Open Access Article
Open Access ArticleTriazine diphosphonium tetrachloroferrate ionic liquid immobilized on functionalized halloysite nanotubes as an efficient and reusable catalyst for the synthesis of mono-, bis- and tris-benzothiazoles†
Farzaneh Gholamhossein Zadeha,
Beheshteh Asadia,
Iraj Mohammadpoor-Baltork *a,
Shahram Tangestaninejad
*a,
Shahram Tangestaninejad a,
Valiollah Mirkhania,
Majid Moghadam
a,
Valiollah Mirkhania,
Majid Moghadam a and
Akbar Omidvarb
a and
Akbar Omidvarb
aDepartment of Chemistry, Catalysis Division, University of Isfahan, Isfahan 81746-73441, Iran. E-mail: imbaltork@sci.ui.ac.ir
bDepartment of Physical Chemistry, Faculty of Chemistry, University of Isfahan, Isfahan, 81746-73441, Iran
First published on 25th October 2023
Abstract
Aminopropyl-1,3,5-triazine-2,4-diphosphonium tetrachloroferrate immobilized on halloysite nanotubes [(APTDP)(FeCl4)2@HNT] was prepared and fully characterized using different techniques such as FT-IR, thermogravimetric analysis (TGA), SEM/EDX, elemental mapping, TEM, ICP-OES, and elemental analysis (EA). This nanocatalyst was found to be highly effective for synthesis of various benzothiazole derivatives in excellent yields under solvent-free conditions. Furthermore, bis- and tris-benzothiazoles were smoothly synthesized from dinitrile and trinitrile in the presence of this catalytic system. High yields and purity, easy work up procedure, high catalytic activity (high TON and TOF) and easy recovery and reusability of the catalyst make this method a useful and important addition to the present methodologies for preparation of these vital heterocyclic compounds.
Introduction
Ionic liquids (ILs) have attracted increasing interest in the last decades, owing to expanded range of applications and potential advantages including tunable polarity, nonflammability, negligible vapor pressure, large liquid range, and wide solubility.1 Among them, phosphonium-based ILs are remarkable worthy choices for catalysis because of their desirable properties such as high thermal stability, ionic conductivity and electrochemical windows as well as low viscosity.2 However, the need for relatively large amounts of these catalysts, the difficulty of separation from the reaction mixture and non reusability1b can be considered the drawbacks of the ILs which limit their practical applications. These restrictions can be overcome by the heterogenization of homogeneous catalysts on solid supports. In this context, halloysite nanotubes (HNTs) with a molecular formula of Al2Si2O5(OH)4·2H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of Al/Si) have attracted considerable attention because of their suitable advantages such as large surface area, biocompatibility, ease of reusability, low price, environmental friendliness, high mechanical and thermal stability, and resistibility against organic solvents.3
1 ratio of Al/Si) have attracted considerable attention because of their suitable advantages such as large surface area, biocompatibility, ease of reusability, low price, environmental friendliness, high mechanical and thermal stability, and resistibility against organic solvents.3
Therefore, HNTs have emerged as a highly efficient supports in the fields of catalysis,4 pharmaceutical studies,5 industry,6 and food safety.7
Benzothiazoles are useful building blocks for a number of naturally occurring products8 and pharmaceuticals with remarkable biological activities such as anticancer,9 antitumor,10 anti-diabetic,11 and anti-Alzheimer drugs.12 Therefore, the development of new methods for the synthesis of benzothiazole derivatives can create a fascinating window in chemistry. For instance, it was reported that benzothiazoles skeletons could be obtained through the condensation of 2-aminothiophenol and aryl/alkyl nitriles using ZnO-nanoparticles as a catalyst.13 The reaction of 2-aminothiophenol with orthoesters in the presence of catalytic amounts of Bi(III) salts, has been also reported for the synthesis of benzothiazoles under solvent-free conditions.14 This structure could also be generated from o-phenylenediamine and N-substituted formamides (C1 sources) via a one-pot protocol catalyzed by zinc in the presence of poly(methylhydrosiloxane).15 Yu et al., have also reported the Brønsted acid-catalyzed synthetic method for benzothiazoles under metal and solvent-free conditions.16 Moreover, one-pot syntheses of these heterocyclic compounds has been described through condensation reactions employing Cu(OAc)2.17 However, some of these methods suffer from disadvantages such as acidic or alkaline conditions, high temperature (up to 120 °C) and long reaction times (18 h). Consequently, the development of simple and environmentally benign synthetic methods for the synthesis of such fine heterocycles is of practical importance and still in demand.
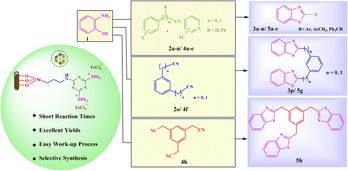
Prompted by these results and in continuation of our efforts to develop novel heterogeneous catalytic systems,18 herein, we wish to report aminopropyl-1,3,5-triazine-2,4-diphosphonium tetrachloroferrate immobilized on halloysite nanotubes as an efficient and easy reusable catalyst for the synthesis of mono-, bis- and tris-benzothiazoles under solvent-free and green conditions (Scheme 1).
Results and discussion
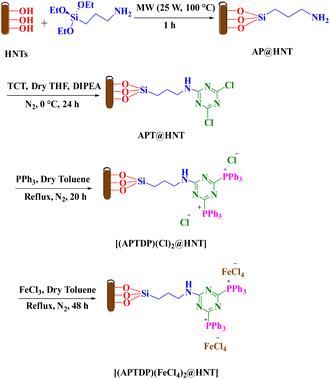
Synthesis and characterization of aminopropyl-1,3,5-triazine-2,4-diphosphonium tetrachloroferrate immobilized on halloysite nanotubes [(APTDP)(FeCl4)2@HNT]
The synthetic pathway of nanocatalyst [(APTDP)(FeCl4)2@HNT] is depicted in Scheme 2. First, AP@HNT was synthesized under microwave irradiation according to our previously reported method.18b Then, the reaction of 1,3,5-trichlorotriazine (TCT) with the surface-attached aminopropyl of AP@HNT was carried out for substitution of one of the chlorine atoms, to yield APT@HNT18b which in turn was converted to [(APTDP)(Cl)2@HNT] upon reaction with PPh3. Finally, [(APTDP)(Cl)2@HNT] reacted with FeCl3 to produce [(APTDP)(FeCl4)2@HNT] (Scheme 2).These processes were monitored by FT-IR, thermogravimetric analysis (TGA), SEM/EDX, elemental mapping, TEM, ICP-OES, and elemental analysis (EA).
