Open Access Article
Open Access ArticleEffect of conjugation length on fluorescence characteristics of carbon dots†
Jianen Zhanga,
Mingjun Chena,
Xiaojie Rena,
Weicai Shia,
Tao Yina,
Tao Luoa,
Youshi Lanb,
Xu Li *a and
Li Guan
*a and
Li Guan *a
*a
aKey Laboratory of High-precision Computation and Application of Quantum Field Theory of Hebei Province, College of Physics Science and Technology, Hebei University, Baoding 071002, PR China. E-mail: lguan@hbu.edu.cn; lixcn@sina.com
bDepartment of Radiochemistry, China Institute of Atomic Energy, Beijing 102413, PR China
First published on 18th September 2023
Abstract
The influence of sp2- and sp3-hybridized carbon coexisting in carbon cores on fluorescence characteristics of carbon dots (CDs) was revealed by density functional theory calculations. Based on the constructed coronene-like structures, the fluorescence emission spectra, transition molecular orbital pairs and several physical quantities describing the distribution of electrons and holes were investigated. The results indicate that due to the interaction between sp2 and sp3 carbon atoms, two main factors including the hyperconjugative effect and the separation of sp2 domain by sp3 carbon atoms can regulate the fluorescence wavelength. By analyzing the transition molecular orbital pairs, it was found that the fluorescence wavelength has a close correlation with the conjugation length, suggesting that the conjugation length can predict the shift of the emission spectra of CDs. The theoretical results provide a comprehensive understanding of fluorescence mechanism and help to synthesize CDs with expected fluorescence wavelength.
1. Introduction
After fullerene nanoballs, carbon nanotubes and graphene, carbon dots (CDs) have become a new hot topic in the research of carbon-based nanomaterials.1–3 CDs have many peculiar characteristics, including low cost, high stability, easy surface modification, low toxicity, good biocompatibility and tunable photoluminescence, leading to a wide range of applications of CDs.4–11 In recent years, the relationship between the structure and fluorescence properties of CDs has been widely explored to meet the requirements of practical applications. Many research works have attempted to make the atomic structure of CDs clear and found that CDs mainly consist of carbon cores and surface chemical functional groups.12–16 Carbon cores are formed by embedding sp2-hybridized graphene fragments in a sp3-hybridized carbon network.17,18 Therefore, there are two main methods to regulate the fluorescence emission wavelength of CDs. One is to change the size of the carbon cores, the other is to modify surface functional groups of CDs.19–21 The main purpose of changing the size of the carbon cores is to change the range of the sp2 carbon domains, which can determine the energy gap of CDs.22,23 Hence, many previous investigations focused on the structures of carbon cores and their influence on fluorescence properties. Experimentally, polycyclic aromatic hydrocarbons (PAHs) are mostly used to construct the carbon cores composed by sp2-hybridized carbon atoms.24–27 Recently, some researchers realized the fluorescence shift of CDs by changing hybridization types of carbon.28–31 Lu et al.32 regulated the external pressure to achieve an irreversible change from yellow (557 nm) to blue-green (491 nm) emission of CDs. To explain this phenomenon, N-doping polyaromatic structures were produced to model the N-doping CD structure, and first-principles calculations performed at the B3LYP/6-31G(d) level proved that the fluorescence blueshift originated from the transformation of sp2 into sp3-hybridized domains under high pressure. Rukhlenko et al.33 simulated the CD structure by pairs of coupled polycyclic aromatic molecules and concluded that with the increase of the sp2-hybridized carbon domains in sp3-hybridized amorphous core, the valence electrons become more delocalized and thus the fluorescence redshift occurs by using a simple quantum chemical approach. Wang et al.19 changed the number of sp2 hybridized benzene rings in the sp3 carbon network to modulate the sp2/sp3 ratio and demonstrated that the conversion of sp3-hybridized to sp2-hybridized leads to the emission redshift of CDs using first-principles calculations at the B3LYP/6-31G(d) level. Recently, Xu et al.34 used acetic acid to promote the formation of sp3 carbons during the synthesis of CDs. They designed a variety of sp2 hybridized benzene ring structures to evaluate the effect of conjugated rings on the emission wavelength and found as the number of conjugated rings increases from 1 to 25, the calculated energy gap energy decreases resulting from electron delocalization within the sp2 domain using first-principles calculations performed at the B3LYP/6-31G(d) level.The above-mentioned theoretical and experimental works have found that the proportion of sp2- and sp3-hybridized carbon has a regulating effect on the fluorescence properties of CDs. However, the influence of sp2-conjugated domains surrounded by sp3 carbon core on fluorescence emission still needs further discussion. In this work, coronene-like structures consisting of sp2-hybridized benzene rings and sp3-hybridized carbons were constructed. The electronic structures and fluorescence emission spectra were calculated by using density functional theory (DFT) and time-dependent density functional theory (TD-DFT) methods. Firstly, the relationship between the geometry configuration of carbon cores and fluorescence spectra was investigated and the interaction between sp2- and sp3-hybridized carbon atoms was discussed in details. Then, the correlation of the conjugation length and the fluorescence emission was revealed by analyzing the transition molecular orbitals. This work illustrates the effect of sp2 and sp3 hybridized carbon on the fluorescence emission of CDs and provides a theoretical guidance for the controlled synthesis of CDs.
