Open Access Article
Open Access ArticleMoringa oleifera Lam.: a comprehensive review on active components, health benefits and application†
Xinyue Su‡
a,
Guanzheng Lu‡a,
Liang Yea,
Ruyu Shia,
Maomao Zhua,
Xinming Yua,
Zhiyong Li*b,
Xiaobin Jia*a and
Liang Feng *a
*a
aSchool of Traditional Chinese Pharmacy, China Pharmaceutical University, Nanjing 211198, P. R. China. E-mail: jiaxiaobin2015@163.com; wenmoxiushi@163.com
bInstitute of Chinese Materia Medica, China Academy of Chinese Medical Sciences, Beijing 100700, P. R. China. E-mail: lizhiyong7899@sina.com
First published on 15th August 2023
Abstract
Moringa oleifera Lam. is an edible therapeutic plant that is native to India and widely cultivated in tropical countries. In this paper, the current application of M. oleifera was discussed by summarizing its medicinal parts, active components and potential mechanism. The emerging products of various formats such as drug preparation and product application reported in the last years were also clarified. Based on literature reports, the unique components and biological activities of M. oleifera need to be further studied. In the future, a variety of new technologies should be applied to the development of M. oleifera products, to enrich the varieties of dosage forms, improve the bitter taste masking technology, and make it better for use in the fields of food and medicine.
Introduction
Moringa oleifera Lam. (M. oleifera), also known as drumstick or horseradish, is a perennial tree that belongs to the Moringaceae family.1 It is considered to be a medicine food homology plant with great medicinal values and was introduced into Yunnan province, China, in the 1960.2 M. oleifera, acquainted with “Tree of Wonders”, “Tree of Life” and “Diamond of Plants”, is widely cultivated for its drought resistance, rapid growth and nutrient-rich properties in African and Asian countries.3 (Fig. 1). M. oleifera is a remarkable plant with multiple edible parts, each offering unique biological activities. Its leaves, fruit pods, fruits, seeds, flowers, and roots all contribute to its versatility and potential benefits. And in India and Africa, it is commonly used to treat diabetes, skin diseases, hypoimmunity, arthritis, cancer, and so on.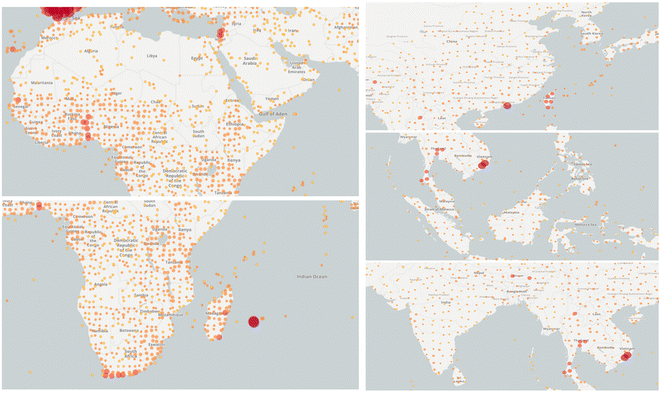 | ||
| Fig. 1 Key distribution areas of M. oleifera worldwide (the dots indicate the presence of M. oleifera in this area. Images were obtained from https://www.gbif.org/). | ||
The safety of M. oleifera is closely related to its ingredients, therefore, an increasing number of scholars have focused on the identification of the active compounds for the investigation of its pharmacological effects and potential mechanisms which are crucial in the process of M. oleifera application of drugs and food products development. It is of great significance and value to explore the relationship between its ingredients and efficacy, the determination of key ingredients, the selection of different dosage forms and the toxicity evaluation of M. oleifera on the human body. Fortunately, studies on M. oleifera have been conducted and numerous new active ingredients were found, leading to the developments of new preparation methods, pharmacological efficacies and toxicological effects of M. oleifera. What's more, the underlying molecular mechanism of M. oleifera action is also being revealed.4
This article summarized the past 10 years of research on the medicinal parts, active ingredients, health benefits and the mechanisms, dosage forms and applications of M. oleifera. In addition, we also use UPLC-Q-TOF-MS, network pharmacology and molecular docking to analyse the components and pharmacological mechanisms. This article summarizes and explains the chemical composition and biological activity and mechanisms of action of M. oleifera. It predicts trends in the expansion of M. oleifera products and formulation design with a focus on innovation and diversification, aiming to provide theoretical support and research ideas for subsequent studies. In this way, this paper provides not only a basic research summary but also a comprehensive future research plan for M. oleifera. Furthermore, the challenges and future trends of M. oleifera are summarized to provide a research approach for the further development and application.
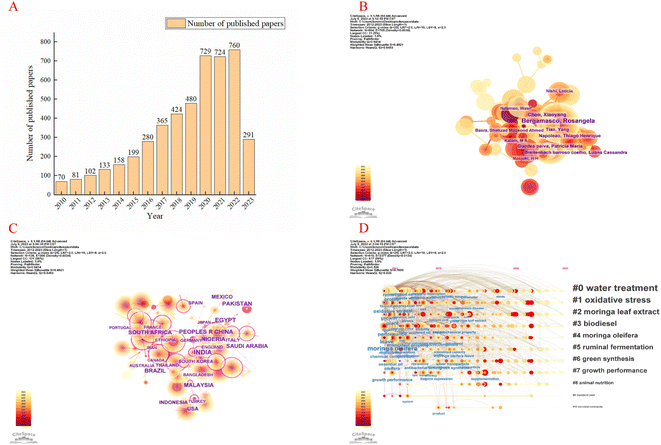
M. oleifera related article acquisition based on CiteSpace
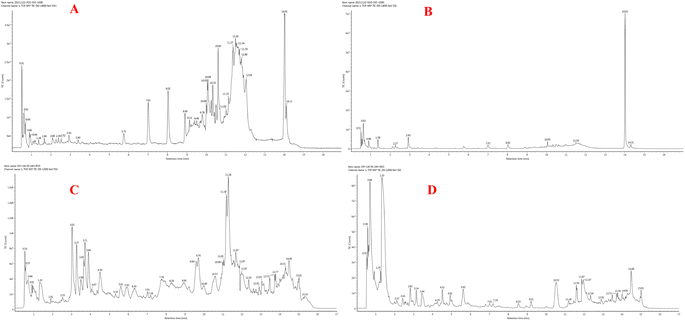
This study regards the Web of Science Core Collection as a data-collection platform according to data resources required in CiteSpace. The bibliometric search strategy can be described as the following: topics = (“Moringa oleifera Lam. OR Moringa oleifera OR Moringa”), time span = 2012–2023, and language = English.5 We used the 6.1.R6 version of CiteSpace software to analyse the current hot spots of M. oleifera.We searched 5249 publications related to M. oleifera. (Fig. 2A) Bergamasco Rosangela was the most prolific author with most publications, i.e., with 60 articles (Fig. 2B). The India, Egypt and China were the leading country and the top institution in this field of study, with 725, 372 and 348 articles, respectively (Fig. 2C). There was active cooperation between institutions, countries, and authors. Hot topics focused on Moringa oleifera leave, Moringa oleifera extract, antioxidant activity, and oxidative stress (Fig. 2D).
Medicinal parts of M. oleifera
The whole plant of M. oleifera including leaf, fruit pod, fruit, seed, flower and root, all have a variety of biological activities, and can even play medicinal roles in preventing and treating diseases.The leaves of M. oleifera are rich in resources and easily harvested which mainly contain active components such as polyphenols, flavonoids, phenylpropanoids, terpenoids, fatty acids, alkanes, sterols, as well as minerals and vitamins.6 Among them, polyphenols and flavonoids are the main bioactive constituents, with antioxidant activity and anticancer properties,7 antisepsis,8 anti-inflammatory,9 anti-hypertension, anti-diabetes, anti-spasm, reduce metal toxicity,10 reduces plasma and liver lipids and affects obesity-related reproductive diseases11 and anti-epilepsy,9 affect reproductive diseases12 and other activities.
The seeds of M. oleifera are mainly composed of protein, fatty and flavonoids, and essential amino acids required by the human body.13–15 Furthermore, the isothiocyanates contained in M. oleifera seeds also play a crucial role in anti-inflammatory, antioxidant, antibacterial and anticancer.16 And the essential oil has coagulant effect that can accelerate wound healing.17
While previous research on M. oleifera mainly focused on the leaves and seeds, there has been limited discourse on its stems. Methanol reflux method and column tomography were used to extract four compounds, namely cholest-5-en-3-ol, stigmasterol, gamma-sitosterol, and tricosanoic acid, from the stem of M. oleifera. These fractions and the methanol extract showed strong antifungal activity against Rhizoctonia solani and Fusarium oxysporum.18 The resulting fractions derived from different solvents such as hexane, benzene, chloroform, ethyl acetate, acetone and water have been found to have activities against Rhizoctonia solani and Fusarium oxysporum. M. oleifera flowers and fruits are rich in carbohydrates, proteins, organic acids, flavonoids and phenols. Singhal et al. recorded that tocopherols, ascorbic acid, carotenoids and flavonoids, are antioxidants and have the ability to eliminate reactive oxygen species (ROS).19
Chemical components of M. oleifera
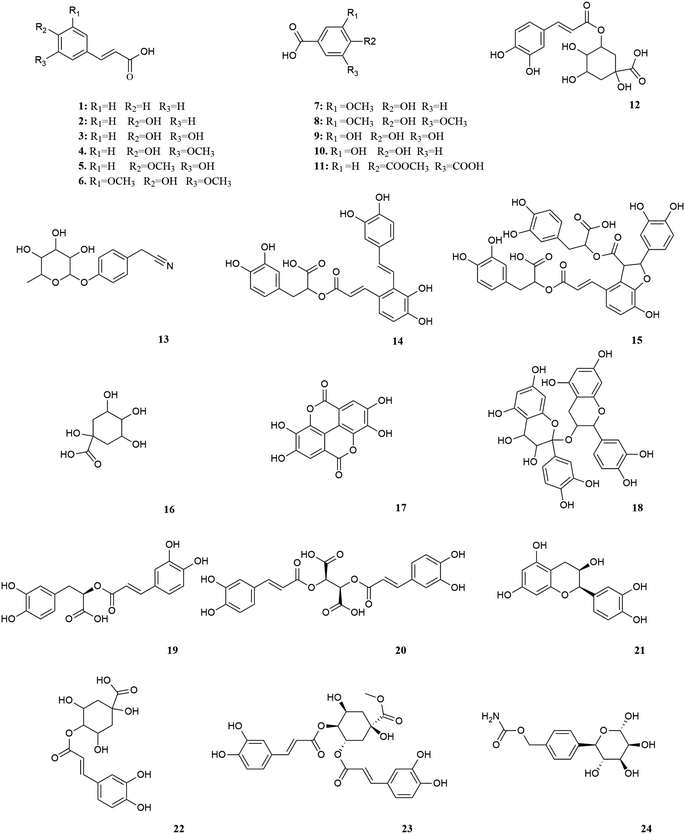
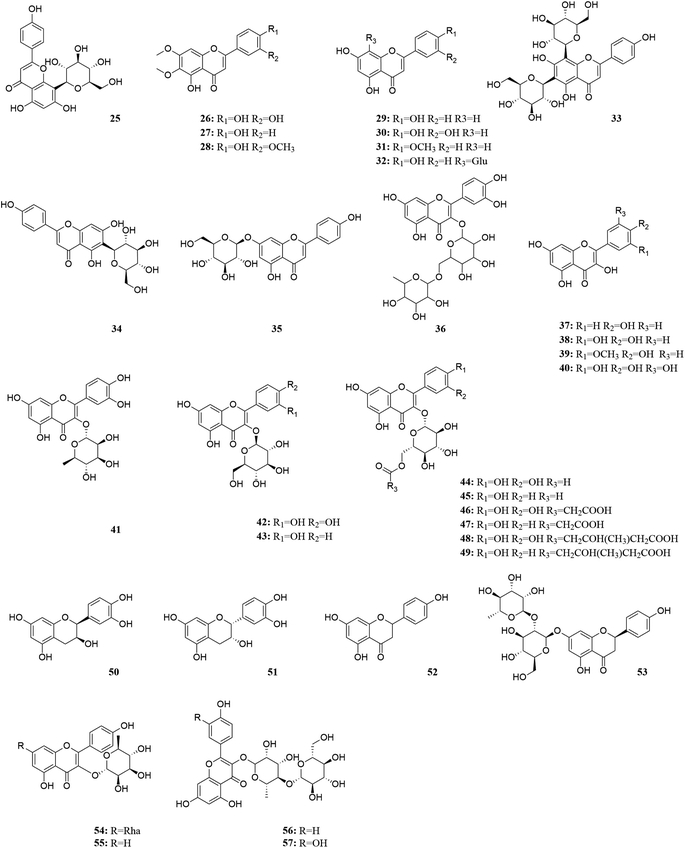
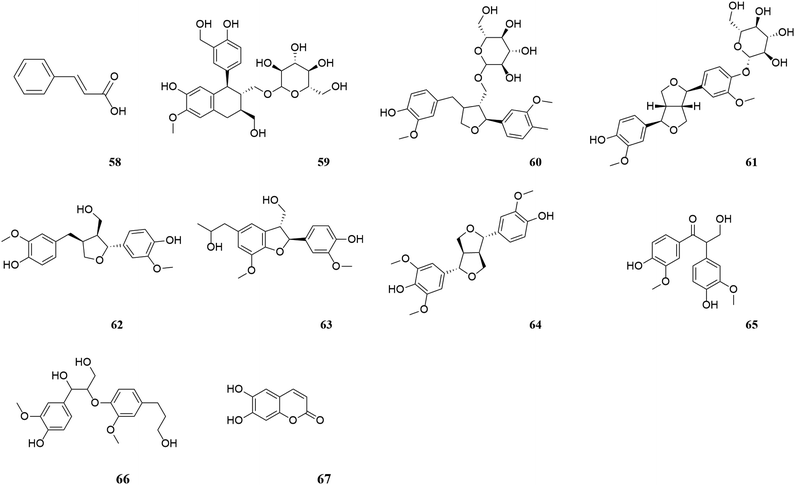
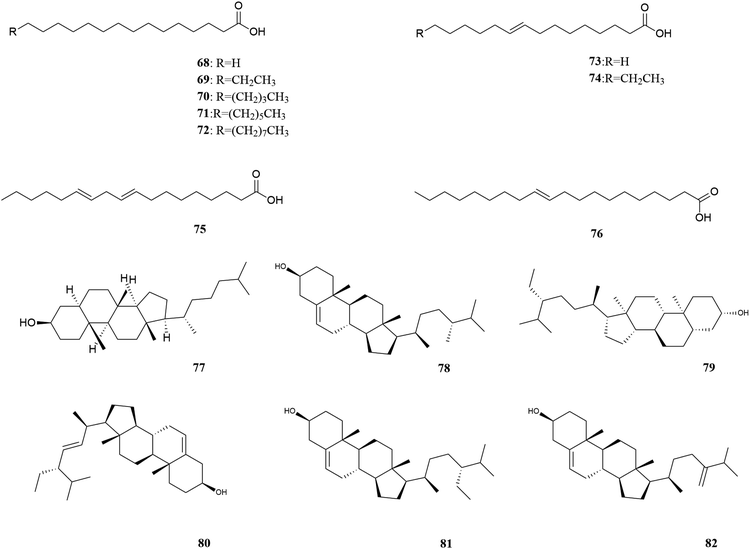
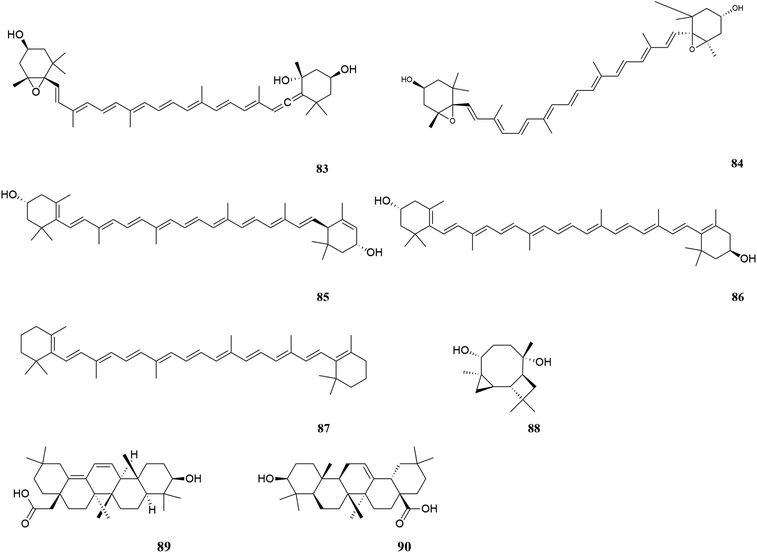
M. oleifera contains a variety of functional active ingredients, which underlie its use as a dietary supplement and functional food. The reported functional active ingredients and their specific effects in M. oleifera are summarized through comprehensive literature review by ChemDraw 19.0 (Fig. 3–8, Table 1).| Number | Components | Bioactivities | Diseases/tissues | Effects and function | Ref. |
|---|---|---|---|---|---|
| 1 | Polyphenols | Antioxidant | Hypertension/neurodegeneration | Free radical scavenging activities | 27 and 28 |
| Inhibition of free radical, lipid peroxidation, cholinergic and monoaminergic activity | 29 | ||||
| Diabetes | Inhibition of α-amylase activity | 30 and 31 | |||
| Enhance sexual function and the male reproductive system | Scavenged DPPH radical, ABTS radical and H2O2 and suppressed the formation of LPO and AGEs | 32 | |||
| Anti-aging | Reduce lipid peroxidation and lipofuscin pigmentation and increase serotonin and antioxidant enzymes in the brain of aged rats | 33 | |||
| Other | 27 and 34 | ||||
| Anticancer | Caspase-dependent and caspase-independent apoptotic pathways mediated by mitochondrial ROS are involved | 35 | |||
| Metabolic syndrome | Improve hyperglycemia, insulin resistance, inflammation, carbohydrate and lipid metabolism, non-alcoholic fatty liver | Reduce food intake, water intake and body weight, lower blood glucose levels and improve inflammation | 1 and 9 | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| 1 | Polyphenols | Antibacterial | S. aureus, Bacillus cereus | Bacterial cell damage and leakage of intracellular substances | 36 and 37 |
| Anti-inflammatory | Arthritis | Scavenging of free radicals, inhibition of protein denaturation, membrane stabilization and anti-proteinase activity | 6, 9 and 38 | ||
| 2 | Flavonoids | Anticancer/cytotoxic activity | Cancer (colon cancer, lung cancer) | By working directly on visceral mass and restoring mRNA expression of leptin, resistin, and adiponectin genes | 27 |
| Antioxidant | Hypolipidemic effects | Improve body weight, atherogenic index and insulin resistance in obese rats | 30 | ||
