Open Access Article
Open Access ArticleStereocontrolled synthesis of some novel functionalized heterocyclic amino ester and amide derivatives with multiple stereocenters†
Anas Semghouliab,
Attila M. Remete*a and
Loránd Kiss *b
*b
aInstitute of Pharmaceutical Chemistry, University of Szeged, Eötvös u. 6, H-6720 Szeged, Hungary
bInstitute of Organic Chemistry, Stereochemistry Research Group, Research Centre for Natural Sciences, Magyar tudósok krt. 2, H-1117 Budapest, Hungary. E-mail: kiss.lorand@ttk.hu; remete.attila.mario@szte.hu; Tel: +36-30-1600354
First published on 27th July 2023
Abstract
The synthesis of some novel functionalized fused-ring β-amino lactones and lactams with multiple chiral centers has been attempted from readily available strained bicyclic β-amino acids via a stereocontrolled synthetic route. The key step was ring-rearrangement metathesis of allyl/propargyl esters or N-allylated/N-propargylated amides of (oxa)norbornene β-amino acids. The RRM transformations [ring-opening metathesis (ROM)/ring-closing metathesis (RCM) or ring-opening metathesis (ROM)/ring-closing enyne metathesis (RCEYM)] have been investigated using some commercially available catalysts. Importantly, the procedure used in this synthetic process does not affect the configurations of the chiral centers. This means that the structure of the starting (oxa)norbornene β-amino acids predetermines the configuration of the formed products.
Introduction
During olefin metathesis, olefins (alkenes) are cleaved into alkylidene fragments, which are subsequently reassembled to new olefins. This process has a number of attractive features. First of all, commercially available Ru-based metathesis catalysts (Fig. 1) possess good functional group tolerance and they are robust (tolerate oxygen and moisture to an extent). Furthermore, metathesis transformations are stereocontrolled, because they only affect the sp2 carbons of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds, while chiral sp3 centers are untouched. Finally, metathesis processes are versatile. The most important subtypes are ring-opening metathesis (ROM), ring-opening metathesis polymerization (ROMP), ring-closing metathesis (RCM), acyclic diene metathesis polymerization (ADMET), cross metathesis (CM), cross enyne metathesis (CEYM), ring-closing enyne metathesis (RCEYM), and ring-rearrangement metathesis (RRM). With everything taken into account, it is not surprising that olefin metathesis became a powerful and increasingly applied tool in organic synthesis during the last decades.1–11
