also be formulated if we consider the conversion of pentoses from hemicellulose to 2-F.
| Matrices | Chemical composition (%, referred to dry matter) | 5-HMF yielda (kg) | 2-F yielda (kg) | ||||
|---|---|---|---|---|---|---|---|
| Cellulose | Hemicelluloses | Lignin | Proteins | Inorganics | |||
| a Theoretical yield (kg based on the conversion of 1 ton of dry biomass). | |||||||
| Forest biomass and grasses | |||||||
| Spruce | 45.0 | 18.4 | 27.6 | 1.1 | 1.0 | 350.0 | 53.8 |
| Beechwood | 41.9 | 18.2 | 21.6 | 0.6 | 0.4 | 325.9 | 24.1 |
| Hybrid poplar | 39.0 | 17.0 | 25.0 | 3.3 | 2.0 | 303.3 | 103.9 |
| Eucalyptus | 46.7 | 17.3 | 28.0 | 0.6 | 0.6 | 363.2 | 65.4 |
| Pine | 38.6 | 20.5 | 29.2 | 0.6 | 4.7 | 300.2 | 36.8 |
| Spruce barks | 24.1 | 12.9 | 36.8 | 1.8 | 5.7 | 187.4 | 51.5 |
| Willow | 34.6 | 17.8 | 18.8 | 3.2 | 0.1 | 269.1 | 115.2 |
| Birchwood | 42.7 | 26.4 | 16.9 | 3.0 | 0.1 | 332.1 | 144.7 |
| Black locust | 45.0 | 19.0 | 29.0 | 3.6 | 0.8 | 350.0 | 119.7 |
| Fresh grass | 18.6 | 27.7 | 4.4 | 26.7 | 12.8 | 144.7 | 36.8 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
| Agricultural and agri-food residues | |||||||
| Wheat straw | 40.8 | 35.3 | 8.2 | 2.9 | 7.4 | 317.3 | 152.8 |
| Corn stovers | 37.5 | 18.7 | 13.4 | 4.7 | 5.7 | 291.7 | 119.7 |
| Sugarcane bagasse | 42.1 | 21.5 | 24.0 | 0.9 | 1.6 | 327.4 | 124.2 |
| Sugar beet pulp | 28.5 | 28.1 | 9.7 | 9.4 | 5.7 | 221.7 | 135.0 |
| Ryegrass | 22.1 | 25.6 | 4.7 | 8.6 | 8.5 | 171.9 | 130.6 |
| Olive kernels | 37.7 | 22.8 | 29.1 | 4.4 | 2.7 | 293.2 | 129.9 |
| Oat hulls | 30.0 | 34.0 | 13.2 | 3.6 | 6.1 | 233.3 | 137.0 |
| Oat straw | 37.6 | 23.3 | 12.9 | 5.3 | 2.2 | 292.4 | 135.0 |
| Fescue | 31.3 | 15.2 | 19.4 | 7.3 | 11.5 | 243.4 | 97.3 |
| Peach kernels | 21.1 | 22.1 | 40.5 | 1.6 | 2.9 | 164.1 | 123.5 |
| Apple pomace (cider industry) | 23.3 | 24.9 | 15.4 | 3.5 | 1.6 | 181.2 | 51.8 |
| Grape pomace (wine industry) | 15.8 | 7.9 | 20.3 | 11.2 | 13.4 | 122.5 | 87.7 |
| Orange peels (juice industry) | 11.1 | 12.6 | 1.0 | 7.8 | 4.2 | 86.3 | 25.5 |
| Destarched wheat bran | 14.8 | 47.7 | 8.1 | 12.2 | 1.7 | 115.1 | 209.9 |
| Spent coffee grounds | 12.4 | 39.1 | 23.9 | 17.4 | 1.3 | 96.4 | 28 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
| Energy crops | |||||||
| Miscanthus | 48.5 | 20.1 | 22.4 | 1.4 | 4.3 | 377.2 | 111 |
| Hemp (woody core) | 48.0 | 12.0 | 28.0 | 3.0 | 2.0 | 373.3 | 31.2 |
| Switchgrass | 33.5 | 26.5 | 18.1 | 5.3 | 6.4 | 260.6 | 90.2 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
| Residues from non-food sectors | |||||||
| Sawdust | 45.1 | 28.1 | 24.2 | 1.3 | 1.2 | 350.8 | 45.2 |
| Oak barks | 24.0 | 13.0 | 25.0 | 1.1 | 9.0 | 186.7 | 55.1 |
| Organic household waste | 14.8 | 9.8 | 3.7 | 15.8 | 11.8 | 115.1 | 1.2 |
| Garden waste | 20.3 | 16.9 | 17.5 | 9.6 | 31.3 | 157.9 | 74.9 |
| Mowing residues | 38.3 | 31.0 | 6.4 | 4.7 | 7.7 | 297.9 | 36.2 |
The analysis in Table 1 suggests that wood and its by-products as well as specific crops (such as hemp or miscanthus) or agricultural residues (cereal straws or sugarcane bagasse) offer timely potential to produce furan compounds in significant quantities. Either softwoods or hardwoods can offer (theoretically) up to one third of their weight in 5-HMF, while some by-products of the food industry seem, however, less suitable.
While the total hemicellulose content within a lignocellulosic material is a key factor to consider, it is worth mentioning that the very specific chemical nature of these hemicelluloses is also variable between the different materials presented in Table 1. The hemicellulose structure can be “homogeneous” or “heterogeneous” depending on the diversity (or not) of the “monomers” (oses) which constitute them. Amongst these “monomers”, the most representative are pentoses (xylose and arabinose), hexoses (glucose, mannose, and galactose), hexuronic acids (4-O-methyl-D-glucuronic acid, D-glucuronic acid, and D-galacturonic acid) and acetyl groups.39 Based on the combination of the above building blocks, hemicellulose can be classified as xylan-based (homoxylans, glucuronoxylans, arabinoxylans, and glucuronoarabinoxylans/arabinoglucuronoxylans), mannan-based (homomannans, galactomannans, glucomannan, and galactoglucomannan), xyloglucan and galactans.39 As evidenced in Table 1, hemicellulose contents in wood could reach 18 to 30 wt%. Hardwoods are usually composed of xylans, while hemicelluloses in softwoods are mostly mannan and galactoglucomannan-type. Agricultural biomasses (cereal straws) and fresh grasses exhibit the highest amounts of hemicelluloses, mostly xylans and arabinoxylans.40
Based on these theoretical results, variable yields of 5-HMF were observed, ranging from 86 kg/ton dry raw biomass to more than 350 kg/ton dry raw biomass. These high yields were detected in rich cellulose matrices such as spruce, eucalyptus, miscanthus, and hemp sawdust. Concerning 2-F formation from hemicellulose, only pentoses were considered to calculate the theoretical yield, which oscillated between 1.2 and 209 kg/ton dry raw biomass. The most interesting lignocellulosic feedstocks to produce 2-F are pentose rich hemicellulose matrices such as destarched wheat bran, hybrid poplar, willow, birchwood, and black locust.
In addition to this purely quantitative perspective, two additional points are equally important. On the one hand, they concern the availability of the lignocellulosic resource and its ability to be mobilized, and on the other hand the “technical” accessibility of the carbohydrate components within these lignocellulosic matrices. If the first point is related to economic, political and often territorial strategies, the second point is related to purely technical aspects linked to the efficiency of the pretreatment phases, which we will discuss below.
4. Role of the pretreatment in the “lignocellulose-to-furan” pathway
In order to transform lignocellulosic matrices into 5-HMF and/or 2-F, the prerequisite remains the pretreatment step whose purpose is to separate and individualize the main lignocellulosic components. Once the structural polysaccharides are isolated, subsequent hydrolysis steps (chemical or enzymatic) are then implemented to obtain the hexoses and/or pentoses that will be converted into furan derivatives (Fig. 3). Numerous pretreatment strategies are referenced in the state of the art and include chemical (acids or bases under aqueous conditions, and/or more or less innovative solvents including ionic liquids (ILs)), mechanical, and biological methods or a combination of several of these approaches. Each pretreatment method can be specific to the raw material and/or the targeted applications.41 The formation of simple furanic compounds such as 5-HMF or 2-F during the pretreatment phases has been demonstrated for decades. This formation has often been avoided, especially because 5-HMF (and 2-F) is a known inhibitor of alcoholic fermentation and its presence decreases bioethanol yields.42 It is recognized that pretreatment methods involving acids, whether solvent (or ionic liquid) methods with acid catalysts or hydrothermal methods, are the most likely to generate these furan species. Therefore, it is these particular pretreatment methods that are currently being used to produce specifically 5-HMF and 2-F.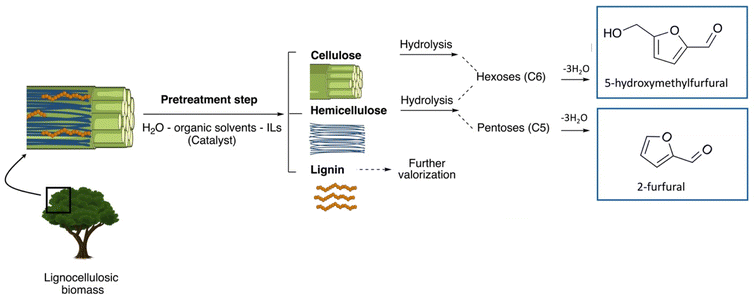 | ||
| Fig. 3 Multi-step conversion of lignocellulosic resources into furanic compounds. ILs stands for ionic liquids. | ||
4.1. Hydrothermal pretreatments
Pretreatment methods that take place in the aqueous phase (with or without the addition of acid catalysts) are known under the scientific term of “hydrothermal pretreatments” (HT). They are classified as “physicochemical pretreatment” and encompass two distinct processes, namely, liquid hot water (LHW) and steam explosion (SE).43 HT is recognized as a “green” process due to the absence (or minimum use) of toxic chemicals or added catalysts and was initially developed for the second-generation bioethanol production, with the aim to remove hemicellulose and partially degrade the robust lignocellulosic structure, leading ultimately to enhanced enzyme hydrolysis of cellulose to glucose. Considering the operational conditions of these processes, LHW utilizes water as the only solvent at elevated temperatures, in the range of 130–270 °C, for a time period of ca. 15 to 180 min depending on temperature.44–49Similar to LHW, SE uses steam instead of water, under pressure (<50 bar) for relatively short reaction times (ca. up to 1–10 min) in the temperature range 160–240 °C, followed by a fast explosive decompression of the high-pressure steam.43,50–52
HT pretreatment of lignocellulosic biomass has been applied on the pilot and industrial scale mainly for the production of second generation bioethanol considering also the “side” streams of hemicellulose and lignin, with Inbicon in Denmark and SEKAB in Sweden being two representative examples.53,54
Hemicellulose deconstruction during LHW is carried out via first-order reactions, catalyzed by hydronium ions, produced due to the subcritical state of water (T < 374 °C, P < 221 bar).57 Hemicellulose polymer (H) is initially depolymerised into oligomers (O), which with further breakdown are converted to monomers (M) and degradation products (DP). The reaction pathways and reaction steps for solubilization and hydrolysis of hemicellulose can be described by eqn (1a) and (1b).58
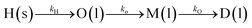 | (1a) |
| Xylan → XOSH → XOSL → xylose → furfural → DP | (1b1) |
| Arabinan → AOS → arabinose → furfural → DP | (1b2) |
More specifically, hemicellulose in biomass is partially acetylated and, under hydrothermal conditions, acetic acid is formed via hydrolysis of ester bonds.59 The in situ produced acetic acid promotes hydrolytic reactions of hemicellulose and to a lesser extent of cellulose into xylan and glucan oligomers, which can be further hydrolyzed to form xylose and glucose, respectively (Fig. 4, step 1). Under the mildly acidic hydrothermal conditions (notably for pH ∼ 2.5–3.5), C5 sugars (xylose and arabinose) can be dehydrated towards furfural formation, which can be further converted to formic acid via hydrolytic fission of the aldehyde group or to 2-furoic acid via oxidation (Fig. 4, step 2).47,60,61
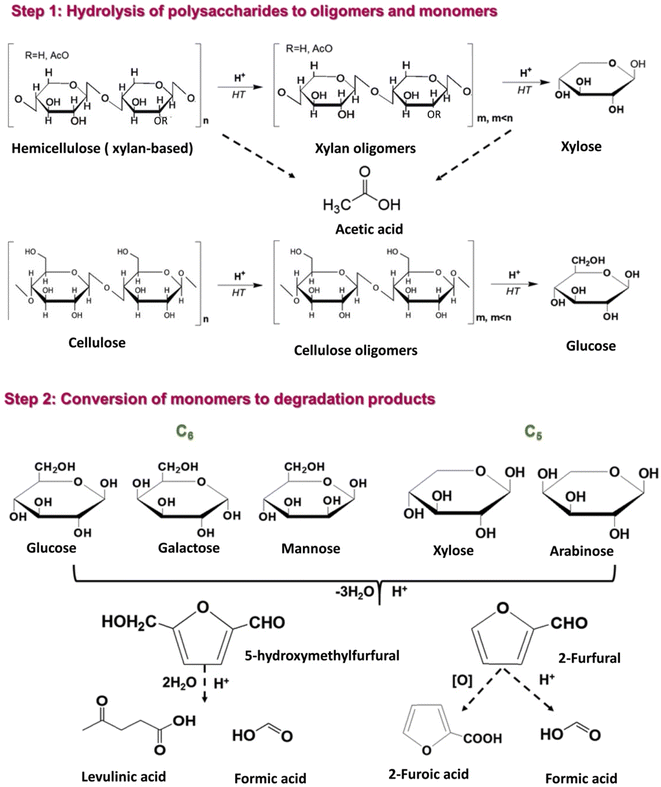 | ||
| Fig. 4 Reaction pathways in the hydrothermal treatment of lignocellulosic biomass towards sugars and degradation products. | ||
Similarly, 5-HMF can be produced via dehydration of C6 sugars (glucose, galactose, and arabinose) and can be further converted into levulinic and formic acids (Fig. 4, step 2).18
The extent of these reactions, as well as the biomass solubilization degree and hemicellulose removal, is controlled by the residence time and temperature of the pretreatment. Their combined effect can be expressed by the severity factor Ro (eqn (2)).62–64 During HT pretreatment, water auto-ionizes into hydronium ions, leading to a pH drop, increasing the severity factor leading to promoting acetyl and uronic group cleavage, and thus improving hydrolysis reactions.65
When small amounts of acid catalysts (i.e., H2SO4 and HCl) are used to facilitate hemicellulose hydrolysis, the modified severity factor is expressed by eqn (3), where t is the residence time (min) and T is the temperature (°C).66–68
| Ro = t·e(T−100)/14.75 | (2) |
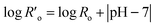 | (3) |
In addition to temperature, time and use of small amounts of acids, the liquid-to-solid (biomass) ratio (LSR) may have a relatively small effect on biomass solubilization and a more pronounced impact on liquid product selectivity and yields, as discussed below.69,70 Furthermore, considering the biomass particle size effects in LHW, they are correlated with heat and mass transfer limitations and it was shown that the particle size affects only sugar extraction and hemicellulose removal from biomass and not their thermal degradation in the liquid fraction.71,72
It should be highlighted that biomass particle size is a less important parameter than temperature and time and its effect is more profound in combination with that of LSR.