| Diabetes | Induced insulin secretion | 30 and 31 | |||
| Myocardial damage | Decreased the apoptotic markers Bax, cytochrome-c and iNOS expression, induced NO level, and increased BCl2 markers | 30 | |||
| Reduce metal toxicity | Protect yeast cells against As(III) toxicity, likely through its role in decreasing As(III) accumulation and As(III)-induced ROS production, the hydroxyl and carboxyl groups of gallic acid appear to play a critical role in chelating As(III) inhibition of the accumulation of toxic metals in the body | ||||
| 2 | Flavonoids | Antihypertensive effects | Hypertension | ACE inhibition test showed ACE inhibition activity | 30 |
| Enhance sexual function and the male reproductive system | Enhanced courtship behavior and reproductive function | 12 | |||
| Antiviral | COVID-19 | High docking binding affinity for non-structural proteins nsp9 and nsp10 of COVID-19 | 39 | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| 2 | Flavonoids | Regulate intestinal flora | Regulate the proportion of digestive tract flora | 40 | |
| Promote the proliferation and migration of fibroblasts | Promote wound healing | Induced fibroblast proliferation and cell migration | 41 | ||
| 3 | Fatty acids and plant sterols | Antioxidant | Hypolipidemic effects | Improved body weight, total cholesterol, triglyceride and LDL levels in obese rats | 30 and 42 |
| Anticancer | Apoptotic cells can be significantly induced by changing mitochondrial membrane potential in EAC cell lines | 43 | |||
| 4 | Glucosinolates and isothiocyanate | Antihypertensive effects | Hypertension | Reduce vascular oxidation in spontaneously hypertensive rats | 30 |
| Anticancer | MCF-7, HepG2 and HCT-116 | Inhibition of IL-3 – induced STAT5 target gene expression. The isothiocyanate group of moringin should be necessary for its chemoprotective activities | 42, 44 and 45 | ||
| Antioxidant | Diabetes | Lower body weight, reduce obesity, improve glucose tolerance, reduce inflammatory gene expression, increase antioxidant gene expression improved glucose tolerance and reduced obesity, inflammation and oxidative stress. Antibiotic-like recombination regulates the gut microbiome | 46 | ||
| Anti-inflammatory | |||||
| Antioxidant | Skin photoaging | It is beneficial to the uptake of HaCaT cells, thus improving the activity of antioxidant enzymes, clearing UVB-induced ROS, protecting skin from damage, and reducing the expression of MMP-1, MMP-3 and MMP-9 caused by radiation-induced photoaging | 16 | ||
| Antibacterial | L. monocytogenes | Damage the integrity of cell walls and membranes, stimulate oxidative stress, interfere with energy metabolism and DNA replication | 47 | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| 5 | Polysaccharides | Antioxidant | DPPH radical scavenging activity removes excess free radicals from the system and protects them from oxidative damage | 48–51 | |
| Antidiabetic | α-Amylase inhibition and α-glucosidase inhibition | ||||
| Regulate intestinal flora | Increased villus height and mucosal thickness in the ileum, colon, and duodenum, as well as villus height and crypt depth ratio in the ileum. Boost the activity of digestive enzymes. Improved the variety of the gut microbiota in mice | 48, 51 and 52 | |||
| Hypolipemia | Hyperlipidaemia | Effective bile acid sequestrants | 53 | ||
| Immunomodulatory effect | Enhanced pinocytic rate and increased production of ROS, NO, IL-6, and TNF-α levels | 48, 51 and 54 | |||
| Anti-inflammatory | Rheumatoid arthriti, inflammatory bowel disease, asthma, and pancreatitis | Inhibited the production of IL-6 and TNF-α | 51 | ||
| Anticancer | Human ovarian cancer | Induce apoptosis of cancer cells and suppress cancer cell proliferation | 49 | ||
| Antibacterial | Wound healing | Promote wound contraction and internal tissue growth. It has highly potent and durable antibacterial activity against infectious pathogens in wounds | 24 | ||
| 6 | Others | Antibacterial | S. aureus | Irreversible membrane damage is caused to S. aureus cells by increasing membrane permeability, leading to the release of intracellular nucleotide pools | 55 |
| Anti-inflammatory | Inhibit DPPH and ABTS free radicals, and inhibit the production of NO | 56 | |||
| Antioxidant | |||||
| Antihypertensive effects | Hypertension | Reduction in systolic and diastolic blood pressure in spontaneously hypertensive rats | 57 | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| 6 | Others | Antibacterial | H1N1 virus | Inhibition of virus replication in host cells has a protective effect on infected cytopathies caused by IAVS | 58 and 59 |
According to research conducted by Tekayev et al. (2010) and Tesfaye et al. (2022), moringa leaves, flowers, seeds, and other nutritional parts are plentiful in multiple nutrients and can be utilized as both food and medicine.20,21 In order to further explore and analyze the functional components and mechanism of the main nutrient parts of M. oleifera, the dry leaves and seeds (MOL and MOS) of M. oleifera were analysed by UPLC-Q-TOF-MS. Manual calibration was performed to verify the accurate mass-to-charge ratio, secondary fragment ion information and literature data for each compound, identifying 33 MOL and 45 MOS components, respectively. Under the proposed analysis conditions, the total ion flow diagram of M. oleifera in positive and negative ion mode are shown in Fig. 9.
The compound of MOL includes 13 flavonoids, 5 phenolic acids, 6 amino acids, 3 phenylpropanoids, 2 phytosterols, 2 terpenoids (2 triterpenoid saponins) and 2 vitamins which are detailed in Table 2. The information of MOS obtained by identification is shown in the table below and includes 19 flavonoids, 11 phenolic acids, 5 amino acids, 7 phenylpropanoids, 1 terpenoid (1 triterpenoid saponin), 2 vitamins.
| No. | t/R (min) | Ion type | M/Z | Error | The total number of pieces (ppm) | Formula | Compound name | Source | |
|---|---|---|---|---|---|---|---|---|---|
| Actual value | Theoretical value | ||||||||
| 1 | 0.56 | [M − H]− | 173.1042 | 174.1117 | −1.4 | 156.0773 (C6H10N3O2), 131.0829 (C5H11N2O2) | C6H14N4O2 | Arginine | MOL |
| 2 | 0.56 | [M − H]− | 154.0624 | 155.0695 | 1.2 | 137.0361 (C6H5N2O2), 93.0449 (C5H5N2) | C6H9N3O2 | Histidine | MOL |
| 3 | 0.59 | [M + HCOO]− | 264.109 | 219.1107 | 0.6 | 146.0461 (C5H8NO4), 114.0196 (C4H4NO3), 88.0413 (C3H6NO2) | C9H17NO5 | Pantothenic acid | MOL |
| 4 | 0.60 | [M − H]− | 146.0461 | 147.0532 | 1.8 | 102.0564 (C4H8NO2), 88.0413 (C3H6NO2), 74.0254 (C2H4NO2) | C5H9NO4 | Glitamic acid | MOL |
| 5 | 0.60 | [M − H]− | 118.0512 | 119.0582 | 2 | 104.0358 (C3H6NO3), 102.0564 (C4H8NO2), 74.0254 (C2H4NO2) | C4H9NO3 | Threonine | MOL |
| 6 | 0.60 | [M − H]− | 132.0305 | 133.0375 | 1.7 | 114.0196 (C4H4NO3), 88.0404 (C3H6NO2), 74.0248 (C2H4NO2) | C4H7NO4 | Aspartic acid | MOL |
| 7 | 0.77 | [M − H]− | 191.0561 | 192.0634 | 0.1 | 119.0352 (C4H7O4), 101.0252 (C4H5O3), 95.0143 (C5H3O2) | C7H12O6 | Quinic acid | MOL |
| 8 | 1.60 | [M + HCOO]− | 326.1248 | 281.1263 | 0.9 | 173.0449 (C10H7NO2), 164.0706 (C9H10NO2), 147.0454 (C9H7O2) | C14H19NO5 | Tyrosine | MOL |
| 9 | 2.27 | [M − H]− | 353.0875 | 354.0951 | −0.8 | 179.0350 (C9H7O4), 161.0244 (C9H5O3), 133.0293 (C8H5O2) | C16H18O9 | 4-O-Caffeoylqunic acid | MOL |
| 10 | 2.27 | [M − H]− | 179.0345 | 180.0423 | −2.7 | 161.0244 (C9H5O3), 133.0293 (C8H5O2), 109.0300 (C6H5O2) | C9H8O4 | Caffeic acid | MOL |
| 11 | 2.76 | [M − H]− | 163.0406 | 164.0473 | 3 | 119.0502 (C8H7O), 93.0346 (C6H5O) | C9H8O3 | trans-4-Hydroxycinnamic acid | MOL |
| 12 | 2.93 | [M + Na]+ | 543.1843 | 520.1945 | 1.2 | 423.1434 (C24H23O7), 305.0985 (C16H17O6) | C26H32O11 | (+)-Pi-noresinol-4-O-β-D-glucopyranoside | MOL |
| 13 | 2.96 | [M + HCOO]− | 375.0707 | 330.074 | −3.8 | 195.0299 (C9H7O5), 179.0354 (C9H7O4), 161.0242 (C9H5O3) | C17H14O7 | Cirsiliol | MOL |
| 14 | 3.40 | [M + Na]+ | 421.3462 | 398.3549 | 4.9 | 343.3362 (C25H43) | C28H46O | 24-Methylenecholesterol | MOL |
| 15 | 3.40 | [M − H]− | 593.1507 | 594.1585 | −0.9 | 473.1089 (C23H21O11), 323.551 (C18H11O6), 161.0237 (C9H5O3) | C27H30O15 | Vicenin 2 | MOL |
| 16 | 4.31 | [M + HCOO]− | 324.1091 | 279.1107 | 0.6 | 132.0455 (C8H6NO), 89.0256 (C3H5O3) | C14H17NO5 | Niazirin | MOL |
| 17 | 5.34 | [M − H]− | 609.1458 | 610.1534 | −0.6 | 300.0275 (C15H8O7), 301.0336 (C15H9O7), 151.0043 (C7H3O4) | C27H30O16 | Rutin | MOL |
| 18 | 5.41 | [M − H]− | 431.0985 | 432.1057 | 0.3 | 323.0528 (C18H11O6), 117.0345 (C8H5O) | C21H20O10 | Cosmosiin | MOL |
| 19 | 5.77 | [M − H]− | 463.0881 | 464.0955 | −0.2 | 300.0275 (C15H8O7), 243.0299 (C13H7O5), 151.0041 (C7H3O4) | C21H20O12 | Isoquercitrin | MOL |
| 20 | 6.95 | [M + HCOO]−, [M − H]− | 567.2081 | 522.2101 | 0 | 341.1402 (C20H21O5), 311.0917 (C18H15O5) | C26H34O11 | (+)-Isolariciresinol-3a-O-β-D-glucopyranoside | MOL |
| 21 | 6.49 | [M − H]− | 549.0877 | 550.0959 | −1.6 | 505.0968 (C23H21O13), 300.0273 (C15H8O7), 151.0416 (C8H7O3) | C24H22O15 | Quercetin-3-O-(6-malondiyl)-β-D-glucopyranoside | MOL |
| 22 | 11.61 | [M + Na]+ | 491.3506 | 468.3604 | 2 | 412.3362 (C28H44O2), 361.2872 (C27H37) | C31H48O3 | 3β-Hydroxy-oleano-11,13 (18)-diene-28-carboxylic acid | MOL |
| 23 | 7.02 | [M − H]− | 447.0937 | 448.1006 | 0.9 | 284.0327 (C15H8O6), 113.0617 (C6H9O2) | C21H20O11 | Quercitrin | MOL |
| 24 | 4.24 | [M − H]− | 447.0926 | 448.0998 | 0.9 | 327.0522 (C17H11O7), 297.0401 (C16H9O6) | C21H20O11 | Astragalin | MOL |
| 25 | 8.09 | [M + H]+ | 435.3604 | 412.3705 | 1.5 | 321.3120 (C22H41O), 209.2257 (C15H29) | C29H48O | Stigmastanol | MOL |
| 26 | 8.09 | [M − H]− | 591.1352 | 592.1428 | −0.6 | 489.1038 (C23H21O12), 284.0328 (C15H8O6), 227.0350 (C13H7O4) | C27H28O15 | Kaempferol-3-O-hydroxymethyl glutaryl hexanoside | MOL |
| 27 | 8.10 | [M − H]− | 533.0935 | 534.101 | −0.4 | 489.1039 (C23H21O12), 285.0412 (C15H9O6) | C24H22O14 | Kaempferol-3-O-malondiacylhexanoside | MOL |
