Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Sequestration of steroidal estrogen in aqueous samples using an adsorption mechanism: a systemic scientometric review†
Ajibola A. Bayode
 *a,
Chijioke Olisah
b,
Stephen Sunday Emmanuel
*a,
Chijioke Olisah
b,
Stephen Sunday Emmanuel
 c,
Morenike Oluwabunmi Adesina
c,
Morenike Oluwabunmi Adesina
 d and
Daniel Terlanga Koko
d and
Daniel Terlanga Koko
 a
a
aDepartment of Chemical Sciences, Faculty of Natural Sciences, Redeemer's University, P.M.B. 230, 232101, Ede, Nigeria. E-mail: bayodea@run.edu.ng
bInstitute for Coastal and Marine Research, Nelson Mandela University, P. O Box 77000, Gqeberha 6031, South Africa
cDepartment of Industrial Chemistry, Faculty of Physical Sciences, University of Ilorin, P.M.B. 1515, Ilorin, Nigeria
dDepartment of Chemistry, Faculty of Natural and Applied Sciences, Lead City University, Nigeria
First published on 26th July 2023
Abstract
Steroidal estrogens (SEs) remain one of the notable endocrine disrupting chemicals (EDCs) that pose a significant threat to the aquatic environment in this era owing to their interference with the normal metabolic functions of the human body systems. They are currently identified as emerging contaminants of water sources. The sources of SEs are either natural or synthetic active ingredients in oral contraceptive and hormonal replacement therapy drugs and enter the environment primarily from excretes in the form of active free conjugate radicals, resulting in numerous effects on organisms in aquatic habitats and humans. The removal of SEs from water sources is of great importance because of their potential adverse effects on aquatic ecosystems and human health. Adsorption techniques have gained considerable attention as effective methods for the removal of these contaminants. A systemic review and bibliometric analysis of the application of adsorption for sequestration were carried out. Metadata for publications on SE removal utilizing adsorbents were obtained from the Web of Science (WoS) from January 1, 1990, to November 5, 2022 (107 documents) and Scopus databases from January 1, 1949, to November 5, 2022 (77 documents). In total, 137 documents (134 research and 4 review articles) were used to systematically map bibliometric indicators, such as the number of articles, most prolific countries, most productive scholars, and most cited articles, confirming this to be a growing research area. The use of different adsorbents, include activated carbon graphene-based materials, single and multi-walled carbon nanotubes, biochar, zeolite, and nanocomposites. The adsorption mechanism and factors affecting the removal efficiency, such as pH, temperature, initial concentration, contact time and adsorbent properties, were investigated in this review. This review discusses the advantages and limitations of different adsorbents, including their adsorption capacities, regenerative potential, and cost-effectiveness. Recent advances and innovations in adsorption technology, such as functionalized materials and hybrid systems, have also been highlighted. Overall, the bibliographic analysis provides a comprehensive overview of the adsorption technique for the removal of SEs from other sources, serving as a valuable resource for researchers and policymakers involved in the development of efficient and sustainable strategies to mitigate the effects of these emerging contaminants.
1. Introduction
The persistent increase in anthropogenic activities has led to the indiscriminate release of chemicals that contaminate waterbodies and negatively affect the aquatic ecosystem owing to high toxicity and persistence in the environment.1,2 These chemicals are of natural and synthetic origin and include endocrine disrupting chemicals with concentrations ranging from ng L−1 to g L−1 in aquatic bodies.3,4“Endocrine disrupting chemicals according to the definition by the World Health Organization (WHO) are chemicals that interfere with the normal function of humans and wildlife endocrine system by blocking or mimicking the way hormones control metabolism, growth, and body function”. EDCs include polyfluorinated alkyl,5,6 pharmaceuticals,7–10 steroid estrogens,2,11 pesticides,12 and personal care products.13,14
Over the past few decades, the occurrence of steroid estrogens (SEs), a class of emerging contaminants in the environment and aquatic environment, has been of serious concern because of rapid urbanization and industrialization,2,15 posing deleterious health challenges and environmental concerns, endangering all forms of life due to the persistent, toxic and estrogenic nature of EDCs.16–19
Steroid estrogens are a group of hormones classified as naturally occurring and synthetically developed. The naturally occurring SEs are estrone (E1), 17-β-estradiol (E2), and estriol (E3), which play a vital role in acting as the female sex hormone responsible for the development and regulation of the female reproductive system and the secondary sex character,4,20 while the synthetically developed estrogen 17-α ethinylestradiol (EE2) is the active ingredients in oral contraceptive and hormone replacement therapy drugs, which is approved for use in the treatment of menopause symptoms, hypoestrogenism and prevention of osteoporosis by the food and drug administration (FDA).4,21
Steroid estrogens enter the environment via different pathways. Humans and animals largely excrete these estrogens via urine and faeces as active free forms of glucuronide conjugate and sulfate conjugates, which are deconjugated back in the environment or in their original form as the body metabolizes only 24–48% of the oral contraceptive dose in the body. Animals excrete more steroid estrogen than humans.4,20 Other sources of steroid estrogens in the environment include animal waste, run offs from farms and effluents from wastewater treatment plants3,4,20,22
Steroid estrogens are known to have a negative effect on human and aquatic life forms when present even at very low concentrations.2,18,20 They have been linked to an increase in testicular, breast, and ovarian cancer, obesity, infertility, hormonal imbalance, low sperm count in adult males, fibroid, and endometriosis in adult females.3,23,24 In aquatic animals, it causes feminization of the fishes, impaired vision, and clover disease, while in plants, it causes reduced growth and seeding inhibition,25,26 as shown in Fig. 1. Therefore, water treatment has become a critical issue globally, which is essential for improving the stability of ecology and human health.
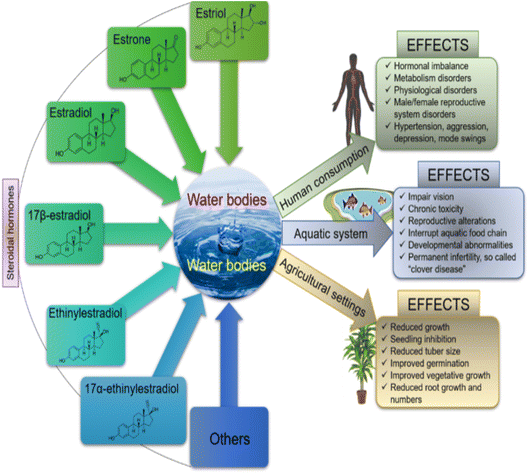 | ||
| Fig. 1 Schematic representation showing the effect of SEs on humans, plants and aquatic life22 (open access). | ||
To sequestrate these SEs in water, the conventional water treatment system cannot cater for their removal2,20 because many of the chemicals have not been included in the wastewater treatment legislation (Directive 2000/60/EC, Directive 2008/56/EC, and Directive 2013/39/EU),27,28 making researchers explore other types of treatment technologies that have been developed. Different types of treatment technologies have been developed, such as advanced oxidation processes,15 chlorination,29 coagulation–flocculation,30 electrocatalysis,31 photocatalysis,2,20 adsorption,11,18,32 membrane technology,33,34 and reverse osmosis.35,36 The major shortcomings of these technologies are the high cost of operation and the production of secondary by-products, which may be more toxic and complex.37,38
According to research reported by various scientists over the years, adsorption techniques have proven to be one of the most promising approaches for removing SEs from water because they are cheap, easy, environmentally benign and do not lead to the production of secondary by-products.27,38
This paper presents a review of the use of adsorption technology for the removal of SEs from water; it comprehensively examines the old status, current status and the future of this research topic through a detailed systemic review and large-scale bibliometric analysis performed based on information search on the ISI web of science search engine using the keywords “steroid estrogen (s)”, “removal”, “adsorbents”, article citation, author's collaboration, country, annual outputs, etc., with the majority of published research works spanning the last 50 years (1973–2022).
2. Chemistry of estrogen and reactions in water
The term “estrogens” refers to a group of biologically active hormones formed in humans and animals by the testes, adrenal cortex, placenta, and ovary.39 They belong to a class of steroid chemicals known as major female sex hormones because of their significance in the estrus cycle. Steroid estrogens can be classified as either natural or synthetic hormones, and when present in excess in the living organism, they can serve as endocrine disruptors (EDCs).40 The cyclopentane phenanthrene ring serves as the building block for the chemical structure of estrogens, as shown in Fig. 2. The additional carbon contributes to the formation of the estran (C18) structure.41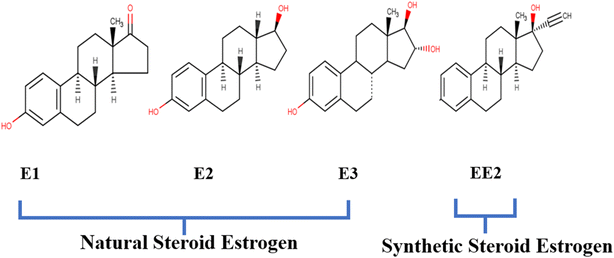 | ||
| Fig. 2 Structure of SEs20 (open access). | ||
Natural estrogens, commonly known as the C18 steroidal group, have four rings: one cyclopentane, two cyclohexanes and one phenolic ring.42 They regulate the development of secondary female sex traits and, in conjunction with the gestagens, regulate all reproductive processes in women. Natural estrogens have different amounts and configurations of hydroxyl groups, such as estrone (E1), 17β estradiol (E2), estriol (E3), and estetrol (E4).43 Synthetic estrogens, however, are a pharmaceutical substance used as an oral contraceptive and hormonal replacement therapy. They are also utilized to stimulate animal growth. 17-ethinyloestradiol is the most commonly known synthetic estrogen.44
To determine the fate of steroidal estrogen chemicals in soil and water systems, it is essential to understand their physiochemical characteristics. The dispersion of organic contaminants in the aqueous phase and other solids is frequently regarded as a function of the partitioning between the organic and aqueous phases.45 The proportion of a compound's concentration under equilibrium conditions in n-octanol and water at a specific temperature is known as water partition coefficient (Kow).45 Large molecules and compounds with a high log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow > 5 are easily adsorbed to sediments and are mostly removed by coagulation. Because estrogens have a substantial log
Kow > 5 are easily adsorbed to sediments and are mostly removed by coagulation. Because estrogens have a substantial log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow, it is expected that they are absorbed onto the solid phases.45
Kow, it is expected that they are absorbed onto the solid phases.45
In general, free or unconjugated estrogens are not highly water soluble. Most SEs are usually moderately hydrophobic (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow = 2.4–4.0); E2 is the most hydrophobic of the estrogens, whereas EE2 is the least soluble. At neutral pH, aqueous solubility progresses from E1 (one OH group) to E2 (two OH groups), and then EE2 with the addition of ethinyl groups at the 17-position on the D ring; solubility appears to be the same at pH 4.45,46 However, solubility can be affected by pH because, for instance, estrogen's relative solubility is higher at pH 10.47
Kow = 2.4–4.0); E2 is the most hydrophobic of the estrogens, whereas EE2 is the least soluble. At neutral pH, aqueous solubility progresses from E1 (one OH group) to E2 (two OH groups), and then EE2 with the addition of ethinyl groups at the 17-position on the D ring; solubility appears to be the same at pH 4.45,46 However, solubility can be affected by pH because, for instance, estrogen's relative solubility is higher at pH 10.47
Estrogens are hydroxylated in the liver. The conversion of the hydroxyl (OH) groups into ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O results in the transformation of E2 to E1, which is further converted to E3 because of subsequent changes.48 Subsequently, sulphate and glucuronic derivatives are produced after esterification, which is later excreted with urine or bile. Most often, estrogens eliminated with bile are partly reabsorbed in the colon, while the remaining substances are expelled from the body system with faeces;45 they enter the natural environment alongside excrement.49
O results in the transformation of E2 to E1, which is further converted to E3 because of subsequent changes.48 Subsequently, sulphate and glucuronic derivatives are produced after esterification, which is later excreted with urine or bile. Most often, estrogens eliminated with bile are partly reabsorbed in the colon, while the remaining substances are expelled from the body system with faeces;45 they enter the natural environment alongside excrement.49
SEs are not volatile and are therefore relatively short-lived in the environment. Consequently, they are unlikely to penetrate the atmosphere in considerable quantities and to be transported a great distance from the place of emission.50
3. Methodology of data retrieval and processing