Considering the effect of temperature and time, under mild treatment conditions (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ro ≤ 2.76, corresponding to ≤150 °C and short time of 15 min), low solubilization of beechwood sawdust was observed (≤6 wt%), while under harsher conditions (log
Ro ≤ 2.76, corresponding to ≤150 °C and short time of 15 min), low solubilization of beechwood sawdust was observed (≤6 wt%), while under harsher conditions (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ro ≥ 4.14, 170 °C, 120–180 min) the solubilization was increased substantially (to about 40 wt%) and complete removal of hemicellulose occurred at log
Ro ≥ 4.14, 170 °C, 120–180 min) the solubilization was increased substantially (to about 40 wt%) and complete removal of hemicellulose occurred at log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ro = 4.69, corresponding to 220 °C and 15 min.47 Regarding product yields, a maximum xylan recovery (60 wt%) in the liquid fraction was determined at 80 wt% hemicellulose removal, which was obtained at moderate severities (log
Ro = 4.69, corresponding to 220 °C and 15 min.47 Regarding product yields, a maximum xylan recovery (60 wt%) in the liquid fraction was determined at 80 wt% hemicellulose removal, which was obtained at moderate severities (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ro = 3.8–4.1). Higher severities led to xylose degradation product formation, mainly furfural and formic acid.47 Differentiations have been observed in the LHW pretreatment between softwood and hardwood biomass. More specifically, by increasing log
Ro = 3.8–4.1). Higher severities led to xylose degradation product formation, mainly furfural and formic acid.47 Differentiations have been observed in the LHW pretreatment between softwood and hardwood biomass. More specifically, by increasing log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ro from 3.3 to 4.7, the hardwoods, grapevine prunings and poplar branches, showed higher solubilization ranging from 28 to 48% and 23.5 to 40%, respectively, while the softwood pine sawdust was more resistant to hydrothermal pretreatment with solubilization ranging from 19 to 34%.59 The recovery of xylan from poplar and grapevine and of mannan/galactan from pine in the liquid products showed a maximum of about 60% at moderate severities (log
Ro from 3.3 to 4.7, the hardwoods, grapevine prunings and poplar branches, showed higher solubilization ranging from 28 to 48% and 23.5 to 40%, respectively, while the softwood pine sawdust was more resistant to hydrothermal pretreatment with solubilization ranging from 19 to 34%.59 The recovery of xylan from poplar and grapevine and of mannan/galactan from pine in the liquid products showed a maximum of about 60% at moderate severities (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ro 3.8–4.1), corresponding to 70–85% hemicellulose removal. By comparing the above results of the two relevant works, it can be suggested that the solubilization/removal of hemicellulose is similar for different types of hardwood biomass (forest and agricultural) and higher than that in softwood biomass (pine), while the recovery of hemicellulose in the liquids reaches a similar maximum and in the same severity region.47,59
Ro 3.8–4.1), corresponding to 70–85% hemicellulose removal. By comparing the above results of the two relevant works, it can be suggested that the solubilization/removal of hemicellulose is similar for different types of hardwood biomass (forest and agricultural) and higher than that in softwood biomass (pine), while the recovery of hemicellulose in the liquids reaches a similar maximum and in the same severity region.47,59
A wide variety of forestry and agricultural residues have been hydrothermally treated in neat water: olive tree prunings with 37.6–45.3% biomass solubilization at log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ro = 3.06–4.83 (ref. 73) and 34.4% solubilization at 3.79,68 cotton stalks with 33% biomass solubilization and 82% xylan removal at 4.29 (ref. 74) bagasse with biomass solubilization of 14.8–27.6% at 2.8–4.8 (ref. 75) and xylan removal of 34.5% and 80% at 3.83 and 4.42,76 eucalyptus sawdust with 0–48.2% biomass solubilization at 2.07–5.60 and 77.7% xylan removal at 3.3,77 maple wood with 80% xylan removal at 4.25,78 corn cobs with 10–40% biomass solubilization at 3.06–4.43 (ref. 79) and 74.1% xylan solubilization at 4.13,80 poplar with 1.9–23% biomass solubilization and 6.8–62.8% xylan removal at 3.06–3.76 (ref. 81) and 50–77% xylan solubilization at 3.6–4.2,46 hazelnut shells with 35.4% biomass solubilization at 4.44,82 hazelnut tree prunings with 14–36% biomass solubilization and 43.2–98.5% hemicellulose removal at 3.24–4.89,83 brewer's spent grains with 64.06% xylan solubilization at 4.13,80 corn husks with 42% xylan solubilization at 4.13,80 wheat straws with 16.5–45.7% biomass solubilization at 3.36–5.14 (ref. 84) and 39.26% xylan solubilization at 4.13 (ref. 80) and almond shells with 39.3% biomass solubilization at 3.79 (ref. 68). A correlation of pretreatment conditions (temperature and time) with biomass solubilization and hemicellulose/xylan removal, based on the above previously reported works, can be observed in Fig. 5.
Ro = 3.06–4.83 (ref. 73) and 34.4% solubilization at 3.79,68 cotton stalks with 33% biomass solubilization and 82% xylan removal at 4.29 (ref. 74) bagasse with biomass solubilization of 14.8–27.6% at 2.8–4.8 (ref. 75) and xylan removal of 34.5% and 80% at 3.83 and 4.42,76 eucalyptus sawdust with 0–48.2% biomass solubilization at 2.07–5.60 and 77.7% xylan removal at 3.3,77 maple wood with 80% xylan removal at 4.25,78 corn cobs with 10–40% biomass solubilization at 3.06–4.43 (ref. 79) and 74.1% xylan solubilization at 4.13,80 poplar with 1.9–23% biomass solubilization and 6.8–62.8% xylan removal at 3.06–3.76 (ref. 81) and 50–77% xylan solubilization at 3.6–4.2,46 hazelnut shells with 35.4% biomass solubilization at 4.44,82 hazelnut tree prunings with 14–36% biomass solubilization and 43.2–98.5% hemicellulose removal at 3.24–4.89,83 brewer's spent grains with 64.06% xylan solubilization at 4.13,80 corn husks with 42% xylan solubilization at 4.13,80 wheat straws with 16.5–45.7% biomass solubilization at 3.36–5.14 (ref. 84) and 39.26% xylan solubilization at 4.13 (ref. 80) and almond shells with 39.3% biomass solubilization at 3.79 (ref. 68). A correlation of pretreatment conditions (temperature and time) with biomass solubilization and hemicellulose/xylan removal, based on the above previously reported works, can be observed in Fig. 5.
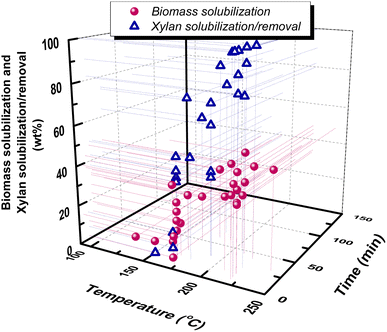 | ||
| Fig. 5 Correlation of xylan solubilization/removal and biomass solubilization with hydrothermal (LHW) pretreatment temperature and time. Data are from ref. 46, 47, 59, 68, 73–75, 77–85. | ||
Liquid to solid ratio (LSR) in LHW pretreatment can be varied in the range of 1–20 but usually is around 10.70 An increase of solid concentration can raise acetic acid concentration and thus enhance the autohydrolysis mechanism. In general, biomass solubilization is slightly affected by LSR, while product distribution in the pretreatment liquor seems to exhibit differentiations. Under mild pretreatment conditions, at 160 °C for 45 min (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ro = 3.42), an increase of LSR from 1 to 11 resulted in a decrease of xylose and xylan concentrations but an increase of xylose and acetic acid weight percentages (on biomass) in the liquor.70 Garrote et al. studied the mild autohydrolysis of eucalyptus wood at 175 °C for 60 min (log
Ro = 3.42), an increase of LSR from 1 to 11 resulted in a decrease of xylose and xylan concentrations but an increase of xylose and acetic acid weight percentages (on biomass) in the liquor.70 Garrote et al. studied the mild autohydrolysis of eucalyptus wood at 175 °C for 60 min (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ro = 3.99) and found that an increase of LSR from 6 to 10 resulted in a decrease of xylose and xylan concentration, as well as a decrease of acetic acid, furfural and 5-HMF concentration in the liquors.69 Fujimoto et al. studied the effect of solid concentration of the same wood under two pretreatment conditions.86 At log
Ro = 3.99) and found that an increase of LSR from 6 to 10 resulted in a decrease of xylose and xylan concentration, as well as a decrease of acetic acid, furfural and 5-HMF concentration in the liquors.69 Fujimoto et al. studied the effect of solid concentration of the same wood under two pretreatment conditions.86 At log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ro = 4.22, an increase of solid concentration (decrease of LSR) resulted in a decrease of xylose yield but an increase of acetic acid, furfural and 5-HMF, while at log
Ro = 4.22, an increase of solid concentration (decrease of LSR) resulted in a decrease of xylose yield but an increase of acetic acid, furfural and 5-HMF, while at log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ro = 4.52, an increase of solid concentration resulted in a decrease of xylose and furfural yields but almost in similar acetic acid and 5-HMF.86 A correlation of biomass solubilization and hemicellulose/xylan removal with log
Ro = 4.52, an increase of solid concentration resulted in a decrease of xylose and furfural yields but almost in similar acetic acid and 5-HMF.86 A correlation of biomass solubilization and hemicellulose/xylan removal with log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ro and LSR, based on the above previously reported works, can be observed in Fig. 6.
Ro and LSR, based on the above previously reported works, can be observed in Fig. 6.
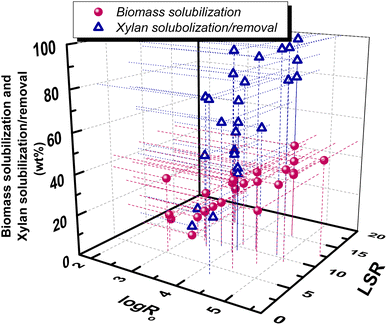 | ||
Fig. 6 Correlation of xylan solubilization/removal and biomass solubilization with hydrothermal pretreatment conditions, expressed by log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ro and LSR. Data are from ref. 46, 47, 59, 68, 73–75, 77–85. Ro and LSR. Data are from ref. 46, 47, 59, 68, 73–75, 77–85. | ||
Steam explosion (SE) is also one of the most studied pretreatment processes of lignocellulosic biomass feedstocks. The parameters affecting the SE process are the biomass particle size, residence time and temperature. Although hemicellulose removal is indeed enhanced at elevated temperatures, it can simultaneously lead to sugar degradation.87 Higher severities achieved at 270 °C for 1 min (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ro = 5.01) resulted in optimal hemicellulose solubilization towards degradation products, while lower temperature and longer residence time (190 °C, 10 min) avoided their formation.50 Similar to LHW, the SE process has been applied to a vast variety of lignocellulosic feedstocks. In the SE pretreatment of banana rachis, an increase of the severity factor from 2.97 to 3.78 resulted in hemicellulose and amorphous cellulose solubilization towards monosaccharides.88 Similar results were obtained for tall fescue grass, which was pretreated in a wide range of severities log
Ro = 5.01) resulted in optimal hemicellulose solubilization towards degradation products, while lower temperature and longer residence time (190 °C, 10 min) avoided their formation.50 Similar to LHW, the SE process has been applied to a vast variety of lignocellulosic feedstocks. In the SE pretreatment of banana rachis, an increase of the severity factor from 2.97 to 3.78 resulted in hemicellulose and amorphous cellulose solubilization towards monosaccharides.88 Similar results were obtained for tall fescue grass, which was pretreated in a wide range of severities log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ro = 1.4–4.2. A gradual increase of hemicellulose removal was observed and at the highest severity, all the hemicellulose has been extracted in the liquid fraction.89 SE of olive tree prunings led to 34–51% biomass solubilization at log
Ro = 1.4–4.2. A gradual increase of hemicellulose removal was observed and at the highest severity, all the hemicellulose has been extracted in the liquid fraction.89 SE of olive tree prunings led to 34–51% biomass solubilization at log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ro = 3.83–4.71, attributed to hemicellulose and extractives, while a higher hemicellulose sugar recovery of 35.6% was obtained at a severity of 4.12.90
Ro = 3.83–4.71, attributed to hemicellulose and extractives, while a higher hemicellulose sugar recovery of 35.6% was obtained at a severity of 4.12.90
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ro = 2.28 combined with 1% w/v H2SO4.94 Careful selection of pretreatment temperature, time and acid concentration can tune the composition of the liquid fraction. Indicatively, in sulfuric acid catalyzed HT pretreatment of poplar, an increase of temperature from 130 to 180 °C increased xylan degradation from 70 to 91%, initially towards xylose (T < 140 °C) and further towards furanics (T > 140 °C) and acids (T > 200 °C).95 Similarly, in acetic acid catalyzed HT pretreatment of the same wood, the higher acetic acid concentration (10% v/v) and the higher pretreatment time (50 min) resulted in 87.8% xylan removal in liquor, mainly in the form of xylose (46.4%), while at lower acid concentration (5% v/v) and shorter time (30 min), xylan removal was equal to 71.3% toward xylo-oligosaccharides (55.8%) with lower xylose yield (32.9%).81
Ro = 2.28 combined with 1% w/v H2SO4.94 Careful selection of pretreatment temperature, time and acid concentration can tune the composition of the liquid fraction. Indicatively, in sulfuric acid catalyzed HT pretreatment of poplar, an increase of temperature from 130 to 180 °C increased xylan degradation from 70 to 91%, initially towards xylose (T < 140 °C) and further towards furanics (T > 140 °C) and acids (T > 200 °C).95 Similarly, in acetic acid catalyzed HT pretreatment of the same wood, the higher acetic acid concentration (10% v/v) and the higher pretreatment time (50 min) resulted in 87.8% xylan removal in liquor, mainly in the form of xylose (46.4%), while at lower acid concentration (5% v/v) and shorter time (30 min), xylan removal was equal to 71.3% toward xylo-oligosaccharides (55.8%) with lower xylose yield (32.9%).81
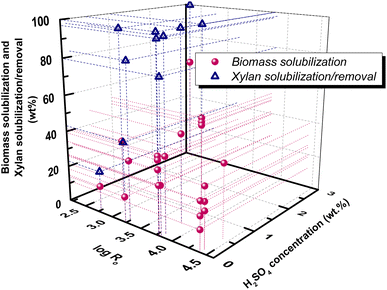 | ||
Fig. 7 Correlation of biomass solubilization with log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ro in dilute acid (H2SO4, wt%) HT pretreatment. Data are collected from ref. 68, 75, 83, 93–95. Ro in dilute acid (H2SO4, wt%) HT pretreatment. Data are collected from ref. 68, 75, 83, 93–95. | ||
Redox environment was also pointed out as an important parameter in auto-hydrolysis and dilute-acid pretreatment of biomass. Addition of O2 improved xylan removal in the liquid whose composition proved to be affected by the severity of the conditions. Under the most severe conditions in the presence of O2, xylan was removed towards the formation of furanics (furfural, 2-furoic acid, and 5-HMF) and organic acids (acetic acid, formic acid and levulinic acid), resulting also in lower glucan recovery in the solid fraction.75 Alternatively to the classic heating, microwave heating has been applied in the acetic acid catalyzed corn stover leading to higher heating rate and high glucose yield in the solid fraction.96
In SE pretreatment the addition of acid catalysts or the impregnation of wood chips with acids prior to pretreatment is more often, allowing for significantly decreased residence temperature and time, and inducing improved hydrolysis rate, decreased degradation products and increased removal of hemicellulose and lignin.87 Addition of 1 wt% H2SO4 in the pretreatment of almond shells, olive tree prunings and grapevine prunings resulted in similar biomass solubilization (53.5%, 50.4% and 49.9%, respectively) but with different hemicellulose recovery percentages (14.19%, 27.68% and 43.31%, respectively) in the liquid fraction.68 Immersion of wheat straw in 0.2% H2SO4 prior to SE resulted in 36–48% biomass solubilization at log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ro = 2.95–4.24 with increased recovery of hemicellulose in the liquid fraction, while glucose remains in the solid.97 Apart from sulfuric acid, other inorganic acids have been also utilized in the catalyzed SE pretreatment and H3PO4 resulted in slightly higher sugarcane bagasse solubilization compared to H2SO4.66
Ro = 2.95–4.24 with increased recovery of hemicellulose in the liquid fraction, while glucose remains in the solid.97 Apart from sulfuric acid, other inorganic acids have been also utilized in the catalyzed SE pretreatment and H3PO4 resulted in slightly higher sugarcane bagasse solubilization compared to H2SO4.66
The one-step (in situ) aimed production of furanics from raw biomass feedstocks during HT pretreatment in neat water is rarely referred in the literature, despite its evident usefulness. The HT pretreatment of lignocellulosic feedstocks results in relatively low yields of furfural and 5-HMF, in the range of 0.05–2 wt% on initial biomass, which are usually not considered as the primary target but as degradation by-products.64,73,107
