Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The construction of a heterostructured RGO/g-C3N4/LaCO3OH composite with enhanced visible light photocatalytic activity for MO degradation†
Deng Guab,
Yuanjin Wanga,
Zhiman Lianga,
Yanting Doua,
Zhenhe Xu *a,
Jiqi Zheng*a,
Yaguang Sun
*a,
Jiqi Zheng*a,
Yaguang Sun b,
Fu Ding
b,
Fu Ding *b and
Yu Gao*a
*b and
Yu Gao*a
aCollege of Environmental and Chemical Engineering, Dalian University, Dalian 116622, China. E-mail: xuzh@syuct.edu.cn; jiqizheng@yeah.net; gaoy777@126.com
bKey Laboratory of Inorganic Molecule-Based Chemistry of Liaoning Province, Shenyang University of Chemical Technology, Shenyang, 110142, China. E-mail: dingfu@syuct.edu.cn
First published on 19th May 2023
Abstract
The construction of a heterojunction and the introduction of a cocatalyst can both promote the transfer of photogenerated electrons, which are effective strategies to enhance photocatalytic efficiency. In this paper, a ternary RGO/g-C3N4/LaCO3OH composite was synthesized by constructing a g-C3N4/LaCO3OH heterojunction and introducing a non-noble metal cocatalyst RGO through hydrothermal reactions. TEM, XRD, XPS, UV-vis diffuse reflectance spectroscopy, photo-electrochemistry and PL tests were carried out to characterize the structures, morphologies and carrier separation efficiencies of products. Benefiting from the boosted visible light absorption capability, reduced charge transfer resistance and facilitated photogenerated carrier separation, the visible light photocatalytic activity of the ternary RGO/g-C3N4/LaCO3OH composite was effectively improved, resulting in a much increased MO (methyl orange) degradation rate of 0.0326 min−1 compared with LaCO3OH (0.0003 min−1) and g-C3N4 (0.0083 min−1). Moreover, by combining the results of the active species trapping experiment with the bandgap structure of each component, the mechanism of the MO photodegradation process was proposed.
1. Introduction
Environmental pollution is one of the main challenges for the rapid development of a green economy.1 Photocatalysis based on semiconductors is considered as a promising technology with wide application prospects to address environmental problems.2 However the development of photocatalysts with low cost, high stability and efficiency remains a challenge.3 Though rare earth-based materials including YVO4,4 CeO2 (ref. 5) and LaCO3OH6 have attracted wide attention in photocatalysis, their application is still limited because of their large bandgaps, leading to a poor ability in visible light absorption.7 The construction of heterojunctions,8,9 the introduction of cocatalysts10 and elemental doping11 are effective strategies to decrease the bandgaps of rare earth photocatalysts and hence improve the utilization efficiency of visible light and accelerate the kinetics of photocatalytic process.LaCO3OH is a rare earth hydroxyl carbonate with an excellent luminescence property, showing a great potential in photocatalytic pollutants degradation and H2 evolution.12,13 LaCO3OH has a relatively broad bandgap of 4.0 eV, which could inhibit the recombination of photogenerated carriers.14 On the other side, as a result of the broad bandgap, it can only absorb ultraviolet light in photocatalytic reactions. Based on recent research, the visible light absorption capability of LaCO3OH can be boosted by constructing heterojunction.15 Under visible light irradiation, pure LaCO3OH showed a negligible photocatalytic activity, while heterostructured g-C3N4/LaCO3OH synthesized through a one-step hydrothermal reaction exhibited a substantially enhanced NO removal ratio of 30.3% after 30 min.16 g-C3N4, a kind of semiconductor photocatalyst, has drawn wide attention in vast fields because of its visible light response and layered structure, which can provide rich surface chemistry and shorten the electron transfer pathways.17 While there are only few reports about g-C3N4/LaCO3OH heterojunction as the catalyst for the photodegradation of organic dyes at present. Wang et al.13 synthesized a g-C3N4/LaCO3OH heterostructure for photocatalytic NO removal, which showed an improved NO removal ratio of 30.3% compared with pristine g-C3N4 (19.3%) after 30 min visible light irradiation. Similar composite also shown considerable improvement in N2 photofixation (8.2 mM g−1 h−1) compared to g-C3N4 (2.1 mM g−1 h−1).18 While there is still no report about the application of g-C3N4/LaCO3OH heterojunction in the photodegradation of dyes. The introduction of cocatalyst such as noble metal (Pt19 and Ag20), non-noble metal (Ni21) and non-metal material such as graphene oxide (GO) can restrain the recombination of photogenerated carriers and enhance visible light absorption capacity.22 Among all these cocatalysts, GO has fascinating properties including good conductivity, high carrier mobility and large exposed specific surface, which can provide abundant active sites for the photocatalytic process.23 Therefore, for the first time, we proposed to introduce reduced graphene oxide (RGO) into g-C3N4/LaCO3OH heterojunction and result in a heterostructured ternary composite for photocatalytic degradation of the organic dye. Benefitting from the extended light absorption in visible light region along with accelerated separation kinetics of charge carriers, the constructed RGO/g-C3N4/LaCO3OH composite can serve as the catalyst for the photodegradation of methyl orange (MO) under visible light irradiation with a superior performance than each component.