We retrieved metadata on SE removal using adsorbents from the Web of Science (WoS) and Scopus databases. The former is hosted on the Clarivate Analytics platform, a leading citation database. The WoS contains a diverse range of multidisciplinary literature, particularly those related to biological, physical, and life sciences, where the theme of the current study lies.51 Inaugurated in 2004 by Elsevier, the Scopus databases represent a comprehensive information system with exhaustive citation metrics and abstracts of a range of disciplines.52 Adopting the guideline stipulated in Olisah et al. (2022), we mapped out research trends from retrospective encoded data on SE removal using adsorbents from the WoS and Scopus databases. Only studies related to the research areas were retrieved. To identify studies conducted on SE removal using adsorbents, a search string TI = (“estrogen”) AND TI = (removal) AND TI = (“adsorbent*) was used to retrieve articles indexed in the WoS database from January 1, 1990, to November 5, 2022. Only “Article” (n = 103), “Review Article” (n = 3) and “Book Chapters” (n = 1) were targeted. Document types, such as “Proceeding Papers” (n = 3) and “Early Access” (n = 2), were excluded. After exclusion, a total of 107 documents were identified. Similar search terms were inserted into the TITLE-ABS-KEY Scopus database module focusing on “Article” (n = 74), “Review” (n = 2), and “Book Chapter” (n = 1) published from January 1, 1949, to November 5, 2022, while “Conference Paper” (n = 3) was excluded. This yielded a total of 77 articles.A total of 107 WoS and 77 indexed Scopus documents were downloaded in Bibtex format and uploaded into the RStudio application (Version 1.4.1106; 2009–2021) for bibliometric processing. Duplicate documents from both databases were combined as one with the R code “h < -duplicated Matching (M, Field = “TI”, tol = 0.95”), giving a total of 137 documents that were used to systematically map bibliometric indicators, such as the number of articles, most prolific countries, most productive scholar, and most cited articles. All codes for statistical bibliometric and statistical analysis (Kolmogorov–Smirnoff (K–S) p-value, goodness of fit, and β-coefficient) were adopted from Aria and Cuccurullo (2017). Bibliometric coupling was done using the equation D = C × CT, where C represents the bipartite network and D represents the symmetrical matrix. Two articles are considered bibliometrically coupled if a cited reference is shown in both articles.53 Country collaborations were mapped using the following set of parameters: n = 2 and label size = 14. Combined metadata from the WoS and Scopus databases were downloaded in CSV format and uploaded on the VoS Viewer App (version 1.6.15 © 2009–2020) for thematic classification using the keyword plus and author's keywords. We used linear and polynomial models to analyse research trends; however, we chose the latter as a predictive growth model owing to its reasonable R2 value.54
3.1 Publication trends
A total of 137 articles were retrieved from the merged databases from 1949 to 2022 (Table S1†), of which 133 were research articles and four were review articles. The studies were conducted by 476 authors with document/author, author/document, and co-author/document ratios of 0.29, 3.47, and 4.91, respectively. All publications were multiple authored and retrieved from 75 journal sources. The retrieved documents in the research focus area accumulated an average citation/document and average citation per year of 5.51 and 3.69, respectively, with a collaboration index of 3.47. The first publication on SE removal was published in 1972, and document production from then until 2009 was relatively steady, with only six years recording at least one publication (1973, 2003, 2005, 2007, 2008, and 2009), as shown in Fig. 3. Peak numbers were recorded in 2019 with 21 articles, followed by 2020 and 2022 with 17 and 18 articles, respectively.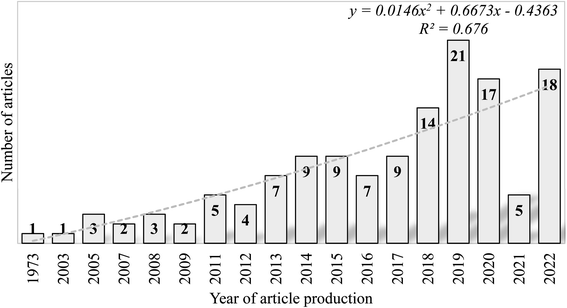 | ||
| Fig. 3 Annual publication frequencies of research involving the removal of SEs from the environment published from 1949 to 2020. | ||
The presence of endocrine-disrupting chemicals in our environment has become a global menace because of their potential to affect terrestrial and aquatic lifeforms adversely. Many of these EDCs are plasticizers, polyestrogens, pesticides, and pharmaceuticals. Emerging contaminants, such as synthetic SEs (17-α ethinyl estradiol – analogue of natural estrogen – 17β-estradiol), have received significant interest from scholars owing to their persistent nature in the environment. This compound, together with estrone and estriol, has been confirmed to be the most potent and ubiquitous environmental estrogen present in various female contraceptives and hormone replacement therapy.55 Our studies revealed that about 88% of most articles on SE removal have been published in the last decade. In this era, researchers were beginning to understand only the adverse effects of these compounds and to explore scientific methods for their removal. Many international initiatives and funding schemes inaugurated to tackle water pollution may be responsible for the high research output in the past few decades. These include the National Institute of Food and Agriculture National Integration Water Quality Program, which was established in 2015 to improve water quality across the United States (https://www.nifa.usda.gov/), and the approval of USD 114 million by OPEC in 2020 for the water and sanitation sector in Africa, Europe, Asia, Latin America and the Caribbean (https://opecfund.org/focus-areas/water-sanitation). The adoption of the Sustainable Development Goals (SDG) by the world's leaders, particularly those linked to water pollution (SDG 16 and SDG 14), may also have spurred research in this area in the last decade. The association between the number of articles and the year of production fitted into a polynomial model, which generated an R2 of 0.7, thus indicating a strong relationship between both indices. We employed Lotka's inverse square law of author predictive to assess authorship distribution dynamics.56 Coupled with other statistical tools (Kolmogorov-Smirnoff goodness of fit = 0.93, p > 0.01 and β-coefficient = 2.35), Lotka's distribution revealed that Lotka's law does not fit the literature of SE removal. An annual growth rate of 6.07% further suggests that a slow output of literature in this research area is likely in subsequent years.
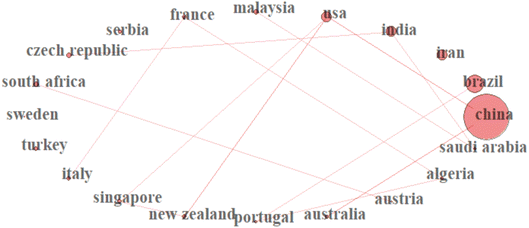
3.2 Most productive countries and citation analysis
This study prioritizes this bibliometric indicator to identify the most productive countries in the considered subject area. As shown in Table S2,† countries were ranked based on the number of articles and the citation metrics produced by authors affiliated with the institutions of these countries. China had the highest publications (n = 47), accounting for 34.3% of the total articles, followed by Brazil (n = 17; 12.4%), Iran (n = 11; 8.03%), India (n = 8; 5.84%) and the USA (n = 6; 4.38%). China also topped the chart on citation metrics with 1172 citations, followed by the USA (n = 442), Spain (n = 357), India (n = 342), and the United Kingdom (n = 325). It is important to note that 50% of the top countries in the research on SE removal are developed countries. The presence of cutting-edge facilities, scientific advancement, government involvement in scientific research, and adequate funding may be responsible for their presence. With population growth and economic progress, China has gradually become one of the largest users of pharmaceuticals.57 Various SE compounds have been widely detected in their environments, and their removal has been deeply concerned by researchers.58,59 In China, over 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 of 36 different pollutants contaminated the environment in 2013, and in 2015, the State Council of China established the “Water Pollution Prevention and Control Action Plan” to tackle the water pollution crisis in the country.60,61 The position of China in the number one spot may also be attributed to the efforts made by the Chinese government to remove pharmaceuticals.62 The findings of this study align with a similar study that also ranked China first when it comes to eliminating pharmaceutical and personal care products (PPCPs). The importance of collaboration in scientific research cannot be over-emphasized as it fosters research progression and makes actualizing a set of research objectives easier.63 Fig. 4 depicts the corporate network of 20 of the most productive countries in the subject area. The line thickness between countries reflects the collaboration frequencies, while the red circle nodes show the number of countries. China had the highest collaboration strength, mainly with the USA and Australia, followed by Brazil, the USA, and India.
000 of 36 different pollutants contaminated the environment in 2013, and in 2015, the State Council of China established the “Water Pollution Prevention and Control Action Plan” to tackle the water pollution crisis in the country.60,61 The position of China in the number one spot may also be attributed to the efforts made by the Chinese government to remove pharmaceuticals.62 The findings of this study align with a similar study that also ranked China first when it comes to eliminating pharmaceutical and personal care products (PPCPs). The importance of collaboration in scientific research cannot be over-emphasized as it fosters research progression and makes actualizing a set of research objectives easier.63 Fig. 4 depicts the corporate network of 20 of the most productive countries in the subject area. The line thickness between countries reflects the collaboration frequencies, while the red circle nodes show the number of countries. China had the highest collaboration strength, mainly with the USA and Australia, followed by Brazil, the USA, and India.
Citation analysis was prioritised in this study to identify the most influential articles among all documents indexed in the WoS and Scopus databases. The top 10 most cited articles are listed in Table S3.† Pan et al. (2018) topped the retrieved records with “Adsorption and hysteresis of bisphenol A and 17α-ethinyl estradiol on carbon nanomaterials”, accumulating a total citation of 366 with an average citation rate of 24.4% per year.64 This was followed by “Removal of estrone and 17β-estradiol from water by adsorption” and “Determining estrogenic steroids in Taipei waters and removal in drinking water treatment using high-flow solid-phase extraction and liquid chromatography/tandem mass spectrometry” in the second (n = 172; 9.56%) and third (n = 151; 9.44%) spots, respectively.
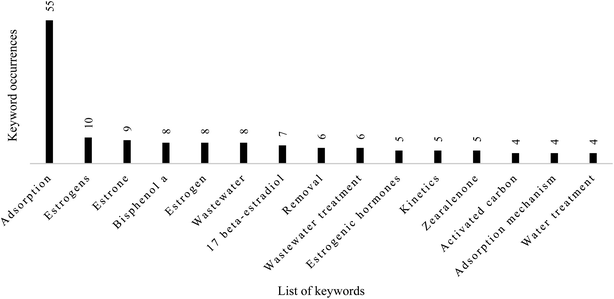
3.3 Classification of thematic areas based on keyword occurrences
Keywords represent one of the most essential contents during the submission of a manuscript for publication. Categorizing and analysing keywords can effectively capture the research area most important to researchers. The analysis of the occurrence of keywords has become a common practice when performing bibliometric studies. A total of 417 authors' keywords were found in the 137 articles retrieved for this study, thus reflecting a diversified author preference. Fig. 5 displays the top 15 keywords used in articles published from 1949 to November 2022. Adsorption, removal, and estrogens are the words used to retrieve articles in the hybrid database, so they can be ignored. As shown in Fig. 4, keywords in the top 15 are “estrone” (n = 9, 2.16), “Bisphenol A”, “wastewater” (n = 8, 1.92%), “17 beta-estradiol” (n = 7, 1.68), “wastewater treatment” (n = 6, 1.44%), and “estrogen hormones” “kinetics”, and “zearalenone” (n = 5, 1.20%). Others include “activated carbon”, “adsorption mechanism” and “water treatment” (n = 4, 0.96%).As shown in Fig. 6, imprinted polymer, biochar, and activated carbon/charcoal are the commonly used adsorbents for removing SEs from contaminated water. In general, activated carbon/charcoal has become the most widely used adsorption material for removing pharmaceuticals owing to its large surface area, chemical stability, and developed physical structure.65 This material's low cost and high removal efficiency properties may be responsible for its frequent usage in the treatment of water contaminated with SEs.
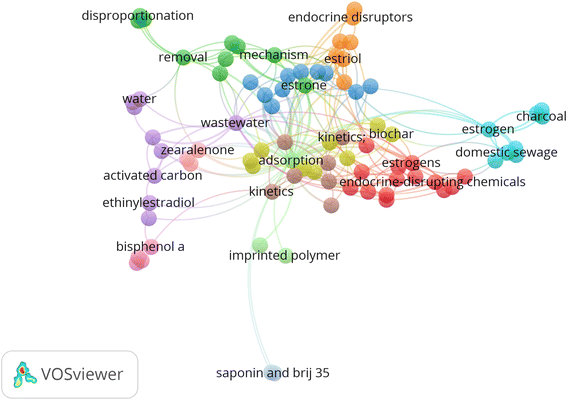 | ||
| Fig. 6 Co-occurrence network of the most frequently used keywords in articles associated with SE removal. | ||
4. Adsorption technique for the removal of SEs in water
Over the years, steroidal estrogens (SEs) have been considered widespread water contaminants, as shown in Fig. 7, and the most lethal kind of EDC owing to their strong affinity for nuclear receptors;18,66,67 thus, their remediation from the aquatic ecosystem is of primary and growing concern owing to their deadly effect on creatures and eco-networking.18,68 Notably, SEs have been labelled as contaminants of emerging concern because they are popularly recognized to engender deleterious changes in marine habitats when present even at relatively close-to-zero concentrations.18,66,68–72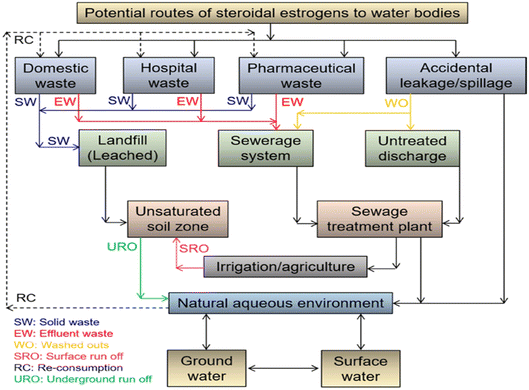 | ||
| Fig. 7 Sources and routes of SE contamination in the aquatic system.70 Reused with permission from Elsevier (order no: 5577471429890). | ||
Interestingly, in recent times, different functional materials, such as MOF, nano adsorbents, unmodified biomass, activated carbon (AC), clays, and biochar (BC), have been used as adsorbents for the adsorptive removal of various types of SE in water. The pragmatic efficiency of these materials in recently conducted SE's adsorption studies is holistically discussed in this section based on their percentage efficiency and adsorption capacity, which were approximated to two decimal points, as depicted in Table 1.