As can be observed in Fig. 8, the concentration of furfural in the pretreatment liquor may reach up to 5 mg ml−1 in a wide range of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ro = 2.5–4.7, thus indicating that other parameters in addition to temperature and time, such as biomass type, may have an important effect. The concentration of 5-HMF is even lower, not exceeding 1 mg ml−1 in most cases. The addition of homogeneous catalysts in the hydrothermal pretreatment process can improve the in situ production of furanics. Dilute inorganic acids under severe conditions of LHW pretreatment resulted in higher furanic production.94,95,108
Ro = 2.5–4.7, thus indicating that other parameters in addition to temperature and time, such as biomass type, may have an important effect. The concentration of 5-HMF is even lower, not exceeding 1 mg ml−1 in most cases. The addition of homogeneous catalysts in the hydrothermal pretreatment process can improve the in situ production of furanics. Dilute inorganic acids under severe conditions of LHW pretreatment resulted in higher furanic production.94,95,108
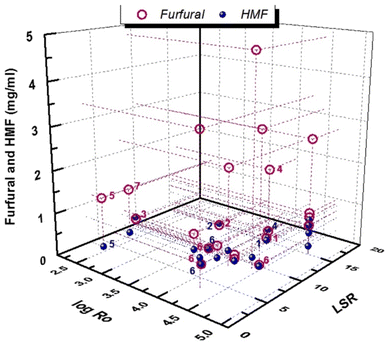 | ||
Fig. 8 Furanic production via LHW pretreatment. Numbers correspond to acid concentrations: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5% v/v H2SO4, 2 0.5% v/v H2SO4, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1%v/v H2SO4, 3 1%v/v H2SO4, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.6 w/v H2SO4, 4 1.6 w/v H2SO4, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 g kg−1 CH3COOH, 5 400 g kg−1 CH3COOH, 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 wt% H2SO4, 6 3 wt% H2SO4, 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 wt% H2SO4, and 7 0.5 wt% H2SO4, and 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3% w/v H2SO4. Purple numbers correspond to furfural and blue numbers to 5-HMF. Data are from ref. 47, 59, 75, 76, 78–80, 85, 92, 94, 95, 108, 114 and 115. 3% w/v H2SO4. Purple numbers correspond to furfural and blue numbers to 5-HMF. Data are from ref. 47, 59, 75, 76, 78–80, 85, 92, 94, 95, 108, 114 and 115. | ||
However, intense hydrothermal conditions in the presence or absence of dilute acids, at ca. log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ro > 4, usually lead to enhanced “loss” of biomass carbon as humins or even gaseous products.47,48,109
Ro > 4, usually lead to enhanced “loss” of biomass carbon as humins or even gaseous products.47,48,109
Aiming to avoid mineral acids, metal chlorides can be utilized as catalysts in the HT pretreatment, which under optimal reaction conditions can convert almost 98% of corn stover hemicellulose towards furfural production (25% wt% of liquid product) without significantly affecting cellulose and lignin.110 In broader research, poppy stalk carbohydrates were converted to furfural and 5-HMF in neat water over a wide range of metal chlorides and among them, CuCl2 was the most active, resulting in 79.9% furfural and 12.2% 5-HMF production yields (based on xylose and glucose of raw biomass) under optimized conditions.111 In general, hydrothermal pretreatment under intense conditions, low LSR and addition of inorganic acids may enhance the production of furfural and 5-HMF, but care of biomass degradation to humins should be taken. To this end, direct furfural and 5-HMF production from lignocellulosic biomass can be further enhanced using co-solvents, which can also minimize the humin formation. The most promising co-solvents are γ-valerolactone (GVL), tetrahydrofuran (THF) and dioxane, which can partially replace water in the hydrothermal pretreatment and enhance the furanic production in the presence or absence of catalysts.25,29,112,113
Another important parameter considering the viable production of furanic compounds from lignocellulosic biomass is their separation and recovery from aqueous pretreatment solutions. Generally, adsorption, distillation, precipitation or solid/liquid phase extraction is applied to selectively recover 5-HMF and furfural. High costs derived by large amounts of solvent consumption and scaling issues led to most suitable processes. 5-HMF recovery from aqueous solutions via extraction has been studied with many solvents, but methyl isobutyl ketone (MIBK) and 2-methyltetrahydrofuran (2-MTHF) gained great interest due to the high recovery yields of 5-HMF.116,117 Similarly, furfural can also be recovered from aqueous solutions either via classic extraction separation or CO2 assisted phase separation.118–120 Considering a low cost and environmentally friendly solvent, 2-pentanone is an alternative for furfural separation, providing low separation load in case of downstream conversion of furfural to fuels, because 2-pentanone can also be converted to hydrocarbon fuels.121 Another interesting approach is the recovery of both furfural, 5-HMF and acetic acid from aqueous solutions with polyethylenimine, a soluble polyelectrolyte, which was successfully applied in biomass pretreatment slurries to remove the fermentation inhibitors.122 Similarly, furanics and acetic acid can be removed from the hydrolysate via sorption on activated charcoal and anion exchange resins.108
4.2. Neoteric solvents as reaction media for pretreatment and conversion of lignocellulosic matrices
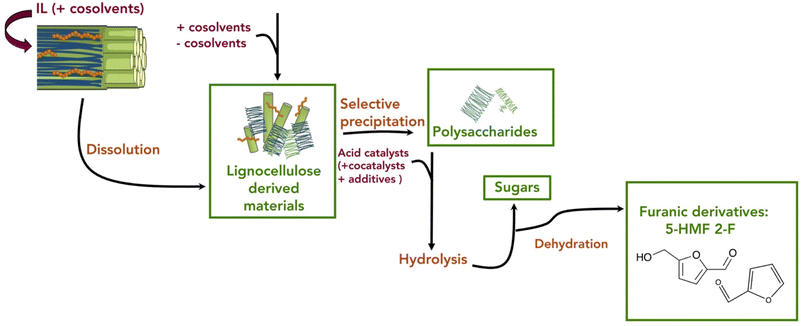
Neoteric solvents have gained interest in the last decade and have been deeply investigated as alternative media for the pretreatment and the conversion of the lignocellulosic feedstock into furanic derivatives. In particular, the use of ionic liquids (ILs), deep eutectic solvents (DES) and supercritical fluids is discussed, highlighting the advantages, strengths, and opportunities of their use in this field. These neoteric solvents present unique properties in comparison with traditional solvents, allowing not only the development of more efficient processes in terms of diversity of materials and catalytic efficiency but also the reduction of the environmental impact for the key treatments and synthetic transformation required to transform lignocellulose materials into high-added value products.The pretreatment of the biomass is a key step for its further conversion into chemical products. In this regard, ILs are among the most efficient solvents for the dissolution of lignocellulosic biomass (i.e., wood chips, straw, cornhusks, etc.).134 Hence, ILs offer a simple alternative to conventional pretreatment methods. Imidazolium based ILs represent the most common type of ILs but pyridinium, phosphonium, morpholinium and ammonium derived ILs have also been evaluated for biomass pretreatment.135,136 While they are generally considered to be ecofriendly compounds due to their non-volatility and non-flammability, the green calcification of the ILs should be carefully considered as some of them present potential toxicity and biodegradability issues.137
Nevertheless, ILs are efficient media for the fractionation of biomass into its components, some of them being a suitable feedstock (i.e., polysaccharides and sugars) for the production of 5-HMF. The typical initial step is the heating of the biomass in the presence of the ILs. The generated non-covalent interactions with the structural elements of the involved biomass help to disrupt the non-covalent interactions between lignocellulose components, leading to its dissolution without significant degradation.138 Different solvents (i.e., acetone–water mixtures, alcohol, or dilute acid) are added to the mixture or removed from it (i.e., by evaporation) in order to induce the precipitation of the different components of the lignocellulosic biomass.139 This type of process requires, for every step, a well-designed separation and purification of the product from the IL-phase, as well as an energy-efficient recycling of the IL, if a potential industrial application of ILs for biomass pretreatment is envisaged (Fig. 9) as well as in the perspective of a green chemistry approach.
The initial reports of biomass dissolution dealt with cellulose. Although the first report on the dissolution of cellulose in a mixture of 1-ethylpyridinium chloride and a nitrogen-containing base dates back to 1934,140 it was not until the seminal work of Rogers et al. that the potential of room temperature ILs for cellulose dissolution was fully revealed.141 The IL-dissolved cellulose could be regenerated without severe degradation by addition of water or other precipitating solvents (e.g., ethanol or acetone). In general, regarding the structure of the cation, decreasing the length of the alkyl chains (decreasing their hydrophobic character) or introducing allyl chains improved the dissolution power of the ILs.142 On the other hand, anions like Cl−, HCOO− and OAc− provided good dissolution capacity. These anions are relatively small and good hydrogen bond acceptors, favouring the formation of hydrogen bonds with the acidic hydrogen atoms of the hydroxyl groups of the polysaccharides. In imidazolium cations, the proton on the C2 position of the ring is relatively acidic and can also form hydrogen bonds with the oxygen atoms in cellulose (Fig. 10).143,144 Possible additional interactions (i.e., van der Waals interactions and hydrophobic effects) have been also claimed to occur between the cation and the cellulose.145,146 The use of an additional organic solvent with the ILs can induce co-solvency and anti-solvency effects.35
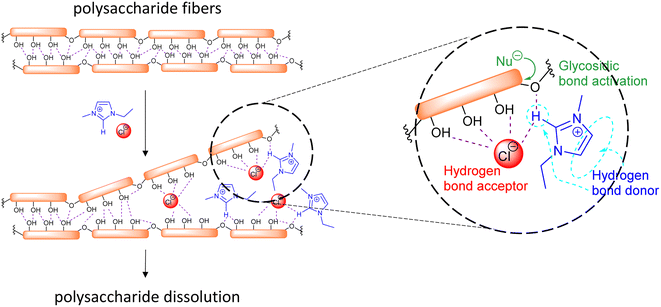 | ||
| Fig. 10 Schematic representation of the main interactions involved in the dissolution and activation for hydrolysis of polysaccharide fiber in ILs. | ||
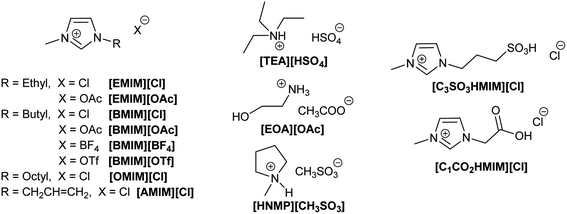
Later studies have demonstrated that ILs can be used to process different types of lignocellulosic biomass, such as agricultural residues, dedicated energy crops and forest biomass.147,148 As for cellulose, ILs' structure and properties can be fine-tuned to improve the capacity for dissolving lignocellulosic biomass from different sources (Fig. 11).32,149,150 The most commonly used ILs for lignocellulosic pretreatment are [EMIM][OAc] (1-ethyl-3-methylimidazolium acetate), [AMIM][Cl] (1-allyl-3-methylimidazolium chloride) and [BMIM][Cl] (1-butyl-3-methylimidazolium chloride).10 The appropriate design of the IL can lead to a reduction of the required dissolution temperature, price, ecotoxicological profile and viscosity, which are key factors to enable their industrial implementation. A simple to prepare, biocompatible and low cost (∼$1 per kg) protic IL such as ethanolamine acetate has been reported for the direct production of biofuels via one-pot synthesis.151 Protic ionic liquids are a subset of ILs produced through the combination of a Brønsted acid and a Brønsted base.152 In this example, after pretreatment of switch grass, the whole slurry is directly used for simultaneous saccharification and fermentation with commercial enzyme cocktails and wild type yeast strains, generating 70% of the theoretical ethanol yield. On the other hand, the so called ionoSolv process based on the use of triethylammonium hydrogen sulfate ([TEA][HSO4]) as a protic ionic liquid has been implemented for the fractionation of even highly recalcitrant feedstocks and for separation of cellulose-rich pulp and lignin fractions.153,154 In addition, the easy recycling, high yields, and low pretreatment times facilitate potential commercialization of this process.155
Apart from the fractionation of the biomass, ILs can also be used as solvents for the depolymerisation of polysaccharides like cellulose, hemicellulose, inulin or starch into the corresponding sugars.32 As mentioned above, the basic anion from the IL disrupts the crystallinity of the polysaccharide that is transformed into an amorphous structure by interfering with the hydrogen bonding network, enhancing its dissolution but also facilitating its modification. On the other hand, the interaction between the cation and the glycosidic moieties of the polysaccharide makes the carbohydrates more susceptible to hydrolysis. Thus, the addition of an acid catalyst, either homogeneous (i.e., sulfuric, acetic, oxalic acids, etc.) or heterogeneous (Amberlyst, zeolite, MOF, etc.), to the polysaccharide dissolved in the IL facilitates the catalytic hydrolysis of the glycosidic bonds linking the sugar monomers in the polysaccharide, leading to its depolymerisation into the corresponding monosaccharide.156 Alternatively, different task-specific ILs displaying a built-in acid function in the cation (e.g., –SO3H and –COOH) or/and in the anion (e.g., HSO4−) have been designed to play a dual role as a solvent for the dissolution of the polysaccharide and as an acid catalyst for its hydrolysis into sugars.128,157
Lewis acidic cations such as those in metal chlorides are also suitable catalysts for the dehydration of fructose to 5-HMF.160 Zhang and co-workers demonstrated that metal chlorides (i.e., CrCl2) dissolved in 1-alkyl-3-methylimidazolium chloride can efficiently catalyse the synthesis of 5-HMF from fructose, while other less potentially harmful metal chlorides in [EMim]Cl showed poor activity or very low selectivity to 5-HMF.161 From the tested imidazolium ILs displaying different N-alkyl chain lengths (octyl, butyl and ethyl) the one with the shorter chain ([EMIM][Cl]) provided the higher yield (73% 5-HMF yield at 120 °C).
5-HMF can also be obtained from other sugars, although the efficiency of the reaction is significantly affected by the nature of the feedstock. When sucrose is used (a disaccharide of glucose and fructose) the sugar is nearly quantitatively transformed into 5-HMF and unreacted glucose.87 Indeed, to convert glucose to 5-HMF, longer reaction times and higher reaction temperatures are required. Two different reactions are needed to convert glucose into 5-HMF: (i) isomerization of glucose into fructose, and (ii) dehydration of fructose to 5-HMF. The coordination of the ILs with a metal salt enables the proton transfer at the isomerization required to convert glucose into fructose.162 The nature of the ILs defines again the reaction conditions (temperature and time) and the yield obtained. In general, ILs containing imidazolium cations combined with nucleophilic anions such as halides (e.g., Cl− or Br−) led to more efficient conversions of sugars into 5-HMF.32
Zhao and co-workers achieved a significant conversion of cellulose and glucose, with ca. 60% and 90% isolated yields, respectively, mediated by CrCl3/[BMIM][Cl] under microwave irradiation (MW).163 Microwave heating allowed for a remarkable reduction in the time required (from hours to minutes). Thus, the combined use of MW and ILs can lead to a fast and more cost-effective process for the conversion to 5-HMF in comparison with conventional heating.164,165 Because of the high dielectric constant and the ionic nature of the ILs, the reaction is heated up quickly, volumetrically and simultaneously leading to a more efficient heating.166 For instance, up to 97% yield of 5-HMF can be obtained from fructose in [BMIM][Cl], in the absence of catalyst, in only three minutes under microwave irradiation.167 The combination of [BMIM][Cl]/CrCl3 yielded ca. 70% of 5-HMF from glucose in only thirty seconds, which represents a yield significantly higher than the one obtained under conventional heating (ca. 48% 5-HMF yield). With this system, a 5-HMF yield of 54% was obtained for cellulose conversion at 150 °C during 10 min of reaction time, with the [BMIM][Cl]/CrCl3 being recyclable for six cycles of use.168
J. P. Hallett and co-workers have reported the efficient use of [BMIM][OTf] for the synthesis of 5-HMF from fructose. The water content plays an important role in stabilising the obtained 5-HMF and in suppressing side reactions. Thus, high yields (>80% 5-HMF yield) from high substrate loadings (14% w) can be obtained in a short reaction time (<10 min), even with the use of non-coordinative ILs and halide anion free ILs.169
The use of ILs as reaction media can also decrease the operational temperature required for the dehydration of sugars to 5-HMF with a reduction in the energy requirements and operational costs. In general, under conventional conditions temperatures as high as 100 to 300 °C are needed. However, when ILs are used, much lower temperatures, in the range of 80–140 °C or lower, can be usually applied, which constitutes a major advantage from the energetic point of view. Indeed, Smith, Qi and co-workers have reported an efficient catalytic system for the conversion of fructose into 5-hydroxymethylfurfural at room temperature.170 Reactions proceeded smoothly and efficiently with 78–82% 5-HMF yields at 25 °C after pre-dissolving the fructose into [BMIM][Cl] and subsequently adding a small amount of a co-solvent (i.e., acetone, DMSO, ethanol, etc.) and using Amberlyst-15 as the catalyst. The transformation of fructose to 5-HMF can also be achieved with a high yield (>90% HMF yield) at room temperature using multicatalytic systems such as [BMIM][Cl]/WCl6,171 or a mixture of [BMIM][Cl] and an acid IL like [HNMP][CH3SO3] (N-methyl-2-pyrrolidonium methyl sulfonate).172 This reduction of the temperature can allow for the continuous extraction of 5-HMF by using organic solvents with low boiling points. This can simplify the implementation of large-scale biphasic continuous systems enabling the 5-HMF separation and recovery by solvent distillation and the recycling of the catalytic IL-phase for subsequent catalytic cycles.
