Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Ti3C2 MXenes-based catalysts for the process of α-pinene isomerization
Bartosz Środa *a,
Anna G. Dymerska
*a,
Anna G. Dymerska a,
Piotr Miądlicki
a,
Piotr Miądlicki b,
Agnieszka Wróblewska
b,
Agnieszka Wróblewska b and
Beata Zielińska
b and
Beata Zielińska a
a
aDepartment of Nanomaterials Physicochemistry, Faculty of Chemical Technology and Engineering, West Pomeranian University of Technology in Szczecin, Piastów Ave. 42, 71-065 Szczecin, Poland. E-mail: bartosz.sroda@zut.edu.pl
bDepartment of Catalytic and Sorbent Materials Engineering, Faculty of Chemical Technology and Engineering, West Pomeranian University of Technology in Szczecin, Piastów Ave. 42, 71-065 Szczecin, Poland
First published on 16th October 2023
Abstract
In this study, the catalytic performance of Ti3C2 MXene materials in the reaction of α-pinene isomerization was demonstrated. The influence of etching agents (HF, HF/H2SO4, and HF/HCl; weight ratios of mixed acids: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 1
3, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, and 1
4, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) on removing Al atoms from MAX phase, creation of an accordion-like structure typical for MXenes and catalytic activity of the produced samples have been revealed. The MXene HF obtained by MAX phase HF treatment exhibited the highest activity (conversion of α-pinene 74.65 mol%), while materials produced with the mixed acids (HF/H2SO4 and HF/HCl) showed a significant reduction in the conversion of α-pinene (on average about 28-fold). However, these last samples displayed an increase of about 10 mol% in the selectivity to the most desirable product-camphene. The high activity of MXene HF is a result of a high concentration of acid sites (11.62 mmol g−1) – the concentration of acid sites in the samples obtained by MAX phase mixed acids treatment was about 2.5–5.5 times smaller. This work proposes possible mechanisms for the α-pinene isomerization reaction on the MXene HF and on the MXene HF/H2SO4 X
5) on removing Al atoms from MAX phase, creation of an accordion-like structure typical for MXenes and catalytic activity of the produced samples have been revealed. The MXene HF obtained by MAX phase HF treatment exhibited the highest activity (conversion of α-pinene 74.65 mol%), while materials produced with the mixed acids (HF/H2SO4 and HF/HCl) showed a significant reduction in the conversion of α-pinene (on average about 28-fold). However, these last samples displayed an increase of about 10 mol% in the selectivity to the most desirable product-camphene. The high activity of MXene HF is a result of a high concentration of acid sites (11.62 mmol g−1) – the concentration of acid sites in the samples obtained by MAX phase mixed acids treatment was about 2.5–5.5 times smaller. This work proposes possible mechanisms for the α-pinene isomerization reaction on the MXene HF and on the MXene HF/H2SO4 X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Y and MXene HF/HCl X
Y and MXene HF/HCl X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Y in connection with their structure.
Y in connection with their structure.
1. Introduction
After the discovery of graphene in 2004, scientists tried to find other two-dimensional (2D) materials. In 2011, a novel class of 2D materials termed MXenes attracted great interest from researchers due to their outstanding properties and range of potential applications. The general equation of MXene is Mn+1XnTx, where M is an early transmission metal, X is carbon and/or nitrogen, n = 1, 2, 3 and Tx is a surface functional group (–F,![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, –OH). One of the most popular MXenes is titanium(IV) carbide (Ti3C2Tx), which resembles layers in its structure.1 Ti3C2Tx has been tested in multiple application places. For instance, they can be tested as microwave-absorbing materials due to their positive dielectric loss ability. Their excellent flexibility and large surface area make them great candidates mainly for catalytic processes and supercapacitors. Moreover, MXenes are potential candidates as electrocatalysts in hydrogen evolution reactions or water splitting. However, in conventional heterogeneous catalysis used in organic chemistry, MXene materials are currently barely investigated.2
O, –OH). One of the most popular MXenes is titanium(IV) carbide (Ti3C2Tx), which resembles layers in its structure.1 Ti3C2Tx has been tested in multiple application places. For instance, they can be tested as microwave-absorbing materials due to their positive dielectric loss ability. Their excellent flexibility and large surface area make them great candidates mainly for catalytic processes and supercapacitors. Moreover, MXenes are potential candidates as electrocatalysts in hydrogen evolution reactions or water splitting. However, in conventional heterogeneous catalysis used in organic chemistry, MXene materials are currently barely investigated.2
One of the directions of application of MXenes in heterogeneous catalysis may be the isomerization of α-pinene – the terpene compound of natural origin. Three main isomers of pinene occurring in nature were described: α-, β- and δ-pinene (each structural isomer has 2 enantiomeric forms (±)). Among these 3 isomers, α-pinene is the most abundant in nature. It has the smell of pine and is characterized by a fresh, forest aroma. The main source of α-pinene is turpentine, which is obtained from the resin of coniferous trees, mainly pine, by the steam distillation or gasoline extraction of pine stumps. α-Pinene is also found in essential oils obtained from pine, rosemary, cumin, thyme, basil, eucalyptus, and orange peels.3–5 α-Pinene is of great interest to scientists due to its biologically active properties (it is used as a fragrance in cosmetics, and in medicine for the treatment of respiratory infections or dissolution of kidney stones), as well as due to the possibility of obtaining derivatives of this terpene compound which also have numerous applications, including applications in medicine. The great advantage of α-pinene as the raw material for organic syntheses is that it is a relatively easily available and cheap raw material, and above all reproducible. This means that the syntheses carried out with the participation of α-pinene meet the principles of green chemistry and sustainable development. In addition, the method of isomerization of α-pinene proposed in this work does not require the use of a solvent, which is also beneficial for the environment and reduces the cost of this process.
The main α-pinene derivatives that are obtained in the isomerization process are camphene and limonene. Camphene is applied in the syntheses of many chemicals, for example, toxaphene (insecticide),6 and synthetic camphor, which is an important fragrance,7 and moreover, the reaction of camphene with guaiacol leads to valuable cyclohexanol products.8 We should also mention camphene applications in medicine, e.g. in the treatment of cancer.9 Limonene also found numerous applications in medicine, cosmetics, and organic synthesis. For example, the mixture of limonene, α-pinene, and 1,8-cineole shows a therapeutic activity similar to the standard antibiotics used in the treatment of respiratory diseases.10 The disproportionation reaction of limonene causes the formation of a mixture of p-cymene and p-menthane.11 Limonene is also converted to (−)-carvone, which is used as the peppermint flavor.12 Research is being conducted on the use of limonene as an additive for the production of plastics that will have contact with food (polyethylene, polystyrene, and polylactic acid).13 Other compounds which are formed in smaller amounts in the process of α-pinene isomerization (terpinolene, α-terpinene, γ-terpinene, and p-cymene) are commercially important compounds widely used in perfume, food, and organic industries and also in medicine.14
In the industrial isomerization of α-pinene, titanium oxide (TiO2) is used as the catalyst. The process is carried out at temperatures above 155 °C for 24 hours. The main products in this process are obtained: camphene, limonene, and tricyclene (the total selectivity of these compounds amounts to 70%).15 In addition, very good results present the work of Arata et al. They used TiO2 as a catalyst in the α-pinene isomerization process and achieved the conversion of this terpene compound amounted to 93%, at the temperature of 100 °C and for the reaction time of 2 hours but the main product of this process was trans-pinocarveol and its selectivity was 80%.16 The J. E. Sanchez-Velandia et al. obtained NT-TiO2-wc catalyst. NT-TiO2-wc was characterized by the conversion of α-pinene 99% and the selectivity of camphene 67%, for the reaction time amounted to 45 minutes.17 A major problem associated with the implementation of the process of isomerization of α-pinene on the industrial scale is the deactivation of the heterogeneous catalyst and the long reaction time. Therefore, the search for catalysts that would be characterized by a higher activity was started, which would shorten the reaction time, and at the same time, their use may allow camphene to be obtained with greater selectivity. These new catalysts should be catalysts that can be used many times in the isomerization process and also these materials should be easily regenerated – examples of such catalysts may be zeolite catalysts (natural origin or synthesized in a laboratory) or materials with a structure similar to the structure of zeolites, which are durable and relatively easy to regenerate. Studies related to the search for new, active catalysts for the α-pinene isomerization process were carried out, among others, with the following heterogeneous catalysts: Ga-SBA-15,18 PW/SBA-15,19 Ga-MCM-41,20 SO4/ZrO4/HMS,21 ferrite-type zeolites,22 acid treated natural zeolites,23 TCA/Y-zeolite,24 heat-treated natural zeolites25 natural clays.26 Very good results were obtained by conducting the studies on the natural zeolite–clinoptilolite, which was modified by washing with sulfuric acid solutions of appropriate concentrations (0.01–2 M). In this case, the conducting of the process for a very short time – 4 minutes and at the temperature of 70 °C, allowed for the complete conversion of α-pinene, and the selectivity of camphene and limonene amounted to 50 mol% and 30 mol%, respectively.27
In our previous study,28 multilayered and exfoliated Ti3C2 MXenes are proposed as a new class of catalysts for the reaction of α-pinene isomerization. Here, 100 mol% conversion of α-pinene in 7 h of process and the selectivity resulting in ∼60 mol% toward camphene formation was revealed. For that time, it was the highest selectivity and conversion reported in the current state of the art. Such promising results motivated us to continue research on Ti3C2Tx MXenes as catalysts in conventional heterogeneous catalysis, especially in the process of α-pinene isomerization. In the present work, Ti3C2Tx MXenes were synthesized by removing aluminum from MAX phase by etching in a mineral acids mixture (HF/HCl and HF/H2SO4). Here, the influence of HF/HCl and HF/H2SO4 weight ratios (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 1
3, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, and 1
4, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) on the physicochemical properties and catalytic activity in the isomerization of α-pinene of the produced samples was studied. The obtained results were compared to the sample synthesized by etching Al from MAX phase with 48% hydrofluoric acid.
