Open Access Article
Open Access ArticleSynthesis of sulfur-containing benzo[b]pyrrolo[2,1-c][1,4]oxazine-3,9-diones: blue light promoted radical cyclization process†
Feng Pan‡
ac,
Haohu Li‡a,
Xiaohua Wang‡d,
Liwen Luoa,
Yanfei Lin*b,
Qingkai Yua,
Wenlin Xie*c and
Lianpeng Zhang *a
*a
aYunnan Provincial Key Laboratory of Wood Adhesives and Glued Products, Southwest Forestry University, Kunming 650224, Yunnan, China. E-mail: lpz@zju.edu.cn
bCollege of Biological, Chemical Sciences and Engineering, Jiaxing University, Jiaxing 314001, Zhejiang, China. E-mail: 11219008@zju.edu.cn
cSchool of Chemistry and Chemical Engineering, Hunan University of Science and Technology, Xiangtan 411201, Hunan, China. E-mail: xwl2000zsu@163.com
dTongji Zhejiang College, Jiaxing 314051, China
First published on 15th May 2023
Abstract
The selective and controllable construction of spio-tricyclic skeletons through visible light promoted radical cyclization still remains challenging. Herein, a general and convenient protocol for the blue light-promoted radical-mediated cascade spiro-cyclization/Michael addition of N-arylpropiolamides with thiophenols under metal-free conditions was developed. In this protocol, commercially available hydrochloric acid was employed as the cheap promoter and air as the sustainable oxidant. In addition, many functional groups tolerate the reaction conditions and produce a series of sulfur-containing benzo[b]pyrrolo[2,1-c][1,4]oxazine-3,9-diones.
Introduction
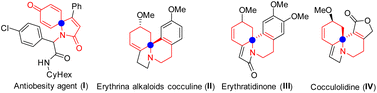
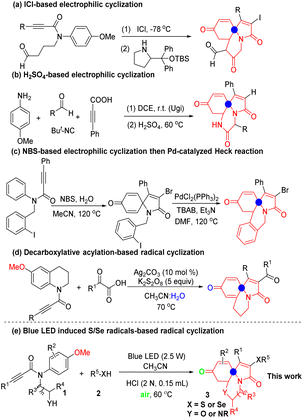
It is well known that spirocyclic compounds can be used in photochromism,1 organic optoelectronics,2 and medicinal chemistry,3 and have also played an important role in organic synthesis.4 Antiobesity agent (I) is a selective histamine-3 receptor (H3R) modulator5 (Scheme 1). Among the spirocyclic compounds, N-containing spiro-products show important bioactivity as anticancer, antimicrobial and anti-inflammatory agents.6 In recent years, the Erythrina alkaloid cocculine II has attracted considerable attention because of its wide range of biological properties.7 Furthermore, some biologically active spirocycles with hypnotic, hypotensive, and CNS activity are found in numerous active molecules, such as the erythrina, aspidosperma, and strychnos families.8Because of its unique properties, tremendous efforts have been made to construct the spirotricyclic derivatives in the past ten years.9 Among them, the dearomatization of arenes is an effective method for the synthesis of spiro compounds. In 2011, a catalytic enantioselective method for synthesizing fused spiro-tricyclic compounds has been developed. In this reaction, Gaunt and co-workers10 developed ICl-based electrophilic dearomatization of anisidine derivatives (Scheme 2a). In 2016, Srivastava and the co-workers reported a diastereo- and regioselective Ugi/ipso-cyclization/aza-michael cascade reaction to the synthesis of alkaloid-mimicking tricyclic skeletons11 (Scheme 2b). In the reaction, the use of H2SO4 played an important role in the dearomative cyclization.
On the other hand, transition metal promoted dearomatization has become one of the most popular reaction models with great achievements. In 2012, Li developed a general method for the synthesis of spiro[4,5]trienones by the intramolecular ipso-halocyclization of 4-(p-unsubstituted-aryl)-1-alkynes12 (Scheme 2c). Subsequently, the azaquaternary tricyclic skeletons could be obtained via Pd-catalyzed intramolecular Heck reaction. In 2020, Reddy and the co-workers reported a Ag-catalyzed oxidative ipso-cyclization via decarboxylative acylation/alkylation to construct pyrrolo-[2,1-j]-quinolone13 (Scheme 2d), which was a challenging framework found in tricyclic marine alkaloids.14
It is worth noting that Qiu and co-workers have paid more attention to the green and mild construction of spio-tricyclic skeletons over the past years.15 Considering that our group's main research interest is the controllable synthesis of nitrogen-containing heterocycles based on the selective functionalization of alkynes.16 So, in this paper, we would like to share a blue light-promoted17 radical-mediated spiro-tricyclization of N-arylpropiolamides with thio(sele)phenols under metal-free conditions (Scheme 2e).