C bonds, while chiral sp3 centers are untouched. Finally, metathesis processes are versatile. The most important subtypes are ring-opening metathesis (ROM), ring-opening metathesis polymerization (ROMP), ring-closing metathesis (RCM), acyclic diene metathesis polymerization (ADMET), cross metathesis (CM), cross enyne metathesis (CEYM), ring-closing enyne metathesis (RCEYM), and ring-rearrangement metathesis (RRM). With everything taken into account, it is not surprising that olefin metathesis became a powerful and increasingly applied tool in organic synthesis during the last decades.1–11
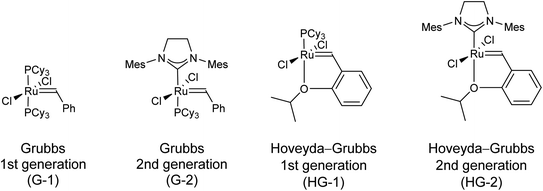 | ||
| Fig. 1 The most commonly used commercially available Ru-based metathesis catalysts. Cy = cyclohexyl, Mes = 2,4,6-trimethylphenyl. | ||
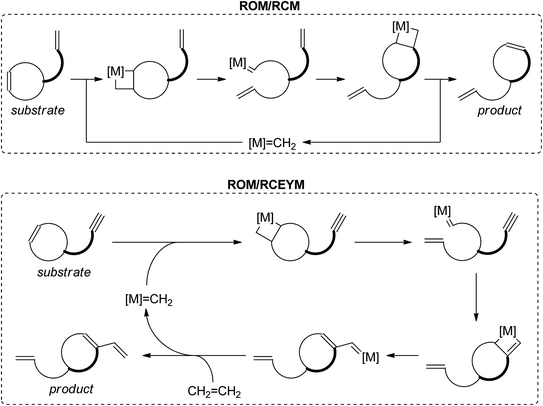
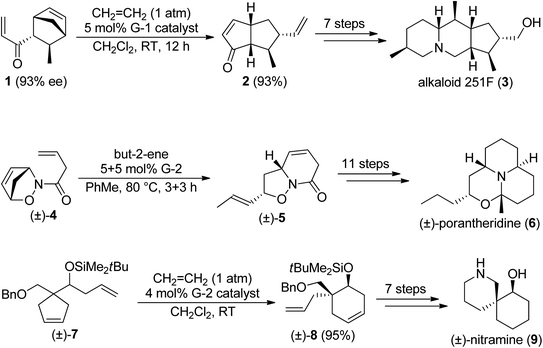
Ring-rearrangement metathesis (RRM) is a domino process, where ring-opening metathesis is followed by closure of a new ring via another olefin metathesis process. The most common RRM sequences are ROM/RCM and ROM/RCEYM (see Scheme 1). Ring-rearrangement metathesis excels in fast and efficient generation of structurally complex frameworks.8–11 Scheme 2 depicts the synthesis of some natural products via RRM.10
Lactones or lactams are widespread amongst natural products. These compounds possess high structural diversity and a wide range of bioactivities, being a very important compound class. Not surprisingly, their synthesis received great attention in pharmaceutical and medicinal chemistry. Scheme 3 depicts some syntheses which utilize olefin metathesis for ring closure.12,13
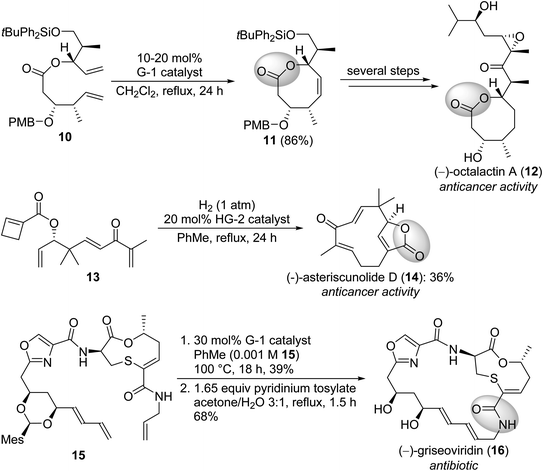 | ||
| Scheme 3 Synthesis of naturally occurring lactams and lactons via olefin metathesis. The lactam/lactone motif is highlighted. PMB = 4-methoxybenzyl, Mes = 2,4,6-trimethylphenyl. | ||
Earlier, our research group applied ring-rearrangement metathesis of easily accessible strained carbocyclic N-allylated or N-propargylated β-amino esters to obtain azaheterocyclic β-amino esters in a stereocontrolled fashion (Scheme 4).14–16
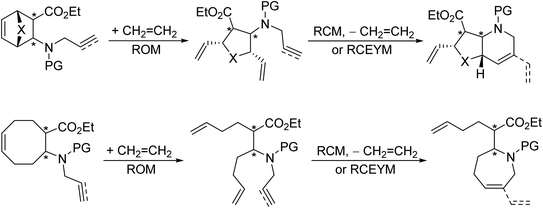 | ||
| Scheme 4 Earlier work: synthesis of azaheterocyclic β-amino esters via ring-rearrangement metathesis. X = O, CH2. PG = Boc, Ts. | ||
To demonstrate the versatility of the method, the aim of the current work was the application of RRM to access β-amino lactones and β-amino lactams. The starting compounds were either allyl/propargyl esters of (oxa)norbornene β-amino acids or the analogous N-allylated/N-propargylated amides (Scheme 5).