2. Models and computational details
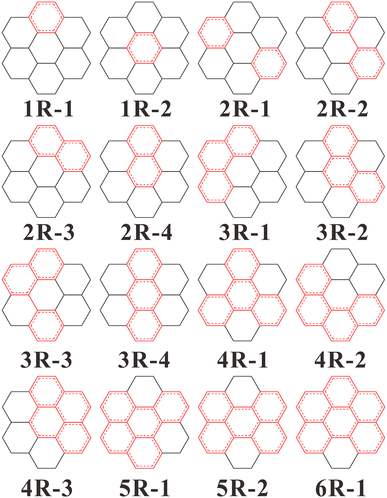
All calculation works were performed by Gaussian 16 package35 and performed by using the range-separated hybrid function ωB97X-D36 with dispersion correction in conjunction with the def2-SVP37 basis set. The polarizable continuum model (PCM) solvation approach was used to calculate the solvation effect in aqueous solution using the self-consistent reaction field method.38 More computational details and the choice of calculation method with basis set are in the ESI.† As shown in Fig. 1, coronene structure was selected to construct various sp2- and sp3-hybridized carbon cores. Here, the size of sp2 carbon domain was controlled by changing the number of sp2-hybridized benzene rings. Single-electron excitation was considered to describe the radiative transitions, and three structures in spin-triplet state were excluded shown in Fig. S1† in the ESI.† The ground-state geometry and electronic structure optimizations were carried out by DFT calculations with no constraints imposed beyond the multiplicity of the electronic state. Frequency calculations were performed to check the local energy minimum. Then the excited-state geometries were further optimized by linear-response TD-DFT method.39 The vertical excitation energy and oscillator intensity of the electronic transitions in fluorescence spectra were also obtained. To verify the validity of theoretical method, the fluorescence emission energy of coronene was firstly calculated, and the theoretical value 3.35 eV is in agreement with the experimental value 2.95 eV.40 The electronic properties analysis of all models were finished by Multiwfn 3.8 software.413. Results and discussion
3.1 Emission properties of CDs
Fig. 2 shows the calculated fluorescence emission spectra of all models. Previously experimental and theoretical works have revealed that the fluorescence wavelength of CDs shows a redshift trend with the increasing content of sp2 carbon domain.19,42 In this work, the content of sp2-hybridized carbon domain can be evaluated as the number of sp2 carbon rings. From structures 1R-i to 6R-i, the content of sp2 carbon domain varies from 25% to 92%. In overall view, the fluorescence wavelengths (250–523 nm) of all models exhibit a rough redshift trend with the increasing content of sp2-hybridized carbon. However, it should be noted that the emission wavelength and the content of sp2 domain are not strictly related. Sometimes, the more content of sp2 domain corresponds to the shorter emission wavelength, or the same content of sp2 domain produces different emission wavelength. The results suggest that the size of sp2 domain is not the only reason for regulating the fluorescence shift.Analyzing the geometry configuration illustrated as Fig. 1, it can be found that structures 1R-1 and 1R-2 with the same content of sp2 domain have different but close fluorescence emission peaks located at 250 nm and 264 nm, respectively. Also, structures 2R-3 and 2R-4, structures 3R-1 and 3R-2 with the same content of sp2 carbon have different but close emission peaks. Observing these structures, one can see that they have the same content and geometry configurations of sp2 domain but different sp3-hybridized carbon surrounding networks. The results mean that although the content of sp2 carbon domain is unchanged, various sp3 surrounding structures can induce the fluorescence emission shift of carbon cores.