| 28 | 8.90 | [M + Na]+ | 631.1258 | 608.1377 | −1.9 | 325.0326 (C17H9O7), 243.0162 (C9H7O8), 201.0444 (C8H9O6) | C27H28O16 | Quercetin - 3-O-hydroxymethyl glutaryl galactoside | MOL |
| 29 | 9.78 | [M + Na]+ | 315.2331 | 314.2246 | 4 | 228.1527 (C16H20O), 176.1186 (C12H16O) | C21H30O2 | Progesterone | MOL |
| 30 | 10.02 | [M + H]+ | 543.221 | 520.2309 | 1.7 | 431.1679 (C23H27O8), 279.1438 (C12H23O7) | C27H36O10 | Lariciresinol-9-O-β-D-glucopyranoside | MOL |
| 31 | 10.09 | [M + Na]+, [M + H]+ | 457.3696 | 456.3604 | 4.3 | 221.1942 (C15H25O), 101.1337 (C7H17) | C30H48O3 | Oleanolic acid | MOL |
| 32 | 10.51 | [M + HCOO]− | 423.1663 | 378.1679 | 0.5 | 165.0194 (C8H5O4), 109.0293 (C6H5O2) | C20H26O7 | 1-(4-Hydroxy-3-methoxyphenyl)-2-[4-(3-hydroxypropyl)-2 | MOL |
| 33 | 11.70 | [M + Na]+ | 453.3682 | 430.3811 | −4.6 | 245.1536 (C16H21O2), 227.2369 (C15H31O), 209.2264 (C15H29) | C29H50O2 | Vitamin E | MOL |
| 34 | 0.60 | [M − H]− | 132.0298 | 133.037 | −3.63 | 115.0031 (C4H3O4), 88.0406 (C3H6NO2), 74.0252 (C2H4NO2) | C4H7NO4 | Aspartic acid | MOS |
| 35 | 0.61 | [M − H]− | 146.0453 | 147.0526 | −3.66 | 102.0558 (C4H8NO2), 88.0406 (C3H6NO2), 74.0252 (C2H4NO2) | C5H9NO4 | Glitamic acid | MOS |
| 36 | 0.90 | [M + HCOO]− | 150.0408 | 105.0426 | 0.23 | 87.0090 (C3H3O3), 59.0147 (C2H3O2) | C3H7NO3 | Serine | MOS |
| 37 | 1.09 | [M + H]+ | 147.1134 | 146.1062 | 4.29 | 135.1137 (C10H15), 128.0944 (C6H12N2O) | C6H14N2O2 | Lysine | MOS |
| 38 | 1.25 | [M + HCOO]− | 326.1223 | 281.1263 | −6.92 | 164.0714 (C9H10NO2), 105.0345 (C7H5O) | C14H19NO5 | Tyrosine | MOS |
| 39 | 1.80 | [M + HCOO]− | 399.0948 | 354.0966 | 3.83 | 209.0442 (C10H9O5), 149.0237 (C8H5O3), 108.0213 (C6H4O2) | C16H18O9 | 4-O-Caffeoyl-quinic acid | MOS |
| 40 | 2.06 | [M − H]− | 218.1029 | 219.1102 | −2.22 | 146.0816 (C6H12NO3), 99.0450 (C5H7O2) | C9H17NO5 | Pantothenic acid | MOS |
| 41 | 2.46 | [M + H]+ | 521.2372 | 520.23 | −1.69 | 405.1908 (C22H29O7), 375.1834 (C21H27O6) | C27H36O10 | Lariciresinol-9-O-β-D-glucopyranoside | MOS |
| 42 | 2.82 | [M − H]− | 353.0867 | 354.094 | −3.2 | 184.0715 (C9H12O4), 143.0357 (C8H6O2), 23.0812 (C12H14O5) | C16H18O9 | Chlorogenic acid | MOS |
| 43 | 2.83 | [M + HCOO]− | 579.0977 | 534.0995 | −2.55 | 169.0495 (C8H9O4), 143.0340 (C6H7O4), 95.0133 (C5H3O2) | C24H22O14 | Kaempferol-3-O-malondiacylhexanoside | MOS |
| 44 | 3.10 | [M + HCOO]− | 243.0502 | 198.052 | −3.48 | 164.0460 (C9H8O3), 134.0370 (C8H6O2) | C9H10O5 | Syringic acid | MOS |
| 45 | 3.22 | [M + HCOO]− | 389.0886 | 344.0904 | 2.15 | 193.0501 (C10H9O4), 178.02724 (C9H6O4) | C18H16O7 | Cirsilineol | MOS |
| 46 | 4.44 | [M + H]+ | 595.1425 | 594.1352 | −3.58 | 167.0353 (C8H7O4), 161.0242 (C9H5O3) | C30H26O13 | Procyanidin | MOS |
| 47 | 4.46 | [M − H]− | 179.0345 | 180.0418 | −2.68 | 161.0238 (C9H5O3), 133.0295 (C8H5O2), 109.0295 (C6H5O2) | C9H8O4 | Caffeic acid | MOS |
| 48 | 7.56 | [M + HCOO]− | 509.0961 | 464.0979 | 4.76 | 299.0196 (C15H8O7), 243.0289 (C13H7O5), 151.0025 (C7H3O4) | C21H20O12 | Isoquercitrin | MOS |
| 49 | 4.68 | [M + HCOO]− | 223.0242 | 178.026 | −2.86 | 125.0237 (C6H5O3), 109.0290 (C6H5O2) | C9H6O4 | Esculetin | MOS |
| 50 | 7.13 | [M − H]−, [+HCOO]− | 609.1529 | 610.1534 | −0.86 | 300.0254 (C15H8O7), 301.0339 (C15H9O7), 167.0322 (C8H3O4) | C27H30O16 | Rutin | MOS |
| 51 | 4.93 | [M − H]−, [+HCOO]− | 289.0715 | 290.0788 | −0.78 | 167.0711 (C9H11O3), 137.0239 (C7H5O3), 108.0214 (C6H4O2), 179.0341 (C9H7O4) | C15H14O6 | (−)-Epicatechin | MOS |
| 52 | 5.57 | [M − H]− | 593.1505 | 594.1578 | −1.09 | 473.1081 (C23H21O11), 323.0546 (C18H11O6), 161.0235 (C9H5O3) | C27H30O15 | Vicenin 2 | MOS |
| 53 | 5.63 | [M + HCOO]−, [−H]− | 324.1087 | 279.1105 | −0.4 | 132.0450 (C8H6NO), 89.0247 (C3H5O3) | C14H17NO5 | Niazirin | MOS |
| 54 | 5.64 | [M + Na]+ | 464.1288 | 441.1395 | −0.29 | 185.0457 (C11H7NO2), 171.0299 (C10H5NO2) | C19H19N7O6 | Folic acid | MOS |
| 55 | 5.70 | [M + HCOO]− | 361.0554 | 316.0572 | −2.94 | 165.0541 (C9H9O3), 122.0367 (C7H6O2), 145.0285 (C9H5O2) | C16H12O7 | Isorhamnetin | MOS |
| 56 | 5.92 | [M − H]− | 447.0914 | 448.0987 | −4.11 | 3270495 (C17H11O7), 297.0397 (C16H9O6) | C21H20O11 | Astragalin | MOS |
| 57 | 5.98 | [M − H]− | 163.0393 | 164.0466 | −4.79 | 119.0500 (C8H7O), 117.0337 (C8H5O), 93.0351 (C6H5O) | C9H8O3 | trans-Cinnamic acid | MOS |
| 58 | 6.66 | [M + HCOO]− | 539.1219 | 494.1237 | 4.48 | 179.0340 (C9H7O4), 151.0389 (C8H7O3), 135.0448 (C8H7O2) | C26H22O10 | Salvianolic acid A | MOS |
| 59 | 6.72 | [M − H]− | 475.0904 | 476.0976 | 4.56 | 304.0604 (C15H12O7), 286.0494 (C15H10O6), 125.0244 (C6H5O3) | C22H20O12 | Kaempferol-3-O-(6′′-acetyl glucoside) | MOS |
| 60 | 6.80 | [M − H]− | 193.0497 | 194.057 | −4.82 | 180.0417 (C9H8O4), 133.0285 (C8H5O2), 108.0221 (C6H4O2) | C10H10O4 | Ferulic acid | MOS |
| 61 | 7.01 | [M + H]+ | 743.2399 | 742.2327 | 0.87 | 473.1683 (C21H29O12), 397.1092 (C18H21O10), 313.0964 (C14H17O8) | C33H42O19 | Troxerutin | MOS |
| 62 | 7.07 | [M − H]− | 317.1028 | 318.11 | −0.97 | 255.0651 (C15H11O4), 135.0081 (C7H3O3), 108.0214 (C6H4O2) | C17H18O6 | Evofolin B | MOS |
| 63 | 6.52 | [M + HCOO]− | 567.2052 | 522.2101 | −2.05 | 451.1628 (C22H27O10), 311.0917 (C18H15O5) | C26H34O11 | (+)-Isolariciresinol-3a-O-β-D-glucopyranoside | MOS |
| 64 | 7.20 | [M − H]− | 431.0983 | 432.1055 | −0.27 | 323.0555 (C18H11O6), 117.0343 (C8H5O) | C21H20O10 | Cosmosiin | MOS |
| 65 | 7.20 | [M − H]− | 431.0983 | 432.1055 | −0.27 | 253.0492 (C15H9O4), 145.0273 (C9H5O2), 93.0347 (C6H5O) | C21H20O10 | Isovitexin | MOS |
| 66 | 7.20 | [M − H]− | 283.0606 | 284.0679 | −2.17 | 151.0029 (C7H3O4), 145.0273 (C9H5O2), 117.0343 (C8H5O) | C16H12O5 | Acacetin | MOS |
| 67 | 7.38 | [M − H]− | 285.0395 | 286.0468 | −3.42 | 152.0113 (C7H14O4), 125.0239 (C6H5O3), 108.0214 (C6H4O2) | C15H10O6 | Luteolin | MOS |
| 68 | 8.37 | [M − H]− | 549.0867 | 550.0939 | −3.53 | 505.0974 (C23H21O13), 300.0269 (C15H8O7), 151.0391 (C8H7O3) | C24H22O15 | Quercetin-3-O-(6-malondiyl)-β-D-glucopyranoside | MOS |
| 69 | 8.89 | [M − H]− | 447.0919 | 448.0992 | −3.14 | 284.0316 (C15H8O6), 93.0349 (C6H5O) | C21H20O11 | Quercitrin | MOS |
| 70 | 9.57 | [M + HCOO]− | 317.0657 | 272.0675 | −3.16 | 162.0296 (C9H6O3), 147.0431 (C9H7O2) | C15H12O5 | Naringenin | MOS |
| 71 | 9.93 | [M + HCOO]− | 359.0756 | 314.0774 | −4.64 | 283.0592 (C16H11O5), 178.0256 (C9H6O4) | C17H14O6 | Cirsimaritin | MOS |
| 72 | 10.03 | [M − H]− | 591.1343 | 592.1416 | −2.07 | 489.1033 (C23H21O12), 284.0312 (C15H8O6), 227.0343 (C13H7O4) | C27H28O15 | Kaempferol-3-O-hydroxymethyl glutaryl hexanoside | MOS |
| 73 | 10.36 | [M + HCOO]− | 433.1487 | 388.1505 | −4.04 | 373.1269 (C20H21O7), 341.1032 (C19H17O6), 181.0484 (C9H9O4) | C21H24O7 | Medioresinol | MOS |
| 74 | 11.01 | [M − H]−, [+HCOO]− | 359.1482 | 360.1555 | −4.91 | 329.1376 (C19H21O5), 314.1159 (C18H18O5), 180.0777 (C10H12O3) | C20H24O6 | (7S,8R)-Dihydrodehydrodiguaiacyl alcohol | MOS |
| 75 | 12.57 | [M + H]+ | 719.1617 | 718.1544 | 1.38 | 535.1587 (C29H27O10), 193.0839 (C22H13O3), 149.0585 (C9H9O2) | C36H30O16 | Salvianolic acid B | MOS |
| 76 | 13.14 | [M + Na]+ | 553.1343 | 530.1451 | 4.86 | 396.10618 (C18H20O10), 237.1079 (C13H17O4) | C26H26O12 | 3,4-Di-O-caffeoylquinic | MOS |
| 77 | 13.40 | [M + Na]+ | 543.184 | 520.1949 | 0.88 | 421.1275 (C24H21O7), 340.1154 (C16H20O8) | C26H32O11 | (+)-Pi-noresinol-4-O-β-D-glucopyranoside | MOS |
| 78 | 13.74 | [M + H]+ | 469.3669 | 468.3596 | −1.62 | 413.3384 (C28H45O2), 361.28765 (C27H37) | C31H48O3 | 3β-Hydroxy-oleano-11,13 (18)-diene-28-carboxylic acid | MOS |
Toxicity of M. oleifera
M. oleifera leaf extracts were tested by WST-1 assay with melanoma cells (A375 human malignant melanoma cell line, A2058 human metastatic melanoma cell line) and normal human dermal fibroblasts (NHDF, WS1).7 The results showed that M. oleifera extract could inhibit the proliferation of both melanoma cells in a dose-dependent manner. However, M. oleifera extract had no significant inhibitory effect on both normal human somatic cells (IC50 > 200 μg mL−1), even at the highest concentration (200 μg mL−1), showed only mild cytotoxicity to normal cells after 48 h.The subacute and acute (5000 mg kg−1) toxic effects of the extract of M. oleifera (40–1000 mg kg−1) on rats were studied. It has been shown that the extract is reasonably safe for consumption.22 Siddiqui et al. studied the cytotoxicity of M. oleifera fruits to human liver cancer, and analysed the risk parameters of mutagenicity, tumgenesis, reproductive and irritability as well as drug toxicity of M. oleifera fruits.23 The results showed that there was no predictive toxicity of the drug candidates, indicating that M. oleifera fruit extract is a safe and potential anticancer drug. The potential of silver nanocomposites (MOS-PS-AGNPs) with M. oleifera seed polysaccharides as an alternative dressing was prepared and investigated. The cytotoxicity of the nanocomposites in vitro was also investigated using mouse fibroblasts (L929). The results showed that MOS-PS-AGNPs of 0–200 μg mL−1 had no obvious cytotoxicity to L929, at higher concentrations (200 μg mL−1), MOS-PS-AGNPs showed slight cytotoxicity and was negligible. In summary, MOS-Ps-AGNPs was non-toxic to fibroblast L929.24
Although M. oleifera and its extracts have been proved to be safe in vivo and in vitro, some studies have shown that M. oleifera may have potential reproductive toxicity. The findings suggest that the methanol seed extract of Moringa stenopetala is not safe to rat embryos and fetuses. Its toxic effects were evidenced by a significant delay in embryonic and fetal development and an increase in fetal resorptions and fetal death.25 The contraceptive ability and uterine activity of water extract of M. oleifera leaf extract were investigated in vivo and in vitro. It was found that water extract could prevent pregnancy, induce abortion and myometrial contraction in rats.26 Hence, M. oleifera are quite safe when used under the certain dose range, and more attention should be paid to their use in special target audiences.
