Open Access Article
Open Access ArticleQuantitative evaluation of H-donating abilities of C(sp3)–H bonds of nitrogen-containing heterocycles in hydrogen atom transfer reaction†
Yan-Hua Fu *a,
Taixuan Jiaa,
Guang-Bin Shen
*a,
Taixuan Jiaa,
Guang-Bin Shen b and
Xiao-Qing Zhuc
b and
Xiao-Qing Zhuc
aCollege of Chemistry and Environmental Engineering, Anyang Institute of Technology, Anyang, Henan 455000, China. E-mail: 20180031@ayit.edu.cn
bSchool of Medical Engineering, Jining Medical University, Jining, Shandong 272000, P. R. China
cThe State Key Laboratory of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin, 300071, China
First published on 30th May 2023
Abstract
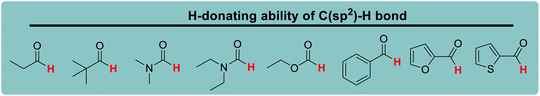
Nitrogen-containing heterocycles are an important class of antioxidants, and their reactivity and selectivity in hydrogen atom reactions have attracted significant interest from chemists. In this work, the kinetics of hydrogen atom transfer reactions from C(sp3)–H bonds of 28 nitrogen-containing heterocycles, oxygen-containing heterocycles, alicyclic amines and cycloalkanes, which were denoted as XH, to the CumO˙ radical, were investigated. The characteristic physical parameter of the substrate, i.e., the thermo–kinetic parameter ΔG≠o(XH), was determined using the kinetic equation [ΔG≠XH/Y = ΔG≠o(XH) + ΔG≠o(Y)] to quantitatively evaluate the H-donating ability of XH. The effects of the substrate structure, substituent attached to the nitrogen atom, and ring size on the H-donating ability were discussed carefully. By comparing the H-donating abilities of cycloalkanes, alicyclic amines and nitrogen/oxygen-containing heterocycles, the influence of the introduction of N, O, or carbonyl groups in the carbon ring on the H-donating ability of C(sp3)–H bond was determined. The electronic, steric and stereo-electronic effects of the groups were also discussed. Herein, we not only quantitatively determined the H-donating ability of the substrate, but also provided ideas for the synthesis of new antioxidants.
Introduction
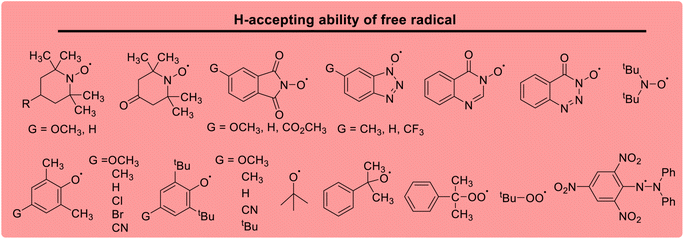
Lactams and other nitrogen-containing heterocyclic compounds such as imidazolidinones, oxazolidinones, and imides are important structural units in a large number of natural products and pharmaceuticals.1–4 In recent years, the selectivity and reactivity of these nitrogen-containing heterocycles as hydrogen atom donors (H-donor), XH, in hydrogen atom transfer reactions (HAT, X–H + Y → X + Y–H) have attracted significant attention from a large number of researchers, gradually becoming a research hotspot.5–12 Accordingly, it is necessary to develop methods to evaluate the H-donating ability of these nitrogen-containing heterocyclic compounds as H-donors using their characteristic physical parameters. Also, it is important to quantitatively study the H-donating abilities and compare the antioxidant activities of antioxidants.In a previous work, a physical parameter, i.e., the thermo–kinetic parameter ΔG≠o, was used to quantitatively evaluate the H-donating ability of H-donors or the H-accepting ability of H-acceptors in the HAT reaction.13 The H-donating abilities of C(sp3)–H bonds in dihydronicotinamide adenine dinucleotide (NADH) derivatives, reductive F420 coenzyme (F420H) derivatives, activated alkanes, amines and amides (Chart 1),13,14 O–H bonds in vitamin C/E, caffeic acid, (+)-catechin and other bioactive molecules, and phenols (Chart 2),15 and C(sp2)–H bonds in aldehydes (Chart 3)16 have been quantitatively evaluated by the thermo–kinetic parameter ΔG≠o(XH). The H-accepting abilities of three regular types of oxygen radicals including the N–O˙ radical, C–O˙ radical, and C–O–O˙ radical and one type of nitrogen radical, 2,2-diphenyl-1-picrylhydrazyl (DPPH˙), were investigated using ΔG≠o(X) (Chart 4).17 In addition, this parameter is also widely used in the hydride transfer reaction18 and electron transfer reaction,19 and is being studied in the proton transfer reaction and Lewis acid–base coupling reaction. It is proposed using a kinetic equation (eqn (1)), which is based on the classical transition state model of chemical reaction, and considers that the fracture and formation of the chemical bonds of reactants in the process of chemical reaction follow the Morse free energy curve variation law.18 In eqn (1), ΔG≠XH/Y is the activation free energy of the HAT reaction X–H + Y → X + Y–H and ΔG≠o(XH) and ΔG≠o(Y) are the thermo–kinetic parameters of XH and Y, respectively.