5) on the physicochemical properties and catalytic activity in the isomerization of α-pinene of the produced samples was studied. The obtained results were compared to the sample synthesized by etching Al from MAX phase with 48% hydrofluoric acid.
2. Experimental section
2.1. Materials
All chemicals used in this work: MAX phase (titanium aluminum carbide, Ti3AlC2, 99% purity, American Elements), hydrofluoric acid (HF, 48% concentration, Sigma-Aldrich), hydrochloric acid (HCl, 35–38% concentration, Sigma-Aldrich), sulfuric acid(VI) (H2SO4, 98% concentration, Sigma-Aldrich), ethanol (EtOH, 96% purity, Sigma-Aldrich) and α-pinene (98%, Sigma-Aldrich) were used as received, without further purification.2.2. Synthesis of multilayered Ti3C2Tx MXenes
Multilayered Ti3C2Tx MXenes were produced via acidic etching aluminum from MAX phase (Ti3C2–Al–Ti3C2–Al–Ti3C2). Here, two different etching agents, HF/HCl and HF/H2SO4 with different weight ratios (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 1
3, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, and 1
4, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5), were used for removing Al atoms from MAX phase. The details of the etching solutions composition and MAX phase quantity are presented in Table 1. Briefly, MAX phase was carefully added to an appropriate mixture of acids and stirred for 24 h at room temperature (RT). After that, the obtained mixture was diluted with deionized water, and the precipitate was separated via centrifugation. The obtained product was washed with deionized water until the pH was ∼7. Finally, the received sample was dried overnight at 50 °C and stored at 5 °C for further analysis.28 The products were referred to as MXene HF/HCl X
5), were used for removing Al atoms from MAX phase. The details of the etching solutions composition and MAX phase quantity are presented in Table 1. Briefly, MAX phase was carefully added to an appropriate mixture of acids and stirred for 24 h at room temperature (RT). After that, the obtained mixture was diluted with deionized water, and the precipitate was separated via centrifugation. The obtained product was washed with deionized water until the pH was ∼7. Finally, the received sample was dried overnight at 50 °C and stored at 5 °C for further analysis.28 The products were referred to as MXene HF/HCl X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Y and MXene HF/H2SO4 X
Y and MXene HF/H2SO4 X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Y, where X
Y, where X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Y means the acids weight ratios. Moreover, a reference sample of Ti3C2Tx MXene was also synthesized according to the same procedure as described above but using 48% HF for etching of Al form Ti3AlC2 (see Table 1). The sample was labeled as MXene HF.
Y means the acids weight ratios. Moreover, a reference sample of Ti3C2Tx MXene was also synthesized according to the same procedure as described above but using 48% HF for etching of Al form Ti3AlC2 (see Table 1). The sample was labeled as MXene HF.
| Sample | HF [mL] | HCl [mL] | H2SO4 [mL] | MAX phase [mg] | Quantity of product [mg] |
|---|---|---|---|---|---|
| MXene HF | 10 | — | — | 590 | 383 |
MXene HF/HCl 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
2 | 5.68 | — | 453 | 312 |
MXene HF/HCl 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
2 | 7.70 | — | 572 | 391 |
MXene HF/HCl 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
2 | 9.48 | — | 677 | 484 |
MXene HF/H2SO4 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
2 | — | 3.70 | 336 | 247 |
MXene HF/H2SO4 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
2 | — | 4.92 | 408 | 295 |
MXene HF/H2SO4 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
2 | — | 6.16 | 481 | 388 |
2.3. Characterization
The morphology of obtained MXenes was characterized via scanning electron microscopy (VEGA3 Tescan). The crystallographic structure of samples was examined via X-ray diffraction (XRD), performed using an AERIS PANalytical X-ray diffractometer with Cu-Kα radiation. Raman spectroscopy (inVia Renishaw; λ = 785 nm) was used for the MXene vibrational signal. Thermogravimetric analysis (TGA) was performed in the temperature range between RT and 1400 °C, with a 10 °C min−1 heating ramp and 100 cm−3 min−1 airflow on an SDT Q600 thermal analyzer. To determine the specific surface area (SSA) and pore size distribution, we utilized the Brunauer–Emmett–Teller (BET) method, which involved conducting N2 sorption measurements following a preliminary sample degassing process using the Micrometrics ASAP 2460 system. The X-ray spectroscopy (XPS) measurements were conducted using Mg Kα (hν = 1253.6 eV) radiation in a Prevac (Poland) system equipped with a Scienta SES 2002 (Sweden) electron energy analyzer operating with constant transmission energy (Ep = 50 eV). The analysis chamber was evacuated to a pressure below 5 × 10−9 mbar.The acid site concentration (As) of the samples was measured using the procedure described by Vilcocq et al.29 Firstly, 20 mg of the appropriate sample was added into 10 cm3 of 0.01 M NaOH solution and mixed at RT for 4 h. Next, the dispersion was filtered off and the pH of the filtrate was determined by titration using 0.01 M HCl in the presence of phenolphthalein as an indicator. The acid sites concentration was calculated from the formula (1) below:
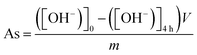 | (1) |
2.4. Isomerization of α-pinene
In the studies on the activity of MXene materials in the isomerization of α-pinene, a 10 cm3 glass reactor with a reflux condenser and magnetic stirrer with heating were used. The reaction mixture consisted of 1 g of α-pinene and 0.05 g of the appropriate catalyst (catalyst content in the reaction mixture amounted to 5 wt%). The reactor was placed in an oil bath and the reaction mixture was stirred at 500 rpm. The isomerization of α-pinene was performed at the temperature of 160 °C and for the reaction time of 6 h.For analysis of the composition of post-reaction mixtures, each post-reaction mixture was centrifuged and the post-reaction solution was dissolved in acetone with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 weight ratio. Qualitative analyses of the post-reaction solutions were performed using a GC-MS method with a ThermoQuest apparatus and a DB-5 column (30 m × 0.25 mm × 0.5 μm). The conditions of analyses were as follows: a helium flow rate of 1 mL min−1, a sample chamber temperature of 200 °C, and a detector temperature of 250 °C. The furnace temperature was held at 50 °C for 2.5 minutes and then increased to 300 °C at a rate of 10 °C min−1.
3 weight ratio. Qualitative analyses of the post-reaction solutions were performed using a GC-MS method with a ThermoQuest apparatus and a DB-5 column (30 m × 0.25 mm × 0.5 μm). The conditions of analyses were as follows: a helium flow rate of 1 mL min−1, a sample chamber temperature of 200 °C, and a detector temperature of 250 °C. The furnace temperature was held at 50 °C for 2.5 minutes and then increased to 300 °C at a rate of 10 °C min−1.
Quantitative analyses were performed using a Thermo Electron FOCUS chromatograph and a ZB-1701 column (30 m × 0.53 mm × 1 μm). The conditions of analyses were as follows: the helium flow rate of 1 mL min−1, the sample chamber temperature of 220 °C, and the detector temperature of 250 °C. The furnace temperature was held at 50 °C for 2 minutes, next it was increased to 100 °C at the rate of 5 °C min−1, and finally the temperature was increased to 200 °C at the rate of 10 °C min−1.
The composition of the post-reaction solutions was determined using the internal normalization method, and the mass balance was used to calculate the conversion of α-pinene and appropriate product selectivity (camphene, limonene, α-terpinene, γ-terpinene, terpinolene, tricyclene, and p-cymene). The summary selectivity of other products, including small amounts of fenchene, polymeric compounds, and oxidation products, was also calculated.