Despite being more challenging than the conversion of fructose, other complex carbohydrates such disaccharides (i.e., sucrose, maltose, lactose and cellobiose)173 and polysaccharides (i.e., inulin, cellulose, chitosan and starch)87 can also be transformed to 5-HMF using ILs. The nature of the ILs, the co-catalyst and the reaction conditions should be properly adjusted depending on the feedstock, as the increase in the degree of polymerization of the feedstock gradually decreases the efficiency of the catalytic system. ILs, being compatible with different catalytic units, also open the possibility to integrate different steps required for the synthesis of furanic derivatives (i.e., dissolution, hydrolysis, isomerization, dehydration, etc.) in a multistep cascade process, where the product from one step becomes the feedstock of the next one without any need of purification processes between them. This integration can save time and energy in pretreatment and isolation steps, increasing profitability and enabling its industrial implementation. For instance, the combination of two metal chlorides (CuCl2/CrCl2) dissolved in [EMIM][Cl] (1-ethyl-3-methylimidazolium chloride) catalyzes the single-step conversion of cellulose to 5-HMF with an unrefined 96% purity among recoverable products (at 55% 5-HMF yield at 80–120 °C).174 The catalytic performance of the CuCl2/CrCl2/[EMIM][Cl] catalytic system can be preserved after the isolation of 5-HMF, keeping the activity for at least three consecutives runs. Some other paired metal catalytic systems have been studied, such as CrCl2/CuCl2, CrCl2/CrCl3, CrCl3/FeCl3, CrCl3/FeCl2, CrCl3/AlCl3, CrCl3/SnCl4, CrCl3/MnCl4, and CrCl3/CuCl2, in this context.175,176
In the search of low-temperature, one-pot processes, Raines and co-workers used ortho-carboxyl-substituted phenylboronic acids as organocatalysts, together with MgCl2·6H2O/H2SO4, to convert cellulose and cellulose-rich municipal waste (i.e., cotton, paper towels, and newspaper) to 5-HMF in [EMIM][Cl] at 105 °C. 5-HMF yields as high as 41% could be achieved with a catalytic system that is devoid of heavy metals.177
A variety of related catalytic systems have been exploited for lignocellulosic biomass conversion to 5-HMF. In these systems, the ILs assist the dissolution and fractionation of the biomass, and as a source of active sites for subsequent transformation of sugars to 5-HMF.17,32,53,72,75 For instance, corn stalk, rice straw and pine wood treated with CrCl3·6H2O/[BMIM][Cl] produced 5-HMF and furfural in 45–52% and 23–31% yields, respectively, after 3 min.178
In summary, IL solvent systems have shown a huge potential for the preparation of 5-HMF from biomass. They can be used for the pretreatment and conditioning of different lignocellulosic materials and for the saccharification of the polysaccharides into the sugars needed for the synthesis of furanic compounds. They also allow the integration of the pretreatment and transformation of different types of natural feedstocks into 5-HMF in a single process. However, there are still some challenges for the acceptance of this technology as a feasible alternative to conventional processes. Among them, we can highlight the reduction of the IL price, the decrease in viscosity, which is an important technical parameter, the improvement in cellulose dissolving capacity, the selection of ILs not providing additional environmental concerns, and the reduction of the conditions required to carry out the corresponding processes at ambient temperature. There is also a need to develop simple strategies for an efficient separation/recovery of all the elements (i.e., products, catalysts, ILs, and co-solvents) used in the process. A simple and efficient separation and reuse of the IL-phase can mitigate the concerns on their cost.179 Although different approaches have been proposed for the isolation of 5-HMF and the recovery of ILs, such as the use of supercritical CO2 or high-vacuum entrained distillation, or the use of membranes, these approaches are still far away from being suitable for large scale 5-HMF production.
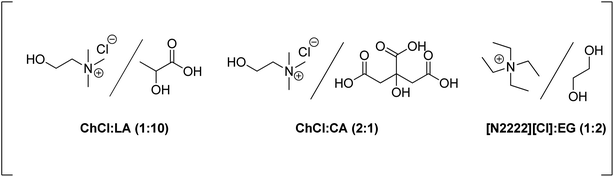
Su and co-workers have developed the dehydration of glucose to 5-HMF under continuous flow conditions using CrCl3−[N2222][Cl]/EG as the catalytic system. A 42% 5-HMF yield was obtained at 150 °C using 10% glucose in only 3.64 min residence time, while under batch conditions only a 10% yield was reached in a much longer period (30 min).187 After the reaction, the products were extracted with ethyl acetate as the solubility of the DES and CrCl3 in ethyl acetate was negligible. The treated catalytic system could be efficiently recycled for four runs.
Catalytic valorization of native biomass has been systematically evaluated in the choline chloride/oxalic acid DES system.188 This acidic DES promotes transformations of structurally branched glucans, fructans, or xylans into glucose (yields up to 68%), fructose (yields up to 60%), xylose (yields up to 73%), 5-HMF (yields up to 14%), or furfural (yields up to 72%). A choline chloride–formic acid–SnCl4·5H2O (ChCl–Fa–SnCl4) catalytic system has been reported for the transformation of herbal residues into furanic derivatives.189 Up to 67% total furfural and xylose yield from herbal residues of Heteropogon contortus (L.) Beauv was reached at 140 °C in 10 min. The same catalytic mixture led to a total yield of 93.8% of 5-HMF and glucose from herbal residues of Anemarrhena asphodeloides Bunge at 120 °C.
A better understanding of the mechanistic aspects for both pretreatment and conversion of the biomass in DES media is required to be able to design more efficient systems. There is also a need to increase the thermal stability of the DES as well as developing efficient ways to isolate/recover the products and for the recycling of the DES-based systems.
Supercritical CO2 (scCO2) presents a low critical pressure and temperature (7.4 MPa and 31.0 °C) in comparison with other supercritical fluids (i.e., 22.1 MPa and 374.2 °C for scH2O). Furthermore, CO2 can be considered as a low cost, nontoxic, non-flammable, readily available solvent and can be easily removed from the final product by simple de-pressurisation.191
scCO2 has proved as a suitable tool to perform the treatment of lignocellulosic biomass prior to biological processing.192,193 Thus, scCO2 has been used for cellulose pretreatment, reducing its crystallinity by ca. 50% and enabling its enzymatic hydrolysis to glucose.194 An enhancement of up to 18–51% in the yield of reducing sugars can be achieved from dilute acid hydrolysis of scCO2-pretreated corncob, cornstalk, and rice straw.195 Other systems included H2SO4-catalyzed cascade hydrolysis–dehydration of rice husk enabled by CO2 extraction, which directly gave furfural in a high yield of around 90%.196
High-pressure CO2 in contact with water produces, in a reversible way, carbonic acid.197–199 This has opened the way to the direct use of this carbonic acid as an environmental friendly catalytic system for the dehydration of carbohydrates to 5-HMF.200 Yields of 5-HMF from fructose, glucose, sucrose, and inulin of ca. 67%, 36%, 50%, and 47%, respectively, at 190 °C and 2 MPa of CO2 in a mixture of 2-propanol/water have been reported.201,202 This evidences the capacity of CO2 to provide an acid catalyst for an environmentally friendly synthesis of 5-HMF from carbohydrates, achieving efficiencies comparable to those obtained with a typical homogeneous or heterogeneous acid catalyst.
Supercritical water (scH2O) is another scF used for processing and transforming lignocellulosic materials.203 Subcritical and supercritical water exhibit unique characteristics, including increased diffusivity and decreased hydrogen bonding and dielectric constant. By varying the temperature and pressure of the water, its dielectric constant (ε) decreases, from ε ∼ 78 under room conditions to ∼6 at the vapor–liquid critical point, increasing dramatically, at this point, the solubility of organic compounds.204 On the other hand, the ion product of supercritical water is two orders of magnitude higher than that at ambient temperature. High concentrations of H+ and OH− can be obtained in SCW, and it is possible to provide a perfect environment for acid- or base-catalysed reactions.205,206 These properties can be exploited to develop hydrothermal pretreatment processes, under sub-critical and supercritical conditions, in the absence or presence of an additional acid catalyst, for the selective production of reducing sugars. A novel technology for the continuous and selective hydrolysis of cellulose and biomass to sugars has been reported based on the use of scH2O in a fast heating and fast cooling reactor. This reactor set-up together with the unique properties of the scH2O allows for the dissolution and hydrolysis of cellulose in a very short reaction time.207,208 At 400 °C and 25 MPa, a total recovery of C-5 sugars was achieved in 0.19 s, while the highest yield of C-6 sugars (65% w/w) was achieved at 0.22 s reaction time. The main hydrolysis product of C-6 and C-5 sugars was glycolaldehyde (20% w/w), while 5-HMF production was highly inhibited (yields lower than 0.5% w/w). The process for the hydrolysis of biomass in scH2O has been scaled-up from laboratory to pilot plant scale. Sugar beet pulp and wheat bran were used to validate the scaling up. The larger particle size (250 μm vs. ≤150 μm) used for the pilot plant scale slows down the hydrolysis. This reduction increased the selectivity and reduced sugar degradation (degradation yield < 15%). For sugar beet pulp, the yields for sugars were 55% and 66%, respectively for pilot plant and lab experiments, respectively. However, the selectivity reached values around 90% for both sugar beet pulp and wheat bran in the pilot plant.209
Low yields of 5-HMF have been reported for the treatment of fructose in supercritical water in the presence of 10 mM H2SO4 (35% selectivity, 80% conversion, 25 MPa, 180 °C, 600 s residence time). The substitution of scH2O by supercritical acetone in the presence of water (acetone![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water 90
water 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) resulted in higher yields of 5-HMF (77% selectivity, 98% conversion, 20 MPa, 180 °C, 120 s residence time).210 Alternatively, when the reaction was carried out in scMeOH, the corresponding furfural ether was obtained (methoxymethylfurfural, MMF, 79% selectivity, 99% conversion, 20 MPa, 240 °C, 2 s residence time), while the reaction in subcritical acetic acid (scAcOH) afforded the corresponding furfural-ester although in lower yields (5-acetoxymethylfurfural, AMF, 38% selectivity, 98% conversion, 20 MPa, 180 °C, 120 s residence time).211
10) resulted in higher yields of 5-HMF (77% selectivity, 98% conversion, 20 MPa, 180 °C, 120 s residence time).210 Alternatively, when the reaction was carried out in scMeOH, the corresponding furfural ether was obtained (methoxymethylfurfural, MMF, 79% selectivity, 99% conversion, 20 MPa, 240 °C, 2 s residence time), while the reaction in subcritical acetic acid (scAcOH) afforded the corresponding furfural-ester although in lower yields (5-acetoxymethylfurfural, AMF, 38% selectivity, 98% conversion, 20 MPa, 180 °C, 120 s residence time).211
4.3. Critical assessment of potential of pretreatment technologies for carbohydrate extraction and furanic compound formation
A comparison between the different pretreatment approaches based on the carbohydrate recovery and furan yield is summarized in Table 2. The hydrothermal pretreatment is cheap and effective for hemicellulose extraction by selective hydrolysis of ether bonds, resulting in a high sugar recovery. LHW and steam explosion do not require any chemical recovery after the process, but low product concentrations are recuperated without catalysts. Regulating different parameters (temperature, time, LSR, and dilute acid catalyst), the formation of 2-furfural can be promoted. This treatment can be applied in a wide range of biomass types, such as hardwood and softwood forestry and agricultural residues as well as food industries and food wastes.| Pretreatment | Main effects on biomass structure | Carbohydrate removal/recovery | Key furanic compounds yields | |
|---|---|---|---|---|
| Hydrothermal | LHW | -Complete hemicellulose removal | -Hemicellulose removal up to (100 wt%)47,59,83,84 | -Low yield of 5-HMF and furfural in catalyst-free conditions (0.05–2 wt%)64,72 |
| -Structural changes on cellulose | ||||
| Steam | -Fiber separation | -Xylan recovery (50–82 wt%)47,59 | ||
| -Auto-hydrolysis and partial. Solubilization of the hemicelluloses | -With catalysts conditions, CuCl2, up to 79.9% furfural and 12.2% 5-HMF production yields111 | |||
| -Reduction of cellulose crystallinity | ||||
| Dilute acid | -High recovery of sugars from hemicellulose | -Hemicellulose recovery up to (98%)94 | Furfural yield up to (11.09%)212 | |
| -Low recovery of sugar from cellulose | -Xylan removal up to (87.8%)81 | |||
| -Enhance cellulose accessibility to enzymes | ||||
| -Conversion of soluble hemicelluloses into fermentable sugars | ||||
| Neoteric | Ionic liquids | -Dissolution of cellulose and hemicellulose | -Carbohydrate's removal (60–90%)163 | -Catalyst-free conditions: 27% 5-HMF yield159 |
| -With catalyst conditions: 54–80% 5-HMF yields159,161,168,169 | ||||
| DES | -Liquid phase is rich in hemicellulose sugar | -Glucose yields (32–94%)185,213 | -5-HMF yields (42–78%)187,214 | |
| -Cellulose is in pretreated solid phase | -Xylose yield up to (73%)188 | -Furfural yield up to (70%)188,189 | ||
| Supercritical fluids | -Promote hemicellulose decomposition and decrease cellulose crystallinity | -Glucose yields (14–51%)192 | -5-HMF yields (18–67%)201,202 |
Supercritical fluid pretreatment is attractive because it is non-toxic and nonflammable to extract carbohydrates and transform them into furanic compounds. This method is effective for high cellulose and hemicellulose content biomass such as wheat straw, sugar beet pulp, oat hulls, apple pomace, and garden waste. However, similarly to hydrothermal treatment, it requires high temperature and pressure.
In ionic liquid media, depending on the based solvent, complex polysaccharides can be solubilized by breaking β-O 4-bonds, thus enabling their application to a wide range of lignocellulosic biomass feedstocks, by fine tuning the treatment parameters and catalysis to achieve optimum lignocellulosic fractionation and conversion. One major advantage of ILs is the fact that they do not require high pressure, can be recyclable, and when adequately selected they can have low toxicity. However, this solvent has severe drawbacks that may hamper upscaling, the fact that they are not easy to prepare and some of them are considered toxic.