| ΔG≠XH/Y = ΔG≠o(XH) + ΔG≠o(Y) | (1) |
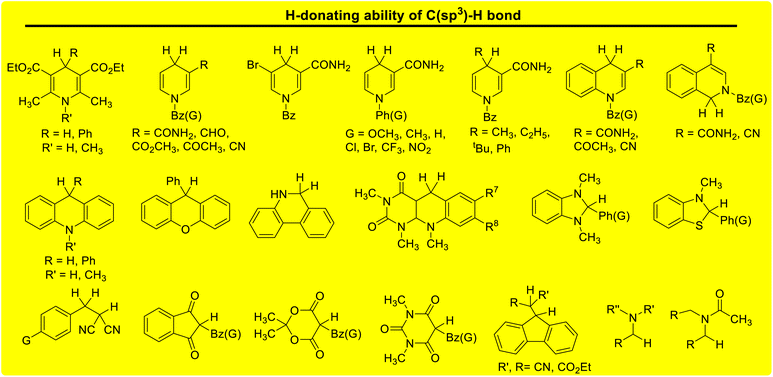 | ||
| Chart 1 H-donating abilities of C(sp3)–H bonds in NADH derivatives, F420H derivatives, activated alkanes, amines and amides examined in previous works. | ||
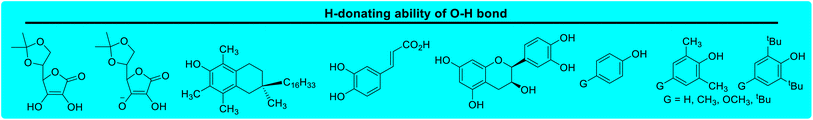 | ||
| Chart 2 H-donating abilities of O–H bonds in vitamin C/E, caffeic acid, (+)-catechin and other bioactive molecules, and phenols examined in previous works. | ||
Herein, the H-donating abilities of 28 nitrogen-containing heterocycles, oxygen-containing heterocycles, alicyclic amines and cycloalkanes, XH, in HAT reactions were studied using ΔG≠o(XH). The parent structures and types of H-donors examined in this work are shown in Scheme 1. HAT reactions between XH with the cumyloxyl radical [PhC(CH3)2O˙, CumO˙] in acetonitrile (MeCN) at 298 K were constructed, and the kinetics of these HAT reactions were investigated.5–10 The effect of substrate structure, the insertion of heteroatoms, the ring size, and the substituent attached to the nitrogen atom on the H-donating abilities of these substrates was discussed in detail.
 | ||
| Scheme 1 Parent structures and types of nitrogen-containing heterocycles and other H-donors examined in this work. | ||
Results and discussion
Determination of ΔG≠o(XH) values of the studied H-donors XH. According to previous research, the HAT reactions between CumO˙ and nitrogen-containing heterocycles predominantly occur from the endocyclic α-C(sp3)–H bonds that are α to nitrogen.5–10 The reaction sites of these substrates are shown in Scheme 1, where the hydrogen atoms involved in the HAT reactions are labelled red. The results of the kinetic studies for the HAT reactions from the ɑ-C(sp3)–H bonds of the studied H-donors to the CumO˙ radical in acetonitrile at 298 K are listed in the ESI.† The activation free energies, ΔG≠XH/Y, of the HAT reactions, which were obtained using the Eyring equation [k2 = (kBT/h)exp(−ΔG≠/RT)], are listed in Table S1.† Given that the thermo–kinetic parameter of CumO˙ [ΔG≠o(CumO˙) = −41.63 kcal mol−1] is already available in our previous work,17 the ΔG≠o(XH) of the ɑ-C(sp3)–H bonds in these 28 H-donors were determined using eqn (1) [ΔG≠o(XH) = ΔGXH/CumO˙≠ − ΔG≠o(CumO˙)]; (Table 1).| XH | Structure | ΔG≠o(XH)a (kcal mol−1) | XH | Structure | ΔG≠o(XH)a (kcal mol−1) |
|---|---|---|---|---|---|
| a ΔG≠o(XH) values are derived from eqn (1), with units of kcal mol−1. | |||||
| 1H |  |
51.50 | 2H |  |
50.41 |
| 3H | 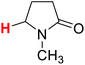 |
50.00 | 4H | 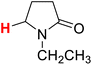 |
49.99 |
| 5H | 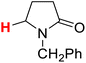 |
50.39 | 6H | 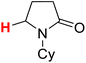 |
50.10 |
| 7H | 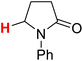 |
50.51 | 8H | 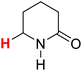 |
50.29 |
| 9H | 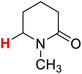 |
50.11 | 10H | 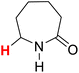 |
50.96 |
| 11H | 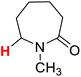 |
50.36 | 12H | 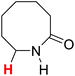 |
51.15 |
| 13H | 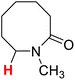 |
50.82 | 14H | 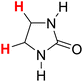 |
49.58 |
| 15H | 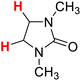 |
48.84 | 16H | 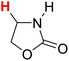 |
50.96 |
| 17H | 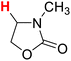 |
50.48 | 18H | 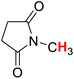 |
52.26 |
| 19H | 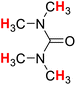 |
49.83 | 20H | 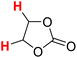 |
52.80 |
| 21H | 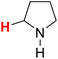 |
48.04 | 22H | 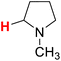 |
47.78 |
| 23H | 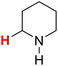 |
48.13 | 24H | 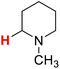 |
48.05 |
| 25H |  |
50.92 | 26H | 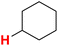 |
50.84 |
| 27H | 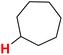 |
50.43 | 28H |  |
50.20 |
Visual comparison of ΔG≠o(XH) values of ɑ-C(sp3)–H bonds in 28 nitrogen-containing heterocycles and other H-donors