Results and discussion
At the initial stage of the investigations, the synthesized N-(2-hydroxyethyl)-N-(4-methoxyphenyl)-3-phenylpropiolamide 1a and the purchased 4-methylbenzenethiol 2a were chosen as model substrates to optimize the reaction conditions using blue LED as the light source (Table 1). Firstly, the influence of the blue LED power on the reaction is explored and 2.5 W was the most effective light power among the tested 1.5 W, 2.5 W, and 3.5 W (entries 1–3). To our delight, when the reaction temperature was raised to 50 °C, the expected product 1-phenyl-2-(p-tolylthio)-5,6,7a,8-tetrahydro-3H,9H-benzo[b]pyrrolo[2,1-c][1,4]oxazine-3,9-dione 3a was obtained in 47% yield (entry 4, Table 1).| Entry | Blue LDE (x (W)) | Solvent | Additive (y (mL)) | T (°C) | Yield 3a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b (%) b (%) |
|---|---|---|---|---|---|
| a Standard conditions: 1a (0.2 mmol), 2a (0.4 mmol), air, in 2 mL of CH3CN were stirred under blue LED, 12 h.b Isolated yield based on 1a.c 2a (1.5 equiv) was used.d 2a (2.5 equiv) was used.e 2a (1.0 equiv) was used. | |||||
| 1 | 1.5 | MeCN | — | r.t. | 33 |
| 2 | 2.5 | MeCN | — | r.t. | 36 |
| 3 | 3.5 | MeCN | — | r.t. | 31 |
| 4 | 2.5 | MeCN | — | 50 | 47 |
| 5 | 2.5 | MeCN | — | 60 | 56 |
| 6 | 2.5 | MeCN | — | 70 | 49 |
| 7c | 2.5 | MeCN | — | 60 | 62 |
| 8d | 2.5 | MeCN | — | 60 | 40 |
| 9e | 2.5 | MeCN | — | 60 | 43 |
| 11c | 2.5 | MeOH | — | 60 | 16 |
| 12c | 2.5 | DMF | — | 60 | 12 |
| 13c | 2.5 | THF | — | 60 | 47 |
| 14c | 2.5 | Toluene | — | 60 | 51 |
| 15c | 2.5 | MeCN | 1.1 N HCl (0.15) | 60 | 68 |
| 16c | 2.5 | MeCN | 2.0 N HCl (0.15) | 60 | 76 |
| 17c | 2.5 | MeCN | 3.0 N HCl (0.15) | 60 | 60 |
| 18c | 2.5 | MeCN | 2.0 N HCl (0.10) | 60 | 65 |
| 19c | 2.5 | MeCN | 2.0 N HCl (0.20) | 60 | 67 |
| 20c | 2.5 | MeCN | 2.0 N HCl (0.25) | 60 | 70 |
| 21c | 2.5 | MeCN | NaCl (1.0 equiv) | 60 | 35 |
| 22c | 2.5 | MeCN | 2.0 N HCl (0.15) | r.t. | 47 |
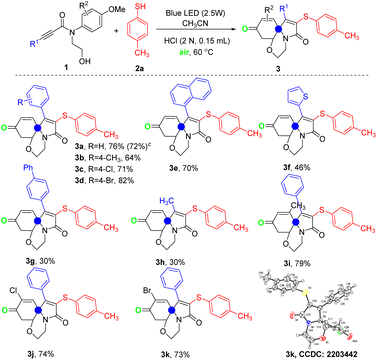
Further, the reaction temperature was increased to 60 °C and 70 °C, and it was found that the optimum temperature of this reaction was 60 °C with the desired product 3a obtained in 56% yield (entries 4–6). In order to further optimize the reaction results, the effect of the amount of 2a on the reaction was evaluated. As shown in entries 7−9, a better result was obtained by using 1.5 equiv of 2a, giving the desired product 3a in 62% yield. Changing the reaction solvent to MeOH, DMF, THF, or toluene has no good effect on the reaction (entries 11–14). When adding 0.15 mL 1.1 N HCl to this reaction, a 68% yield was achieved (entry 15, Table 1). Encouraged by this result, the effect of the additive HCl to this reaction was further explored. Replacing 1.1 N HCl to 2.0 N HCl or 3.0 N HCl, 2.0 N HCl was the optimal choice, leading to the desired product 3a in 76% yield (entries 15−17, Table 1). In addition, increasing or decreasing the amount of 2.0 N HCl led to a decrease in the 3a yield (entries 18–20, Table 1). When NaCl was used as the chlorine radical source, there was no significant increase in the yield of product 3a (entry 21). Next, with the addition of 0.15 mL of 2.0 N HCl, the reaction temperature was lowered to room temperature and the yield was found only 45% (entry 22). Finally, the standard reaction conditions were established as follows: a mixture of 1a (0.2 mmol), 2a (0.3 mmol), and 0.15 mL 2.0 N HCl in 2 mL MeCN were stirred under 2.5 W blue LED at 60 °C in air.