Herein, ternary composite RGO/g-C3N4/LaCO3OH heterojunction was synthesized through hydrothermal reactions and its photocatalytic activity was evaluated by using it as the catalyst in the degradation of MO under visible light irradiation. The photocatalysis mechanism was investigated and the migration pathway of photogenerated carriers was proposed. This work is excepted to provide a novel strategy to enhance the photocatalytic activity of rare-earth based semiconductors with broad bandgaps for the photodegradation of organic dyes.
2. Experimental section
2.1 Materials
Analytical grade chemicals were used directly in the experiments. Graphite powder, K2S2O8, H2SO4, HCl, KMnO4 and BaCl2 were purchased from Sinopharm, China. Urea, La(NO3)3·6H2O and MO were provided by Aladdin, China.g-C3N4 was synthesized through the method in the literature.24 10.0 g urea was calcined at 250 °C for 1 h, then 350 °C for 2 h and 550 °C for another 2 h in a tube furnace. The obtained pale yellow powder was washed with distilled water then dried at 60 °C for 12 h.
X wt% g-C3N4/LaCO3OH (X represents the mass ratio of g-C3N4, X = 15.0, 25.0, 35.0) was synthesized through a hydrothermal reaction. For 15.0 wt% g-C3N4/LaCO3OH, 50.0 mg g-C3N4 and 671.6 mg La(NO3)3·6H2O were dispersed in 35 mL distilled water and stirred for 3 h. After adjusting pH to 8.0–8.5 using 0.1 M NaOH aqueous solution, the obtained suspension was transferred to 100 mL autoclave and heated at 160 °C for 6 h. After washed with distilled water for 3 times, the product was dried at 60 °C for 12 h. Pure LaCO3OH was also produced via a reported method for comparison.14
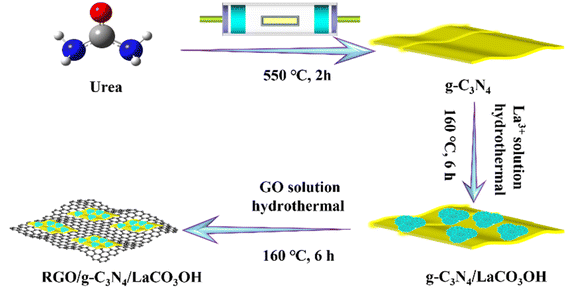
RGO/g-C3N4/LaCO3OH was obtained through a hydrothermal reaction using g-C3N4/LaCO3OH and GO as raw materials, as shown in Scheme 1, and the latter was produced by the Hummers' method.10 For Y wt% RGO/g-C3N4/LaCO3OH (Y represents the mass ratio of RGO, X = 0.6, 0.8, 1.0), 50.0 mg 25.0 wt% g-C3N4/LaCO3OH was dispersed in 35.0 mL distilled water, in which 400.0 μL GO suspension (1.0 mg mL−1) was added. The obtained mixture was heated at 160 °C for 6 h in an autoclave, and the product was washed and dried in the same way as the synthesis of g-C3N4/LaCO3OH sample.