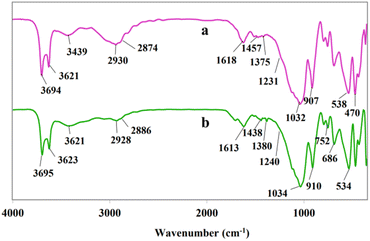
The FT-IR spectrum of halloysite nanotubes revealed a number of characteristic peaks at 3694 and 3623, 910, 1034 and 463–537 cm−1 which can be ascribed to OH groups, Al–OH bending, Si–O stretching and Si–O bending vibrations, respectively (Fig. 1a). The result of this analysis is in agreement with the previous work.18b In addition, the presence of bands at 3431 and 3356 cm−1 (NH2 stretching vibration), 2933 and 2880 cm−1 (C–H stretching vibration), and 1654–1581 cm−1 (NH2 bending vibration) confirms the synthesis of AP@HNT (Fig. 1b).18b As can be seen in Fig. 1c, the FT-IR spectrum of APT@HNT displayed characteristic bands at around 3432 cm−1 (NH stretching vibration), 1617 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1514 cm−1 (NH bending vibration), 1312 and 1228 cm−1 (C–N stretching vibration), confirming that the TCT was covalently immobilized onto the surface of the nano-clay through formation of C–N bond, but it was not possible to assign the C–Cl band of TCT at 1035–1100 cm−1, due to interference of the Si–O stretching band at 1000–1100 cm−1. Furthermore, the absorption band at 1469 and 1457 cm−1 in Fig. 1d and e may approve that the PPh3 is attached.
N), 1514 cm−1 (NH bending vibration), 1312 and 1228 cm−1 (C–N stretching vibration), confirming that the TCT was covalently immobilized onto the surface of the nano-clay through formation of C–N bond, but it was not possible to assign the C–Cl band of TCT at 1035–1100 cm−1, due to interference of the Si–O stretching band at 1000–1100 cm−1. Furthermore, the absorption band at 1469 and 1457 cm−1 in Fig. 1d and e may approve that the PPh3 is attached.
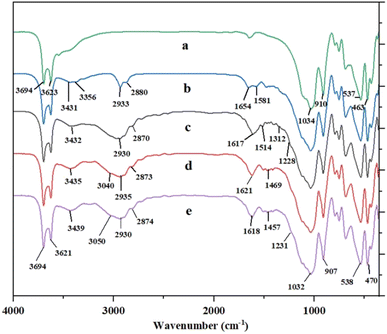 | ||
| Fig. 1 The FT-IR spectra of: (a) HNT, (b) AP@HNT, (c) APT@HNT, (d) [(APTDP)(Cl)2@HNT] and (e) [(APTDP)(FeCl4)2@HNT]. | ||
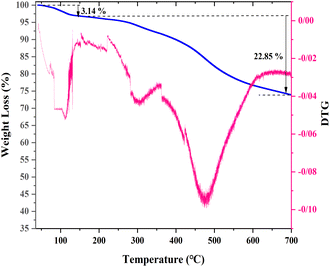
Fig. 2 shows the TGA analysis of the [(APTDP)(FeCl4)2@HNT] sample, which provides information about the thermal stability of this nanocatalyst. As seen in the TGA curve, the initial weight loss (∼3.14%) is related to the removal of water molecules within the range of 30–180 °C. the second weight loss (∼22.85%) corresponds to the departure of linker and organic moieties, was occurred at around 180–680 °C. Based on these results, the aminopropyl-1,3,5-triazine-2,4-diphosphonium tetrachloroferrate ionic liquid has covalently been linked to nano-clay and can be applied in a wide range of temperatures.
The morphology of the surfaces of HNTs and [(APTDP)(FeCl4)2@HNT] were examined using scanning electron microscopy (SEM) (Fig. 3a and b). It is noteworthy that the morphology of the two samples is diverse, and also, the geometric shape of the prepared nanoparticles is tubular without aggregation. Furthermore, in order to confirm the presence of Fe and other elements in the final catalyst, the energy dispersive X-ray (EDX) analysis was applied, which obviously illustrated the presence of C, N, P, Cl, O, Al, Si and Fe in catalyst texture (Fig. 3c).
 | ||
| Fig. 3 SEM images of: (a) HNTs, (b) [(APTDP)(FeCl4)2@HNT], and (c) SEM/EDX spectrum of [(APTDP)(FeCl4)2@HNT]. | ||
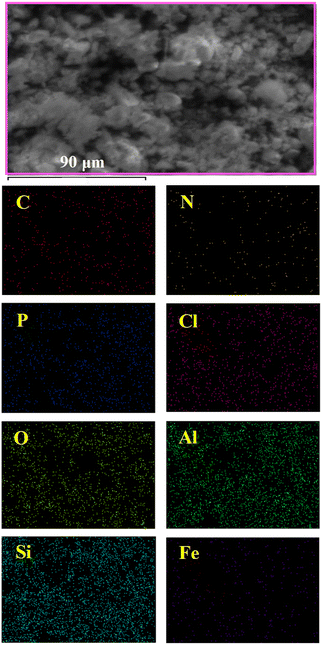
EDX elemental mapping was also indicated that the nanocatalyst was successfully formed owing to the uniform scattering of C, N, P, Cl, O, Al, and Si, and also, the Fe complex was homogeneously dispersed on the functionalized halloysite nanotubes, without any aggregation (Fig. 4). This figure supports the SEM/EDX results (Fig. 3).
To confirm and determine the nanostructure of [(APTDP)(FeCl4)2@HNT], we performed transmission electron microscopy (TEM) technique (Fig. 5). Based on the results of this analysis, the average particle size of [(APTDP)(FeCl4)2@HNT] is about 50–80 nm (Fig. 5c) with a well-defined tubular structure.