A visual comparison of the ΔG≠o(XH) values of the ɑ-C(sp3)–H bonds in 28 nitrogen-containing heterocycles and other H-donors in acetonitrile at 298 K is shown in Scheme 2. As discussed in our previous reports,13–16 ΔG≠o(XH) can be used as the characteristic physical parameter of XH to quantitatively evaluate its H-donating ability. The smaller the ΔG≠o(XH) value of XH, the stronger its H-donating ability. According to Scheme 2, the ΔG≠o(XH) values of the studied substrates range from 47.78 kcal mol−1 for 1-methylpyrrolidine (22H) to 52.80 kcal mol−1 for ethylene carbonate (20H). For these nitrogen-containing heterocycles, their H-donating abilities follow the order of alicyclic amines (21H–24H) > 2-imidazolidinones (14H–15H) > lactams (1H–13H). The H-donating abilities of two 2-oxazolidinones (16H–17H) are at the downstream level of the studied lactams. It can be seen that the H-donating abilities of alicyclic amines are the strongest among these nitrogen-containing heterocycles, and the introduction of amide [–C(N–R)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O], carbonyl (–C
O], carbonyl (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) or ester [–C(O)
O) or ester [–C(O)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)] groups in the ɑ-site of the nitrogen atom on the ring reduces the H-donating ability of the alicyclic amine. In addition, it can be seen that the H-donating abilities of cycloalkanes (25H–28H) are about 2 kcal mol−1 weaker than that of the alicyclic amines and are at the middle and downstream level of the investigated lactams. The H-donating ability of tetramethylurea (19H) is between the studied lactams and 2-imidazolidinones, belonging to the medium ability of H-donor. It is worth noting that ethylene carbonate (20H) has the weakest H-donating ability, followed by N-methylsuccinimide (18H). By comparing the H-donating abilities of 14H, 16H and 20H, it can be found that the introduction of an N atom on the ring increases the H-donating ability of the compound, while the introduction of an O atom decreases the H-donating ability of the compound.
O)] groups in the ɑ-site of the nitrogen atom on the ring reduces the H-donating ability of the alicyclic amine. In addition, it can be seen that the H-donating abilities of cycloalkanes (25H–28H) are about 2 kcal mol−1 weaker than that of the alicyclic amines and are at the middle and downstream level of the investigated lactams. The H-donating ability of tetramethylurea (19H) is between the studied lactams and 2-imidazolidinones, belonging to the medium ability of H-donor. It is worth noting that ethylene carbonate (20H) has the weakest H-donating ability, followed by N-methylsuccinimide (18H). By comparing the H-donating abilities of 14H, 16H and 20H, it can be found that the introduction of an N atom on the ring increases the H-donating ability of the compound, while the introduction of an O atom decreases the H-donating ability of the compound.
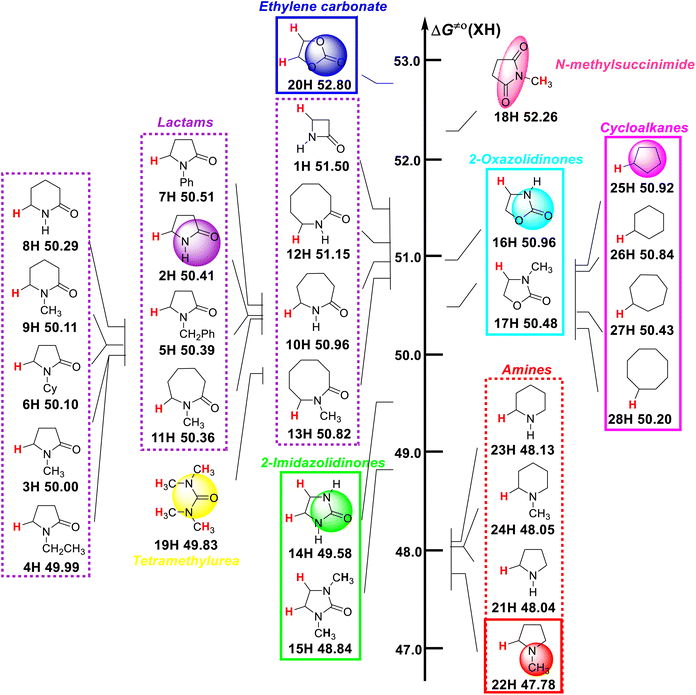 | ||
| Scheme 2 Visual comparison of ΔG≠o(XH) of ɑ-C(sp3)–H bonds in 28 nitrogen-containing heterocycles and other H-donors in MeCN at 298 K, with units of kcal mol−1. | ||
Effect of substrate structure on H-donating ability
As can be seen in Scheme 2, the structure of the substrate has a great influence on its H-donating ability. Thus, to study the effect of the substrate structure on its H-donating ability, the thermo–kinetic parameters ΔG≠o(XH) of the studied five-membered-ring compounds are listed in Scheme 3. Except for N-methylsuccinimide (18H), the reaction sites of the substrates in the HAT reactions are the endocyclic α-C(sp3)–H bonds, given that there is no endocyclic α-C(sp3)–H in 18H. The H-donating abilities of these five-membered-ring compounds follow the order of pyrrolidine (21H) > 2-imidazolidinone (14H) > cyclopentane (25H) > 2-pyrrolidone (2H) > 2-oxazolidinone (16H) > N-methylsuccinimide (18H) > ethylene carbonate (20H). The scale of ΔG≠o(XH) is 4.76 kcal mol−1. This large range indicates that the substrate structure has a great influence on the H-donating ability.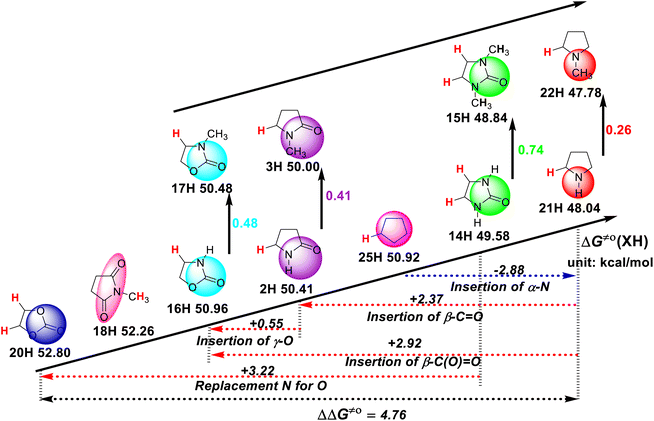 | ||
| Scheme 3 Effect of substrate structure on ΔG≠o(XH) of ɑ-C(sp3)–H bonds in five-membered-ring compounds in MeCN at 298 K, with units of kcal mol−1. | ||
