Open Access Article
Open Access ArticleEvaluation of the catalytic activity of Zn-MOF-74 for the alcoholysis of cyclohexene oxide†
J. Gabriel Flores‡
ab,
Juan L. Obeso‡ cd,
V. Martínez-Jiméneza,
Nancy Martín-Guareguae,
Alejandro Islas-Jácomee,
Eduardo González-Zamora
cd,
V. Martínez-Jiméneza,
Nancy Martín-Guareguae,
Alejandro Islas-Jácomee,
Eduardo González-Zamora e,
Héctor Serrano-Espejele,
Britney Mondragón-Rodrígueze,
Carolina Leyva
e,
Héctor Serrano-Espejele,
Britney Mondragón-Rodrígueze,
Carolina Leyva c,
D. A. Solís-Casadosf,
Ilich A. Ibarra
c,
D. A. Solís-Casadosf,
Ilich A. Ibarra d,
Ricardo A. Peralta
d,
Ricardo A. Peralta *e,
Julia Aguilar-Pliego*b and
José Antonio de los Reyes*a
*e,
Julia Aguilar-Pliego*b and
José Antonio de los Reyes*a
aDepartamento de Ingeniería de Procesos e Hidráulica, División de Ciencias Básicas e Ingeniería, Universidad Autónoma Metropolitana-Iztapalapa, 09340, Ciudad de México, Mexico. E-mail: jarh@xanum.uam.mx
bÁrea de Química Aplicada, Departamento de Ciencias Básicas, Universidad Autónoma Metropolitana-Azc Apotzalco, 02200, Ciudad de México, Mexico. E-mail: apj@azc.uam.mx
cInstituto Politécnico Nacional, CICATA U. Legaria, Laboratorio Nacional de Ciencia, Tecnología y Gestión Integrada del Agua (LNAgua), Legaria 694 Irrigación, Miguel Hidalgo, CDMX, Mexico
dLaboratorio de Fisicoquímica y Reactividad de Superficies (LaFReS) Instituto de Investigaciones en Materiales, Universidad Nacional Autónoma de México, Circuito Exterior s/n, CU, Coyoacán, 04510, Ciudad de México, Mexico
eDepartamento de Química, División de Ciencias Básicas e Ingeniería, Universidad Autónoma Metropolitana-Iztapalapa, 09340, Ciudad de México, Mexico. E-mail: rperalta@izt.uam.mx
fCentro Conjunto de Investigación en Química Sustentable UAEM-UNAM, Carretera Toluca-Atlacomulco Km 14.5, Unidad San Cayetano, Toluca 50200, Estado de México, Mexico
First published on 11th September 2023
Abstract
In the present work, nanocrystalline Zn-MOF-74 is shown to be a heterogeneous catalyst for the acid-catalyzed ring-opening alcoholysis of cyclohexene oxide. The results corroborated that accessible open metal sites within the material are critical conditions (Zn(II) Lewis acid sites) for this reaction. Zn-MOF-74 was tested at three different temperatures (30, 40, and 50 °C) for the alcoholysis reaction. Furthermore, the cyclohexene oxide conversion was 94% in less than two days. A comparison of the catalytic activity with different crystal sizes of Zn-MOF-74 and the homogenous phase, zinc acetate, was conducted. Zn-MOF-74 exhibited excellent catalytic cyclability for three cycles without losing its activity. The material showed chemical stability by retaining its crystalline structure after the reaction and cyclability process.
