Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Determination of lindane in surface water samples and its degradation by hydrogen peroxide and persulfate assisted TiO2-based photocatalysis†
Sanaullah Khan a,
Javed Ali Khan
a,
Javed Ali Khan *b,
Noor S. Shahc,
Murtaza Sayed
*b,
Noor S. Shahc,
Murtaza Sayed d,
Muhammad Ateeqb,
Sabah Ansar
d,
Muhammad Ateeqb,
Sabah Ansar e,
Grzegorz Boczkaj
e,
Grzegorz Boczkaj fg and
Umar Farooqhi
fg and
Umar Farooqhi
aDepartmen of Chemistry, Women University Swabi, 23430, Pakistan
bDepartment of Chemistry, Abdul Wali Khan University Mardan, Mardan 23200, Pakistan. E-mail: javedkhan@awkum.edu.pk; khanjaved2381@gmail.com; Fax: +92-937-542189; Tel: +92-937-929122
cDepartment of Environmental Sciences, COMSATS University Islamabad, Vehari Campus, 61100, Pakistan
dRadiation Chemistry Laboratory, National Centre of Excellence in Physical Chemistry, University of Peshawar, Peshawar 25120, Pakistan
eDepartment of Clinical Laboratory Sciences, College of Applied Medical Sciences, King Saud University, P.O. Box 10219, Riyadh, 11433, Saudi Arabia
fDepartment of Sanitary Engineering, Faculty of Civil and Environmental Engineering, Gdansk University of Technology, G. Narutowicza St. 11/12, 80-233, Gdansk, Poland
gEkoTech Center, Gdansk University of Technology, G. Narutowicza St. 11/12, 80-233, Gdansk, Poland
hDepartment of Chemistry, COMSATS University Islamabad, Abbottabad-Campus, 22060, Abbottabad, Pakistan
iBeijing National Laboratory for Molecular Sciences, State Key Laboratory of Molecular Reaction Dynamics, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, China
First published on 10th July 2023
Abstract
Organochlorine pesticides (OCPs) have been used extensively as insecticides and herbicides. This study investigates the occurrence of lindane in surface water from the Peshawar valley (i.e., Peshawar, Charsadda, Nowshera, Mardan and Swabi districts of Khyber Pakhtunkhwa, Pakistan). Out of 75 samples tested (i.e., 15 samples from each district), 13 samples (including 2 from Peshawar, 3 from Charsadda, 4 from Nowshera, 1 from Mardan, and 3 from Swabi) are found to be contaminated with lindane. Overall, the detection frequency is 17.3%. The maximum concentration of lindane is detected in a water sample from Nowshera and found to be 2.60 μg L−1. Furthermore, the degradation of lindane in the water sample from Nowshera, containing the maximum concentration, is investigated by simulated solar-light/TiO2 (solar/TiO2), solar/H2O2/TiO2 and solar/persulfate/TiO2 photocatalysis. The degradation of lindane by solar/TiO2 photocatalysis is 25.77% after 10 h of irradiation. The efficiency of the solar/TiO2 process is significantly increased in the presence of 500 μM H2O2 and 500 μM persulfate (PS) (separately), represented by 93.85 and 100.00% lindane removal, respectively. The degradation efficiency of lindane is lower in natural water samples as compared to Milli-Q water, attributed to water matrix effect. Moreover, the identification of degradation products (DPs) shows that lindane follows similar degradation pathways in natural water samples as the one in Milli-Q water. The results show that the occurrence of lindane in surface waters of Peshawar valley is a matter of great concern for human beings and the environment. Interestingly, H2O2 and PS assisted solar/TiO2 photocatalysis is an effective method for the removal of lindane from natural water.
1. Introduction
Pesticides – synthesized organic compounds – have dramatically promoted agricultural production worldwide via pest control.1 Besides, pesticides are used in a number of non-agricultural applications such as livestock, forest industry, sprays and domestic products.2 Worldwide, about 2.5 million tons of pesticides are used annually and alarmingly, this number increases with the passage of time.3 Being an agricultural country, the largest source of economy in Pakistan is the agriculture sector. For instance, in 2004, about 23.3% of the GDP and 42.1% of the labor force in Pakistan come from the agriculture sector.4 A large quantity of pesticides is regularly used in Pakistan, aiming at an increased agricultural production – demanded for the high population of the country. Due to widespread application, pesticide residues may enter the water environment, mainly via direct runoff, air deposition, leaching, long-range transportation, equipment washings, disposal of containers, and as effluents from the manufacturing industries.5–7 A high frequency of pesticide residues was found in surface and ground waters as well as in the shallow drinking wells, especially in agriculturally developed areas of Pakistan.3,7–10 Moreover, a large number of different pesticides were frequently detected in river waters of Pakistan, such as river Kabul, Ravi and Indus.11–13Among various classes of pesticides, organochlorine pesticides (OCPs) are used in relatively high quantity due to their higher efficacy. In the present study, lindane was selected as a model pollutant and representative of OCPs. Lindane (also called hexachlorocyclohexane, γ-HCH) – an organochlorine pesticide – has been extensively used against a large number of pests in the last several decades.14 Lindane has also been widely used in livestock, horticulture, forestry, pharmaceuticals and personal care products.2 It is used as a biocide for enhancing durability of indoor materials such as wood, leather, wool and cotton.15 Presently, it is used as an active ingredient in a variety of personal hygiene products such as lotions, creams and shampoos for the treatment of lice and scabies.16,17 Lindane is recognized as a highly chlorinated organic pollutant in the environment, and is considered as a possible neurotoxin, endocrine disruptor and a possible human carcinogen.2,17–19 Due to the excessive applications of lindane for multiple purposes during the last several decades, followed by its persistency as well as long-range transportation, it was frequently detected in the water environments in many regions around the world, including Europe,20 USA,21 Africa,22 China,23 India24 and Pakistan.7 Lindane was found in the far away regions of the world as well, i.e., Arctic and Antarctica.25 The large-scale applications, high persistency, bioaccumulation and magnification tendency as well as high toxicity especially to the non-target species are some of the major issues concerned with the presence of lindane in the aquatic environment. Considering the toxic nature of lindane, a thorough investigation of its distribution in the water environment is of immense importance for environmental sustainability and public health welfare. Meanwhile, there is an urgent need for the development of environmentally friendly and sustainable methods for the removal of organochlorine pesticides from water. In this regard, advanced oxidation processes (AOPs), particularly TiO2 photocatalysis is a promising technology.26–28
This study investigated the determination of lindane in surface water of Peshawar valley, comprising of five districts, i.e., Peshawar, Nowshera, Charsadda, Mardan and Swabi, of Khyber Pakhtunkhwa province, Pakistan. The validation of the extraction and analysis methods was done by using precision, accuracy, relative recovery, limit of detection (LOD) and limit of quantification (LOQ). Meanwhile, the removal of lindane from real water by an environmentally friendly and sustainable advanced oxidation process, i.e., solar light assisted-TiO2 photocatalysis was investigated. The effect of hydrogen peroxide (H2O2) and persulfate (PS, S2O82−) on the efficiency of solar/TiO2 photocatalysis in the natural water was investigated. Moreover, the radical scavenger tests were performed to investigate the role of various reactive species towards degradation of lindane by solar/TiO2 photocatalysis. Finally, the degradation products (DPs) of lindane by solar/H2O2/TiO2 and solar/PS/TiO2 photocatalysis in the natural water sample of Nowshera was identified by gas chromatography/mass spectrometry (GC-MS).
2. Materials and methods
2.1 Materials
Lindane (C6H6Cl6, 97%), sodium persulfate and titanium(IV) isopropoxide (TTIP, 97%) were purchased from Sigma-Aldrich. Hydrogen peroxide (H2O2, 50% v/v) was obtained from Fisher Scientific. Commercial grade plastic bottles (100 mL) were used for the collection of water samples. All the chemicals were used as received. Milli-Q water (resistivity = 18.2 MΩ cm) was used for preparation of standard solutions of lindane while obtaining the calibration curve.2.2 Synthesis of TiO2 films
The synthesis of TiO2 photocatalyst was performed using a sol–gel method. The preparation of film types of TiO2 photocatalyst was carried out by a dip-coating technique using a TiO2 solution. The synthesis method and characterizations of TiO2 film can be seen in our previous paper.292.3 Sample collection
The water samples were collected from different sites of district Peshawar, Charsadda, Nowshera, Mardan and Swabi of Khyber Pakhtunkhwa, Pakistan.Peshawar is the capital of Khyber Pakhtunkhwa province of Pakistan. In Pakistan, it is the sixth most populated city. It is the habitant of over 2.3 million people. People use water of (tube)wells for drinking and other domestic purposes. For irrigation purposes, mainly canal and river water are used. In Peshawar, the samples were collected from Taru Jabba, Tarnab, Chamkani, Wadpagga, Bakhshi Pull, Mathra, Tahkal, Pishtakhara, Saddar, Palosi, Nasir Bagh, Industrial Estate Hayatabad, Karkhano, Bara and Badaber.