According to Scheme 3, it is again proven that the introduction of an N atom in the five-membered-ring increases the H-donating ability of the compound by 2.88 kcal mol−1, which can be obtained by comparing 21H and 25H. In the case of the stereo-electronic effect, HAT is the most rapid when the C(sp3)–H bond being broken can be conjugated with π electrons or lone pair electrons. Thus, the H-donating ability of pyrrolidine (21H) is stronger than that of cyclopentane (25H) given that the C(sp3)–H bond of pyrrolidine being broken can be eclipsed with the α nitrogen lone pair electrons. However, the introduction of the carbonyl group (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) decreases the H-donating ability by 2.37 kcal mol−1, which can be obtained by comparing 2H and 21H. Specifically, the introduction of an ester group [–C(O)
O) decreases the H-donating ability by 2.37 kcal mol−1, which can be obtained by comparing 2H and 21H. Specifically, the introduction of an ester group [–C(O)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)] decreases the H-donating ability by 2.92 kcal mol−1, which can be obtained by comparing 16H and 21H. The replacement of the nitrogen atom with an oxygen atom decreases the H-donating ability by 3.22 kcal mol−1, which can be obtained by comparing 20H and 14H. According to the above-mentioned analysis, the influence of the introduction of a nitrogen atom, carbonyl and ester groups in the five-membered rings on the H-donating abilities can be determined quantitatively. Simultaneously, it can be seen that the introduction of a carbonyl group on the ring reduces the H-donating ability, and the introduction of an oxygen atom on the ring further reduces the H-donating ability, given that the two electron-withdrawing groups, i.e., carbonyl and ester groups, reduce the electron density of the reaction center at the transition state when the substrates react with the electrophilic HAT reagent CumO˙, which reduces the stability of the transition state. The introduction of an oxygen atom on the ring further reduces the electron density of the reaction center, and thus the ethylene carbonate (20H) has the weakest H-donating ability among these five-membered-ring substrates.
O)] decreases the H-donating ability by 2.92 kcal mol−1, which can be obtained by comparing 16H and 21H. The replacement of the nitrogen atom with an oxygen atom decreases the H-donating ability by 3.22 kcal mol−1, which can be obtained by comparing 20H and 14H. According to the above-mentioned analysis, the influence of the introduction of a nitrogen atom, carbonyl and ester groups in the five-membered rings on the H-donating abilities can be determined quantitatively. Simultaneously, it can be seen that the introduction of a carbonyl group on the ring reduces the H-donating ability, and the introduction of an oxygen atom on the ring further reduces the H-donating ability, given that the two electron-withdrawing groups, i.e., carbonyl and ester groups, reduce the electron density of the reaction center at the transition state when the substrates react with the electrophilic HAT reagent CumO˙, which reduces the stability of the transition state. The introduction of an oxygen atom on the ring further reduces the electron density of the reaction center, and thus the ethylene carbonate (20H) has the weakest H-donating ability among these five-membered-ring substrates.
Moreover, the introduction of a methyl group to the nitrogen atom also enhances the H-donating ability, which can be explained by the electron-donating effect of the methyl group. Although the introduction of a methyl group increases the steric hindrance of the reaction, the electronic effect caused by its introduction can completely offset the increase in steric hindrance. The increase in H-donating ability ΔΔG≠o induced by the methyl group on the nitrogen atom in different series is also different, as shown in Scheme 3. It can be found that the lower the electron density of the reaction center in the transition state, the more obvious the H-donating ability of C(sp3)–H increased by the introduction of a methyl group, as the substrates react with the electrophilic HAT reagent CumO˙. The increased values of H-donating ability ΔΔG≠o caused by the introduction of a methyl group to the nitrogen atom in each five-membered-ring series follow the order of 2-oxazolidinones (ΔΔG≠o = 0.48 kcal mol−1) > 2-pyrrolidones (0.41 kcal mol−1) > 2-imidazolidinones (0.74/2 = 0.37 kcal mol−1) > pyrrolidines (0.26 kcal mol−1).