Health benefits of M. oleifera
Although M. oleifera is commonly used as a functional food, its pharmacological action is non-negligible. It is capable of preventing or treating diseases such as lung cancer,4 breast carcinoma,92 diabetes,93 periodontitis,94 acute pancreatitis95 and the others. The compounds mentioned above in M. oleifera contribute to the antioxidant, anti-inflammatory, anti-cancer, antibacterial and antiviral activities. Next, we will summarize the functions in detail (Table 3).| Effect | Disease | Subjects | Model | Dose | Components | Mechanism of action | Functional properties | Ref. | |
|---|---|---|---|---|---|---|---|---|---|
| Antioxidant and anti-inflammatory effect | Paw edema and peritonitis | Female Swiss (Mus musculus) mice | In vivo | 15 mg kg−1, 30 mg kg−1 | M. oleifera flowers trypsin inhibitor (MoFTI) | Reduced leukocyte migration, plasma extravasation (attributed to lower protein content), and the levels of NO and pro-inflammatory (tumor necrosis factor-α, interleukin IL-6, and IL-17A) and anti-inflammatory (IL-4 and IL-10) cytokines | Promote anti-inflammatory activity | 60 | |
| — | Chang liver cells | In vitro | 2 mg mL−1, 4 mg mL−1, 6 mg mL−1, 250 mg mL−1 | M. oleifera seeds protein hydrolysate (FM3) | Increasing the activities of endogenous antioxidant enzymes SOD and CAT, and scavenging intracellular ROS | Protect hepatocytes from oxidative stress damage. May be used as potential antioxidants or protective agents against oxidative damage to cells | 61 | ||
| Diabetic nephropathy | HRMC cell, Sprague Dawley rats | In vitro, in vivo | 200 mg kg−1 | M. oleifera seeds extract (MOS) | Antioxidant and anti-renal fibrosis activities by increasing the activity of GSK-3β and the expression of Nrf2 and HO-1 | Delay the progression of diabetic nephropathy by improving renal function and reducing pathophysiological changes | 62 | ||
| Male reproductive system health | Wistar male rats | in vivo | 0.55 mg kg−1, 1.10 mg kg−1 and 2.20 mg kg−1 | M. oleifera leaves tea (total phenols, flavonoids, and antioxidants) | Increase sertoli cells and the total spermatogenic cells; scavenged DPPH radical, ABTS radical and H2O2, and inhibit the formation of LPO and AGEs | Rich total phenols, flavonoids, and antioxidants could enhance sexual function and the male reproductive system. Increased the courtship behavior, seminiferous tubule diameter, epithelium height, epithelium area, type A spermatogonia, and spermatogonia efficiency | 32 | ||
| Rat paw edema | RAW 264.7 murine cell, Sprague Dawley rats | In vitro, in vivo | 1 μM, 5 μM, 33 mg kg−1 | M. oleifera seed extract (MSE) | MIC-1 decreased inflammatory signaling (NO production, gene expression of iNOS, IL-1β, IL-6) and promoted detoxification (gene expression of NQO1, HO1, GSTP1) in LPS-stimulated murine macrophages | Anti-inflammatory and antioxidant properties in vivo and in vitro | 63 | ||
| — | Murine macrophage cell line RAW 246.7 | In vitro | 50 μg mL−1, 100 μg mL | M. oleifera leaves protein hydrolysate (MOPH) | Peptides remove DDPH and ABTS radicals and exhibit strong ORAC determination activity. Increase in the number of exposed amino acid residues and promote an increase in scavenging activity; inhibits NO production and acts as an anti-inflammatory | Antioxidant and anti-inflammatory activity | 56 | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||||
| Antioxidant and anti-inflammatory effect | Hepatic inflammation | Healthy adult male mice | In vivo | 100 mg kg−1, 200 mg kg−1, 400 mg kg−1 | M. oleifera leaves extract (MOLE) | Modulated TLR4/NF-κB pathway, suppressed TLR4 and NF-κB gene expression and as well as TLR4 and NF-κB-p65 protein expression. Protects from apoptotic cell death via antioxidative and anti-inflammatory impacts with consequent attenuation of DNA-induced genotoxicity | Protective role against hepatotoxicity | 64 | |
| Ageing | Wistar albino rats | In vivo | 200 mg per kg per body weight | M. oleifera leaves (MOAE) | Reduced lipid peroxidation and lipofuscin pigmentation increased serotonin and antioxidant enzymes | Protective effect on age-related cerebral oxidative stress | 65 | ||
| Stomach and forestomach ulceration | E. coli, P. aeruginosa, S. aureus, Sprague-Dawley male rats | In vivo, in vitro | 100–500 μg mL−1, 100 mg kg−1 | M. oleifera leaves extract | Hydrochloric acid secretion neutralization effect in gastric mucosa, decreased MDA level and increasing antioxidant enzyme activity (CAT, SOD, GPx) | Anti-ulcer effect, protect the gastric ulcer | 66 | ||
| Wistar albino male rats | In vivo | 200 mg per kg body wt | M. oleifera aqueous extract | Reduces oxidative stress-induced DNA damage by improving NF-kB and TNF-α, restoring lead disturbance, preserving hepatocyte integrity and reducing serum liver enzyme activity | Alleviated lead-induced adverse effects | 67 | |||
| Antitumor effect | Human breast cancer cells | Breast cancer cells, T-47D and MDA-MB-231 | in vitro | 0.25 μg mL−1, 5 μg mL−1, 10 μg mL−1, 15 μg mL−1, 20 μg mL−1, 40 μg mL−1, 80 μg mL−1, 100 μg mL−1 | M. oleifera leaves methanolic extract (MOME) | Antiproliferative effect on T-47D and MDA-MB-231. The phosphatidylserine exposure on the membrane of the cells confirmed the mitochondria mediated intrinsic apoptosis induction in the MOME treated cells | Contributes to cell cycle arrest and inhibits the proliferation of breast cancer cells | 68 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||||
| Antitumor effect | MDA-MB-231 cells | MDA-MB-231 cells, Female mice | In vitro, in vivo | A high-fat diet (HFD) supplemented with 0.6% w/w MC | M. oleifera seeds concentrate (MC) | Decreased fasting blood glucose and increased insulin sensitivity in mice after obesity attack. Decreased protein expression of CD31 | Protected against high-fat diet- and chemotherapy-induced increases in fasting glucose and improved insulin sensitivity | 69 | |
| A549 lung and SNO oesophageal cancer | A549 lung and SNO oesophageal cancer cells | In vitro | 1.575–393.83 μg mL−1 | AuNP synthesized from MO aqueous leaf extracts (MLAuNP) | Increased caspase activity in SNO cells and PS externalization, ΔΨm, caspase-9, caspase-3/7 activities, and decreased ATP levels in A549 cells. Increased, p53 mRNA and protein levels, SRp30a (P = 0.428), Bax, Smac/DIABLO and PARP-1 24 kDa fragment levels and protein levels and activated alternate splicing with caspase-9a splice variant. Decreased Bcl-2, Hsp70, Skp2, Fbw7α, c-myc Mrna | No cytotoxic to PBMCs, pro-apoptotic properties were confirmed in A549 and SNO cells | 70 | ||
| The human pancreatic epithelioid carcinoma | PANC-1 cell, immune deficient athymic CD-1 nude mice | In vivo | 0.5, 1.0, and 1.5 mg g−1, 200 μL per mouse | M. oleifera aqueous leaves extract | Induction of apoptosis. Decreased expression of the pro-apoptotic protein Bcl-2, and downregulated the key component of DNA repair pathways PARP-1 and the NF-κB-related proteins IκB-α, p65-subunit, and COX-2 | Antiproliferative and antiangiogenic effects, decreased pancreatic cancer cell survival and metastatic activity and inhibited tumor growth | 71 | ||
| Colorectal cancer | CD-1 male (ICR) mice | In vivo | 2.5% w/w and 5% w/w | M. oleifera leaves powder | Reduce the activity of harmful faecal enzymes (β-glucosidase, β-glucuronidase, tryptophanase and urease) | Diminish colonic lesions and decrease the activity of fecal harmful enzymes such as β-glucosidase, β-glucuronidase, tryptophanase and urease | 72 | ||
| Hepatocellular carcinoma | Wistar male rats | In vivo | 500 mg kg−1 | M. oleifera leaf ethanol extract (MOLEE) | Reduce Bcl-2 and Bcl-xL and the up-regulation of Bax and caspase 3, and reduced the expression level of β-arrestin-2 mRNA, thus promoting apoptosis signal transduction | Against DEN-induced HCC by exerting antioxidant and apoptotic activities | 73 | ||
| Sarcoma | Sarcoma 180 cells, Swiss female mice | In vitro, in vivo | 24.6–25 μg mL−1, 15 or 30 mg kg−1 | M. oleifera flower trypsin inhibitor (MoFTI) | Upregulation of pro-apoptotic proteins and the downregulation of anti-apoptotic proteins (e.g., Bcl-2 and Bcl-xl), caspase activation, and cytochrome c release. Induce ROS production and DNA fragmentation, suppress extracellular-signal-regulated kinase (ERK) activity, disrupt the cell cycle, and cause mitochondrial membrane damage | Cytotoxic to sarcoma 180 cells, reduce in tumor weight and antiangiogenic effect | 74 | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||||
| Gut protective and regulation of intestinal flora effect | Hymenolepiasis nana | Female Swiss albino mice | In vivo | 400 mg per kg body weight | M. oleifera leaves extract | Decreased TGF-β, IFN-y and MMC counts versus increased GC counts, T-helper cell type 2 (Th2) cytokines and IgA level achieve protection against H. nana infections | Anti-inflammatory and induce protection against H. nana infection | 75 | |
| Adiposity | C57BL/6J male mice | In vivo | Dietary supplementation with 0.34%MIC-1 (0.73%MSE) | M. oleifera seed extract (MSE) | Activate Nrf2 and its downstream targets and/or regulation of gut microbiota, MSE activates Nrf2 and its downstream targets and/or modulates the gut microbiota. MIC-1 has antibiotic properties, making it an, affecting host metabolism by regulating energy balance, glucose metabolism, lipid metabolism, and inflammation | Reduce body weight, obesity and inflammatory gene expression, improve glucose tolerance, and increase antioxidant gene expression | 46 | ||
| — | C57BL/6 mice | In vivo | 40 mg kg−1, 60 mg kg−1 | M. oleifera polysaccharides | Inhibit the expression levels of IL-6 and TNF-α, affecting immune repertoire and change immune state | Regulate the intestinal flora, increase the relative abundance of beneficial bacteria and reduce the relative abundance of harmful bacteria, promoting intestinal health | 54 | ||
| — | C57BL/7 mice | In vivo | 20 mg kg−1, 40 mg kg−1, 61 mg kg−1 | M. oleifera polysaccharides | Reduced glucose, total cholesterol, and malondialdehyde. Improved superoxide dismutase and catalase in serum. Modulates the microbiological balance of the gut by increasing the abundance of good bacteria and decreasing the abundance of bad bacteria in the gut | Improve serum index, intestinal morphology, caecal microflora in mice and the villi length and crypt depth in both ileum and jejunum; increased the ratio of villi length to crypt depth in jejunum. Affecting the function of microbiota | 52 | ||
| — | Stool samples from healthy participants | In vitro | The stool suspension was loaded into sterile anaerobic jars containing 50 mg polysaccharide samples | M. oleifera root polysaccharides (MRP) | Improve the intestinal environment by producing short-chain fatty acids by intestinal flora fermentation, and maintain intestinal homeostasis by controlling the composition of intestinal flora | Increased the content of beneficial microflora and decreased the abundance of harmful microflora | 76 | ||
| Neuroprotective effect | Glutamate-induced DNA damage in RGCs | RGCs cells | In vitro | 10 μg mL−1, 50 μg mL−1,45 μg mL | M. oleifera seeds extract | Increase neuronal cell viability and minimal cellular injury, extend branches in neurons, modulate axonal development, and promote synaptogenesis | Neuroprotective effects | 77 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||||
| Neuroprotective effect | Neurodegenerative | Periodontal tissue cell | In vitro | 0.5 μM | Moringin was isolated from M. oleifera seeds | Increased the expression of genes involved in neuron cortical development and in particular in neuron belonging to upper and deep cortical layers, involved in osteogenesis and adipogenesis | Promote and accelerate cortical neuronal differentiation of hPDLSCs to improve stem cell therapy for neurological diseases | 78 | |
| — | PC12 cells | In vitro | 0.01 μM, 0.1 μM, 1 μM, 10 μM, 50 μM, and 100 μM | Pyrrole-2-carbaldehydes from M. oleifera seeds (pyrrolemorines A–G) | Against oxygen-glucose deprivation/reperfusion injury in PC12 cells by regulating NF-κb and Nrf2 | Pyrrolemorines A, E, and pyrrolemarumine displayed neuroprotective activities, and ttenuate PC12 cell damage induced by oxygen glucose deprivation | 79 | ||
| Peripheral nerve injury (PNI) | Adult male albino mice (BALB/c) | In vivo | 200 mg per kg body weight | M. oleifera leaves extract | Promoting effects of M. oleifera might be attributed to the flavonoid class | Prove a valuable target for the discovery of effective and affordable intervention against peripheral nerve injuries | 80 | ||
| Ameliorate metabolic syndrome effect | NAFLD | ICR mice, L02 cells | In vivo, in vitro | 50 mg kg−1, 100 mg kg−1, and 200 mg kg−1, 0.2, 0.4, 0.6, 0.8, and 1 mM | M. oleifera seed extract containing 1-O-(4-hydroxy-methylphenyl)-α-L-rhamno-pyranoside (GR) | Inhibits lipid accumulation in L1 cells by regulating AMPK/mTOR/SREBP-02 and PPARα pathways. GR anti-inflammatory can promote PPARα and AMPK, inhibiting downstream mTOR/SREBP-1, activating lipoprotein lipase and changing gene coding enzyme to induce transcription and participate in lipid and lipoprotein metabolism, improve the release of intracellular reactive oxygen species, reduce the accumulation of oil and TG, and promote lipid metabolism | Decreased serum fat content, inhibited liver injury, and increased antioxidant mechanism | 81 | |
| Diabetes | Wister male rats | In vivo | 500 mg kg−1, 250 mg kg−1 and 500 mg kg−1 | M. oleifera leaves methanolic leave extracts (MO) | Reduced inflammation and oxidative stress, reducing hyperglycemia-related liver damage | Reduced fasting plasma ALAT, ASAT, GGT, increased plasma albumin, and restored the damage hepatocellular architecture | 82 | ||
| Diabetes | Pre-diabetic subjects aged 40 to 70 years who have not used medication to control glycemic | In vivo | 1600 mg per day | M. oleifera leaves capsule | TNF-α to be a key factor to identify potential respondents | The beneficial effect on blood glucose control in pre-diabetic patients | 83 | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||||
| Ameliorate metabolic syndrome effect | Diabetes and NAFLD | C57BL/6 mice | In vivo | 250 mg kg−1 | M. oleifera fermentation extract (FM) | Reduced high fat diet-induced ER stress, oxidative stress, lipid toxicity of quadriceps muscle and proinflammatory cytokine mRNA expression in the liver, epididymal adipose tissue, and quadriceps of HFD-fed mice, glucose intolerance and NAFLD in HFD-induced obesity by reducing ER stress, oxidative stress, and inflammation | Antidiabetic effects and can be an effective dietary supplement for treating hyperglycemia and NAFLD | 84 | |
| T2DM | UCD T2DM rats | In vivo | 202 mg per kg per day, 94 mg per kg per day | M. oleifera seeds extract | Reduced inflammatory markers (KC/GRO and TNF-α). MS has anti-inflammatory activity by activating Nrf2 pathway | Delayed onset of diabetes in rat models of ucd T2DM | 85 | ||
| Hypertensive | Wistar Kyoto male rats | In vivo | 750 mg per day per rat | M. oleifera (MOI) seed powder | Decreased the level of free 8-isoprostaglandin circulation and the expressions of iNOS and NF-κB protein were decreased, up-regulated the expressions of p22phox and p47phox and SOD2 | Vascular antioxidant, anti-inflammatory, and endothelial protective effects in SHR. Combat cardiovascular diseases associated with oxidative stress and inflammation | 86 | ||
| Other | Reproductive protection | NIH Swiss female and male mice | In vivo | Diet supplemented with 4% MOL, diet supplemented with 8% MOL | M. oleifera leave (MOL) powder | Decreased serum MDA in both male and female mice, reduced the rate of sperm abnormality in male, and the expression of Bax | Affecting reproductive | 87 | |
| — | NZW rabbits | In vivo | 5 g kg−1, 10 g kg−1 and 15 g kg−1 | M. oleifera leaves extract | Attenuate phytoestrogenic pituitary–gonadal axis, and reduced serum gonadotropin (FSH and LH) and oestrogen concentration in does | Female rabbits: reductions in serum FSH, LH, and estrogen concentrations. Male rabbits: increased, FSH and LH, and decreased the serum testosterone concentration. Improved semen volume, sperm count and motility were significantly | 88 | ||
| — | Sprague Dawley male rats | In vivo | 800 mg kg−1 | M. oleifera aqueous extract | Reduces the oxidative stress in a unilateral cryptorchidism induced rats, and attenuate histopathological damages, HSP expression and germ cell apoptosis | Increased apoptotic cells in the undescended testes. Decreased pathological damages, oxidative stress, expression level of HSP70 and apoptosis | 20 | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||||
| Other | Promote wound healing | P. aeruginosa, S. aureus, and E. coli, Swiss albino male mice | In vivo, in vitro | 100 mg mL−1, 10% hydrogel was formulated using 10 g of M. oleifera n-hexane seed extract and 90 g of gel | M. oleifera seeds extract | Antioxidant, antimicrobial, and wound healing activitie. Gram-positive and Gram-negative bactericidal potential | Could be used as potential herbal wound healing agents | 89 | |