Introduction
The process of opening epoxide rings has attracted attention because these species can produce relevant chemicals for multiple applications in industry.1,2 For this transformation, it is necessary to use chemical agents that induce nucleophilic attack, such as amines,3,4 alcohols,5,6 water,7 and phenols.8Ring-opening of cyclohexene oxide produces an important chemical precursor for synthesising products used in the pharmaceutical industry.9 Cyclohexene alcoholysis is a reaction that occurs under relatively mild and safe conditions but with poor conversion when a catalyst is not present.10 For this reason, strong acids, Lewis bases, or Lewis acids are commonly applied as heterogeneous catalysts to ensure faster product production. Another advantage is that the reaction can be carried out, with high efficiency, under mild conditions, such as atmospheric pressure and a temperature of 50 °C or less.11,12
Research on obtaining better performance in this reaction mainly focuses on heterogeneous catalysts with Lewis acid sites in zeolites, mixed oxides, alumina, and others due to their presumed chemical and thermal stabilities and recyclability. Zeolite Sn-Beta-50 has been synthesized at high temperatures and presented conversions of 90.2% at 40 °C.13 On the other hand, materials possessing Brønsted acid sites, such as graphene oxide with sulphur molecules, exhibited a conversion of approximately 97%.12 However, conventional synthesis of this type of catalyst is a process that demands high temperatures, uses toxic reagents-solvents, and normally involves a very highly energetic process to clean the catalyst.14 Thus, developing novel catalysts that require considerably less aggressive and safer synthesis methods are necessary.
Metal–Organic Frameworks (MOFs) are relatively new materials with increasing relevance in pollutant adsorption processes15,16 and heterogeneous catalysis.17–21 These applications are possible due to the high porosity, facile synthesis, and versatility of these materials, allowing the design of customized MOF materials according to the application of interest.22,23 In catalysis, MOFs are commonly used as Lewis acid sites because some materials possess labile solvent molecules that can be removed via thermal or chemical activation to form open metal sites (OMS). Our group has previously reported catalytic activity with these sites to perform different reactions.24–26 Furthermore, the inherent properties of MOF materials can allow for outstanding performances as catalysts. The well-dispersion of active sites and diverse morphologies of MOFs are desirable for improved interaction of the reagents with the material.27 The well-arrangement of the framework makes a high dispersion of the active sites, thereby reducing a poisoning concern of the active sites.28 Furthermore, the bonding strength between metal ligands is sufficient to generate robust structures, which is ideal for catalytic applications.29
MOFs have been used to open epoxide rings with alcohols as heterogeneous catalysts. For example, UiO-type MOFs and MOF-808, with Brønsted acid sites, showed 40% and 100% conversion, respectively.10 MOFs possessing Lewis acid sites and exhibiting high catalytic activity have also been reported; for instance, Fe(BTC) combined with styrene oxide yielded a 93% conversion in methanol at 40 °C for 1 h.30 Moreover, a Cu-based MOF presented a remarkably 100% conversion of cyclohexene oxide with methanol.9
MOF-74 has been described as a honeycomb-like material containing a pore size of about 12 nm. A mixture of divalent metal ions and 2,5-dihydroxyterephthalic acid forms the framework.31 Each metal ion is coordinated with three O atoms from the carboxylate linker and two from two μ2-OH groups. At the same time, the remanent molecule is a labile water molecule coordinated to the metallic centre, which can be readily removed to generate an OMS.32 The structure of the Zn-MOF-74 with the open metal site is shown in Fig. 1. Furthermore, MOF-74 can be constructed with different metallic centres such as Co(II), Cu(II), Mg(II), Mn(II), Ni(II), and Zn(II),32 allowing the variation of the Lewis acidity strength. These materials have been demonstrated to have high efficiency as heterogeneous catalysts in different oxidation reactions.33 Our research group has developed the synthesis of MOF-74 at room temperature in methanol, which guarantees a faster and safer process, in addition to the possibility of real-time synthesis analysis and the minimization of the washing steps.25,34 Zn-MOF-74 shows relatively high thermal stability and possesses Zn(II) OMS. Moreover, Zn-MOF-184, which is isoreticular to MOF-74, has shown the best catalytic activity of its family as a Lewis acid in the opening of epoxides for CO2 addition.35 Thus, Zn-MOF-74 was our best candidate to evaluate its activity as a catalyst in the alcoholysis of a cyclohexene oxide reaction. In the present work, we report Zn-MOF-74 synthesized at room temperature in methanol for the open ring reaction of cyclohexene with methanol, evaluated at different temperatures, and compared with Zn-MOF-74 synthesized by the conventional method.