2.2 Characterization
An X-ray diffractometer (XRD, Bruker D8 Advance) with Cu Kα radiation was used to characterize the products' structures. X-ray photoelectron spectroscopy (XPS) was performed on Thermal ESCALAB 250Xi. The morphologies, microstructures, elemental mappings and the corresponding energy-dispersive X-ray spectroscopy (EDS) of the products were recorded by transmission electron microscopy (TEM, JEOL JEM 2010 EX). UV-vis diffuse reflectance spectra were tested on a Shimadzu UV-2500 spectrophotometer using BaSO4 as the reference. Photoluminescence spectra (PL) were characterized by a F4500 Hitachi spectrophotometer with an excitation wavelength of 330 nm. The photoelectrochemical performance and electrochemical impedance spectroscopy (EIS) of the products were performed on a CHI 660E workstation using a three electrode system in 0.2 M Na2SO4 aqueous solution. The counter electrode was Pt wire and the reference electrode was saturated calomel electrode. For the working electrode, 10 mg product was dispersed in 1.0 mL ethanol and the suspension was dropped on fluorine-doped tin oxide (FTO) glass.2.3 Photocatalytic tests
Photocatalytic performances of the obtained products were evaluated through the degradation of MO under visible light irradiation (λ > 420 nm), which was provided by a Xe lamp with a filter. The photograph of the lamp and the transmission spectrum of the filtered light were shown in Fig. S1.† 10 mg sample and 20 mL MO solution (10 mg L−1) was added in to a quartz reactor, whose temperature was maintained at 30 °C. After stirring in darkness for 1 h to establish the adsorption–desorption equilibrium, the suspension was exposed in visible light. The concentrations of MO under different irritation times were then calculated based on the intensities of the peak at 465 nm in UV-vis spectra.3. Results and discussion
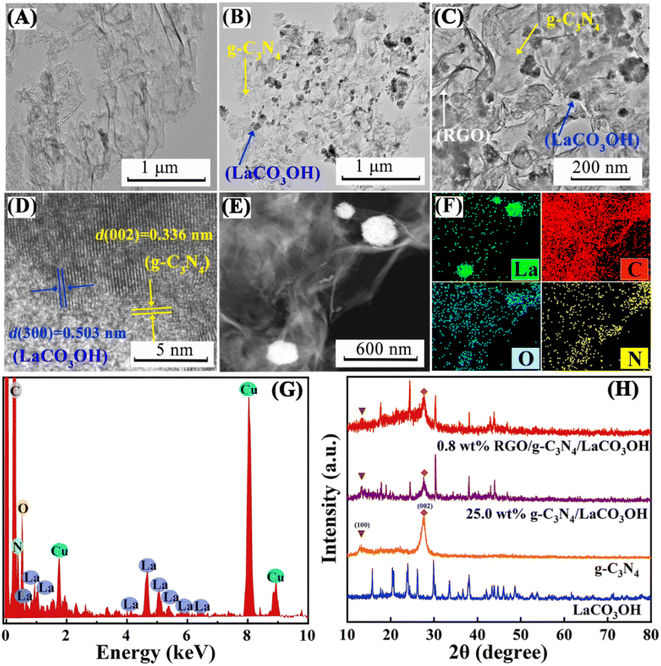
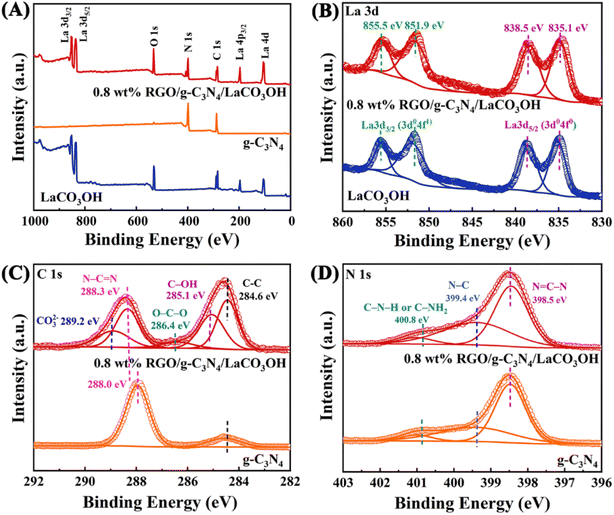
As shown in Scheme 1, ternary RGO/g-C3N4/LaCO3OH heterojunction was synthesized through a two-step hydrothermal process. It can be observed in Fig. 1A that the synthesized g-C3N4 composes of 2D nanosheets with smooth and uniform surface. g-C3N4/LaCO3OH heterojunction was fabricated by the in situ growth of LaCO3OH on the surface of g-C3N4 via the hydrothermal reaction. In this process, g-C3N4 served as a self-sacrificial agent to produce CO32− and OH− due to the weaker binding energy of C chain. Then La3+ ions adsorbed on g-C3N4 reacted with the generated CO32− and OH− to produced LaCO3OH, resulting in the in situ construction of g-C3N4/LaCO3OH heterojunction.13,18 The TEM image (Fig. 1B) shows that LaCO3OH nanoparticles with diameters of 20–50 nm are evenly dispersed on g-C3N4. Then RGO was further introduced as the cocatalyst to obtain ternary RGO/g-C3N4/LaCO3OH (Fig. 1C). In the high-resolution TEM image, the heterostructure interface between the (3 0 0) plane of LaCO3OH and (0 0 2) plane of g-C3N4 can be clearly observed (Fig. 1D), suggesting the construction of g-C3N4/LaCO3OH heterojunction. The scanning TEM image, corresponding elemental mappings and EDS spectrum (Fig. 1E–G) verify that RGO/g-C3N4/LaCO3OH are composed of La, C, O and N element, and La mainly distributed in the nanoparticles, which is consistent with the TEM results. The XRD patterns of LaCO3OH, g-C3N4, g-C3N4/LaCO3OH and RGO/g-C3N4/LaCO3OH are shown in Fig. 1H. For the synthesized LaCO3OH, its XRD pattern agrees well with hexagonal LaCO3OH (JCPDS No. 49-0981).14 