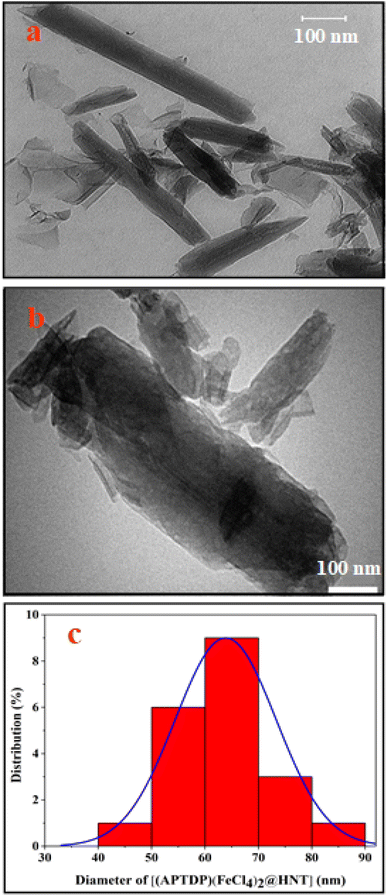 | ||
| Fig. 5 TEM image of: (a) HNT, (b) [(APTDP)(FeCl4)2@HNT] and (c) particle size distribution for [(APTDP)(FeCl4)2@HNT]. | ||
The FT-IR, TGA, SEM/EDX, elemental mapping, and TEM information show that the [(APTDP)(FeCl4)2@HNT] catalyst was shaped well by using this process. Besides, the ferric content of the catalyst was measured by ICP analysis and showed a value of 0.60 mmol g−1 of the [(APTDP)(FeCl4)2@HNT] of nanocatalyst. This is in good agreement with the values of elemental analysis, which was found to be: C, 11.44; H, 2.69; and N, 1.56. Based on the nitrogen content, the amount of IL immobilized onto the HNT was determined about 0.58 mmol g−1 of the nanocatalyst.
Synthesis of benzothiazole derivatives catalyzed by [(APTDP)(FeCl4)2@HNT]
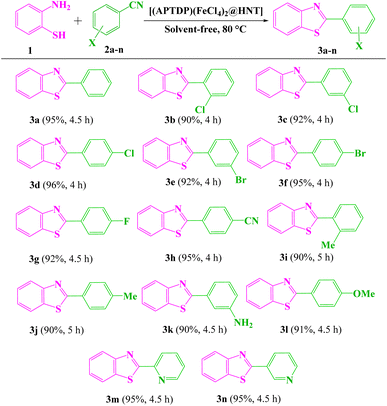
We initiated our studies by using 2-aminothiophenol 1 (1.0 mmol) and benzonitrile 2a (1.0 mmol) as model substrates for optimization of reaction parameters; the results are summarized in Table 1. The model reaction was first performed in the absence of catalyst under solvent-free conditions at 80 °C. No product was obtained under this condition (Table 1, entry 1). In order to achieve the most appropriate conditions, various Lewis and Brønsted acids, such as ZnCl2, FeCl3, InCl3, BiCl3, ZnO, NaHSO4, Fe(HSO4)3, Al(HSO4)3, p-TSA, NaFeCl4, NaFeCl4@HNT, HNT, [(APTDP)(Cl)2@HNT] and [(APTDP)(FeCl4)2@HNT] were examined (Table 1, entries 2–15). Amongst them [(APTDP)(FeCl4)2@HNT] proved to be superior, producing 3a in 95% yield (Table 1, entry 15). Notably, the activity of [(APTDP)(FeCl4)2@HNT] can be attributed to the isolation of catalytic active sites on the high surface area of halloysite nanotube. A survey of the amount of [(APTDP)(FeCl4)2@HNT] revealed that 1.5 mol% is the best choice (Table 1, entry 15), with no improvement upon increasing the catalyst loading to 2 mol% (Table 1, entries 15–17). For temperature optimization (Table 1, entries 18–20), when the reaction was performed at 80 °C, significant improvement was observed with maximum yield of 3a (Table 1, entry 15). In accordance with Table 1, the optimal reaction conditions were 2-aminothiophenol (1.0 mmol) and benzonitrile (1.0 mmol) in the presence of [(APTDP)(FeCl4)2@HNT] (1.5 mol%, 25 mg) at 80 °C under solvent-free conditions.| Entry | Catalyst (mol%) | T (°C) | Time (h) | Yieldb (%) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a Experimental conditions: 2-aminothiophenol (1.0 mmol), benzonitrile (1.0 mmol) and [(APTDP)(FeCl4)2@HNT] (1.5 mol%, 25 mg) at 80 °C under solvent-free conditions.b Isolated yield. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1 | — | 80 | 12 | 0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2 | ZnCl2 (1.5) | 80 | 4.5 | 50 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3 | FeCl3 (1.5) | 80 | 4.5 | 50 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4 | InCl3 (1.5) | 80 | 4.5 | 0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5 | BiCl3 (1.5) | 80 | 4.5 | 5 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6 | ZnO (1.5) | 80 | 4.5 | 20 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7 | NaHSO4 (1.5) | 80 | 4.5 | 10 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 8 | Fe(HSO4)3 (1.5) | 80 | 4.5 | 20 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 9 | Al(HSO4)3 (1.5) | 80 | 4.5 | 20 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 10 | p-TSA (1.5) | 80 | 4.5 | 35 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 11 | NaFeCl4 (1.5) | 80 | 4.5 | 65 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 12 | NaFeCl4@HNT (25 mg) | 80 | 4.5 | 40 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 13 | HNT (25 mg) | 80 | 4.5 | 0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 14 | [(APTDP)(Cl)2@HNT] (25 mg) | 80 | 4.5 | 0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 15 | [(APTDP)(FeCl4)2@HNT] (1.5) | 80 | 4.5 | 95 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 16 | [(APTDP)(FeCl4)2@HNT] (0.8) | 80 | 4.5 | 60 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 17 | [(APTDP)(FeCl4)2@HNT] (2) | 80 | 4.5 | 95 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 18 | [(APTDP)(FeCl4)2@HNT] (1.5) | 25 | 4.5 | 5 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 19 | [(APTDP)(FeCl4)2@HNT] (1.5) | 50 | 4.5 | 45 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 20 | [(APTDP)(FeCl4)2@HNT] (1.5) | 100 | 4.5 | 95 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
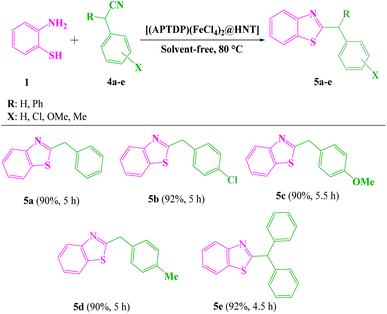
With the optimized experimental conditions in hand, the scope and generality of this methodology was checked by varying the aryl nitrile derivatives (2a–l) containing various electron-withdrawing and donating substituents (Scheme 3). The corresponding products 3a–l were obtained in 90–96% yields within 4–5 h. Significantly, heterocyclic nitriles such as 2- and 3-cyanopyridine yielded the expected products (3m, n) in 95% yields (Scheme 3). However, reactions with aliphatic nitriles, such as pentanenitrile and heptanenitrile did not give the corresponding products under the identical reaction conditions. Furthermore, 2-aminophenyl disulfide (instead of 2-aminothiophenol) did not take part in the related reactions under optimum conditions. It is also noteworthy that benzothiazoles were accessible on gram scales. On a 10 mmol scale, benzonitrile reacted with 2-aminothiophenol in the presence of [(APTDP)(FeCl4)2@HNT] (0.15 mol%) at 80 °C, to afford 2-phenylbenzo[d]thiazole 3a in 70% yield after 8 h. We further examined the scope of arylacetonitriles for this reaction (Scheme 4). Interestingly, phenylacetonitrile, 4-chlorophenylacetonitrile, 4-methoxyphenylacetonitrile and 4-methylphenylacetonitrile gave desired products 5a–d in 90–92% yields. Besides, diphenylacetonitrile provided the corresponding product 5e in 92% yield.