In Fig. 2, another interesting result is that different contents of sp2 carbon domains possibly causes similar fluorescence wavelengths. For example, structure 2R-1 exhibits an emission peak at 251 nm, which is very close to the peak position of structure 1R-1 or 1R-2. Comparing their benzene ring configurations, one can see that although structure 2R-1 has two sp2-hybridized rings, the two rings are separated by sp3-hybridized carbon atoms, suggesting that the two benzene rings are essentially independent. Therefore, the completely-separated sp2 carbon domains in carbon core behave as smaller individual domains, which finally determines the fluorescence wavelength. This phenomenon has been observed in experimental measurement, where the fluorescence emission was dictated by isolated small sp2 domains.43 Nonetheless, it should be noted that the sp2 carbon domains in some cases seem to be separated, but the benzene rings are factually linked by C–C single bonds. For example, the sp2 carbon domains in structures 2R-2 and 3R-3 are not completely separated, which leads to the fluorescence emission energies of the two structures different from any smaller individual domains such as structure 1R-1 or 2R-4. Thus, the conclusion is not suitable for describing the separated sp2 domains linked by C–C single bonds.
As we all know, zigzag edges and arm-chair edges are two typical edges of sp2 carbon domain in CDs. In the present models, structures 2R-4, 3R-4 and 5R-2 have zigzag edges and exhibit long-wavelength fluorescence emission compared to other structures with the same content of sp2 domain. By contrast, structures 3R-1, 4R-1 and 5R-1 have arm-chair edges and exhibit short-wavelength fluorescence emission among all structures with the same content of sp2 domain. The results indicate that when the content of sp2 domains remains unchanged, the structures with zigzag edges exhibit long-wavelength emission compared with the ones with arm-chair edges, which is consistent with the theoretical results.42 For those structures with both edge features, the fluorescence emission peaks are located between the two situations mentioned above. From the perspective of aromatic stabilization, the zigzag and arm-chair structures exhibit obvious differences, and the former is more susceptible to chemical reaction and has lower aromatic stabilization than the latter.44 The calculated spectra in Fig. 2 indicate that when the content of sp2 domain is the same, the higher the stability of CDs, the shorter the fluorescence wavelength.
3.2 Electron excitation characters of CDs
In order to give a deeper understand of the excitation properties of CDs, the transition energy, oscillator strength, fluorescence wavelength and the configuration interaction contributions of molecular orbitals mainly involved in electronic transitions are summarized shown as Table 1. The highest occupied molecular orbital (HOMO)–the lowest unoccupied molecular orbital (LUMO) transition contributions of 9 models (2R-2, 2R-3, 2R-4, 3R-3, 3R-4, 4R-3, 5R-1, 5R-2 and 6R-1) approach 100%, which indicates that the fluorescence emissions of these structures are mainly dominated by the HOMO to LUMO transitions. The electron transitions of the remaining 7 structures are contributed by 2–3 groups of orbitals pairs near HOMO and LUMO.| Structure | Transition energy (eV) | Oscillator strength (f) | λem (nm) | Configuration interaction contribution (%) | |||||
|---|---|---|---|---|---|---|---|---|---|
| 1R-1 | 4.96 | 0.022 | 250 | H → L | 62.7% | H−1 → L+1 | 30.7% | ||
| 1R-2 | 4.70 | 0.008 | 264 | H−1 → L | 54.7% | H → L+1 | 39.1% | ||
| 2R-1 | 4.93 | 0.021 | 251 | H → L+1 | 51.3% | H−2 → L | 19.9% | H−1 → L+1 | 11.9% |
| 2R-2 | 3.66 | 0.664 | 339 | H → L | 97.8% | ||||
| 2R-3 | 3.59 | 0.289 | 340 | H → L | 98.9% | ||||
| 2R-4 | 3.65 | 0.360 | 345 | H → L | 98.9% | ||||
| 3R-1 | 3.88 | 0.018 | 319 | H → L+1 | 61.6% | H−1 → L | 33.4% | ||
| 3R-2 | 3.79 | 0.058 | 327 | H → L+1 | 48.8% | H → L | 21.3% | H−1 → L | 19.4% |
| 3R-3 | 3.09 | 0.556 | 401 | H → L | 98.2% | ||||
| 3R-4 | 2.69 | 0.333 | 460 | H → L | 99.5% | ||||
| 4R-1 | 3.87 | 0 | 320 | H → L | 49.4% | H-1 → L+1 | 46.1% | ||
| 4R-2 | 3.70 | 0.005 | 335 | H → L+1 | 55.6% | H-1 → L | 36.9% | ||
| 4R-3 | 3.14 | 0.970 | 395 | H → L | 98.1% | ||||
| 5R-1 | 3.34 | 0.817 | 371 | H → L | 96.0% | ||||
| 5R-2 | 2.37 | 0.636 | 523 | H → L | 98.7% | ||||
| 6R-1 | 2.90 | 0.617 | 428 | H → L | 97.8% | ||||
The hole–electron distribution is introduced by analyzing the weights of electronic transitions of molecular orbitals.45,46 According to Kasha's rule, the first excited state of singlet state is usually the critical state for emitting fluorescence.47 Therefore, the hole–electron distribution is applied to evaluate the electronic emission process from the first excited state to the ground state. Table 2 shows that the values of the relevant physical quantities describing the distribution of electrons and holes for CDs, and the explanations and formulas for these physical quantities are shown in the ESI.† It can be found that the average value of charge transfer distance (D) for all structures is 0.11 Å and the maximum value is 0.433 Å. Due to the C–C bond length is about 1.4 Å, so the small charge transfer distance indicates that the centers of mass of electrons and holes are very close. Sr index measures the degree of electron–hole overlap, and the calculated minimum value 0.82 means that the distributions of holes and electrons mostly overlap. Therefore, according to the values of D and Sr, it can be judged that the fluorescence emissions of all structures belong to local excitation.48,49 Moreover, all the overall spatial distribution scope Δσ are close to 0 and the electron–hole separation degree t are negative, which implies that there is no significant separation of the hole and electron. In a word, the above analysis gives a clear explanation that the excitation of all models belongs to a local excitation dominated by π–π* transitions, which suggests that one effective approach for regulating fluorescence spectrum of CDs is to change the π-conjugated sp2 carbon domains.