| Diabetic foot ulcers | Wister male rats | In vivo | 0.5%, 1%, and 2% w/w aqueous fraction | M. oleifera leaves extract | Decreased wound size, improved wound contraction, and tissue regeneration, as well as downregulation of inflammatory mediators, such as TNF-α, IL-1β, IL-6, inducible nitric oxide synthase, and cyclooxygenase-2, and upregulate an angiogenic marker vascular endothelial growth factor in wound tissue | Vicenin-2 active compound may accelerate wound healing in hyperglycemic condition | 90 | ||
| Bone protection | Early osteoblasts resulting from neonatal rats | In vitro, in vivo | 25 μg mL−1, 50 μg mL−1, 100 μg mL−1 and 150 μg mL−1, 1 g kg−1 | M. oleifera polysaccharides | Reduced apoptosis and intracellular ROS levels in glucocorticoid-induced femoral necrosis. Decreased the expression of TNF-α and IL-6 genes with polysaccharide, and increased the expression of OCN, RUNX2 and COL-1 genes in cartilage tissue, protecting femoral head necrosis | Controlling and regenerating osteoblasts cells, and preventing necrosis of the femoral head | 91 | ||
Antioxidant and anti-inflammatory effect
The oxidative stress derives from the imbalance between the generation of ROS and antioxidant system, commonly leading to inflammation and consequently to a variety of diseases such as pancreatitis,96 periodontitis,97 arthritis,98 acute kidney injury,99 colitis100 and so on. Previous studies have suggested that the flavonoids and polyphenols contained in M. oleifera, such as rutin and myricetin, are the main active ingredients that exert the antioxidant and anti-inflammatory functions.101 In addition, isothiocyanates, which are abundant in the seeds of M. oleifera, also exhibit these effects both in vitro and in vivo.63 The specific mechanism of the anti-inflammatory effect of MO may be through regulating the gene expression levels of inflammatory cytokines. These cytokines include interleukin-6 (IL-6), interleukin-17A (IL-17A), interleukin-4 (IL-4), interleukin-10 (IL-10), nitric oxide (NO), inducible nitric oxide synthase (iNOS) and interleukin-1β (IL-1β). In addition, the gene expression of antioxidant factor (quinone oxidoreductase 1 (NQO1), heme oxygenase 1 (HO-1), catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GPX) and antioxidant enzyme (GSTP1)) is regulated to play the role of antioxidant (Fig. 10).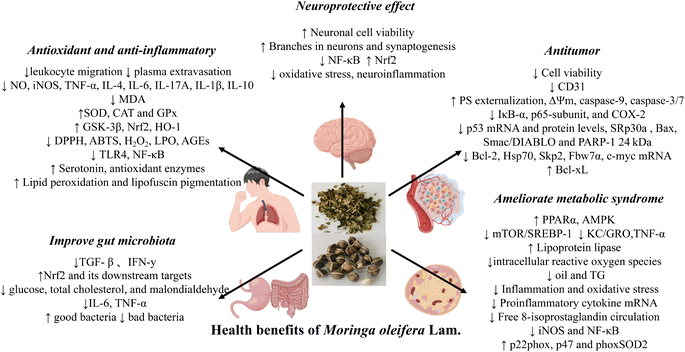 | ||
| Fig. 10 Summary of the potential health benefits of M. oleifera. (Images were obtained from http://figdraw.com/). | ||
Antitumor effect
Cancer remains a major health concern with chemotherapy previously prescribed having significant side effects. The plant-based therapies have become a hot research topic in recent years. M. oleifera contains a variety of bioactive substances that have positive effects on the treatment for cancer.M. oleifera leaves can fight against variety classes cancer cells. M. oleifera leaves water extract (MOE) has significant anticancer effect on melanoma cells in vitro, while it has almost no effect on normal human fibroblasts. The specific mechanisms of action involve mitochondrial-mediated Cleaved-Caspase and Caspase apoptosis pathway, indicating its potential for skin cancer treatment.102
Alkaloids, which are nitrogen-containing organic compounds, have demonstrated antitumor effects. M. oleifera alkaloids have been shown to inhibit PC3 cell proliferation and migration by inhibiting cyclooxygenase-2-mediated (COX-2-mediated) Wnt/β-catenin signaling pathway through in vivo and in vitro experiments. Lung cancer is one of the common malignant tumors. Studies have found that M. oleifera alkaloids also have therapeutic effects on lung cancer. Further experiment showed that M. oleifera can inhibit the proliferation and migration of human non small cell lung cancer cell (A549) cells by inhibiting the mechanisms related to activation of Janus kinase2/Signal Transducer and Activator of Transcription 3 (JAK2/STAT3) pathway, and induce cell apoptosis and cell cycle arrest, further highlighting its potential in preventing and treating lung cancer.4
Improve gut microbiota effect
The importance of gut microbiota in human health has been revealed by numerous studies in recent years. The imbalance of intestinal flora is associated with some diseases, such as diabetes, obesity, chronic kidney disease and colitis. M. oleifera seed extract (MSE) was given to male C57BL/6J mice for 16S of fecal/cecum samples after 12 weeks of feeding together with normal diet and high-fat diet rRNA gene sequencing and quantitative Polymerase Chain Reaction (PCR) showed major changes in intestinal microbiota and significant reduction of bacterial load, similar to antibiotic response, suggesting that M. oleifera seed extract can improve metabolic health through antibiotic-like recombination of intestinal microbiota, meanwhile, MSE supplementation can reduce body weight, obesity and inflammatory gene expression, improve glucose tolerance, and increase antioxidant gene expression.46 Wang et al. extracted the polysaccharide component MOS-2-A from M. oleifera leaves.103 Through experiments, they verified that MOS-2-A could reduce the number of pathogenic bacteria by increasing the number of beneficial bacteria, significantly improve the diversity of intestinal flora in mice, improve the structure of intestinal flora, and help maintain the integrity of intestinal mucosa, promote digestion and promote intestinal health. M. oleifera leaves induce the production of acetic acid, propionic acid and n-butyric acid after gastrointestinal digestion and colonic fermentation in vitro, resulting in a decrease in pH and promoting the growth of beneficial colonic bacteria.40 In addition, more experimental results reveal that M. oleifera has the ability to regulate intestinal for the treatment of diseases.Neuroprotective effect
Herbs therapy is widely used in the treatment of many diseases due to its low toxicity and good curative effect. M. oleifera has a variety of functions, including protective effects on the nervous system, due to its rich active ingredients.M. oleifera is rich in flavonoids and polyphenols, which have neuroprotective effects. Di-(2-ethylhexyl) phthalate (DEHP) at specific concentrations can produce neurotoxic effects after prenatal and postnatal exposure. In rats, prenatal exposure to DEHP can disrupt the development of the central nervous system and reduce brain weight. It was found that M. oleifera extract can restore the activity of mitochondrial respiratory chain complex by regulating and reducing the formation of ROS, prevent oxidative injury by regulating Nrf2/HO-1 expression, and inhibit endoplasmic reticulum stress (ER stress) response to prevent neuron injury and protect neurons SH-SY5Y cells were protected from DEHP-induced apoptosis and maintained mitochondrial membrane permeability and caspase-3 activation, thus realizing the neuroprotective effect of M. oleifera extract on DEHP injury. M. oleifera seeds are rich in glucosinolates, such as niazimicin (NZ), the main bioactive substance.104 Abdelsayed et al. found that NZ in M. oleifera seeds can play a neuroprotective role by affecting oxidative stress marker glutathione (GSH), malondialdehyde (MDA) inflammatory mediators NF-κB and NO neurotransmitters, dopamine and 5-hydroxytryptamine, and brain fatty acids (FA) levels in AlCl3-induced dementia rats.105
Ameliorate metabolic syndrome effect
Metabolic syndrome is a group of clinical syndromes with hypertension, central obesity, diabetes mellitus, hyperlipidemia and abnormal glucose metabolism. In modern times, people's life pressure, irregular work and rest, unreasonable diet structure and other factors lead to the low age of metabolic syndrome diseases, and become one of the main causes of death.106 And studies have shown that M. oleifera can play a significant role in the treatment of these diseases.Niazirin, a phenol glycoside isolated from M. oleifera seeds, could improve insulin resistance, hyperglycemia, hyperlipidemia, and non-alcoholic fatty liver disease. And in-depth investigation showed that niazirin can reduce the accumulation of gluconeogenesis and lipid, improve glycolysis and lipid oxidation by activating the AMP-Activated Protein Kinase (AMPK) pathway. These findings suggest that niazirin could serve as an effective treatment for metabolic syndrome.1
Additional studies have shown that M. oleifera leaf hydrolysate (MOLH), which is rich in phenols and peptides, can improve hyperuricemia and metabolic disorders.107 To verify the therapeutic effect of 1-O-(4-hydroxy-methylphenyl)-α-L-rhamno-pyranoside (GR) in M. oleifera seeds on non-alcoholic fatty liver disease (NAFLD), in vitro and in vivo experiments were established. The results showed that GR could significantly reduce intracellular fat deposition and ROS content, up-regulate AMPK and peroxisome proliferator-activated receptor (PPARα), down-regulate Mechanistic Target of Rapamycin (mTOR) and sterol regulatory element binding transcription factor 1 (SREBP-1) to achieve liver lipid homeostasis. GR could also reduce serum fat content in high-fat diet mice, inhibit liver injury, and increase antioxidant mechanism, indicating that GR has hypolipidemia and liver protection activities. MOE water extract can drive vasodilation to reduce arterial blood pressure by stimulating endothelium-derived NO, providing a new method for the treatment of hypertension.108
Other effects
Herbs therapy is widely used in the treatment of many diseases due to its low toxicity and good curative effect. Because the therapy affects reproductive physiology and improves certain infertility problems by acting on the hypothalamic–pituitary–gonadal axis.109 M. oleifera is commonly prescribed by herbalists in Nigeria and other tropical countries to treat male infertility and female reproductive diseases.Traditional plant medicines have often been used for wound treatment since ancient times. Besides the use of chemical drugs, high prices, side effects and other problems, herbal remedies have attracted attention.110 M. oleifera is widely used in wound treatment due to its low side effects and excellent antioxidant and antibacterial activity. In addition to the above functions, M. oleifera can also be used for bone protection,91 promote coagulation111 and so on.
Mechanism and components of M. oleifera in preventing and treating diseases
M. oleifera has been extensively studied for its pharmacological activity and function, and the mechanism of action of M. oleifera should be further elucidated at the molecular level. In this paper, from a network pharmacology perspective, we have explored the potential targets and molecular mechanisms of action of the major components of the Moringa oleifera leaves (MOL) against major diseases.Combined with literature review and pharmacological action review results, cancer,7 hypertension,112,113 diabetes93 and arthritis114 were selected as potential treatment diseases of MOL. Refer to Table 2 for the results of UPLC-Q-TOF-MS analysis of the chemical composition of the MOL.
Metascape data platform was used to analyse the Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment of MOL for diabetes, hypertension, arthritis and tumor intersections. The results showed that the main biological processes were icosanoid metabolic process, unsaturated fatty acid metabolic process, fatty acid metabolic process. Molecular functional targets are mainly related to heme binding, tetrapyrrole binding and oxidoreductase activity, acting on paired donors, with incorporation or reduction of molecular oxygen. The main enrichment items of the targets in cellular component are membrane raft, membrane microdomain and plasma membrane raft.
The enrichment results of KEGG pathway were sorted according to Partition coefficient value (−log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P), and the top results were selected to draw a chart (P < 0.05)115 (Fig. 11 and S1 to S4†). The results showed that the arthritis pathway was mainly enriched in: chemical carcinogenesis–DNA adducts, drug metabolism-cytochrome P450, linoleic acid metabolism, et al. In collagen-induced arthritis of rats, the linoleic acid metabolic pathway was significantly altered before and after treatment with 2-deoxy-D-glucose, suggesting that the linoleic acid metabolic pathway is closely related to the treatment of arthritis and may serve as a potential target.116 And studies showed that the bezoar bile acid in the rheumatoid arthritis (RA) rats' body of the small molecule can affect the metabolic pathways of steroid hormone biosynthesis, which led to a decline in immune function in rats, by taking the Atractylodes DC., and the metabolic pathway to normal levels, which can be speculated that by steroid hormones in the treatment of RA.45
P), and the top results were selected to draw a chart (P < 0.05)115 (Fig. 11 and S1 to S4†). The results showed that the arthritis pathway was mainly enriched in: chemical carcinogenesis–DNA adducts, drug metabolism-cytochrome P450, linoleic acid metabolism, et al. In collagen-induced arthritis of rats, the linoleic acid metabolic pathway was significantly altered before and after treatment with 2-deoxy-D-glucose, suggesting that the linoleic acid metabolic pathway is closely related to the treatment of arthritis and may serve as a potential target.116 And studies showed that the bezoar bile acid in the rheumatoid arthritis (RA) rats' body of the small molecule can affect the metabolic pathways of steroid hormone biosynthesis, which led to a decline in immune function in rats, by taking the Atractylodes DC., and the metabolic pathway to normal levels, which can be speculated that by steroid hormones in the treatment of RA.45
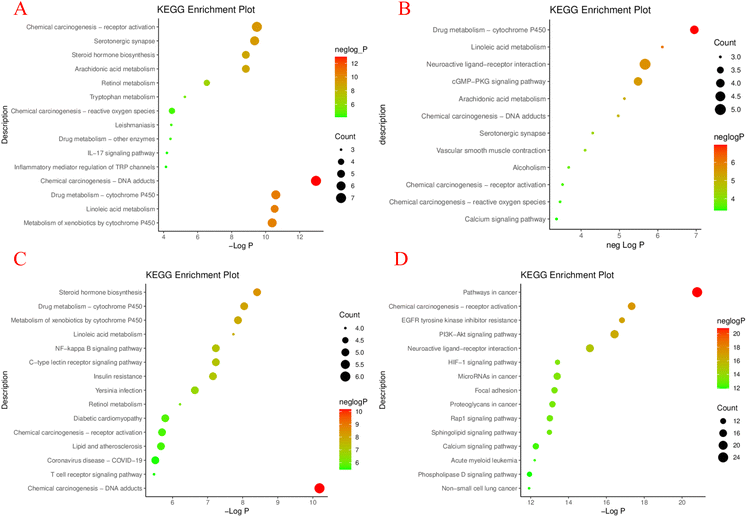 | ||
| Fig. 11 The common target of M. oleifera and 4 diseases obtained by the above screening was imported into the Metascape Data platform (https://metascape.org/gp/index.html).117 The species was set to be “Homo sapiens”, and GO function enrichment analysis and KEGG pathway enrichment was performed. GO functional analysis includes biological process (BP, biological process), cellular component (CC, cellular component), molecular function (MF, molecular function). It is visualized as histogram and bubble chart by the ImageGP (https://www.bic.ac.cn/ImageGP/).115 KEGG enrichment analysis of potential targets of MOL in treating 4 diseases: arthritis (A), hypertension (B), diabetes (C), tumor (D). | ||
Study on the major pathways of hypertension has shown that the haploidy insufficiency of glucocorticoid receptor in rats can cause the disorder of linoleic acid metabolism pathway.57 Another study on the mechanism of melatonin-mediated hypotension, at 18 h, melatonin acted on the (cGMP-PKG) signaling pathway involved in NO synthesis, suggesting that the regulation of blood pressure could be achieved through the cGMP-PKG signaling pathway.118
According to KEGG pathway analysis of diabetes, there are drug metabolism-cytochrome P450, linoleic acid metabolism and Neuroactive ligand–receptor interaction, et al. Hogan et al. used quantitative reverse transcription Quantitative Real-time polymerase chain reaction (QRT-PCR) to identify differentially regulated gene expression of islet amyloid, which is associated with islet physiology and pathology of type II diabetes mellitus, and found that it contains steroidogenic acute regulatory protein (STAR protein), which is part of the synthetic response group of steroid hormone metabolism. It can be inferred that the pathway of steroid hormone metabolism is closely related to diabetes.119 Resveratrol plays a protective role in the treatment of inflammation-induced vascular injury in type II diabetes by regulating NF-κB signaling pathway, suggesting that this signaling pathway has significant potential for type II diabetes and its complications.120
The results of KEGG pathway enrichment analysis suggested that amphiphysin (AMPH1), a protein at the nerve endings, plays an inhibitory role in ovarian cancer. Studies have demonstrated that AMPH1 inhibits the growth of tumor cells by inhibiting the growth of phosphatidylinositol 3 kinase/protein kinase B (PI3K/AKT) signaling pathway in ovarian cancer.121 Sadegh et al. studied the carcinogenic or anti-cancer effects of miRNA in cancer and apoptosis, and speculated that manipulating miRNA expression level might be a potential method to treat cancer.122
Based on the MOL composition-disease target-pathway network, combined with literature records and degree values, the main active components and targets of the MOL for different diseases were screened for molecular docking validation.123 It is generally believed that the lower the conformational stability energy of the ligand and the receptor, the greater the possibility of interaction, based on the choice of the degree value of the top core target in the corresponding active ingredient for docking as shown in Fig. S5.† The results show that there is a strong interaction between the target and the compound which score between −10.5 ∼ −9.6 kcal mol−1. The core compound of arthritis, diabetes and tumour is rutin. The docking results with the lowest binding energies for the four diseases are shown in Fig. S6† respectively.