2.5. Results and discussion
SEM images of MAX phase, MXene HF, MXene HF/H2SO4 X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Y, and MXene HF/HCl X
Y, and MXene HF/HCl X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Y are presented in Fig. 1A–I. MAX phase (Fig. 1A) is composed of Ti3AlC2 particles with irregular shapes. The reference MXene HF (Fig. 1B and C) presents a layered structure with clearly marked spacing between nanosheets. Here, a clear difference between the samples obtained after mixed acids (HF/H2SO4 and HF/HCl) and HF treatment is observed. MXene HF/H2SO4 X
Y are presented in Fig. 1A–I. MAX phase (Fig. 1A) is composed of Ti3AlC2 particles with irregular shapes. The reference MXene HF (Fig. 1B and C) presents a layered structure with clearly marked spacing between nanosheets. Here, a clear difference between the samples obtained after mixed acids (HF/H2SO4 and HF/HCl) and HF treatment is observed. MXene HF/H2SO4 X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Y samples (Fig. 1D–F) do not show layered structures characteristic of MXenes. Their morphology is similar to the MAX phase (Fig. 1A). Whereas, MXene HF/HCl X
Y samples (Fig. 1D–F) do not show layered structures characteristic of MXenes. Their morphology is similar to the MAX phase (Fig. 1A). Whereas, MXene HF/HCl X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Y (Fig. 1G–I) exhibit a lamellar structure without clearly marked spacing between the nanosheets. It indicates that using a mixture of HF with HCl or H2SO4 with studied weight ratios for removing Al form Ti3AlC2 does not cause the creation of accordion-like structure characteristics for MXenes.
Y (Fig. 1G–I) exhibit a lamellar structure without clearly marked spacing between the nanosheets. It indicates that using a mixture of HF with HCl or H2SO4 with studied weight ratios for removing Al form Ti3AlC2 does not cause the creation of accordion-like structure characteristics for MXenes.
 | ||
Fig. 1 SEM images of MAX phase (A), MXene HF (B and C), MXene HF/H2SO4 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (D), MXene HF/H2SO4 1 3 (D), MXene HF/H2SO4 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (E), MXene HF/H2SO4 1 4 (E), MXene HF/H2SO4 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 (F). MXene HF/HCl 1 5 (F). MXene HF/HCl 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (G), MXene HF/HCl 1 3 (G), MXene HF/HCl 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (H), MXene HF/HCl 1 4 (H), MXene HF/HCl 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 (I). 5 (I). | ||
Fig. 2 presents XRD patterns of MAX phase, MXene HF, MXene HF/H2SO4 X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Y, and MXene HF/HCl X
Y, and MXene HF/HCl X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Y. The reference MXene HF exhibits reflections at 9.0, 18.2, 27.7, 37.2, 40.7, and 60.7°, typical for Ti3C2.30,31 All MXene HF/H2SO4 X
Y. The reference MXene HF exhibits reflections at 9.0, 18.2, 27.7, 37.2, 40.7, and 60.7°, typical for Ti3C2.30,31 All MXene HF/H2SO4 X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Y do not show characteristic reflections of Ti3C2. Here, peaks located at 9.5, 19.1, 33.9, 36.7, 38.8, 41.7, 48.3, 56.3 and 60.1° related to MAX phase are observed (see pattern of MAX phase). It is also seen that MXene HF/H2SO4 X
Y do not show characteristic reflections of Ti3C2. Here, peaks located at 9.5, 19.1, 33.9, 36.7, 38.8, 41.7, 48.3, 56.3 and 60.1° related to MAX phase are observed (see pattern of MAX phase). It is also seen that MXene HF/H2SO4 X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Y exhibit lower intensity peaks than the MAX phase, which indicates that the structure of the MAX phase has been harmed by the reaction with the acids mixture. The materials synthesized via removing Al from MAX phase by etching in HF/HCl mixture (MXene HF/HCl X
Y exhibit lower intensity peaks than the MAX phase, which indicates that the structure of the MAX phase has been harmed by the reaction with the acids mixture. The materials synthesized via removing Al from MAX phase by etching in HF/HCl mixture (MXene HF/HCl X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Y) show several reflections at 7.7, 18.1, 26.9, 35.4, 40.2, 61.0° characteristics of Ti3C2 MXene. However, considering MXene HF/HCl X
Y) show several reflections at 7.7, 18.1, 26.9, 35.4, 40.2, 61.0° characteristics of Ti3C2 MXene. However, considering MXene HF/HCl X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Y samples with reference MXene HF, a clear shift of all peaks toward lower angles is detected. It is due to the presence of residual MAX phase in the samples.32,33 As the concentration of HF in the HF/HCl mixture decreases, the intensity of peaks derived from Ti3C2 also decreases, which indicates that the MXenes have not fully developed and give a weaker signal with the same measurement parameters.34
Y samples with reference MXene HF, a clear shift of all peaks toward lower angles is detected. It is due to the presence of residual MAX phase in the samples.32,33 As the concentration of HF in the HF/HCl mixture decreases, the intensity of peaks derived from Ti3C2 also decreases, which indicates that the MXenes have not fully developed and give a weaker signal with the same measurement parameters.34
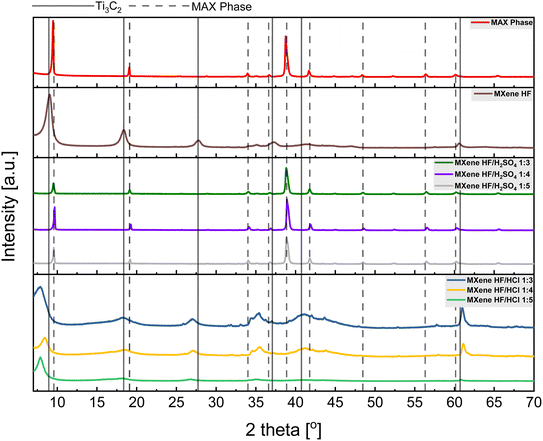 | ||
Fig. 2 XRD patterns of MAX phase, MXene HF, MXenes HF/H2SO4 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 1 3, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, 1 4, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 and MXenes HF/HCl 1 5 and MXenes HF/HCl 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 1 3, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, 1 4, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5. 5. | ||
Raman spectra of MAX phase, MXene HF, MXene HF/H2SO4 X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Y and MXene HF/HCl X
Y and MXene HF/HCl X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Y are shown in Fig. 3. The spectrum of MAX phase shows four peaks at ∼201, 269, 631 and 662 cm−1, which correspond to Ti–Al vibrations.35 MXene HF exhibits five sharp modes located at ∼126, 207, 364, 621 and 716 cm−1. The modes observed at ∼126, 364, 621 and 716 cm−1 are related to carbon and titanium vibrations. The peak located at ∼207 and ∼716 cm−1 indicates proper removal of Al from precursor (MAX phase).36 The spectra of MXene HF/H2SO4 X
Y are shown in Fig. 3. The spectrum of MAX phase shows four peaks at ∼201, 269, 631 and 662 cm−1, which correspond to Ti–Al vibrations.35 MXene HF exhibits five sharp modes located at ∼126, 207, 364, 621 and 716 cm−1. The modes observed at ∼126, 364, 621 and 716 cm−1 are related to carbon and titanium vibrations. The peak located at ∼207 and ∼716 cm−1 indicates proper removal of Al from precursor (MAX phase).36 The spectra of MXene HF/H2SO4 X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Y present modes for both MAX phase and MXene. All MXene HF/H2SO4 X
Y present modes for both MAX phase and MXene. All MXene HF/H2SO4 X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Y show four peaks at ∼123, 202, 367, and 733 cm−1, as in the case of MXene HF sample. Moreover, the modes located at ∼202 and ∼733 cm−1 are the results of the partial removal of the Al from the precursor (MAX phase).37 MXene HF/HCl X
Y show four peaks at ∼123, 202, 367, and 733 cm−1, as in the case of MXene HF sample. Moreover, the modes located at ∼202 and ∼733 cm−1 are the results of the partial removal of the Al from the precursor (MAX phase).37 MXene HF/HCl X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Y exhibit four peaks at ∼121, 201, 363 and 725 cm−1, characteristic for Ti3C2 MXene. Here, it is also seen that all modes have shifted values in respect to MXene HF, which means, not fully formed MXene structure.38 The results from Raman are consistent with the results from SEM and XRD.
Y exhibit four peaks at ∼121, 201, 363 and 725 cm−1, characteristic for Ti3C2 MXene. Here, it is also seen that all modes have shifted values in respect to MXene HF, which means, not fully formed MXene structure.38 The results from Raman are consistent with the results from SEM and XRD.