| Structure | D | Sr | Δσ | t |
|---|---|---|---|---|
| 1R-1 | 0.058 | 0.91 | 0.044 | −1.261 |
| 1R-2 | 0.124 | 0.89 | 0.181 | −1.395 |
| 2R-1 | 0.038 | 0.89 | 0.032 | −1.767 |
| 2R-2 | 0.197 | 0.88 | −0.006 | −1.511 |
| 2R-3 | 0.006 | 0.92 | 0.112 | −0.978 |
| 2R-4 | 0.074 | 0.82 | 0.114 | −1.107 |
| 3R-1 | 0.433 | 0.86 | 0.027 | −1.322 |
| 3R-2 | 0 | 0.89 | −0.037 | −1.585 |
| 3R-3 | 0.012 | 0.89 | 0.031 | −1.548 |
| 3R-4 | 0.155 | 0.85 | 0.043 | −1.556 |
| 4R-1 | 0.267 | 0.88 | 0.020 | −1.865 |
| 4R-2 | 0.009 | 0.91 | 0.011 | −2.094 |
| 4R-3 | 0.362 | 0.87 | −0.026 | −1.535 |
| 5R-1 | 0.024 | 0.88 | 0.055 | −2.132 |
| 5R-2 | 0 | 0.87 | 0.089 | −2.491 |
| 6R-1 | 0.098 | 0.86 | 0.063 | −2.006 |
3.3 The relationship between conjugation length and fluorescence emission
In order to further describe the fluorescence properties of carbon core structures, all the orbital pairs mainly involved in electronic transitions were calculated. Fig. 3 shows main transition molecular orbital pairs for the fluorescence emission process of the coronene-like structures. According to the distribution of molecular orbitals, one can see that the sp2 carbon domain is the main source of fluorescence emission, and the sp3 carbon atoms adjacent to sp2 domain also contribute to the electronic transition process, whereas the sp3 carbon atoms away from the sp2 domains have a lesser immediate impact on the fluorescence.The sp2 carbon domains are constructed from PAHs, and hence the fluorescence emission of carbon core is closely related to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds. The conjugation length is defined as the number of C
C double bonds. The conjugation length is defined as the number of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds on the shortest path between two terminal carbon atoms.50 For conjugated molecules, sp2 carbon domain belongs to a π-conjugated system, and its conjugation length is helpful to understand the optical properties.51 The relationship between conjugation length and fluorescence emission is significant for understanding the fluorescence characteristics and synthetizing CDs with tunable luminescence. For simple PAHs, the conjugation length can be directly given by evaluating C
C bonds on the shortest path between two terminal carbon atoms.50 For conjugated molecules, sp2 carbon domain belongs to a π-conjugated system, and its conjugation length is helpful to understand the optical properties.51 The relationship between conjugation length and fluorescence emission is significant for understanding the fluorescence characteristics and synthetizing CDs with tunable luminescence. For simple PAHs, the conjugation length can be directly given by evaluating C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds via valence bond theory. However, CDs have complex structures containing PAHs and sp3 carbon atoms, and a reasonable conjugation length needs to be estimated by molecular orbitals calculations.
C double bonds via valence bond theory. However, CDs have complex structures containing PAHs and sp3 carbon atoms, and a reasonable conjugation length needs to be estimated by molecular orbitals calculations.