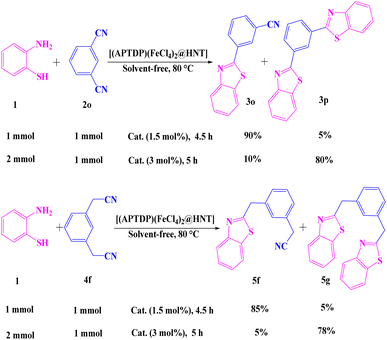
To our delight, the [(APTDP)(FeCl4)2@HNT] was applied as an efficient catalyst for the selective synthesis of mono- and bis-benzothiazoles (Scheme 5). In this respect, dinitriles such as 1,3-dicyanobenzene and 1,3-phenylenediacetonitrile were used. Fortunately, the corresponding mono-benzonitrile 3o and mono-methylphenylacetonitrile 5f were selectively synthesized using a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of starting materials, indicating that mono-products are of vital importance to provide other useful functional groups. It is also worth noting that the corresponding bis-derivatives 3p and 5g were selectively obtained in high yields, when a 2
1 molar ratio of starting materials, indicating that mono-products are of vital importance to provide other useful functional groups. It is also worth noting that the corresponding bis-derivatives 3p and 5g were selectively obtained in high yields, when a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of starting materials was used (Scheme 5).
1 molar ratio of starting materials was used (Scheme 5).
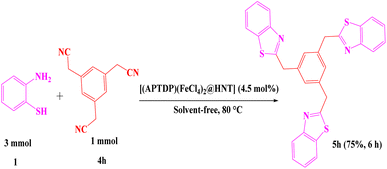
In another approach for the synthesis of tris-benzothiazole, the reaction of 2-aminothiophenol 1 (3 mmol) with 2,2′,2′′-(benzene-1,3,5-triyl)triacetonitrile 4h (1 mmol) proceeded smoothly to give the corresponding symmetric tris-benzothiazole 5h in 75% yield under solvent-free conditions (Scheme 6).
In the following, the competitive reaction of 2-aminothiophenol (1 mmol) with aromatic nitrile (4-methylbenzonitrile) (1 mmol) and aliphatic nitrile (heptanenitrile) (1 mmol) was examined under the same conditions. The results showed that 4-methylbenzonitrile was transformed to the corresponding benzothiazole 3j in the presence of heptanenitrile. This outcome clearly indicated the selectivity performance of [(APTDP)(FeCl4)2@HNT] catalyst (Scheme 7).
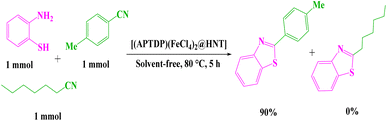 | ||
| Scheme 7 Competitive reaction of 2-aminothiophenol with 4-methylbenzonitrile and heptanenitrile in the presence of [(APTDP)(FeCl4)2@HNT]. | ||
The structure of the products was identified by their melting points, spectral data, and elemental analysis.
Although several mechanisms have been proposed for this reaction, we tried to provide the best path using density functional theory (DFT) calculations. Geometry and frequency calculations were carried out using B3LYP19 functional implemented in Gaussian 09.20 The van der Waals (vdW) interaction is performed by the semiempirical long-range DFT-D3 method.21 All configurations were fully optimized with default convergence criteria. Besides, frequency calculations were performed to ensure that there are no imaginary frequencies for minima. First, we investigated the electronic properties of 2-aminothiophenol (structure 1) and benzothiazole derivative (structure 3a). Fig. 6 represents the most stable geometries of the 1 and 3a structures together with bond length parameters.
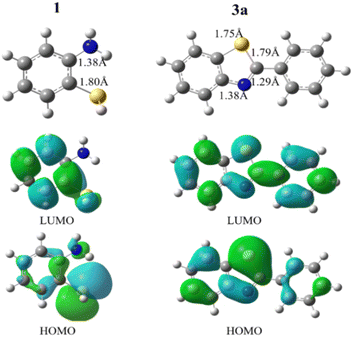 | ||
| Fig. 6 Optimized geometries and spatial distributions of frontier molecular orbitals of the 1 and 3a structures. The blue and green colors stand for electrons and holes, respectively. | ||
The results show that the 3a has a longer C–N bond with 1.38 Å and a smaller C–N bond with a distance of 1.29 Å. Also, the C–S bond lengths in the 1 and 3a structures are about 1.80 Å and 1.75 Å, respectively. The natural bond orbital (NBO) analysis reveals that the nitrogen atoms carry the negative charge of respectively, 0.79e and 051e in the 1 and 3a structures. Also, the results show a negative charge of 0.09e and a positive charge of 0.21e for the S atoms in the 1 and 3a structures, respectively. As frontier molecular orbital analysis shows, the HOMOs of the 1 and 3a structures are mostly localized on the S and N atoms, while the LUMOs are approximately distributed along the whole molecular framework (Fig. 6). The calculated HOMO–LUMO energy gaps for the 1 and 3a structures are 5.24 eV and 4.46 eV, respectively. In order to explore the favorable mechanisms, we have examined two possible mechanisms for the model reaction between [(APTDP)(FeCl4)2@HNT] and benzonitrile (Scheme 8).