Charsadda – the eighty fifth-largest city of Pakistan – is located in the Peshawar Valley. Its population is over 1.6 million. It is about 29 km away from Peshawar and located at an altitude of 276 metres. In Charsadda, the main source of irrigation is water of Jindi, Kabul and Swat rivers. The samples were collected from Sardaryab, Gulabad, Sarwani, Tarka Kalay, Kandar, Dheri Sikander Khan, Ambadher, Mirchakai Kalay, Rajar, Fazalabad, Akbarabad, Sardheri, Manga Dargai, Qala Korona and Dab Banda.
Nowshera is the 9th largest city in Khyber Pakhtunkhwa and 78th in Pakistan. Located on Kabul River, Nowshera lies in the Peshawar Valley and is approximately 43 km east of Peshawar. About 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 095 acre land in Nowshera is irrigated by the river Kabul. District Nowshera covers a total area of 1748 km2 and the population is 1.518 million. Nowshera is an industrially developed area in Khyber Pakhtunkhwa and it has a number of industries, mainly located in the Industrial estate Nowshera, comprising of around 40 units, and acquiring 108 acres area. Particularly, a DDT factory was established in Nowshera (Amangarh) in 1963, which, however, was closed down in 1994. District Nowshera has a vast agricultural land as well, e.g., Nowshera Kalan and Pabbi, the latter being agriculturally more developed than the former. In this study, the sampling area included Pabbi, Jallozai, Pashtun Garhi, Azakhel, Miskeen Khel, Nowshera Kalan, Amangarh, Kabul River, Khat Kalay, Cantt, Tuheed Abad, Islamabad Koruna, Risalpur, Turlandi and Akora Khattak.
095 acre land in Nowshera is irrigated by the river Kabul. District Nowshera covers a total area of 1748 km2 and the population is 1.518 million. Nowshera is an industrially developed area in Khyber Pakhtunkhwa and it has a number of industries, mainly located in the Industrial estate Nowshera, comprising of around 40 units, and acquiring 108 acres area. Particularly, a DDT factory was established in Nowshera (Amangarh) in 1963, which, however, was closed down in 1994. District Nowshera has a vast agricultural land as well, e.g., Nowshera Kalan and Pabbi, the latter being agriculturally more developed than the former. In this study, the sampling area included Pabbi, Jallozai, Pashtun Garhi, Azakhel, Miskeen Khel, Nowshera Kalan, Amangarh, Kabul River, Khat Kalay, Cantt, Tuheed Abad, Islamabad Koruna, Risalpur, Turlandi and Akora Khattak.
Mardan – after Peshawar – is the second largest city of Khyber Pakhtunkhwa having 1632 square km area and 2.25 million population. In Pakistan, Mardan is one of the best agricultural areas. Due to its land suitability for cultivation of tobacco and sugar cane, Mardan is known as the land of tobacco and sugar cane. Major crops grown in Mardan are maize, rice, sugarcane, wheat, and mustard. Main source of irrigation is canal water however, tube wells are also used. In the present study, surface water samples were collected from Nawan Killi, Toru, Rashakai, Mohabat Abad, Ghalla Dher, Faqir Abad, Mayar, Gujar Garhi, Janday, Seri Bahlol, Saro Shah, Bakhshali, Charguli, Shankar and Rustam.
Swabi district is located on the bank of the river Indus, 70 km westward of Islamabad. The total area of district Swabi is 1550 km2 and the population is 1.625 million. District Swabi is one of agriculturally more developed areas of Khyber Pakhtunkhwa and is especially known for production of high-quality tobacco and maize. The major sources of irrigation water in district Swabi are the two main canals, i.e., the upper Swat canal and the Stefa canal. The water samples were collected from Shewa, Dagai, Ayaub Khan Kalay, Yar Hussain, Gumbat, Dobian, Ismalia, Adina, Kalu Khan, Sadbar Abad, Serai, Ambar, Saleem Khan, Shera Ghund and Tarakai.
All the samples were stored at 5 °C in clean plastic bottles before analysis. Prior to the SPME, the samples were filtered through 0.45 μm filter paper in order to remove particulate matter, if any.
2.4 SPME extraction
Solid phase micro-extraction (SPME) technique developed by Pawliszyn30 is a solvent-free alternative for the extraction of organic compounds from their aqueous solution. SPME is an easy to use, time saving, and easily automated technique.31 It is increasingly used in environmental applications, especially for the analysis of volatile organic compounds (VOCs),32 halocarbons,33 pesticides,34 and polychlorinated benzenes (PCBs).35 For extraction of lindane from water samples, SPME technique was used. The SPME was based on employing a poly(dimethylsiloxan)-divinylbenzene (PDMS-DVB) fiber coating protected by a metallic needle and operated by CTC Analytics Combi PAL auto-sampler. The water samples were placed in 20 mL glass vials with magnetic crimp-top caps capped with silicones PTFE lined septa. The lindane was extracted from the samples by holding the SPME fiber in the glass vials for 2 min. After extraction, the SPME fiber was injected into the GC inlet for thermal desorption, holding time was 2 min. The SPME fiber was washed with Milli-Q water after each run by dip-injecting method to remove any contaminants.2.5 Degradation experiment
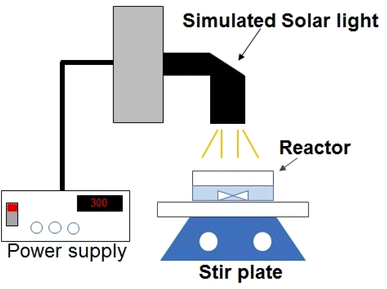
Degradation of lindane was performed in selected real water sample where its maximum concentration (i.e., 2.60 μg L−1) was detected, i.e., in water sample of Nowshera (Amangarh, N7). The physicochemical characteristics of this water sample are shown in Table S1, ESI.† The degradation experiments were carried out in a batch photoreactor, i.e., a 100 mL beaker containing 50 mL of the water sample collected from Nowshera. The schematic diagram of the photoreactor is shown in Fig. 1. A TiO2 film was used as a photocatalyst. The reaction solution was illuminated by simulated solar light. The simulated solar light was achieved by a 300 W Xenon lamp (Newport, Oriel Instrument) with light irradiance (Ee) of 4.71 × 10−2 W cm−2, as measured by Newport broadband radiant power meter.2.6 Lindane quantification and its degradation products identification
Gas chromatography-micro-cell electron capture detector (GC-μECD) is a modern analytical sophisticated technique frequently employed for the detection and quantification of organochlorine compounds in aqueous solution.36 The ECD is highly sensitive towards detecting the halogenated organic compounds at trace levels in environmental and biological samples.37The samples were analyzed for quantification of lindane by using gas chromatography (Agilent 1200 N series, USA) coupled with micro-cell electron capture detector (GC-μECD) in a splitless mode. For separation purposes, HP-5 capillary column (30 m × 0.25 mm I.D. and 0.25 μm film thickness) was used. The programming of oven temperature was: 50 °C (2 min hold) to 150 °C at increasing rate of 10 °C min−1 (3 min hold), and then further to 250 °C at increasing rate of 20 °C min−1 (5 min hold). The GC injector and detector temperatures were set at 220 and 350 °C, respectively. Ultrapure N2 gas at a flow rate of 1.5 mL min−1 was used as a carrier gas.
Degradation products (DPs) of lindane were identified by gas chromatography/mass spectrometry (GC-MS; Agilent 6890) having an HP-5MS (5% phenyl methylsiloxane) capillary column (30 m × 0.25 μm). The temperature of the MS source and quadrapole was set at 230 and 150 °C, respectively. Electron impact ionization mode (EI+) set at 70 eV was used for obtaining mass spectra of the DPs. The m/z range was set from 50 to 550. DPs were identified by comparison to online NIST mass spectral library installed in the GC/MS. The rest of the conditions were same as of the GC-μECD.
2.7 Method validation
Calibration of GC-μECD was made using standard lindane solutions at seven different concentrations, i.e., 0.05, 0.10, 0.50, 1.00, 3.00, 6.00, and 10.0 μg L−1, by plotting peak area (a.u) versus concentration. The concentration of lindane in the water samples was determined by using calibration curve (via least square method using straight line equation obtained from the calibration plot).Limit of detection (LOD), i.e., the minimum concentration of an analyte that can be differentiated from the blank or background signal with a certain limit of confidence,38 was determined at a signal to noise (S/N) ratio of 3. The limit of quantification (LOQ), i.e., the smallest concentration of analyte that can be determined quantitatively with certainty38 was determined at S/N ratio of 10.