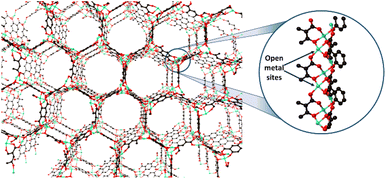 | ||
| Fig. 1 Structure of porous Zn-MOF-74 containing OMS within the structure. Atom label: green: zinc, black: carbon, and red: oxygen. | ||
Results and discussion
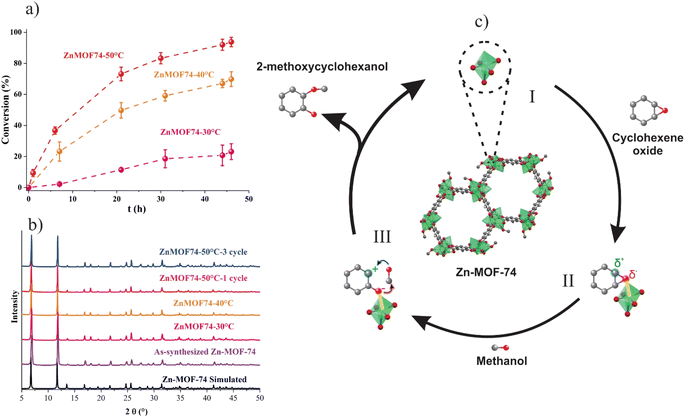
The basic characterization of Zn-MOF-74 synthesized at room temperature was performed with the following techniques: PXRD is shown in Fig. 2b and S1,† which are in a good relationship with the simulated structure of Zn-MOF-74 for room temperature and conventional synthesis. The BET surface area was calculated from N2 adsorption isotherms (Fig. S2†) in conventional and room temperature Zn-MOF-74 synthesis. The data yielded surface areas of 837 and 737 m2 g−1 for conventional and room temperature Zn-MOF-74 synthesis respectively. These values are in agreement with the different synthesis methodologies in this family of MOFs.36 Furthermore, Zn-MOF-74 exhibits high thermal stability at 350 °C by TGA (Fig. S3†). SEM analysis (Fig. S4†) was performed to observe the difference in the crystal size of Zn-MOF-74 synthesized at room temperature and conventional methods. Also, high-resolution XPS was conducted to corroborate the Zn2+ state in the materials (Fig. S5†). The survey analysis displays the contribution of Zn, C, and O atoms. The O 1s spectra show the peaks for Zn–O and C–O bonds. The spectra for Zn 2p exhibit the characteristic two bands related to Zn 2p3/2 and 2p1/2, showing a separation of 23.15 eV, which is in line with the Zn2+ oxidation state.37Our first attempt in the alcoholysis reaction using Zn-MOF-74 was with a reaction temperature of 30 °C in methanol. However, the epoxide conversion was slow and thereby, we decided to increase the temperature of the reaction. According to the graphs of cyclohexene oxide conversion as a function of time (Fig. 2a), the conversion increases with the reaction temperature. This behavior is similar to the study of ring-opening of styrene oxide with benzoic acid (i.e., the conversion increased, and the time of reaction decreased when the reaction temperature rose).38 Therefore, in this type of reaction, the catalytic activity is associated with the reaction temperature. As shown in Fig. 2a, the Zn-MOF-74 catalyst transforms practically all the cyclohexene within two days at 50 °C.
More than 83% of the conversion was obtained in the first 30 hours of reaction; the rate became slower afterward. To guarantee the stability of the catalysts after each reaction, Zn-MOF-74 was separated from the reaction mixture by centrifugation and was analysed by PXRD; the obtained diffractograms are shown in Fig. 2b and demonstrated that the crystalline structure was retained. Therefore, the material can be used as a heterogeneous catalyst since it is stable under the reaction conditions. Recyclability experiments of Zn-MOF-74 were carried out with the sample that achieved the best conversions. Table 1 and Fig. S6† show that the conversion was not decreased, demonstrating that the catalyst is regenerated. In addition, the stability of Zn-MOF-74 after the third cycle was determined by PXRD (Fig. 2b). To confirm that there is no leaching of the catalyst, a lixiviation test was carried out at 50 °C, where the catalyst was removed after 21 hours of reaction through centrifugation. Subsequently, the reaction mixture was kept at the same temperature until 46 hours of reaction. The conversion did not increase significantly compared to after 21 hours of reaction, indicating the absence of leaching.
