Open Access Article
Open Access ArticleBenzoate-based thermally activated delayed fluorescence materials†
Liang Zhang *a,
Wenjing Zhua,
Kangkang Gaoa,
Yun Wua,
Yani Lua,
Chao Shuaia,
Penghui Zhanga,
Huicheng Lia and
Chuan-Feng Chen
*a,
Wenjing Zhua,
Kangkang Gaoa,
Yun Wua,
Yani Lua,
Chao Shuaia,
Penghui Zhanga,
Huicheng Lia and
Chuan-Feng Chen *bc
*bc
aCollege of Petrochemical Engineering, Longdong University, Qingyang 745000, China. E-mail: zhang_liang1113@126.com
bBeijing National Laboratory for Molecular Sciences, CAS Key Laboratory of Molecular Recognition and Function, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, China. E-mail: cchen@iccas.ac.cn
cUniversity of Chinese Academy of Sciences, Beijing 100049, China
First published on 14th July 2023
Abstract
Compounds PTZ-MBZ (methyl 3-(10H-phenothiazin-10-yl)benzoate) and DMAC-MBZ (methyl 3-(9,9-dimethylacridin-10(9H)-yl)benzoate) were conveniently synthesized, and they exhibited TADF properties with lifetimes of 0.80 and 2.17 μs, respectively. The spatially separated highest occupied molecular orbital and lowest unoccupied molecular orbital resulted in a very small singlet–triplet energy gap of 0.0152 eV and 0.0640 eV, respectively. Thermally activated delayed fluorescence materials with short lifetime could be used as promising luminescent materials for organic light-emitting diodes.
Thermally activated delayed fluorescence (TADF) materials1–3 have aroused increasing interest, and thus have also become the third-generation organic luminescent materials4–6 after fluorescent and phosphorescent materials.7 As the third-generation organic luminescent materials, TADF materials display a special radiative transition via reverse intersystem crossing (RISC) of excitons from the lowest triplet (T1)8–10 to the lowest singlet (S1) state,11 and thus obtain a high theoretical quantum yield of 100%.12–14 Generally, TADF molecules are designed by introducing a twisted angle between acceptor and donor moieties15,16 to avoid the overlap between lowest unoccupied molecular orbital (LUMO) and the highest occupied molecular orbital (HOMO).17–20
In general, the lifetime of a TADF material is in a range of hundreds of microseconds as a result of its endothermic RISC process.21–25 Most TADF materials reveal long delayed fluorescent lifetime,26–29 which lead to enhance the triplet-related nonradiative process,30–34 such as triplet–triplet annihilate (TTA), triplet-polaron annihilate (TPA), and severely efficiency roll-off at high current density. So far, various electron-accepting moieties have been applied as receptors for the design and construction of TADF molecules. However, benzoate group has rarely been used as receptor to study their TADF property.
Herein, we report two novel TADF emitters PTZ-MBZ (methyl 3-(10H-phenothiazin-10-yl)benzoate) and DMAC-MBZ (methyl 3-(9,9-dimethylacridin-10(9H)-yl)benzoate) with benzoate as electron acceptor (Fig. 1), which not only showed TADF property in films, but also exhibited short lifetime. Especially, thermally activated delayed fluorescence materials with short lifetime would be used as the promising luminescent materials for the organic light-emitting diode.
Compound DMAC-MBZ was conveniently synthesized by palladium catalyzed cross-coupling reactions of methyl 3-bromobenzoate with 9,9-dimethyl-9,10-dihydroacridine. Compound PTZ-MBZ was synthesized using similar synthetic steps. The compounds PTZ-MBZ and DMAC-MBZ could be easily synthesized in good yields. Their structures were confirmed by 1H NMR, 13C NMR, HRMS spectra.