With the optimal conditions established, the general utilization of the photomediated spiro-tricyclization of N-hydroxylethyl-N-arylpropiolamides with thio(sele)phenols was probed. As shown in Table 2, diverse N-arylpropionamides with different substituents in the alkynyl moiety were all tolerated, and the yields of the corresponding products 3a–d were satisfactory (64−82% yields). In addition, the 1.0 mmol experiment of 1a was carried out under standard conditions to obtain the desired product 3a in 72% yield. Furthermore, the substituent R1 could be replaced by 1-naphthyl, 2-thiophenyl, or 1,1′-biphenyl and the corresponding products 3e, 3f, and 3g were observed in 70%, 46%, and 30% yields, respectively. Unfortunately, when R1 is methyl, the corresponding product 3h was obtained with only 30% yield. Next, the influence of phenyl substituents R2 on the reaction of N-arylpropamides was studied. It can be seen from the results that substrates containing methyl, chlorine, or bromine substituted could react smoothly and give the corresponding products 3i–k in good yields. The structure of compound 3k (CCDC: 2203442†) was identified by X-ray single-crystal diffraction (see the ESI† for details).
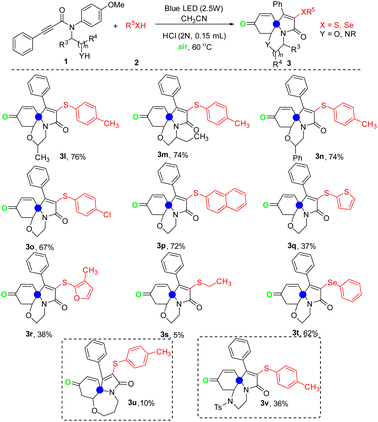
In order to extend the universality of the reaction substrates, the substrate with secondary alcohol or other primary alcohol was synthesized as the starting material (Table 3). The results showed that the substrate with secondary alcohol or other primary alcohol was also compatible for the reaction, leading to the corresponding products 3l, 3m, and 3n in 76%, 74%, and 74% yields, respectively. Next, the influence of different thioalcohols on the reaction was investigated. Using 4-chlorobenzenethiol or naphthalene-2-thiol as the reaction substrate, the reaction proceeded smoothly and the corresponding products 3o and 3p were obtained in good yields. Unfortunately, using heterocyclic mercaptan and ethanethiol as the reaction substrates, the corresponding products could only be obtained in low yields of 5–38%. Happily, when phenylselenol was used as the substrate, the corresponding product 3t was obtained in 62% yield under standard conditions. Notably, the reaction using the substrate with 3-propanol was retarded, leading to the corresponding product 3u in only 10% yield. Finally, changing the primary alcohol to Ts protected amine, the reaction was still going on smoothly and the expected product 3v was achieved in 36% yield.