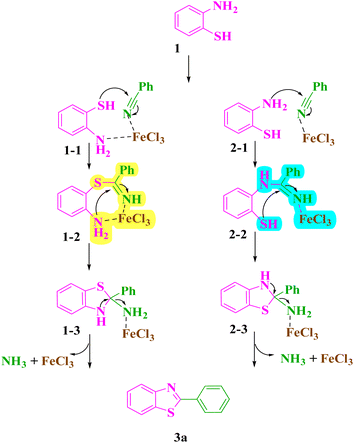 | ||
| Scheme 8 Two mechanisms (1–1 to 3a (left) and 2–1 to 3a (right)) for the synthesis of benzothiazole derivative (3a). | ||
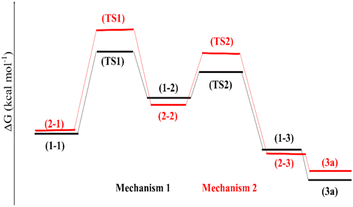
The use of (APTDP)(FeCl4)2 instead of [(APTDP)(FeCl4)2@HNT] was due to its lower steric factor which is more appropriate for theoretical studies. Table 2 gathered the Gibbs reaction energies of the two mechanisms, and the corresponding free energy profiles are represented in Fig. 7. Moreover, the geometries of the stationary points are shown in Fig. 8.
| Mechanism 1 | Mechanism 2 | ||
|---|---|---|---|
| ΔG (kcal mol−1) | ΔG (kcal mol−1) | ||
| (1–1) → TS1 | 5.7 | (2–1) → TS1 | 7.5 |
| TS → (1–2) | −3.5 | TS → (2–2) | −3.8 |
| (1–2) → TS2 | 2.4 | (2–2) → TS2 | 3.5 |
| TS → (1–3) | −8.3 | TS → (2–3) | −7.9 |
| (1–3)→(3a) | −4.6 | (2–3) → (3a) | −2.3 |
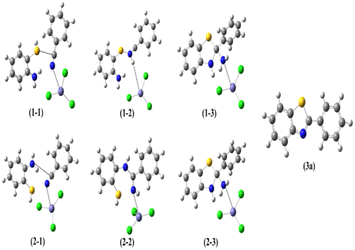 | ||
| Fig. 8 Structures of stationary points corresponding to the two proposed mechanisms in Scheme 8. | ||
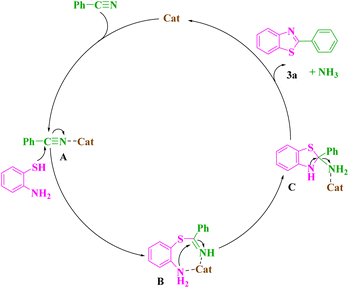
The Gibbs formation energies of the TS1 for the mechanisms 1 and 2 are, 5.7 and 7.5 kcal mol−1, respectively, that reveal a higher difference as compared to the TS2 steps. These reactions involve the formation of a C–S and C–N bonds between [(APTDP)(FeCl4)2@HNT] and benzonitrile. Furthermore, the calculated Gibbs reaction energies for the formation of (1–3) and (2–3) complexes in the mechanisms 1 and 2 are, −8.3 kcal mol−1 and −7.9 kcal mol−1, respectively Finally, the Gibbs reaction energy for the dissociation of (1–3) to the (3a) in mechanism 1 is significantly lower than the similar step in the mechanism 2. According to these differences, the Gibbs free energy profiles for the two mechanisms predict the preference of mechanism 1 versus the mechanism 2. However, the differences of the Gibbs free energy values in two mechanisms are small, and due to the computational uncertainty, the mechanism 2 would be predicted to be competitive with the mechanism 1. Consequently, the most probable mechanism was proposed on the basis of theoretical study using DFT simulation method (Scheme 9). First, the benzonitrile is activated by the catalyst to give A and intermediate B by nucleophilic attack of thiol group of 2-aminothiophenol to the carbon of benzonitrile. Next, intramolecular cyclization of B in the presence of the catalyst afforded the intermediate C. Finally, elimination of NH3 from C furnishes the corresponding benzothiazole 3 and releases the catalyst for the next run.
To show the quality and reactivity of [(APTDP)(FeCl4)2@HNT], a comparison with those of some previously reported catalysts for the synthesis of 3a is presented in Table 3. As can be seen, our catalytic system is superior to the previously documented methods in terms of catalyst loading, time, yield, TON and TOF.
| Catalyst/conditions | Catalyst loading | Time (h) | Yielda (%) | TONb | TOFc (h−1) | Ref. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a Isolated yield.b Turn-over number.c Turn-over frequency. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Zn(OAc)2·2H2O, PMHS, 120 °C, solventless | 5 mol% | 18 | 91 | 18.2 | 1.01 | 15 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| TfOH, 100 °C | 2 mol% | 12 | 94 | 47.0 | 3.91 | 16 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cu(OAc)2, Et3N/EtOH, 70 °C | 10 mol% | 6 | 86 | 8.6 | 1.43 | 17 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CoSAs-NPs/NC (0.25), 150 °C, 10 h, PhCl (2 mL) | 2.5 mol% | 10 | 92 | 36.8 | 3.68 | 23 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| [(APTDP)(FeCl4)2@HNT]/solvent-free, 80 °C | 1.5 mol% | 4.5 | 95 | 63.3 | 14.07 | This work | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Furthermore, the green chemistry metrics such as atom-economy (AE), carbon efficiency (CE), reaction mass efficiency (RME), and optimum efficiency (OE) concepts, and E-factor, have been embraced by the academic community and the chemical industry.22 It is of great importance that the favourable green chemistry metrics like smaller E-factor (0.14) and higher atom economy (92.57), carbon efficiency (95%), reaction mass efficiency (87.72%), and optimum efficiency (94.76%) were observed for our optimized protocol (Table 4).