| Adsorbent | ASE | HAC (mg g−1) | HAE (%) | OP | OT (°C) | AT (min.) | Adsorption mechanism | Best fit isotherm | Best fit kinetics | References |
|---|---|---|---|---|---|---|---|---|---|---|
| a Where AT = adsorption time, OT = optimum temperature, OP = optimum pH, HAE = highest adsorption efficiency, HAC = highest adsorption capacity, ASE = adsorbed steroidal estrogens, FI = Freundlich isotherm, LI = Langmuir isotherm, PFO = pseudo-first-order, PSO = pseudo-second-order, DM = dual mode AC = activated carbon, BC = biochar, HC = hydrochar, NP/NPs = nanoparticle(s), SWCNTs = single-walled carbon nanotubes, CNTs = carbon nanotubes, MWCNTs = multi-walled carbon nanotubes, rGO = reduced graphene oxide, GO = graphene oxide, HEMA = 2-hydroxyethyl methacrylate, MAPA = N-methacryloyl-L-phenylalanine, UF = ultra-filtration, and * = SE in multicomponent system. HAC was taken from LI. | ||||||||||
| AC-alginate biopolymer | EE2 | 0.53 | 84.00 | 3 | — | 30 | — | FI | — | 86 |
| Fe3O4@SiO2@MPS | E2 | 27.35 | 92.10 | 6 | 30 | 120 | H-Bonding | FI | PFO | 74 |
| Rice husk silica | E1 | 0.56 × 10−4 | 93.10 | 4 | — | 60 | — | LI | PFO | 82 |
| E2 | 0.62 × 10−4 | 95.50 | 4 | — | 60 | — | LI | PFO | ||
| GO-magnetic rice straw BC | E2 | 46.22 | — | 7 | 25 | 1200 | H-Bonding, electrostatic and π–π interactions, chemisorption | FI | PSO | 94 |
| Mg3Al-layered double hydroxide intercalated with Na-dodecyl sulfate | E2 | — | 94.00 | 7 | 25 | 45 | Hydrophobic interactions, and H-bonding | FI | PSO | 83 |
| Rice husk | E1 | 2.70 | >98 | 7 | 25 | 60 | H-Bonding | LI | PFO | 85 |
| E2 | 1.65 | >98 | 7 | 25 | 120 | H-Bonding | LI | PSO | ||
| PFO | ||||||||||
| E3 | 1.00 | >77 | 7 | 25 | 120 | H-Bonding | LI | PFO | ||
| Rice husk | E1* | 2.64 | — | 7 | 25 | 60 | H-Bonding | LI | PFO | 85 |
| E2* | 1.59 | — | 7 | 25 | 120 | H-Bonding | LI | PFO | ||
| E3* | 0.89 | — | 7 | 25 | 120 | H-Bonding | LI | PFO | ||
| N-Propyl-mesoporous silica | E1 | 88.38 | >70 | 7 | 25 | 60 | Hydrophobic, hydrogen-bonding, and dipole–dipole interactions | LI | PSO | 96 |
| E2 | 86.91 | 70 | 7 | 25 | 120 | LI | PSO | |||
| EE2 | 119.87 | >80 | 7 | 25 | 120 | LI | PSO | |||
| β-Cyclodextrin polymers | E2 | 126.16 | >99 | 7 | 25 | — | Hydrophobic, and hydrogen-bonding interactions | LI | PSO | 97 |
| EE2 | 145.81 | >99 | 7 | 25 | — | Hydrophobic, and hydrogen-bonding interactions | LI | PSO | ||
| γ-Cyclodextrin polymers | E2 | 198.46 | >99 | 7 | 25 | — | Hydrophobic, and hydrogen-bonding interactions | LI | PSO | 97 |
| EE2 | 216.87 | >99 | 7 | 25 | — | Hydrophobic, and hydrogen-bonding interactions | LI | PSO | ||
| Poly(HEMA-MAPA) microparticles | E2 | 98.4 | >90 | — | 25 | 150 | Hydrophobic interactions and physisorption | LI | — | 98 |
| BC pellet | E1 | 6.39 | 55 | 25 | 15 | Hydrophobic interactions and chemisorption | FI | PSO | 99 | |
| E2 | 10.43 | 5 | 25 | 15 | Hydrophobic interactions and chemisorption | FI | PSO | |||
| E3 | 4.80 | 5 | 25 | 15 | Hydrophobic interactions and chemisorption | FI | PSO | |||
| Soybean hulls | E1 | 2.59 | 100 | 7 | 25 | 60 | H-bonding | LI | PFO | 100 |
| Physisorption | ||||||||||
| Hydrophobic interactions | ||||||||||
| E2 | 2.24 | 100 | 7 | 25 | 120 | H-bonding | LI | PSO | ||
| Physisorption | ||||||||||
| Hydrophobic interactions | ||||||||||
| E3 | 0.86 | 100 | 7 | 25 | 120 | H-Bonding | LI | PFO | ||
| Hydrophobic interactions | PSO | |||||||||
| Physisorption | ||||||||||
| Soybean hulls | E1* | 2.56 | 100 | 7 | 25 | 60 | H-Bonding | LI | PFO | 100 |
| Hydrophobic interactions | ||||||||||
| Physisorption | ||||||||||
| E2* | 1.98 | 100 | 7 | 25 | 120 | H-Bonding | LI | PFO | ||
| Hydrophobic interactions | ||||||||||
| Physisorption | ||||||||||
| E3* | 0.84 | 100 | 7 | 25 | 120 | H-Bonding | LI | PFO | ||
| Hydrophobic interactions | ||||||||||
| Physisorption | ||||||||||
| Macadamia nutshell AC | E1 | 22.00 | 85 | 7 | 25 | 120 | H-Bonding | LI | PSO | 102 |
| Physisorption | ||||||||||
| Hydrophobic interactions | ||||||||||
| Chemisorption | ||||||||||
| Electrostatic interactions | ||||||||||
| E2 | 22.00 | 82 | 7 | 25 | 120 | H-bonding | LI | PSO | ||
| Electrostatic interactions | ||||||||||
| Physisorption | ||||||||||
| Hydrophobic interactions | ||||||||||
| Chemisorption | ||||||||||
| Mesoporous imprinted polymer | E1 | 371.20 | 100 | 9 | — | 20 | Intra-particle diffusion | LI | PSO | 91 |
| Chemisorption | ||||||||||
| E3 | 323.10 | 100 | 9 | — | 20 | Intra-particle diffusion | LI | PSO | ||
| Chemisorption | ||||||||||
| Mesoporous non-imprinted polymer | E1 | 285.30 | 100 | 9 | — | 20 | Intra-particle diffusion | LI | PSO | 91 |
| Chemisorption | ||||||||||
| E3 | 237.40 | 100 | 9 | — | 20 | Intra-particle diffusion | LI | PSO | ||
| Chemisorption | ||||||||||
| Boron nitride nanosheets | E1 | 249.15 | 96.34 | — | 25 | — | Hydrophobic, and π–π interactions | LI | PSO | 103 |
| van der Waal's | ||||||||||
| Yeast biomass | E1 | 0.93 | >90 | 10 | — | 20 | Electrostatic interactions | FI | PSO | 106 |
| EE2 | 21.41 | >90 | 10 | — | 20 | Electrostatic interactions | FI | PSO | ||
| Modified zeolites | E1 | 41.67 | — | 5 | 25 | Distribution effects and surface adsorption | LI | PSO | 107 | |
| E2 | 23.87 | — | 5 | 25 | — | Distribution effects and surface adsorption | LI | PSO | ||
| Thermally activated sludge | E2 | 8.75 × 10−3 | — | 5.5 | — | 360 | Chemisorption | FI | Elovich | 114 |
| EE2 | 14.56 × 10−3 | — | 5.5 | — | 240 | Chemisorption | LI/FI | PFO | ||
| KOH activated sludge | E2 | 17.90 × 10−3 | — | 5.5 | — | 240 | Chemisorption | LI/FI | PFO | 114 |
| EE2 | 0.44 × 10−3 | — | 5.5 | — | 360 | Chemisorption | LI | Elovich | ||
| H2SO4 activated sludge | E2 | 16.42 × 10−3 | — | 5.5 | — | 360 | Chemisorption | LI/FI | PFO | 114 |
| EE2 | 4.23 × 10−3 | — | 5.5 | — | 420 | Chemisorption | LI | Elovich | ||
| Fe mining tailing CNT | EE2 | 21.60 | — | 6 | 25 | 120 | π–π interactions | FI | PSO | 118 |
| Corn straw BC | EE2 | 1.70 | — | 7 | 25 | 480 | Chemisorption | FI | PSO | 117 |
| H-Bonding | ||||||||||
| Mesoporous carbons | EE2 | 157.00 | 99 | 7 | — | 45 | Physisorption | FI | PSO | 109 |
| Plastic-char | EE2 | 82.30 | — | 7 | 25 | 600 | Covalent and π–π interaction | FI | PSO | 115 |
| Chemisorption | ||||||||||
| Attapulgite | E2 | 28.12 | — | 3.5 | 25 | — | H-Bonding | LI | PSO | 116 |
| Chemisorption | ||||||||||
| Electrostatic and π–π interaction | ||||||||||
| Sulfur-doped g-C3N4 | E2 | 33.38 | 100 | 7 | 25 | 45 | — | — | PSO | 130 |
| Walnut shell BC | E1 | 21.36 | >45 | 4 | 25 | 120 | — | FI | PSO | 111 |
| E2 | 17.74 | 50 | 4 | 25 | 120 | FI | PSO | |||
| E3 | 25.07 | 40 | 4 | 25 | 120 | FI | PSO | |||
| Uncarbonized Fe/Ni NPs (using eucalyptus leaf extract) | E2 | 35.00 | 77.60 | 6 | 40 | 30 | H-Bonding | FI | PSO | 127 |
| π–π interaction | ||||||||||
| Intraparticle diffusion | ||||||||||
| Fe3+-saturated montmorillonite | E2 | — | 93.00 | 6 | 40 | 30 | — | — | — | 119 |
| FeNPs/rGO (using green tea extract) | EE2 | 11.34 | 56.10 | 6.5 | 40 | — | π–π interaction | LI | PSO | 131 |
| PFO | ||||||||||
| rGO@Fe NPs (using green tea extract) | EE2 | — | 99.90 | 6 | 40 | — | — | — | — | 120 |
| Untreated bentonite | EE2 | 5.16 | — | 7 | 25 | — | Chemisorption | LI and DM | PSO | 132 |
| Na-bentonite | EE2 | 5.21 | — | 7 | 25 | — | Chemisorption | LI and DM | PSO | 132 |
| Trp-Na-bentonite | EE2 | 7.40 | — | 7 | 25 | — | Chemisorption | LI and DM | PSO | 132 |
| Fe–Na- bentonite | EE2 | 6.28 | — | 7 | 25 | — | Chemisorption | LI and DM | PSO | 132 |
| Fe-MIL-101-NH2 | E2 | 202.02 | — | — | 37 | — | H-bonding and π–π interaction | LI | PSO | 133 |
| EE2 | 229.89 | — | — | 37 | — | H-bonding and π–π interaction | LI | PSO | ||
| E3 | 205.85 | — | — | 37 | — | H-Bonding and π–π interaction | LI | PSO | ||
| Carbonized montmorillonite/carboxymethyl cellulose | E2 | 138.95 | — | 5 | 25 | 480 | H-Bonding and π–π interaction | FI | Ritchie nth-order kinetic model | 104 |
| Pore-filling | ||||||||||
| Hydrophobic partitioning | ||||||||||
| van der Waals interaction | ||||||||||
| Lotus seedpod BC | E2 | 147.12 | — | — | 17 | 1200 | Chemisorption | LI | PSO | 90 |
| Electrostatic and π–π interaction | ||||||||||
| K2FeO4 modified Lotus seedpod BC | E2 | 121.18 | — | — | 35 | 240 | Micropore filling | LI | PSO | 121 |
| Electrostatic and π–π interaction | ||||||||||
| Chemisorption | ||||||||||
| Fe–Mn binary oxide/MWCNT | E2 | 47.25 | 86.16 | 7 | 25 | 360 | H-Bonding and π–π interaction | LI | PSO | 122 |
For instance, Prokić et al.73 comparatively examined the adsorption of three SEs from water using unmodified and chemically modified AC clothes (ACC). From the study, applied chemical (HNO3, HCl, and KOH) modification improved the specific surface area and the content of architectural oxygen functional moieties of the ACC, which elevate its adsorption capacity up to 30% with the one modified with HNO3, resulting in the highest adsorption efficiency for E1, E2, and EE2, as shown in Fig. 8. The adsorption followed PSO and fitted well with the Freundlich isotherm model, which models the physisorption mechanism.73 The remarkable adsorption efficiency (>80%) recorded for the ACC (HNO3) was consistent with 83.1% recorded for the removal of EE2 using hydrothermally carbonized and steam AC fabricated from palm kernel shells by.68
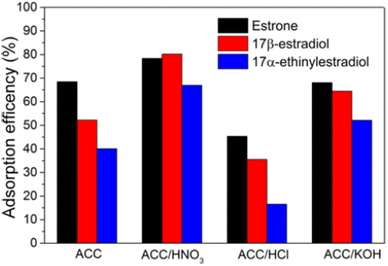 | ||
| Fig. 8 Adsorption efficiency of unmodified and chemically modified AC clothes.73 Reused with permission from Springer (order number: 501826394). | ||
Although68 reported PSO kinetics in their research, the thermodynamic result demonstrates that the adsorption operation was predominantly chemisorption, which somewhat agrees with the report on the adsorption of E2 using magnetic molecularly imprinted polymers74 and 3-D covalently linked GO and rGO-methoxyl ether polyethylene glycol functionalized silica75 but differs from 73 that claimed physisorption. Furthermore, Akpotu et al.75 empirically pointed out that the −ve ΔG° obtained proved the spontaneity and thermodynamic propitiousness of the adsorption course, which improved with an uptick in SE temperature, while the +ve ΔH° and ΔS° figures obtained for the adsorbing material illustrate that the adsorption operation was endothermic and improved randomness/disorderliness between the adsorbent/adsorbate interfaces.