The precision, accuracy and relative recoveries of the optimized method was investigated at three concentration levels (2.5, 5.0 and 10.0 μg L−1) in a triplicate manner. The precision was measured in terms of relative standard deviation (RSD). The intra-day precision and accuracy were determined by analyzing the lindane samples at mentioned concentrations (in triplicate) on the same day, while the inter-day precision and accuracy were obtained by analyzing the samples on three different days. The accuracy of the optimized method was calculated by using the following equation:
 | (1) |
To assess the effect of matrices on the concentration of lindane in the field water, the relative recoveries were obtained for a set of six samples, prepared in the field water. Of the six samples used for relative recoveries, three samples did not contain any detectable quantity of lindane, while the remaining three samples do contain some detectable amount of lindane. The relative recoveries were determined by using eqn (1), but in this case lindane solution was prepared in field water rather than Milli-Q water, as in the case of accuracy measurement.
2.8 Toxicity evaluation
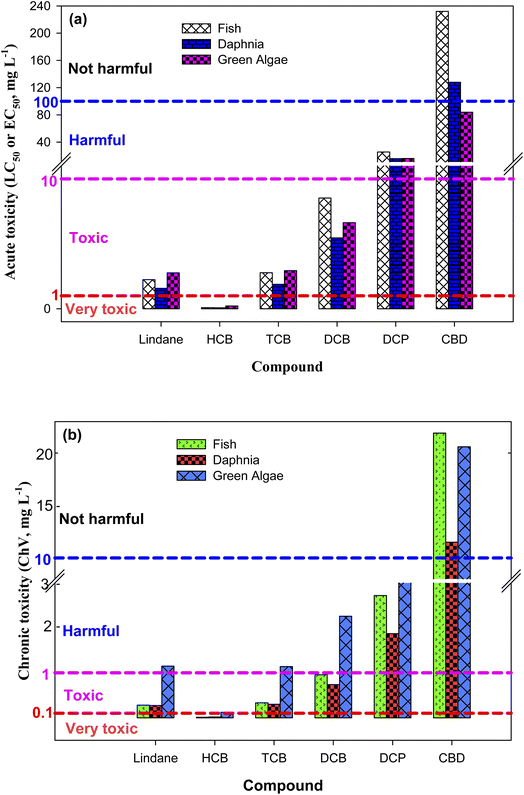
To examine the toxicity of lindane and its detected DPs, the acute (LC50/EC50) and chronic toxicities (ChV) were calculated using the Ecological Structure Activity Relationship (ECOSAR) program – an effective-prediction tool of the substances' toxicities towards three aquatic organisms (i.e., fish, daphnia and green algae).39 The acute toxicity is represented by LC50 and EC50. LC50 is the concentration of toxicant which results in the death of 50% fish and daphnia after 96 and 48 h contact times, respectively.40 Similarly, EC50 is the concentration of toxicant which results in the 50% growth inhibition of green algae after 96 h contact time.40 According to the European Union criteria, a compound is considered as: (i) “not harmful” if LC50/EC50 > 100 mg L−1, (ii) “harmful” if LC50/EC50 = 10 to 100 mg L−1, (iii) “toxic” if LC50/EC50 = 1 to 10 mg L−1, and (iv) “very toxic” if LC50/EC50 < 1 mg L−1.41 Similarly, in accordance of the Chinese hazard evaluation criteria, a compound could be classified as: (i) “not harmful” if ChV > 10 mg L−1, (ii) “harmful” if ChV = 1 to 10 mg L−1, (iii) “toxic” if ChV = 0.1 to 1 mg L−1, and (iv) “very toxic” if ChV < 0.1 mg L−1.413. Results and discussion
3.1 Method validation
A suitable method was developed for the analysis of lindane using GC-μECD according to the parameters shown in Section 2.6. The detector showed a linear response with R2 = 0.997 in the concentration range of 0.05 to 10.0 μg L−1 as shown in Table S2, ESI.† The LOD and LOQ determined at S/N of 3 and 10 were found to be 0.008 μg L−1 and 0.037 μg L−1, respectively (Table S2†). As shown in Table 1, very precise, accurate and more reproducible results were obtained under both intra-day and inter-day conditions.| Analyte | Nominal concentration (μg L−1) | Intra-day response | Inter-day response | ||||
|---|---|---|---|---|---|---|---|
| Measured concentration (mean ± SD) | Precision (RSD) | Accuracy (%) | Measured concentration (mean ± SD) | Precision (RSD) | Accuracy (%) | ||
| Lindane | 2.5 | 2.64 ± 0.27 | 10.29 | 105.37 | 2.37 ± 0.28 | 11.91 | 94.78 |
| 5.0 | 4.82 ± 0.25 | 5.15 | 96.36 | 5.03 ± 0.26 | 5.12 | 100.55 | |
| 10.0 | 9.20 ± 0.71 | 7.68 | 92.00 | 9.27 ± 0.39 | 4.21 | 92.70 | |
The relative recoveries of lindane by SPME-GC-μECD method in six different field water samples ranged from 83 to 114% (Table 2). The good RSD (%) values were also achieved for lindane analysis by the stated method as shown in Table 2.
| Lindane concentration (μg L−1) | Field water sample | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P1 | C1 | N1 | N8 | M1 | S1 | |||||||
| Relative recovery | RSD | Relative recovery | RSD | Relative recovery | RSD | Relative recovery | RSD | Relative recovery | RSD | Relative recovery | RSD | |
| a P1 (Taru Jabba, Peshawar); C1 (Sardaryab, Charsadda); N1 (Pabbi, Nowshera); N8 (Kabul River, Nowshera); M1 (Nawan Killi, Mardan); S1 (Shewa, Swabi). | ||||||||||||
| 2.5 | 88.18 | 8.98 | 110.57 | 7.87 | 88.58 | 13.18 | 114.16 | 2.64 | 103.37 | 16.04 | 99.57 | 17.48 |
| 5.0 | 98.46 | 9.16 | 101.05 | 11.28 | 95.66 | 14.84 | 103.45 | 8.99 | 96.56 | 3.11 | 92.06 | 7.77 |
| 10.0 | 83.31 | 9.78 | 88.40 | 8.09 | 99.69 | 7.14 | 99.15 | 2.56 | 88.05 | 5.17 | 100.34 | 5.31 |
3.2 Determination of lindane in collected water samples
Table 3 show the concentration of lindane in water samples of district Peshawar, Charsadda, Nowshera, Mardan and Swabi. In Peshawar, only 2 samples (out of 15) were contaminated with lindane, corresponding to a detection frequency of 13.3%. The lindane concentration varied in the range of 0 (i.e., non-detectable, ND) to 0.63 μg L−1. Detection frequency was found to be 20.0% for Charsadda water samples where lindane was detected in 3 out 15 samples. Here, the maximum concentration was found to be 1.01 μg L−1 in water collected from Mirchakai Kalay. As for as district Nowshera is concerned, here lindane was found in 4 samples corresponding to detection frequency of 26.7%. This is the maximum detection frequency among the studied districts. The maximum concentration detected in water samples of Nowshera district was 2.60 μg L−1. This is the maximum concentration found among the tested water samples of all the five districts. Luckily, only one water sample collected from Mardan district contains lindane at a concentration level of 0.22 μg L−1. However, in water samples of district Swabi, 3 samples were observed to have lindane with a maximum concentration of 0.28 μg L−1.| Sample ID | Collection spot | Concentration (μg L−1) | Sample ID | Collection spot | Concentration (μg L−1) |
|---|---|---|---|---|---|
| Peshawar | |||||
| P1 | Taru Jabba | ND | P9 | Saddar | ND |
| P2 | Tarnab | 0.63 | P10 | Palosi | ND |
| P3 | Chamkani | ND | P11 | Nasir Bagh | ND |
| P4 | Wadpagga | ND | P12 | Hayatabad | ND |
| P5 | Bakhshi Pull | ND | P13 | Karkhano | ND |
| P6 | Mathra | 0.32 | P14 | Bara | ND |
| P7 | Tahkal | ND | P15 | Badaber | ND |
| P8 | Pishtakhara | ND | |||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Charsadda | |||||
| C1 | Sardaryab | 0.25 | C9 | Rajar | ND |
| C2 | Gulabad | ND | C10 | Fazalabad | ND |
| C3 | Sarwani | ND | C11 | Akbarabad | ND |
| C4 | Tarka Kalay | ND | C12 | Sardheri | ND |
| C5 | Kandar | ND | C13 | Manga Dargai | ND |
| C6 | Dheri Sikander Khan | 0.56 | C14 | Qala Korona | ND |
| C7 | Ambadher | ND | C15 | Dab Banda | ND |
| C8 | Mirchakai Kalay | 1.01 | |||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Nowshera | |||||
| N1 | Pabbi | ND | N9 | Khat Kalay | ND |
| N2 | Jallozai | 0.71 | N10 | Cantt | ND |
| N3 | Pashtun Garhi | ND | N11 | Tuheed Abad | 0.64 |
| N4 | Azakhel | ND | N12 | Islamabad Koruna | ND |
| N5 | Miskeen Khel | ND | N13 | Risalpur | ND |
| N6 | Nowshera Kalan | ND | N14 | Turlandi | ND |
| N7 | Amangarh | 2.60 | N15 | Akora Khattak | ND |
| N8 | Kabul River | 0.32 | |||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Mardan | |||||
| M1 | Nawan Killi | ND | M9 | Janday | ND |
| M2 | Toru | ND | M10 | Seri Bahlol | ND |
| M3 | Rashakai | ND | M11 | Saro Shah | ND |
| M4 | Mohabat Abad | ND | M12 | Bakhshali | ND |
| M5 | Ghalla Dher | ND | M13 | Charguli | ND |
| M6 | Faqir Abad | ND | M14 | Shankar | ND |
| M7 | Mayar | ND | M15 | Rustam | 0.22 |
| M8 | Gujar Garhi | ND | |||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Swabi | |||||
| S1 | Shewa | 0.14 | S9 | Kalu Khan | ND |
| S2 | Dagai | ND | S10 | Sadbar Abad | ND |
| S3 | Ayaub Khan Kalay | ND | S11 | Serai | ND |
| S4 | Yar Hussain | ND | S12 | Ambar | ND |
| S5 | Gumbat | ND | S13 | Saleem Khan | 0.28 |
| S6 | Dobian | ND | S14 | Shera Ghund | ND |
| S7 | Ismalia | ND | S15 | Tarakai | 0.19 |
| S8 | Adina | ND | |||