It has been reported that the MOF synthesis temperature affects the crystal size obtained; this generally occurs at higher temperatures,39,40 generating a larger crystal size under certain considerations, which is why the conventional synthesis of Zn-MOF-74 generates a material of larger crystal size than the synthesis at room temperature. Considering this, Zn-MOF-74 was synthesized under conventional conditions and tested for the alcoholysis of cyclohexene oxide at 50 °C for 46 hours with a conversion of nearly 51.1%, representing almost half of Zn-MOF-74 synthesized at room temperature (93.8%). The difference in the catalytic activity can be attributed to the crystal size of the material because the synthesis at room temperature generates nanocrystal materials. It has been observed, precisely with this family of materials, that nanocrystalline MOF-74 exhibits better catalytic activity because its size allows better interaction with the reagents, as demonstrated with oxidation reactions.33 Finally, the precursor metal salt of MOF-74 (zinc acetate) was utilized to perform the reaction in a homogeneous system, with a conversion of 26.2% at 50 °C for 46 hours. The low conversion observed can be due to the ligand dissociation of the Zn(II) complex and, ultimately, to metal decomposition behavior reported in the Cu(NO3)2 salt.25 Conversely, a MOF-type system is more stable since it is heterogeneous. Recovering the catalyst in a homogeneous system is also a challenge. To rationalize the result obtained with Zn-MOF-74, we compare our catalyst with various materials shown in Table 2. The time of conversion of cyclohexene oxide using (near to 100%) Zn-MOF-74, at 50 °C, is higher than that of other catalysts reported at a similar temperature as sulphated yttria–zirconia,41 H-Mont-3,42 Cu-MOF,9 and Cu(BF4)2·nH2O.2 Another factor is the solvent type employed, which can harm the environment, such as CH2Cl2 or the solvent employed during the catalyst synthesis.2 Furthermore, heating during the synthesis process results in materials requiring more energy consumption. Other catalysts such as Zr-UiO66-NHCH2 Naph-OH43 and Ga-MOF44 present good conversion, which could be due to the higher temperature of the reaction. As a comparison, the thermal stability of Zr-UiO66-NHCH2 Naph-OH (285 °C) is roughly similar to Zn-MOF-74 (350 °C). Although the UiO-66 family displays chemical stability under aqueous and acid conditions against Zn-MOF-74.45,46 However, the MOF-74 family exhibits stability under organic solvents, which is necessary for catalytic reactions. In addition, the solvent exchange (in this case, methanol) avoids the material's activation, saving steps and energy costs. On the other hand, the conversion of Zn-MOF-74 at 50 °C is superior (73.2% at 21 hours) to that reported with graphene oxide at similar conditions.11 Also, using methanol as a nucleophile; may be due to the efficient renewal of Zn (Zn-MOF-74) in the catalytic cycle and the acid strength of the metal centre since this influences the opening of the epoxide.