In the XRD pattern of g-C3N4, two typical peaks at 13.20° and 27.54° are corresponding to the (1 0 0) and (0 0 2) plane, respectively.16 The typical peaks of both LaCO3OH and g-C3N4 can be observed in the g-C3N4/LaCO3OH and RGO/g-C3N4/LaCO3OH composite with good crystallinities, indicating that the crystal structures of these two components were maintained during the hydrothermal processes. With the introduction of RGO, the (0 0 2) peak of g-C3N4 broaden because of the interlayer stacking between 2D RGO and g-C3N4 sheets.25 All the above results clarified that ternary RGO/g-C3N4/LaCO3OH heterojunction was successfully constructed.XPS spectra are used to further study the constitution and surface chemical states of the products. In the survey spectrum of RGO/g-C3N4/LaCO3OH (Fig. 2A), La, C, O and N elements can be identified, which is in accordance with the results of EDS. As shown in Fig. 2B, the spectrum of La 3d can be divided into two pairs of peaks. Peaks at 851.9 and 835.1 eV can be assigned to La 3d3/2 and 3d5/2 of La3+, respectively, and peaks at 855.5 and 838.5 eV are two corresponding shakeup satellite peaks.14 C 1s spectrum of RGO/g-C3N4/LaCO3OH can be fitted into five peaks considering three different sources (Fig. 2C). Peaks at 286.4, 285.1 and 286.4 eV can be indexed to O–C–O, C–OH and C–C in RGO, respectively. Peaks at 289.2 and 288.3 eV stem from CO32− in LaCO3OH and N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N in g-C3N4, respectively. Compared with g-C3N4, the binding energy of N–C
N in g-C3N4, respectively. Compared with g-C3N4, the binding energy of N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N in RGO/g-C3N4/LaCO3OH increased by 0.3 eV because of the electron transfer at the interfaces of the composite.6 For N 1s spectrum, peaks at 400.8, 399.4 and 398.5 eV are attributed to C–N–H (or C–NH2), N–C and N
N in RGO/g-C3N4/LaCO3OH increased by 0.3 eV because of the electron transfer at the interfaces of the composite.6 For N 1s spectrum, peaks at 400.8, 399.4 and 398.5 eV are attributed to C–N–H (or C–NH2), N–C and N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–N in g-C3N4, respectively (Fig. 2D).17 The groups such as –OH and –NH2 can provide abundant active sites for the photodegradation of MO.
C–N in g-C3N4, respectively (Fig. 2D).17 The groups such as –OH and –NH2 can provide abundant active sites for the photodegradation of MO.
UV-vis spectra of LaCO3OH and g-C3N4 were measured to evaluate their band gap structures (Fig. 3A). The light absorption edge of g-C3N4 is 460 nm, indicating its small bandgap and good response to the visible light. While LaCO3OH exhibits a light absorption edge of 300 nm, demonstrating its large bandgap along with poor visible light absorption capability. For g-C3N4/LaCO3OH and RGO/g-C3N4/LaCO3OH, light absorption edges are located at 475 and 500 nm respectively, indicating the improved visible light absorption capability benefiting from the construction of heterojunction and the introduction of cocatalyst RGO. The band gaps Eg (eV) of LaCO3OH and g-C3N4 can be calculated based on the Kubelka–Munk function:
| αħv = A(ħv − Eg)n/2 | (1) |
| ECB = ECB − Eg | (2) |
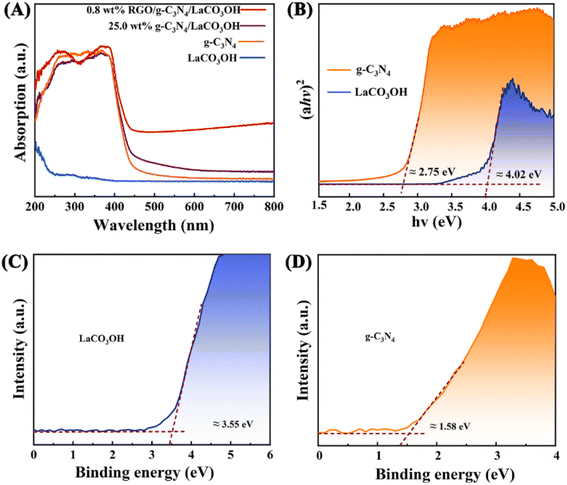 | ||
| Fig. 3 (A) The UV-vis spectra of LaCO3OH, g-C3N4, g-C3N4/LaCO3OH and RGO/g-C3N4/LaCO3OH; (B) the bandgap energy diagram and the valence band (XPS) spectra of the (C) LaCO3OH and (D) g-C3N4. | ||
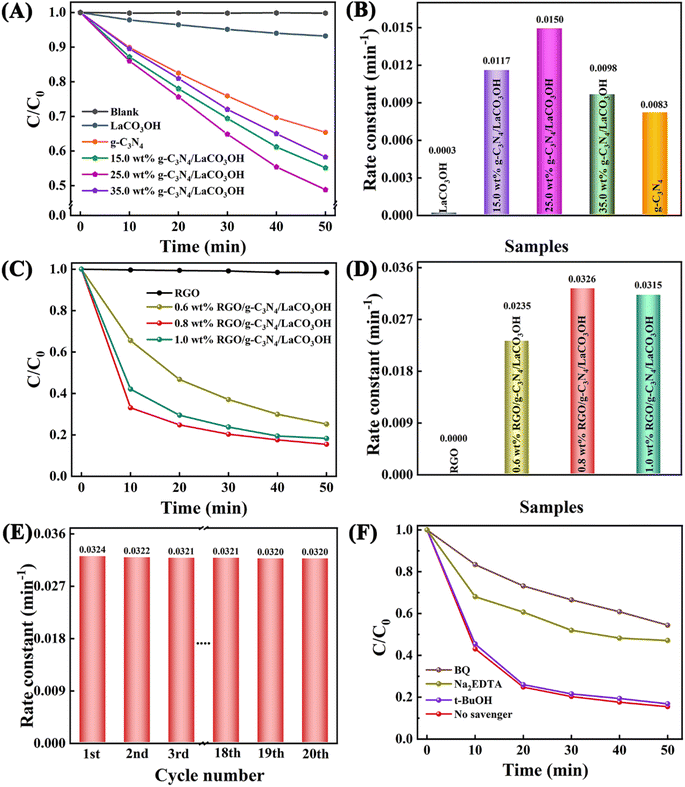