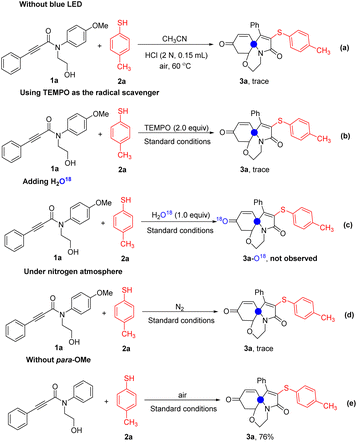
In order to shed light on the mechanism of this reaction, some control experiments were carried out (Scheme 3). First of all, the reaction could not be carried out normally without the blue LED (Scheme 3a). Next, the expected product 3a could not be detected when 2.0 equiv of TEMPO (2,2,6,6-tetramethyl-1-piperidinyloxy) was added to the reaction system (Scheme 3b). From this result, the reaction probably underwent a radical pathway. Furthermore, when adding 1.0 equiv of H2O18 to the standard conditions, the O18 atom was not observed by LC-MS analysis in the product, which perhaps indicated that the oxygen of carbonyl was not derived from the water (Scheme 3c). Finally, the reaction did not work under N2 atmosphere may be implied that the oxygen of carbonyl group came from the oxygen in the air (Scheme 3d). If the reaction was carried out with N-phenylproiolamides without a para-methoxy substituent on the N-substituted aryl moiety, the desired product 3a was still obtained in 76% yield, which indicated that oxygen of carbonyl was derived from the air (Scheme 3e).
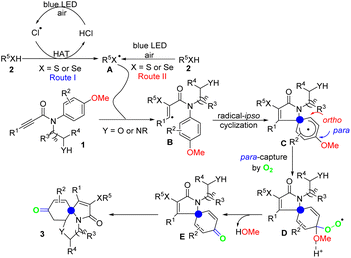
Based on the control experiments aforementioned above and the previous reports,15,18 a plausible mechanism was proposed in Scheme 4. Initially, 1O2 (E = 2.2 V) reacted with Cl− (E = 1.36 V) producing chlorine radical in the presence of blue LED and air.18b Then chlorine radical undergoes HAT with thio(sele)phenol to give sulfur(selenium) radical A (route I).18a Of course, the sulfur(selenium) radical A could also be offered directly by the blue LED and air (route II). Intermediate B was formed through the α-addition of N-arylpropiolamides 1 to sulfur(selenium) radical A. Then, the key intermediate C was provided by radical ipso-cyclization. Next, the intermediate C was captured by molecular oxygen in para-site fashion to offer the intermediate D. Subsequently, under the action of proton acid, the key intermediate E was obtained through electron rearrangement, while releasing a molecule of methanol. When there was no methoxy group present in the reaction substrate, the required product 3a was still obtained in 76% yield (Scheme 3e), indicating that intermediate C could still be captured by molecular oxygen, leading to electron rearrangement to obtain intermediate E. Finally, assisted by the additives, the intermediate E undergoes an intramolecular Michael-addition reaction, thus providing the final products 3.
Conclusions
In conclusion, we have developed a general and convenient protocol for the blue LED-promoted chlorine radical-mediated spiro-tricyclization of N-arylpropiolamides with thiophenols under metal-free conditions. In this reaction, hydrochloric acid is used as the cheap promoter and air as the sustainable oxidant. Many functional groups tolerate the reaction conditions and produce a series of sulfur-containing benzo[b]pyrrolo[2,1-c][1,4]oxazine-3,9-diones with high efficiency and specific selectivity. Preliminary mechanistic studies indicated that the reaction undergoes LED-promoted chlorine radical-mediated spiro-cyclization and intramolecular Michael-addition reaction. Further studies on the visible light induced radical cyclization are currently ongoing.Experimental
General methods and materials
Unless stated otherwise, reactions were conducted in dried glassware. Commercially available reagents and solvents were used as received. 300–400 mesh silica gel was used for flash column chromatography. Visualization on TLC was achieved by the use of UV light (254 nm). 400 MHz and 100 MHz were used to record the 1H NMR and 13C NMR spectra. Chemical shifts (δ ppm) were reported in parts per million, referring to either the internal standard of TMS or the residue of the deuterated solvents. The splitting pattern was described as follows: s for singlet, d for doublet, t for triplet, q for quartet, and m for multiplet. Coupling constants were reported in Hz. The high-resolution mass spectrum (HRMS) was performed on waters Xevo G2-S QTof mass spectrometer. The crystal of 3k was measured on an Agilent Technologies Gemini single-crystal diffractometer and the solvent system for crystal growth is dichloromethane and petroleum ether.Typical procedure for the synthesis of compound 3a