With a similar mechanism, deep eutectic solvents have the same advantages over ILs since they tend to be cheaper and easier to formulate. Furthermore, DESs can degrade the physical structure of the biomass with minimal energy consumption during pretreatment, comparable with the chemical and physical methods in terms of energy consumption and conversion efficiency. This treatment type can be applied on biomass enriched in cellulose, such as spruce, beechwood, hybrid poplar, birchwood, black locust, fescue, miscanthus, hemp, and sawdust. The drawback of their high viscosity requires a high consideration on a pilot scale together with cost effective strategies for the recovery and reuse of the DESs in multiple cycles without compromising their performance.
5. Circular biorefinery as a transitional object of the bioeconomy
Integrating lignocellulosic biomass as an alternative non-fossil resource is an incentive for developing a bio-based economy. Biorefineries are based on renewable feedstocks that can be assembled from several supply categories, available at low cost in a short time throughout the year.From an environmental point of view, the oil refinery is a main contributor to carbon dioxide and air pollution. Conversely, biomass-based biorefineries utilize a wide range of feedstocks: (i) cultivated biomass, whose growth contributes to carbon capture from the atmosphere and storage for varying periods of time,215,216 and (ii) exploited biomass which was earlier left untouched or burned for energy needs, causing a significant amount of greenhouse emission, and therefore its exploitation contributes to waste management and reduction in polluting gas emissions.217
Unlike petroleum-based refineries, where the process is costly due to the needed oxidation step to synthesize hydrocarbons, in biorefineries, renewable feedstocks are naturally rich in oxygen and converted into functional intermediates with lower energy.217
Shifting toward green and sustainable biorefineries is a prospect for the future circular bioeconomy. It can start with integrating biomass-based refineries with current petrochemical refineries by exploiting the current infrastructure, thus minimizing the startup cost of the biorefinery. In addition to that, several processes, notably chemically catalyzed reactions already used in petroleum refineries, can be adapted for biorefineries.
Technoeconomic assessment is a needed tool to design novel circular biorefinery schemes. This analysis starts with an economic analysis of process scalability from the laboratory to pilot and industrial scales. Based on the laboratory study, the equipment cost can be estimated with the scaling exponent for each equipment (eqn (4))218 and adjusted to the base year using the Chemical Engineering Plant Cost Index (CEPCI) expressed by (eqn (5)).219
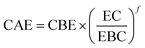 | (4) |
 | (5) |
The pretreatment method cost is directly associated with capital expenditure (CAPEX) and operational expenditures (OPEX). CAPEX consists of start-up and fixed costs (FC), which englobes equipment. Regarding hydrothermal and supercritical fluid treatment, the reactor vessel is the most expensive in capital cost.220 For example, in LHW pretreatment (14.33 × 106 L, at 180 °C, 10 min, and 20% solid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water ratio), a fixed capital investment of (US$ 102 × 106) represents 28.17%.219,221 In subcritical conditions, the pretreatment of lignocellulosic biomass for industrial processes of the order of 310 tons of feedstock daily represents US$ 27.4 × 106 of the FC, and 35% (US$ 9.6 × 106) is associated with the reactor.219,222 However, in the case of the ILs and DESs, the capital cost of the reactor is expected to be lower than that of supercritical fluids and hydrothermal treatment, which require special instrumentation for high temperatures and excessive pressure.155
water ratio), a fixed capital investment of (US$ 102 × 106) represents 28.17%.219,221 In subcritical conditions, the pretreatment of lignocellulosic biomass for industrial processes of the order of 310 tons of feedstock daily represents US$ 27.4 × 106 of the FC, and 35% (US$ 9.6 × 106) is associated with the reactor.219,222 However, in the case of the ILs and DESs, the capital cost of the reactor is expected to be lower than that of supercritical fluids and hydrothermal treatment, which require special instrumentation for high temperatures and excessive pressure.155
OPEX are subcategorized into direct production costs, including raw material, waste steam charges, and fixed operating costs, including labor and general expenses.223 In the case of supercritical fluid and hydrothermal pretreatment, the energy demand dominates as the major operating cost and represents around 70% of OPEX.224 For example, the needed heat required for lignocellulosic biomass treatment under these conditions varied between 610 kJ kg−1225 and 1096 × 106 kcal day−1.222 However, the hydrothermal process is carried out without using chemicals or catalysts.219 And among supercritical fluids, scCO2 presents the lowest cost compared to other supercritical fluids.139
Unlike HT and ScFs, OPEX in IL and DES-based biorefineries concerns mainly chemicals. ILs suffer from high costs ranging from $20 to 101 kg−1. A bulk-scale of 1-methylimidazolium hydrogen sulfate was studied in order to decrease the cost index ratio to 1.47 and an exchange rate of 1.1 $ €−1.155 However, DESs are cheaper than ILs and easily recyclable, where operating costs dropped significantly from $1573.56 million to $1060.03 million when DES recycling time increased from 5 to 20.226
Optimizing the total cost in biorefineries is recommended depending on the base treatment type. For example, in hydrothermal conditions using higher solid loading can improve energy efficiency,221 and implementation of renewable energy, like wind farms, can contribute to achieving lower prices of electricity.227 Concerning DES and IL-based pretreatment, some improvements can be made by reducing costs and loading, and increasing recycling.226,228
6. Conclusions and outlook
It is an unquestionable fact that fossil resources available for the world population are facing environmental, economic, and social challenges. As an alternative, biomass constitutes an inexhaustive source for platform molecules such as 5-hydroxymethylfurfural and 2-furfural. Both of them can be derived from lignocellulosic carbohydrates to form new chemicals and bio-based polymers. Therefore, a screening of chemical identification and quantification of major molecules of four categories of LCB, including forest biomass and grass, agricultural and agri-food residue, energy crops, and residues from non-food sectors, showed that these renewable resources revealed a considerable potential within the European context to be exploited for furanic compound formation aiming at their use in the bioplastic industry.In a green chemistry context, an updated overview of ecofriendly pretreatment technologies was comparatively reported to efficiently convert them into furans. Regarding the hydrothermal pretreatment in neat water, hemicellulose is completely solubilized and recovered in the liquid fraction. Solubility of biomass, hemicellulose removal and liquid fraction composition are strongly related to the severity and the process parameters, mainly temperature and time. Furfural and 5-HMF yields are increased under more severe conditions, but attention should be paid to avoid carbon loss due to humin formation. Alternatively, the formed humins can be upgraded by recovering them followed by added-value material production. Aiming to increase the isolation of hemicellulose as xylose monomers in the liquid fraction, dilute acid pretreatment at relatively lower temperature is preferred, coupled with controlled downstream dehydration of xylose to furfural. Contrarily, neoteric solvents solubilize carbohydrates and result in high furanic compound yield.
Lignocellulosic biomass is an attractive renewable source to be exploited for bio-based molecule production for new polymers and additives. However, there are challenges for the wide-scale commercialization of these value-added products to satisfy the community demands.
As a starting premise, it is recommended that future investigations for further industrial development take into account the following gaps:
• Tackle the whole value chain system including (i) feedstock cultivation, harvesting, collection, transport, and storage; (ii) processes concerning biomass pretreatment technologies and monosaccharide extraction; (iii) intermediate molecule production notably 5HMF and 2-F, (iv) and finally desired products such as chemicals, biomaterials, and biofuels.
• Ensure sustainable biomass supply regarding geolocation, storage, and transport logistics.
• Manage development practices of standardized process techniques to achieve the most adequate process conversion.
• Establish different combined approaches using efficient green processes to fulfil sustainable production.
• Provide an action plan to adapt commercialization and eventually application of produced furanic products.
Abbreviations
| 2-F | 2-Furfural |
| 2-MTHF | 2-Methyltetrahydrofuran |
| 5-HMF | 5-Hydroxymethylfurfural |
| AOS | Arabino-oligosaccharides |
| BFDCA | 2,2′-Bifuran-5,5′-dicarboxylic acid |
| BHF | 2,5-Bis(hydroxymethyl)furan |
| ChCl | Choline chloride |
| CMF | 5-(Chloromethyl)furfural |
| DES | Deep eutectic solvents |
| DFF | Diformylfuran |
| EMF | 5-Ethoxymethylfurfural |
| FA | Furfuryl alcohol |
| FDCA | Furan-2,5-dicarboxylic acid |
| GVL | γ-Valerolactone |
| HBA | Hydrogen-bond acceptor |
| HBD | Hydrogen-bond donor |
| HT | Hydrothermal pretreatments |
| ILs | Ionic liquids |
| LCB | Lignocellulosic biomass |
| LHW | Liquid hot water |
| LSR | Liquid-to-solid ratio |
| MIBK | Methyl isobutyl ketone |
| PEF | Poly(ethylene 2,5-furanoate) |
| PET | Poly(ethylene terephthalate) |
| scCO2 | Supercritical CO2 |
| ScFs | Supercritical fluids |
| SE | Steam explosion |
| THF | Tetrahydrofuran |
| XOSH | Xylo-oligosaccharides |
| XOSL | Xylo-oligosaccharides |
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This publication is supported by COST Action FUR4Sustain—European network of FURan based chemicals and materials FOR a Sustainable development, CA18220, supported by COST (European Cooperation in Science and Technology). This work was developed within the scope of CICECO—Aveiro Institute of Materials (UIDB/50011/2020 & UIDP/50011/2020) & LA/P/0006/2020, financed by national funds through the FCT—Fundação para a Ciência e a Tecnologia/MEC (PIDDAC). This research is also sponsored by FEDER funds through the program COMPETE—Programa Operacional Factores de Competitivid-ade—and by national funds through the FCT under the project UID/EMS/00285/2020. The FCT is also acknowledged for the re-search contract under Scientific Employment Stimulus to A. F. S. (CEECIND/02322/2020). Our final words go to our dearly remembered college and friend Anna Aladjadjiyan.References
- P. Manzanares, The role of biorefinering research in the development of a mpder n bioeconomy, Acta Innov., 2020, 37, 47–56 Search PubMed.
- A. Gandini, T. M. Lacerda, A. J. F. Carvalho and E. Trovatti, Progress of Polymers from Renewable Resources: Furans, Vegetable Oils, and Polysaccharides, Chem. Rev., 2016, 116, 1637–1669 CrossRef CAS PubMed.
- R. J. Van Putten, et al., Hydroxymethylfurfural, a versatile platform chemical made from renewable resources, Chem. Rev., 2013, 113, 1499–1597 CrossRef CAS PubMed.
- T. Scapini, et al., Hydrothermal pretreatment of lignocellulosic biomass for hemicellulose recovery, Bioresour. Technol., 2021, 342, 126033 CrossRef CAS PubMed.
- H. A. Ruiz, et al., Engineering aspects of hydrothermal pretreatment: From batch to continuous operation, scale-up and pilot reactor under biorefinery concept, Bioresour. Technol., 2020, 299, 122685 CrossRef CAS PubMed.
- R. Wang, et al., Hydrothermal CO2-assisted Pretreatment of Wheat Straw for Hemicellulose Degradation Followed with Enzymatic Hydrolysis for Glucose Production, Waste Biomass Valorization, 2021, 12, 1483–1492 CrossRef CAS.
- M. A. Martín-Lara, et al., Liquid Hot Water Pretreatment and Enzymatic Hydrolysis as a Valorization Route of Italian Green Pepper Waste to Delivery Free Sugars, Foods, 2020, 9, 1640 CrossRef PubMed.
- S. Meenakshisundaram, A. Fayeulle, E. Leonard, C. Ceballos and A. Pauss, Fiber degradation and carbohydrate production by combined biological and chemical/physicochemical pretreatment methods of lignocellulosic biomass – A review, Bioresour. Technol., 2021, 331, 125053 CrossRef CAS PubMed.
- C. H. Kuo and C. K. Lee, Enhanced enzymatic hydrolysis of sugarcane bagasse by N-methylmorpholine-N-oxide pretreatment, Bioresour. Technol., 2009, 100, 866–871 CrossRef CAS PubMed.
- K. C. Badgujar and B. M. Bhanage, Factors governing dissolution process of lignocellulosic biomass in ionic liquid: Current status, overview and challenges, Bioresour. Technol., 2015, 178, 2–18 CrossRef CAS PubMed.
- J. G. Lynam, N. Kumar and M. J. Wong, Deep eutectic solvents' ability to solubilize lignin, cellulose, and hemicellulose; thermal stability; and density, Bioresour. Technol., 2017, 238, 684–689 CrossRef CAS PubMed.
- E. Scelsi, A. Angelini and C. Pastore, Deep Eutectic Solvents for the Valorisation of Lignocellulosic Biomasses towards Fine Chemicals, Biomass, 2021, 1, 29–59 CrossRef.
- M. K. Akalın, K. Tekin and S. Karagöz, Supercritical fluid extraction of biofuels from biomass, Environ. Chem. Lett., 2017, 15, 29–41 CrossRef.
- S. Peleteiro, S. Rivas, J. L. Alonso, V. Santos and J. C. Parajó, Furfural production using ionic liquids: A review, Bioresour. Technol., 2016, 202, 181–191 CrossRef CAS PubMed.
- A. A. Rosatella, S. P. Simeonov, R. F. M. Frade and C. A. M. Afonso, 5-Hydroxymethylfurfural (HMF) as a building block platform: Biological properties, synthesis and synthetic applications, Green Chem., 2011, 13, 754–793 RSC.
- M. E. Zakrzewska, E. Bogel-Łukasik and R. Bogel-Łukasik, Ionic liquid-mediated formation of 5-hydroxymethylfurfural-A promising biomass-derived building block, Chem. Rev., 2011, 111, 397–417 CrossRef CAS PubMed.
- M. G. Davidson, S. Elgie, S. Parsons and T. J. Young, Production of HMF, FDCA and their derived products: a review of life cycle assessment (LCA) and techno-economic analysis (TEA) studies, Green Chem., 2021, 23, 3154–3171 RSC.
- M. Kammoun, et al., Hydrothermal dehydration of monosaccharides promoted by seawater: fundamentals on the catalytic role of inorganic salts, Front. Chem., 2019, 7, 132 CrossRef CAS PubMed.
- T. Istasse and A. Richel, Mechanistic aspects of saccharide dehydration to furan derivatives for reaction media design, RSC Adv., 2020, 10, 23720–23742 RSC.
- A. A. Marianou, et al., Effect of Lewis and Brønsted acidity on glucose conversion to 5-HMF and lactic acid in aqueous and organic media, Appl. Catal., A, 2018, 555, 75–87 CrossRef CAS.
- Z. Xue, M. G. Ma, Z. Li and T. Mu, Advances in the conversion of glucose and cellulose to 5-hydroxymethylfurfural over heterogeneous catalysts, RSC Adv., 2016, 6, 98874–98892 RSC.
- T. Zhang, et al., Advance in constructing acid catalyst-solvent combinations for efficient transformation of glucose into 5-Hydroxymethylfurfural, Mol. Catal., 2020, 498, 111254 CrossRef CAS.
- L. Zhu, X. Fu, Y. Hu and C. Hu, Controlling the Reaction Networks for Efficient Conversion of Glucose into 5-Hydroxymethylfurfural, ChemSusChem, 2020, 13, 4812–4832 CrossRef CAS PubMed.
- B. R. Caes, R. E. Teixeira, K. G. Knapp and R. T. Raines, Biomass to Furanics: Renewable Routes to Chemicals and Fuels, ACS Sustainable Chem. Eng., 2015, 3, 2591–2605 CrossRef CAS.
- P. Zhou and Z. Zhang, One-pot catalytic conversion of carbohydrates into furfural and 5-hydroxymethylfurfural, Catal. Sci. Technol., 2016, 6, 3694–3712 RSC.
- B. Saha and M. M. Abu-Omar, Advances in 5-hydroxymethylfurfural production from biomass in biphasic solvents, Green Chem., 2014, 16, 24–38 RSC.
- R. Mariscal, P. Maireles-Torres, M. Ojeda, I. Sádaba and M. López Granados, Furfural: a renewable and versatile platform molecule for the synthesis of chemicals and fuels, Energy Environ. Sci., 2016, 9, 1144–1189 RSC.
- F. Delbecq, et al., Hydrolysis of hemicellulose and derivatives-a review of recent advances in the production of furfural, Front. Chem., 2018, 6, 146 CrossRef PubMed.
- Y. Luo, et al., The production of furfural directly from hemicellulose in lignocellulosic biomass: A review, Catal. Today, 2019, 319, 14–24 CrossRef CAS.