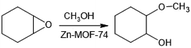
The mechanism for the ring-opening of cyclohexene oxide reaction has been proposed previously in the literature,42 as is shown in Fig. 2c. The first reaction stage (step I) involves access to unsaturated metal sites, which can be achieved by activating MOF-74 that removing solvent molecules coordinated to the metal centre.47 It is worth mentioning that the synthesized Zn-MOF-74 is submerged in methanol, the same reagent as the nucleophile for the alcoholysis reaction. Thus, the activation in our system occurs automatically within the reaction system without requiring more energy, either for heating or vacuum. Subsequently (step II), the OMS acts as Lewis acid and generates an interaction with the oxygen of the epoxide, producing partial charges in the structure.42 This behavior has been observed in similar systems, for example, in cycloaddition reactions of CO2 to epoxides openings for adding aromatic amines.48 In the third stage (step III), the opening of the epoxide and the addition of methanol occur through a nucleophilic attack of the oxygen of the methanol to the epoxide carbon with a partial positive charge.49 At the same time, the oxygen of the epoxide ends its interaction with the OMS and attacks the methanol carbon. Finally, 2-methoxycyclohexanol is produced and released. Then Zn-MOF-74 is returned to the reaction system with reusable Zn(II) complexes that can perform further catalytic cycles. The excellent performance of this material demonstrated the advantages of heterogeneous systems since they allow an efficient interaction of the reagents with the catalyst, preventing the metal from leaching within the material and simply separating the catalyst from the reaction mixture.
Experimental
Synthesis of Zn-MOF-74 at room temperature
Zn-MOF-74 was synthesized following the method reported at room temperature with a molar ratio of 2 metal: 1 linker.34 Two solutions were prepared: solution 1 of 1 mmol of 2,5-dihydroxyterephthalic acid (DHTP) and 6.6 mL of methanol was added by drip, solution 2 of 2 mmol of zinc acetate dihydrate (Zn(CH3COO)2·2H2O) and 3.3 mL of methanol, solution 1 was added to solution 2 by slow drip under continuous stirring at room temperature for 18 hours. Subsequently, the Zn-MOF-74 crystals were separated by centrifuging and washed with methanol three times to remove the excess reactant within the pores of the MOF. The material was stored submerged in methanol.Synthesis of Zn-MOF-74 conventional
Zn-MOF-74 was synthesized under the conventional method at high temperatures.40 In a covered glass tube of 20 mL were added 2.9 of zinc acetate dihydrate, 1 mmol of DHTP, 2 mL of water, and 38 mL of DMF. This mixture was heated at 100 °C for 20 hours. Later, the Zn-MOF-74 crystals were separated by centrifuging and washed two times with DMF, and after three times with methanol, the material was stored submerged in methanol.Gas chromatography experiments
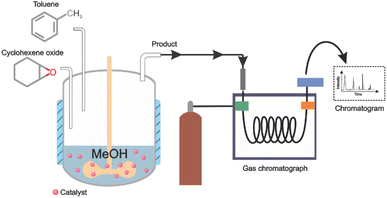
Gas chromatography (GC) was performed using a Carbowax column (25 m × 0.25 mm ID and 0.25 μm) with a He flow of 2.5 mL min−1, FID 250 °C, heating ramps of 15 °C min−1 from 80 to 180 °C and of 10 °C min−1 from 180 to 210 °C.Power X-ray (PXRD) diffraction experiments
Materials were dried before diffraction measurements. The analyzes were carried out on a Bruker AXD D8 Advance diffractometer operated at 160 W with a Cu lamp Kα1 (λ = 1.5406 A).Thermogravimetric analysis (TGA)
Thermal stability was determined by TGA, with a heating rate of 20 °C min−1 under airflow from 30 to 600 °C.Isotherms of adsorption/desorption of N2
This analysis was performed to know the apparent surface area of conventional and room temperature Zn-MOF-74. N2 sorption isotherms were performed on a Belsorp analyser under vacuum (10−3 bar), with the pressure of P/P0 from 0 to 1 and 77 K. Both Zn-MOF-74 were activated at 150 °C for 16 h (under 10−3 bar).Scanning electron microscopy (SEM)