Additionally,76 reported the performance of single-walled carbon nanotubes (SWCNTs) for the adsorption of EE2 from seawater and brackish water. A 95% efficiency recorded was attributed to hydrophobic interactions and π–π electron (π–π e−).76 The adsorbent performance of SWCNTs is in a very close range with 93% reported for the adsorption of E2 using magnetic molecularly imprinted polymers fabricated on the surface of Fe3O4.74 This mechanism is consistent with those cited in several previous adsorption studies77–81 using CNTs, which considered a large specific surface area with evenly distributed hydrophobic sites for organic contaminant adsorption.
Similar to the high adsorption efficiency reported in the foregoing paragraph,82 used rice husk silica also achieved excellent adsorption of 93.10% and 95.50% for E1 and E2 in 1 h at pH 4, respectively. It is noted that the adsorption efficacy of E1 and E2 reduced with an uptick in pH from 4 to 9 because with an upsurge in ambient pH, the composition of hydroxyl ions surges in the environment. Because the exterior surface of the adsorbing material has a +ve charge, the silica tends to absorb the copious volume of O2; thus, hydroxyl ions are adsorbed on the adsorbent at alkaline pH, which decreases the sorption capacity, i.e. the hydroxyl group present on the surface of the rice husk silica makes it −ve and creates a repulsive force between the adsorbing material and SE anionic molecules. As shown in Fig. 9, the dependency of adsorption efficiency on pH is parallel to that expounded by.83,84 Both teams82,83 also alluded to the fact that as the reaction time increases, the adsorption efficiency also increases and does not differ from what85,86 opined in their studies. Abdel-Gawad and Abdel-Aziz86 further investigated the effect of stirring on adsorption efficiency, and they observed that the percentage adsorption (84–85%) did not considerably change when the stirring tempo was increased from 200 to 500 rpm compared with 100 rpm (80%) even though the EE2 diffusion to the surface of the AC-alginate biopolymer was accelerated by the amplified stirring frequency. Notably, according to,85 the LI's qmax revealed that the E3 was the most temperature-sensitive adsorbate, with a reduction of 76.92% at 35 °C and 56.59% at 45 °C relative to the E3's qmax at 25 °C. For the E2, the qmax declined from 97.82% at 35 °C to 80.96% at 45 °C, but the E1 showed negligible sensitivity to the temperature upswing, exhibiting qmax of 98.85% at 35 °C and 96.60% at 45 °C. Additionally, the multi-component system (E1 + E2 + E3) exhibited similar traits displayed for the monocomponent system (Individual SE) as per the adsorption isotherm with a very close range of adsorption capacity (Table 1) under the same experimental conditions.85
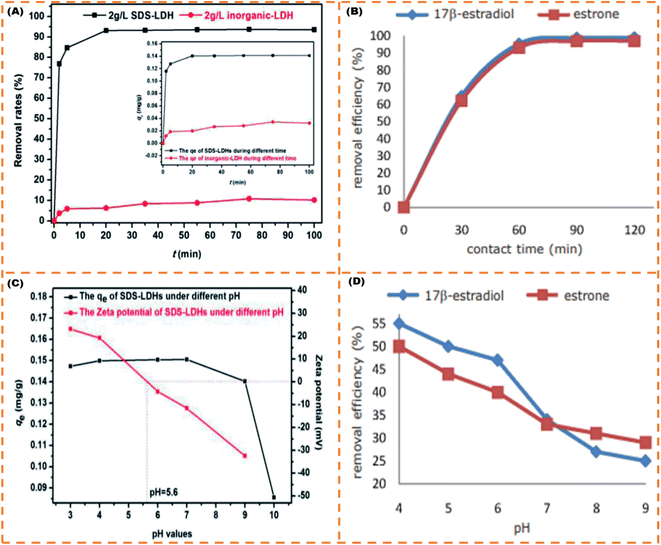 | ||
| Fig. 9 Contact time effect (A) and pH effect (C) on the removal efficiency of E2,83 contact time effect (B) and pH effect (D) on the removal efficiency of E1 and E2.82 (Open access). | ||
To corroborate the unravelling of the mystery of the dependency of SE adsorptive operation on pH accentuated in the foregoing paragraph, Jiang et al.87 pointed out that there are three plausible explanations for how pH affects adsorption: (i) uptick in pH inhibits the formation of H-bond owing to the cleavage of functional groups; (ii) elevating pH might lead to an upsurge in the breakdown of the hydrophobic neutral SE into hydrophilic SE and then stimulate water cluster materialization around the polar spots of adsorbing material, leading to a reduction in hydrophobic interaction; and (iii) by increasing pH, various charged SEs would repel one another more electrostatically and limit/weaken the adhesion between them and the adsorbing material. In addition, most reports in Table 1 empirically confirm that the number of electrostatic charges, which the ES contributes to the adsorption process, is strongly governed by pH. This can be easily explained using pHzc (when the surface of the adsorbent material is uncharged). In other words, there is no net charge associated with the functional group charge.88–91 For example, as the pH of the solution increases, the surface potential becomes highly negative (when the pH is higher than pHzc), intensifying the electrostatic effect. When the pH falls below pHzc, the surface charge turns positive, making it challenging to adsorb positively charged SE molecules.88,91,92
Dong's team93 magnetically modified BC was employed for the removal of E2. The improved BC yielded excellent E2 adsorption with exceptional magnetic separation capability and powerful magnetic responsivity, as shown in Fig. 10. It was empirically established that the installation of Fe particles on the surface of BC provided supplementary binding spots, which in turn enabled efficient and quick access of E2 to the sorption spots and thus resulted in effective estrogen uptake in a shorter time compared to that reported by Liu et al.94 and Yin et al.88 using magnetically modified rice straw BC and its analogues supported by graphene oxide, resulting in effective estrogen uptake in 20 h and 5 h, respectively. However, they88,94 affirmed that the sorption efficiency of modified BC was much higher compared to that of unmodified BC. A similar scenario was observed for the removal of E1 using unmodified Litchi chinensis Sonn BC and its modified Ca and Fe–Mn-impregnated analogues.95 Notably, as shown in Fig. 11, both the modified and unmodified BC showed parallel adsorption kinetic profiles for the removal of E1, but the best adsorption efficiency of 91.50% was achieved by Fe–Mn BC in 2 hours, which was much better than the one achieved by BC (60.1%) and Ca-BC (73.2%). It was accentuated that Fe and Mn can oxidize the reducing entities on the BC surface, which may be the main reason for their high surface area and more adsorption sites, yielding outstanding performance95,96 in their SE adsorption experiment used N-propyl functionalized spherical mesoporous silica to accomplish improved and selective sorption of E1, E2, and EE2. In this study, apart from the fundamental function of hydrophobic interaction, the input of the carbonylic lone pair e− on Carbon 17 of E1 was pragmatically confirmed to create stronger H-bonding with silicon OH and boosted the dipole–dipole interaction between E1 and the adsorbent than E2 and EE2. In addition, from another study, it was clear that E2 had a better adsorption capacity than EE2 using graphene nanosheets as the adsorbent, which might be strongly linked to E2's stronger hydrophobicity than EE2's.87 A somewhat synonymous greater adsorption efficiency of E2 than EE2 was observed when β-cyclodextrin polymers and γ-cyclodextrin polymers were used as adsorbents for rapid endothermic adsorptive operation.97 Compared to most adsorbents reported in Table S4,† both β-cyclodextrin and γ-cyclodextrin polymers came to the fore because of their high adsorption capacity and jaw-dropping removal efficiency. Such strong sorption capability could be attributed to the mesoporous configuration of the polymer and the exceptional packing ability of cyclodextrin, which can stimulate or help the SE to be implanted into the cyclodextrin hollow swiftly and form the host–guest inclusion complex, as shown in Fig. 12.97
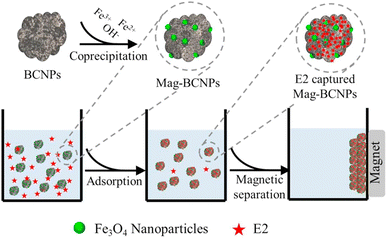 | ||
| Fig. 10 E2 adsorption process and magnetic separation.93 Reused with permission from Elsevier (order number: 557491148583). | ||
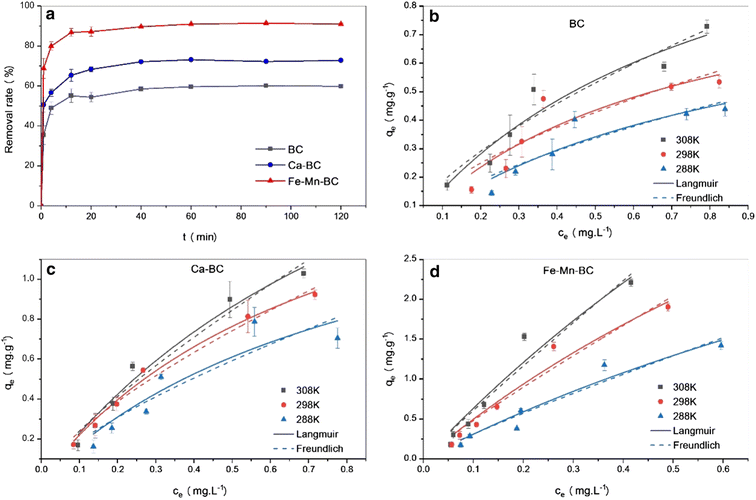 | ||
| Fig. 11 Adsorption performance and kinetic characteristics of modified and unmodified Litchi chinensis Sonn. BC for the aqueous removal of E1.95 Reused with permission from Springer (order number: 501826371). | ||
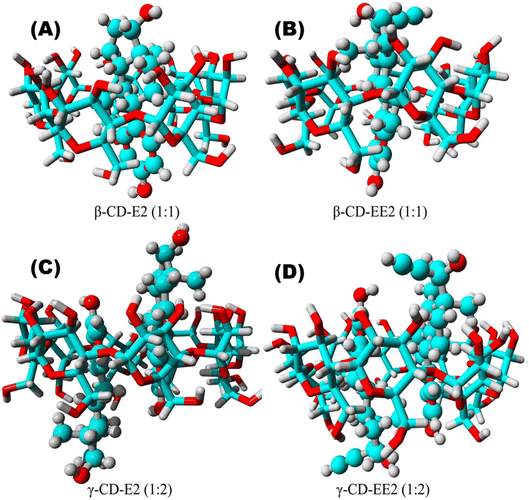 | ||
| Fig. 12 Models of the (A) β-cyclodextrin polymers-E2, (B) β-cyclodextrin polymers-EE2, (C) γ-cyclodextrin polymers-E2, and (D) γ-cyclodextrin polymers-EE2 complexes.97 Reused with permission from Elsevier (order number: 5577501114986). | ||
Kireç research group98 synthesized L-phenylalanine-containing poly(HEMA-MAPA) microparticles by microemulsion polymerization and utilized it as an adsorbent for the adsorption of E2 from aqueous solution. The microparticle adsorbent was reported to have a good adsorption capacity of 98.4 mg g−1 for E2 through hydrophobic interaction, while the adsorption operation fitted the Langmuir isotherm than Freundlich and Temkin. It was observed that the adsorption mechanism has a single layer and uniform surface that occurs spontaneously through physisorption, and the amount of adsorption increases as the temperature and ionic intensity increase.99 differs in their report as it was observed that the sorption mechanism of E2 and its collimates (E1 and E3) occur spontaneously through the chemisorption and fit well with Freundlich than Langmuir isotherm when nanoscale zero-valent iron-supported biochar pellets were used as the adsorbing material. More specifically, as shown in Table 1 and Fig. 13, the nZVI-BC pellet performed better than the BC pellet with E2 coming to the fore, followed by E1 and E3 based on the adsorption capability of the two adsorbing materials.99
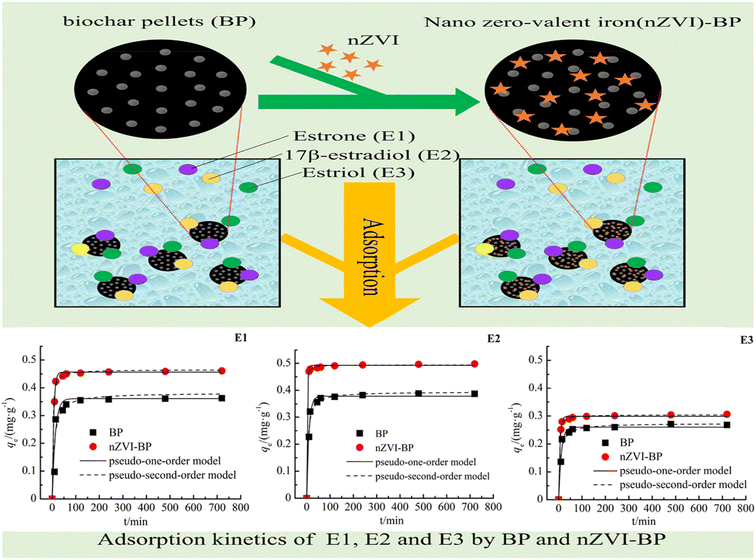 | ||
| Fig. 13 Adsorption kinetics of E1, E2, and E3 by BP and nZVI-BP.99 (Open access). | ||