Effect of substituent attached to nitrogen atom on H-donating ability
The substituent attached to the nitrogen atom also affects the H-donating ability of the ɑ-C(sp3)–H bonds in XH. As shows in Scheme 4, the ΔG≠o(XH) values of the six N-substituted 2-pyrrolidone series is listed in order. The scale of ΔG≠o(XH) is 0.52 kcal mol−1. The H-donating abilities of these six substrates follow the order of –C2H5 > –CH3 > –Cy > –CH2Ph > –H > –Ph. It can be seen that when the substituent attached to the nitrogen atom is alkyl, cycloalkyl or benzyl group, the H-donating ability of 2-pyrrolidone is enhanced, whereas when the substituent attached to the nitrogen atom is a phenyl group, the H-donating ability is weakened. Given that the alkyl, cycloalkyl and benzyl groups are electron-donating substituents, they are more conducive to the stability of the transition state (see Scheme 5) when the substrate reacts with the electrophilic HAT reagent CumO˙, and thus these substituents enhance the H-donating abilities of the substrates. The phenyl group is conjugated with the lone pair electrons of the nitrogen atom and exhibits an electron-withdrawing conjugation effect, and thus it is not conducive to the stability of the transition state, weaking the H-donating ability of 7H.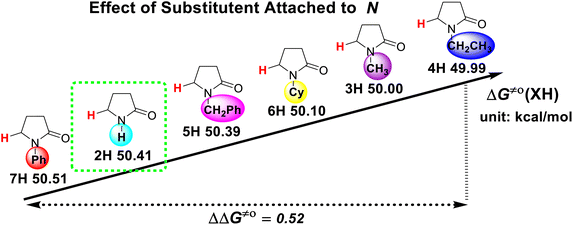 | ||
| Scheme 4 Effect of substituent attached to nitrogen atom on ΔG≠o(XH) of ɑ-C(sp3)–H bonds in 2-pyrrolidone series in MeCN at 298 K, with units of kcal mol−1. | ||
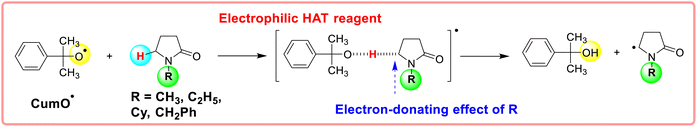 | ||
| Scheme 5 Electron-donating effect of substituents (alkyl and cycloalkyl groups) on the stability of transition state when the substrate reacts with the electrophilic HAT reagent CumO˙. | ||
Effect of ring size on H-donating ability
The effect of the ring size on the H-donating ability of the ɑ-C(sp3)–H bond in lactams and cycloalkanes is also discussed. As can be seen in Scheme 6, in the cycloalkane series, the H-donating ability increases with an increase in the carbon ring size. Comparing cycloalkanes and lactams with the same ring size, it is interesting to find that the H-donating abilities of the five-membered and six-membered cycloalkanes are weaker than that of the corresponding lactams, while the seven-membered and eight-membered cycloalkanes are stronger than that of the corresponding lactams. Meanwhile, in the N–H lactam series, the H-donating ability does not increase with an increase in the ring size like in the cycloalkane series, but follows the order of four-membered ring (1H) < eight-membered ring (12H) < seven-membered ring (10H) < five-membered ring (2H) < six-membered ring (8H). However, the H-donating abilities of the N-methyl lactam series increase with a decrease in the ring size.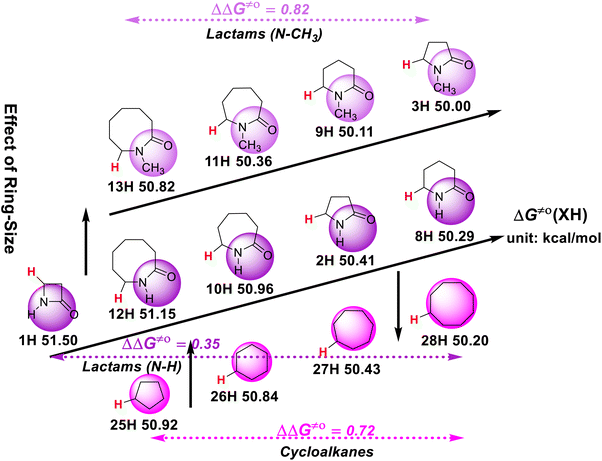 | ||
| Scheme 6 Effect of ring-size on ΔG≠o(XH) of ɑ-C(sp3)–H bonds in lactams and cycloalkanes in MeCN at 298 K, with units of kcal mol−1. | ||
To explore the reasons for the effect of ring size on H-donating ability in the different series, the thermodynamic bond dissociation free energy ΔGo(XH), kinetic intrinsic resistance energy ΔG≠XH/X and thermo–kinetic parameter ΔG≠o(XH) of these three series are listed in Table 2. According to the definition of ΔG≠o(XH), it consists of 1/2 the sum of ΔGo(XH) and ΔG≠XH/X.13–16 In this work, ΔGo(XH) was calculated using the iBonD HM method15c,16,20–22 to evaluate the thermodynamic H-donating potential of the substrate. ΔG≠XH/X was determined using the definition ΔG≠o(XH) {ΔG≠o(XH) = 1/2[ΔG≠XH/X + ΔGo(XH)]} and employed to evaluate the kinetic H-donating ability of the substrate.13–16
| XH | Structure | ΔG≠o(XH)a (kcal mol−1) | ΔGo(XH)b (kcal mol−1) | ΔG≠XH/Xc (kcal mol−1) |
|---|---|---|---|---|
| a ΔG≠o(XH) values are derived from eqn (1).b ΔGo(XH) values are obtained by the iBonD HM method in this work.15c,16,20–22c ΔG≠XH/X values are derived from ΔG≠o(XH) = 1/2[ΔG≠XH/X + ΔGo(XH)]. | ||||
| 1H | 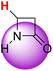 |