Morphology and crystal size studies were carried out in an ultrahigh resolution Philips XL 30 SEM instrument with a tungsten filament.Alcoholysis of cyclohexene
This reaction was evaluated in a 10 mL covered glass reactor at three different temperatures (30, 40, 50 °C), each one by triplicate, with the following amounts of reagents: 3.0 mmol cyclohexene oxide, 0.3 mmol toluene (internal standard), 75 mmol methanol (nucleophile) and 0.03 mmol of a catalyst under continuous stirring of 250 rpm (Fig. 3). An Aliquot of 0.1 mL was taken and dissolved in 1 mL of acetonitrile to be analysed by GC. After each reaction, the catalyst was separated from the reaction mixture by centrifugation and washed 2 times with methanol.Conclusions
Zn-MOF-74 has become an efficient heterogeneous catalyst for the acid-catalysed ring-opening alcoholysis of cyclohexene oxide. The reaction is governed by thermodynamics, being at 50 °C the best temperature employed, which yields the highest conversion; this effect may be because the catalyst regenerates faster and the reactants access the unsaturated Zn(II) sites within the material. Under the best conditions, a conversion greater than 73% was obtained in the first 21 hours and around 94% in less than two days of reaction. The nanocrystalline Zn-MOF-74 material, synthesized at room temperature, showed higher activity than the large crystal size due to diffusional effects. The material showed no degradation, leaching, or decrease in its catalytic activity in 3 reaction cycles. Therefore, it is clear that the porous Zn-based-MOF material is an efficient catalyst for alcoholysis reactions, allowing its reuse without losing catalytic activity, resulting in an exciting platform to perform heterogeneous catalysis.Author contributions
J. Gabriel Flores and Juan L. Obeso: conceptualization, investigation, formal analysis, and writing – Héctor Serrano-Espejel and Britney Mondragón-Rodríguez, characterization of the material – original draft. V. Martínez-Jiménez and Nancy Martín-Guaregua: formal analysis and editing. Alejandro Islas-Jácome, Eduardo González-Zamora and Julia Aguilar-Pliego: writing – review and editing. D. A. Solís-Casados: XPS experiments and analysis. Carolina Leyva, Ilich A. Ibarra, Ricardo A. Peralta, and José Antonio de los Reyes: conceptualization, supervision, project administration, and resources.Conflicts of interest
There are no conflicts to declare.Acknowledgements
J. G. Flores, thanks to CONAHCYT for the postdoctoral position (687839). J. L. O. thanks CONAHCYT for the PhD fellowship (1003953). We thank U. Winnberg (Euro Health) for scientific discussions and G. Ibarra-Winnberg for scientific encouragement. We thank N. S. Portillo-Vélez for her help in the XPS analysis.References
- Z. Yan, C. Du, G. Luo and J. Deng, React. Chem. Eng., 2021, 6, 2159–2169 RSC.
- J. Barluenga, H. Vázquez-Villa, A. Ballesteros and J. M. González, Org. Lett., 2002, 4, 2817–2819 CrossRef CAS PubMed.
- M. Halder, A. Singha Roy and K. Sen, J. Indian Chem. Soc., 2021, 98, 100056 CrossRef CAS.
- B. Tang, W. Dai, X. Sun, G. Wu, N. Guan, M. Hunger and L. Li, Green Chem., 2015, 17, 1744–1755 RSC.
- D. B. G. Williams and M. Lawton, Org. Biomol. Chem., 2005, 3, 3269 RSC.
- Y. Feng, M. E. Lydon and C. W. Jones, ChemCatChem, 2013, 5, 3636–3643 CrossRef CAS.
- W. Dai, C. Wang, B. Tang, G. Wu, N. Guan, Z. Xie, M. Hunger and L. Li, ACS Catal., 2016, 6, 2955–2964 CrossRef CAS.
- Y.-S. Kim, X.-F. Guo and G.-J. Kim, Chem. Commun., 2009, 4296 RSC.
- Y. Jiang, J. Huang, M. Hunger, M. Maciejewski and A. Baiker, Catal. Sci. Technol., 2015, 5, 897–902 RSC.
- Y. Liu, R. C. Klet, J. T. Hupp and O. Farha, Chem. Commun., 2016, 52, 7806–7809 RSC.
- A. Dhakshinamoorthy, M. Alvaro, P. Concepción, V. Fornés and H. Garcia, Chem. Commun., 2012, 48, 5443 RSC.