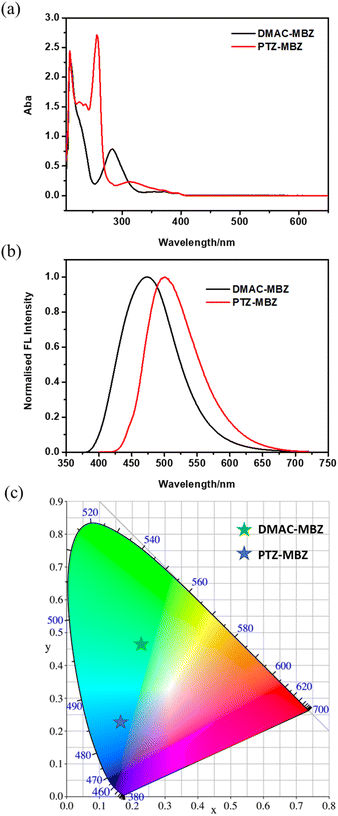
The UV-vis absorption spectra of PTZ-MBZ and DMAC-MBZ in tetrahydrofuran were shown in Fig. 2a. Owing to their similar structures, the absorption bands of the two compounds are similar. These compounds showed a broad absorption band 258, 284 and 316 nm, which were assigned to π–π* transitions. Their absorption band range 395 nm and 396 nm can be assigned to the strong intermolecular charge transfer (ICT) between the phenothiazine donor moieties to benzoate acceptor group. As shown in Fig. 2b, we can achieve color regulation by regulating the strength of ICT transition. The maximum fluorescence emission peaks are red-shifted on increase of the donor's electron donating ability, and are 501 and 474 nm for PTZ-MBZ and DMAC-MBZ, respectively. Absolute PLQYs of PTZ-MBZ and DMAC-MBZ in films were 74.02 and 55.12%, respectively. The chromatic coordinates of these luminescent compounds have also been studied (Fig. 2c). For PTZ-MBZ and DMAC-MBZ, their fluorescence CIE coordinates were found to be at (0.1666, 0.2238) and (0.2299, 0.4591), respectively.
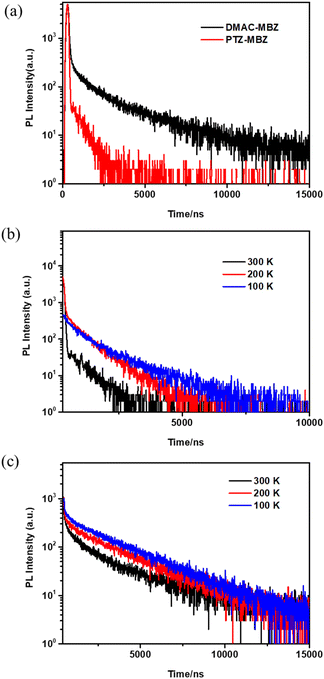
The transient PL spectra of PTZ-MBZ and DMAC-MBZ in films were further conducted to determine whether the triplet excited states were involved in the delayed luminescence. As shown in Fig. 3a, it was found that the DF lifetimes of PTZ-MBZ and DMAC-MBZ in neat films at room temperature were 0.80 and 2.17 μs, respectively. It was found that these compounds displayed a distinctive microsecond-scaled delayed relaxation at room temperature, which implied the TADF properties of the emitters. Moreover, the temperature-dependent transient PL of PTZ-MBZ and DMAC-MBZ (Fig. 3b and c) was then investigated. The ratio of the delayed component gradually increased from 100 K to 300 K, demonstrating the typical characteristics of TADF for these emitters.
The density functional theory (DFT) calculation for all molecules were performed using GAUSSIAN 09W package.35 All the molecules were optimized following the functional of with Becke-3-Yang-Parr (B3LYP) combined with the basis set of def2-SVP (Ahlrich split-valence basis set with polarization functions on heavy atoms). Note that, the dispersion corrections for the non-bonding vdW interaction were carried out through the Grimme approach using atom pair-wise additive schemes, so-called DFT-D3 method. Finally, the excited states of all optimized structures were further investigated at the accuracy level of wB97XD/TZVP. The optimized geometry and the electron density distribution of PTZ-MBZ and DMAC-MBZ were investigated by density functional theory calculations (Fig. 4). Five compounds all showed the separated HOMO and LUMO distributions on their optimized geometries. The HOMOs are predominantly located on the electron-donating subunit, whereas the LUMOs are distributed over the electron-withdrawing benzoate group. The frontier molecular orbital of PTZ-MBZ and DMAC-MBZ showed small overlap mainly on the donor and acceptor units, resulting in the appreciable ΔEST values, evidenced by the calculated ΔEST values of 0.0152 eV and 0.0640 eV, respectively. Small ΔEST values led to high efficiency of the RISC, which could induce the TADF capability.