51.50 | 91.40 | 11.60 |
| 2H | 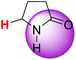 |
50.41 | 86.30 | 14.52 |
| 3H | 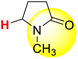 |
50.00 | 85.70 | 14.30 |
| 8H | 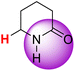 |
50.29 | 85.10 | 15.48 |
| 9H | 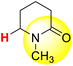 |
50.11 | 85.00 | 15.22 |
| 10H | 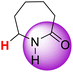 |
50.96 | 87.90 | 14.02 |
| 11H | 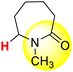 |
50.36 | 85.10 | 15.63 |
| 12H | 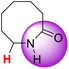 |
51.15 | 88.40 | 13.91 |
| 13H | 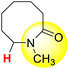 |
50.82 | 89.30 | 12.33 |
| 25H |  |
50.92 | 90.60 | 11.24 |
| 26H |  |
50.84 | 93.60 | 8.07 |
| 27H | 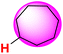 |
50.43 | 89.10 | 11.75 |
| 28H | 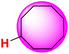 |
50.20 | 91.20 | 9.21 |
The ΔGo(XH) and ΔG≠XH/X values of the ɑ-C(sp3)–H bonds in the lactams and cycloalkanes in MeCN at 298 K are shown in Scheme 7. According to Scheme 7, it can be seen that the reduction of ΔGo(XH) from cyclopentane (25H) to 2-pyrrolidone (2H) (4.30 kcal mol−1) and from cyclohexane (26H) to 2-piperidinone (8H) (8.50 kcal mol−1) caused by the introduction of an amide group fully offsets the increase in ΔG≠XH/X (3.28 kcal mol−1, 7.41 kcal mol−1, respectively), which means that the thermodynamic advantage caused by the introduction of an amide group can fully compensate for the kinetic disadvantage. Therefore, 2-pyrrolidone and 2-piperidinone have stronger H-donating abilities than that of cyclopentane and cyclohexane. However, from cycloheptane (27H) to 2-azepanone (10H) and from cyclooctane (28H) to 2-azocanone (12H), the reduction in ΔGo(XH) caused by the introduction of an amide group (1.20 kcal mol−1, 0.90 kcal mol−1, respectively) is not enough to offset the increase in ΔG≠XH/X (2.27 kcal mol−1, 4.70 kcal mol−1, respectively), which means that the thermodynamic advantage caused by the introduction of an amide group cannot fully compensate for the kinetic disadvantage. Therefore, 2-azepanone and 2-azocanone have weaker H-donating abilities than cycloheptane and cyclooctane. The thermo–kinetic parameter ΔG≠o(XH) of azetidinone (1H) is 51.50 kcal mol−1. The ΔG≠o(1H) value is the largest among the lactam series, which means azetidinone has the weakest H-donating ability. According to Scheme 7, 1H has the biggest ΔGo(XH) value (91.40 kcal mol−1) and smallest ΔG≠XH/X (11.60 kcal mol−1) among the lactam series. Although 1H has the smallest intrinsic resistance energy due to its small structure, its C(sp3)–H bond is the most difficult to break the thermodynamics. This means that 1H has the strongest kinetic H-donating ability and the weakest thermodynamic H-donating potential. Because its kinetic H-donating advantage (4.03 kcal mol−1) is not enough to make up for its thermodynamic H-donating disadvantage (6.40 kcal mol−1), its actual H-donating ability in the HAT reaction is ultimately the weakest among the lactam series.
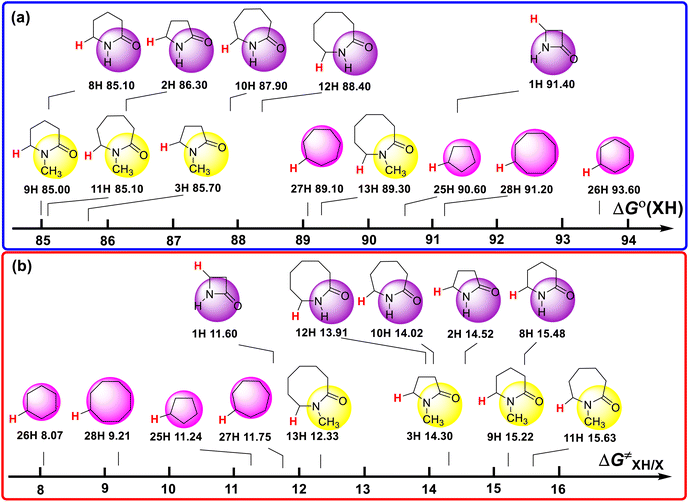 | ||
| Scheme 7 (a) ΔGo(XH) and (b) ΔG≠XH/X values of ɑ-C(sp3)–H bonds in lactams and cycloalkanes in MeCN at 298 K, with units of kcal mol−1. | ||
In the N–H lactam series, the ΔGo(XH) values follow the order of 2-azetidinone (1H) > 2-azocanone (12H) > 2-azepanone (10H) > 2-pyrrolidone (2H) > 2-piperidinone (8H). The scale of ΔGo(XH) is 6.30 kcal mol−1. The ΔG≠XH/X values follow the order of 2-azetidinone (1H) < 2-azocanone (12H) < 2-azepanone (10H) < 2-pyrrolidone (2H) < 2-piperidinone (8H). The scale of ΔG≠XH/X is 3.88 kcal mol−1. The order of the thermodynamic H-donating potentials of these lactams is opposite to the order of their kinetic H-donating abilities. However, the increasing slope of thermodynamic H-donating potential is larger than the decreasing slope of kinetic H-donating ability. Therefore, the order of H-donating abilities of this series in HAT reactions is consistent with the order of the thermodynamic H-donating potentials.