- G. Kumar, G. Kumar and R. Gupta, RSC Adv., 2016, 6, 21352–21361 RSC.
- B. Tang, W. Dai, G. Wu, N. Guan, L. Li and M. Hunger, ACS Catal., 2014, 4, 2801–2810 CrossRef CAS.
- J. Liang, Z. Liang, R. Zou and Y. Zhao, Adv. Mater., 2017, 29, 1701139 CrossRef PubMed.
- X.-D. Song, S. Wang, C. Hao and J.-S. Qiu, Inorg. Chem. Commun., 2014, 46, 277–281 CrossRef CAS.
- J. L. Obeso, H. Viltres, C. V. Flores, A. López-Olvera, A. R. Rajabzadeh, S. Srinivasan, I. A. Ibarra and C. Leyva, J. Environ. Chem. Eng., 2023, 11, 109872 CrossRef CAS.
- R. A. Perlata, M. T. Huxley, Z. Shi, Y.-B. Zhang, C. J. Sumby and C. J. Doonan, Chem. Commun., 2020, 56, 15313–15316 RSC.
- J. L. Obeso, A. López-Olvera, C. V. Flores, R. A. Peralta, I. A. Ibarra and C. Leyva, Chem. Commun., 2023, 59, 3273–3276 RSC.
- R. A. Peralta, M. T. Huxley, J. D. Evans, T. Fallon, H. Cao, M. He, X. S. Zhao, S. Agnoli, C. J. Sumby and C. J. Doonan, J. Am. Chem. Soc., 2020, 142, 13533–13543 CrossRef CAS PubMed.
- J. L. Obeso, D. R. Amaro, C. V. Flores, A. Gutiérrez-Alejandre, R. A. Peralta, C. Leyva and I. A. Ibarra, Coord. Chem. Rev., 2023, 485, 215135 CrossRef CAS.
- A. Dhakshinamoorthy, M. Alvaro and H. Garcia, Catal. Sci. Technol., 2011, 1, 856 RSC.
- H. Furukawa, K. E. Cordova, M. O'Keeffe and O. M. Yaghi, Science, 2013, 341, 1230444 CrossRef PubMed.
- A. Corma, H. García and F. X. Llabrés i Xamena, Chem. Rev., 2010, 110, 4606–4655 CrossRef CAS PubMed.
- J. G. Flores, J. Aguilar-Pliego, N. Martin-Guaregua, I. A. Ibarra and M. Sanchez-Sanchez, Catal. Today, 2022, 394–396, 295–303 CrossRef CAS.
- J. G. Flores, E. Sánchez-González, A. Gutiérrez-Alejandre, J. Aguilar-Pliego, A. Martínez, T. Jurado-Vázquez, E. Lima, E. González-Zamora, M. Díaz-García, M. Sánchez-Sánchez and I. A. Ibarra, Dalton Trans., 2018, 47, 4639–4645 RSC.
- R. A. Peralta, P. Lyu, A. López-Olvera, J. L. Obeso, C. Leyva, N. C. Jeong, I. A. Ibarra and G. Maurin, Angew. Chem., Int. Ed., 2022, 61, e202210857 CrossRef CAS PubMed.
- H. Konnerth, B. M. Matsagar, S. S. Chen, M. H. G. Prechtl, F.-K. Shieh and K. C.-W. Wu, Coord. Chem. Rev., 2020, 416, 213319 CrossRef CAS.
- T. A. Wezendonk, V. P. Santos, M. A. Nasalevich, Q. S. E. Warringa, A. I. Dugulan, A. Chojecki, A. C. J. Koeken, M. Ruitenbeek, G. Meima, H.-U. Islam, G. Sankar, M. Makkee, F. Kapteijn and J. Gascon, ACS Catal., 2016, 6, 3236–3247 CrossRef CAS.
- C. Healy, K. M. Patil, B. H. Wilson, L. Hermanspahn, N. C. Harvey-Reid, B. I. Howard, C. Kleinjan, J. Kolien, F. Payet, S. G. Telfer, P. E. Kruger and T. D. Bennett, Coord. Chem. Rev., 2020, 419, 213388 CrossRef CAS.