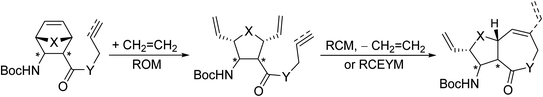 | ||
| Scheme 5 Current goal: synthesis of β-amino lactones and β-amino lactams through ring-rearrangement metathesis. X = CH2, O; Y = O, NH. | ||
Results and discussion
Initially, we focused on the application of the ROM/RCM protocol. First, N-protected diexo amino acid (±)-17 (ref. 17) was subjected to O-allylation. The resulting ester (±)-18 was then treated with various metathesis catalysts in the presence of ethylene.18 Unfortunately, olefin metathesis stopped after the ring opening step, with the only product being amino ester (±)-19 (Scheme 6). The most efficient catalyst was G-1, but all four catalysts provided the product in good yields. Treating isolated (±)-19 with metathesis catalysts in CH2Cl2 at room temperature or under reflux failed to trigger RCM. N-Allylated amide (±)-20, obtained via DCC-mediated coupling of compound (±)-17 with allylamine, behaved in a way similar to its ester analogue (±)-18 (Scheme 6), but its ROM was the most efficient with HG-1 catalyst.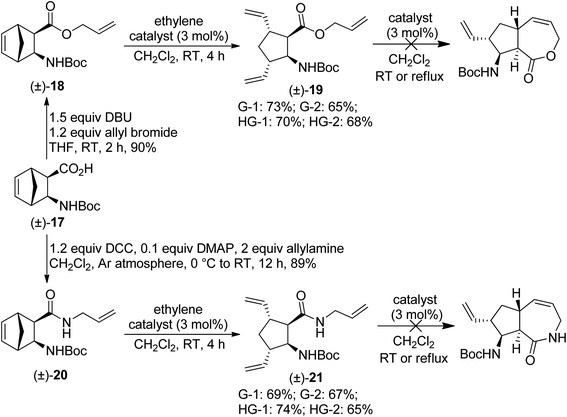 | ||
| Scheme 6 Attempted ring-rearrangement metathesis of diexo norbornene β-amino acid derivatives (±)-18 and (±)-20. | ||
Our next substrates, ester (±)-23 and amide (±)-25 were prepared from N-Boc-protected diendo amino acid (±)-22 (ref. 17) via the methods shown on Scheme 6. Unfortunately, the desired RRM process did not occur, only ring-opened products (±)-24 and (±)-26 were formed. The best yields were achieved with G-2 catalyst, but other catalysts also provided the products in similar good yields (Scheme 7). Treatment of isolated (±)-24 and (±)-26 with metathesis catalysts failed to trigger the desired RCM process.
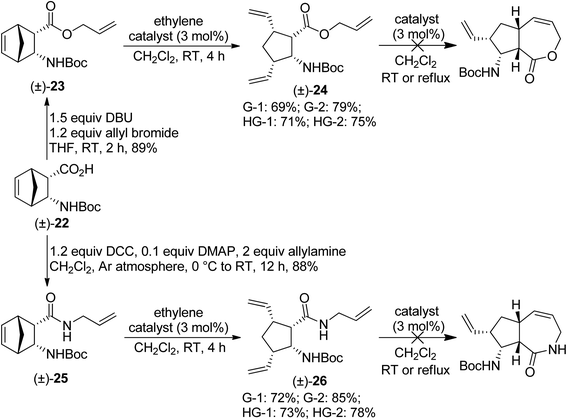 | ||
| Scheme 7 Attempted ring-rearrangement metathesis of diendo norbornene β-amino acid derivatives (±)-23 and (±)-25. | ||
Afterwards, compound (±)-27 (ref. 19) was used to synthesize oxanorbornene β-amino acid derivatives (±)-28 and (±)-30. Again, when compounds (±)-28 and (±)-30 were subjected to olefin metathesis, the transformation stopped after the ROM step, and attempts to force RCM of products (±)-29 and (±)-31 were unsuccessful (Scheme 8).