In the N-methyl lactam series, the ΔGo(XH) values follow the order of 1-methyl-2-azocanone (13H) > 1-methyl-2-pyrrolidone (3H) > 1-methyl-2-azepanone (11H) > 1-methyl-2-piperidinone (9H). The ΔG≠XH/X values follow the order of 1-methyl-2-azocanone (13H) < 1-methyl-2-pyrrolidone (3H) < 1-methyl-2-piperidinone (9H) < 1-methyl-2-azepanone (11H). The order of the thermodynamic H-donating potentials of these lactams is not consistent with the order of their kinetic H-donating abilities. The thermodynamic H-donating disadvantage of 13H completely offsets its kinetic H-donating advantage, making its H-donating ability in the HAT reaction weakest among the N-methyl lactam series. Although the thermodynamic H-donating potential and kinetic H-donating ability of 3H are not the strongest in this series, the H-donating ability of 3H in the HAT reaction is the strongest, combining the results of thermodynamics and kinetics. This is why the H-donating abilities of the N-methyl lactam series increase with a decrease in the ring size.
Conclusions
Herein, the H-donating abilities of 28 nitrogen-containing heterocycles, oxygen-containing heterocycles, alicyclic amines and cycloalkanes, which were denoted as XH, in HAT reactions were researched by using the characteristic physical parameter of XH, i.e., the thermo–kinetic parameter ΔG≠o(XH). The following conclusions were drawn.(1) For these nitrogen-containing heterocycles, their H-donating abilities follow the order of alicyclic amines (21H–24H) > 2-imidazolidinones (14H–15H) > lactams (1H–13H). The H-donating abilities of two 2-oxazolidinones (16H–17H) are at the downstream level of the studied lactams. The introduction of a nitrogen atom on the ring increases the H-donating ability of the substrate, while the introduction of an oxygen atom or carbonyl group (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) decreases the H-donating ability.
O) decreases the H-donating ability.
(2) The introduction of a methyl group to the nitrogen atom enhances the H-donating abilities of these heterocycles. In addition, when the substituent attached to the nitrogen atom is an electron-donating group, such as alkyl, cycloalkyl or benzyl group, the H-donating ability is enhanced, whereas when the substituent attached to the nitrogen atom is an electron-withdrawing group, such as a phenyl group, the H-donating ability is weakened.
(3) In the cycloalkane series, the H-donating ability increases with an increase in the carbon ring size. In the N–H lactam series, the H-donating ability does not increase with an increase in the ring size like in the cycloalkane series, but follows the order of four-membered ring (1H) < eight-membered ring (12H) < seven-membered ring (10H) < five-membered ring (2H) < six-membered ring (8H). The H-donating abilities of the N-methyl lactam series increase with a decrease in the ring-size. The thermodynamic bond dissociation free energy ΔGo(XH) and kinetic intrinsic resistance energy ΔG≠XH/X were determined to explain these phenomena.
Experimental
Kinetic measurements
The kinetics of the HAT reactions from the C(sp3)–H bonds of alkanes and alkane derivatives to CumO˙ radical in acetonitrile at 298 K were quoted from the literature.5–10 LFP experiments were carried out with a laser kinetic spectrometer using the third harmonic (355 nm) of a Q-switched Nd:YAG laser, delivering 8 ns pulses. Argon- or nitrogen-saturated acetonitrile solution of dicumyl peroxide (1.0 M) was employed. The kinetics of the HAT reactions were conveniently monitored using an Applied Photophysics SX.18MV-R stopped-flow system (a brief introduction of the equipment is shown in the ESI†), which was thermostated at 298 K under strict anaerobic conditions in dry acetonitrile. The method of the kinetic measurement was pseudo-first-order method. The concentration of the substrate was maintained at more than 20-fold excess of the radical to attain pseudo-first-order condition. The second-order rate constants (k2) were derived from the plots of the pseudo-first-order rate constants versus the concentrations of the excessive reactants. In each case, it was confirmed that the rate constants derived from three to five independent measurements agreed within an experimental error of ±5%.Thermodynamic measurements
The bond dissociation free energies ΔGo(XH) were determined using the iBonD HM prediction methods: Light GBM and SPOC descriptors with RMSE = 1.82, r2 = 0.980 and Mean Absolute Errors (MAE) = 1.03 (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 train test split).15c,16,20–22
5 train test split).15c,16,20–22
Conflicts of interest
The authors declare no competing financial interests.Acknowledgements
Financial support from PhD Research Startup Foundation of Anyang Institute of Technology (BSJ2019032), the Cross-Regional R&D Cooperation Project of Henan Provincial Central Guiding Local Science and Technology Development (Z20221343016) and the Major Science and Technology Project (2022A02GX008) supported by the government of Anyang city.References
- (a) M. Chen, F. Liu and P. Dong, Angew. Chem., Int. Ed., 2018, 57, 3815–3819 CrossRef CAS PubMed; (b) R. W. Sabnis, ACS Med. Chem. Lett., 2022, 13, 1556–1557 CrossRef CAS PubMed.
- (a) S. P. Swain and S. Mohanty, ChemMedChem, 2019, 14, 291–302 CrossRef CAS PubMed; (b) Q. Li, X. Fang, R. Pan, H. Yao and A. Lin, J. Am. Chem. Soc., 2022, 144(15), 11364–11376 CrossRef CAS PubMed.
- (a) H. Lu, H. Wang, H. Zhao and D. Zhang, Future Med. Chem., 2022, 14(15), 1149–1165 CrossRef CAS PubMed; (b) G. F. S. Fernandes, C. B. Scarim, S.-H. Kim, J. Wu and D. Castagnolo, RSC Med. Chem., 2023, 14, 823–847 RSC.