- J. S. Luterbacher, et al., Nonenzymatic sugar production from biomass using biomass-derived γ-valerolactone, Science, 2014, 343, 277–280 CrossRef CAS PubMed.
- A. F. Sousa, et al., Recommendations for replacing PET on packaging, fiber, and film materials with biobased counterparts, Green Chem., 2021, 23, 8795–8820 RSC.
- A. F. Sousa, et al., Biobased polyesters and other polymers from 2,5-furandicarboxylic acid: a tribute to furan excellency, Polym. Chem., 2015, 6, 5961–5983 RSC.
- N. Eid, B. Ameduri and B. Boutevin, Synthesis and Properties of Furan Derivatives for Epoxy Resins, ACS Sustainable Chem. Eng., 2021, 9, 8018–8031 CrossRef CAS.
- T. P. Kainulainen, P. Erkkilä, T. I. Hukka, J. A. Sirviö and J. P. Heiskanen, Application of Furan-Based Dicarboxylic Acids in Bio-Derived Dimethacrylate Resins, ACS Appl. Polym. Mater., 2020, 2, 3215–3225 CrossRef CAS.
- D. Zhang and M. J. Dumont, Advances in polymer precursors and bio-based polymers synthesized from 5-hydroxymethylfurfural, J. Polym. Sci., Part A: Polym. Chem., 2017, 55, 1478–1492 CrossRef CAS.
- X. Kong, et al., Catalytic conversion of 5-hydroxymethylfurfural to some value-added derivatives, Green Chem., 2018, 20, 3657–3682 RSC.
- M. Kammoun, H. Ayeb, T. Bettaieb and A. Richel, Chemical characterisation and technical assessment of agri-food residues, marine matrices, and wild grasses in the South Mediterranean area: A considerable inflow for biorefineries, Waste Manage., 2020, 118, 247–257 CrossRef CAS PubMed.
- S2Biom Project Grant Agreement no. 608622 D8.2 Vision for 1 billion dry tonnes lignocellulosic biomass as a contribution to biobased economy by 2030 in Europe Delivery of sustainable supply of non-food biomass to support a ‘resource-efficient’ Bioeconomy in Europe, 2016 Search PubMed.
- X. Zhou, W. Li, R. Mabon and L. J. Broadbelt, A Critical Review on Hemicellulose Pyrolysis, Energy Technol., 2017, 5, 52–79 CrossRef CAS.
- F. H. Isikgor and C. R. Becer, Lignocellulosic biomass: a sustainable platform for the production of bio-based chemicals and polymers, Polym. Chem., 2015, 6, 4497–4559 RSC.
- A. K. Kumar and S. Sharma, Recent updates on different methods of pretreatment of lignocellulosic feedstocks: a review, Bioresour. Bioprocess., 2017, 4, 1–19 CrossRef PubMed.
- S. Montipó, et al., Integrated production of second generation ethanol and lactic acid from steam-exploded elephant grass, Bioresour. Technol., 2018, 249, 1017–1024 CrossRef PubMed.
- J. Baruah, et al., Recent trends in the pretreatment of lignocellulosic biomass for value-added products, Front. Energy Res., 2018, 6, 141 CrossRef.
- F. Carvalheiro, L. C. Duarte, F. Gírio and P. Moniz, Hydrothermal/Liquid Hot Water Pretreatment (Autohydrolysis): A Multipurpose Process for Biomass Upgrading, Biomass Fractionation Technol. a Lignocellul. Feed. Based Biorefinery, 2016, pp. 315–347, DOI:10.1016/B978-0-12-802323-5.00014-1.
- Y. Liao, et al., The role of pretreatment in the catalytic valorization of cellulose, Mol. Catal., 2020, 487, 110883 CrossRef CAS.
- M. Li, et al., The effect of liquid hot water pretreatment on the chemical-structural alteration and the reduced recalcitrance in poplar, Biotechnol. Biofuels, 2017, 10, 1–13 CrossRef PubMed.
- C. K. Nitsos, K. A. Matis and K. S. Triantafyllidis, Optimization of Hydrothermal Pretreatment of Lignocellulosic Biomass in the Bioethanol Production Process, ChemSusChem, 2013, 6, 110–122 CrossRef CAS PubMed.
- B. Yang, L. Tao and C. E. Wyman, Strengths, challenges, and opportunities for hydrothermal pretreatment in lignocellulosic biorefineries, Biofuels, Bioprod. Biorefin., 2018, 12, 125–138 CrossRef CAS.
- X. Zhuang, et al., Liquid hot water pretreatment of lignocellulosic biomass for bioethanol production accompanying with high valuable products, Bioresour. Technol., 2016, 199, 68–75 CrossRef CAS PubMed.
- V. B. Agbor, N. Cicek, R. Sparling, A. Berlin and D. B. Levin, Biomass pretreatment: Fundamentals toward application, Biotechnol. Adv., 2011, 29, 675–685 CrossRef CAS PubMed.
- H. Z. Chen and Z. H. Liu, Steam explosion and its combinatorial pretreatment refining technology of plant biomass to bio-based products, Biotechnol. J., 2015, 10, 866–885 CrossRef CAS PubMed.
- A. Duque, P. Manzanares, I. Ballesteros and M. Ballesteros, Steam Explosion as Lignocellulosic Biomass Pretreatment, Biomass Fractionation Technol. a Lignocellul. Feed. Based Biorefinery, 2016, pp. 349–368, DOI:10.1016/B978-0-12-802323-5.00015-3.
- J. Larsen, M. Ø. Haven and L. Thirup, Inbicon makes lignocellulosic ethanol a commercial reality, Biomass Bioenergy, 2012, 46, 36–45 CrossRef CAS.
- C. Xiros, et al., Toward a sustainable biorefinery using high-gravity technology, Biofuels, Bioprod. Biorefin., 2017, 11, 15–27 CrossRef CAS.
- D. Steinbach, A. Kruse and J. Sauer, Pretreatment technologies of lignocellulosic biomass in water in view of furfural and 5-hydroxymethylfurfural production-A review, Biomass Convers. Biorefin., 2017, 7, 247–274 CrossRef CAS.
- P. Gullón, A. Romaní, C. Vila, G. Garrote and J. C. Parajó, Potential of hydrothermal treatments in lignocellulose biorefineries, Biofuels, Bioprod. Biorefin., 2012, 6, 219–232 CrossRef.
- M. Möller, P. Nilges, F. Harnisch and U. Schröder, Subcritical Water as Reaction Environment: Fundamentals of Hydrothermal Biomass Transformation, ChemSusChem, 2011, 4, 566–579 CrossRef PubMed.
- F. Carvalheiro, G. Garrote, J. C. Parajó, H. Pereira and F. M. Gírio, Kinetic Modeling of Breweryapos;s Spent Grain Autohydrolysis, Biotechnol. Prog., 2005, 21, 233–243 CrossRef CAS PubMed.
- C. K. Nitsos, T. Choli-Papadopoulou, K. A. Matis and K. S. Triantafyllidis, Optimization of hydrothermal pretreatment of hardwood and softwood lignocellulosic residues for selective hemicellulose recovery and improved cellulose enzymatic hydrolysis, ACS Sustainable Chem. Eng., 2016, 4, 4529–4544 CrossRef CAS.
- B. Danon, G. Marcotullio and W. De Jong, Mechanistic and kinetic aspects of pentose dehydration towards furfural in aqueous media employing homogeneous catalysis, Green Chem., 2014, 16, 39–54 RSC.
- A. P. Dunlop, Furfural Formation and Behavior, Ind. Eng. Chem., 1948, 40, 204–209 CrossRef CAS.
- A. T. W. M. Hendriks and G. Zeeman, Pretreatments to enhance the digestibility of lignocellulosic biomass, Bioresour. Technol., 2009, 100, 10–18 CrossRef CAS PubMed.
- R. P. Overend and E. Chornet, Fractionation of lignocellulosics by steam-aqueous pretreatments, Philos. Trans. R. Soc., A, 1987, 321, 523–536 CrossRef CAS.
- M. Pedersen, A. Viksø-Nielsen and A. S. Meyer, Monosaccharide yields and lignin removal from wheat straw in response to catalyst type and pH during mild thermal pretreatment, Process Biochem., 2010, 45, 1181–1186 CrossRef CAS.
- L. C. Duarte et al., Comparison of Two Posthydrolysis Processes of Brewery's Spent Grain Autohydrolysis Liquor to Produce a Pentose-Containing Culture Medium, Proc. Twenty-Fifth Symp. Biotechnol. Fuels Chem., held May 4–7, 2003, Breckenridge, CO 1041–1058, 2004, DOI:10.1007/978-1-59259-837-3_85.
- D. H. Fockink, J. H. Sánchez and L. P. Ramos, Comprehensive analysis of sugarcane bagasse steam explosion using autocatalysis and dilute acid hydrolysis (H3PO4 and H2SO4) at equivalent combined severity factors, Ind. Crops Prod., 2018, 123, 563–572 CrossRef CAS.
- M. A. Kabel, G. Bos, J. Zeevalking, A. G. J. Voragen and H. A. Schols, Effect of pretreatment severity on xylan solubility and enzymatic breakdown of the remaining cellulose from wheat straw, Bioresour. Technol., 2007, 98, 2034–2042 CrossRef CAS PubMed.
- C. Nitsos, L. Matsakas, K. Triantafyllidis, U. Rova and P. Christakopoulos, Investigation of different pretreatment methods of Mediterranean-type ecosystem agricultural residues: characterisation of pretreatment products, high-solids enzymatic hydrolysis and bioethanol production, Biofuels, 2018, 9, 545–558 CrossRef CAS.
- G. Garrote, H. Domínguez and J. C. Parajó, Mild autohydrolysis: an environmentally friendly technology for xylooligosaccharide production from wood, J. Chem. Technol. Biotechnol., 1999, 74, 1101–1109 CrossRef CAS.
- M. E. Vallejos, M. D. Zambon, M. C. Area and A. A. Da Silva Curvelo, Low liquid–solid ratio (LSR) hot water pretreatment of sugarcane bagasse, Green Chem., 2012, 14, 1982–1989 RSC.
- H. A. Ruiz, M. H Thomsen and H. L. Trajano, Hydrothermal Processing in Biorefineries, 2017, DOI:10.1007/978-3-319-56457-9.
- H. A. Ruiz, et al., Evaluation of a hydrothermal process for pretreatment of wheat straw—effect of particle size and process conditions, J. Chem. Technol. Biotechnol., 2011, 86, 88–94 CrossRef CAS.
- C. Cara, I. Romero, J. M. Oliva, F. Sáez and E. Castro, Liquid Hot Water Pretreatment of Olive Tree Pruning Residues, Appl. Biochem. Biotechnol., 2007, 379–394, DOI:10.1007/978-1-60327-181-3_33.
- K. Dimos, et al., Effect of Various Pretreatment Methods on Bioethanol Production from Cotton Stalks, Ferment, 2019, 5, 5 CrossRef CAS.
- D. Ilanidis, S. Stagge, L. J. Jönsson and C. Martín, Effects of operational conditions on auto-catalyzed and sulfuric-acid-catalyzed hydrothermal pretreatment of sugarcane bagasse at different severity factor, Ind. Crops Prod., 2021, 159, 113077 CrossRef CAS.
- M. Michelin, E. Ximenes, M. de Lourdes Teixeira de Moraes Polizeli and M. R. Ladisch, Effect of phenolic compounds from pretreated sugarcane bagasse on cellulolytic and hemicellulolytic activities, Bioresour. Technol., 2016, 199, 275–278 CrossRef CAS PubMed.
- H. Inoue, S. Yano, T. Endo, T. Sakaki and S. Sawayama, Combining hot-compressed water and ball milling pretreatments to improve the efficiency of the enzymatic hydrolysis of eucalyptus, Biotechnol. Biofuels, 2008, 1, 1–9 CrossRef PubMed.
- Y. Kim, E. Ximenes, N. S. Mosier and M. R. Ladisch, Soluble inhibitors/deactivators of cellulase enzymes from lignocellulosic biomass, Enzyme Microb. Technol., 2011, 48, 408–415 CrossRef CAS PubMed.
- A. Deng, J. Ren, H. Li, F. Peng and R. Sun, Corncob lignocellulose for the production of furfural by hydrothermal pretreatment and heterogeneous catalytic process, RSC Adv., 2015, 5, 60264–60272 RSC.
- M. Michelin and J. A. Teixeira, Liquid hot water pretreatment of multi feedstocks and enzymatic hydrolysis of solids obtained thereof, Bioresour. Technol., 2016, 216, 862–869 CrossRef CAS PubMed.
- P. Wen, T. Zhang, J. Wang, Z. Lian and J. Zhang, Production of xylooligosaccharides and monosaccharides from poplar by a two-step acetic acid and peroxide/acetic acid pretreatment, Biotechnol. Biofuels, 2019, 12, 1–13 CrossRef PubMed.
- L. López, S. Rivas, A. Moure, C. Vila and J. C. Parajó, Development of Pretreatment Strategies for the Fractionation of Hazelnut Shells in the Scope of Biorefinery, Agron, 2020, 10, 1568 CrossRef.
- K. Sabanci and A. O. Buyukkileci, Comparison of liquid hot water, very dilute acid and alkali treatments for enhancing enzymatic digestibility of hazelnut tree pruning residues, Bioresour. Technol., 2018, 261, 158–165 CrossRef CAS PubMed.
- J. A. Pérez, et al., Optimizing Liquid Hot Water pretreatment conditions to enhance sugar recovery from wheat straw for fuel-ethanol production, Fuel, 2008, 87, 3640–3647 CrossRef.
- F. Cebreiros, L. Clavijo, E. Boix, M. D. Ferrari and C. Lareo, Integrated valorization of eucalyptus sawdust within a biorefinery approach by autohydrolysis and organosolv pretreatments, Renewable Energy, 2020, 149, 115–127 CrossRef CAS.
- S. Fujimoto, S. Inoue and M. Yoshida, High solid concentrations during the hydrothermal pretreatment of eucalyptus accelerate hemicellulose decomposition and subsequent enzymatic glucose production, Bioresour. Technol. Rep., 2018, 4, 16–20 CrossRef.
- L. Capolupo and V. Faraco, Green methods of lignocellulose pretreatment for biorefinery development, Appl. Microbiol. Biotechnol., 2016, 100, 9451–9467 CrossRef CAS PubMed.
- M. F. Tiappi Deumaga, et al., Fractionation and Structural Characterization of Hemicellulose from Steam-Exploded Banana Rachis, Waste Biomass Valorization, 2018, 11, 2183–2192 CrossRef.
- G. Maniet, et al., Effect of steam explosion treatment on chemical composition and characteristic of organosolv fescue lignin, Ind. Crops Prod., 2017, 99, 79–85 CrossRef CAS.
- M. Barbanera, C. Buratti, F. Cotana, D. Foschini and E. Lascaro, Effect of Steam Explosion Pretreatment on Sugar Production by Enzymatic Hydrolysis of Olive Tree Pruning, Energy Procedia, 2015, 81, 146–154 CrossRef CAS.
- X. Zhuang, Q. Yu, Z. Yuan, X. Kong and W. Qi, Effect of hydrothermal pretreatment of sugarcane bagasse on enzymatic digestibility, J. Chem. Technol. Biotechnol., 2015, 90, 1515–1520 CrossRef CAS.
- J. Xu, M. H. Thomsen and A. B. Thomsen, Investigation of acetic acid-catalyzed hydrothermal pretreatment on corn stover, Appl. Microbiol. Biotechnol., 2010, 86, 509–516 CrossRef CAS PubMed.
- G. Dimitrellos, G. Lyberatos and G. Antonopoulou, Does Acid Addition Improve Liquid Hot Water Pretreatment of Lignocellulosic Biomass towards Biohydrogen and Biogas Production?, Sustainability, 2020, 12, 8935 CrossRef CAS.
- J. A. Rojas-Chamorro, I. Romero, J. C. López-Linares and E. Castro, Brewer's spent grain as a source of renewable fuel through optimized dilute acid pretreatment, Renewable Energy, 2020, 148, 81–90 CrossRef CAS.