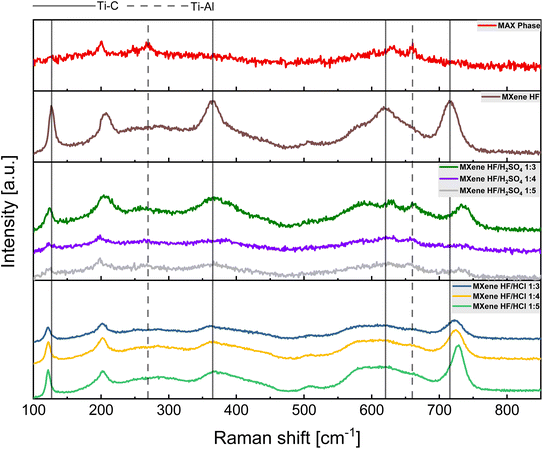 | ||
Fig. 3 Raman spectra of MAX phase, MXene HF, MXenes HF/H2SO4 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 1 3, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, 1 4, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 and MXenes HF/HCl 1 5 and MXenes HF/HCl 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 1 3, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, 1 4, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5. 5. | ||
The TGA results of MAX phase, MXene HF, MXene HF/H2SO4 X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Y, and MXene HF/HCl X
Y, and MXene HF/HCl X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Y are presented in Fig. 4. For MAX phase the total weight increase of ∼48.7 wt% is observed. Here, two significant steps of mass increase are detected in the TGA curve. The first one (∼307–624 °C; ∼9.1 wt%) represents the oxidation of titanium (formation of TiO2), and the second one (∼658–971 °C; ∼27.5 wt%) represents the oxidation of aluminum (formation of Al2O3). After heating from ∼1000 to 1400 °C aluminum titanium oxide (AlTiO) is formed.39,40 MXene HF shows a significant mass increase at ∼310 °C (∼7.4 wt%) followed by a clear mass decrease (∼378–942 °C; ∼12.8 wt%). At ∼1000 °C, the stabilization of the mass of the sample is observed. These mass changes are associated with a parallel process of oxidation of Ti3C2 and detachment of MXene HF surface functional groups. The total MXene HF mass decreased up to −5.7 wt%, which is in agreement with the literature.41 For MXene HF/H2SO4 X
Y are presented in Fig. 4. For MAX phase the total weight increase of ∼48.7 wt% is observed. Here, two significant steps of mass increase are detected in the TGA curve. The first one (∼307–624 °C; ∼9.1 wt%) represents the oxidation of titanium (formation of TiO2), and the second one (∼658–971 °C; ∼27.5 wt%) represents the oxidation of aluminum (formation of Al2O3). After heating from ∼1000 to 1400 °C aluminum titanium oxide (AlTiO) is formed.39,40 MXene HF shows a significant mass increase at ∼310 °C (∼7.4 wt%) followed by a clear mass decrease (∼378–942 °C; ∼12.8 wt%). At ∼1000 °C, the stabilization of the mass of the sample is observed. These mass changes are associated with a parallel process of oxidation of Ti3C2 and detachment of MXene HF surface functional groups. The total MXene HF mass decreased up to −5.7 wt%, which is in agreement with the literature.41 For MXene HF/H2SO4 X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Y two steps of mass increase, like to MAX phase, are detected. For MXene HF/H2SO4 1
Y two steps of mass increase, like to MAX phase, are detected. For MXene HF/H2SO4 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, MXene HF/H2SO4 1
5, MXene HF/H2SO4 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, and MXene HF/H2SO4 1
4, and MXene HF/H2SO4 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 the final mass increases are ∼42.5, 41.4, and 40.6 wt%, which is lower by 6.2, 7.3 and 8.1%, respectively, than for MAX phase. MXene HF/HCl X
3 the final mass increases are ∼42.5, 41.4, and 40.6 wt%, which is lower by 6.2, 7.3 and 8.1%, respectively, than for MAX phase. MXene HF/HCl X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Y samples display a significant mass increase, up to ∼624 °C (∼17.9 wt%) manifests oxidation of MXene.42 Next, the clear mass loss (up to ∼971 °C) corresponds to the decomposition of MXenes.41 The final mass increased by ∼4.1, 6.2, and 11.0 wt% for MXene HF/HCl 1
Y samples display a significant mass increase, up to ∼624 °C (∼17.9 wt%) manifests oxidation of MXene.42 Next, the clear mass loss (up to ∼971 °C) corresponds to the decomposition of MXenes.41 The final mass increased by ∼4.1, 6.2, and 11.0 wt% for MXene HF/HCl 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, MXene HF/HCl 1
3, MXene HF/HCl 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, MXene HF/HCl 1
4, MXene HF/HCl 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, respectively. Moreover, to identify the product produced after TGA measurement, the ash of MAX phase, MXene HF, MXene HF/HCl 1
5, respectively. Moreover, to identify the product produced after TGA measurement, the ash of MAX phase, MXene HF, MXene HF/HCl 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 and MXene HF/H2SO4 1
3 and MXene HF/H2SO4 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
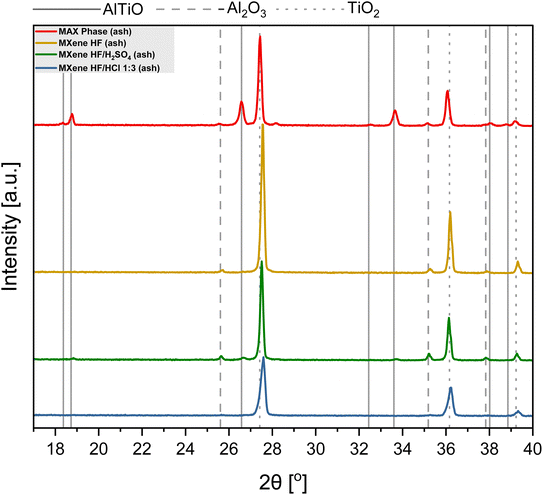
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 was examined via XRD (Fig. 5). The ash obtained after MAX phase thermal treatment is a mixture of AlTiO (18.3, 18.7, 26.6, 28.2, 32.5, 33.6, 38.0, and 38.8°; card no: 01-076-8799), Al2O3 (25.6, 35.1, and 37.8°; card no: 01-088-4954) and TiO2 (27.4, 36.0, and 39.2°; card no: 01-083-3673). The ash of MXene HF exhibits characteristic reflections of TiO2. Additionally, a trace amount of Al2O3 is also detected. XRD pattern of MXene HF/H2SO4 1
3 was examined via XRD (Fig. 5). The ash obtained after MAX phase thermal treatment is a mixture of AlTiO (18.3, 18.7, 26.6, 28.2, 32.5, 33.6, 38.0, and 38.8°; card no: 01-076-8799), Al2O3 (25.6, 35.1, and 37.8°; card no: 01-088-4954) and TiO2 (27.4, 36.0, and 39.2°; card no: 01-083-3673). The ash of MXene HF exhibits characteristic reflections of TiO2. Additionally, a trace amount of Al2O3 is also detected. XRD pattern of MXene HF/H2SO4 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 after TGA shows reflections corresponding to three phases such as TiO2, AlTiO, and Al2O3. Moreover, it is also seen that the intensity of AlTiO and Al2O3 reflections is much lower in comparison to that presented in MAX phase after TGA. It means that partial etching of Al from MAX phase occurred in HF/H2SO4. MXene HF/HCl 1
3 after TGA shows reflections corresponding to three phases such as TiO2, AlTiO, and Al2O3. Moreover, it is also seen that the intensity of AlTiO and Al2O3 reflections is much lower in comparison to that presented in MAX phase after TGA. It means that partial etching of Al from MAX phase occurred in HF/H2SO4. MXene HF/HCl 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 after the TGA shows reflections at 27.4, 36.0, and 39.2° corresponding to TiO2 (card no.: 01-083-3673). There is also trace amount of Al2O3 in the ash of MXene HF/HCl 1
3 after the TGA shows reflections at 27.4, 36.0, and 39.2° corresponding to TiO2 (card no.: 01-083-3673). There is also trace amount of Al2O3 in the ash of MXene HF/HCl 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.
3.