To a test tube were added N-(2-hydroxyethyl)-N-(4-methoxyphenyl)-3-phenylpropiolamide 1a (0.20 mmol), 4-methylbenzenethiol 2a (0.30 mmol, 1.50 equiv), CH3CN (2.00 mL), and then HCl (0.15 mL, 2.00 mol L−1) was added to the mixture. Then the mixture was stirred under air at 60 °C under 2.5 W blue LED irradiation for 12 hours until the complete consumption of the substrate. The mixture was treated with saturated NaHCO3 (2.0 mL) and extracted with EA (2.0 mL × 3). The combined organic layer was dried with anhydrous Na2SO4. The solvent was removed under vacuum and the residue was purified by silica gel chromatography, using a mixture of petroleum ether/ethyl acetate to give the desired product 3a.Characterization data
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is supported by the Natural Science Foundation of China (22161043 and 21801096), Yunnan Fundamental Research Project (202201AU070071 and 202301AT070224), “High-level Talent Introduction Program” Project of Yunnan Province (YNQR-QNRC-2019-065), Department of Education of Zhejiang Province (Y202147909), the Scientific Research Foundation of Hunan Provincial Education Department (22A0348), and the Start up Funding of Southwest Forestry University (112126), also Supported by the 111 Project (D21027).Notes and references
- For selected examples, see: (a) C. Tonnelé and F. Castet, Photochem. Photobiol. Sci., 2019, 18, 2759 CrossRef PubMed; (b) T. H.-C. Fung, C.-L. Wong, W.-K. Tang, M.-Y. Leung, K.-H. Low and V. W.-W. Yam, Chem. Commun., 2022, 58, 4231 RSC; (c) J. Liu, A. K.-W. Chan, M. Ng, E. Y.-H. Hong, N. M.-W. Wu, L. Wu and V. W.-W. Yam, Organometallics, 2019, 38, 3542 CrossRef CAS; (d) A. V. Chernyshev, N. A. Voloshin, I. A. Rostovtseva, O. P. Demidov, K. E. Shepelenko, E. V. Solov'eva, E. B. Gaeva and A. V. Metelitsa, Dyes Pigm., 2020, 178, 108337 CrossRef CAS; (e) J. Karpiuk, P. Gawryś, E. Karpiuk and K. Suwińska, Chem. Commun., 2019, 55, 8414 RSC.
- For selected examples: (a) T. P. I. Saragi, T. Spehr, A. Siebert, T. Fuhrmann-Lieker and J. Salbeck, Chem. Rev., 2007, 107, 1011 CrossRef CAS PubMed; (b) R. O. Al-Kaysi, I. Gallardo and G. Guirado, Molecules, 2008, 13, 1282 CrossRef CAS PubMed; (c) C.-Y. Chank, Y.-C. Wong, M.-Y. Chan, S.-H. Cheung, S.-K. So and V. W.-W. Yam, ACS Appl. Mater. Interfaces, 2016, 8, 24782 CrossRef PubMed; (d) Q. Wang, X. Jiao, C. Liu, K. Huang, S. He, L. Zhao and X. Zeng, Org. Biomol. Chem., 2018, 16, 7609 RSC.
- (a) Y.-J. Zheng and C. M. Tice, Expert Opin. Drug Discovery, 2016, 11, 831 CrossRef PubMed; (b) K. Hiesinger, D. Daŕin, E. Proschak and M. Krasavin, J. Med. Chem., 2021, 64, 150 CrossRef CAS PubMed; (c) M. Benabdallah, O. Talhi, F. Nouali, N. Choukchou-Braham, K. Bachari and A. M. S. Silva, Curr. Med. Chem., 2018, 25, 3748 CrossRef CAS PubMed; (d) A. A. Kirichok, I. O. Shton, I. M. Pishel, S. A. Zozulya, P. O. Borysko, V. Kubyshkin, O. A. Zaporozhets, A. A. Tolmachev and P. K. Mykhailiuk, Chem.–Eur. J., 2018, 24, 5444 CrossRef CAS PubMed.
- (a) R. Saruengkhanphasit, D. Collier and I. Coldham, J. Org. Chem., 2017, 82, 6489 CrossRef CAS PubMed; (b) M. Luo, R. Yuan, X. Liu, L. Yu and W. Wei, Chem.–Eur. J., 2016, 22, 9797 CrossRef CAS PubMed; (c) Y.-C. Wang, J.-B. Liu, H. W. Zhou, W. Xie, P. Rojsitthisak and G. Qiu, J. Org. Chem., 2020, 85, 1906 CrossRef CAS PubMed; (d) N. Lv, Y. Liu, C. Xiong, Z. Liu and Y. Zhang, Org. Lett., 2017, 19, 4640 CrossRef CAS PubMed; (e) A. Ren, B. Lang, J. Lin, P. Lu and Y. Wang, J. Org. Chem., 2017, 82, 10953 CrossRef CAS PubMed; (f) Y. Yu, Y. Chen, W. Huang, W. Wu and H. Jiang, J. Org. Chem., 2017, 82, 9479 CrossRef CAS PubMed.