- A. Dhakshinamoorthy, M. Alvaro and H. Garcia, Chem.–Eur. J., 2010, 16, 8530–8536 CrossRef CAS PubMed.
- N. L. Rosi, J. Kim, M. Eddaoudi, B. Chen, M. O'Keeffe and O. M. Yaghi, J. Am. Chem. Soc., 2005, 127, 1504–1518 CrossRef CAS PubMed.
- Ü. Kökçam-Demir, A. Goldman, L. Esrafili, M. Gharib, A. Morsali, O. Weingart and C. Janiak, Chem. Soc. Rev., 2020, 49, 2751–2798 RSC.
- D. Ruano, M. Díaz-García, A. Alfayate and M. Sánchez-Sánchez, ChemCatChem, 2015, 7, 674–681 CrossRef CAS.
- J. G. Flores, M. Díaz-García, I. A. Ibarra, J. Aguilar-Pliego and M. Sánchez-Sánchez, J. Solid State Chem., 2021, 298, 122151 CrossRef CAS.
- Y. B. N. Tran, P. T. K. Nguyen, Q. T. Luong and K. D. Nguyen, Inorg. Chem., 2020, 59, 16747–16759 CrossRef CAS PubMed.
- M. Díaz-García, Á. Mayoral, I. Díaz and M. Sánchez-Sánchez, Cryst. Growth Des., 2014, 14, 2479–2487 CrossRef.
- M. Claros, M. Setka, Y. P. Jimenez and S. Vallejos, Nanomaterials, 2020, 10, 471 CrossRef CAS PubMed.
- M. N. S. Rad and S. Behrouz, Mol. Diversity, 2013, 17, 9–18 CrossRef CAS PubMed.
- K. A. S. Usman, J. W. Maina, S. Seyedin, M. T. Conato, L. M. Payawan, L. F. Dumée and J. M. Razal, NPG Asia Mater., 2020, 12, 58 CrossRef CAS.
- T. A. Mulyati, R. Ediati and A. Rosyidah, Indones. J. Chem., 2015, 15, 101–107 CrossRef CAS.
- S. S. Kahandal, S. R. Kale, S. T. Disale and R. V. Jayaram, Catal. Sci. Technol., 2012, 2, 1493 RSC.
- D. Bhuyan, L. Saikia and D. K. Dutta, Appl. Catal., A, 2014, 487, 195–201 CrossRef CAS.
- A. Das, N. Anbu, M. Sk, A. Dhakshinamoorthy and S. Biswas, ChemCatChem, 2020, 12, 1789–1798 CrossRef CAS.
- Z. Xue, J. Jiang, M.-G. Ma, M.-F. Li and T. Mu, ACS Sustain. Chem. Eng., 2017, 5, 2623–2631 CrossRef CAS.
- X. Liu, N. K. Demir, Z. Wu and K. Li, J. Am. Chem. Soc., 2015, 137, 6999–7002 CrossRef CAS PubMed.
- S. Zuluaga, E. M. A. Fuentes-Fernandez, K. Tan, F. Xu, J. Li, Y. J. Chabal and T. Thonhauser, J. Mater. Chem. A, 2016, 4, 5176–5183 RSC.
- J. A. Botas, G. Calleja, M. Sánchez-Sánchez and M. G. Orcajo, Int. J. Hydrogen Energy, 2011, 36, 10834–10844 CrossRef CAS.
- W.-Y. Gao, Y. Chen, Y. Niu, K. Williams, L. Cash, P. J. Perez, L. Wojtas, J. Cai, Y.-S. Chen and S. Ma, Angew. Chem., Int. Ed., 2014, 53, 2615–2619 CrossRef CAS PubMed.
- G. D. Yadav and S. Singh, Tetrahedron Lett., 2014, 55, 3979–3983 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Characterization and catalysis. See DOI: https://doi.org/10.1039/d3ra03122e |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2023 |