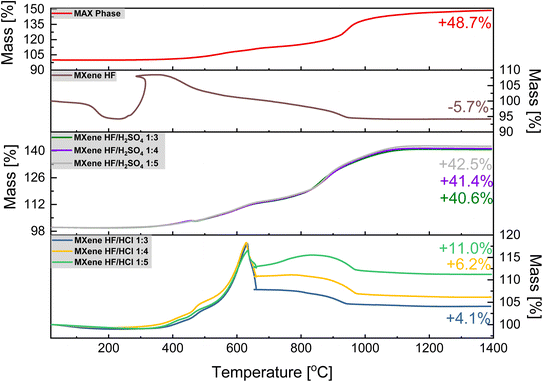 | ||
Fig. 4 TGA curves of MAX phase, MXene HF, MXenes HF/H2SO4 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 1 3, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, 1 4, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 and MXenes HF/HCl 1 5 and MXenes HF/HCl 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 1 3, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, 1 4, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5. 5. | ||
The N2 adsorption/desorption isotherms of MAX phase, MXene HF, MXene HF/H2SO4 X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Y, and MXene HF/HCl X
Y, and MXene HF/HCl X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Y conducted at 77 K are presented in Fig. 6. All studied materials exhibited an IV-type isotherm with H3-type hysteresis loop, indicating multimolecular adsorption.43 The specific surface area (SSA) of the samples was calculated using Brunauer–Emmett–Teller (BET) theory (Table 2). The SSA of MXene HF is 2.74 m2 g−1. For MXene HF/H2SO4 1
Y conducted at 77 K are presented in Fig. 6. All studied materials exhibited an IV-type isotherm with H3-type hysteresis loop, indicating multimolecular adsorption.43 The specific surface area (SSA) of the samples was calculated using Brunauer–Emmett–Teller (BET) theory (Table 2). The SSA of MXene HF is 2.74 m2 g−1. For MXene HF/H2SO4 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3; 1
3; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 and 1
4 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, SSA is 2.64, 6.04, and 6.37 m2 g−1, respectively. The SSA of MXene HF/HCl X
5, SSA is 2.64, 6.04, and 6.37 m2 g−1, respectively. The SSA of MXene HF/HCl X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Y is 0.62 (1
Y is 0.62 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), 1.66 (1
3), 1.66 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) and 1.91 (1
4) and 1.91 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) m2 g−1. The specific surface area of the materials produced by HF/HCl etching is significantly lower than that obtained after HF and HF/H2SO4 treatment. MXene HF/H2SO4 1
5) m2 g−1. The specific surface area of the materials produced by HF/HCl etching is significantly lower than that obtained after HF and HF/H2SO4 treatment. MXene HF/H2SO4 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 exhibits the highest BET-SSA (6.37 m2 g−1) compared to all other samples.
5 exhibits the highest BET-SSA (6.37 m2 g−1) compared to all other samples.
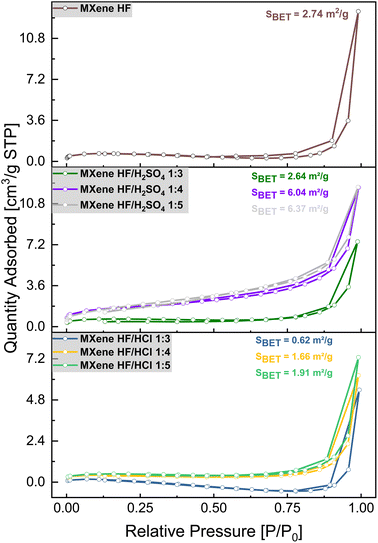 | ||
Fig. 6 Nitrogen adsorption/desorption isotherms of MXene HF, MXene HF/H2SO4 X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Y and MXene HF/HCl X Y and MXene HF/HCl X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Y. Y. | ||
| Sample | Acid sites concentration [mmol g−1] | Specific surface area [m2 g−1] |
|---|---|---|
| MXene HF | 11.62 | 2.74 |
MXene HF/H2SO4 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
2.21 | 2.64 |
MXene HF/H2SO4 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
3.05 | 6.04 |
MXene HF/H2SO4 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
3.12 | 6.37 |
MXene HF/HCl 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
4.74 | 0.62 |
MXene HF/HCl 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
4.65 | 1.66 |
MXene HF/HCl 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
4.50 | 1.91 |
The acid site concentration (As) of MXene HF, MXene HF/H2SO4 X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Y, and MXene HF/HCl X
Y, and MXene HF/HCl X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Y is presented in Table 2. The reference MXene HF shows As of 11.62 mmol g−1. For both MXene HF/HCl X
Y is presented in Table 2. The reference MXene HF shows As of 11.62 mmol g−1. For both MXene HF/HCl X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Y and MXene HF/H2SO4 X
Y and MXene HF/H2SO4 X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Y. As values are significantly lower in comparison to MXene HF. For the samples produced via HF/HCl etching, the value of As is kept in the following order: MXene HF/HCl 1
Y. As values are significantly lower in comparison to MXene HF. For the samples produced via HF/HCl etching, the value of As is kept in the following order: MXene HF/HCl 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (4.74 mmol g−1) > MXene HF/HCl 1
3 (4.74 mmol g−1) > MXene HF/HCl 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (4.65 mmol g−1) > MXene HF/HCl 1
4 (4.65 mmol g−1) > MXene HF/HCl 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 (4.50 mmol g−1). It means that the higher HF content in the HF/HCl mixture the higher concentration of acid sites. The acid site concentration for MXene HF/H2SO4 1
5 (4.50 mmol g−1). It means that the higher HF content in the HF/HCl mixture the higher concentration of acid sites. The acid site concentration for MXene HF/H2SO4 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 1
3, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 and 1
4 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 was 2.21, 3.05 and 3.12 mmol g−1, respectively. Here, an opposite correlation for MXene HF/HCl X
5 was 2.21, 3.05 and 3.12 mmol g−1, respectively. Here, an opposite correlation for MXene HF/HCl X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Y was observed, along with the lower content of HF in HF/H2SO4 mixture, the higher concentration of acid sites is detected.
Y was observed, along with the lower content of HF in HF/H2SO4 mixture, the higher concentration of acid sites is detected.
In the next stage of the studies, the catalytic activity of MXene HF, MXene HF/H2SO4 X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Y, and MXene HF/HCl X
Y, and MXene HF/HCl X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Y in the reaction of isomerization of α-pinene was examined. The obtained results are presented in Fig. 7.
Y in the reaction of isomerization of α-pinene was examined. The obtained results are presented in Fig. 7.
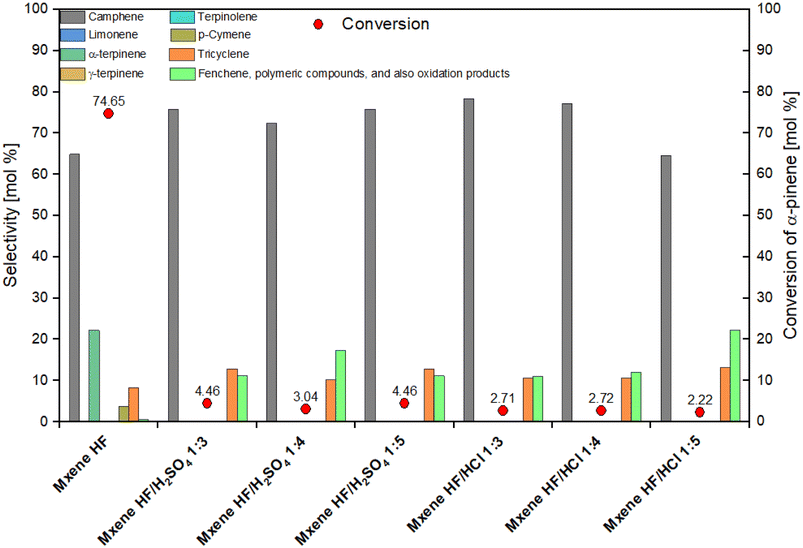 | ||
Fig. 7 Results of the catalytic tests for the isomerization of α-pinene for MXene HF, MXene HF/H2SO4 X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Y, and MXene HF/HCl X Y, and MXene HF/HCl X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Y. Y. | ||
It is seen from Fig. 7 that taking into account the value of conversion of α-pinene the highest activity showed MXene HF (conversion of α-pinene amounted to 74.65 mol%). All MXene HF/HCl X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Y and MXene HF/H2SO4 X
Y and MXene HF/H2SO4 X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Y showed a significant, approximately 28-fold, decrease in activity. At the same time, the samples of MXene HF/H2SO4 X
Y showed a significant, approximately 28-fold, decrease in activity. At the same time, the samples of MXene HF/H2SO4 X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Y were characterized by a slightly higher activity than the materials of MXene HF/HCl X
Y were characterized by a slightly higher activity than the materials of MXene HF/HCl X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Y. The conversion of α-pinene is 4,46, 3.04 and 4.46 mol% for MXene HF/H2SO4 1
Y. The conversion of α-pinene is 4,46, 3.04 and 4.46 mol% for MXene HF/H2SO4 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, MXene HF/H2SO4 1
3, MXene HF/H2SO4 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, and MXene HF/H2SO4 1
4, and MXene HF/H2SO4 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, respectively. On the other hand, the increase of about 8–10 mol% in the selectivity of transformation of α-pinene to camphene and also the increase of about 2–4 mol% in the selectivity of transformation to tricyclene was detected for the samples synthesized by the treatment with the mixtures of 2 acids. In particular, the increase in selectivity of the transformation to the first of these compounds is advantageous as camphene is the preferred product of this reaction. It should also be noted that only in the isomerization of α-pinene performed on MXene HF, α-terpinene (selectivity 22.27 mol%) and p-cymene (selectivity 3.88 mol%) were formed. Moreover, a significant, 20 to 40-fold increase in the selectivity of α-pinene transformation to other products was observed on catalysts which were obtained by the treatment with the mixtures of 2 acids. Other products can be such products as fenchene, polymeric compounds, and also oxidation products. The highest increase in the amount of these compounds was noted for the MXene HF/HCl 1
5, respectively. On the other hand, the increase of about 8–10 mol% in the selectivity of transformation of α-pinene to camphene and also the increase of about 2–4 mol% in the selectivity of transformation to tricyclene was detected for the samples synthesized by the treatment with the mixtures of 2 acids. In particular, the increase in selectivity of the transformation to the first of these compounds is advantageous as camphene is the preferred product of this reaction. It should also be noted that only in the isomerization of α-pinene performed on MXene HF, α-terpinene (selectivity 22.27 mol%) and p-cymene (selectivity 3.88 mol%) were formed. Moreover, a significant, 20 to 40-fold increase in the selectivity of α-pinene transformation to other products was observed on catalysts which were obtained by the treatment with the mixtures of 2 acids. Other products can be such products as fenchene, polymeric compounds, and also oxidation products. The highest increase in the amount of these compounds was noted for the MXene HF/HCl 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 (more than 40 times). It is also visible that the lowest selectivity of transformation to camphene was simultaneously obtained on this sample (64.62 mol%) – this is the value comparable to the value obtained on the MXene HF.