Preparation formulations of M. oleifera
M. oleifera has numerous functions that are expected to be used to treat human diseases. Therefore, the selection of preparation formulation of M. oleifera should not be ignored. M. oleifera has been developed into granules, tablets, capsules, external preparation and other different preparation formulations such as nanoparticles. Additionally, the ingredient of M. oleifera can also be used as preparation excipients in the development of current drugs.Tablet and capsules
A tablet is a solid preparation of flake or profile-flake formed after uniform mixing of drug and excipient. Commonly, tablets are known to enhance drug dissolution and bioavailability, and has the advantages of stable quality, accurate dose, easy to take and carry. As for the choice of adhesive during the preparation of M. oleifera tablet, researchers carried on a detailed study with different adhesives including corn starch, gelatin and microcrystalline cellulose (MCC) and the water extract of M. oleifera leaves preparation of tablets, the formula were characterized using various parameters, such as the chemical and physical properties (bulk density tap water content, density ratio, Karr index ash value strength (fragility and crushing strength) and release properties (disintegration and dissolution time tests)).124,125 The results showed that compared with MCC and corn starch, the tablets with gelatin as binder had the lowest brittleness and disintegration time, and the crushing strength was within the acceptable range (36 kgF). The comprehensive results showed that gelatin was the best adhesive for M. oleifera tablets. On the other hand, capsules are composed of is an active content with the appropriate auxiliary materials, either in the hollow hard capsule filling or seal in soft capsule material. M. oleifera have bitter taste, is not convenient to make direct oral powder, sealed with hard capsule or hollow capsule material, preparation into capsule not only can hide M. oleifera bad smell at the same time also can according to different drug purposes to achieve the location of drug release. M. oleifera leaves alcohol extract was prepared and standardized hard gelatin capsules (400 mg per capsule), in order to study the anti-obesity effect of M. oleifera leaves.126 Fifteen female participants aged 45–55 years with a body mass index (BMI) of 29–34 kg m−2 were selected for the test. The mean BMI total cholesterol (TC) and lowdensity lipoprotein (LDL) were significantly reduced in obese patients after taking M. oleifera capsules for eight weeks (P < 0.05). Moreover, M. oleifera capsules can also be used as a natural emulsion to increase the volume of breast milk.127New preparation technology
The components of M. oleifera have been found to have low solubility bioavailability in drug preparation and poor compliance in common preparations due to their specific smell and taste. To improve the application and promotion of M. oleifera preparations, new preparation formulations have been gradually applied in it. Isothiocyanate extracted from M. oleifera (MITC) has excellent anti-skin photoaging effect, but its application is limited due to its extremely low water solubility, low bioavailability and easy degradation.16 Therefore, they prepared amphiphilic hyaluronic acid (HA) conjugated with ceramide (CE) to modify nanoliposomes for MITC (HACE/MITC NPs) delivery. The results showed that the entrapment of MITC in nanoliposomes improves its stability, efficacy, and skin permeation. In addition, M. oleifera extract has been found to have mosquito repellent effect and can be used as a safe and cost-effective substitute for N,N-diethyl-3-methylbenzamide (DEET). Gelatin nanoparticles coated with M. oleifera bioactive extract were used to improve its availability.128 Moreover, silver nanoparticles synthesized from M. oleifera leaf extract have also been found to have good anti-leishmania activity.129 The treated fabric repellency to Culex pipiens mosquitoes showed stable 100% repellency for 6 days for all treated fabrics and ranged from 50 to 90% repellency after 12 days. Green synthesis of silver nanoparticles has been shown to have anticancer activity, Althomali et al. took M. oleifera leaf extract as the reducing agent and stabilizer of synthesize AgNPs, and the synthesized compound showed superior anticancer activity against human cancer cell line HTC116 and SW480 than M. oleifera leaf extract.130 This result implies that AgNPs synthesized by M. oleifera extract could be an ideal strategy to combat colon cancer.Preparations for external use and others
In order to find a way to prevent 2019-nCoV through personal hygiene, researcher found that various components in M. oleifera leaves had antibacterial effects, for example: polyphenols, terpenoids, saponins, and so on.131 The hand sanitizer is extracted by osmosis, and after processing, it can meet hand sanitizer quality standards and bacteriostatic standards. The hydrogel prepared with hexane extract of M. oleifera seeds has significant wound healing activity.89 M. oleifera seed n-hexane water gel preparation can be used as a skin repair replacement therapy during wound healing. A membrane consisting of normalized water extract of oil tea leaf was then prepared for wound healing, which enhanced the proliferation and migration properties of human dermal fibroblasts and keratinocytes.132 Study investigated the potential of M. oleifera seed polysaccharide (MOS-PS) and its composite with MOS-PS-AgNPs as an alternative material for wound dressing.24 In addition, oral treatment of a predominantly Leishmania infected mouse model using silver nanoparticles biosynthesized from M. oleifera leaf extract (AG-NPs) was found to significantly reduce the mean size of skin lesions of leishmaniasis.133 It was proved that the nanoparticles synthesized from M. oleifera leaf extract had good anti-leishmania activity.Pharmaceutical adjunct
Moringa oleifera is available in various dosage forms and due to its diverse composition, it can serve multiple purposes such as an emetic, a dissolvent, an adhesive, and a drug carrier. M. oleifera powder, although only slightly soluble in water, has the capability to expand and form a highly viscous solution, which can be used as an adhesive in the preparation of tablets.134 The coagulated protein was extracted from M. oleifera, and its interface properties and interaction with sodium dodecyl sulfate (SDS) were studied. The results showed that the protein extracted from M. oleifera seeds had significant surfactant behavior and could be used as a surfactant. Therefore, moringa gum powder extracted from M. oleifera can also be used as disintegrating agent. On the basis of the research, moringa gum has potential as a binder and sustained-release agent in tablets.135 Moreover, in vitro drug release studies have evaluated the sensitivity of moringa gum to colonic bacteria and tablets containing it have shown a drug release rate of 90.46%. According to the results, moringa gum could be used as a potential carrier for colonic specific drug delivery.136Future trends
On the one hand, herbal medicine can fully mobilize the human immune system through multi-target and multi-way synergistic effect, and play a radical cure effect. On the other hand, extensive toxicity experiments have proved its safety. Based on this, in recent years, medicinal plants and herbs have gradually become a treatment strategy for a variety of diseases. According to traditional ancient books, M. oleifera has multiple functions and plays a key role in curing and preventing diseases. As a medicinal and edible plant, M. oleifera has attracted extensive attention from countries around the world. How to study M. oleifera, an exotic species, with the help of traditional Chinese medical theory, and explain the pharmacological action and mechanism of its medicinal substance has become an opportunity and challenge for us to face. At the same time, the development of dietary supplements and adjuncts is inextricably linked to the design of prepared formulations, the selection of appropriate preparations, which can improve the stability and safety, in addition, enhance their efficacy. At present, M. oleifera only has common dosage forms such as granules, tablets, and capsules. Among the new preparations, only nano preparations have been studied, and the other preparation types are still to be developed. In addition, the development of current preparation types should start from the biopharmaceutical properties of M. oleifera itself, such as clarifying the solubility and permeability of relevant medicinal substances. In combination with different dosage forms, such as nano-pharmaceuticals, to improve the safety and stability of the in vivo bioavailability of the preparations.M. oleifera used in health products
Beyond its considerable medicinal properties, M. oleifera is also widely utilized in food industry, natural resource preservation efforts, cosmeceuticals and other health product applications. Studies have demonstrated the nutritional benefits of incorporating M. oleifera leaves and buds into food products as a rich source of vitamins, minerals, and protein.137 For instance, adding moringa bud powder (MSP) to conventional pasta has been shown to bolster the content of protein, lipid, fiber and minerals, while the content of carbohydrate showed a trend of decline. The addition of MSP also increases the levels of thiamine, riboflavin, gamma-aminobutyric acid, glucosinolate and antioxidant activity in pasta. Based on the sensory scores, the inclusion of MSP is a technical approach that can improve the nutritional value and biological activity of traditional pasta without compromising consumer acceptance.138 From the perspective of nutrition, researchers have also developed a cheese product containing M. oleifera leaf powder. The aim is to increase the ash, protein, fiber and fat content of the product with the powder, so that cheese can be used as an alternative food in the fight against malnutrition.139 M. oleifera is also widely used in baked goods. In response to consumer demand for organic food, moringa bread was developed by incorporating natural plant moringa leaves into baking product recipes. Studies have found that moringa bread products are more beneficial to human health because they are higher in nutrients and antioxidant activity than regular bread products, which contain less fiber and bioactive substances.140 Besides, the addition of M. oleifera leaf to biscuits can also increase the iron and protein content of the product, while providing essential amino acids to the human body.141 It is worth noting also M. oleifera contains large amounts of flavonoids and phenols, which have good antioxidant activity, so it can be used in the development of cosmeceuticals like body washes and lotions, as it is gentle on skin and helps to reduce irritation. For example, several studies have developed moringa-containing lotions by exploiting the properties of moringa, which can be used externally on the skin and have the properties of natural plant ingredients 64. In addition to its ingredient properties, the addition of M. oleifera has the advantage of reducing skin irritation and improving the safety of the body wash.142 As well as developing excellent emollient products using M. oleifera seed oil. Finally, M. oleifera's biocoagulant capabilities make it a promising tool in natural resource management for water purification purposes. M. oleifera seed can remove turbidity and natural organic material to bring surface water up to standard requirements and it has the advantages of low-cost, simple operation, high efficiency and health safety.143Summary
M. oleifera is a kind of plant which can be used both as medicine and food. In this review the medicinal component of M. oleifera and the chemical components of each component of M. oleifera in combination with their functions was summarized, finding that MOL and MOS are currently the most widely used. It was found that the main components of M. oleifera are flavonoids and polyphenols. These bioactive compounds exhibit promising antioxidant, anti-inflammatory, anti-tumor, improve gut microbiota antibacterial, neuroprotective antioxidant, ameliorate metabolic syndrome and other activities. Based on the pharmacological action and molecular mechanism was used to analyse the mechanism of M. oleifera, which may play a key role through PI3K-Akt signaling pathway in treating cancer and other diseases. Finally, the application of M. oleifera preparation, products and other format is reviewed. However, there are still some key questions that need to be answered through further studies: firstly, as an edible product, it is important to address the bitterness of M. oleifera for better future development. Next, although there have been several studies on the composition and function of M. oleifera, a more in-depth study of the unique composition, including structural and pharmacological aspects, is needed. Thirdly, the underlying mechanism of the MOL function is obtained from data calculations and simulation methods. To further confirm their correctness, in vitro and in vivo experiments should be designed for validation. Fourthly, it is found that research on M. oleifera preparation formulations mainly focused on traditional tablets, granules and capsules, etc. The preparation is relatively monotonous, and how to improve M. oleifera's bitter taste needs further research. In addition, the database used in this study is still constantly updated, so further research is needed to clarify the specific mechanism of M. oleifera on diseases, so as to better guide the development of functional foods and complementary drugs.Abbreviations
| M. oleifera | Moringa oleifera Lam. |
| MOL | Moringa oleifera Lam. leaves |
| MOS | Moringa oleifera Lam. seed |
| UPLC-Q-TOF-MS | Ultra High Performance Liquid Chromatography-Quadrupole-Time of Flight-Mass Spectrometry |
| ROS | Reactive oxygen species |
| NHDF | Normal human dermal fibroblasts |
| MOS-PS-AGNPs | Silver nanocomposites with M. oleifera seed polysaccharides |
| IL-6 | Interleukin 6 |
| IL-17A | Interleukin 17A |
| IL-4 | Interleukin4 |
| IL-10 | Interleukin10 |
| INOS | Inducible Nitric Oxide Synthase |
| IL-1β | Interleukin1β |
| NQO1 | Quinone oxidoreductase 1 |
| HO-1 | Heme oxygenase 1 |
| CAT | Catalase |
| SOD | Superoxide dismutase |
| GPX | Glutathione peroxidase |
| COX-2 | Cyclooxygenase-2 |
| A549 | Human non small cell lung cancer cell |
| JAK2 | Janus kinase2 |
| STAT3 | Signal Transducer and Activator of Transcription 3 |
| PCR | Polymerase Chain Reaction |
| MSE | M. oleifera seed extract |
| cGMP-PKG | cGMP-dependent protein kinase |
| MOs-2-a | A polysaccharide separated from M. oleifera leaf |
| DEHP | Di-(2-ethylhexyl) phthalate |
| Nrf2 | Factor erythro2-related factor 2 |
| ER stress | Endoplasmic reticulum stress |
| NZ | Niazimicin |
| GSH | Glutathione |
| MDA | Malondialdehyde |
| NF-κB | Nuclear factor kappa-B |
| FA | Fatty acids |
| AMPK | AMP-activated Protein Kinase |
| MOLH | M. oleifera leaf hydrolysate |
| GR | 1-O-(4-Hydroxy-methylphenyl)-α-L-rhamno-pyranoside |
| PPARα | Peroxisome proliferator-activated receptor |
| mTOR | Mechanistic Target Of Rapamycin |
| SREBP-1 | Sterol regulatory element binding transcription factor 1 |
| GO | Gene ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
−log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P value P value | Partition coefficient value |
| RA | Rheumatoid arthritis |
| QRT-PCR | Quantitative Real-time polymerase chain reaction |
| STAR protein | Steroidogenic acute regulatory protein |
| AMPH1 | Amphiphysin |
| PI3K/AKT | Phosphatidylinositol 3 kinase/protein kinase B |
| MCC | Microcrystalline cellulose |
| TC | Total cholesterol |
| LDL | Lowdensity lipoprotein |
| MITC | Isothiocyanate extracted from M. oleifera |
| HA | Hyaluronic acid |
| CE | Conjugated with ceramide |
| DEET | N,N-Diethyl-3-methylbenzamide |
| MOS-PS | M. oleifera seed polysaccharide |
| AG-NPs | M. oleifera leaf extract |
| SDS | Sodium dodecyl sulfate |
| MSP | Moringa bud powder |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| ABTS | 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) |
| LPO | Lactoperoxidase |
| AGEs | Advanced glycation end-products |
| Bax | BCL2-associated X protein |
| BCl2 | B-Cell lymphoma-2 |
| ACE | Angiotensin I-converting enzyme |
| IL-3 | Interleukin-3 |
| STAT5 | Signal Transducer and Activator of Transcription 5 |
| UVB | Ultraviolet radiation b |
| MMP-1 | Matrix metalloproteinase 1 |
| MMP-3 | Matrix metalloproteinase 3 |
| MMP-9 | Matrix metalloproteinase 9 |
| TNF-α | Tumor necrosis factors-α |
| IAVS | Influenza A viruses |
| GSK-3β antibody | Glycogen synthase kinase-3β |
| DDPH | 2,2-Diphenyl-1-picrylhydrazyl |
| ORAC | Oxygen Radical Absorption Capacity |
| TLR4 | Toll-like receptor 4 |
| T-47D | Human breast ductal carcinoma cells |
| CD31 | Platelet endothelial cell adhesion molecule-1 |
| Smac | Second mitochondria-derived activator of caspases |
| HSP 70 | Heat shock protein 70 |
| Skp2 | S-Phase kinase-associated protein 2 |
| Fbw7α | F-Box and WD repeat domain-containing7α |
| INF-κB | Nuclear factor kappa-B inhibitor |
| ERK | Extracellular-signal-regulated kinase |
| TGF-β | Transforming growth factor beta |
| IFN-y | Interferon-gamma |
| Th2 | T-Helper cell type 2 |
| NAFLD | Non-alcoholic fatty liver disease |
| HFD | Histone fold domain |
| p22phox | Cytochrome b-245, alpha polypeptide |
| p47phox | Phospho-Ser359 |
| RUNX2 | Runt-related transcription factor 2 |
| COL-1 | Collagentypel 1 |
Author contributions
Xinyue Su, Guanzheng Lu, Liang Ye Maomao Zhu and Xinming Yu collected and analysed the literature data. Xinyue Su and Guanzheng Lu completed the network pharmacology and UPLC-Q-TOF-MS. Xinyue Su and Ruyu Shi was also responsible for writing and submission of this manuscript. Liang Ye and Xinming Yu revised the format of the article. Xinbin Jia, Zhiyong Li and Liang Feng participated in reviewing and proofreading this manuscript. All authors read and approved the final manuscript. All data were generated inhouse, and no paper mill was used. All authors agree to be accountable for all aspects of work ensuring integrity and accuracy.Conflicts of interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service or company that could be construed as influencing the position.Acknowledgements
This work was supported by Key research projects on modernization of traditional Chinese medicine, National Key R&D Program (2018YFC1706900). “Double First-Class” University project of China Pharmaceutical University (CPU2018GF07, CPU2018PZQ19). The protein targets of the active substances in M. oleifera were retrieved from the Swiss Target Predition database (http://www.swisstargetprediction.ch/).144 Diseases-related human genes were collected from three databases, namely, Online Mendelian Inheritance in Man (OMIM, http://www.omim.org/), Therapeutic Target Database (TTD, https://db.idrblab.org/ttd/),145 and DrugBank (https://www.drugbank.ca/). The PPI network of M. oleifera and diseases intersection targets were obtained on the String11.5 platform (http://string-db.org/).146 The gene ontology (GO) and pathway enrichment analyes were conducted using the functional annotation tool of Metascape Data platform (https://metascape.org/gp/index.html).117 The compound-target and target-pathway networks were generated using Cytoscape 3.7.1.147 The 3D structure of the key target proteins was downloaded from PDB database (http://www.wwpdb.org/).148References
- Y. Bao, J. Xiao, Z. Weng, X. Lu, X. Shen and F. Wang, Food Chem., 2020, 311, 125948 CrossRef CAS PubMed.