Fig. 3 shows that in HOMO, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds are in bonding states and C–C single bonds are in anti-bonding states, whereas in LUMO, the bonding and anti-bonding states are reverse. If a six-membered ring demonstrates the molecular orbital characteristics of benzene, its conjugation length is 2. Based on the calculated occupied orbitals of models given in Fig. 3, the values of conjugation lengths are determined by evaluating the number of C
C double bonds are in bonding states and C–C single bonds are in anti-bonding states, whereas in LUMO, the bonding and anti-bonding states are reverse. If a six-membered ring demonstrates the molecular orbital characteristics of benzene, its conjugation length is 2. Based on the calculated occupied orbitals of models given in Fig. 3, the values of conjugation lengths are determined by evaluating the number of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds on the shortest path between two terminal carbon atoms and the configuration interaction contribution is considered in the present calculations.
C double bonds on the shortest path between two terminal carbon atoms and the configuration interaction contribution is considered in the present calculations.
In order to make clear the influences of sp3 carbon atoms on fluorescence wavelength, the fluorescence spectra of corresponding PAHs without sp3-hybridized carbon atoms were calculated and the geometric structures of corresponding PAHs are shown in Fig. S2 in the ESI.† According to the transition molecular orbital pairs of all coronene-like and PAHs structures, the conjugation lengths were evaluated and Fig. 4 gives the relationship between the conjugation length N and fluorescence wavelength. In Fig. 4, one can see that the conjugation length of any coronene-like structure is equal to that of corresponding PAHs, suggesting that when the sp2 carbon domain is fixed, the sp3 carbon network has no substantial influence on the conjugation length. For two groups of structures, the change trend of the emission wavelength is consistent with that of the conjugation length, suggesting that fluorescence emission is highly correlated with the conjugation length N. The longer conjugation length, the longer fluorescence wavelength, and vice versa. The results demonstrate that besides PAHs containing sp2-hybridized carbon, the conjugation length also is suitable to characterize the fluorescence wavelength of carbon core structures with sp2- and sp3-hybridized carbon.
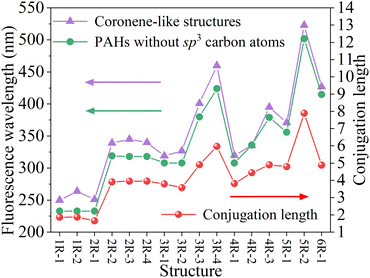 | ||
| Fig. 4 The relationship between conjugation length and fluorescence wavelength of the coronene-like structures and the corresponding PAHs without sp3 carbon atoms. | ||
Additionally, shown as in Fig. 4, the emission wavelengths of all coronene-like structures are always longer than those of corresponding PAHs. The differences between two groups of fluorescence wavelengths are in the range of 11–36 nm. The result indicates that when the sp2 carbon domain keeps unchanged, the surrounding sp3 carbon atoms can cause a little fluorescence redshift of carbon cores. Combined with the analysis of molecular orbital pairs in Fig. 3, it is found that the influence of sp3 carbon atoms mainly originates from the hyperconjugative effect. This conclusion also can explain the small wavelength difference between the structures with the same content of sp2 carbon such as 1R-1 and 1R-2 shown as Fig. 2.
The conjugation length of π-conjugated system does not increase infinitely. When the conjugation length is short, the fluorescence wavelength and the conjugation length show a linear relationship.50 With the increase of sp2 carbon domain and conjugation length, the fluorescence wavelength gradually reaches a limit value, that is, the fluorescence emission no longer undergoes red shift.52,53 The longest conjugation length was defined as effective conjugation length.50,54 Based on the relationship between the fluorescence emission energy and the reciprocal of conjugation length 1/N, Kuhn fit and linear fit can be applied to obtain the effective conjugation length of conjugated polymers.50 Details about the Kuhn fit are in the ESI.† In order to further characterize the effective conjugation length of CDs, Fig. 5 gives that fluorescent emission energy versus 1/N of coronene-like structures and the corresponding PAHs. According to the analysis of electron–hole distribution in Table 2, and it is concluded that the electronic transition of coronene-like structures belongs to local excitation, suggesting that Kuhn fit is suitable to describe these systems. One can see that when the value of 1/N is greater than about 0.1, the fluorescence emission energies of all the structures display a linear relationship with 1/N, which further explains the linear relationship between the size of sp2 carbon domain and fluorescence wavelength observed in previous research works about CDs.42