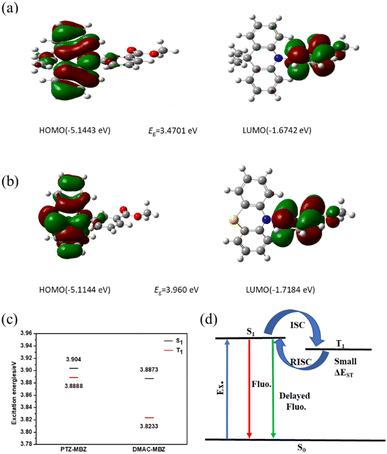 | ||
| Fig. 4 Calculated spatial distributions of the HOMO and LUMO energy densities of (a) DMAC-MBZ and (b) PTZ-MBZ. (c) Excited state energy diagram. (d) Schematic energy levels diagram. | ||
In conclusion, we have conveniently synthesized compound PTZ-MBZ and DMAC-MBZ, and found that it exhibited TADF properties with the lifetime of 0.80 and 2.17 μs, respectively. By connecting donor group with benzoate unit, realizing effective separation between HOMO and LUMO. The negligible overlap between HOMO and LUMO enables its CT character and a small ΔEST. The small ΔEST facilitate the fast RISC process and reduce the delayed fluorescence lifetime. Thermally activated delayed fluorescence materials with short lifetime would be used as the promising luminescent materials for the organic light emitting diode.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Doctoral Start-up Founds from Longdong University (No. XYBY202015), the Youth Science Foundation of Gansu Province (No. 21JR7RM194), the Youth Science and Technology Foundation of Qingyang City (No. QY-STK-2022A-007) and Higher Education Innovation Ability Improvement project of Gansu Province (No. 2022A-131).Notes and references
- H. Uoyama, K. Goushi, K. Shizu, H. Nomura and C. Adachi, Nature, 2012, 492, 234–238 CrossRef CAS PubMed.
- X. Y. Cai and S. J. Su, Adv. Funct. Mater., 2018, 28, 1802558 CrossRef.
- Y. Liu, X. Wu, Y. Chen, L. Chen, H. Li, S. Wang, H. Tian, H. Tong and L. Wang, J. Mater. Chem. C, 2019, 7, 9719–9725 RSC.
- Q. Wang, Q. S. Tian, Y. L. Zhang, X. Tang and L. S. Liao, J. Mater. Chem. C, 2019, 7, 11329–11360 RSC.
- X. Liang, Z.-L. Tu and Y.-X. Zheng, Chem.–Eur. J., 2019, 25, 5623–5642 CrossRef CAS.
- J. Xu, X. Zhu, J. Guo, J. Fan, J. Zeng, S. Chen, Z. Zhao and B. Z. Tang, ACS Mater. Lett., 2019, 1, 613–619 CrossRef CAS.
- Y. Tao, R. Chen, H. Li, J. Yuan, Y. Wan, H. Jiang, C. Chen, Y. Si, C. Zheng, B. Yang, G. Xing and W. Huang, Adv. Mater., 2018, 30, 1803856 CrossRef PubMed.
- T. Huang, X. Song, M. Cai, D. Zhang and L. Duan, Mater. Today Energy, 2021, 21, 100705 CrossRef CAS.
- H. Xu, Z. Li, D. Yang, C. Han, B. Zhao, H. Wang, P. Ma, P. Chang and D. Ma, Angew. Chem., Int. Ed., 2021, 60, 14846–14851 CrossRef.
- M. Ma, J. Li, D. Liu, Y. Mei and R. Dong, ACS Appl. Mater. Interfaces, 2021, 13, 44615–44627 CrossRef CAS.
- D. Liu, M. Zhang, H. Chen, D. Ma, W. Tian, K. Sun, W. Jiang and Y. Sun, J. Mater. Chem. C, 2021, 9, 1221–1227 RSC.
- L. Poulard, S. Kasemthaveechok, M. Coehlo, R. A. Kumar, L. Frédéric, P. Sumsalee, T. d'Anfray, S. Wu, J. Wang, T. Matulaitis, J. Crassous, E. Zysman-Colman, L. Favereau and G. Pieters, Chem. Commun., 2022, 58, 6554–6557 RSC.
- Z. G. Wu, H. B. Han, Z. P. Yan, X. F. Luo, Y. Wang, Y. X. Zheng, J. L. Zuo and Y. Pan, Adv. Mater., 2019, 31, 1900524 CrossRef PubMed.
- H. Liu, H. Liu, J. Fan, J. Guo, J. Zeng, F. Qiu, Z. Zhao and B. Z. Tang, Adv. Opt. Mater., 2020, 8, 2001027 CrossRef CAS.
- Z. Qiu, W. Xie, Z. Yang, J. H. Tan, Z. Yuan, L. Xing, S. Ji, W. C. Chen, Y. Huo and S. J. Su, Chem. Eng. J., 2021, 415, 128949 CrossRef CAS.