The photocatalytic performance of the obtained heterostructured products were assessed by the photodegradation of MO under the visible light irradiation. After 50 min, the degradation of MO without catalysts is negligible, as shown in Fig. 4A. For pure LaCO3OH, the photodegradation activity is low with a MO removal ratio of only 6.8% after 50 min because of its poor visible light absorption capability, which is proved by its UV-vis spectrum. Constructing g-C3N4/LaCO3OH heterojunction can significantly improve the MO removal ratio. Among the composites with different mass ratios of g-C3N4, 25% g-C3N4/LaCO3OH exhibited the highest activity with a calculated photodegradation rate constant of 0.0150 min−1 (Fig. 4B), which is 50.0 and 1.8 times higher than LaCO3OH and g-C3N4 respectively. Such results confirmed that the construction of heterojunction could be conducive to the improvement of photocatalytic activity compared with each component.
Whereas, though the MO removal ratio has been effectively improved, the photodegradation of MO only 51.2%. To further improve the activity of the photocatalyst and shorten the reaction time, RGO was introduced into the composite as the cocatalyst. Photocatalytic performances of ternary RGO/g-C3N4/LaCO3OH composite with different mass ratios of RGO is shown in Fig. 4C. Though RGO itself shows no activity in the photodegradation of MO as expected, the introduction of RGO into 25% g-C3N4/LaCO3OH can still further enhance its photocatalytic performance. When the reaction time decreased to 50 min, the MO removal ratio of 0.8% RGO/g-C3N4/LaCO3OH can be remarkably improved to 84.5%, which is the highest among ternary composites with different mass ratios, and is much improved compared with 25% g-C3N4/LaCO3OH (51.2%). The corresponding photodegradation rate constant of 0.8% RGO/g-C3N4/LaCO3OH is 0.0326 min−1, which is 2.17 times higher than that of 25% g-C3N4/LaCO3OH (Fig. 4D). Such improvement arises from the introduction of cocatalyst RGO, which can serve as the transfer medium for the photogenerated carriers, thus can accelerate their transfer kinetics and restrain their recombination, leading to an increased rate of photocatalytic reaction.
The high stability of ternary RGO/g-C3N4/LaCO3OH composite was clarified by the cycling test carried out using 0.8% RGO/g-C3N4/LaCO3OH as the catalyst. As shown in Fig. 4E, the rate constant remains basically stable during 20 cycles. The slight decrease was generated by the sample loss during the washing and drying process after each cycle. TEM images shows that after the long-time cycling, there is no obvious change in the microstructure of the RGO/g-C3N4/LaCO3OH composite, confirming its high structural stability (Fig. S2†). Moreover, the active species trapping experiment was taken to analyze the active species during the photocatalytic degradation process (Fig. 4F). There is no significant change on MO removal ratio with the existence of t-BuOH, demonstrating that ˙OH radical does not involved in the photodegradation reaction. While with the addition of 1,4-benzoquinone (BQ) and Na2EDTA, the MO removal ratio declined from 84.5% to 45.6% and 52.9% respectively, indicating that both active species ˙O2− and h+ play a critical role in the photodegradation process. The above characterizations show that compared with single components, RGO/g-C3N4/LaCO3OH exhibits an enhanced photocatalytic activity for MO degradation, which is owing to the construction of heterojunction and the introduction of cocatalyst RGO.