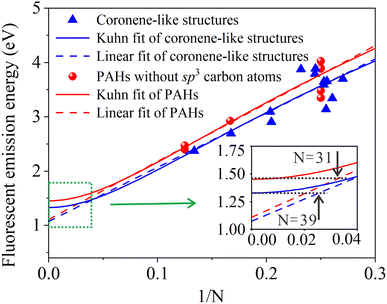 | ||
| Fig. 5 Fluorescent emission energy versus 1/N of coronene-like structures and corresponding PAHs. Curves: Kuhn fit. Dash lines: linear fit. | ||
Additionally, the present simulation in Fig. 5 shows that when 1/N gradually approaches 0, the fluorescence emission energy reaches a minimum value. The result tells us that with the increase of sp2 carbon domain, the fluorescence wavelength will reach a limit. For the coronene-like structures, the minimum fluorescence emission energy 1.33 eV can be obtained by Kuhn fit and linear fit, the effective conjugation length is 39, and the corresponding size of carbon core is 10.4 nm. For PAHs structures, the fitting minimum fluorescence emission energy is 1.45 eV, correspondingly, the effective conjugation length is 31 and the effective size of PAHs is 8.3 nm. The results are in good agreement with the fitting minimum emission energy 1.53 eV, the effective conjugation length 36, and the effective size 9.6 nm obtained from experimental data of PAHs,44 as shown in Fig. S3 in the ESI.† The results suggest that effective conjugation length can be helpful to predict the minimum fluorescence emission energy and effective size of carbon cores. Moreover, compared with PAHs and coronene-like structures, it is found that when sp2 carbon domains are surrounded by sp3 carbon, the fluorescence redshift can be induced, hence the emission energy of coronene-like structures is always less than that of PAHs structure. In a word, the conjugated length has a dominant effect on the fluorescence wavelength shift of carbon core, which helps to further analyze the relationship between the sp2 carbon domain and fluorescence spectrum.
4. Conclusions
In order to further understand the fluorescence characteristics of CDs, the coronene-like structures with various sp2-hybridized benzene rings and sp3 carbon network were constructed, and the electronic properties and fluorescence spectra were calculated by DFT and TD-DFT methods. The results revealed that when the content of sp2 domains in carbon cores is the same, the influence of sp3 carbon atoms is mainly reflected in the hyperconjugative effect and the separation of sp2 domain by sp3 carbon atoms, which causes a small fluorescence shift of carbon cores. The sp2 domains with zigzag edges have longer fluorescence wavelength compared with the ones with arm-chair edge due to lower aromatic stabilization.Analyzing the correlation between the conjugation length and fluorescence emission, it was found that the longer the conjugation length, the longer the fluorescence wavelength. Related to the size of sp2 carbon domains, the conjugation length is more convenient to predict the fluorescence emission shift trend of CDs. Moreover, based on the relationship between the fluorescence emission energy and 1/N, Kuhn fit and linear fit can be applied to predict the effective conjugation length and effective fluorescence size of CDs. This work provides a deeper understand about the influence of sp3 carbon atoms and the conjugation length of sp2 domain on the fluorescence properties of CDs.
Author contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was partly funded by the National Natural Science Foundation of China (No. 62075055, No. 62175075, No. 61974009), the Natural Science Foundation of Hebei Province (No. 2020201030), the Central Project Guide Local Science and Technology for Development of Hebei Province (226Z1103G, 216Z1104G), and the Interdisciplinary research project of Hebei University (No. DXK202012). We also appreciate High Performance Supercomputer Center of Hebei University.References
- S. Li, L. Li, H. Tu, H. Zhang, D. S. Silvester, C. E. Banks, G. Zou, H. Hou and X. Ji, Mater. Today, 2021, 51, 188–207 CrossRef CAS.
- J. Liu, R. Li and B. Yang, ACS Cent. Sci., 2020, 6, 2179–2195 CrossRef CAS PubMed.
- N. Baig, I. Kammakakam and W. Falath, Mater. Adv., 2021, 2, 1821–1871 RSC.
- J. Yu, X. Yong, Z. Tang, B. Yang and S. Lu, J. Phys. Chem. Lett., 2021, 12, 7671–7687 CrossRef CAS PubMed.
- I. S. Raja, S. J. Song, M. S. Kang, Y. B. Lee, B. Kim, S. W. Hong, S. J. Jeong, J. C. Lee and D. W. Han, Nanomaterials, 2019, 9, 1214 CrossRef CAS PubMed.
- J. Du, N. Xu, J. Fan, W. Sun and X. Peng, Small, 2019, 15, e1805087 CrossRef PubMed.
- S. Y. Lim, W. Shen and Z. Gao, Chem. Soc. Rev., 2015, 44, 362–381 RSC.
- G. Li, C. C. Lin, W.-T. Chen, M. S. Molokeev, V. V. Atuchin, C.-Y. Chiang, W. Zhou, C.-W. Wang, W.-H. Li, H.-S. Sheu, T.-S. Chan, C. Ma and R.-S. Liu, Chem. Mater., 2014, 26, 2991–3001 CrossRef CAS.