Honorio et al.100 explored the adsorption performance of soybean hull biosorbent for the adsorption of steroidal estrogen (E1, E2, and E3) in both single component (SC) and multiple components (MC (E1 + E2 + E3)) systems from swine manure (biofertilizer) effluent. From the adsorption evaluation, the best SE removal was performed at 25 °C and pH 7. For the SC and MC systems, equilibrium was attained in 60 minutes for E1 and 120 minutes for E2 and E3, and the PSO model did a good job of describing the kinetics. Models of MC equilibrium suggested that there was no contest between the steroidal estrogens. The optimum adsorption capabilities using LI for E1, E2, and E3 in the MC system were 2.560 mg g−1, 1.978 mg g−1, and 0.835 mg g−1, respectively. This is relatively in a very tight range with adsorption capacity recorded in SC, as shown in Table 1. Thermodynamically, the adsorption was physisorption in nature and favourably spontaneous through H-bonding, as depicted in Fig. 14. Honorio et al.101 achieved analogous performance in the E1, E2, and E3 adsorption using rice husk adsorbent and reported no contest between the SE in the SC and MC systems, within comparable equilibrium times (60 minutes for E1 and 120 minutes for E2 and E3), and this is closely consistent with the adsorptive removal of E1 and E2 in MC system investigated using Macadamia nutshell AC within an equilibrium time of 90 min and adsorption capacity of 22 mg g−1.102 Elias et al. and Honorio et al.100,102 empirically pointed out that E1 has a higher interaction capacity owing to the two e− pairs available to generate H-bonding shown in Fig. 14 with the biomass OH functional groups, while E2 and E3 have a poorer interaction ability owing to the protonated active spot. Investigators100,102 also underscore that the E3 adjacent OH groups perhaps thwart the interaction with biomass compared to E1 and E2. However, Elias et al.102 differ by reporting that both LI and FI suit the experimental data of E1 and E2 adsorbed by nutshell AC, and this event was attributed to the complex mechanism. In addition, the E1 adsorption by few-layered boron nitride nanosheets103 and E1 and E3 adsorption onto a mesoporous molecularly imprinted polymer91 presented thermodynamic characteristics in parallel to that of.100 The authors91,100,102,103 reported that in physisorption, where the adsorption is caused because of the electrostatic interactions, the ΔG° is around −20 kJ mol−1, while in chemisorption, the ΔG° ranges from −84 to −40 kJ mol−1 and adsorption occurs because of e− transfer, e− exchange or e− sharing.100,102 further accentuated that owing to the hydrophobic profile of SE, during the sorption operation, there is an imbalance between the surface of the adsorbent and its internal forces, fostering the operation to molecular adsorption by the van der Walls force among many other interactions reported in Table 1. Furthermore, according to Ahmed et al.,92 during the adsorption process, the SE phenolic moiety can create a resonating stabilized configuration by transferring e− to the benzene ring. The improved electron density of the benzene ring enables it to function as a powerful e− giver; at highly acidic pH (pH ≤ 2), the hydroxyl and ketonic functional groups are protonated, and the SE is in their cationic form.92 The findings above corroborate the findings of other researchers who have equally accentuated that interactions occur between the phenolic moiety of SE and the e− acceptor groups tied to adsorbent,95,104,105 and this results in efficacious adsorption.
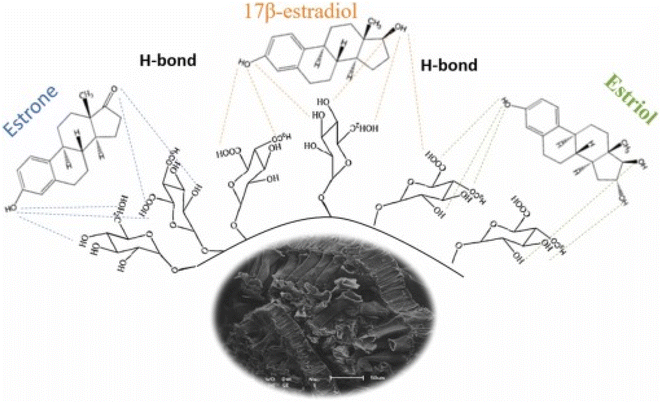 | ||
| Fig. 14 H-Bonding interaction of E1, E2, and E3 with the biosorbent (soybean hull).100 Reused with permission from Elsevier (order number: 5577511310171). | ||
Analogue to the SE adsorptive studies juxtaposed in the foregoing paragraph, Debs et al.106 and Zhong et al.107 evaluated the adsorption of E1 and E2 using yeast biomass from an ethanol industry and HDTMA-modified zeolites, respectively. The PFO model described the adsorption studies,106,107 and Langmuir models best represented the equilibrium data, which indicates that the biosorbent is dynamically regular and that the SE-biosorbent interactions are uniformly distributed to the surface of the biosorbent.100,106,107 Furthermore, contrary to some authors,85,100–102 no-competition reports exist on the multi-component system (E1 + E2 + E3/EE/EE2) adsorption capacity. Debs et al. and Zhong et al.106,107 reported mixed associative/competitive sorption.
Sobhanardakani team108 performed an empirical adsorptive study using CeO2 decorated on CuFe2O4 nanofibers. A remarkable adsorption efficiency of 97.50% was achieved at 50 °C as against 70.50%, which was achieved at 20 °C. This trend of an increase in temperature leading to an increase in adsorption efficiency is contrary to that of,100–102 where the studies by the authors agreed that the adsorption operation lost efficiency with an increase in temperature. Sobhanardakani team108 established that the enhancement of EE2 adsorption with an increase in temperature is attributed to the improvement of the interaction frequency between EE2 moieties, and the adsorption spots with temperature uptick reveals that EE2 adsorption onto nanofibers adsorbent an endothermic operation in contrast to the exothermic process reported by.100–102 Conversely, the isotherm study revealed that the Elovich model could best describe the equilibrium data, and the findings also demonstrated that the adsorption operation followed the LI and FI models.108 The maximum adsorption efficiency (∼98%) observed by108 is comparable to the one (99%) reported for EE2 by109 using mesoporous carbons and another one (99%) reported by Tagliavini et al.110 for the adsorption of E2 in cellulose ultrafiltration membrane setup coupled with polymer-based spherical AC produced by carbonization and steam activation of cross-linked polystyrene. However, the efficiency is higher than the one (50%) reported by111 using walnut shell BC and the one (<95%) reported by112,113 using cellulose nanofiltration and ultrafiltration membranes setup coupled with polymer-based spherical AC.
Martins et al.114 studied the use of physically modified and chemically activated sludge from a water treatment plant as an adsorbent for the removal of E2 and EE2. The effect of the two activating agents (KOH and H2SO4) used on the adsorptive capacity of the sludge adsorbent was comparatively explored with that of the physically modified (through heat treatment) one. E2 and EE2 gave better adsorption capacities at the lowest adsorbent dosage with a reduction in adsorption capacities as the dosage increased. It can, however, be noted that the chemically activated sludge stood out in the adsorption of both SEs when physically modified compared to the one activated with KOH coming to the fore in all, except for the 0.5 g dosage where H2SO4 managed to come to the fore for EE2 adsorption with varied equilibrium times between 180 and 420 min. It was holistically established that as the adsorbent dose increases, the availability of higher energy spots diminishes, with a greater percentage of low energy sites occupied, leading to a reduced qe value. The smaller mass allows all active spots to be accessible and the adsorption on the surface to be promptly saturated. The increase in the diffusion route length caused by the overlapping of adsorption spots as a function of the increase in the adsorbent dose and the active spots that remained unsaturated following adsorption are additional factors in the decrease in qe.114 The adsorption equilibrium time obtained by ref. 114 is within the commonly reported range,115–118 while the isotherm model is similar to that of ref. 115 and 116. In addition, Yin research group116 observed a reduction in adsorption capacity with an increase in pH and examined the influence of various background electrolytes on the adsorption operation of E2 by adding different anions and cations under different concentrations into the reaction system. Attapulgite/BC was shown to have better adsorption efficacy when monovalent cations (Na+ and K+) were present, and its adsorption capacity only marginally diminished as the concentration increased from 0.01 to 0.1 g L−1. However, the ability of E2 to adsorb was hampered by the presence of divalent cations (Ca2+ and Mg2+). Owing to the strong polarization strength of the divalent cations, which was larger when they were present, the squeezing-out effect may be used to elucidate these phenomena. Moreover, owing to the effects of background electrolyte anions, such as Cl, NO3, SO42−, and PO43−, on E2 adsorption onto attapulgite/BC, anions were found to have no noticeable effects on the adsorption of E2 from an aqueous medium. The adsorption capacity of attapulgite/BC diminished marginally in the presence of anions. This behavior might be explained by the electrostatic attraction of anions to the −vely charged attapulgite/BC surface, which diminished the material's ability to adsorb E2.116 Other research groups119–122 also obtained in another study that the presence of the two cations is common in effluents (Ca2+ and Na+); effluents did not significantly influence SE removal efficiency when Fe3+-saturated montmorillonite, binary oxide of Fe–Mn/MWCNT, K2FeO4 modified Lotus seedpod BC and green tea bio-fabricated rGO@Fe NPs were employed. A few findings18,90 have also alluded to the fact that natural organic matter carries an overall −ve charge owing to a lot of carboxyl and phenolic moieties in its structure, which makes it exhibit a negligible competitive effect with infinitesimal variation in adsorption capacity90,93,121,123 or form interactions (NOM-SE and NOM-adsorbent) that considerably diminish the adsorption of SE; this is because of the polar functionalities that are generated on the surface of the BC following bonding with NOM, which stimulates the materialization of water clusters around the adsorbent surface through a far-reaching H-bonding network and leads to lose of adsorbent hydrophobicity and SE-adsorbent hydrophobic interaction.94,95,123
It is imperative to say that in our view, different adsorbing materials respond differently to different activating agents, and this accounts for variation in the adsorbing capacity/efficiency of different chemically activated adsorbents reported.
Going forward, the advent of the fabrication of biogenic NPs using biological entities (plant and microorganisms)124–126 has also resulted in jaw-dropping adsorption records of many organic pollutants, including steroid estrogens.