- N. Bennour, H. Mighri, T. Bouhamda, M. Mabrouk, E. Apohan, O. Yesilada, H. Küçükbay and A. Akrout, Prep. Biochem. Biotechnol., 2021, 51, 1018–1025 CrossRef CAS PubMed.
- F. Wang, Y. Bao, C. Zhang, L. Zhan, W. Khan, S. Siddiqua, S. Ahmad, E. Capanoglu, K. Skalicka-Woźniak, L. Zou, J. Simal-Gandara, H. Cao, Z. Weng, X. Shen and J. Xiao, Crit. Rev. Food Sci. Nutr., 2021, 1–25 CrossRef PubMed.
- J. Xie, L. J. Peng, M. R. Yang, W. W. Jiang, J. Y. Mao, C. Y. Shi, Y. Tian and J. Sheng, J. Evidence-Based Complementary Altern. Med., 2021, 2021, 5591687 Search PubMed.
- H. Yang, X. Shao and M. Wu, Sustainability., 2019, 11, 4908 CrossRef.
- F. Al Juhaimi, K. Ghafoor, I. A. M. Ahmed, E. E. Babiker and M. M. Özcan, J. Food Meas. Char., 2017, 11, 1745–1751 CrossRef.
- B. H. Do, N. S. Hoang, T. P. T. Nguyen, N. Q. C. Ho, T. L. Le and C. C. Doan, Nutr. Cancer, 2021, 73, 869–888 CrossRef CAS PubMed.
- S. Chandrashekar, R. Vijayakumar, R. Chelliah, E. B.-M. Daliri, I. H. Madar, G. Sultan, M. Rubab, F. Elahi, S.-J. Yeon and D.-H. Oh, Molecules, 2021, 26, 2080 CrossRef CAS PubMed.
- L. R. Managa, E. S. du Toit and G. Prinsloo, Molecules, 2021, 26, 2298 CrossRef CAS PubMed.
- K. Kerdsomboon, W. Chumsawat and C. Auesukaree, Chemosphere, 2021, 270, 128659 CrossRef CAS PubMed.
- F. Wang, Y. Bao, C. Zhang, L. Zhan, W. Khan, S. Siddiqua, S. Ahmad, E. Capanoglu, K. Skalicka-Woźniak, L. Zou, J. Simal-Gandara, H. Cao, Z. Weng, X. Shen and J. Xiao, Crit. Rev. Food Sci. Nutr., 2021, 62, 3873–3897 CrossRef PubMed.
- J. Laoung-on, K. Saenphet, C. Jaikang and P. Sudwan, Plants, 2021, 10, 2019 CrossRef CAS PubMed.
- B. S. Ogunsina, C. Radha and R. S. Govardhan Singh, Int. J. Food Sci. Technol., 2010, 45, 2433–2439 CrossRef CAS.
- M. M. Özcan, S. Afr. J. Bot., 2020, 129, 25–31 CrossRef.
- F. Al Juhaimi, K. Ghafoor, E. E. Babiker, B. Matthäus and M. M. Özcan, J. Food Biochem., 2017, 41, e12322 CrossRef.
- Y. Wang, Q. Ouyang, X. Chang, M. Yang, J. He, Y. Tian and J. Sheng, Drug Delivery, 2022, 29, 871–881 CrossRef CAS PubMed.
- Â. Fernandes, A. Bancessi, J. Pinela, M. I. Dias, Â. Liberal, R. C. Calhelha, A. Ćirić, M. Soković, L. Catarino, I. C. F. R. Ferreira and L. Barros, Food Chem., 2021, 341, 128229 CrossRef PubMed.
- J. Punia and R. Singh, Orient. J. Chem., 2018, 34, 1589–1596 CrossRef CAS.
- A. K. Singhal, E. E. Jarald, A. Showkat and A. Daud, Int. J. Pharm. Invest., 2012, 2, 48–51 CrossRef CAS PubMed.
- M. Tekayev, N. Bostancieri, K. A. S. M. Saadat, M. Turker, M. Yuncu, H. Ulusal, H. Cicek and K. Arman, Gene, 2019, 688, 140–150 CrossRef CAS PubMed.
- A. Tesfaye, A. Anjulo, A. Fekadu, K. Beyene, A. Girma, B. Gemeda, G. Temesgen, F. Wolde, E. Mulugeta and A. Manilal, PLoS One, 2022, 17, e0274678 CrossRef CAS PubMed.
- I. J. Asiedu-Gyekye, S. Frimpong-Manso, C. Awortwe, D. A. Antwi and A. K. Nyarko, J. Toxicol., 2014, 2014, 786979 CrossRef CAS PubMed.
- S. Siddiqui, S. Upadhyay, I. Ahmad, A. Hussain and M. Ahamed, J. Food Biochem., 2021, 45, e13720 CrossRef CAS PubMed.
- H. M. Mehwish, G. Liu, M. S. R. Rajoka, H. Cai, J. Zhong, X. Song, L. Xia, M. Wang, R. M. Aadil, M. Inam-Ur-Raheem, Y. Xiong, H. Wu, M. I. Amirzada, Q. Zhu and Z. He, Int. J. Biol. Macromol., 2021, 184, 144–158 CrossRef CAS PubMed.
- D. Teshome, C. Tiruneh and G. Berihun, BioMed Res. Int., 2021, 2021, 5291083 Search PubMed.
- A. F. Attah, J. O. Moody, M. A. Sonibare, H. H. Salahdeen, O. O. Akindele, P. O. Nnamani, O. A. Diyaolu and Y. Raji, S. Afr. J. Bot., 2020, 129, 255–262 CrossRef CAS.
- N. Bennour, H. Mighri, T. Bouhamda, M. Mabrouk, E. Apohan, O. Yesilada, H. Küçükbay and A. Akrout, Prep. Biochem. Biotechnol., 2021, 51, 1018–1025 CrossRef CAS PubMed.
- S. Tariq, H. Umbreen, R. Noreen, C. Petitbois, K. Aftab, F. A. Alasmary, A. S. Almalki and M. A. Mazid, BioMed Res. Int., 2022, 2022, 4987929 Search PubMed.
- O. H. Oyeniran, A. O. Ademiluyi and G. Oboh, J. Food Biochem., 2021, 45, e13401 CAS.
- F. Alia, M. Putri, N. Anggraeni and M. R. A. A. Syamsunarno, Front. Pharmacol., 2022, 12, 724439 CrossRef PubMed.
- W. Khan, R. Parveen, K. Chester, S. Parveen and S. Ahmad, Front. Pharmacol., 2017, 8, 577 CrossRef PubMed.
- J. Laoung-on, K. Saenphet, C. Jaikang and P. Sudwan, Plants, 2021, 10, 2019 CrossRef CAS PubMed.
- A. Nair D, T. J. James, S. L. Sreelatha, B. J. Kariyil and S. N. Nair, Nat. Prod. Res., 2021, 35, 6216–6222 CrossRef CAS PubMed.
- O. I. Fantoukh, Y. H. Wang, A. Parveen, M. F. Hawwal, G. A. Al-Hamoud, Z. Ali, A. G. Chittiboyina and I. A. Khan, Planta Med., 2021, 87, 417–427 CrossRef CAS PubMed.
- B. H. Do, N. S. Hoang, T. P. T. Nguyen, N. Q. C. Ho, T. L. Le and C. C. Doan, Nutr. Cancer, 2021, 73, 869–888 CrossRef CAS PubMed.
- F. Dhawi, S. E.-B. H. E. Aly and A. M. Hamed, Foods, 2020, 9, 1157 CrossRef CAS PubMed.
- P. Sharma, J. Wichaphon and W. Klangpetch, Int. J. Food Microbiol., 2020, 332, 108770 CrossRef CAS PubMed.
- A. Saleem, M. Saleem and M. F. Akhtar, S. Afr. J. Bot., 2020, 128, 246–256 CrossRef CAS.
- S. Muhammad, S. H. Hassan, A. G. Al-Sehemi, H. A. Shakir, M. Khan, M. Irfan and J. Iqbal, Chem. Phys. Lett., 2021, 767, 138379 CrossRef CAS PubMed.
- Z. Dou, C. Chen and X. Fu, Food Funct., 2019, 10, 5070–5079 RSC.
- T. Rodríguez-García, B. H. Camacho-Díaz, A. R. Jiménez-Aparicio, J. Santaolalla-Tapia, S. Evangelista-Lozano and M. L. Arenas-Ocampo, Rev. Bras. Farmacogn., 2021, 31, 302–309 CrossRef.
- Y.-Y. Wu, Y.-M. Xu and A. T. Y. Lau, Molecules, 2021, 26, 7512 CrossRef CAS PubMed.
- D. Barhoi, P. Upadhaya, S. N. Barbhuiya, A. Giri and S. Giri, J. Am. Coll. Nutr., 2021, 40, 70–85 CrossRef CAS PubMed.
- C. Michl, F. Vivarelli, J. Weigl, G. R. De Nicola, D. Canistro, M. Paolini, R. Iori and A. Rascle, PLoS One, 2016, 11, e0157430 CrossRef PubMed.
- Y. Liu, B. Zhang and Q. Cai, Biomed. Pharmacother., 2020, 131, 110554 CrossRef CAS PubMed.
- A. Jaja-Chimedza, L. Zhang, K. Wolff, B. L. Graf, P. Kuhn, K. Moskal, R. Carmouche, S. Newman, J. M. Salbaum and I. Raskin, J. Funct. Foods, 2018, 47, 376–385 CrossRef CAS PubMed.
- Y. Wen, W. Li, R. Su, M. Yang, N. Zhang, X. Li, L. Li, J. Sheng and Y. Tian, Front. Microbiol., 2022, 13, 925291 CrossRef PubMed.
- W. Wang, J. Jin, H. Xu, Y. Shi, M. Boersch and Y. Yin, J. Ethnopharmacol., 2022, 285, 114894 CrossRef PubMed.
- Y. N. Feng and X. F. Zhang, Int. J. Biol. Macromol., 2020, 157, 267–275 CrossRef CAS PubMed.
- T. B. He, Y. P. Huang, Y. Huang, X. J. Wang, J. M. Hu and J. Sheng, Int. J. Biol. Macromol., 2018, 112, 126–133 CrossRef CAS PubMed.
- K. Sharma, M. Kumar, R. Waghmare, R. Suhag, O. P. Gupta, J. M. Lorenzo, P. S. Radha, N. Rais, V. Sampathrajan, C. Thappa, T. Anitha, A. A. S. Sayed, B. A. Abdel-Wahab, M. Senapathy, R. Pandiselvam, A. Dey, S. Dhumal, R. Amarowicz and J. F. Kennedy, Int. J. Biol. Macromol., 2022, 209, 763–778 CrossRef CAS PubMed.
- H. Tian, Y. Liang, G. Liu, Y. Li, M. Deng, D. Liu, Y. Guo and B. Sun, Int. J. Biol. Macromol., 2021, 182, 595–611 CrossRef CAS PubMed.
- Y. Yang, M. Zhao and L. Lin, Int. J. Food Sci. Technol., 2020, 55, 1539–1546 CrossRef CAS.
- Z. Wen, H. Tian, Y. Liang, Y. Guo, M. Deng, G. Liu, Y. Li, D. Liu and B. Sun, Int. J. Biol. Macromol., 2022, 198, 135–146 CrossRef CAS PubMed.
- Q. Zhao, L. He, X. Wang, X. Ding, L. Li, Y. Tian and A. Huang, J. Agric. Food Chem., 2022, 70, 6123–6133 CrossRef CAS PubMed.
- S. Avilés-Gaxiola, J. León-Félix, Y. B. Jiménez-Nevárez, M. A. Angulo-Escalante, R. Ramos-Payán, J. Colado-Velázquez and J. B. Heredia, S. Afr. J. Bot., 2021, 141, 466–473 CrossRef.
- P. E. Vanderriele, Q. Wang, A. M. Mérillat, F. Ino, G. Aeschlimann, X. Ehret, D. Ancin Del Olmo, V. Ponce de León, U. I. Scholl, D. V. Winter, A. Odermatt, E. Hummler and S. N. Verouti, Int. J. Mol. Sci., 2021, 22, 13218 CrossRef CAS PubMed.
- Y. Xiong, M. S. Riaz Rajoka, M. Zhang and Z. He, Nat. Prod. Res., 2022, 36, 974–983 CrossRef CAS PubMed.
- Y. Xiong, M. S. R. Rajoka, H. M. Mehwish, M. Zhang, N. Liang, C. Li and Z. He, Int. Immunopharmacol., 2021, 95, 107561 CrossRef CAS PubMed.
- L. L. d. S. Patriota, D. d. B. M. Ramos, M. Gama e Silva, A. C. L. A. dos Santos, Y. A. Silva, A. d. O. Marinho, L. C. B. B. Coelho, P. M. G. Paiva, E. V. Pontual, R. L. Mendes and T. H. Napoleão, S. Afr. J. Bot., 2021, 143, 474–481 CrossRef CAS.
- L.-l. Liang, S.-y. Cai, M. Gao, X.-m. Chu, X.-y. Pan, K.-K. Gong, C.-w. Xiao, Y. Chen, Y.-q. Zhao, B. Wang and K.-l. Sun, J. Funct. Foods, 2020, 64, 103698 CrossRef CAS.
- Y. Wen, Y. Liu, Q. Huang, R. Liu, J. Liu, F. Zhang, S. Liu and Y. Jiang, Phytomedicine, 2022, 95, 153856 CrossRef CAS PubMed.
- A. Jaja-Chimedza, B. L. Graf, C. Simmler, Y. Kim, P. Kuhn, G. F. Pauli and I. Raskin, PLoS One, 2017, 12, e0182658 CrossRef PubMed.
- S. M. Fathy and M. S. Mahmoud, Food Sci. Hum. Wellness, 2021, 10, 383–391 CrossRef CAS.
- D. A. Nair, T. J. James, S. L. Sreelatha, B. J. Kariyil and S. N. Nair, Nat. Prod. Res., 2021, 35, 6216–6222 CrossRef PubMed.
- W. Dalhoumi, F. Guesmi, A. Bouzidi, S. Akermi, N. Hfaiedh and I. Saidi, Saudi J. Biol. Sci., 2022, 29, 103284 CrossRef CAS PubMed.
- M. E. Abdel Fattah, H. M. Sobhy, A. Reda and H. M. A. Abdelrazek, Environ. Sci. Pollut. Res., 2020, 27, 43028–43043 CrossRef CAS PubMed.
- U. F. Mohd Fisall, N. Z. Ismail, I. A. Adebayo and H. Arsad, Mol. Biol. Rep., 2021, 48, 4465–4475 CrossRef CAS PubMed.
- E. R. M. Zunica, S. Yang, A. Coulter, C. White, J. P. Kirwan and L. A. Gilmore, Nutrients, 2021, 13, 2923 CrossRef CAS PubMed.