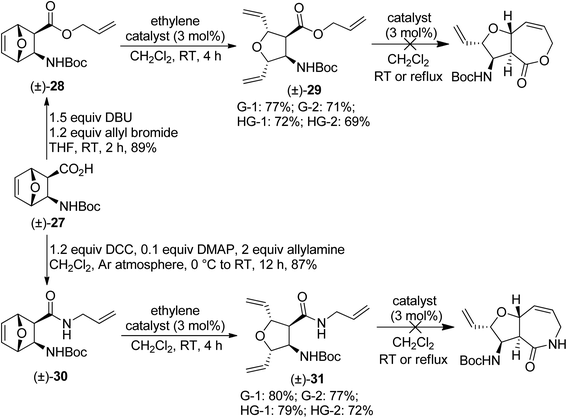 | ||
| Scheme 8 Attempted ring-rearrangement metathesis of diexo oxanorbornene β-amino acid derivatives (±)-28 and (±)-30. | ||
Taking into consideration the failure of the ROM/RCM protocol, we turned our attention towards the ROM/RCEYM pathway. First, diexo ester (±)-32 was prepared by O-propargylation of compound (±)-17. Its ring-rearrangement metathesis yielded various products. With second generation catalysts, only ring opening took place, and compound (±)-33 was formed as the sole product. In contrast, first generation catalysts provided compound (±)-34 as the main product, formed by cross enyne metathesis between (±)-33 and one molecule of ethylene. ROM/CEYM product (±)-34 was accompanied with small amounts of ROM product (±)-33 and the desired RRM product (±)-35 (Scheme 9). G-1 catalyst gave compound (±)-35 in the highest, but still low yield.
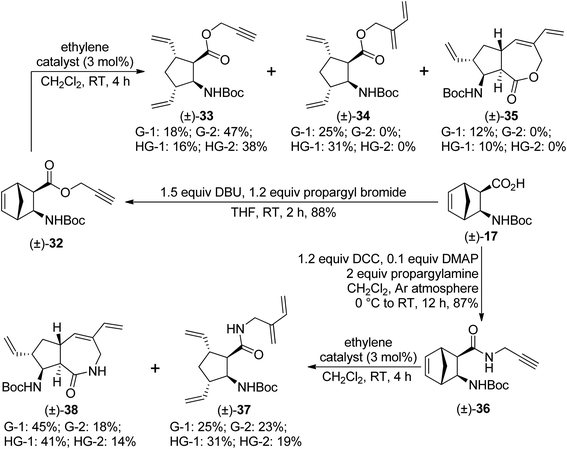 | ||
| Scheme 9 Ring-rearrangement metathesis of diexo norbornene β-amino acid derivatives (±)-32 and (±)-36. | ||
Then, diexo amide (±)-36, prepared via DCC-mediated amidation of compound (±)-17 with propargylamine, was subjected to ring-rearrangement metathesis. Mixtures of ROM/CEYM product (±)-37 and the desired ROM/RCEYM product (±)-38 were obtained. With second generation catalysts, compound (±)-37 was the main product, while first generation catalysts (especially G-1) preferred formation of compound (±)-38 (Scheme 9).
We continued our work with diendo analogues. Ester (±)-39 behaved similarly to its analogue (±)-32. With second generation catalysts, only ROM product (±)-40 was formed, while first generation catalysts provided ROM/CEYM product (±)-41 as the main product, (±)-40 as the minor product, and small amounts of the desired RRM product (±)-42 (Scheme 10). HG-1 catalyst delivered compound (±)-42 in the highest yield. However, amide (±)-43 behaved in a different manner in comparison with its stereoisomer (±)-36. Specifically, with all catalysts, a mixture of ROM product (±)-44, ROM/CEYM product (±)-45, and RRM product (±)-46 was formed, and their yields decreased in the order (±)-44 > (±)-45 > (±)-46. The highest yield of the desired compound (±)-46 was achieved with G-1 catalyst (Scheme 10).