For instance, Gong et al.127 studied the use of carbonized green bimetallic Fe/Ni NPs biosynthesized using eucalyptus leaf extract to adsorb E2 from an aqueous solution. The carbonized biogenic Fe/Ni NPs efficiently adsorbed E2 in less than half an hour according to the batch adsorbent studies, with optimum adsorption performance of 98.3%. Compared to other adsorption efficiencies of other adsorbents reported in Table 1, this outstanding efficiency by the biosynthesized NPs was partly attributed to their improved specific surface area and better porous architecture following carbonization; the BET study revealed that the surface area of green Fe/Ni NPs improved from 31.99 m2 g−1 before carbonization to 57.57 m2 g−1 upon carbonization. In addition, as shown in Fig. 15, the surface of carbonized-Fe/Ni NPs still has many oxygen-containing functional groups derived from the bioactive compounds present in the eucalyptus leaf extract, which may have a combinatory effect to expedite better E2 adsorptive removal. Unfortunately, only 48.7% and 51.8% of E2 were removed from pig runoffs and domestic sewage, respectively, when the same novel biogenic NPs were applied in a real-life scenario. However, the authors127 justified this low removal percentage by considering the massive volumes of dissolved organic materials that are often present in raw effluents, which hypothetically influence the adsorptive removal of E2 in a negative way. This is due to the presence of highly electron-loaded functional groups, such as phenolic and aniline, in dissolved organic materials, resulting in a high propensity to participate in various environmental reactions and thus effortlessly react with the Fe3+ surface. Thus, one of the causes of the decrease in E2 adsorption effectiveness in both pig runoffs and domestic sewage compared to that reported for ultrapure water is the presence of dissolved organic matter in raw effluents.127 This justification is consistent with what was earlier established by Qin's group119 in their studies using Fe3+-saturated montmorillonite, even though the efficiency (93%) reported is less than the 98.30% and 99% reported for carbonized biogenic Fe/Ni NPs127 and green tea bio-fabricated rGO@Fe NPs, respectively.120
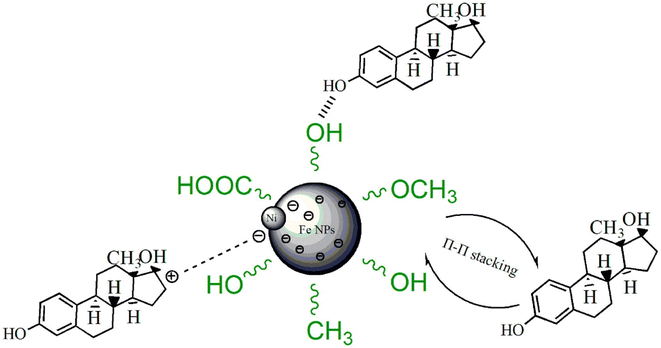 | ||
| Fig. 15 Possible mechanism of carbonized biogenic Fe/Ni NP combinatorial adsorption of E2.127 Reused with permission from Elsevier (order number: 5577531212233). | ||
To summarise, as seen in the adsorption efficiency discussion in this concluded section, it is obvious that in the chemistry of SE adsorption, the major challenge is how to select the most promising types of adsorbents, as per low cost, benignness, high adsorption capacity, high adsorption rate, and rapid kinetics; to overcome these challenges, a grounded understanding of the SE adsorption mechanism is required. Moreover, understanding the SE adsorption mechanism offers a win–win insight into designing an outstanding desorption strategy for the recovery of adsorbed SE and the recyclability of spent adsorbents.128 However, in a bid to identify the SE adsorption mechanism(s) (particularly the interactions occurring at the adsorbent/SE interface) to easily overcome those aforementioned challenges and take the SE adsorption process to the next level, this also becomes another real challenge.128 In general, amid all the holistic and pragmatic explanations of SE adsorption presented in the foregoing paragraphs, its mechanisms are not fully understood because many interactions are possible, as shown in Fig. 16 (ref. 18, 88, 92, 93, 104, 105, 123) and Table 1. These interactions are largely governed by the experimental settings (temperature, kinetics, adsorbent dose, pH, and background electrolytes), the SE profile (mixability/dissolution rate, molecular weight, and pKa), and the adsorbent profile (surface architectural functional moieties and surface area). Thus, this conclusively left us with an intriguing query that overlaps with that of Crini et al.128 about whether all the aforementioned interactions have to be considered to fully comprehend the SE adsorption mechanism. In our view, this question's response is somewhat complicated. Depending on the adsorbent architectural makeup, the SE makeup and its characteristics, the solution pH, temperature, and ionic strength, it is feasible that more than one of these interactions can occur concurrently in the adsorption process of SE, as shown in the report of various literature presented in Table 1.
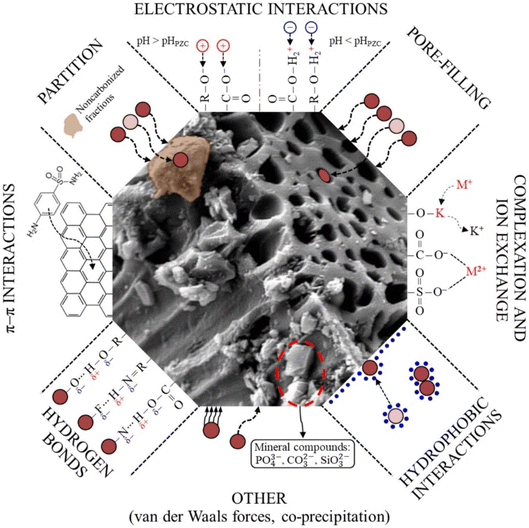 | ||
| Fig. 16 Plausible mechanistic adsorbent-SE interactions.129 Reused with permission from Elsevier (order number: 557740119124). | ||
5. Desorption and reusability
Adsorbents after adsorbing contaminants may still be potentially harmful to the ecological system because adsorption is merely a physicochemical operation that encompasses the mass transfer of a solute from the liquid phase to the surface of the adsorbing material; thus, the regeneration and recyclability of spent adsorbent is a proficient mechanism towards achieving a practicable, reliable, effective, industrial and eco-economical SE adsorptive remediation technique.75,93,108,120,134 The regeneration entails removing spent adsorbent from the reaction mixture and then removing loaded SE from the surface using an eluting reagent or instrument, such as a centrifuge.75,134 An ethanol solution was employed as an eluting solvent by Beri et al.68 to remove EE2 from palm kernel shell AC. The palm kernel shell AC was recovered from the regeneration solution by filtration, washed with water, and dried at 80 °C for 720 minutes. The number of adsorption–regeneration cycles was used to test reusability. The result revealed that the regeneration process was not effective enough, and the desorption of EE2 reduced to 32% after four cycles.68 This significant reduction in adsorption capacity is in parallel with what was reported when distilled water and acetonitrile were used in four cycles of adsorption–desorption of rice husk silica and E1 and E2.82 The high activation energy (60.4 kJ mol−1) of EE2 sorption, which suggests that chemisorption is the primary process occurring on the palm kernel shell AC adsorbent, may be used to justify the dramatic decrease in the EE2 sorption process after the fourth cycle. Notably, according to the authors,68 chemisorbed SE is considerably tougher to desorb from the absorbing material than a physisorbed one. However, it was opined that increasing the temperature of the regeneration process should enhance the removal of EE2.68,74 explored a regeneration study on the E2 loaded on Fe3O4@SiO2@MPS by applying different eluting solvents, such as methanol, ethanol, acetone, and acetonitrile. It was observed that the optimal removal of E2 was at best for methanol, followed by ethanol, then acetonitrile and acetone. It was then observed for the methanol desorption experiment that the binding ability of Fe3O4@SiO2@MPS for E2 hardly decreased after about five times of reuse.In another study by Tang et al.,97 after six cycles of using the β-cyclodextrin polymer and γ-cyclodextrin polymer adsorbents, the desorption proficiency of the regenerated β-cyclodextrin polymer and γ-cyclodextrin polymer was sustained above 99%, and almost no reduction was witnessed after five adsorption-regeneration successions, thereby making β- and γ-cyclodextrin polymers appropriate and efficacious polymeric adsorbing materials for E2 and EE2 removal from aqueous solutions.
Furthermore, magnetization is a value-added strategy that is included for the simple reclamation of the used adsorbing material. In various studies, magnetically engineered adsorbents have been employed by researchers for the sorption of SE and have shown remarkable reusability.88,93,94 For example, in a study conducted by Dong's team,93 ozone treatment, which broke down the adsorbed E2 on the surface of adsorbents, was used to regenerate BC/Fe2O3NPs. Each cycle involved the reaction of 1 mg L−1 of E2 and 0.1 mg mL−1 of BC/Fe2O3NPs for 25 min at 25 °C. BC/Fe2O3NPs were retrieved by magnet separation after reaching adsorption equilibrium. This was done after purging with an ozone gas (10 g h−1) for 10 minutes and washing with deionized water three times. The subsequent E2 adsorption process by the renewed BC/Fe2O3NPs required the addition of a new E2 solution. As shown in Table 2, there was no considerable reduction in the sorption efficiency of the BC/Fe2O3NP adsorbent after five sorption-regeneration series. A similar insignificant diminution in the sorption efficiency of adsorbents after five adsorption-regeneration cycles was observed by other researchers.88,108,109,116 Table 2 provides an overview of desorption and recycling in the available studies. Various eluting reagents, such as NaOH,18 HCl,108 HNO3,116 ethanol,74,94,109,116 methanol,74,91 acetic acid,91 acetonitrile,74,82 and acetone74 have been used to desorb different SEs from spent adsorbing materials.
| Adsorbent | SE | Eluent | % Adsorbed (at n = 1) | No. of cycles | % Adsorbed after n cycles | References |
|---|---|---|---|---|---|---|
| BC/Fe2O3NPs | E2 | Magnet + ozone gas | >90 | 5 | 89.26 | 93 |
| SBA-rGO-mPEG | E2 | acidic ethanol (adjusted with 0.1 M HCl) | 90 | 4 | 86 | 75 |
| Palm kernel shells AC | EE2 | 20 mL of 4 mg−1 of ethanol | 54.60 | 5 | 32.30 | 68 |
| Fe3O4@SiO2@MPS | E2 | 10 mL methanol | 98 | 5 | >85 | 74 |
| Rice husk silica | E1 | Double-distilled water and acetonitrile | 93.10 | 4 | 5 | 82 |
| E2 | Double-distilled water and acetonitrile | 95.50 | 4 | 3.5 | ||
| GO-magnetic rice straw BC | E2 | 100 mL ethanol | 100 | 5 | 87 | 94 |
| Activated magnetic rice straw BC | E2 | 100 mL ethanol deionized water | 100 | 5 | >80 | 88 |
| β-Cyclodextrin polymers | E2 | 10 mL ethanol | >99 | 5 | >99 | 97 |
| EE2 | 10 mL ethanol | >99 | 5 | >99 | ||
| γ-Cyclodextrin polymers | E2 | 10 mL ethanol | >99 | 5 | >99 | 97 |
| EE2 | 10 mL ethanol | >99 | 5 | >99 | ||
| Poly(HEMA-MAPA) microparticles | E2 | Acetonitrile![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol (70 methanol (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30; v/v) 30; v/v) |
— | 10 | 92.70 | 98 |
| Mesoporous imprinted polymer | E1 | 1.25 mL of MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetic acid (9 acetic acid (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) 1, v/v) |
100 | 5 | 84.60 | 91 |
| E3 | 1.25 mL of MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetic acid (9 acetic acid (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) 1, v/v) |
100 | 5 | 84.6 | ||
| CeO2/CuFe2O4 nanofibers | EE2 | 0.1 mol L−1 HNO3 | 97.50 | 6 | >92 | 108 |
| Mesoporous carbons | EE2 | 10 mL ethanol | 100 | 10 | >85 | 109 |
| Attapulgite-rice straw BC | E2 | 100 mL ethanol | 99 | 5 | >89 | 116 |
| Milli-Q water | ||||||
| rGO@Fe NPs (using green tea extract) | EE2 | Centrifugation | 99.40 | 5 | 64.40 | 120 |
6. Outlook
The adsorption technique for the removal of various contaminants, including steroid estrogens, in water is a very promising area that has been explored by several researchers. Research in this area could focus on developing sustainable adsorbents with enhanced selectivity, specificity and adsorption capacity for SE removal either by modifying the existing adsorbents to improve their properties or by synthesizing materials tailored towards SEs removal.For future studies, an in-depth study is required to understand the mechanism involved in SE adsorption and to explore the potential of combining it with other water treatment techniques. Furthermore, there is a need to evaluate the practical feasibility and scalability of adsorption techniques for large-scale applications, cost-effectiveness, regeneration of spent adsorbents and the development of efficient adsorption systems for real-life water treatment applications.
Because the in situ implementation of the commonly used chemical regeneration method is not yet feasible, the management of the eluted product during the chemical regeneration of the spent adsorbents should be considered for total environmental security.
7. Conclusion
This systemic scientometric review provided a comprehensive analysis of the sequestration of SEs in aqueous samples using the adsorption mechanism by examining and analyzing a wide range of scientific publications (articles, review articles, and book chapters) on SE removal from the Web of Science (WoS) database from January 1, 1990, to November 5, 2022, and the Scopus database from January 1, 1949, to November 5, 2022, which are leading citation databases. A total of 137 documents was used to methodically map bibliometric indicators, such as the number of articles, most prolific countries, most productive scholars, and most cited articles. It emphasizes the diverse range of adsorbents utilized, including activated carbon, zeolites, biochars, and MOFs, revealing the potential of these adsorbents in efficiently removing SEs and mitigating their adverse effects on human health and aquatic ecosystems. Various factors influencing the adsorption process, such as the initial concentration, pH, temperature, and physicochemical properties of the adsorbent, were studied to decipher the adsorption mechanism. The mechanisms are mostly observed to be physisorption, hydrophobic interaction, π–π interaction, H-bonding, chemisorption, or pore filling. One significant relevance accrues to the use of adsorbents is the desorption and reusability concept, which involves the regeneration of the spent absorbent by removing the loaded SEs on the surface using an eluting agent or centrifuge. Overall, the sequestration of SEs through adsorption is a promising approach with significant potential for environmental remediation and also helps researchers with an advanced understanding of refined adsorption techniques that will contribute to the protection of water resources and the wellbeing of the human population and ecosystem.Abbreviations
| SEs: | Steroid estrogens |
| EDC: | Endocrine-disrupting chemicals |
| WHO: | World Health Organization |
| E1: | Estrone |
| E2: | 17-β-Estradiol |
| E3: | Estriol |
| EE2: | 17-α ethinylestradiol |
| (Kow): | Partition coefficient |
| NOEL: | No-observed-adverse-effect |
| SDG: | Sustainable development goals |
| OPEC: | Organisation of petroleum exporting countries |
| WoS: | Web of science |
| GO: | Graphene oxide |
| CNT: | Carbon nanotube |
| rGO: | Reduced graphene oxide |
Author contributions
Ajibola. A. Bayode: conceptualization, supervision, software, writing – original draft, review, and editing. Chijioke Olisah: writing – original draft, software, review, and editing. Stephen Sunday Emmanuel: writing – original draft, review, and editing. Morenike Oluwabunmi Adesina: writing – original draft. Koko Daniel Terlanga: writing – original draft.Conflicts of interest
There are no conflict to declare.Acknowledgements
Ajibola. A. Bayode thanks the Department of Chemical Sciences, Redeemer's University Ede. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.References
- B. Almazrouei, D. Islayem, F. Alskafi, M. K. Catacutan, R. Amna, S. Nasrat, B. Sizirici and I. Yildiz, Emerg. Contam., 2023, 100210 CrossRef CAS.