- H. Yang, K. Wang, F. Xu, R. C. Sun and Y. Lu, H2SO4-catalyzed hydrothermal pretreatment of triploid poplar to enhance enzymatic hydrolysis, Ind. Eng. Chem. Res., 2012, 51, 11598–11604 CrossRef CAS.
- C. Katsimpouras, P. Christakopoulos and E. Topakas, Acetic acid-catalyzed hydrothermal pretreatment of corn stover for the production of bioethanol at high-solids content, Bioprocess Biosyst. Eng., 2016, 39, 1415–1423 CrossRef CAS PubMed.
- M. Linde, E. L. Jakobsson, M. Galbe and G. Zacchi, Steam pretreatment of dilute H2SO4-impregnated wheat straw and SSF with low yeast and enzyme loadings for bioethanol production, Biomass Bioenergy, 2008, 32, 326–332 CrossRef CAS.
- F. Sibilla and P. D. de María, Integrating White Biotechnology in Lignocellulosic Biomass Transformations: From Enzyme-Catalysis to Metabolic Engineering, Role Catal. Sustain. Prod. Bio-Fuels Bio-Chemicals, 2013, pp. 445–466, DOI:10.1016/B978-0-444-56330-9.00013-9.
- C. K. Nitsos, C. M. Mihailof, K. A. Matis, A. A. Lappas and K. S. Triantafyllidis, The Role of Catalytic Pretreatment in Biomass Valorization Toward Fuels and Chemicals, Role Catal. Sustain. Prod. Bio-Fuels Bio-Chemicals, 2013, pp. 217–260, DOI:10.1016/B978-0-444-56330-9.00007-3.
- A. W. Bhutto, et al., Insight into progress in pre-treatment of lignocellulosic biomass, Energy, 2017, 122, 724–745 CrossRef CAS.
- J. U. Hernández-Beltrán, et al., Insight into Pretreatment Methods of Lignocellulosic Biomass to Increase Biogas Yield: Current State, Challenges, and Opportunities, Appl. Sci., 2019, 9, 3721 CrossRef.
- F. Ahmad, E. L. Silva and M. B. A. Varesche, Hydrothermal processing of biomass for anaerobic digestion – A review, Renewable Sustainable Energy Rev., 2018, 98, 108–124 CrossRef CAS.
- C. K. Nitsos, P. A. Lazaridis, A. Mach-Aigner, K. A. Matis and K. S. Triantafyllidis, . Enhancing Lignocellulosic Biomass Hydrolysis by Hydrothermal Pretreatment, Extraction of Surface Lignin, Wet Milling and Production of Cellulolytic Enzymes, ChemSusChem, 2019, 12, 1179–1195 CrossRef CAS PubMed.
- L. Senila, et al., Bioethanol Production from Vineyard Waste by Autohydrolysis Pretreatment and Chlorite Delignification via Simultaneous Saccharification and Fermentation, Mol, 2020, 25, 2606 CrossRef CAS PubMed.
- S. M. Kim, B. S. Dien and V. Singh, Promise of combined hydrothermal/chemical and mechanical refining for pretreatment of woody and herbaceous biomass, Biotechnol. Biofuels, 2016, 9, 1–15 CrossRef.
- M. A. Kougioumtzis, et al., Production of 5-HMF from Cellulosic Biomass: Experimental Results and Integrated Process Simulation, Waste Biomass Valorization, 2018, 9, 2433–2445 CrossRef CAS.
- M. P. García-Aparicio, et al., Effect of inhibitors released during steam-explosion pretreatment of barley straw on enzymatic hydrolysis, Appl. Biochem. Biotechnol., 2006, 129, 278–288 CrossRef PubMed.
- M. Miura, Y. Shimotori, H. Nakatani, A. Harada and M. Aoyama, Bioconversion of Birch Wood Hemicellulose Hydrolyzate to Xylitol, Appl. Biochem. Biotechnol., 2015, 176, 947–955 CrossRef CAS PubMed.
- F. Shiyang, et al., A Review of Lignocellulose Change During Hydrothermal Pretreatment for Bioenergy Production, Curr. Org. Chem., 2016, 20, 2799–2809 CrossRef.
- J. Yi, T. He, Z. Jiang, J. Li and C. Hu, AlCl3 catalyzed conversion of hemicellulose in corn stover, Chin. J. Catal., 2013, 34, 2146–2152 CrossRef CAS.
- E. Z. Hoşgün, One-pot hydrothermal conversion of poppy stalks over metal chloride catalysts, Biomass Convers. Biorefin., 2021, 11, 2703–2710 CrossRef.
- A. Mittal, H. M. Pilath and D. K. Johnson, Direct Conversion of Biomass Carbohydrates to Platform Chemicals: 5-Hydroxymethylfurfural (HMF) and Furfural, Energy Fuels, 2020, 34, 3284–3293 CrossRef CAS.
- L. Zhang, G. Xi, J. Zhang, H. Yu and X. Wang, Efficient catalytic system for the direct transformation of lignocellulosic biomass to furfural and 5-hydroxymethylfurfural, Bioresour. Technol., 2017, 224, 656–661 CrossRef CAS PubMed.
- I. C. Roberto, S. I. Mussatto and R. C. L. B. Rodrigues, Dilute-acid hydrolysis for optimization of xylose recovery from rice straw in a semi-pilot reactor, Ind. Crops Prod., 2003, 17, 171–176 CrossRef CAS.
- N. Suriyachai, et al., Efficiency of catalytic liquid hot water pretreatment for conversion of corn stover to bioethanol, ACS Omega, 2020, 5, 29872–29881 CrossRef CAS PubMed.
- L. C. Blumenthal, et al., Systematic Identification of Solvents Optimal for the Extraction of 5-Hydroxymethylfurfural from Aqueous Reactive Solutions, ACS Sustainable Chem. Eng., 2016, 4, 228–235 CrossRef CAS.
- E. C. Sindermann, A. Holbach, A. D. Haan and N. Kockmann, Single stage and countercurrent extraction of 5-hydroxymethylfurfural from aqueous phase systems, Chem. Eng. J., 2016, 283, 251–259 CrossRef CAS.
- J. P. Lange, E. Van Der Heide, J. Van Buijtenen and R. Price, Furfural—A Promising Platform for Lignocellulosic Biofuels, ChemSusChem, 2012, 5, 150–166 CrossRef CAS PubMed.
- A. R. C. Morais, M. D. D. J. Matuchaki, J. Andreaus and R. Bogel-Lukasik, A green and efficient approach to selective conversion of xylose and biomass hemicellulose into furfural in aqueous media using high-pressure CO2 as a sustainable catalyst, Green Chem., 2016, 18, 2985–2994 RSC.
- A. R. C. Morais and R. Bogel-Lukasik, Highly efficient and selective CO2 -adjunctive dehydration of xylose to furfural in aqueous media with THF, Green Chem., 2016, 18, 2331–2334 RSC.
- J. An, G. Lee, J. M. Ha and M. J. Park, Process analysis for biphasic dehydration of xylose: effects of solvents on the purification of furfural, Biofuels, 2019, 13, 63–67 CrossRef.
- B. Carter, P. C. Gilcrease and T. J. Menkhaus, Removal and recovery of furfural, 5-hydroxymethylfurfural, and acetic acid from aqueous solutions using a soluble polyelectrolyte, Biotechnol. Bioeng., 2011, 108, 2046–2052 CrossRef CAS PubMed.
- T. Welton, Room-Temperature Ionic Liquids. Solvents for Synthesis and Catalysis, Chem. Rev., 1999, 99, 2071–2083 CrossRef CAS PubMed.
- J. P. Hallett and T. Welton, Room-temperature ionic liquids: Solvents for synthesis and catalysis. 2, Chem. Rev., 2011, 111, 3508–3576 CrossRef CAS PubMed.
- N. A. S. Ramli and N. A. S. Amin, Catalytic Conversion of Carbohydrate Biomass in Ionic Liquids to 5-Hydroxymethyl Furfural and Levulinic Acid: A Review, BioEnergy Res., 2020, 13, 693–736 CrossRef CAS.
- S. A. Forsyth, J. M. Pringle and D. R. MacFarlane, Ionic Liquids—An Overview, Aust. J. Chem., 2004, 57, 113–119 CrossRef CAS.
- J. Song, H. Fan, J. Ma and B. Han, Conversion of glucose and cellulose into value-added products in water and ionic liquids, Green Chem., 2013, 15, 2619–2635 RSC.
- A. M. Asim, et al., Acidic ionic liquids: Promising and cost-effective solvents for processing of lignocellulosic biomass, J. Mol. Liq., 2019, 287, 110943 CrossRef CAS.
- A. Chinnappan, C. Baskar and H. Kim, Biomass into chemicals: green chemical conversion of carbohydrates into 5-hydroxymethylfurfural in ionic liquids, RSC Adv., 2016, 6, 63991–64002 RSC.
- S. Peleteiro, A. M. da Costa Lopes, G. Garrote, R. Bogel-Łukasik and J. C. Parajó, Manufacture of furfural in biphasic media made up of an ionic liquid and a co-solvent, Ind. Crops Prod., 2015, 77, 163–166 CrossRef CAS.
- S. Peleteiro, A. M. Da Costa Lopes, G. Garrote, J. C. Parajó and R. Bogel-Łukasik, Simple and efficient furfural production from xylose in media containing 1-butyl-3-methylimidazolium hydrogen sulfate, Ind. Eng. Chem. Res., 2015, 54, 8368–8373 CrossRef CAS.
- A. V. Carvalho, A. M. Da Costa Lopes and R. Bogel-ŁUkasik, Relevance of the acidic 1-butyl-3-methylimidazolium hydrogen sulphate ionic liquid in the selective catalysis of the biomass hemicellulose fraction, RSC Adv., 2015, 5, 47153–47164 RSC.
- A. M. Da Costa Lopes, R. M. G. Lins, R. A. Rebelo and R. M. Łukasik, Biorefinery approach for lignocellulosic biomass valorisation with an acidic ionic liquid, Green Chem., 2018, 20, 4043–4057 RSC.
- A. S. Amarasekara, Ionic Liquids in Biomass Processing, Isr. J. Chem., 2019, 59, 789–802 CrossRef CAS.
- M. Zavrel, D. Bross, M. Funke, J. Büchs and A. C. Spiess, High-throughput screening for ionic liquids dissolving (ligno-)cellulose, Bioresour. Technol., 2009, 100, 2580–2587 CrossRef CAS PubMed.
- K. Rajan, T. Elder, N. Abdoulmoumine, D. J. Carrier and N. Labbé, Understanding the in situ state of lignocellulosic biomass during ionic liquids-based engineering of renewable materials and chemicals, Green Chem., 2020, 22, 6748–6766 RSC.
- J. Flieger and M. Flieger, Ionic liquids toxicity—benefits and threats, Int. J. Mol. Sci., 2020, 21, 1–41 Search PubMed.
- A. Brandt, J. Gräsvik, J. P. Hallett and T. Welton, Deconstruction of lignocellulosic biomass with ionic liquids, Green Chem., 2013, 15, 550–583 RSC.
- P. Halder, et al., Progress on the pre-treatment of lignocellulosic biomass employing ionic liquids, Renewable Sustainable Energy Rev., 2019, 105, 268–292 CrossRef CAS.
- C. Graenacher, Cellulose solution, US Pat.1943176, 1934, available at: https://patents.google.com/patent/US1943176A/en. accessed 2nd June, 2022 Search PubMed.
- R. P. Swatloski, S. K. Spear, J. D. Holbrey and R. D. Rogers, Dissolution of cellose with ionic liquids, J. Am. Chem. Soc., 2002, 124, 4974–4975 CrossRef CAS PubMed.
- C. Verma, et al., Dissolution of cellulose in ionic liquids and their mixed cosolvents: A review, Sustainable Chem. Pharm., 2019, 13, 100162 CrossRef.
- Y. Zhao, X. Liu, J. Wang and S. Zhang, Effects of Cationic Structure on Cellulose Dissolution in Ionic Liquids: A Molecular Dynamics Study, ChemPhysChem, 2012, 13, 3126–3133 CrossRef CAS PubMed.
- J. Zhang, et al., NMR spectroscopic studies of cellobiose solvation in EmimAc aimed to understand the dissolution mechanism of cellulose in ionic liquids, Phys. Chem. Chem. Phys., 2010, 12, 1941–1947 RSC.
- H. Liu, K. L. Sale, B. M. Holmes, B. A. Simmons and S. Singh, Understanding the interactions of cellulose with ionic liquids: a molecular dynamics study, J. Phys. Chem. B, 2010, 114, 4293–4301 CrossRef CAS PubMed.
- B. Mostofian, J. C. Smith and X. Cheng, Simulation of a cellulose fiber in ionic liquid suggests a synergistic approach to dissolution, Cellulose, 2014, 21, 983–997 CrossRef CAS.
- I. Kilpeläinen, et al., Dissolution of wood in ionic liquids, J. Agric. Food Chem., 2007, 55, 9142–9148 CrossRef PubMed.
- D. A. Fort, et al., Can ionic liquids dissolve wood? Processing and analysis of lignocellulosic materials with 1-n-butyl-3-methylimidazolium chloride, Green Chem., 2007, 9, 63–69 RSC.
- A. Xu and F. Wang, Carboxylate ionic liquid solvent systems from 2006 to 2020: thermal properties and application in cellulose processing, Green Chem., 2020, 22, 7622–7664 RSC.
- H. Abushammala and J. Mao, A Review on the Partial and Complete Dissolution and Fractionation of Wood and Lignocelluloses Using Imidazolium Ionic Liquids, Polym, 2020, 12, 195 CAS.
- J. Sun, et al., One-pot integrated biofuel production using low-cost biocompatible protic ionic liquids, Green Chem., 2017, 19, 3152–3163 RSC.
- T. L. Greaves and C. J. Drummond, Quantitative glucose release from softwood after pretreatment with low-cost ionic liquids, Chem. Rev., 2008, 108, 206–237 CrossRef CAS PubMed.
- F. J. V. Gschwend, et al., Quantitative glucose release from softwood after pretreatment with low-cost ionic liquids, Green Chem., 2019, 21, 692–703 RSC.
- F. J. V. Gschwend, F. Malaret, S. Shinde, A. Brandt-Talbot and J. P. Hallett, Rapid pretreatment of Miscanthus using the low-cost ionic liquid triethylammonium hydrogen sulfate at elevated temperatures, Green Chem., 2018, 20, 3486–3498 RSC.
- A. Brandt-Talbot, et al., An economically viable ionic liquid for the fractionation of lignocellulosic biomass, Green Chem., 2017, 19, 3078–3102 RSC.
- Z. Zhang, J. Song and B. Han, Catalytic Transformation of Lignocellulose into Chemicals and Fuel Products in Ionic Liquids, Chem. Rev., 2017, 117, 6834–6880 CrossRef CAS PubMed.
- A. M. Da Costa Lopes and R. Bogel-Lukasik, Acidic Ionic Liquids as Sustainable Approach of Cellulose and Lignocellulosic Biomass Conversion without Additional Catalysts, ChemSusChem, 2015, 8, 947–965 CrossRef CAS PubMed.
- T. Ståhlberg, W. Fu, J. M. Woodley and A. Riisager, Synthesis of 5-(Hydroxymethyl)furfural in Ionic Liquids: Paving the Way to Renewable Chemicals, ChemSusChem, 2011, 4, 451–458 CrossRef PubMed.
- C. Lansalot-Matras and C. Moreau, Dehydration of fructose into 5-hydroxymethylfurfural in the presence of ionic liquids, Catal. Commun., 2003, 4, 517–520 CrossRef CAS.
- Y. W. Chen and H. V. Lee, Recent progress in homogeneous Lewis acid catalysts for the transformation of hemicellulose and cellulose into valuable chemicals, fuels, and nanocellulose, Rev. Chem. Eng., 2020, 36, 215–235 CrossRef CAS.
- H. Zhao, J. E. Holladay, H. Brown and Z. C. Zhang, Metal chlorides in ionic liquid solvents convert sugars to 5-hydroxymethylfurfural, Science, 2007, 316, 1597–1600 CrossRef CAS PubMed.