- J. Liao, M. Wang, F. Lin, Z. Han, B. Fu, D. Tu, X. Chen, B. Qiu and H. R. Wen, Nat. Commun., 2022, 13, 2090 CrossRef CAS PubMed.
- L. Wu, X. Tian, K. Deng, G. Liu and M. Yin, Opt. Mater., 2015, 45, 28–31 CrossRef CAS.
- H. Zheng, Z. Zhao, J. B. Phan, H. Ning, Q. Huang, R. Wang, J. Zhang and W. Chen, ACS Appl. Mater. Interfaces, 2020, 12, 2145–2151 CrossRef CAS PubMed.
- H. J. Yoo, B. E. Kwak and D. H. Kim, Carbon, 2021, 183, 560–570 CrossRef CAS.
- F. Yuan, Z. Wang, X. Li, Y. Li, Z. Tan, L. Fan and S. Yang, Adv. Mater., 2017, 29, 1604436 CrossRef PubMed.
- X. Miao, D. Qu, D. Yang, B. Nie, Y. Zhao, H. Fan and Z. Sun, Adv. Mater., 2018, 30, 1704740 CrossRef PubMed.
- H. Li, S. Han, B. Lyu, T. Hong, S. Zhi, L. Xu, F. Xue, L. Sai, J. Yang, X. Wang and B. He, Chin. Chem. Lett., 2021, 32, 2887–2892 CrossRef CAS.
- J. Deb, D. Paul and U. Sarkar, J. Phys. Chem. A, 2020, 124, 1312–1320 CrossRef CAS PubMed.
- J. E. Abraham and M. Balachandran, J. Fluoresc., 2022, 32, 887–906 CrossRef PubMed.
- E. A. Stepanidenko, I. A. Arefina, P. D. Khavlyuk, A. Dubavik, K. V. Bogdanov, D. P. Bondarenko, S. A. Cherevkov, E. V. Kundelev, A. V. Fedorov, A. V. Baranov, V. G. Maslov, E. V. Ushakova and A. L. Rogach, Nanoscale, 2020, 12, 602–609 RSC.
- B. Wang, J. Yu, L. Sui, S. Zhu, Z. Tang, B. Yang and S. Lu, Adv. Sci., 2020, 8, 2001453 CrossRef PubMed.
- P. Li, S. Xue, L. Sun, X. Zong, L. An, D. Qu, X. Wang and Z. Sun, Light: Sci. Appl., 2022, 11, 298 CrossRef CAS PubMed.
- H. Ding, X.-X. Zhou, J.-S. Wei, X.-B. Li, B.-T. Qin, X.-B. Chen and H.-M. Xiong, Carbon, 2020, 167, 322–344 CrossRef CAS.
- L. Jiang, H. Ding, M. Xu, X. Hu, S. Li, M. Zhang, Q. Zhang, Q. Wang, S. Lu, Y. Tian and H. Bi, Small, 2020, 16, 2000680 CrossRef CAS PubMed.
- Z. Sun, F. Yan, J. Xu, H. Zhang and L. Chen, Nano Res., 2021, 15, 414–422 CrossRef.
- E. V. Kundelev, N. V. Tepliakov, M. Y. Leonov, V. G. Maslov, A. V. Baranov, A. V. Fedorov, I. D. Rukhlenko and A. L. Rogach, J. Phys. Chem. Lett., 2020, 11, 8121–8127 CrossRef CAS PubMed.
- L. F. Melia, S. D. Barrionuevo and F. J. Ibañez, J. Chem. Educ., 2022, 99, 745–751 CrossRef CAS.
- M. Fu, F. Ehrat, Y. Wang, K. Z. Milowska, C. Reckmeier, A. L. Rogach, J. K. Stolarczyk, A. S. Urban and J. Feldmann, Nano Lett., 2015, 15, 6030–6035 CrossRef CAS PubMed.
- E. V. Kundelev, N. V. Tepliakov, M. Y. Leonov, V. G. Maslov, A. V. Baranov, A. V. Fedorov, I. D. Rukhlenko and A. L. Rogach, J. Phys. Chem. Lett., 2019, 10, 5111–5116 CrossRef CAS PubMed.
- X. Duan, Z. Ao, H. Zhang, M. Saunders, H. Sun, Z. Shao and S. Wang, Appl. Catal., B, 2018, 222, 176–181 CrossRef CAS.
- C. Hu, T. J. Lin, Y. C. Huang, Y. Y. Chen, K. H. Wang and K. Y. Andrew Lin, Environ. Res., 2021, 197, 111008 CrossRef CAS PubMed.
- M. Cao, X. Zhao and X. Gong, Small, 2022, 18, e2106683 CrossRef PubMed.