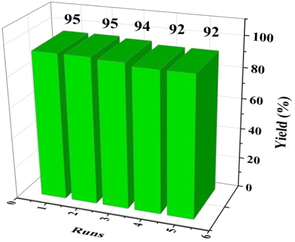
The recovery and reusability of a heterogeneous catalyst is an important feature from safety and economy point of view. For this purpose, the reusability of [(APTDP)(FeCl4)2@HNT] was probed using the model reaction under the optimized conditions (Fig. 9). In this respect, the mixture was cooled to room temperature at the end of each reaction and diluted with ethyl acetate. The catalyst was separated by centrifugation, washed several times with ethyl acetate, then, dried in a vacuum oven, and finally, reused in next run. The results revealed that the catalyst could be reused for four times without noticeable change in its catalytic activity (Fig. 9). Comparison of the FT-IR spectra of fresh and recovered catalysts showed no obvious changes in the structure of the catalyst and the characteristic bands. This clearly exposed that the catalyst is stable under the reaction conditions and can be recovered and reused (Fig. 10).
Conclusions
To summarize, a benign and facile protocol for the synthesis of benzothiazole derivatives through the reaction of 2-aminothiophenol and aryl nitriles using [(APTDP)(FeCl4)2@HNT], as a green catalyst, has been developed. The key achievement of this study is the selective synthesis of mono-, bis- and tris-benzothiazoles from dinitrile and trinitrile in high yields and purity. Excellent yields, short reaction times, easy work-up procedure, waste-free process combined with recovery and reusability of the catalyst, mild conditions, and avoiding hazardous organic solvents are other advantages of the present method.Experimental section
General
The chemicals used in this work were purchased from Fluka and Merck chemical companies. HNTs were purchased from Sigma-Aldrich (Germany) chemical company. The properties of HNTs are as follows: diameter = 30–70 nm, length = 1–3 μm, cation exchange capacity = 8.0 meq g−1, density = 2.53 g cm−3, average hydrodynamic diameter = 399.7 nm, specific surface area (as,BET = 39.156 m2 g−1), total pore volume = 0.214 cm3 g−1 and elemental compositions = Al2Si2O5(OH)4·2H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of Al/Si). Melting points were determined with a Stuart Scientific SMP2 apparatus. FT-IR spectra was recorded on a Jasco 6300 in the range of 400–4000 cm−1. 1H and 13C NMR (400 and 100 MHz) spectra were recorded on a Bruker Avance 400 MHz spectrometer using DMSO-d6 and CDCl3 as solvent. Elemental analysis was performed on a LECO, CHNS-932 analyzer. Thermogravimetric analysis (TGA) was carried out on a Mettler TG50 instrument under air flow at a uniform heating rate of 20 °C min−1 in the range 30–700 °C. The TGA instrument was re-calibrated at frequent intervals with standards; the accuracy was always better than ±2.0%. The scanning electron microscope measurement was carried out on a Hitachi S-4700 scanning electron microscope (SEM). The transmission electron microscopy (TEM) was carried out on a Philips CM10 instrument operating at 100 kV.
1 ratio of Al/Si). Melting points were determined with a Stuart Scientific SMP2 apparatus. FT-IR spectra was recorded on a Jasco 6300 in the range of 400–4000 cm−1. 1H and 13C NMR (400 and 100 MHz) spectra were recorded on a Bruker Avance 400 MHz spectrometer using DMSO-d6 and CDCl3 as solvent. Elemental analysis was performed on a LECO, CHNS-932 analyzer. Thermogravimetric analysis (TGA) was carried out on a Mettler TG50 instrument under air flow at a uniform heating rate of 20 °C min−1 in the range 30–700 °C. The TGA instrument was re-calibrated at frequent intervals with standards; the accuracy was always better than ±2.0%. The scanning electron microscope measurement was carried out on a Hitachi S-4700 scanning electron microscope (SEM). The transmission electron microscopy (TEM) was carried out on a Philips CM10 instrument operating at 100 kV.
General procedure for the preparation of [(APTDP)(FeCl4)2@HNT]
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) petroleum ether/ethyl acetate, 5
petroleum ether/ethyl acetate, 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), the mixture was cooled to room temperature and ethyl acetate (5 mL) was added. Then, the catalyst was separated by centrifugation and washed with ethyl acetate (3 × 5 mL). The organic residue was purified by column chromatography on silica gel (petroleum ether/ethyl acetate) to give the pure product in 90–96% yields (Scheme 3, 3a–n and Scheme 4, 5a–e).
1), the mixture was cooled to room temperature and ethyl acetate (5 mL) was added. Then, the catalyst was separated by centrifugation and washed with ethyl acetate (3 × 5 mL). The organic residue was purified by column chromatography on silica gel (petroleum ether/ethyl acetate) to give the pure product in 90–96% yields (Scheme 3, 3a–n and Scheme 4, 5a–e).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) petroleum ether/ethyl acetate, 5
petroleum ether/ethyl acetate, 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Upon completion, the work-up was done as defined for synthesis of mono-benzothiazoles and the desired products were obtained in 78–80% yields (Scheme 5, 3p and 5g).
1). Upon completion, the work-up was done as defined for synthesis of mono-benzothiazoles and the desired products were obtained in 78–80% yields (Scheme 5, 3p and 5g).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) petroleum ether/ethyl acetate, 5
petroleum ether/ethyl acetate, 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), the work-up was carried out as described for synthesis of mono-benzothiazoles and the corresponding product was gained in 75% isolated yield (Scheme 6, 5h).
1), the work-up was carried out as described for synthesis of mono-benzothiazoles and the corresponding product was gained in 75% isolated yield (Scheme 6, 5h).Abbreviations
| HNTs | Halloysite nanotubes |
| ILs | Ionic liquids |
| APTS | (3-Aminopropyl)triethoxysilane |
| TCT | 1,3,5-Trichlorotriazine |
| DIPEA | N,N-Diisopropylethylamine |
| THF | Tetrahydrofuran |
| PPh3 | Triphenylphosphine |
| p-TSA | p-Toluenesulfonic acid |
| TLC | Thin layer chromatography |
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are grateful to the Research Council of the University of Isfahan for financial support of this work.Notes and references
- (a) C. S. Buettner, A. Cognigni, C. Schröder and K. Bica-Schröder, J. Mol. Liq., 2022, 347, 118160–118191 CrossRef CAS; (b) V. S. Sivasankarapillai, A. Sundararajan, E. C. Easwaran, M. Pourmadadi, A. Aslani, R. Dhanusuraman, A. Rahdar and G. Z. Kyzas, J. Mol. Liq., 2023, 382, 121846–121856 CrossRef; (c) R. Goutham, P. Rohit, S. S. Vigneshwar, A. Swetha, J. Arun, K. P. Gopinath and A. Pugazhendhi, J. Mol. Liq., 2022, 349, 118150–118160 CrossRef CAS; (d) K. Dong, X. Liu, V. Dong, X. Zhang and S. Zhang, Chem. Rev., 2017, 117, 6636–6695 CrossRef CAS PubMed; (e) C. Dai, J. Zhang, C. Huang and Z. Lei, Chem. Rev., 2017, 117, 6929–6983 CrossRef CAS PubMed; (f) B. Wang, L. Qin, T. Mu, Z. Xue and G. Gao, Chem. Rev., 2017, 117, 7113–7131 CrossRef CAS PubMed.