In Pakistan, pesticides are mostly used for agricultural purposes. Thus, the main cause of lindane in surface water could possibly be the run-off waters from agricultural fields. An outlook of pesticides consumption in Pakistan during 1997–2006 is provided in Table S3, ESI.†42 Other uses of lindane include: as a biocide agent for making wood, leather, wool and cotton durable, and as an anti-scabies and anti-lice agent in personal hygiene products such as lotions, creams and shampoos. Consequently, the effluents of wood, paper and pulp, leather and cotton industries may possibly contribute to the entrance of lindane in surface waters. Besides, domestic wash-off and drainage waters can have a minor contribution to the presence of lindane in water bodies if the lotions, creams and shampoos used in homes contain lindane as an active ingredient. In fact, due to the wide range applications of lindane, there could be numerous causes of lindane entrance into water bodies. Because of its persistent and non-biodegradable nature, lindane can accumulate in surface waters. Thus, minor contributions from several sources can lead to lindane concentration in surface waters at detectable and quantifiable level. As a result, 17.3% water samples were found to have lindane at quantifiable level. Among the five districts, the detection frequency of lindane in surface waters follows the order: Nowshera > Charsadda = Swabi > Peshawar > Mardan with detection frequency of 26.7, 20.0, 20.0, 13.3 and 6.7%, respectively. Moreover, the highest concentration of lindane in 75 tested water samples was also detected in Nowshera where the surface water of Amangarh has 2.60 μg L−1 of lindane. This concentration is beyond the maximum acceptable level (MAL) for a single pesticide in surface water, i.e., 1.0 μg L−1, according to parametric guideline values of European Union.43 Of note, the European Union has set the maximum permissible limit in drinking water as 0.1 μg L−1 for an individual pesticide and 0.5 μg L−1 for total pesticides.44
A DDT factory was established in Amangarh, district Nowshera, which annually produced 700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 kg of technical grade dichlorodiphenyltrichloroethane (DDT) from 1963 to 1994.45 This DDT factory was abandoned after the ban on production and uses of persistent organic pollutants (POPs) by the Stockholm convention 2001.46 Even after officially shut down, the factory was kept operational for several years.45 Though the factory has been demolished, higher concentration of DDT has been detected in soil and water samples several years after the factory closure.47 The inappropriate dumping of the DDT and its raw materials in the factory site will keep contaminated the soil and water with DDT in the vicinity of the factory. Though the DDT factory was producing only DDT, the presence of lindane in the water sample of Amangarh could be due to the formation of lindane as a byproduct during the synthesis of DDT. Another possibility is the large-scale applications of pesticides in the surrounding area for agricultural purposes. Irrespective of the sources of lindane in the surface waters, its presence in water bodies could pose serious threats to human beings and other living animals. Hence, development of cheap and effective remediation technologies is urgently needed to tackle this problem efficiently.
000 kg of technical grade dichlorodiphenyltrichloroethane (DDT) from 1963 to 1994.45 This DDT factory was abandoned after the ban on production and uses of persistent organic pollutants (POPs) by the Stockholm convention 2001.46 Even after officially shut down, the factory was kept operational for several years.45 Though the factory has been demolished, higher concentration of DDT has been detected in soil and water samples several years after the factory closure.47 The inappropriate dumping of the DDT and its raw materials in the factory site will keep contaminated the soil and water with DDT in the vicinity of the factory. Though the DDT factory was producing only DDT, the presence of lindane in the water sample of Amangarh could be due to the formation of lindane as a byproduct during the synthesis of DDT. Another possibility is the large-scale applications of pesticides in the surrounding area for agricultural purposes. Irrespective of the sources of lindane in the surface waters, its presence in water bodies could pose serious threats to human beings and other living animals. Hence, development of cheap and effective remediation technologies is urgently needed to tackle this problem efficiently.
3.3 Photocatalytic degradation of lindane in real water
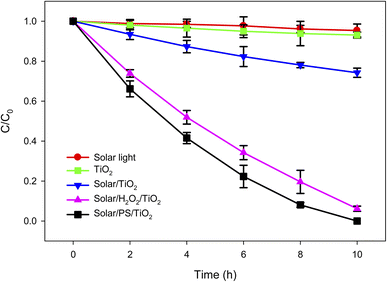
The lindane concentration found in the real water (RW) sample of Amangarh (i.e., [lindane] = 2.60 μg L−1), was the highest concentration of lindane in the studied region of Peshawar valley. This water sample was subjected to simulated solar light-assisted TiO2 photocatalysis (solar/TiO2) (Fig. 2). The results showed that solar/TiO2 process can achieve 25.77% of lindane degradation, at treatment time of 10 h, in RW sample of Amangarh (see the physicochemical properties of this water sample in Table S1, ESI†).Activation of semiconductor photocatalysts such as TiO2 in aqueous solutions by light photons could lead to the generation of reactive species in the form of hydroxyl radicals (˙OH) and superoxide radical anions (O2˙−) as shown in reactions (2)–(5).48–50 These reactive species are generally responsible for the degradation of target organic pollutants such as lindane, in the present case.
| TiO2 + hν → hVB+ (valence band hole) + eCB− (conduction band electron) | (2) |
| eCB− + O2 → O2˙− | (3) |
| hVB+ + H2O → ˙OH + H+ | (4) |
| hVB+ + OH− → ˙OH | (5) |
Since lindane degradation/removal by solar light and TiO2 was only 4.6 and 6.9%, respectively, at 10 h of treatment (Fig. 2), ˙OH and O2˙− could possibly contribute to the degradation of lindane by solar/TiO2 photo-catalysis to achieve 25.77% degradation in same treatment time. The degradation of lindane by ˙OH was previously reported in the literature using photo-Fenton process,51 photocatalysis52 and UV/H2O2.53
Since the solar/TiO2 photocatalytic degradation of lindane in Amangarh water was relatively low (25.77% degradation in 10 h), hydrogen peroxide (H2O2) and persulfate (PS, S2O82−) was added (500 μM each one) – separately – to the solar/TiO2 system to accelerate the lindane degradation (Fig. 2). It can be seen that the degradation of lindane was increased from 25.77% to 93.85 and 100.00% in presence of H2O2 and PS, corresponding to an increase in kobs from 0.0309 h−1 to 0.1891 and 0.2383 h−1, respectively. This increase in photo-catalytic degradation efficiency of lindane by H2O2 and PS was due to the production of additional ˙OH in solar/H2O2/TiO2 (reactions (6) and (7)) and SO4˙− and ˙OH in solar/PS/TiO2 processes (reactions (8)–(10)).54 Both H2O2 and PS are good electron acceptors, thus, effectively scavenge the photogenerated electrons (eCB−), (reactions (6) and (8)), thus suppressing the recombination rate of hVB+ and eCB−, thereby enhancing the concentration of hVB+, subsequently increasing the concentration of ˙OH at the TiO2 surface.
| H2O2 + eCB− → ˙OH + HO− | (6) |
| H2O2 + hv → 2 ˙OH (λ = 254 nm, ϕ = 1.0) | (7) |
| S2O82− + eCB− → SO42− + SO4˙− | (8) |
| S2O82− + hv → 2 SO4˙− (λ = 254 nm, ϕ = 1.8) | (9) |
| SO4˙− + H2O → SO42− + ˙OH + H+ | (10) |
Moreover, the results further indicated that PS has higher enhancement effect on the photo-catalytic removal efficiency of lindane as compared to H2O2. The higher removal of lindane by solar/PS/TiO2 as compared to by solar/H2O2/TiO2 could be attributed to: (i) higher quantum yield of SO4˙− from S2O82− photolysis (i.e., 1.8) as compared to the quantum yield of ˙OH from H2O2 photolysis (i.e., 1.0); (ii) lower reactivity of natural organic matter (NOM) with SO4˙− than ˙OH, suggesting lower scavenging efficiency of NOM for SO4˙− as compared to ˙OH (reactions (11) and (12));55 (iii) higher redox potential of SO4˙− than ˙OH under neutral conditions; and (iv) longer half-life of SO4˙− than ˙OH (i.e., 30–40 μs for SO4˙− and < 1 μs for ˙OH).