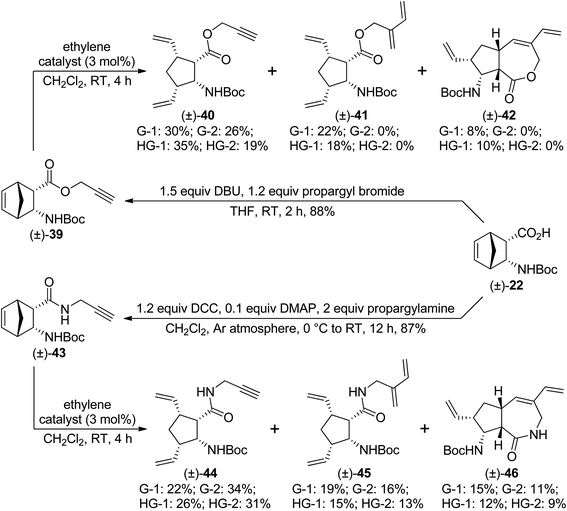 | ||
| Scheme 10 Ring-rearrangement metathesis of diendo norbornene β-amino acid derivatives (±)-39 and (±)-43. | ||
Finally, oxanorbornene derivatives (±)-47 and (±)-50 were subjected to metathesis reactions. The former was a propargyl ester, while the latter was an N-propargylated amide. Unfortunately, the desired RRM products were not formed. Only ROM products (as minor products) and ROM/CEYM products (as major products) were isolated. Treatment of the isolated ROM products [(±)-48 and (±)-51] with metathesis catalysts failed to trigger RCM (Scheme 11).
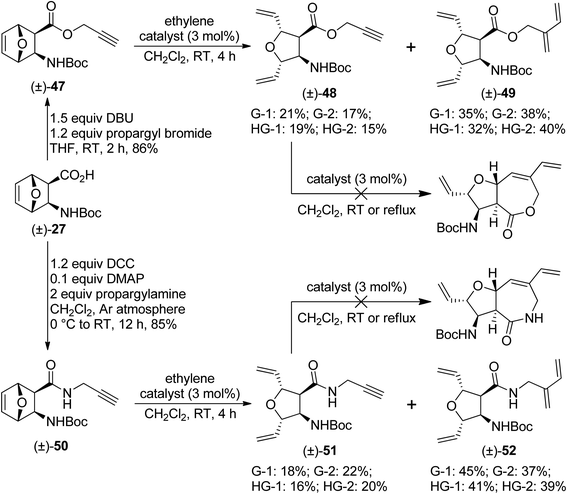 | ||
| Scheme 11 Attempted ring-rearrangement metathesis of oxanorbornene β-amino acid derivatives (±)-47 and (±)-50. | ||
Conclusions
Synthesis of β-amino lactams and lactons with an 5,7-fused ring system was attempted via ring-rearrangement metathesis of various (oxa)norbornene β-amino esters and amides.Allyl esters (±)-18, (±)-23, and (±)-28 as well as N-allylated amides (±)-20, (±)-25, and (±)-30 failed to produce ROM/RCM reaction, only ring-opening metathesis occurred. ROM of diexo compounds (±)-18, (±)-20, (±)-28, and (±)-30 was usually the most efficient with G-1 catalyst, while G-2 catalyst provided the best yield in ROM of diendo compounds (±)-23 and (±)-25.
Metathesis reactions of propargyl esters of norbornene β-amino acids (±)-32 and (±)-39 provided highly catalyst-dependent outcome in most cases. With second generation catalysts, only ROM products were formed. In contrast, first generation catalysts provided three-component product mixtures, including the desired RRM products.
Metathesis reactions of oxanorbornene β-amino acid propargyl ester (±)-47 and related N-propargylated amide (±)-50 failed to deliver ROM/RCEYM products. Instead, mixtures of ROM and ROM/CEYM products were formed with the latter providing the major products.
In metathesis reactions of N-propargylated amides of norbornene β-amino acids, every catalyst provided RRM products. In the case of diexo compound (±)-36, metathesis led to a ROM/CEYM product (main product with second generation catalysts) and an RRM product (main product with first generation catalysts). Subjecting diendo compound (±)-42 to olefin metathesis yielded three-component product mixtures. Yields of the RRM products were lower than those of ROM and ROM/CEYM products.
To sum up, synthesis of 5,7-fused ring systems via ring-rearrangement metathesis proved to be much more difficult than previous syntheses of 5,6-fused ring systems. The probable reason is the slightly strained nature of the seven-membered ring, which greatly hinders ring closure. Further studies are planned to fully investigate the capabilities and limitations of ring-rearrangement of cycloalkene β-amino acid derivatives.