- A. A. Bayode, E. M. Vieira, R. Moodley, S. Akpotu, A. S. S. de Camargo, D. Fatta-Kassinos and E. I. Unuabonah, Chem. Eng. J., 2021, 420, 127668 CrossRef CAS.
- M. Adeel, X. Song, Y. Wang, D. Francis and Y. Yang, Environ. Int., 2017, 99, 107–119 CrossRef CAS PubMed.
- M. Bilal, K. Rizwan, M. Adeel, D. Barceló, Y. A. Awad and H. M. Iqbal, Environ. Pollut., 2022, 306, 119373 CrossRef CAS PubMed.
- L. P. Wackett, Microb. Biotechnol., 2022, 15, 773–792 CrossRef CAS PubMed.
- S. M. Khumalo, B. F. Bakare and S. Rathilal, Appl. Sci., 2022, 12, 12196 CrossRef CAS.
- J. P. Bavumiragira and H. Yin, Sci. Total Environ., 2022, 153635 CrossRef CAS.
- R. Sridharan, B. Monisha, P. S. Kumar and K. V. Gayatahri, Chemosphere, 2022, 133731 CrossRef CAS.
- A. H. Khan, N. A. Khan, M. Zubair, M. A. Shaida, M. S. Manzar, A. Abutaleb, M. Naushad and J. Iqbal, Environ. Res., 2022, 204, 112243 CrossRef CAS PubMed.
- A. Adewuyi, Water, 2020, 12, 1551 CrossRef CAS.
- M. M. S. Yazdan, R. Kumar and S. W. Leung, Ecologies, 2022, 3, 206–224 CrossRef.
- O. T. Ore, A. O. Adeola, A. A. Bayode, D. T. Adedipe and P. N. Nomngongo, Environ. Chem. Ecotoxicol., 2023, 5, 9–23 CrossRef CAS.
- A. S. Adeleye, J. Xue, Y. Zhao, A. A. Taylor, J. E. Zenobio, Y. Sun, Z. Han, O. A. Salawu and Y. Zhu, J. Hazard. Mater., 2022, 424, 127284 CrossRef CAS PubMed.
- A. Priya, L. Gnanasekaran, S. Rajendran, J. Qin and Y. Vasseghian, Environ. Res., 2022, 204, 112298 CrossRef CAS PubMed.
- D. Azizi, A. Arif, D. Blair, J. Dionne, Y. Filion, Y. Ouarda, A. G. Pazmino, R. Pulicharla, V. Rilstone and B. Tiwari, Environ. Res., 2022, 207, 112196 CrossRef CAS PubMed.
- Q. Liu, Y. Zhou, J. Lu and Y. Zhou, Chemosphere, 2020, 241, 125043 CrossRef CAS PubMed.
- M. O. Alfred, M. O. Omorogie, O. Bodede, R. Moodley, A. Ogunlaja, O. G. Adeyemi, C. Günter, A. Taubert, I. Iermak, H. Eckert, I. D. A. Silva, A. S. S. de Camargo, A. de Jesus Motheo, S. M. Clarke and E. I. Unuabonah, Chem. Eng. J., 2020, 398, 125544 CrossRef.
- C. Peiris, S. Nawalage, J. J. Wewalwela, S. R. Gunatilake and M. Vithanage, Environ. Res., 2020, 191, 110183 CrossRef CAS PubMed.
- A. Adewuyi and W. J. Lau, in Handbook of nanotechnology applications, Elsevier, 2021, pp. 67–97 Search PubMed.
- A. A. Bayode, D. M. dos Santos, M. O. Omorogie, O. D. Olukanni, R. Moodley, O. Bodede, F. O. Agunbiade, A. Taubert, A. S. de Camargo and H. Eckert, J. Water Process. Eng., 2021, 40, 101865 CrossRef.
- K. He, E. Hain, A. Timm and L. J. Blaney, Sci. Total Environ., 2021, 764, 142871 CrossRef CAS PubMed.
- M. Bilal and H. M. N. Iqbal, Sci. Total Environ., 2019, 690, 447–459 CrossRef CAS PubMed.
- R. F. Salla, F. U. Gamero, R. Z. Rissoli, S. E. Dal-Medico, L. M. Castanho, C. d. S. Carvalho, E. C. M. Silva-Zacarin, A. L. Kalinin, F. C. Abdalla and M. J. Costa, Chemosphere, 2016, 144, 1862–1868 CrossRef CAS PubMed.
- M. Giulivo, M. L. de Alda, E. Capri and D. Barceló, Environ. Res., 2016, 151, 251–264 CrossRef CAS PubMed.
- K. E. Arnold, A. R. Brown, G. T. Ankley and J. P. Sumpter, Philos. Trans. R. Soc. B, 2014, 369, 20130569 CrossRef PubMed.
- E. Rose, K. A. Paczolt and A. G. Jones, Evol. Appl., 2013, 6, 1160–1170 CrossRef CAS PubMed.
- L. Zhao, J. Deng, P. Sun, J. Liu, Y. Ji, N. Nakada, Z. Qiao, H. Tanaka and Y. Yang, Sci. Total Environ., 2018, 627, 1253–1263 CrossRef CAS PubMed.
- R. Kumar, M. Qureshi, D. K. Vishwakarma, N. Al-Ansari, A. Kuriqi, A. Elbeltagi, A. Saraswat and Engineering, Case Stud. Chem. Environ. Eng., 2022, 6, 100219 CrossRef CAS.
- J. Liang, Y. Luo, B. Li, S. Liu, L. Yang, P. Gao, L. Feng, Y. Liu, Z. Du and L. Zhang, Front. Environ. Sci. Eng., 2022, 16, 126 CrossRef CAS.
- M. M. Cook, E. M. Symonds, B. Gerber, A. Hoare, E. S. Van Vleet and M. Breitbart, Water, 2016, 8, 128 CrossRef.
- L. Tian, M. Zhu, L.-S. Zhang, L.-J. Zhou, J.-P. Fan, D.-S. Wu and J.-P. Zou, J. Environ. Chem. Eng., 2022, 10, 107414 CrossRef CAS.
- H. Xu, Y. Han, G. Wang, P. Deng and L. Feng, Environ. Technol. Innov., 2022, 28, 102870 CrossRef CAS.
- R. Lyubimenko, A. Turshatov, A. Welle, P. G. Weidler, B. S. Richards and A. I. Schäfer, Chem. Eng. J., 2023, 451, 138449 CrossRef CAS.
- M. Tagliavini, Removal of steroid hormones from water by permeate-side polymer-based spherical activated carbon assisted membrane filtration, Karlsruher Institut für Technologie (KIT), 2022 Search PubMed.
- C. G. Moreira, L. C. de Souza, T. C. Castor Neto, G. Gomes, D. M. Bila and F. V. Fonseca, Environ. Technol., 2022, 1–13 CrossRef PubMed.
- C. G. Moreira, T. C. Neto, D. M. Bila and F. V. Fonseca, Int. J. Environ. Res., 2023, 17, 1 CrossRef CAS.
- A. A. Bayode, M. T. Folorunso, B. Helmreich and M. O. Omorogie, ACS Omega, 2023, 7956 CrossRef CAS PubMed.
- A. A. Bayode, F. O. Agunbiade, M. O. Omorogie, R. Moodley, O. Bodede and E. I. Unuabonah, Environ. Sci. Pollut. Res., 2020, 27, 9957–9969 CrossRef CAS.
- L. Olena, L. Tetyana, N. Mykola and P. V. Pysarenko, Water Res., 2018, 180 Search PubMed.
- B. Almazrouei, D. Islayem, F. Alskafi, M. K. Catacutan, R. Amna, S. Nasrat, B. Sizirici and I. Yildiz, Emerging Contam., 2023, 100210 CrossRef CAS.
- M. Ciślak, I. Kruszelnicka, J. Zembrzuska and D. Ginter-Kramarczyk, Water Res., 2022, 119413 Search PubMed.
- T. Huanyu, S. Jianghong, G. Wei, Z. Jiawei, G. Hui and W. Yunhe, Environ. Res., 2022, 113849 CrossRef PubMed.
- S. Lu, K. Zhu, H. Guo, H. Wang, N. Zhang, A. Alsaedi, T. Hayat, Y. Li and Y. Sun, Chem. Eng. J., 2019, 368, 598–605 CrossRef CAS.
- P. Ghosh and A. Saha, Chem. Biol. Interface, 2019, 9(5), 225–233 CAS.
- M. Adeel, X. Song, Y. Wang, D. Francis and Y. Yang, Environ. Int., 2017, 99, 107–119 CrossRef CAS.
- Z. Syed, M. Sogani, A. Dongre, A. Kumar, K. Sonu, G. Sharma and A. B. Gupta, Sci. Total Environ., 2021, 780, 146544 CrossRef CAS PubMed.
- X. Bai, Fate and transport of an estrogen conjugate 17β-estradiol-17-sulfate in soil-water systems, North Dakota State University, 2013 Search PubMed.
- M. Królik and H. Milnerowicz, Adv. Clin. Exp. Med., 2012, 21, 535–543 Search PubMed.
- L. Racz and R. K. Goel, J. Environ. Monit., 2010, 12, 58–70 RSC.
- P. Okkerman, C. Groshart, A. Pijnenburg and Rapportnr, Chemical study on estrogens, 2001, vol. 28, p. 2001 Search PubMed.
- K. Li, J. Rollins and E. Yan, Scientometrics, 2018, 115, 1–20 CrossRef PubMed.
- C. Olisah, O. O. Okoh and A. I. Okoh, Heliyon, 2018, 4, e00964 CrossRef PubMed.
- M. M. Kessler, Am. Doc., 1963, 14, 10–25 CrossRef.
- Y. Zou, Y. Luo, J. Zhang, N. Xia, G. Tan and C. Huang, Medicine, 2019, 98 Search PubMed.
- L. Racz and R. K. Goel, J. Environ. Monit., 2010, 12, 58–70 RSC.
- T. C. Ekundayo and A. I. Okoh, PLoS One, 2018, 13, e0207655 CrossRef PubMed.
- M. Xu, H. Huang, N. Li, F. Li, D. Wang and Q. Luo, Ecotoxicol. Environ. Saf., 2019, 175, 289–298 CrossRef CAS PubMed.
- W. Jiang, Y. Yan, M. Ma, D. Wang, Q. Luo, Z. Wang and S. K. Satyanarayanan, J. Environ. Sci., 2012, 24, 320–328 CrossRef CAS.
- K. Rao, B. Lei, N. Li, M. Ma and Z. Wang, J. Environ. Sci., 2013, 25, 1164–1171 CrossRef CAS PubMed.
- Z. Yan, H. Yang, H. Dong, B. Ma, H. Sun, T. Pan, R. Jiang, R. Zhou, J. Shen and J. Liu, Environ. Pollut., 2018, 239, 223–232 CrossRef CAS PubMed.
- Y. Zhang, B. Wang, G. Cagnetta, L. Duan, J. Yang, S. Deng, J. Huang, Y. Wang and G. Yu, Water Res., 2018, 140, 291–300 CrossRef CAS PubMed.
- Y. Zhao, C. Zhang, Z. Yang, Y. Yang, N. Huang, J. E. Arku, G. Mao and Y. Wang, J. Water Process. Eng., 2021, 41, 102004 CrossRef.
- N. A. Vasilevsky, M. Hosseini, S. Teplitzky, V. Ilik, E. Mohammadi, J. Schneider, B. Kern, J. Colomb, S. C. Edmunds and K. Gutzman, Account. Res., 2021, 28, 23–43 CrossRef PubMed.
- B. Pan, D. Lin, H. Mashayekhi and B. Xing, Environ. Sci. Technol., 2008, 42, 5480–5485 CrossRef CAS.
- L. Nielsen, M. J. Biggs, W. Skinner and T. J. Bandosz, Carbon, 2014, 80, 419–432 CrossRef CAS.
- P. K. Singh, R. Bhatacharjya, J. Lakshmi, I. S. Thakur and A. Tiwari, Environ. Monit. Assess., 2022, 820 Search PubMed.
- C. L. S. Vilela, J. P. Bassin and R. S. Peixoto, Environ. Pollut., 2018, 235, 546–559 CrossRef CAS.
- K. Y. V. Beri, D. P. Barbosa, M. Zbair, S. Ojala and S. B. de Oliveira, Energy Nexus, 2021, 1, 100009 CrossRef CAS.