5 (more than 40 times). It is also visible that the lowest selectivity of transformation to camphene was simultaneously obtained on this sample (64.62 mol%) – this is the value comparable to the value obtained on the MXene HF.
The above-mentioned observations can be explained by taking into account the structure of MXene HF sample and the samples of catalysts obtained by the treatment with the mixture of 2 acids. For MXene HF, which is characterized by the accordion-like structure with clearly marked spacing between layers, the formation of bicyclic products: camphene and tricyclene, and monocyclic products: α-terpinene and p-cymene, was characteristic. A slight selectivity of the transformation to “other products” (fenchene, polymeric compounds, and also oxidation products) was also observed for MXene HF. In the case of the catalysts obtained by the treatment with the mixture of 2 acids, we observe the formation of only bicyclic products and the increase in the amount of products such as fenchene, polymeric compounds, and also oxidation products. These products are characterized by much larger molecules (often very spatially expanded), requiring more space for reactions involving them to occur. Looking at the structure of these catalysts (MXene HF/H2SO4 X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Y samples do not show layered structure and MXene HF/HCl X
Y samples do not show layered structure and MXene HF/HCl X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Y samples exhibit the lamellar structure without clearly marked spacing between the nanosheets) and the MXene HF catalyst structure, it can be assumed that in the case of the MXene HF, the formation of monocyclic products was related to the diffusion of α-pinene molecules into narrow spaces between the layers of this catalyst. Probably the diffusion of α-pinene molecules was difficult and only some of the α-pinene molecules reached there. It should also be taken into account that limitations in the diffusion to the area between the layers may also be related to the presence of anions in these areas (e.g. Cl− and F−) that were not completely washed out from the catalyst after synthesis. These anions may additionally limit the diffusion of α-pinene molecules to the area between the layers and block their access to the active sites. Due to the small space between the layers, the formation of monocyclic products and their further transformations (formation of p-cymene) was favored. However, the remaining molecules of α-pinene remained on the surface of the layered catalyst particles and the α-pinene transformation process probably took place on them. Due to much smaller steric constraints on the surface of large, layered MXene HF catalyst particles, it was possible to obtain large, bicyclic products such as camphene and tricyclene. In the case of the MXene HF/H2SO4 X
Y samples exhibit the lamellar structure without clearly marked spacing between the nanosheets) and the MXene HF catalyst structure, it can be assumed that in the case of the MXene HF, the formation of monocyclic products was related to the diffusion of α-pinene molecules into narrow spaces between the layers of this catalyst. Probably the diffusion of α-pinene molecules was difficult and only some of the α-pinene molecules reached there. It should also be taken into account that limitations in the diffusion to the area between the layers may also be related to the presence of anions in these areas (e.g. Cl− and F−) that were not completely washed out from the catalyst after synthesis. These anions may additionally limit the diffusion of α-pinene molecules to the area between the layers and block their access to the active sites. Due to the small space between the layers, the formation of monocyclic products and their further transformations (formation of p-cymene) was favored. However, the remaining molecules of α-pinene remained on the surface of the layered catalyst particles and the α-pinene transformation process probably took place on them. Due to much smaller steric constraints on the surface of large, layered MXene HF catalyst particles, it was possible to obtain large, bicyclic products such as camphene and tricyclene. In the case of the MXene HF/H2SO4 X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Y and MXene HF/HCl X
Y and MXene HF/HCl X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Y catalysts the lack of spatial limitations related to the size of the reaction space (as was for reactions taking place between layers) made it possible to obtain bicyclic products and, at the same time, steric factors did not force the formation of monocyclic products. Also, the formation of fenchene, polymeric products, and oxidation products with higher selectivity is associated with lower steric restrictions on the surface of the catalyst particles. Moreover, due to the significantly reduced number of acid centers on the surface of particles of MXene HF/H2SO4 X
Y catalysts the lack of spatial limitations related to the size of the reaction space (as was for reactions taking place between layers) made it possible to obtain bicyclic products and, at the same time, steric factors did not force the formation of monocyclic products. Also, the formation of fenchene, polymeric products, and oxidation products with higher selectivity is associated with lower steric restrictions on the surface of the catalyst particles. Moreover, due to the significantly reduced number of acid centers on the surface of particles of MXene HF/H2SO4 X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Y and MXene HF/HCl X
Y and MXene HF/HCl X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Y catalysts, the conversion of α-pinene was significantly reduced. MXene HF was characterized by the concentration of acid sites amounted to 11.62 mmol g−1, but for MXene HF/HCl X
Y catalysts, the conversion of α-pinene was significantly reduced. MXene HF was characterized by the concentration of acid sites amounted to 11.62 mmol g−1, but for MXene HF/HCl X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Y and MXene HF/H2SO4 X
Y and MXene HF/H2SO4 X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Y, the concentration of acid sites was about 2.5–5.5 times smaller (see Table 2). At the same time, it can also be noted that although the MXene HF/HCl X
Y, the concentration of acid sites was about 2.5–5.5 times smaller (see Table 2). At the same time, it can also be noted that although the MXene HF/HCl X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Y samples were characterized by a slightly higher concentration of acid sites, their activity in the isomerization of α-pinene was lower than the MXene HF/H2SO4 X
Y samples were characterized by a slightly higher concentration of acid sites, their activity in the isomerization of α-pinene was lower than the MXene HF/H2SO4 X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Y materials (taking into account the conversion of α-pinene), which were characterized by almost 2 times greater specific surface area. This difference in the activity of samples obtained by the treatment with the mixtures of two acids may therefore probably be due to the different availability of acid sites on the particles of these materials. Probably in MXene HF/H2SO4 X
Y materials (taking into account the conversion of α-pinene), which were characterized by almost 2 times greater specific surface area. This difference in the activity of samples obtained by the treatment with the mixtures of two acids may therefore probably be due to the different availability of acid sites on the particles of these materials. Probably in MXene HF/H2SO4 X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Y acid sites are more accessible.
Y acid sites are more accessible.