More photoelectrochemical characterizations including EIS tests, transient photocurrent responses and PL spectroscopy were performed to study the separation efficiency of photogenerated charge carriers. The Nyquist plots (Fig. 5A) demonstrates that 0.8% RGO/g-C3N4/LaCO3OH has the smallest semicircle radius in the high-frequency region compared with that of g-C3N4/LaCO3OH, g-C3N4 and LaCO3OH, indicating its lowest charge transfer resistance benefited from the construction of the heterojunction and the introduction of RGO, leading to the highest separation efficiency of photogenerated electron–hole pairs.26 Transient photocurrent curves during five on/off cycles are shown in Fig. 5B. 0.8% RGO/g-C3N4/LaCO3OH generates a stable and highest photocurrent of 1.52 μA cm−2, which is 1.78 and 4 times higher compared with 25% g-C3N4/LaCO3OH (0.85 μA cm−2) and g-C3N4 (0.38 μA cm−2), indicating the heterostructured ternary composite can effectively accelerate the charge transfer process. In PL spectra, the lower PL intensity means the lower recombination rate and longer lifetime of the photogenerated carriers.27 As shown in Fig. 5C, compared with g-C3N4 and 25% g-C3N4/LaCO3OH, the PL intensity of 0.8% RGO/g-C3N4/LaCO3OH is much lower, confirming that cocatalyst RGO plays an important role in the restriction of photogenerated carriers' recombination. Overall, the synergistic effect of heterostructure and cocatalyst RGO endows RGO/g-C3N4/LaCO3OH composite an improved photocatalytic activity by reducing the charge transfer resistance, facilitating the separation of charge carries and lowering their recombination rate.
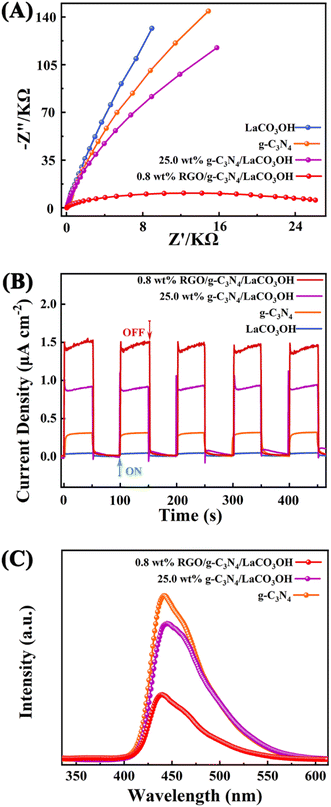 | ||
| Fig. 5 (A) The Nyquist plots of RGO, g-C3N4, LaCO3OH and RGO/g-C3N4/LaCO3OH in the frequency range from 106 Hz to 0.1 Hz; (B) transient photocurrent responses and (C) the PL spectra. | ||
According to above characterizations, the mechanism of the MO photodegradation process using RGO/g-C3N4/LaCO3OH composite as the catalyst was proposed, as shown in Fig. 6. With the irradiation of visible light, g-C3N4 with a smaller bandgap of 2.75 eV can be excited and produce photogenerated electrons and holes, while LaCO3OH does not response to visible light due to its large bandgap (4.02 eV). The electrons generated on the VB of g-C3N4 (1.58 eV) can be excited and separate with holes, then transfer to the CB of g-C3N4 (−1.17 eV). As g-C3N4 has a more negative CB compared with LaCO3OH (−0.47 eV), the photogenerated electrons will transfer to the CB of LaCO3OH driven by the interfacial electric field force, thus the recombination of photogenerated electrons and holes can be retarded. The introduction of RGO as cocatalyst can not only enhance the light absorption efficiency but also provide abundant active sites for the photocatalytic reaction because of the enlarged surface area and facilitated charge transfer kinetics of RGO. The main reactions in the photodegradation process of MO are as follows:
| g-C3N4 + ħv → g-C3N4 (h+) + LaCO3OH/g-C3N4 (e−) |
| LaCO3OH/g-C3N4 (e−) + ħv → 2D-RGO (e−) |
| LaCO3OH/g-C3N4 (e−) + 2D-RGO (e−) + O2 → (˙O2−) |
| (˙O2−) + MO → CO2 + H2O |
| g-C3N4 (h+) + MO → CO2 + H2O |
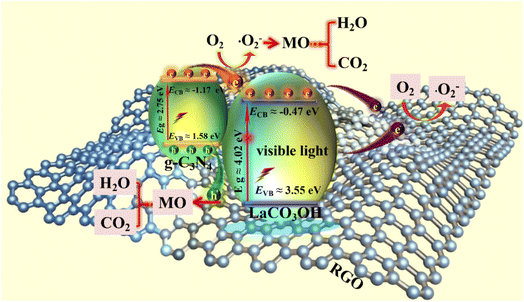 | ||
| Fig. 6 The schematic of the proposed mechanism of RGO/g-C3N4/LaCO3OH composites for MO photodegradation under visible light irradiation. | ||