- (a) S. T. Hemp, M. Zhang, M. H. Allen Jr., S. Cheng, R. B. Moore and T. E. Long, Macromol. Chem. Phys., 2013, 214, 2099–2107 CrossRef CAS; (b) K. Tsunashima, E. Niwa, S. Kodama, M. Sugiya and Y. Ono, J. Phys. Chem. B, 2009, 113, 15870–15874 CrossRef CAS PubMed; (c) K. Tsunashima, S. Kodama, M. Sugiya and Y. Kunugi, Electrochim. Acta, 2010, 56, 762–766 CrossRef CAS; (d) K. Tsunashima, A. Kawabata, M. Matsumiya, S. Kodama, R. Enomoto, M. Sugiya and Y. Kunugi, Electrochem. Commun., 2011, 13, 178–181 CrossRef CAS; (e) J. Luo, O. Conrad and I. F. Vankelecom, J. Mater. Chem., 2012, 22, 20574–20579 RSC; (f) U. A. Rana, R. Vijayaraghavan, M. Walther, J. Sun, A. A. Torriero, M. Forsyth and D. R. MacFarlane, Chem. Commun., 2011, 47, 11612–11614 RSC; (g) A. C. Barsanti, C. Chiappe, T. Ghilardi and C. S. Pomelli, RSC Adv., 2014, 4, 38848–38854 RSC.
- (a) M. J. Saif, H. M. Asif and M. Naveed, J. Chil. Chem. Soc., 2018, 63, 4109–4125 CrossRef CAS; (b) X. Ding, H. Wang, W. Chen, J. Liu and Y. Zhang, RSC Adv., 2014, 4, 41993–41996 RSC; (c) L. Duan, W. Huang and Y. Zhang, RSC Adv., 2015, 5, 6666–6674 RSC; (d) Z. Hajizadeh and A. Maleki, Mol. Catal., 2018, 460, 87–93 CrossRef CAS.
- (a) N. Bálsamo, S. Mendieta, A. Heredia and M. Crivello, Mol. Catal., 2020, 481, 110290–110299 CrossRef; (b) V. Bertolino, G. Cavallaro, G. Lazzara, S. Milioto and F. Parisi, Langmuir, 2017, 33, 3317–3323 CrossRef CAS PubMed; (c) S. Sadjadi, M. M. Heravi, M. Malmir and B. Masoumi, Appl. Organomet. Chem., 2018, 32, 4113–4125 CrossRef; (d) S. Sadjadi, M. M. Heravi, M. Malmir and F. G. Kahangi, Appl. Clay Sci., 2018, 162, 192–203 CrossRef CAS; (e) S. Sadjadi, M. Malmir and M. M. Heravi, Appl. Clay Sci., 2019, 168, 184–195 CrossRef CAS; (f) S. Sadjadi, G. Lazzara, M. M. Heravi and G. Cavallaro, Appl. Clay Sci., 2019, 182, 105299–105313 CrossRef CAS; (g) B. Eftekhari far and M. Nasr-Esfahani, Appl. Organomet. Chem., 2020, 34, 5406–5419 CrossRef.
- (a) X. Ding, H. Wang, W. Chen, J. Liu and Y. Zhang, RSC Adv., 2014, 4, 41993–41996 RSC; (b) L. Yu, Y. Zhang, B. Zhang and J. Liu, Sci. Rep., 2014, 4, 4551–4556 CrossRef PubMed; (c) J. Kurczewska, M. Cegłowski, B. Messyasz and G. Schroeder, Appl. Clay Sci., 2018, 153, 134–143 CrossRef CAS; (d) Z. Long, Y. P. Wu, H. Y. Gao, Y. F. Li, R. R. He and M. Liu, Bioconjugate Chem., 2018, 29, 2606–2618 CrossRef CAS PubMed; (e) S. Ramanayaka, B. Sarkar, A. T. Cooray, Y. S. Ok and M. Vithanage, J. Hazard. Mater., 2020, 384, 121301–121327 CrossRef CAS PubMed.
- S. M. Stagnaro, C. Volzone and L. Huck, Procedia Mater. Sci., 2015, 8, 586–591 CrossRef CAS.
- (a) R. K. Deshmukh, L. Kumar and K. K. Gaikwad, Appl. Clay Sci., 2023, 234, 106856–106866 CrossRef CAS; (b) D. Cheikh, H. Majdoub and M. Darder, Appl. Clay Sci., 2022, 216, 106335–106345 CrossRef CAS.
- (a) J. Nishiu, M. Ito, Y. Ishida, M. Kakutani, T. Shibata, M. Matsushita and M. Shindo, Diabetes, Obes. Metab., 2006, 8, 508–516 CrossRef CAS PubMed; (b) L. Leventhal, M. R. Brandt, T. A. Cummons, M. J. Piesla, K. E. Rogers and H. A. Harris, Eur. J. Pharmacol., 2006, 553, 146–148 CrossRef CAS PubMed; (c) W. S. Saari, J. M. Hoffman, J. S. Wai, T. E. Fisher, C. S. Rooney, A. M. Smith, C. M. Thomas, M. E. Goldman and J. A. O'Brien, J. Med. Chem., 1991, 34, 2922–2925 CrossRef CAS PubMed; (d) L. Q. Sun, J. Chen, K. Takaki, G. Johnson, L. Iben, C. D. Mahle, E. Ryan and C. Xu, Bioorg. Med. Chem. Lett., 2004, 14, 1197–1200 CrossRef CAS PubMed; (e) S. Yoshida, S. Shiokawa, K. I. Kawano, T. Ito, H. Murakami, H. Suzuki and Y. Sato, J. Med. Chem., 2005, 48, 7075–7079 CrossRef CAS PubMed.