- (a) A. Ghoshal, A. Kumar, D. Yugandhar, C. Sona, S. Kuriakose, K. Nagesh, M. Rashid, S. K. Singh, M. Wahajuddin, P. N. Yadav and A. K. Srivastava, Eur. J. Med. Chem., 2018, 152, 148 CrossRef CAS PubMed; (b) T. Honda and H. Shigehisa, Org. Lett., 2006, 8, 657 CrossRef CAS PubMed; (c) H. Shigehisa, J. Takayama and T. Honda, Tetrahedron Lett., 2006, 47, 7301 CrossRef CAS.
- (a) Y.-T. Yang, J.-F. Zhu, G. Liao, H.-J. Xu and B. Yu, Curr. Med. Chem., 2018, 25, 2233 CrossRef CAS PubMed; (b) E. Rajaranendar, K. Ramakrishna, K. Govardhan Reddy, D. Nagaraju and N. Y. Reddy, Bioorg. Med. Chem. Lett., 2013, 23, 3954 CrossRef PubMed; (c) G. Bhaskar, Y. Arun, C. Balachandran, C. Salikumar and P. T. Perumal, Eur. J. Med. Chem., 2012, 51, 79 CrossRef CAS PubMed; (d) Y. Arun, G. Bhaskar, C. Balachandran, S. Ignachimuthu and P. T. Perumal, Bioorg. Med. Chem. Lett., 2013, 23, 1839 CrossRef CAS PubMed.
- A. F. Parsons and D. A. J. Williams, Tetrahedron, 2000, 56, 7217 CrossRef CAS.
- (a) K. Sakamoto, E. Tsujii, F. Abe, T. Nakanishi, M. Yamashita, N. Shigematsu, S. Izumi and M. Okuhara, J. Antibiot., 1996, 49, 37 CrossRef CAS PubMed; (b) A. J. Blackman, C. Li, D. C. R. Hockless, B. W. Skelton and A. H. White, Tetrahedron, 1993, 49, 8645 CrossRef CAS; (c) J. Y. Cha, Y. Huang and T. R. R. Pettus, Angew. Chem., Int. Ed., 2009, 48, 9519 CrossRef CAS PubMed.
- (a) C. R. Reddy, D. H. Kolgave, U. Ajaykumar and R. Ramesh, Org. Biomol. Chem., 2022, 20, 6879 RSC; (b) D. Yugandhar and A. K. Srivastava, ACS Comb. Sci., 2015, 17, 474 CrossRef CAS PubMed; (c) Y. He, L. Song, C. Liu, D. Wu, Z. Li, L. V. Meervelt and E. V. V. Eycken, J. Org. Chem., 2020, 85, 15092 CrossRef CAS PubMed; (d) X.-H. Ouyang, R.-J. Song, Y. Li, B. Liu and J.-H. Li, J. Org. Chem., 2014, 79, 4582 CrossRef CAS PubMed; (e) W.-T. Wei, R.-J. Song, X.-H. Ouyang, Y. Li, H.-B. Li and J.-H. Li, Org. Chem. Front., 2014, 1, 484 RSC; (f) X.-H. Yang, X.-H. Ouyang, W.-T. Wei, R.-J. Song and J.-H. Li, Adv. Synth. Catal., 2015, 357, 1161 CrossRef CAS; (g) X.-H. Ouyang, R.-J. Song, B. Liu and J.-H. Li, Chem. Commun., 2016, 52, 2573 RSC; (h) M. Li, R.-J. Song and J.-H. Li, Chin. J. Chem., 2017, 35, 299 CrossRef CAS.
- R. Leon, A. Jawalekar, T. Redert and M. J. Gaunt, Chem. Sci., 2011, 2, 1487 RSC.
- D. Yugandhar, S. Kuriakose, J. B. Nanubolu and A. K. Srivastava, Org. Lett., 2016, 18, 1040 CrossRef CAS PubMed.