| NOM + ˙OH → Products k = 2.23 × 108 L (mol C)−1 s−1 | (11) |
| NOM + SO4˙− → Products k = > 6 × 106 L(mol C)−1 s−1 | (12) |
For better comparison, the results of solar/TiO2, solar/H2O2/TiO2 and solar/PS/TiO2 photocatalytic degradation of lindane in distilled water (DW) – performed in our previous study29 – has been provided in Table 4. Compared to DW results, the RW sample (Amangarh) showed lower removal efficiency of lindane by solar/TiO2 photocatalysis, attributed to the matrix effects, as indicated from the physico-chemical characteristics of the RW samples (Table S1, ESI†). Literature studies showed that water matrix might have neutral, inhibiting or enhancing effect on the degradation efficiency of organic pollutants using various advanced oxidation processes, depending on the nature and concentration of natural water constituents, as well as process and mechanism by which these constituents react within the system.56 The inhibiting effects due to the inorganic ions can be explained via: (i) scavenging/quenching of reactive radicals and/or h+, (ii) conversion of hydroxyl radicals to less reactive radicals, and (iii) adsorption of the ions to the active sites of the catalyst. While the enhancing effect of inorganic ions could be due to the formation of more reactive radicals.56 The presence of NOM and inorganic anions in RW of Amangarh (as shown in Table S1, ESI†) might have lowered the degradation efficiency of lindane, via scavenging of ˙OH/SO4˙− and/or quenching of h+, according to reactions (11)–(14).56–58
| Xn− + h+ → X˙(n−1)− | (13) |
| Xn− + ˙OH/SO4˙− → X˙(n−1)− + OH−/SO42−/H2O | (14) |
 .
.
| Type of water | Pseudo-first-order rate constant (kobs, h−1) | Reference | ||
|---|---|---|---|---|
| Solar/TiO2 | Solar/H2O2/TiO2 | Solar/PS/TiO2 | ||
| Natural water sample of Amangarh | 0.0309 | 0.1891 | 0.2383 | This study |
| Distilled water | 0.0423 | 0.2084 | 0.4948 | 29 |
Our results were consistent with the findings of Yuan et al.59 which showed that the degradation efficiency of sulfamethoxazole by TiO2 photocatalysis was reduced in the presence of Cl− and SO42−. Yap and Lim60 also found the degradation efficiency of bisphenol-A by solar light-assisted N–TiO2/activated carbon photocatalysis was decreased from 82 to 76, 77 and 76% in the presence of 100 mM of each Cl−, NO3− and SO42−, respectively, consistent with our results. Rehman et al.61 found that the degradation efficiency of diclofenac sodium by H2O2/catalyst process was decreased in the presence of NO3− and SO42−. The inhibiting effect of the inorganic ions was attributed to the scavenging of ˙OH. Also, the Cl− might compete with organic compounds for the adsorption sites on the catalyst surface,62 and SO42− showed more adsorption capacity than Cl−.63 Owing to different water matrix constituents, the degradation efficiency of organic pollutants by AOPs could be varied in natural and real water systems, including river, surface, ground, deionized and wastewater. Salimi et al.64 showed that visible light-assisted D-g-C3N4–Bi5O7I photocatalytic degradation efficiency of metronidazole in deionized water was lowered from 89 to 85 and 77% in the tap and wastewater treatment plant effluent, respectively, attributed to radical scavenging by the water constituents such as NO3− and SO42−. Sayed et al.65 showed that the degradation efficiency of norfloxacin by gamma radiation based AOP was low in the surface and ground waters, attributed to radical scavenging effect of water constituents.
3.4 Relative contribution of different reactive species
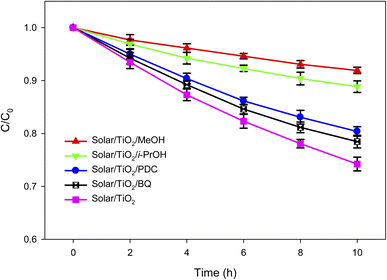
It has been reported that a number of different reactive species (RSs) such as ˙OH, O2˙−, h+, and eCB− can be generated during the irradiation of TiO2 containing aqueous solution by solar or UV light.66,67 These RSs are then responsible for the degradation of organic pollutants. However, the contribution of these RSs towards organic compounds degradation depends on the nature of the target compound and the reaction system. To find out the relative contribution of these RSs towards lindane degradation by solar/TiO2 photo-catalysis, specific scavengers were used during the photocatalytic degradation of lindane (Fig. 3 and Table S4, ESI†).Iso-propanol (i-PrOH), methanol (MeOH), p-benzoquinone (BQ) and potassium dichromate (PDC, K2Cr2O7) were used to quench ˙OH, h+ and ˙OH, O2˙− and eCB−, respectively.68,69 The pseudo-first-order rate constants (kobs) were found to be 0.0309, 0.0126 and 0.0089, 0.0258 and 0.0230 h−1 in the presence of no scavenger, iso-propanol, methanol, BQ and PDC, respectively. The reduction in kobs was calculated to be 71.20% (= (0.0309–0.0089)/0.0309 × 100) and 59.22% (= (0.0309–0.0126)/0.0309 × 100) in the presence of methanol and iso-propanol, respectively. Thus, the contribution of ˙OH towards lindane degradation was 59.22% and that from h+ was 11.97% (= 71.20–59.20%). The reduction in kobs was calculated to be 16.50% (= (0.0309–0.0258)/0.0309 × 100) in presence of BQ, suggesting O2˙− contributed 16.50% towards lindane degradation. Moreover, potassium dichromate reduced the kobs by 25.57% (= (0.0309–0.0230)/0.0309 × 100). Although it can be concluded from these results that eCB− contributed 25.57% towards lindane degradation, this is not direct contribution. The formation of O2˙− in the reaction system was due to the reduction of dissolved O2 by eCB− (reaction (3)), thus this 25.57% contribution also involved the contribution from O2˙−. In summary, the main RS in solar/TiO2 photo-catalysis was ˙OH towards lindane degradation.