- (a) W. Jiang and Z. Wang, J. Am. Chem. Soc., 2022, 144, 14976–14991 CrossRef CAS PubMed; (b) M. Bhunia, C. Sandoval-Pauker, M. G. Jafari, L. N. Grant, M. R. Gau, B. Pinter and D. J. Mindiola, Angew. Chem., Int. Ed., 2022, 61, e202209122 CrossRef CAS PubMed.
- M. Galeotti, C. Trasatti, S. Sisti, M. Salamone and M. Bietti, J. Org. Chem., 2022, 87, 7456–7463 CrossRef CAS PubMed.
- M. Salamone, R. Martella and M. Bietti, J. Org. Chem., 2012, 77, 8556–8856 CrossRef CAS PubMed.
- M. Salamone, V. B. Ortega and M. Bietti, J. Org. Chem., 2015, 80, 4710–4715 CrossRef CAS PubMed.
- M. Bietti, R. Martella and M. Salamone, Org. Lett., 2011, 13, 6110–6113 CrossRef CAS PubMed.
- M. Salamone, M. Galeotti, E. Romero-Montalvo, J. A. v. Santen, B. D. Groff, J. M. Mayer, G. A. DiLabio and M. Bietti, J. Am. Chem. Soc., 2021, 143, 11759–11776 CrossRef CAS PubMed.
- M. Salamone, G. Carboni and M. Bietti, J. Org. Chem., 2016, 81, 9269–9278 CrossRef CAS PubMed.
- (a) X. Han, W. Hao, X.-Q. Zhu and V. D. Parker, J. Org. Chem., 2012, 77, 6520–6529 CrossRef CAS PubMed; (b) X.-Q. Zhu, M.-T. Zhang, A. Yu, C.-H. Wang and J.-P. Cheng, J. Am. Chem. Soc., 2008, 130, 2501–2516 CrossRef CAS PubMed; (c) N.-P. Lei, Y.-H. Fu and X.-Q. Zhu, Org. Biomol. Chem., 2015, 13, 11472–11485 RSC.
- (a) G.-B. Shen, Y.-H. Fu and X.-Q. Zhu, J. Org. Chem., 2020, 85, 12535–12543 CrossRef CAS PubMed; (b) B.-C. Qian, L. Zhang, G.-S. Zhang, Y.-H. Fu, X.-Q. Zhu and G.-B. Shen, ChemistrySelect, 2022, 7, e202204144 CrossRef CAS.
- Y.-H. Fu, G.-B. Shen, Y. Li, L. Yuan, J.-L. Li, L. Li, A.-K. Fu, J.-T. Chen, B.-L. Chen, L. Zhu and X.-Q. Zhu, ChemistrySelect, 2017, 2, 904–925 CrossRef CAS.
- (a) Y.-H. Fu, K. Wang, G.-B. Shen and X.-Q. Zhu, J. Phys. Org. Chem., 2022, 35, e4358 CrossRef CAS; (b) Y.-H. Fu, Z. Wang, Y. Zhang, G.-B. Shen and X.-Q. Zhu, ChemistrySelect, 2022, 7, e202202625 CAS; (c) Y.-H. Fu, C. Geng, G.-B. Shen, K. Wang and X.-Q. Zhu, ACS Omega, 2022, 7, 26416–26424 CrossRef CAS PubMed.
- (a) Y.-H. Fu, G.-B. Shen, K. Wang and X.-Q. Zhu, ChemistrySelect, 2021, 6, 8007–8010 CrossRef CAS; (b) Y.-H. Fu, Z. Wang, K. Wang, G.-B. Shen and X.-Q. Zhu, RSC Adv., 2022, 12, 27389–27395 RSC; (c) Y.-H. Fu, Y. Zhang, F. Wang, L. Zhao, G.-B. Shen and X.-Q. Zhu, RSC Adv., 2023, 13, 3295–3305 RSC.
- Y.-H. Fu, F. Wang, L. Zhao, Y. Zhang, G.-B. Shen and X.-Q. Zhu, ChemistrySelect, 2023, 8, e202204789 CrossRef CAS.
- Y.-H. Fu, G.-B. Shen, K. Wang and X.-Q. Zhu, ACS Omega, 2022, 7, 25555–25564 CrossRef CAS PubMed.
- X.-Q. Zhu, F.-H. Deng, J.-D. Yang, X.-T. Li, Q. Chen, N.-P. Lei, F.-K. Meng, X.-P. Zhao, S.-H. Han, E.-J. Hao and Y.-Y. Mu, Org. Biomol. Chem., 2013, 11, 6071–6089 RSC.
- J.-Y. Zhang, L.-L. Wang and X.-Q. Zhu, ACS Phys. Chem. Au, 2023 DOI:10.1021/acsphyschemau.3c00001.
- http://pka.luoszgroup.com/bde_prediction.
- Q. Yang, Y. Li, J. D. Yang, Y. Liu, L. Zhang, S. Luo and J. P. Cheng, Angew. Chem., Int. Ed., 2020, 59, 19282–19291 CrossRef CAS PubMed.
- http://ibond.nankai.edu.cn/.
Footnote |
| † Electronic supplementary information (ESI) available: The second-order rate constants k2 and activation free energies ΔG≠XH/Y of HAT reactions from nitrogen-containing heterocycles, oxygen-containing heterocycle, alicyclic amines and cycloalkanes XH to CumO˙ radical in acetonitrile at 298 K, and the brief introduction of Applied Photophysics SX.18MV-R stopped-flow were shown. See DOI: https://doi.org/10.1039/d3ra02211k |
| This journal is © The Royal Society of Chemistry 2023 |