To clarify what functional groups may constitute the active centers on which the α-pinene isomerization reaction takes place, XPS analysis was performed for the material with the highest catalytic activity (MXene HF). The sample overview is presented in Fig. 8A. The results confirmed the presence of Ti 2p, C 1s, O 1s, and F 1s on the surface of the MXene HF. The detailed XPS spectra of Ti 2p (Fig. 8B) demonstrate characteristic peaks at ∼458.1, 459.0, 460.4, 462.8, and 464.8 eV which is assigned to Ti2+, Ti–O, C–Ti–Fx, Ti2+, and Ti–O, respectively.44 High-resolution XPS C 1s spectrum is shown in Fig. 8C. MXene HF exhibits an intense peak located at ∼285.0 eV, which is attributed to the C–C bonds. The material shows two additional peaks at ∼287.7 and 289.7 eV, which correspond to C–O and O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds, respectively.44 As depicted in Fig. 8D the spectrum for O 1s is deconvoluted into three components corresponding to the presence of different oxygen species at the surface of MXene HF. The first peak at ∼532.0 eV is ascribed to Ti–O–H bonding. The second peak at ∼533.8 eV is due to adsorbed H2O. The third peak is located at ∼535.0 eV and corresponds to O–Fx bonding.45,46 As depicted in Fig. 8E F 1s region is deconvoluted into 2 peaks at ∼688.3, and 690.0 eV, which is attributed to C–F, and F–C–F, respectively.47
O bonds, respectively.44 As depicted in Fig. 8D the spectrum for O 1s is deconvoluted into three components corresponding to the presence of different oxygen species at the surface of MXene HF. The first peak at ∼532.0 eV is ascribed to Ti–O–H bonding. The second peak at ∼533.8 eV is due to adsorbed H2O. The third peak is located at ∼535.0 eV and corresponds to O–Fx bonding.45,46 As depicted in Fig. 8E F 1s region is deconvoluted into 2 peaks at ∼688.3, and 690.0 eV, which is attributed to C–F, and F–C–F, respectively.47
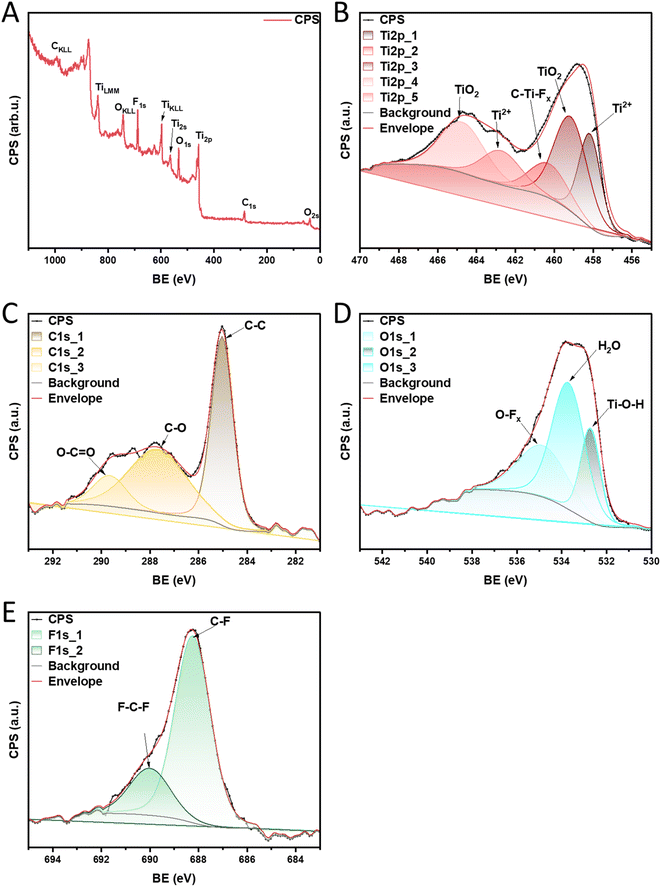 | ||
| Fig. 8 XPS survey spectra of MXene HF (A), high-resolution XPS of elemental: Ti 2p (B), C 1s (C), O 1s (D), and F 1s (E). | ||
The results of tests carried out using the XPS method for the MXene HF catalyst confirmed the presence of Ti–OH groups on the surface of this material, which constitute the active centers of the catalyst (proton source for the α-pinene isomerization reaction). They may, among others, be formed from titanyl groups (Ti![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) in the hydrolysis process. Similarly, C–OH groups present on the surface of the tested catalyst may be the proton source for the isomerization reaction. The C–Ti–Fx, O–Fx, C–F and F–C–F groups also attract attention. They indicate the binding of fluorine in the structure of the tested material, but at the same time, the formation of such groups indicates that protons from HF must also have been bound in some way in the structure. It can be assumed, for example, that HF reacting with the carbonyl group (C
O) in the hydrolysis process. Similarly, C–OH groups present on the surface of the tested catalyst may be the proton source for the isomerization reaction. The C–Ti–Fx, O–Fx, C–F and F–C–F groups also attract attention. They indicate the binding of fluorine in the structure of the tested material, but at the same time, the formation of such groups indicates that protons from HF must also have been bound in some way in the structure. It can be assumed, for example, that HF reacting with the carbonyl group (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group) causes the formation of CF(OH) groups, in which the OH group can be the proton source for the isomerization reaction. Similarly, the titanyl group (Ti
O group) causes the formation of CF(OH) groups, in which the OH group can be the proton source for the isomerization reaction. Similarly, the titanyl group (Ti![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) reacts with HF to form TiF(OH) groups, and here also the OH group can be the proton source for the isomerization reaction. However, a detailed explanation of the mechanism of HF reaction with surface groups and its participation in the formation of acidic centers, which can be the source of a proton for the isomerization reaction, requires further, very detailed research in the future.
O) reacts with HF to form TiF(OH) groups, and here also the OH group can be the proton source for the isomerization reaction. However, a detailed explanation of the mechanism of HF reaction with surface groups and its participation in the formation of acidic centers, which can be the source of a proton for the isomerization reaction, requires further, very detailed research in the future.
3. Conclusion
Ti3C2 MXenes-based materials were synthesized and applied as catalysts in the reaction of α-pinene isomerization. The impact of the etching medium (HF; HF/HCl and HF/H2SO4: weight ratios: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 1
3, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, and 1
4, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) on the composition, morphology, and structure of the produced samples was investigated. During the catalytic tests, the highest activity showed MXene HF, the sample with layered structure and clearly marked spacing between nanosheets, which was obtained by the treatment of MAX phase only with HF (conversion of α-pinene achieved a value of 74.65 mol%). The catalytic tests also showed that materials produced by the treatment of MAX phase with HF/H2SO4 mixtures were slightly more active than the samples obtained by HF/HCl treatment. The latter materials were characterized by the lower concentration of acid sites, but probably these acid sites were more accessible to α-pinene molecules. In general, it can be said that using HF/H2SO4 and HF/HCl mixtures for removing Al form MAX phase led to a significant reduction in the conversion of α-pinene (on average about 28-fold), while increasing by about 10 mol% in the selectivity of the transformation to the most desirable product – camphene. To summarize, further modifications of the method of aluminum etching from MAX phase should go towards such a selection of compounds used for etching and their mutual ratio that, while maintaining the increased selectivity of transformation to camphene, significantly increase the conversion of α-pinene.
5) on the composition, morphology, and structure of the produced samples was investigated. During the catalytic tests, the highest activity showed MXene HF, the sample with layered structure and clearly marked spacing between nanosheets, which was obtained by the treatment of MAX phase only with HF (conversion of α-pinene achieved a value of 74.65 mol%). The catalytic tests also showed that materials produced by the treatment of MAX phase with HF/H2SO4 mixtures were slightly more active than the samples obtained by HF/HCl treatment. The latter materials were characterized by the lower concentration of acid sites, but probably these acid sites were more accessible to α-pinene molecules. In general, it can be said that using HF/H2SO4 and HF/HCl mixtures for removing Al form MAX phase led to a significant reduction in the conversion of α-pinene (on average about 28-fold), while increasing by about 10 mol% in the selectivity of the transformation to the most desirable product – camphene. To summarize, further modifications of the method of aluminum etching from MAX phase should go towards such a selection of compounds used for etching and their mutual ratio that, while maintaining the increased selectivity of transformation to camphene, significantly increase the conversion of α-pinene.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research has received funding from National Science Centre (Poland) with grant PRELUDIUM BIS 2 number (2020/39/O/ST5/01340). The raw data will be placed in a repository accessible to anyone.References
- K. Lim and R. Garrick, et al., Fundamentals of MXene synthesis, Nat. Synth., 2022, 1(8), 601–614 CrossRef.
- X. Li, et al., MXene chemistry, electrochemistry and energy storage applications, Nat. Rev. Chem, 2022, 6, 389–404 CrossRef PubMed.
- B. Salehi, S. Upadhyay, I. E. Orhan, A. K. Jugran, L. D. Jayaweera Sumali, D. A. Dias, F. Sharopov, Y. Taheri, N. Martins, N. Baghalpour, C. William Cho and J. Sharifi-Rad, Therapeutic Potential of α- and β-Pinene: A Miracle Gift of Nature, Biomolecules, 2019, 9, 738, DOI:10.3390/biom9110738.
- M. Allenspach and C. Steuer, α-Pinene: A never-ending story, Phytochemistry, 2021, 190, 112857, DOI:10.1016/j.phytochem.2021.112857.
- R. J. Nyamwihura and I. V. Ogungbe, The pinene scaffold: its occurrence, chemistry, synthetic utility, and pharmacological importance, RSC Adv., 2022, 12, 11346, 10.1039/d2ra00423b.
- M. Coelhan and M. Maurer, Synthesis of Two Major Toxaphene Components and Their Photostabilities, J. Agric. Food Chem., 2005, 53(26), 10105–10112, DOI:10.1021/jf051451b.
- C. Glaser, US Pat.US875062 A, Maryland, 31 Dec, 1907 Search PubMed.
- L. Bintang, Y. Jinquan, E. Aiqun, Z. Ping and X. Shude, Study on selective alkylation of guaiacol with camphene over H-Mordenite, Chin. J. Org. Chem., 1995, 15(3), 318–322 Search PubMed.
- N. Girola, C. R. Figueiredo, C. F. Farias, R. A. Azevedo, A. K. Ferreira, S. F. Teixeira, T. M. Capello, E. G. A. Martins, A. L. Matsuo, L. R. Travassos and J. H. G. Lago, Camphene isolated from essential oil of Piper cernuum (Piperaceae) induces intrinsic apoptosis in melanoma cells and displays antitumor activity in vivo, Biochem. Biophys. Res. Commun., 2015, 467(4), 928–934, DOI:10.1016/j.bbrc.2015.10.041.