3.5 Identification of degradation products
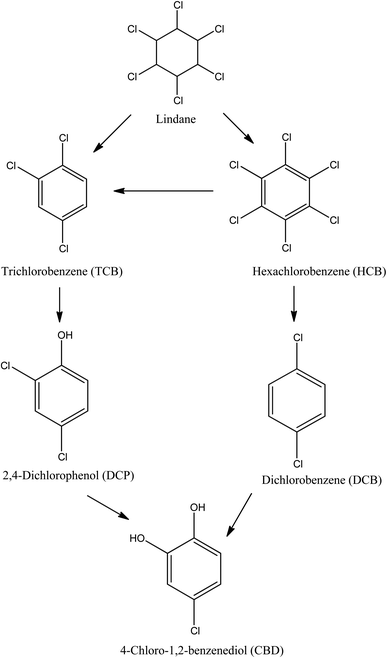
The solar/H2O2/TiO2 and solar/PS/TiO2 photocatalysis of lindane in real water of Amangarh was investigated for the identification of degradation products (DPs). Additional lindane was spiked into the real water sample to bring the concentration from 2.60 to 500 μg L−1 so that the DPs could be produced in easily detectable levels. Solar/H2O2/TiO2 resulted in the formation of five DPs namely: (i) hexachlorobenzene (HCB), (ii) trichlorobenzene (TCB), (iii) dichlorobenzene (DCB), (iv) 2,4-dichlorophenol (DCP), and (v) 4-chloro-1,2-benzenediol (CBD) (Scheme 1). Whereas, only the first three DPs, i.e., (i) HCB, (ii) TCB and (iii) DCB, were detected in solar/PS/TiO2 photocatalysis. The formation of HCB occurs via dehydrogenation reaction (i.e., removal of hydrogen), TCB and DCP produced via dehydrochlorination reaction (i.e., removal of hydrogen chloride) and DCP and CBD formed via dechlorination–hydroxylation reactions. These results indicated that both ˙OH and SO4˙− are capable of dehydrogenation and dechlorination reactions which led to the formation of HCB, TCB and DCB. Previously, hydrogen abstraction (removal of hydrogen) and dechlorination reactions of organic compounds by ˙OH and SO4˙− have been reported.70 Although hydroxylation reaction by SO4˙− has also been reported,71,72 the results of the present study – formation of DCP and CBD in solar/H2O2/TiO2 – indicated that hydroxylation reaction is the predominant property ˙OH. Moreover, it can also be concluded that there is relatively lower concentration of ˙OH in solar/PS/TiO2 (produced via reaction (10)) than in solar/H2O2/TiO2, otherwise DCP and CBD could be produced in solar/PS/TiO2. These detected DPs were identified in previous studies involving lindane degradation by ˙OH and/or SO4˙− based AOPs.52,54,71–73 Interestingly, the results showed that although NOM and inorganic ions, i.e., NO3−, SO42− and Cl−, had reduced the degradation efficiency of lindane, they had not affected the degradation mechanism of lindane.3.6 Toxicity evaluation of lindane and its detected degradation products
It has been reported that degradation of some organic compounds initially increases the toxicity of the treated solution.74,75 This is because some of the DPs are sometimes more toxic than the parent compound. In such cases, the contaminated water could be treated for an extended period of time – to achieve complete degradation of DPs along with target compound – rather than to merely focus the removal of the target compound. To find out whether lindane removal could efficient for the water decontamination or its complete mineralization (degradation of lindane along with its organic products) is inevitable for detoxification of lindane polluted water, the aquatic toxicity of lindane and its detected DPs was determined using the ECOSAR program. The calculated results are depicted in Fig. 4 and Table S5, ESI.† The acute toxicity results showed that the LC50 values of lindane, HCB, TCB, DCB, DCP and CBD for fish are 2.24, 0.08, 2.77, 8.52, 25.5 and 232.0 mg L−1, respectively. These results indicated that HCB is more toxic than lindane whereas all other products are less toxic than lindane. Generally, the toxicity increases with increasing the number of chlorine atoms in a compound. Thus, the relatively lower toxicity of TCB, DCB, DCP and CBD than lindane could be attributed to the removal of chlorine atoms from these DPs. Since both lindane and HCB possess equal number of chlorine atoms, the higher toxicity of HCB than lindane could be assigned to the aromatic nature of HCB. The acute toxicity of lindane and its detected DPs towards fish follows the order: HCB > lindane > TCB > DCB > DCP > CBD, in agreement with the direct relationship between toxicity and number of chlorine atoms. Similar results – in the same order – were found for daphnia and green algae. In terms of chronic toxicity, lindane and its DPs followed the same order as of acute toxicity (Fig. 4 and Table S5†), except for lindane and TCB toxicity towards green algae, where TCB (ChV = 1.14 mg L−1) was a little more toxic than lindane (ChV = 1.15 mg L−1). It can be concluded from the toxicity results that lindane degradation by solar/H2O2/TiO2 and solar/PS/TiO2 are effective technologies for the detoxification of lindane contaminated waters as most of the DPs are less toxic than lindane except HCB which is produced at initial stages of the reaction but subsequently degraded as the reaction proceeds.4. Conclusions
The occurrence of lindane in the surface water of Peshawar valley (i.e., Peshawar, Charsadda, Nowshera, Mardan and Swabi districts) was investigated. Out of the 75 samples tested (i.e., 15 samples from each district), 13 samples were found to be contaminated with lindane (i.e., 17.3% detection frequency). In most of the contaminated samples, lindane concentration exceeded the maximum acceptable level (MAL) for a single pesticide in surface water, i.e., 1 μg L−1, indicating an issue of concern for the environment and living organisms including human beings. The maximum lindane concentration (i.e., 2.60 μg L−1) was detected in water sample of Nowshera, attributed to the location of a sealed pesticide factory in this region, established in 1963, but closed down in 1994.The current study can be extended to other regions in order to get a clear baseline data for the entire country on assessment of lindane and other organochlorine pesticides. The habitants of Amangarh (Nowshera), where the closed pesticide factory is located, must be alarmed on the health hazards associated with lindane contamination. It was concluded that solar light assisted TiO2 photocatalysis was an effective method for the removal of lindane from the real water samples. The efficiency of solar/TiO2, solar/H2O2/TiO2 and solar/PS/TiO2 processes were slightly lowered in natural water samples as compared to Milli-Q water due to the water constituents scavenging effects. However, the photocatalytic degradation pathways of lindane were apparently unaffected by the natural water constituents as similar DPs were detected in natural water sample as in Milli-Q water – detected in our previous studies. The obtained results could be used to design an Integrated Management Program for controlling the concentration of pesticides in the aquatic environment.
Author contributions
Sanaullah Khan: conceptualization, data curation, formal analysis, investigation, methodology, writing – original draft. Javed Ali Khan: conceptualization, project administration, supervision, funding acquisition, validation, writing – review & editing. Noor S. Shah: conceptualization, data curation, formal analysis, investigation, methodology, resources, writing – original draft. Murtaza Sayed: conceptualization, validation, writing – review & editing. Muhammad Ateeq: software, validation, writing – review & editing. Sabah Ansar: conceptualization, funding acquisition, writing – review & editing. Umar Farooq: conceptualization, writing – review & editing. Grzegorz Boczkaj: conceptualization, writing – review & editing.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
Higher Education Commission (HEC), Pakistan is acknowledged for financial assistance. The authors also thank the Researchers Supporting Project number (RSP2023R169), King Saud University, Riyadh, Saudi Arabia for the financial support. Dr S. Khan acknowledges the financial support from Women University, Swabi, Pakistan.References
- F. P. Carvalho, Agriculture, pesticides, food security and food safety, Environ. Sci. Policy, 2006, 9, 685–692 CrossRef.
- S. Khan, X. He, H. M. Khan, D. Boccelli and D. D. Dionysiou, Efficient degradation of lindane in aqueous solution by iron (II) and/or UV activated peroxymonosulfate, J. Photochem. Photobiol., A, 2016, 316, 37–43 CrossRef CAS.
- A. Azizullah, M. N. K. Khattak, P. Richter and D.-P. Häder, Water pollution in Pakistan and its impact on public health-A review, Environ. Int., 2011, 37, 479–497 CrossRef CAS PubMed.
- R. Begum and G. Yasmeen, Contribution of Pakistani women in agriculture: productivity and constraints, Sarhad J. Agric., 2011, 27, 637–643 Search PubMed.
- I. Mukherjee and M. Gopal, Chromatographic techniques in the analysis of organochlorine pesticide residues, J. Chromatogr. A, 1996, 754, 33–42 CrossRef CAS PubMed.
- J. H. Syed and R. N. Malik, Occurrence and source identification of organochlorine pesticides in the surrounding surface soils of the Ittehad Chemical Industries Kalashah Kaku, Pakistan, Environ. Earth Sci., 2011, 62, 1311–1321 CrossRef CAS.
- M. I. Tariq, S. Afzal, I. Hussain and N. Sultana, Pesticides exposure in Pakistan: A review, Environ. Int., 2007, 33, 1107–1122 CrossRef CAS PubMed.
- S. Ali-Musstjab-Akber-Shah Eqani, R. N. Malik, A. Alamdar and H. Faheem, Status of organochlorine contaminants in the different environmental compartments of Pakistan: A review on occurrence and levels, Bull. Environ. Contam. Toxicol., 2012, 88, 303–310 CrossRef CAS PubMed.
- M. Ali and A. Jabbar, Effect of pesticides and fertilizers on shallow groundwater quality, in Final technical report (Jan. 1990–Sep. 1991), Pakistan Council of Research in Water Resources (PCRWR) Government of Pakistan, 1992 Search PubMed.
- K. Ahad, T. Anwar, I. Ahmad, A. Mohammad, S. Tahir, S. Aziz and U. Baloch, Determination of insecticide residues in groundwater of Mardan Division, NWFP, Pakistan: a case study, WATER SA-PRETORIA-, 2000, 26, 409–412 CAS.
- M. Aamir, S. Khan, J. Nawab, Z. Qamar and A. Khan, Tissue distribution of HCH and DDT congeners and human health risk associated with consumption of fish collected from Kabul River, Pakistan, Ecotoxicol, Environ. Saf., 2016, 125, 128–134 CrossRef CAS PubMed.
- S. Mahboob, F. Niazi, K. AlGhanim, S. Sultana, F. Al-Misned and Z. Ahmed, Health risks associated with pesticide residues in water, sediments and the muscle tissues of Catla catla at Head Balloki on the River Ravi, Environ. Monit. Assess., 2015, 187, 1–10 CrossRef CAS PubMed.
- U. Ali, A. Bajwa, M. J. I. Chaudhry, A. Mahmood, J. H. Syed, J. Li, G. Zhang, K. C. Jones and R. N. Malik, Significance of black carbon in the sediment–water partitioning of organochlorine pesticides (OCPs) in the Indus River, Pakistan, Ecotoxicol. Environ. Saf., 2016, 126, 177–185 CrossRef CAS PubMed.
- J. L. Barber, A. J. Sweetman, D. Van Wijk and K. C. Jones, Hexachlorobenzene in the global environment: emissions, levels, distribution, trends and processes, Sci. Total Environ., 2005, 349, 1–44 CrossRef CAS PubMed.
- J. Regueiro, M. Llompart, C. Garcia-Jares and R. Cela, Development of a high-throughput method for the determination of organochlorinated compounds, nitromusks and pyrethroid insecticides in indoor dust, J. Chromatogr. A, 2007, 1174, 112–124 CrossRef CAS PubMed.