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
The authors gratefully acknowledge financial support from the National Research, Development and Innovation Office of Hungary (NKFIH/OTKA K 142266). The majority of the high-resolution mass spectrometric analysis was performed by Róbert Berkecz. Project no. TKP2021-EGA-32 has been implemented with the support provided by the Ministry of Innovation and Technology of Hungary from the National Research, Development and Innovation Fund, financed under the TKP2021-EGA funding scheme.References
- A. H. Hoveyda and A. R. Zhugralin, Nature, 2007, 450, 243–251, DOI:10.1038/nature06351.
- G. C. Vougioukalakis and R. H. Grubbs, Chem. Rev., 2010, 110, 1746–1787, DOI:10.1021/cr9002424.
- S. Chikkali and S. Mecking, Angew. Chem., Int. Ed., 2012, 51, 5802–5808, DOI:10.1002/anie.201107645.
- Olefin Metathesis: Theory and Practice, ed. K. Grela, John Wiley & Sons, Inc., Hoboken, New Jersey, 2014, DOI:10.1002/9781118711613.
- C. S. Higman, J. A. M. Lummiss and D. E. Fogg, Angew. Chem., Int. Ed., 2016, 55, 3552–3565, DOI:10.1002/anie.201506846.
- D. Hughes, P. Wheeler and D. Ene, Org. Process Res. Dev., 2017, 21, 1938–1962, DOI:10.1021/acs.oprd.7b00319.
- C. Lecourt, S. Dhambri, L. Allievi, Y. Sanogo, N. Zeghbib, R. B. Othman, M.-I. Lannou, G. Sorin and J. Ardisson, Nat. Prod. Rep., 2018, 35, 105–124, 10.1039/c7np00048k.
- N. Holub and S. Blechert, Chem.–Asian J., 2007, 2, 1064–1082, DOI:10.1002/asia.200700072.
- S. Roscales and J. Plumet, Nat. Prod. Commun., 2017, 12, 713–732, DOI:10.1177/1934578X1701200.
- S. Kotha, M. Meshram, P. Khedkar, S. Banerjee and D. Deodhar, Beilstein J. Org. Chem., 2015, 11, 1833–1864, DOI:10.3762/bjoc.11.199.
- E. Bernardi, L. Colombo and M. Serra, Synthesis, 2021, 53, 785–804, DOI:10.1055/s-0040-1705965.
- Natural Lactones and Lactams: Synthesis, Occurrence and Biological Activity, ed. T. Janecki, Wiley-VCH Verlag GmbH & Co., Weinheim, 2014, DOI:10.1002/9783527666911.
- Synthesis of Heterocycles by Metathesis Reactions, Topics in Heterocyclic Chemistry, ed. J. Prunet, Springer International Publishing, Switzerland, 2017, vol. 47, DOI:10.1007/978-3-319-39941-6.
- A. Semghouli, Z. Benke, A. M. Remete, T. T. Novák, S. Fustero and L. Kiss, Chem.–Asian J., 2021, 16, 3873–3881, DOI:10.1002/asia.202100956.
- A. Semghouli, A. M. Remete, T. T. Novák and L. Kiss, Synlett, 2022, 33, 1655–1659, DOI:10.1055/a-1877-3822.
- A. Semghouli, A. M. Remete and L. Kiss, ChemistrySelect, 2022, 7, e202204244, DOI:10.1002/slct.202204244.
- M. Palkó, M. E. Haimer, Z. Kormányos and F. Fülöp, Molecules, 2019, 24, 772, DOI:10.3390/molecules24040772.
- A. H. Hoveyda, Z. Liu, C. Qin, T. Koengeter and Y. Mu, Angew. Chem., Int. Ed., 2020, 59, 22324–22348, DOI:10.1002/anie.202010205.
- P. Canonne, M. Akssira, A. Dahdouh, H. Kasmi and M. Boumzebra, Tetrahedron, 1993, 49, 1985–1992, DOI:10.1016/S0040-4020(01)86298-0.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra03866a |
| This journal is © The Royal Society of Chemistry 2023 |