- B.-X. Tang, Y.-H. Zhang, R.-J. Song, D.-J. Tang, G.-B. Deng, Z.-Q. Wang, Y.-X. Xie, Y.-Z. Xia and J.-H. Li, J. Org. Chem., 2012, 77, 2837 CrossRef CAS PubMed.
- C. R. Reddy, D. H. Kolgave, M. Subbarao, M. Aila and S. K. Prajapti, Org. Lett., 2020, 22, 5342 CrossRef CAS PubMed.
- E. Vessally, M. Babazadeh, K. Didehban, A. Hosseinian and L. Edjlali, Curr. Org. Chem., 2018, 22, 286 CrossRef CAS.
- For selective examples: (a) Y.-C. Wang, K.-K. Huang, X. Lai, Z. Shi, J.-B. Liu and G. Qiu, Org. Biomol. Chem., 2021, 19, 1940 RSC; (b) S. F. Ren, Y.-C. Wang, J.-B. Liu and G. Qiu, Chin. J. Org. Chem., 2021, 41, 3652 CrossRef CAS; (c) Y.-C. Wang, J.-B. Liu, G. Qiu, Y. Yang and H. Zhou, Chin. J. Org. Chem., 2021, 41, 4798 CrossRef CAS; (d) F. Pan, H. Xie, W. Xie, Y.-C. Wang, J.-B. Liu and G. Qiu, Synthesis, 2022, 54, 3105 CrossRef CAS; (e) S. Guo, Z. Zhang, Y. Zhu, Z. Wei, X. Zhang and X. Fan, Org. Chem. Front., 2022, 9, 6598 RSC; (f) K. Huang, J.-N. Li, G. Qiu, W. Xie and J.-B. Liu, RSC Adv., 2019, 9, 33460 RSC; (g) G. Qiu, Z.-F. Chen, W. Xie and H. Zhou, Eur. J. Org. Chem., 2019, 2019, 4327 CrossRef CAS.
- (a) Y. Zhang, T. Liu, L. Liu, H. Guo, H. Zeng, W. Bi, G. Qiu, W. Gao, X. Ran, L. Yang, G. Du and L. Zhang, J. Org. Chem., 2022, 87, 8515 CrossRef CAS PubMed; (b) Y. Zhang, W. Lai, L. Zhang, X. Gao, G. Qiu and H. Zhou, Org. Biomol. Chem., 2021, 19, 1827 RSC; (c) X. Wang, H. Zeng, W. Zhang, H. Guo, T. Jin, S. Shi, X. Jin, N. Qu, L. Liu and L. Zhang, Org. Biomol. Chem., 2022, 20, 7949 RSC; (d) H. Diao, L. Liu, J. Wang, Y. Lin, X. Zhao, H. Zeng, S. Shi, W. Gao, L. Yang, G. Du and L. Zhang, Eur. J. Org. Chem., 2022, 2022, e202200811 CrossRef CAS.
- (a) F.-L. Zeng, X.-L. Chen, K. Sun, H.-L. Zhu, X.-Y. Yuan, Y. Liu, L.-B. Qu, Y.-F. Zhao and B. Yu, Org. Chem. Front., 2021, 8, 760 RSC; (b) W.-C. Yang, M.-M. Zhang and J.-G. Feng, Adv. Synth. Catal., 2020, 362, 4446 CrossRef CAS; (c) C.-H. Ma, L. Zhao, X. He, Y.-Q. Jiang and B. Yu, Org. Chem. Front., 2022, 9, 1445 RSC.
- (a) L. Ding, K. Niu, Y. Liu and Q. Wang, ChemSusChem, 2022, 15, e202200367 CrossRef CAS PubMed; (b) K. Niu, X. Shi, L. Ding, Y. Liu, H. Song and Q. Wang, ChemSusChem, 2022, 15, e202102326 CrossRef CAS PubMed; (c) M. Yang, J. Hua, H. Wang, T. Ma, C. Liu, W. He, N. Zhu, Y. Hu, Z. Fang and K. Guo, J. Org. Chem., 2022, 87, 8445 CrossRef CAS PubMed; (d) T. Liu, Y. Li, L. Jiang, J. Wang, K. Jin, R. Zhang and C. Duan, Org. Biomol. Chem., 2020, 18, 1933 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2203442. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3ra02247a |
| ‡ Equal contributions to this manuscript. |
| This journal is © The Royal Society of Chemistry 2023 |