- U. Heudorf, J. Angerer and H. Drexler, Current internal exposure to pesticides in children and adolescents in Germany: Blood plasma levels of pentachlorophenol (PCP), lindane (γ-HCH), and dichloro (diphenyl) ethylene (DDE), a biostable metabolite of dichloro (diphenyl) trichloroethane (DDT), Int. J. Hyg. Environ. Health, 2003, 206, 485–491 CrossRef CAS PubMed.
- S. Khan, M. Sohail, C. Han, J. A. Khan, H. M. Khan and D. D. Dionysiou, Degradation of highly chlorinated pesticide, lindane, in water using UV/persulfate: Kinetics and mechanism, toxicity evaluation, and synergism by H2O2, J. Hazard. Mater., 2021, 402, 123558 CrossRef CAS PubMed.
- P. Caicedo, A. Schröder, N. Ulrich, U. Schröter, A. Paschke, G. Schüürmann, I. Ahumada and P. Richter, Determination of lindane leachability in soil–biosolid systems and its bioavailability in wheat plants, Chemosphere, 2011, 84, 397–402 CrossRef CAS PubMed.
- S.-L. McManus, C. E. Coxon, K. G. Richards and M. Danaher, Quantitative solid phase microextraction–Gas chromatography mass spectrometry analysis of the pesticides lindane, heptachlor and two heptachlor transformation products in groundwater, J. Chromatogr. A, 2013, 1284, 1–7 CrossRef CAS PubMed.
- D. Fatta, C. Michael, E. D. Georgiou, M. Christodoulidou, A. Achilleos and M. Vasquez, Organochlorine and organophosphoric insecticides, herbicides and heavy metals residue in industrial wastewaters in Cyprus, J. Hazard. Mater., 2007, 145, 169–179 CrossRef CAS PubMed.
- S. J. Larson, R. J. Gilliom and P. D. Capel, Pesticides in streams of the United States: initial results from the national water-quality assessment program, US Department of the Interior, US Geological Survey, 1999, vol. 98, No. 4222 Search PubMed.
- J. O. Babayemi, Overview of levels of organochlorine pesticides in surface water and food items in Nigeria, J. Environ. Earth Sci., 2016, 6, 77–86 Search PubMed.
- C. Jiawei, L. Chen, Y. Zhongfang and W. Jiyuan, Residues and characteristics of organochlorine pesticides in the surface water in the suburb of Beijing, Earth Sci. Front., 2008, 15, 242–247 CrossRef.
- N. Sankararamakrishnan, A. K. Sharma and R. Sanghi, Organochlorine and organophosphorous pesticide residues in ground water and surface waters of Kanpur, Uttar Pradesh, India, Environ. Int., 2005, 31, 113–120 CrossRef CAS PubMed.
- S. Lakaschus, K. Weber, F. Wania, R. Bruhn and O. Schrems, The air-sea equilibrium and time trend of hexachlorocyclohexanes in the Atlantic Ocean between the Arctic and Antarctica, Environ. Sci. Technol., 2002, 36, 138–145 CrossRef CAS PubMed.
- C. Xu, X. He, C. Wang, X. Chen, R. Yuan and W. Dai, Introduction of holes into graphene sheets to further enhance graphene-TiO2 photocatalysis activities, RSC Adv., 2016, 6, 84068–84073 RSC.
- S. Li, X. Ma, L. Liu and X. Cao, Degradation of 2,4-dichlorophenol in wastewater by low temperature plasma coupled with TiO2 photocatalysis, RSC Adv., 2015, 5, 1902–1909 RSC.
- R. Giovannetti, E. Rommozzi, M. Zannotti, C. A. D'Amato, S. Ferraro, M. Cespi, G. Bonacucina, M. Minicucci and A. Di Cicco, Exfoliation of graphite into graphene in aqueous solution: an application as graphene/TiO2 nanocomposite to improve visible light photocatalytic activity, RSC Adv., 2016, 6, 93048–93055 RSC.
- S. Khan, C. Han, M. Sayed, M. Sohail, S. Jan, S. Sultana, H. M. Khan and D. D. Dionysiou, Exhaustive photocatalytic lindane degradation by combined simulated solar light-activated nanocrystalline TiO2 and inorganic oxidants, Catalysts, 2019, 9, 425 CrossRef CAS.
- J. Pawliszyn, Solid phase microextraction: theory and practice, John Wiley & Sons, New York, 1997 Search PubMed.
- J. O'Reilly, Q. Wang, L. Setkova, J. P. Hutchinson, Y. Chen, H. L. Lord, C. M. Linton and J. Pawliszyn, Automation of solid-phase microextraction, J. Sep. Sci., 2005, 28, 2010–2022 CrossRef.
- C. Achten, A. Kolb and W. Püttmann, Sensitive method for determination of methyl tert-butyl ether (MTBE) in water by use of headspace-SPME/GC–MS, Fresenius. J. Anal. Chem., 2001, 371, 519–525 CrossRef CAS PubMed.
- B. Cancho, F. Ventura and M. T. Galceran, Solid-phase microextraction for the determination of iodinated trihalomethanes in drinking water, J. Chromatogr. A, 1999, 841, 197–206 CrossRef CAS PubMed.
- M. Natangelo, S. Tavazzi, R. Fanelli and E. Benfenati, Analysis of some pesticides in water samples using solid-phase microextraction-gas chromatography with different mass spectrometric techniques, J. Chromatogr. A, 1999, 859, 193–201 CrossRef CAS PubMed.
- Y. Yang, D. J. Miller and S. B. Hawthorne, Solid-phase microextraction of polychlorinated biphenyls, J. Chromatogr. A, 1998, 800, 257–266 CrossRef CAS PubMed.
- F. Santos and M. Galceran, The application of gas chromatography to environmental analysis, TrAC, Trends Anal. Chem., 2002, 21, 672–685 CrossRef CAS.
- A. Derouiche, M. R. Driss, J.-P. Morizur and M.-H. Taphanel, Simultaneous analysis of polychlorinated biphenyls and organochlorine pesticides in water by headspace solid-phase microextraction with gas chromatography-tandem mass spectrometry, J. Chromatogr. A, 2007, 1138, 231–243 CrossRef CAS PubMed.
- M. Ribani, C. H. Collins and C. B. Bottoli, Validation of chromatographic methods: Evaluation of detection and quantification limits in the determination of impurities in omeprazole, J. Chromatogr. A, 2007, 1156, 201–205 CrossRef CAS PubMed.
- ECOSAR, http://www.epa.gov/oppt/newchems/tools/21ecosar.htm, 2014 Search PubMed.
- J. A. Khan, X. He, N. S. Shah, M. Sayed, H. M. Khan and D. D. Dionysiou, Degradation kinetics and mechanism of desethyl-atrazine and desisopropyl-atrazine in water with ˙OH and SO4˙− based-AOPs, Chem. Eng. J., 2017, 325, 485–494 CrossRef CAS.
- M. Sayed, A. Arooj, N. S. Shah, J. A. Khan, L. A. Shah, F. Rehman, H. Arandiyan, A. M. Khan and A. R. Khan, Narrowing the band gap of TiO2 by co-doping with Mn2+ and Co2+ for efficient photocatalytic degradation of enoxacin and its additional peroxidase like activity: A mechanistic approach, J. Mol. Liq., 2018, 272, 403–412 CrossRef CAS.
- Economic Survey of Pakistan, Finance Division, Government of Pakistan, Islamabad, 2006, http://www.finance.gov.pk/survey/sur_chap_05-06/02-Agriculture.PDF Search PubMed.
- M. Lopez-Blanco, B. Reboreda-Rodrıguez, B. Cancho-Grande and J. Simal-Gandara, Optimization of solid-phase extraction and solid-phase microextraction for the determination of α-and β-endosulfan in water by gas chromatography–electron-capture detection, J. Chromatogr. A, 2002, 976, 293–299 CrossRef CAS PubMed.
- N. D. G. Chau, Z. Sebesvari, W. Amelung and F. G. Renaud, Pesticide pollution of multiple drinking water sources in the Mekong Delta, Vietnam: Evidence from two provinces, Environ. Sci. Pollut. Res., 2015, 22, 9042–9058 CrossRef CAS PubMed.
- A. Younas, I. Hilber, S. ur Rehman, M. Khwaja and T. D. Bucheli, Former DDT factory in Pakistan revisited for remediation: severe DDT concentrations in soils and plants from within the area, Environ. Sci. Pollut. Res., 2013, 20, 1966–1976 CrossRef CAS PubMed.
- S. Ullah, P. Faiz, M. Aamir, M. A. Sabir and Q. Mahmood, Occurrence and spatio-vertical distribution of DDT in soils of abandoned DDT factory area, Amangarh, Pakistan, SN Appl. Sci., 2019, 1, 1–9 CAS.
- M. R. Jan, J. Shah, M. A. Khawaja and K. Gul, DDT residue in soil and water in and around abandoned DDT manufacturing factory, Environ. Monit. Assess., 2009, 155, 31–38 CrossRef CAS PubMed.