- C. Tiloke, A. Phulukdaree, K. Anand, R. M. Gengan and A. A. Chuturgoon, J. Cell. Biochem., 2016, 117, 2302–2314 CrossRef CAS PubMed.
- L. Hagoel, A. Vexler, L. Kalich-Philosoph, G. Earon, I. Ron, A. Shtabsky, S. Marmor and S. Lev-Ari, Integr. Cancer Ther., 2019, 18, 1534735419828829 CAS.
- M. L. Cuellar-Nuñez, I. Luzardo-Ocampo, R. Campos-Vega, M. A. Gallegos-Corona, E. González de Mejía and G. Loarca-Piña, Food Res. Int., 2018, 105, 159–168 CrossRef PubMed.
- K. M. Sadek, T. K. Abouzed, R. Abouelkhair and S. Nasr, Pharm. Biol., 2017, 55, 1458–1466 CrossRef CAS PubMed.
- L. L. d. S. Patriota, D. d. B. M. Ramos, A. C. L. A. dos Santos, Y. A. Silva, M. Gama e Silva, D. J. L. Torres, T. F. Procópio, A. M. de Oliveira, L. C. B. B. Coelho, E. V. Pontual, D. C. N. da Silva, P. M. G. Paiva, V. M. B. de Lorena, R. L. Mendes and T. H. Napoleão, Food Chem. Toxicol., 2020, 145, 111691 CrossRef CAS PubMed.
- M. Abdel-Latif, G. El-Shahawi, S. M. Aboelhadid and H. Abdel-Tawab, J. Helminthol., 2018, 92, 142–153 CrossRef CAS PubMed.
- L. Jia, X. Peng, Z. Deng, B. Zhang and H. Li, Food Biosci., 2023, 52, 102482 CrossRef CAS.
- M. Amina, R. S. Bhat, A. M. Al-Dbass, N. M. Musayeib, R. Fahmy, L. Alhadlaq and A. El-Ansary, PeerJ, 2021, 9, e11569 CrossRef PubMed.
- L. Romeo, F. Diomede, A. Gugliandolo, D. Scionti, F. Lo Giudice, V. Lanza Cariccio, R. Iori, P. Bramanti, O. Trubiani and E. Mazzon, Sci. Rep., 2018, 8, 9153 CrossRef PubMed.
- Y. Jiang, R. Liu, J. Li, Q. Huang, S. Liu and J. He, Phytochemistry, 2022, 204, 113451 CrossRef CAS PubMed.
- A. Razzaq, S. Ahmad Malik, F. Saeed, A. Imran, A. Rasul, M. Qasim, S. Zafar, S. K. S. Kamran, J. Maqbool, M. Imran, G. Hussain and M. Hussain, Food Sci. Nutr., 2020, 8, 4009–4016 CrossRef CAS PubMed.
- M. Liao, C. Sun, R. Li, W. Li, Z. Ge, M. Adu-Frimpong, X. Xu and J. Yu, Food Chem., 2022, 379, 132087 CrossRef CAS PubMed.
- W. T. Muzumbukilwa, M. Nlooto and P. M. O. Owira, J. Funct. Foods, 2019, 57, 75–82 CrossRef CAS.
- L. E. Díaz-Prieto, S. Gómez-Martínez, I. Vicente-Castro, C. Heredia, E. A. González-Romero, M. D. C. Martín-Ridaura, M. Ceinos, M. J. Picón, A. Marcos and E. Nova, Nutrients, 2022, 14, 1937 CrossRef PubMed.
- H. Joung, B. Kim, H. Park, K. Lee, H.-H. Kim, H.-C. Sim, H.-J. Do, C.-K. Hyun and M.-S. Do, J. Med. Food, 2017, 20, 439–447 CrossRef CAS PubMed.
- C. Waterman, J. L. Graham, C. D. Arnold, K. L. Stanhope, J. H. Tong, A. Jaja-Chimedza and P. J. Havel, Sci. Rep., 2020, 10, 8861 CrossRef CAS PubMed.
- J. I. Randriamboavonjy, M. Rio, P. Pacaud, G. Loirand and A. Tesse, Oxid. Med. Cell. Longevity, 2017, 2017, 4129459 Search PubMed.
- B. Zeng, J. Luo, P. Wang, L. Yang, T. Chen, J. Sun, M. Xie, M. Li, H. Zhang, J. He, Y. Zhang and Q. Xi, Food Sci. Nutr., 2019, 7, 738–746 CrossRef CAS PubMed.
- P. K. Ajuogu, O. O. Mgbere, D. S. Bila and J. R. McFarlane, J. Ethnopharmacol., 2019, 233, 80–86 CrossRef CAS PubMed.
- A. Ali, P. Garg, R. Goyal, G. Kaur, X. Li, P. Negi, M. Valis, K. Kuca and S. Kulshrestha, Plants, 2020, 10, 25 CrossRef PubMed.
- A. A. Muhammad, P. Arulselvan, P. S. Cheah, F. Abas and S. Fakurazi, Drug Des., Dev. Ther., 2016, 10, 1715–1730 CrossRef CAS PubMed.
- B. Zhang, C. Lu, Z. Xu, H. Guo, G. Zhang and Y. Hao, Arabian J. Chem., 2022, 15, 103410 CrossRef CAS.
- I. A. Adebayo, H. Arsad, N. N. S. b. N. M. Kamal and M. R. Samian, S. Afr. J. Bot., 2020, 129, 379–387 CrossRef CAS.
- M. J. Tuorkey, Interv. Med. Appl. Sci., 2016, 8, 109–117 Search PubMed.
- F. Wang, S. Long and J. Zhang, Arch. Oral Biol., 2021, 132, 105280 CrossRef CAS PubMed.
- T. P. Omayone, O. M. Ijomone, S. B. Oloyede, S. T. Okunola, Z. O. Aigoro, V. U. Esukpa and S. O. Dinakin, J. Basic Clin. Physiol. Pharmacol., 2021 DOI:10.1515/jbcpp-2021-0149,000010151520210149.
- I. Buchwalow, J. Schnekenburger, D. Atiakshin, V. Samoilova, E. Wolf, W. Boecker and K. Tiemann, Acta Histochem., 2017, 119, 252–256 CrossRef CAS PubMed.
- Y. Wang, O. Andrukhov and X. Rausch-Fan, Front. Physiol., 2017, 8, 910 CrossRef PubMed.
- P. Lepetsos and A. G. Papavassiliou, Biochim. Biophys. Acta, 2016, 1862, 576–591 CrossRef CAS PubMed.
- A. M. Tomsa, A. L. Alexa, M. L. Junie, A. L. Rachisan and L. Ciumarnean, PeerJ, 2019, 7, e8046 CrossRef PubMed.
- T. Mitani, Y. Yoshioka, T. Furuyashiki, Y. Yamashita, Y. Shirai and H. Ashida, Free Radical Biol. Med., 2017, 106, 355–367 CrossRef CAS PubMed.
- K. H. Alzoubi, N. Q. Rawashdeh, O. F. Khabour, T. El-Elimat, H. Albataineh, H. M. Al-Zghool and F. Q. Alali, J. Mol. Neurosci., 2017, 63, 355–363 CrossRef CAS PubMed.
- B. H. Do, T. P. T. Nguyen, N. Q. C. Ho, T. L. Le, N. S. Hoang and C. C. Doan, Mol. Biol. Rep., 2020, 47, 3675–3689 CrossRef PubMed.
- F. Wang, Y. F. Bao, J. J. Si, Y. Duan, Z. B. Weng and X. C. Shen, J. Med. Food, 2019, 22, 907–918 CrossRef CAS PubMed.
- I. Amara, M. L. Ontario, M. Scuto, G. M. Lo Dico, S. Sciuto, V. Greco, S. Abid-Essefi, A. Signorile, A. T. Salinaro and V. Calabrese, Antioxidants, 2021, 10, 532 CrossRef CAS PubMed.
- E. M. Abdelsayed, D. Medhat, Y. M. Mandour, R. S. Hanafi and A. A. Motaal, J. Food Biochem., 2021, 45, e13992 CrossRef CAS PubMed.
- J. Tabeshpour, B. M. Razavi and H. Hosseinzadeh, Phytother. Res., 2017, 31, 819–837 CrossRef PubMed.
- Y. Tian, L. Lin, M. Zhao, A. Peng and K. Zhao, J. Ethnopharmacol., 2021, 270, 113808 CrossRef CAS PubMed.
- D. Aekthammarat, P. Tangsucharit, P. Pannangpetch, T. Sriwantana and N. Sibmooh, Biomed. Pharmacother., 2020, 130, 110605 CrossRef CAS PubMed.
- K. Ried, Compl. Ther. Med., 2015, 23, 116–128 CrossRef PubMed.
- S. Ning, J. Zang, B. Zhang, X. Feng and F. Qiu, Front. Pharmacol., 2022, 13, 885484 CrossRef CAS PubMed.
- A. Satish, S. Sairam, F. Ahmed and A. Urooj, Pharmacogn. Res., 2012, 4, 44–49 CrossRef CAS PubMed.
- S. M. Ezzat, M. H. E. Bishbishy, N. M. Aborehab, M. M. Salama, A. Hasheesh, A. A. Motaal, H. Rashad and F. M. Metwally, J. Ethnopharmacol., 2020, 251, 112541 CrossRef CAS PubMed.
- K. Ma, Y. Wang, M. Wang, Z. Wang, X. Wang, X. Ju and R. He, Food Funct., 2021, 12, 8994–9006 RSC.
- M. H. Jameel, K. N. A. Karim, A. M. Z. Bin, M. Roziahanim and A. L. M. Vikneswaran, Integr. Med. Res., 2018, 7, 85–94 CrossRef PubMed.
- T. Chen, Y.-X. Liu and L. Huang, iMeta, 2022, 1, e5 Search PubMed.
- H. Wang, N. Zhang, K. Fang and X. Chang, Front. Immunol., 2021, 12, 713799 CrossRef CAS PubMed.
- Y. Zhou, B. Zhou, L. Pache, M. Chang, A. H. Khodabakhshi, O. Tanaseichuk, C. Benner and S. K. Chanda, Nat. Commun., 2019, 10, 1523 CrossRef PubMed.
- G. Shao, S. Zhang, J. Nie, J. Li and J. Tong, J. Toxicol. Environ. Health, Part A, 2017, 80, 1342–1348 CrossRef CAS PubMed.
- M. F. Hogan, Z. Mark, K. N. Harikrishnan, R. Hanah, K. Antony, E. Nathalie, T. A. T. E. Assam and S. E. Kahn, Protein Eng., Des. Sel., 2019, 32, 67–76 CrossRef CAS PubMed.
- X. Zheng, S. Zhu, S. Chang, Y. Cao, J. Dong, J. Li, R. Long and Y. Zhou, Eur. J. Pharmacol., 2013, 720, 147–157 CrossRef CAS PubMed.
- C. Yajun, C. Wenjiao, W. Lihua and Z. Tianying, J. Cell. Mol. Med., 2020, 24, 7652–7659 CrossRef PubMed.
- S. Babashah and M. Soleimani, Eur. J. Cancer, 2011, 47, 1127–1137 CrossRef CAS PubMed.
- O. Trott and A. J. Olson, J. Comput. Chem., 2010, 31, 455–461 CAS.
- J. Muazu and Z. A. Suleiman, J. Pharm. Res. Int., 2014, 4, 2261–2272 Search PubMed.
- K. Al-Khafaji and T. Taskin Tok, Comput. Methods Progr. Biomed., 2020, 195, 105660 CrossRef PubMed.
- S. M. Ezzat, M. H. El Bishbishy, N. M. Aborehab, M. M. Salama, A. Hasheesh, A. A. Motaal, H. Rashad and F. M. Metwally, J. Ethnopharmacol., 2020, 251, 112541 CrossRef CAS PubMed.
- S. Fungtammasan and V. Phupong, PLoS One, 2021, 16, e0248950 CrossRef CAS PubMed.
- N. M. Hani, A. E. Torkamani, M. H. Azarian, K. W. Mahmood and S. H. Ngalim, J. Sci. Food Agric., 2017, 97, 3348–3358 CrossRef CAS PubMed.
- A. A. El-Sayed, A. Amr, O. M. H. M. Kamel, M. M. T. El-Saidi and A. E. Abdelhamid, Cellulose, 2020, 27, 8429–8442 CrossRef CAS.
- A. Althomali, M. H. Daghestani, F. Basil Almukaynizi, S. A. Al-Zahrani, M. A. Awad, N. M. Merghani, W. I. Bukhari, E. M. Ibrahim, S. M. Alzahrani, N. Altowair, A. S. Al-Ghamdi, A. M. AlQahtani, R. Ramadan and R. S. Bhat, Green Process. Synth., 2022, 11, 545–554 CrossRef CAS.
- F. Arifan, R. W. Broto, E. F. Sapatra and A. Pujiastuti, IOP Conf. Ser. Earth Environ. Sci., 2021, 623, 012015 CrossRef.
- C. Y. Chin, J. Jalil, P. Y. Ng and S. F. Ng, J. Ethnopharmacol., 2017, 212, 188–199 CrossRef PubMed.
- M. El-Khadragy, E. M. Alolayan, D. M. Metwally, M. F. S. El-Din, S. S. Alobud, N. I. Alsultan, S. S. Alsaif, M. A. Awad and A. E. Abdel Moneim, Int. J. Environ. Res. Public Health, 2018, 15, 1037 CrossRef PubMed.
- B. Uphadek, M. Shinkar D., B. Patil P. and B. Saudagar R., Int. J. Curr. Pharm. Res., 2018, 10, 13–16 CAS.
- D. S. Panda, N. S. K. Choudhury, M. Yedukondalu, S. Si and R. Gupta, Indian J. Pharm. Sci., 2008, 70, 614–618 CrossRef CAS PubMed.
- A. K. Singhal, E. E. Jarald, A. Showkat and A. Daud, Int. J. Pharm. Invest., 2012, 2, 48–51 CrossRef CAS PubMed.
- A. T. Oyeyinka and S. A. Oyeyinka, J. Saudi Soc. Agric. Sci., 2018, 17, 127–136 Search PubMed.
- K. E. Coello, E. Peñas, C. Martinez-Villaluenga, M. Elena Cartea, P. Velasco and J. Frias, Food Chem., 2021, 360, 130032 CrossRef CAS PubMed.
- K. A. Bermudez-Beltrán, J. K. Marzal-Bolaño, A. B. Olivera-Martínez and P. J. P. Espitia, LWT–Food Sci. Technol., 2020, 123, 109101 CrossRef.
- M. Chakraborty, S. Budhwar and S. Kumar, J. Food Process. Preserv., 2022, 46, e17074 CAS.
- A. Hedhili, S. Lubbers, E. Bou-Maroun, F. Griffon, B. E. Akinyemi, F. Husson and D. Valentin, S. Afr. J. Bot., 2021, 138, 406–414 CrossRef CAS.
- Z. Nizioł-Łukaszewska, D. Furman-Toczek, T. Bujak, T. Wasilewski and Z. Hordyjewicz-Baran, Dermatology Research and Practice, 2020, 2020, 8197902 CrossRef PubMed.
- H. T. Nhut, N. T. Q. Hung, B. Q. Lap, L. T. N. Han, T. Q. Tri, N. H. K. Bang, N. T. Hiep and N. M. Ky, Int. J. Environ. Sci. Technol., 2021, 18, 2173–2180 CrossRef CAS.
- A. Daina, O. Michielin and V. Zoete, Nucleic Acids Res., 2019, 47, W357–w364 CrossRef CAS PubMed.
- Y. Zhou, Y. Zhang, X. Lian, F. Li, C. Wang, F. Zhu, Y. Qiu and Y. Chen, Nucleic Acids Res., 2022, 50, D1398–D1407 CrossRef CAS PubMed.
- C. von Mering, L. J. Jensen, B. Snel, S. D. Hooper, M. Krupp, M. Foglierini, N. Jouffre, M. A. Huynen and P. Bork, Nucleic Acids Res., 2005, 33, D433–D437 CrossRef CAS PubMed.
- M. E. Smoot, K. Ono, J. Ruscheinski, P.-L. Wang and T. Ideker, Bioinformatics, 2011, 27, 431–432 CrossRef CAS PubMed.
- H. Berman, K. Henrick and H. Nakamura, Nat. Struct. Mol. Biol., 2003, 10, 980 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra03584k |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2023 |