- Z. Huang, Z. Bin, R. Su, F. Yang, J. Lan and J. You, Angew. Chem., Int. Ed., 2020, 59, 9992–9996 CrossRef CAS PubMed.
- Z. Lv, J. Hou, J. Yao, Y. Yuan, Y. Qian, J. Zhu, H. Zhao, X. Xiong and C. Jiao, RSC Adv., 2022, 12, 11477–11483 RSC.
- L. Yu, Z. Wu, G. Xie, W. Zeng, D. Ma and C. Yang, Chem. Sci., 2018, 9, 1385–1391 RSC.
- Y. Liu, Y. Chen, H. Li, S. Wang, X. Wu, H. Tong and L. Wang, ACS Appl. Mater. Interfaces, 2020, 12, 30652–30658 CrossRef CAS.
- Q. Li, J. Hu, J. Lv, X. Wang, S. Shao, L. Wang, X. Jing and F. Wang, Angew. Chem., Int. Ed., 2020, 59, 20174–20182 CrossRef CAS PubMed.
- G. Xia, C. Qu, Y. Zhu, J. Ye, K. Ye, Z. Zhang and Y. Wang, Angew. Chem., Int. Ed., 2021, 60, 9598–9603 CrossRef CAS.
- L. Zhang, Y. F. Wang, M. Li, Q. Y. Gao and C.-F. Chen, Chin. Chem. Lett., 2020, 32, 740–744 CrossRef.
- H. J. Kim, H. Kang, J. E. Jeong, S. H. Park, C. W. Koh, C. W. Kim, H. Y. Woo, M. J. Cho, S. Park and D. H. Choi, Adv. Funct. Mater., 2021, 31, 2102588 CrossRef CAS.
- K. Matsuo and T. Yasuda, Chem. Sci., 2019, 10, 10687–10697 RSC.
- Y. Wang, Y. Zhu, X. Lin, Y. Yang, B. Zhang, H. Zhan, Z. Xie and Y. Cheng, J. Mater. Chem. C, 2018, 6, 568–574 RSC.
- A. Nyga, T. Kaihara, T. Hosono, M. Sipala, P. Stachelek, N. Tohnai, S. Minakata, L. E. d. Sousa, P. d. Silva, P. Data and Y. Takeda, Chem. Commun., 2022, 58, 5889–5892 RSC.
- Z. Lv, J. Hou, J. Yao, Y. Yuan, Y. Qian, J. Zhu, H. Zhao, X. Xiong and C. Jiao, RSC Adv., 2022, 12, 11477–11483 RSC.
- H. Gao, Z. Li, Z. Pang, Y. Qin, G. Liu, T. Gao, X. Dong, S. Shen, X. Xie, P. Wang, C.-S. Lee and Y. Wang, ACS Appl. Mater. Interfaces, 2023, 15, 5529–5537 CrossRef CAS.
- H. Xu, B. Zhao, H. Wang, C. Han, P. Ma, Z. Li and P. Chang, Angew. Chem., Int. Ed., 2020, 59, 19042–19047 CrossRef.
- D. G. Congrave, B. H. Drummond, P. J. Conaghan, H. Francis, S. T. E. Jones, C. P. Grey, N. C. Greenham, D. Credgington and H. Bronstein, J. Am. Chem. Soc., 2019, 141, 18390–18394 CrossRef CAS PubMed.
- M. Monka, I. E. Serdiuk, K. Kozakiewicz, E. Hoffman, J. Szumilas, A. Kubicki, S. Y. Park and P. Bojarski, J. Mater. Chem. C, 2022, 10, 7925–7934 RSC.
- C. Leng, S. You, Y. Si, H.-M. Qin, J. Liu, W.-Q. Huang and K. Li, J. Phys. Chem. A, 2021, 125, 2905–2912 CrossRef CAS PubMed.
- B. Huang and O. A. von Lilienfeld, Nat. Chem., 2020, 12, 945–951 CrossRef CAS.
- L. Huang, Y. Sun, G. Zhao, L. Wang, X. Meng, J. Zhou and H. Duan, J. Mol. Struct., 2022, 1255, 132427 CrossRef CAS.
- Y. Wang, W. Wu and K. L. Choy, J. Phys. Org. Chem., 2022, 35, e4386 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis and characterization data of new compounds. See DOI: https://doi.org/10.1039/d3ra03289b |
| This journal is © The Royal Society of Chemistry 2023 |