During this process, N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond of MO was broken under the attack of generated radicals, and aromatic hydrocarbons such as 4-amino-N,N-dimethylaniline and 4-diazenyl-N,N-dimethyl benzenamine were produced. Then the aromatic rings were continuously opened under the attack of reactive oxygen species and converted into carbon dioxide and water eventually.28–30
N bond of MO was broken under the attack of generated radicals, and aromatic hydrocarbons such as 4-amino-N,N-dimethylaniline and 4-diazenyl-N,N-dimethyl benzenamine were produced. Then the aromatic rings were continuously opened under the attack of reactive oxygen species and converted into carbon dioxide and water eventually.28–30
4. Conclusion
In summary, ternary RGO/g-C3N4/LaCO3OH composite was constructed by coupling RGO with heterostructured g-C3N4/LaCO3OH through a hydrothermal reaction, and serve as an effective catalyst for the photodegradation of MO under visible light. Compared with pure g-C3N4, the MO photodegradation rate can be increased from 0.0083 min−1 to 0.0150 min−1 by constructing g-C3N4/LaCO3OH heterojunction, and further boosted to 0.0326 min−1 by introducing RGO as the cocatalyst, which is 3.93 times higher than pure g-C3N4. Such significant improvement is owing to the improved photocatalytic activities stemmed from the combination of g-C3N4/LaCO3OH heterostructure with RGO, which can accelerate the transfer and restrict the recombination of the photogenerated carriers, and prolong their lifetime. This study realized the utilization of LaCO3OH with a broad bandgap in the visible light region as a promising photocatalyst for the MO degradation, and also proposed a novel strategy for the further development of photocatalyst based on semiconductors with wide bandgaps.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by LiaoNing Revitalization Talents Program (XLYC2007166), Joint Funds for Innovation Capability Improvement of Natural Science Foundation of Liaoning Province (2021-NLTS-12-08), Science and Technology Innovation Foundation of Dalian (2022RQ047), Doctoral Startup Foundation of Dalian University (No. 1201012), the Key Task and Local Project in Science & Technology of SYUCT (LDB2019004).References
- J. Yang, D. Wang, H. Han and C. Li, Acc. Chem. Res., 2013, 46, 1900–1909 CrossRef CAS PubMed.
- Q. Z. Zhang, J. J. Deng, Z. H. Xu, M. Chaker and D. L. Ma, ACS Catal., 2017, 7, 6225–6234 CrossRef CAS.
- Z. Lei, W. You, M. Liu, G. Zhou, T. Takata, M. Hara, K. Domen and C. Li, Chem. Commun., 2003, 2142–2143, 10.1039/B306813G.
- Y. Gao, J. Y. Lin, Q. Z. Zhang, H. Yu, F. Ding, B. T. Xu, Y. G. Sun and Z. H. Xu, Appl. Catal., B, 2018, 224, 586–593 CrossRef CAS.
- C. Z. Zhu, Y. T. Wang, Z. F. Jiang, F. C. Xu, Q. M. Xian, C. Sun, Q. Tong, W. X. Zou, X. G. Duan and S. B. Wang, Appl. Catal., B, 2019, 259, 118072 CrossRef CAS.
- T. Lv, Z. N. Xu, W. Hong, G. F. Li, Y. W. Li and L. S. Jia, Chem. Eng. J., 2020, 382, 123021 CrossRef CAS.
- Y. Gao, C. Shi, J. Z. Feng, G. Y. Zhao, H. Yu, Y. F. Bi, F. Ding, Y. G. Sun and Z. H. Xu, RSC Adv., 2017, 7, 54555–54561 RSC.
- Z. H. Xu, M. Quintanilla, F. Vetrone, A. O. Govorov, M. Chaker and D. L. Ma, Adv. Funct. Mater., 2015, 25, 2950–2960 CrossRef CAS.
- Y. Lin, W. Su, X. Wang, X. Fu and X. Wang, Angew. Chem., Int. Ed., 2020, 59, 20919–20923 CrossRef CAS PubMed.