- C. Wijewardana, A. K. Gonzales, C. M. Vogel and N. E. Martinez, Agrosyst., Geosci. Environ., 2022, 5, e20258 CAS.
- K. Patni, C. Pande and T. Joshi, Resilience, Response, and Risk in Water Systems: Shifting Management and Natural Forcings Paradigms, 2020, pp. 151–171 Search PubMed.
- S. Barreca, M. Busetto, L. Colzani, L. Clerici, D. Daverio, P. Dellavedova, S. Balzamo, E. Calabretta and V. Ubaldi, Microchem. J., 2019, 147, 1186–1191 CrossRef CAS.
- J. C. Sousa, A. R. Ribeiro, M. O. Barbosa, C. Ribeiro, M. E. Tiritan, M. F. R. Pereira and A. M. Silva, Sci. Total Environ., 2019, 649, 1083–1095 CrossRef CAS.
- D. Prokić, M. Vukčević, A. Kalijadis, M. Maletić, B. Babić and T. Đurkić, Fibers Polym., 2020, 21, 2263–2274 CrossRef.
- L. Bi, J. Shen, Z. Yao, J. Kang, S. Zhao, P. Yan, B. Wang and Z. Chen, Separations, 2022, 9, 381 CrossRef CAS.
- S. O. Akpotu, I. A. Lawal, B. Moodley and A. E. Ofomaja, Chemosphere, 2020, 246, 125729 CrossRef CAS PubMed.
- L. Joseph, J. Heo, Y.-G. Park, J. R. V. Flora and Y. Yoon, Desalination, 2011, 281, 68–74 CrossRef CAS.
- L. Wang, G. Chen, H. Shu, X. Cui, Z. Luo, C. Chang, A. Zeng, J. Zhang and Q. Fu, J. Chromatogr. A, 2021, 1638, 461889 CrossRef CAS PubMed.
- Y.-H. Ge, H. Shu, X.-Y. Xu, P.-Q. Guo, R.-L. Liu, Z.-M. Luo, C. Chang and Q. Fu, Mater. Sci. Eng., C, 2019, 97, 650–657 CrossRef CAS PubMed.
- X. Dong, J. Yang, X.-T. Zhen, Y. Chen, H. Zheng and J. Cao, J. Pharm. Biomed. Anal., 2020, 188, 113461 CrossRef CAS PubMed.
- Z. Li, L. Sellaoui, D. Franco, M. S. Netto, J. Georgin, G. L. Dotto, A. Bajahzar, H. Belmabrouk, A. Bonilla-Petriciolet and Q. Li, Chem. Eng. J., 2020, 389, 124467 CrossRef CAS.
- M. N. Nguyen, P. B. Trinh, C. J. Burkhardt and A. I. Schäfer, Sep. Purif. Technol., 2021, 264, 118405 CrossRef CAS.
- M. Zarghi, A. Roudbari, S. Jorfi and N. Jaafarzadeh, Chem. Biochem. Eng. Q., 2019, 33, 281–293 CrossRef CAS.
- Y. Kong, Y. Huang, C. Meng and Z. Zhang, RSC Adv., 2018, 8, 31440–31454 RSC.
- L. Jiang, Y. Liu, G. Zeng, S. Liu, W. Que, J. Li, M. Li and J. Wen, Chem. Eng. J., 2018, 343, 371–378 CrossRef CAS.
- J. Ferandin Honorio, M. T. Veit, P. Y. R. Suzaki, P. F. Coldebella, E. Sloboda Rigobello and C. R. G. Tavares, Environ. Technol., 2020, 41, 1075–1092 CrossRef CAS.
- S. A. Abdel-Gawad and H. M. Abdel-Aziz, Appl. Water Sci., 2019, 9, 75 CrossRef.
- L. Jiang, Y. Liu, G. Zeng, S. Liu, X. Hu, L. Zhou, X. Tan, N. Liu, M. Li and J. Wen, Chem. Eng. J., 2018, 339, 296–302 CrossRef CAS.
- Z. Yin, Y. Liu, S. Liu, L. Jiang, X. Tan, G. Zeng, M. Li, S. Liu, S. Tian and Y. Fang, Sci. Total Environ., 2018, 639, 1530–1542 CrossRef CAS PubMed.
- K. O. Iwuozor, E. C. Emenike, C. O. Aniagor, F. U. Iwuchukwu, E. M. Ibitogbe, O. B. Temitayo, P. E. Omuku and A. G. Adeniyi, Curr. Res. Green Sustainable Chem., 2022, 100300 CrossRef CAS.
- N. Liu, Y. Liu, G. Zeng, J. Gong, X. Tan, S. Liu, L. Jiang, M. Li and Z. Yin, J. Environ. Sci., 2020, 87, 10–23 CrossRef CAS PubMed.
- H. L. de Oliveira, B. C. Pires, L. S. Teixeira, L. A. F. Dinali, T. A. do Nascimento and K. B. Borges, Colloids Surf., A, 2020, 590, 124506 CrossRef.
- M. B. Ahmed, J. L. Zhou, H. H. Ngo, M. A. H. Johir and K. Sornalingam, Chem. Eng. J., 2018, 335, 801–811 CrossRef CAS.
- X. Dong, L. He, H. Hu, N. Liu, S. Gao and Y. Piao, Chem. Eng. J., 2018, 352, 371–379 CrossRef CAS.
- S. Liu, M. Li, Y. Liu, N. Liu, X. Tan, L. Jiang, J. Wen, X. Hu and Z. Yin, J. Taiwan Inst. Chem. Eng., 2019, 102, 330–339 CrossRef CAS.
- H.-y. Tao, H. Ge, J. Shi, X. Liu, W. Guo, M. Zhang, Y. Meng and X.-y. Li, Environ. Geochem. Health, 2020, 42, 1601–1615 CrossRef CAS PubMed.
- P. Gao, C. Yang, Z. Liang, W. Wang, Z. Zhao, B. Hu and F. Cui, Chemosphere, 2019, 214, 361–370 CrossRef CAS PubMed.
- P. Tang, Q. Sun, Z. Suo, L. Zhao, H. Yang, X. Xiong, H. Pu, N. Gan and H. Li, Chem. Eng. J., 2018, 344, 514–523 CrossRef CAS.
- O. Kireç, İ. Alacabey, K. Erol and H. Alkan, J. Polym. Eng., 2021, 41, 226–234 CrossRef.
- P. Deng, G. Wang, C. Li, S. Dou and W. Yuan, Water Sci. Technol., 2022, 85, 3259–3270 CrossRef CAS PubMed.
- J. F. Honorio, M. T. Veit, P. Y. R. Suzaki, C. R. G. Tavares, J. C. Z. Barbieri, F. de Oliveira Tavares and E. B. Lied, J. Environ. Chem. Eng., 2022, 10, 107995 CrossRef CAS.
- J. F. Honorio, M. T. Veit, P. Y. R. Suzaki, P. F. Coldebella, E. S. Rigobello and C. R. G. Tavares, Environ. Technol., 2018, 1075–1092 Search PubMed.
- K. D. Elias, I. P. Ejidike, F. M. Mtunzi and V. E. Pakade, J. Chem. Thermodyn., 2021, 3, 100013 Search PubMed.
- G. Liu, Z. Zhang, C. Yan, Y. Wang, X. Ma, P. Gao and Y. Feng, Chemosphere, 2018, 207, 534–542 CrossRef CAS.
- S. Liu, Y. Liu, L. Jiang, G. Zeng, Y. Li, Z. Zeng, X. Wang and Q. Ning, J. Environ. Manage., 2019, 236, 25–33 CrossRef CAS PubMed.
- S.-r. Tian, Y.-g. Liu, S.-b. Liu, G.-m. Zeng, L.-h. Jiang, X.-f. Tan, X.-x. Huang, Z.-h. Yin, N. Liu and J. Li, RSC Adv., 2018, 8, 4273–4283 RSC.
- K. B. Debs, H. D. T. da Silva, M. de Lourdes Leite de Moraes, E. N. V. M. Carrilho, S. G. Lemos and G. Labuto, Environ. Sci. Pollut. Res., 2019, 26, 28419–28428 CrossRef CAS PubMed.
- S. Zhong, S. Zhang, Y. Zhang and C. Li, J. Mater. Sci.: Mater. Electron., 2019, 30, 20410–20419 CrossRef CAS.
- S. Sobhanardakani and R. Zandipak, Sep. Sci. Technol., 2018, 53, 2339–2351 CrossRef CAS.
- R. C. F. Silva, P. S. Pinto and A. P. de Carvalho Teixeira, Chem. Eng. J., 2021, 407, 127219 CrossRef CAS.
- M. Tagliavini, P. G. Weidler, C. Njel, J. Pohl, D. Richter, B. Böhringer and A. I. Schäfer, Water Res., 2020, 185, 116249 CrossRef CAS.
- H. Xu, Y. Han, G. Wang, P. Deng and L. Feng, Environ. Technol. Innovation, 2022, 28, 102870 CrossRef CAS.
- M. Tagliavini and A. I. Schäfer, J. Hazard. Mater., 2018, 353, 514–521 CrossRef CAS PubMed.
- J. Wolters, M. Tagliavini and A. I. Schäfer, J. Membr. Sci., 2019, 592, 117315 CrossRef CAS.
- D. S. Martins, B. R. Estevam, I. D. Perez, J. H. P. Américo-Pinheiro, W. D. Isique and R. F. Boina, J. Environ. Chem. Eng., 2022, 10, 108090 CrossRef CAS.
- C. Lai, H. He, W. Xie, S. Fan, H. Huang, Y. Wang, B. Huang and X. Pan, J. Hazard. Mater., 2022, 423, 127066 CrossRef CAS PubMed.
- Z. Yin, Y. Liu, X. Tan, L. Jiang, G. Zeng, S. Liu, S. Tian, S. Liu, N. Liu and M. Li, Process Saf. Environ. Prot., 2019, 121, 155–164 CrossRef CAS.
- W. Guo, S. Lu, J. Shi and X. Zhao, Ecotoxicol. Environ. Saf., 2019, 174, 363–369 CrossRef CAS PubMed.
- R. C. F. Silva, J. D. Ardisson, A. A. C. Cotta, M. H. Araujo and A. P. de Carvalho Teixeira, Environ. Pollut., 2020, 260, 114099 CrossRef CAS.
- C. Qin, C. Shang and K. Xia, Chemosphere, 2019, 224, 480–486 CrossRef CAS.
- L. Liu, Q. Wang, J. Lin, G. Owens and Z. Chen, Process Saf. Environ. Prot., 2022, 159, 53–60 CrossRef CAS.
- N. Liu, Y. Liu, X. Tan, M. Li, S. Liu, X. Hu, P. Zhang, M. Dai, W. Xu and J. Wen, Sci. Total Environ., 2020, 715, 136723 CrossRef CAS PubMed.
- M.-y. Dai, Y.-g. Liu, G.-m. Zeng, S.-b. Liu and Q.-m. Ning, Environ. Sci. Pollut. Res., 2019, 26, 7614–7626 CrossRef CAS PubMed.
- P. Regkouzas and E. Diamadopoulos, Chemosphere, 2019, 224, 840–851 CrossRef CAS PubMed.
- S. S. Emmanuel and A. A. Adesibikan, Water Environ. Res., 2021, 93, 2873–2882 CrossRef CAS PubMed.
- S. S. Emmanuel, A. A. Adesibikan and O. D. Saliu, Appl. Organomet. Chem., 2023, 37, e6946 CrossRef CAS.
- S. S. Emmanuel, A. A. Adesibikan, O. D. Saliu and E. A. Opatola, Plant Nano Biology, 2023, 3, 100024 CrossRef.
- K. Gong, Y. Lin, P. Wu, X. Jin, G. Owens and Z. Chen, Chemosphere, 2022, 291, 132777 CrossRef CAS PubMed.
- G. Crini, E. Lichtfouse, L. D. Wilson and N. Morin-Crini, Environ. Chem. Lett., 2019, 17, 195–213 CrossRef CAS.
- C. E. Barquilha and M. C. Braga, Bioresour. Technol. Rep., 2021, 15, 100728 CrossRef CAS.
- A. Ghaffar Rana, M. Zahid Hussain, N. Hammond, S. Vlad Luca, R. A. Fischer and M. Minceva, ChemistrySelect, 2022, 7, e202201909 CrossRef CAS.
- L. Liu, J. Lin, G. Owens and Z. Chen, Sep. Purif. Technol., 2022, 283, 120222 CrossRef CAS.
- H. Gallouze, D.-E. Akretche, C. Daniel, I. Coelhoso and J. G. Crespo, Environ. Eng. Sci., 2021, 38, 4–14 CrossRef CAS.
- J. Xu, K. Li, S. Zhang, Y. Chen and L. Ning, ACS Appl. Nano Mater., 2020, 3, 3646–3651 CrossRef CAS.
- J. O. Ighalo, P. A. Sagboye, G. Umenweke, O. J. Ajala, F. O. Omoarukhe, C. A. Adeyanju, S. Ogunniyi and A. G. Adeniyi, Environ. Nanotechnol., Monit. Manage., 2021, 15, 100443 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra02296j |
| This journal is © The Royal Society of Chemistry 2023 |