- S. K. Król, K. Skalicka-Wożniak, M. Kandefer-Szerszeń and A. Stepulak, The biological and pharmacological activity of essential oils in the treatment and prevention of infectious diseases, Postepy Hig. Med. Dosw., 2013, 67, 1000–1007, DOI:10.5604/17322693.1067687.
- M. A. Martın-Luengoa, M. Yatesb, M. J. Martınez Domingo, B. Casala, M. Iglesiasa, M. Estebana and E. Ruiz-Hitzky, Synthesis of p-cymene from limonene, a renewable feedstock, Appl. Catal. B, 2008, 81(3–4), 218–224, DOI:10.1016/j.apcatb.2007.12.003.
- E. E. Royals and S. E. Horne Jr, Conversion of d-Limonene to l-Carvone, J. Am. Chem. Soc., 1951, 73, 5856–5857, DOI:10.1021/ja01156a119.
- M. P. Arrieta, J. Lopez, S. Ferrandiz and M. A. Peltzer, Characterization of PLA-limonene blends for food packaging applications, Polym. Test., 2013, 32(4), 760–768, DOI:10.1016/j.polymertesting.2013.03.016.
- D. Pybus and C. Sell, The Chemistry of Fragrances, The Royal Society of Chemistry, 1999, pp. 24–51 Search PubMed.
- E. Gastao,US Pat.US 2551795 A, Du Pont, 8 May 1951, 10.1039/9781847552044-00024.
- K. Arata and K. Tanabe, Isomerization of α-plnene oxide over solid acids and bases, Chem. Lett., 1979, 8, 1017–1018 CrossRef.
- J. E. Sánchez-Velandia, et al., Selective synthesis of camphene from isomerization of α- and β-pinene over heterogeneous catalysts, Microporous Mesoporous Mater., 2021, 324, 111273 CrossRef.
- F. Launay, B. Jarry and J. L. Bonardet, Catalytic activity of mesoporous Ga-SBA-15 materials in α-pinene isomerisation: Similarities and differences with Al-SBA-15 analogues, Appl. Catal. A: General, 2009, 368(1), 132–138, DOI:10.1016/j.apcata.2009.08.022.
- C. Wu, H. Liu, C. Zhuang and G. Du, Study on Mesoporous PW/SBA-15 for Isomerization of α-Pinene, Appl. Mech. Mater., 2014, 483, 134–137, DOI:10.4028/www.scientific.net/AMM.483.134.
- R. Luque, J. M. Campelo, T. D. Conesa, D. Luna, J. M. Marinad and A. A. Romero, Ga-MCM-41 synthesis and catalytic activity in the liquid-phase isomerisation of α-pinene, Microporous Mesoporous Mater., 2007, 103(1), 333–340, DOI:10.1016/j.micromeso.2007.02.022.
- M. A. Ecormier, A. F. Lee and K. Wilson, High activity, templated mesoporous SO4/ZrO2/HMS catalysts with controlled acid site density for α-pinene isomerisation, Microporous Mesoporous Mater., 2005, 80(1), 305–310, DOI:10.1016/j.micromeso.2005.01.007.
- R. Rachwalik, Z. Olejniczak, J. Jiao, J. Huang, M. Hunger and B. Sulikowski, Isomerization of α-pinene over dealuminated ferrierite-type zeolites, J. Catal., 2007, 252(2), 161–170, DOI:10.1016/j.jcat.2007.10.001.
- E. Unveren, G. Gunduz and F. Cakicioglu-ozkan, Isomerization of Alpha-pinene Over Acid Treated Natural Zeolite, Chem. Eng. Commun., 2005, 192(3), 386–404 CrossRef.
- N. Wijayati, H. D. Pranowo, J. Jumina, T. Triyono and G. H. Chuah, Characterization of ZHY and TCA/ZHY Catalysts for Hydration of α-Pinene, Int. J. Chem. Eng. Appl., 2013, 4(4), 178–182, DOI:10.7763/IJCEA.2013.V4.289.
- O. Akpolat, G. Gunduz, F. Ozkan and N. Besug, General, Isomerization of α-pinene over calcined natural zeolites, Appl. Catal. A, 2005, 265(1), 11–22, DOI:10.1016/j.apcata.2003.12.055.
- G. Gynduz and D. Y. Murzin, Influence of catalyst pretreatment on α-pinene isomerization over natural calys, React. Kinet. Catal. Lett., 2002, 75(2), 231–237 CrossRef.
- P. Miądlicki, A. Wróblewska, K. Kiełbasa, Z. C. Koren and B. Michalkiewicz, Sulfuric acid modified clinoptilolite as a solid green catalyst for solvent-free α-pinene isomerization process, Microporous Mesoporous Mater., 2021, 324, 111266, DOI:10.1016/j.micromeso.2021.111266.
- B. Zielińska, et al., High catalytic performance of 2D Ti3C2Tx MXene in α-pinene isomerization to camphene, Appl. Catal., A, 2020, 604, 117765 CrossRef.
- L. Vilcocq, V. Spinola, P. Moniz, L. C. Duarte, F. Carvalheiro, C. Fernandes and P. Castilho, Catal. Sci. Technol., 2015, 5, 4072–4080 RSC.
- A. Tariq, S. Irfan Ali, D. Akinwande and S. Rizwan, Efficient visible-light photocatalysis of 2D-MXene nanohybrids with Gd3+-and Sn4+-codoped bismuth ferrite, ACS omega, 2018, 3(10), 13828–13836, DOI:10.1021/acsomega.8b01951.
- C. E. Shuck, et al., Scalable synthesis of Ti3C2Tx mxene, Adv. Eng. Mater., 2020, 22(3), 1901241 CrossRef CAS.
- M. Mahmood, et al., Synthesis of ultrathin MnO2 nanowire-intercalated 2D-MXenes for high-performance hybrid supercapacitors, Energy Fuels, 2021, 35(4), 3469–3478 CrossRef CAS.
- M. Anayee, et al., Kinetics of Ti3AlC2 Etching for Ti3C2T x MXene Synthesis, Chem. Mater., 2022, 34(21), 9589–9600 CrossRef CAS.
- T. B. Limbu, et al., Green synthesis of reduced Ti 3 C 2 T x MXene nanosheets with enhanced conductivity, oxidation stability, and SERS activity, J. Mater. Chem. C, 2020, 8(14), 4722–4731 RSC.
- F. Liu, et al., Preparation and methane adsorption of two-dimensional carbide Ti 2 C, Adsorption, 2016, 22, 915–922 CrossRef CAS.
- T. B. Limbu, et al., Green synthesis of reduced Ti 3 C 2 T x MXene nanosheets with enhanced conductivity, oxidation stability, and SERS activity, Journal of Materials Chemistry C, 2020, 8(14), 4722–4731 RSC.
- F. Liu, et al., Preparation and methane adsorption of two-dimensional carbide Ti2C, Adsorption, 2016, 22(7), 915–922 CrossRef CAS.
- J. Li, H. Wang and X. Xiao, Intercalation in two-dimensional transition metal carbides and nitrides (MXenes) toward electrochemical capacitor and beyond, Energy Environ. Mater., 2020, 3, 306–322 CrossRef CAS.
- M. Haftani, et al., Studying the oxidation of Ti2AlC MAX phase in atmosphere: A review, Int. J. Refract. Hard Met., 2016, 61, 51–60 CrossRef CAS.
- I. Ostroman, et al., Highly Reversible Ti/Sn Oxide Nanocomposite Electrodes for Lithium Ion Batteries Obtained by Oxidation of Ti3Al (1-x) SnxC2 Phases, Small Methods, 2023, 2300503 CrossRef PubMed.
- E. P. Simonenko, et al., Gas-Sensitive Properties of ZnO/Ti2CTx Nanocomposites, Micromachines, 2023, 14(4), 725 CrossRef PubMed.
- X. H. Wang and Y. C. Zhou, Oxidation behavior of Ti 3 AlC 2 powders in flowing air, J. Mater. Chem., 2002, 12(9), 2781–2785 RSC.
- Y. Wen, et al., FeNC/MXene hybrid nanosheet as an efficient electrocatalyst for oxygen reduction reaction, RSC adv., 2019, 9(24), 13424–13430 RSC.
- S. Wan, et al., Strong sequentially bridged MXene sheets, Proc. Natl. Acad. Sci., 2020, 117(44), 27154–27161 CrossRef CAS PubMed.
- W. Yang, et al., Covalently sandwiching MXene by conjugated microporous polymers with excellent stability for supercapacitors, Small Methods, 2020, 4(10), 2000434 CrossRef CAS.
- S. A. Shah, et al., Template-free 3D titanium carbide (Ti 3 C 2 T x) MXene particles crumpled by capillary forces, Chem. Commun., 2017, 53(2), 400–403 RSC.
- G. Chen, J. Zhang and S. Yang, Fabrication of hydrophobic fluorinated amorphous carbon thin films by an electrochemical route, Electrochem. Commun., 2008, 10(1), 7–11 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2023 |