- H. Li, S. Yang, S. Saravanamurugan and A. Riisager, Glucose Isomerization by Enzymes and Chemo-catalysts: Status and Current Advances, ACS Catal., 2017, 7, 3010–3029 CrossRef CAS.
- C. Li, Z. Zhang and Z. K. Zhao, Direct conversion of glucose and cellulose to 5-hydroxymethylfurfural in ionic liquid under microwave irradiation, Tetrahedron Lett., 2009, 50, 5403–5405 CrossRef CAS.
- F. Delbecq and C. Len, Recent advances in the microwave-assisted production of hydroxymethylfurfural by hydrolysis of cellulose derivatives—A review, Molecules, 2018, 23, 8 CrossRef PubMed.
- S. Tabasso, D. Carnaroglio, E. Calcio Gaudino and G. Cravotto, Microwave, ultrasound and ball mill procedures for bio-waste valorisation, Green Chem., 2015, 17, 684–693 RSC.
- J. Hoffmann, M. Nüchter, B. Ondruschka and P. Wasserscheid, Ionic liquids and their heating behaviour during microwave irradiation – a state of the art report and challenge to assessment, Green Chem., 2003, 5, 296–299 RSC.
- C. Li, Z. K. Zhao, H. Cai, A. Wang and T. Zhang, Microwave-promoted conversion of concentrated fructose into 5-hydroxymethylfurfural in ionic liquids in the absence of catalysts, Biomass Bioenergy, 2011, 35, 2013–2017 CrossRef CAS.
- X. Qi, M. Watanabe, T. M. Aida and R. L. Smith, Fast Transformation of Glucose and Di-/Polysaccharides into 5-Hydroxymethylfurfural by Microwave Heating in an Ionic Liquid/Catalyst System, ChemSusChem, 2010, 3, 1071–1077 CrossRef CAS PubMed.
- A. Al Ghatta, J. D. E. T. Wilton-Ely and J. P. Hallett, Rapid, High-Yield Fructose Dehydration to 5-Hydroxymethylfurfural in Mixtures of Water and the Noncoordinating Ionic Liquid [bmim][OTf], ChemSusChem, 2019, 12, 4452–4460 CrossRef CAS PubMed.
- X. Qi, M. Watanabe, T. M. Aida and R. L. Smith, Efficient Catalytic Conversion of Fructose into 5-Hydroxymethylfurfural in Ionic Liquids at Room Temperature, ChemSusChem, 2009, 2, 944–946 CrossRef CAS PubMed.
- J. Y. G. Chan and Y. Zhang, Selective Conversion of Fructose to 5-Hydroxymethylfurfural Catalyzed by Tungsten Salts at Low Temperatures, ChemSusChem, 2009, 2, 731–734 CrossRef CAS PubMed.
- J. Zhang, et al., Room-Temperature Ionic Liquid System Converting Fructose into 5-Hydroxymethylfurfural in High Efficiency, ACS Sustainable Chem. Eng., 2015, 3, 3338–3345 CrossRef CAS.
- J. Shi, W. Liu, N. Wang, Y. Yang and H. Wang, Production of 5-hydroxymethylfurfural from monoand disaccharides in the presence of ionic liquids, Catal. Lett., 2014, 144, 252–260 CrossRef CAS.
- Y. Su, et al., Single-step conversion of cellulose to 5-hydroxymethylfurfural (HMF), a versatile platform chemical, Appl. Catal., A, 2009, 361, 117–122 CrossRef CAS.
- H. Abou-Yousef, E. B. Hassan and P. Steele, Rapid conversion of cellulose to 5-hydroxymethylfurfural using single and combined metal chloride catalysts in ionic liquid, J. Fuel Chem. Technol., 2013, 41, 214–222 CrossRef CAS.
- E. Leng, et al., The Direct Conversion of Cellulose into 5-Hydroxymethylfurfural with CrCl3 Composite Catalyst in Ionic Liquid under Mild Conditions, ChemistrySelect, 2019, 4, 181–189 CrossRef CAS.
- B. R. Caes, M. J. Palte and R. T. Raines, Organocatalytic conversion of cellulose into a platform chemical, Chem. Sci., 2012, 4, 196–199 RSC.
- Z. Zhang and Z. K. Zhao, Microwave-assisted conversion of lignocellulosic biomass into furans in ionic liquid, Bioresour. Technol., 2010, 101, 1111–1114 CrossRef CAS PubMed.
- J. Zhou, et al., Recovery and purification of ionic liquids from solutions: a review, RSC Adv., 2018, 8, 32832–32864 RSC.
- Y. Chen and T. Mu, Application of deep eutectic solvents in biomass pretreatment and conversion, Green Energy Environ, 2019, 4, 95–115 CrossRef.
- E. S. Morais, et al., Use of Ionic Liquids and Deep Eutectic Solvents in Polysaccharides Dissolution and Extraction Processes towards Sustainable Biomass Valorization, Mol., 2020, 25, 3652 CrossRef CAS PubMed.
- E. L. Smith, A. P. Abbott and K. S. Ryder, Deep Eutectic Solvents (DESs) and Their Applications, Chem. Rev., 2014, 114, 11060–11082 CrossRef CAS PubMed.
- M. Kammoun, et al., Ultrafast Lignin Extraction from Unusual Mediterranean Lignocellulosic Residues, JoVE, 2021, 169, e61997 Search PubMed.
- B. B. Hansen, et al., Deep Eutectic Solvents: A Review of Fundamentals and Applications, Chem. Rev., 2021, 121, 1232–1285 CrossRef CAS PubMed.
- X. Shen, et al., Facile fractionation of lignocelluloses by biomass-derived deep eutectic solvent (DES) pretreatment for cellulose enzymatic hydrolysis and lignin valorization, Green Chem., 2019, 21, 275–283 RSC.
- S. Hu, et al., Conversion of fructose to 5-hydroxymethylfurfural using ionic liquids prepared from renewable materials, Green Chem., 2008, 10, 1280–1283 RSC.
- H. Zhang, et al., Continuous synthesis of 5-hydroxymethylfurfural using deep eutectic solvents and its kinetic study in microreactors, Chem. Eng. J., 2020, 391, 123580 CrossRef CAS.
- I. Bodachivskyi, U. Kuzhiumparambil and D. B. G. Williams, Catalytic Valorization of Native Biomass in a Deep Eutectic Solvent: A Systematic Approach toward High-Yielding Reactions of Polysaccharides, ACS Sustainable Chem. Eng., 2020, 8, 678–685 CrossRef CAS.
- Q. Yu, et al., Catalytic conversion of herbal residue carbohydrates to furanic derivatives in a deep eutectic solvent accompanied by dissolution and recrystallisation of choline chloride, Cellulose, 2019, 26, 8263–8277 CrossRef CAS.
- H. Li, H. Wu, Z. Yu, H. Zhang and S. Yang, CO2-Enabled Biomass Fractionation/Depolymerization: A Highly Versatile Pre-Step for Downstream Processing, ChemSusChem, 2020, 13, 3565–3582 CrossRef CAS PubMed.
- L. V. Daza Serna, C. E. Orrego Alzate and C. A. Cardona Alzate, Supercritical fluids as a green technology for the pretreatment of lignocellulosic biomass, Bioresour. Technol., 2016, 199, 113–120 CrossRef CAS PubMed.
- E. L. N. Escobar, T. A. da Silva, C. L. Pirich, M. L. Corazza and L. Pereira Ramos, Supercritical Fluids: A Promising Technique for Biomass Pretreatment and Fractionation, Front. Bioeng. Biotechnol., 2020, 8, 252 CrossRef PubMed.
- A. R. C. Morais, A. M. Da Costa Lopes and R. Bogel-Łukasik, Carbon dioxide in biomass processing: Contributions to the green biorefinery concept, Chem. Rev., 2015, 115, 3–27 CrossRef CAS PubMed.
- Y. Zheng, et al., Supercritical carbon dioxide explosion as a pretreatment for cellulose hydrolysis, Biotechnol. Lett., 1995, 17, 845–850 CrossRef CAS.
- Y. F. Liu, P. Luo, Q. Q. Xu, E. J. Wang and J. Z. Yin, Investigation of the effect of supercritical carbon dioxide pretreatment on reducing sugar yield of lignocellulose hydrolysis, Cellul. Chem. Technol., 2014, 48, 89–95 CAS.
- W. Sangarunlert, P. Piumsomboon and S. Ngamprasertsith, Furfural production by acid hydrolysis and supercritical carbon dioxide extraction from rice husk, Korean J. Chem. Eng., 2007, 24, 936–941 CrossRef CAS.
- C. Park and J. Lee, Recent achievements in CO2-assisted and CO2-catalyzed biomass conversion reactions, Green Chem., 2020, 22, 2628–2642 RSC.
- T. Miyazawa and T. Funazukuri, Polysaccharide Hydrolysis Accelerated by Adding Carbon Dioxide under Hydrothermal Conditions, Biotechnol. Prog., 2005, 21, 1782–1785 CrossRef CAS PubMed.
- C. Y. Park, Y. W. Ryu and C. Kim, Kinetics and rate of enzymatic hydrolysis of cellulose in supercritical carbon dioxide, Korean J. Chem. Eng., 2001, 18, 475–478 CrossRef CAS.
- S. Wu, et al., Effect of CO2 on conversion of inulin to 5-hydroxymethylfurfural and propylene oxide to 1,2-propanediol in water, Green Chem., 2010, 12, 1215–1219 RSC.
- H. Lin, Q. Xiong, Y. Zhao, J. Chen and S. Wang, Conversion of carbohydrates into 5-hydroxymethylfurfural in a green reaction system of CO2-water-isopropanol, AIChE J., 2017, 63, 257–265 CrossRef CAS.
- R. Lee, J. Harris, P. Champagne and P. G. Jessop, CO2-Catalysed conversion of carbohydrates to 5-hydroxymethyl furfural, Green Chem., 2016, 18, 6305–6310 RSC.
- H. Weingärtner, E. U. Franck, H. Weingärtner and E. U. Franck, Supercritical Water as a Solvent, Angew. Chem., Int. Ed., 2005, 44, 2672–2692 CrossRef PubMed.
- M. Uematsu and E. U. Frank, Static Dielectric Constant of Water and Steam, J. Phys. Chem. Ref. Data, 1980, 9, 1291 CrossRef CAS.
- P. E. Savage, A perspective on catalysis in sub- and supercritical water, J. Supercrit. Fluids, 2009, 47, 407–414 CrossRef CAS.
- A. Kruse and A. Gawlik, Biomass conversion in water at 330-410 °C and 30-50 MPa. Identification of key compounds for indicating different chemical reaction pathways, Ind. Eng. Chem. Res., 2003, 42, 267–279 CrossRef CAS.
- D. A. Cantero, C. Martínez, M. D. Bermejo and M. J. Cocero, Simultaneous and selective recovery of cellulose and hemicellulose fractions from wheat bran by supercritical water hydrolysis, Green Chem., 2015, 17, 610–618 RSC.
- L. Vaquerizo and M. J. Cocero, Ultrafast heating by high efficient biomass direct mixing with supercritical water, Chem. Eng. J., 2019, 378, 122199 CrossRef CAS.
- C. M. Martínez, T. Adamovic, D. A. Cantero and M. J. Cocero, Scaling up the production of sugars from agricultural biomass by ultrafast hydrolysis in supercritical water, J. Supercrit. Fluids, 2019, 143, 242–250 CrossRef.
- M. Bicker, J. Hirth and H. Vogel, Dehydration of fructose to 5-hydroxymethylfurfural in sub- and supercritical acetone, Green Chem., 2003, 5, 280–284 RSC.
- M. Bicker, D. Kaiser, L. Ott and H. Vogel, Dehydration of d-fructose to hydroxymethylfurfural in sub- and supercritical fluids, J. Supercrit. Fluids, 2005, 36, 118–126 CrossRef CAS.
- Y. Luo, L. Hu, D. Tong and C. Hu, Selective dissociation and conversion of hemicellulose in Phyllostachys heterocycla cv. var. pubescens to value-added monomers via solvent-thermal methods promoted by AlCl3, RSC Adv., 2014, 4, 24194–24206 RSC.
- A. Procentese, et al., Deep eutectic solvent pretreatment and subsequent saccharification of corncob, Bioresour. Technol., 2015, 192, 31–36 CrossRef CAS PubMed.
- Q. Yu, et al., Catalytic conversion of herbal residue carbohydrates to furanic derivatives in a deep eutectic solvent accompanied by dissolution and recrystallisation of choline chloride, Cellulose, 2019, 26, 8263–8277 CrossRef CAS.
- A. Poluzzi, G. Guandalini, F. d'Amore and M. C. Romano, The Potential of Power and Biomass-to-X Systems in the Decarbonization Challenge: a Critical Review, Curr. Sustainable/Renewable Energy Rep., 2021, 8, 242–252 CrossRef CAS.
- P. Patrizio, M. Fajardy, M. Bui and N. M. Dowell, CO2 mitigation or removal: The optimal uses of biomass in energy system decarbonization, iScience, 2021, 24, 102765 CrossRef CAS PubMed.
- B. Kumar and P. Verma, Biomass-based biorefineries: An important architype towards a circular economy, Fuel, 2021, 288, 119622 CrossRef CAS.
- J. A. Okolie, et al., A techno-economic assessment of biomethane and bioethanol production from crude glycerol through integrated hydrothermal gasification, syngas fermentation and biomethanation, Energy Convers. Manage.: X, 2021, 12, 100131 CAS.
- H. A. Ruiz, et al., Advances in process design, techno-economic assessment and environmental aspects for hydrothermal pretreatment in the fractionation of biomass under biorefinery concept, Bioresour. Technol., 2023, 369, 128469 CrossRef CAS PubMed.
- R. Roy, M. S. Rahman and D. E. Raynie, Recent advances of greener pretreatment technologies of lignocellulose, Curr. Res. Green Sustainable Chem., 2020, 3, 100035 CrossRef.
- M. H. Cheng, Z. Wang, B. S. Dien, P. J. W. Slininger and V. Singh, Economic analysis of cellulosic ethanol production from sugarcane bagasse using a sequential deacetylation, hot water and disk-refining pretreatment, Processes, 2019, 7, 642 CrossRef CAS.
- W. G. Sganzerla, D. Lachos-Perez, L. S. Buller, G. L. Zabot and T. Forster-Carneiro, Cost analysis of subcritical water pretreatment of sugarcane straw and bagasse for second-generation bioethanol production: a case study in a sugarcane mill, Biofuels, Bioprod. Biorefin., 2022, 16, 435–450 CrossRef CAS.
- K. Lan, et al., Techno-economic analysis of producing xylo-oligosaccharides and cellulose microfibers from lignocellulosic biomass, Bioresour. Technol., 2021, 340, 125726 CrossRef CAS PubMed.
- T. E. Seiple, R. L. Skaggs, L. Fillmore and A. M. Coleman, Municipal wastewater sludge as a renewable, cost-effective feedstock for transportation biofuels using hydrothermal liquefaction, J. Environ. Manage., 2020, 270, 110852 CrossRef CAS PubMed.
- W. G. Sganzerla, et al., Techno-economic assessment of subcritical water hydrolysis process for sugars production from brewer’s spent grains, Ind. Crops Prod., 2021, 171, 113836 CrossRef CAS.
- J. Zhao, J. Lee and D. Wang, An integrated deep eutectic solvent-ionic liquid-metal catalyst system for lignin and 5-hydroxymethylfurfural production from lignocellulosic biomass: Technoeconomic analysis, Bioresour. Technol., 2022, 356, 127277 CrossRef CAS PubMed.
- A. W. Zimmermann, et al., Techno-Economic Assessment Guidelines for CO2 Utilization, Front. Energy Res., 2020, 8, 1–23 CrossRef.
- P. Halder and K. Shah, Techno-economic analysis of ionic liquid pre-treatment integrated pyrolysis of biomass for co-production of furfural and levoglucosenone, Bioresour. Technol., 2023, 371, 128587 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2023 |