- Y. Gao, B. T. Xu, M. Cherif, H. Yu, Q. Z. Zhang, F. Vidal, X. F. Wang, F. Ding, Y. G. Sun, D. L. Ma, Y. F. Bi and Z. H. Xu, Appl. Catal., B, 2020, 279, 119403 CrossRef CAS.
- B. L. Li, L. L. Li and C. Zhao, Green Chem., 2017, 19, 5412–5421 RSC.
- B. Pan, Q. Xie, H. Wang, J. Zhu, Y. Zhang, W. Su and X. Wang, J. Mater. Chem. A, 2013, 1, 6629–6634 RSC.
- Z. Wang, Y. Huang, L. Chen, M. Chen, J. Cao, W. Ho and S. C. Lee, J. Mater. Chem. A, 2018, 6, 972–981 RSC.
- B. Pan, Q. H. Xie, H. M. Wang, J. Zhu, Y. F. Zhang, W. Y. Su and X. X. Wang, J. Mater. Chem. A, 2013, 1, 6629 RSC.
- S. S. Patil, M. G. Mali, A. Roy, M. S. Tamboli, V. G. Deonikar, D. R. Patil, M. V. Kulkarni, S. S. Al-Deyab, S. S. Yoon, S. S. Kolekar and B. B. Kale, J. Energy Chem., 2016, 25, 845–853 CrossRef.
- Z. Y. Wang, Y. Huang, L. Chen, M. J. Chen, J. J. Cao, W. K. Ho and S. C. Lee, J. Mater. Chem. A, 2018, 6, 972–981 RSC.
- Y. Gao, K. Qian, B. T. Xu, F. Ding, V. Dragutan, I. Dragutan, Y. Sun and Z. Xu, RSC Adv., 2020, 10, 32652–32661 RSC.
- X. Feng, H. Chen, F. Jiang and X. Wang, Chem. Eng. J., 2018, 347, 849–859 CrossRef CAS.
- B. Pan, S. Luo, W. Su and X. Wang, Appl. Catal., B, 2015, 168–169, 458–464 CrossRef CAS.
- J. X. Li, C. Ye, X. B. Li, Z. J. Li, X. W. Gao, B. Chen, C. H. Tung and L. Z. Wu, Adv. Mater., 2017, 29, 1606009–1606014 CrossRef PubMed.
- M. Xiao, L. Zhang, B. Luo, M. Lyu, Z. Wang, H. Huang, S. Wang, A. Du and L. Wang, Angew. Chem., Int. Ed., 2020, 59, 7230–7234 CrossRef CAS PubMed.
- Q. H. Zhu, Z. H. Xu, B. C. Qiu, M. Y. Xing and J. L. Zhang, Small, 2021, 17, 2101070–2101095 CrossRef CAS PubMed.
- S. D. Perera, R. G. Mariano, K. Vu, N. Nour, O. Seitz, Y. Chabal and K. J. Balkus, ACS Catal., 2012, 2, 949–956 CrossRef CAS.
- Z. H. Xu, B. T. Xu, K. Qian, Z. Li, F. Ding, M. Fan, Y. Sun and Y. Gao, RSC Adv., 2019, 9, 25638–25646 RSC.
- Y. Fu, J. Zhu, C. Hu, X. Wu and X. Wang, Nanoscale, 2014, 6, 12555–12564 RSC.
- Z. H. Xu, M. G. Kibria, B. AlOtaibi, P. N. Duchesne, L. V. Besteiro, Y. Gao, Q. Z. Zhang, Z. Mi, P. Zhang, A. O. Govorov, L. Q. Mai, M. Chaker and D. L. Ma, Appl. Catal., B, 2018, 221, 77–85 CrossRef CAS.
- Z. P. Wang, Z. L. Wang, X. D. Zhu, C. Z. Ai, Y. M. Zeng, W. Y. Shi, X. D. Zhang, H. R. Zhang, H. W. Si, J. Li, C. Z. Wang and S. W. Lin, Small, 2021, 17, 2102699–2102710 CrossRef CAS PubMed.
- S. Balasurya, M. K. Okla, I. A. Alaraidh, A. A. Al-ghamdi, A. Mohebaldin, M. A. Abdel-Maksoud, R. F. Abdelaziz, A. M. Thomas, L. L. Raju and S. S. Khan, J. Environ. Manage., 2022, 319, 115674 CrossRef CAS PubMed.
- Z. Zhang, G. Wang, W. Li, L. Zhang, T. Chen and L. Ding, Colloids Surf., A, 2020, 601, 125034 CrossRef CAS.
- V. Subhiksha, A. A. Alatar, M. K. Okla, I. A. Alaraidh, A. Mohebaldin, M. Aufy, M. A. Abdel-Maksoud, L. L. Raju, A. M. Thomas and S. S. Khan, Chemosphere, 2022, 303, 135177 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra02415f |
| This journal is © The Royal Society of Chemistry 2023 |