- J. A. Khan, C. Han, N. S. Shah, H. M. Khan, M. N. Nadagouda, V. Likodimos, P. Falaras, K. O'Shea and D. D. Dionysiou, Ultraviolet-visible light-sensitive high surface area phosphorous-fluorine–co-doped TiO2 nanoparticles for the degradation of atrazine in water, Environ. Eng. Sci., 2014, 31, 435–446 CrossRef CAS.
- B. B. Adormaa, W. K. Darkwah and Y. Ao, Oxygen vacancies of the TiO2 nano-based composite photocatalysts in visible light responsive photocatalysis, RSC Adv., 2018, 8, 33551–33563 RSC.
- R. Giovannetti, E. Rommozzi, M. Zannotti, C. A. D'Amato, S. Ferraro, M. Cespi, G. Bonacucina, M. Minicucci and A. Di Cicco, Exfoliation of graphite into graphene in aqueous solution: an application as graphene/TiO2 nanocomposite to improve visible light photocatalytic activity, RSC Adv., 2016, 6, 93048–93055 RSC.
- I. Nitoi, T. Oncescu and P. Oancea, Mechanism and kinetic study for the degradation of lindane by photo-Fenton process, J. Ind. Eng. Chem., 2013, 19, 305–309 CrossRef CAS.
- S. Khan, C. Han, H. M. Khan, D. L. Boccelli, M. N. Nadagouda and D. D. Dionysiou, Efficient degradation of lindane by visible and simulated solar light-assisted S-TiO2/peroxymonosulfate process: kinetics and mechanistic investigations, Mol. Catal., 2017, 428, 9–16 CrossRef CAS.
- A. M. Nienow, J. C. Bezares-Cruz, I. C. Poyer, I. Hua and C. T. Jafvert, Hydrogen peroxide-assisted UV photodegradation of Lindane, Chemosphere, 2008, 72, 1700–1705 CrossRef CAS PubMed.
- S. Khan, X. He, J. A. Khan, H. M. Khan, D. L. Boccelli and D. D. Dionysiou, Kinetics and mechanism of sulfate radical- and hydroxyl radical-induced degradation of highly chlorinated pesticide lindane in UV/peroxymonosulfate system, Chem. Eng. J., 2017, 318, 135–142 CrossRef CAS.
- F. Rehman, M. Sayed, J. A. Khan, N. S. Shah, H. M. Khan and D. D. Dionysiou, Oxidative removal of brilliant green by UV/S2O82−, UV/HSO5− and UV/H2O2 processes in aqueous media: A comparative study, J. Hazard. Mater., 2018, 357, 506–514 CrossRef CAS PubMed.
- A. R. L. Ribeiro, N. F. Moreira, G. L. Puma and A. M. Silva, Impact of water matrix on the removal of micropollutants by advanced oxidation technologies, Chem. Eng. J., 2019, 363, 155–173 CrossRef.
- J. A. Khan, N. S. Shah and H. M. Khan, Decomposition of atrazine by ionizing radiation: Kinetics, degradation pathways and influence of radical scavengers, Sep. Purif. Technol., 2015, 156, 140–147 CrossRef CAS.
- N. S. Shah, J. A. Khan, M. Sayed, Z. U. H. Khan, J. Iqbal, S. Arshad, M. Junaid and H. M. Khan, Synergistic effects of H2O2 and S2O82− in the gamma radiation induced degradation of congo-red dye: Kinetics and toxicities evaluation, Sep. Purif. Technol., 2020, 233, 115966 CrossRef CAS.
- R. Yuan, Y. Zhu, B. Zhou and J. Hu, Photocatalytic oxidation of sulfamethoxazole in the presence of TiO2: Effect of matrix in aqueous solution on decomposition mechanisms, Chem. Eng. J., 2019, 359, 1527–1536 CrossRef CAS.
- P.-S. Yap and T.-T. Lim, Effect of aqueous matrix species on synergistic removal of bisphenol-A under solar irradiation using nitrogen-doped TiO2/AC composite, Appl. Catal., B, 2011, 101, 709–717 CrossRef CAS.
- F. Rehman, W. Ahmad, N. Parveen, S. K. Zakir, S. Khan and C. Han, The catalytic degradation of the inflammatory drug diclofenac sodium in water by Fe2+/persulfate, Fe2+/peroxymonosulfate and Fe2+/H2O2 processes: A comparative analysis, Water, 2023, 15, 885 CrossRef CAS.
- L. Lin, H. Wang, H. Luo and P. Xu, Photocatalytic treatment of desalination concentrate using optical fibers coated with nanostructured thin films: impact of water chemistry and seasonal climate variations, Photochem. Photobiol., 2016, 92, 379–387 CrossRef CAS PubMed.
- W. Zhang, Y. Li, Y. Su, K. Mao and Q. Wang, Effect of water composition on TiO2 photocatalytic removal of endocrine disrupting compounds (EDCs) and estrogenic activity from secondary effluent, J. Hazard. Mater., 2012, 215, 252–258 CrossRef PubMed.
- M. Salimi, A. Esrafili, H. R. Sobhi, M. Behbahani, M. Gholami, M. Farzadkia, A. J. Jafari and R. R. Kalantary, Photocatalytic degradation of metronidazole using D-g-C3N4-Bi5O7I composites under visible light irradiation: Degradation product, and mechanisms, ChemistrySelect, 2019, 4, 10288–10295 CrossRef CAS.
- M. Sayed, J. A. Khan, L. A. Shah, N. S. Shah, H. M. Khan, F. Rehman, A. R. Khan and A. M. Khan, Degradation of quinolone antibiotic, norfloxacin, in aqueous solution using gamma-ray irradiation, Environ. Sci. Pollut. Res., 2016, 23, 13155–13168 CrossRef CAS PubMed.
- A. Fernandes, M. Gągol, P. Makoś, J. A. Khan and G. Boczkaj, Integrated photocatalytic advanced oxidation system (TiO2/UV/O3/H2O2) for degradation of volatile organic compounds, Sep. Purif. Technol., 2019, 224, 1–14 CrossRef CAS.
- H. Fang, Y. Gao, G. Li, J. An, P.-K. Wong, H. Fu, S. Yao, X. Nie and T. An, Advanced oxidation kinetics and mechanism of preservative propylparaben degradation in aqueous suspension of TiO2 and risk assessment of its degradation products, Environ. Sci. Technol., 2013, 47, 2704–2712 CrossRef CAS PubMed.
- T. An, J. An, Y. Gao, G. Li, H. Fang and W. Song, Photocatalytic degradation and mineralization mechanism and toxicity assessment of antivirus drug acyclovir: Experimental and theoretical studies, Appl. Catal., B, 2015, 164, 279–287 CrossRef CAS.
- H. Tang, S. Chang, K. Wu, G. Tang, Y. Fu, Q. Liu and X. Yang, Band gap and morphology engineering of TiO2 by silica and fluorine co-doping for efficient ultraviolet and visible photocatalysis, RSC Adv., 2016, 6, 63117–63130 RSC.
- J. A. Khan, X. He, N. S. Shah, H. M. Khan, E. Hapeshi, D. Fatta-Kassinos and D. D. Dionysiou, Kinetic and mechanism investigation on the photochemical degradation of atrazine with activated H2O2, S2O82− and HSO5−, Chem. Eng. J., 2014, 252, 393–403 CrossRef CAS.
- X. Zhang, X. Liu, J. Zhao, W. Sun, X. Li, J. Li, J. Zhang and Z. Jing, NaOH-activated persulfate-assisted mechanochemical mechanism and removal of lindane from contaminated soil, J. Environ. Chem. Eng., 2021, 9, 105391 CrossRef CAS.
- L. O. Conte, G. Legnettino, D. Lorenzo, S. Cotillas, M. Prisciandaro and A. Santos, Degradation of lindane by persulfate/ferrioxalate/solar light process: Influential operating parameters, kinetic model and by-products, Appl. Catal., B, 2023, 324, 122288 CrossRef CAS.
- S. Antonaraki, T. M. Triantis, E. Papaconstantinou and A. Hiskia, Photocatalytic degradation of lindane by polyoxometalates: Intermediates and mechanistic aspects, Catal, Today, 2010, 151, 119–124 CrossRef CAS.
- M. Trojanowicz, P. Drzewicz, P. Pańta, W. Głuszewski, G. Nałecz-Jawecki, J. Sawicki, M. H. O. Sampa, H. Oikawa, S. I. Borrely, M. Czaplicka and M. Szewczyńska, Radiolytic degradation and toxicity changes in γ-irradiated solutions of 2,4-dichlorophenol, Radiat. Phys. Chem., 2002, 65, 357–366 CrossRef CAS.
- R. Zona, S. Schmid and S. Solar, Detoxification of aqueous chlorophenol solutions by ionizing radiation, Water Res., 1999, 33, 1314–1319 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra03610c |
| This journal is © The Royal Society of Chemistry 2023 |