- (a) M. Wang, M. Gao, B. H. Mock, K. D. Miller, G. W. Sledge, G. D. Hutchins and Q. H. Zheng, Bioorg. Med. Chem., 2006, 14, 8599–8607 CrossRef CAS PubMed; (b) R. Bazzi, T. D. Bradshaw, J. C. Rowlands, M. F. Stevens and D. R. Bell, Toxicol. Appl. Pharmacol., 2009, 237, 102–110 CrossRef CAS PubMed; (c) K. Wang and F. P. Guengerich, Chem. Res. Toxicol., 2012, 25, 1740–1751 Search PubMed.
- (a) C. G. Mortimer, G. Wells, J. P. Crochard, E. L. Stone, T. D. Bradshaw, M. F. Stevens and A. D. Westwell, J. Med. Chem., 2006, 49, 179–185 CrossRef CAS PubMed; (b) S. Aiello, G. Wells, E. L. Stone, H. Kadri, R. Bazzi, D. R. Bell, M. F. Stevens, C. S. Matthews, T. D. Bradshaw and A. D. Westwell, J. Med. Chem., 2008, 51, 5135–5139 CrossRef CAS PubMed.
- B. L. Mylari, E. R. Larson, T. A. Beyer, W. J. Zembrowski, C. E. Aldinger, M. F. Dee, T. W. Siegel and D. H. Singleton, J. Med. Chem., 1991, 34, 108–122 CrossRef CAS PubMed.
- (a) M. A. Butters, W. E. Klunk, C. A. Mathis, J. C. Price, S. K. Ziolko, J. A. Hoge, N. D. Tsopelas, B. J. Lopresti, C. F. Reynolds III, S. T. DeKosky and C. C. Meltzer, Alzheimer Dis. Assoc. Disord., 2008, 22, 261–268 CrossRef PubMed; (b) W. E. Klunk, B. J. Lopresti, M. D. Ikonomovic, I. M. Lefterov, R. P. Koldamova, E. E. Abrahamson, M. L. Debnath, D. P. Holt, G. F. Huang, L. Shao and S. T. DeKosky, J. Neurosci., 2005, 25, 10598–10606 CrossRef CAS PubMed; (c) B. J. Lopresti, W. E. Klunk, C. A. Mathis, J. A. Hoge, S. K. Ziolko, X. Lu, C. C. Meltzer, K. Schimmel, N. D. Tsopelas, S. T. DeKosky and J. C. Price, J. Nucl. Med., 2005, 46, 1959–1972 CAS.
- K. D. Dhawale, A. P. Ingale, S. V. Shinde, N. M. Thorat and L. R. Patil, Synth. Commun., 2021, 51, 1588–1601 CAS.
- I. Mohammadpoor-Baltork, A. R. Khosropour and S. F. Hojati, Monatsh. Chem., 2007, 138, 663–667 CrossRef CAS.
- D. B. Nale and B. M. Bhanage, Synlett, 2015, 26, 2835–2842 CrossRef CAS.
- X. Yu, Q. Yin, Z. Zhang, T. Huang, Z. Pu and M. Bao, Tetrahedron Lett., 2019, 60, 1964–1966 CrossRef CAS.
- Y. Sun, H. Jiang, W. Wu, W. Zeng and X. Wu, Org. Lett., 2013, 15, 1598–1601 CrossRef CAS PubMed.
- (a) M. Moeini Korbekandi, I. Mohammadpoor-Baltork, M. Moghadam, S. Tangestaninejad, V. Mirkhani, A. Omidvar and B. Notash, ACS Omega, 2023, 8, 15883–15895 CrossRef PubMed; (b) M. Samadani, B. Asadi, I. Mohammadpoor-Baltork, V. Mirkhani, S. Tangestaninejad and M. Moghadam, RSC Adv., 2021, 11, 11976–11983 RSC; (c) M. Azizi, M. Nasr-Esfahani, I. Mohammadpoor-Baltork, M. Moghadam, V. Mirkhani, S. Tangestaninejad and R. Kia, J. Org. Chem., 2018, 83, 14743–14750 CrossRef CAS PubMed; (d) F. Rahmani, I. Mohammadpoor-Baltork, A. R. Khosropour, M. Moghadam, S. Tangestaninejad and V. Mirkhani, Tetrahedron Lett., 2016, 57, 2294–2297 CrossRef CAS; (e) F. Karimi, B. Tighsazzadeh, B. Asadi, I. Mohammadpoor-Baltork, M. Layeghi, V. Mirkhani, S. Tangestaninejad and M. Moghadam, RSC Adv., 2022, 12, 22180–22187 RSC; (f) B. Asadi, I. Mohammadpoor-Baltork, S. Tangestaninejad, M. Moghadam, V. Mirkhani and A. Landarani-Isfahani, New J. Chem., 2016, 40, 6171–6184 RSC; (g) B. Asadi, I. Mohammadpoor-Baltork, V. Mirkhani, S. Tangestaninejad and M. Moghadam, ChemistrySelect, 2020, 5, 7840–7848 CrossRef CAS; (h) T. Ataee-Kachouei, M. Nasr-Esfahani, I. Mohammadpoor-Baltork, V. Mirkhani, M. Moghadam, S. Tangestaninejad and B. Notash, Appl. Organomet. Chem., 2020, 34, 5948–5965 CrossRef.
- A. D. Becke, J. Chem. Phys., 1993, 98, 5648–5652 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson and H. Nakatsuji, Gaussian Inc., Wallingford CT, 2009, p. 139.
- S. J. Grimme, Comput. Chem., 2006, 27, 1787–1799 CrossRef CAS PubMed.
- (a) F. I. McGonagle, H. F. Sneddon, C. Jamieson and A. J. Watson, ACS Sustainable Chem. Eng., 2014, 2, 523–532 CrossRef CAS; (b) A. D. Curzons, D. J. Constable, D. N. Mortimer and V. L. Cunningham, Green Chem., 2001, 3, 1–6 RSC.
- B. Wang, M. Li, S. Zhang, H. Wu, Y. Liao and H. Li, Appl. Catal., B, 2023, 327, 122454–122469 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra05491h |
| This journal is © The Royal Society of Chemistry 2023 |