- W. Ren, J. Bai, T. Cai, S. Li, E. Pang, H. Zhang and Z. Li, Opt. Mater., 2021, 115, 111064 CrossRef CAS.
- S. Lu, G. Xiao, L. Sui, T. Feng, X. Yong, S. Zhu, B. Li, Z. Liu, B. Zou, M. Jin, J. S. Tse, H. Yan and B. Yang, Angew Chem. Int. Ed. Engl., 2017, 56, 6187–6191 CrossRef CAS PubMed.
- N. V. Tepliakov, E. V. Kundelev, P. D. Khavlyuk, Y. Xiong, M. Y. Leonov, W. Zhu, A. V. Baranov, A. V. Fedorov, A. L. Rogach and I. D. Rukhlenko, ACS Nano, 2019, 13, 10737–10744 CrossRef CAS PubMed.
- J. Xu, Q. Liang, Z. Li, V. Y. Osipov, Y. Lin, B. Ge, Q. Xu, J. Zhu and H. Bi, Adv. Mater., 2022, 34, e2200011 CrossRef PubMed.
- M. Frisch, G. Trucks, H. Schlegel, G. Scuseria, M. Robb, J. Cheeseman, G. Scalmani, V. Barone, G. Petersson and H. Nakatsuji, Gaussian 16, revision C.01, Gaussian Inc., Wallingford, CT, 2016 Search PubMed.
- J. D. Chai and M. Head-Gordon, Phys. Chem. Chem. Phys., 2008, 10, 6615–6620 RSC.
- F. Weigend and R. Ahlrichs, Phys. Chem. Chem. Phys., 2005, 7, 3297–3305 RSC.
- S. Miertuš, E. Scrocco and J. Tomasi, Chem. Phys., 1981, 55, 117–129 CrossRef.
- C. Adamo and D. Jacquemin, Chem. Soc. Rev., 2013, 42, 845–856 RSC.
- B. Shi, D. Nachtigallova, A. J. A. Aquino, F. B. C. Machado and H. Lischka, J. Phys. Chem. Lett., 2019, 10, 5592–5597 CrossRef CAS PubMed.
- T. Lu and F. Chen, J. Comput. Chem., 2012, 33, 580–592 CrossRef CAS PubMed.
- M. A. Sk, A. Ananthanarayanan, L. Huang, K. H. Lim and P. Chen, J. Mater. Chem. C, 2014, 2, 6954–6960 RSC.
- C. T. Chien, S. S. Li, W. J. Lai, Y. C. Yeh, H. A. Chen, I. S. Chen, L. C. Chen, K. H. Chen, T. Nemoto, S. Isoda, M. Chen, T. Fujita, G. Eda, H. Yamaguchi, M. Chhowalla and C. W. Chen, Angew Chem. Int. Ed. Engl., 2012, 51, 6662–6666 CrossRef CAS PubMed.
- R. Rieger and K. Müllen, J. Phys. Org. Chem., 2010, 23, 315–325 CrossRef CAS.
- Z. Liu, T. Lu and Q. Chen, Carbon, 2020, 165, 461–467 CrossRef CAS.
- T. Le Bahers, C. Adamo and I. Ciofini, J. Chem. Theory Comput., 2011, 7, 2498–2506 CrossRef CAS PubMed.
- M. Kasha, Discuss. Faraday Soc., 1950, 9, 14–19 RSC.
- U. J. Undiandeye, H. Louis, T. E. Gber, T. C. Egemonye, E. C. Agwamba, I. A. Undiandeye, A. S. Adeyinka and B. I. Ita, J. Indian Chem. Soc., 2022, 99, 100500 CrossRef CAS.
- Y. Zhang, C. Shen, X. Lu, X. Mu and P. Song, Spectrochim. Acta, Part A, 2020, 227, 117687 CrossRef CAS PubMed.
- J. Gierschner, J. Cornil and H. J. Egelhaaf, Adv. Mater., 2007, 19, 173–191 CrossRef CAS.
- B. Milián-Medina and J. Gierschner, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2012, 2, 513–524 Search PubMed.
- H. Okamoto, in Physics and Chemistry of Carbon-Based Materials, 2019, ch. 7, pp. 211–228, DOI:10.1007/978-981-13-3417-7_7.
- F. B. Mallory, K. E. Butler, A. C. Evans, E. J. Brondyke, C. W. Mallory, C. Yang and A. Ellenstein, J. Am. Chem. Soc., 1997, 119, 2119–2124 CrossRef CAS.
- H. Meier, U. Stalmach and H. Kolshorn, Acta Polym., 1997, 48, 379–384 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra05031a |
| This journal is © The Royal Society of Chemistry 2023 |