Open Access Article
Open Access ArticleVisible light photoreforming of greenhouse gases by nano Cu–Al LDH intercalated with urea-derived anions†
Ayat A.-E. Sakr *a,
Dalia R. Abd El-Hafizb,
Osama Elgabrya,
Eman S. Abdullaha,
Mohamed A. Ebiada and
Tamer Zakib
*a,
Dalia R. Abd El-Hafizb,
Osama Elgabrya,
Eman S. Abdullaha,
Mohamed A. Ebiada and
Tamer Zakib
aGas Chromatogarphy Lab, Analysis & Evaluation Division, Egyptian Petroleum Research Institute, Nasr City, Cairo 11727, Egypt. E-mail: ayatsakr@yahoo.com; ayatsakr78@gmail.com; ayatsakr@epri.sci.eg
bCatalysis Lab, Petroleum Refining Division, Egyptian Petroleum Research Institute, Nasr City, P.B. 11727, Cairo, Egypt
First published on 16th November 2023
Abstract
The accumulation of anthropogenic greenhouse gases (GHGs) in the atmosphere causes global warming. Global efforts are carried out to prevent temperature overshooting and limit the increase in the Earth's surface temperature to 1.5 °C. Carbon dioxide and methane are the largest contributors to global warming. We have synthesized copper–aluminium layered double hydroxide (Cu–Al LDH) catalysts by urea hydrolysis under microwave (MW) irradiation. The effect of MW power, urea concentration, and MII/MIII ratios was studied. The physicochemical properties of the prepared LDH catalysts were characterized by several analysis techniques. The results confirmed the formation of the layered structure with the intercalation of urea-derived anions. The urea-derived anions enhanced the optical and photocatalytic properties of the nano Cu–Al LDH in the visible-light region. The photocatalytic activity of the prepared Cu–Al LDH catalysts was tested for greenhouse gas conversion (CH4, CO2, and H2O) under visible light. The dynamic gas mixture flow can pass through the reactor at room temperature under atmospheric pressure. The results show a high conversion percentage for both CO2 and CH4. The highest converted amounts were 7.48 and 1.02 mmol mL−1 g−1 for CH4 and CO2, respectively, under the reaction conditions. The main product was formaldehyde with high selectivity (>99%). The results also show the stability of the catalysts over several cycles. The current work represents a green chemistry approach for efficient photocatalyst synthesis, visible light utilization, and GHGs' conversion into a valuable product.
1 Introduction
The greenhouse gases (GHGs) are those that absorb infrared radiation from the atmosphere, consequently trapping the heat energy that is emitted from the Earth's surface and causing global warming.1 This action results in climate change, that causes irreversible losses and damage to the environmental and human systems.2 Carbon dioxide (CO2) is the largest contributor to global warming. According to the Kyoto Protocol, methane is considered a GHG.3 It is a short-lived greenhouse gas compared with CO2.4 It is a potent greenhouse gas. The anthropogenic accumulation of GHGs in the atmosphere leads to the global warming phenomenon, where the temperature of the Earth's surface is higher than that before the industrial era by ∼1.5 °C. To prevent temperature overshooting and to limit global warming to 1.5 °C, global efforts are carried out in order to get the CO2 net zero emission scenario (NZE), where CO2 emissions decline to zero by 2050.5–7 The Global Methane Pledge is targeted to reduce about 30% of anthropogenic methane emissions by 2030.8,9 This could be done by using a clean energy source and supporting the transition to a low-carbon energy system.7 Reduction of the accumulation of GHGs in the atmosphere could be performed through their capture and utilization.6,10 Utilizing GHGs to produce fuel is considered a sustainable fuel production pathway.11 The main challenge is to overcome the stability and inertness of both CO2 and CH4 and activate them.12–14Photoreforming is a process where valuable products can be produced through the redox reaction of an illuminated photocatalyst. Thus, it utilizes light energy (solar) to produce fuel or chemical feedstock.15 This process is considered an eco-friendly conversion process.16 Photocatalytic partial oxidation of methane could be done using CO2 and/or H2O as soft oxidants. This process produces C1 platform molecules (such as formaldehyde, methanol, and carbon monoxide). Furthermore, higher hydrocarbons than methane (C2+) could be produced and are considered value-added products.10,12
Layered double hydroxide (LDH) or hydrotalcite-like materials are two-dimensional and classified as anionic clays. It is composed of positively charged (brucite-like) layers, with its anion counterpart located in the interlayer space. The layers consist of metal cations that are octahedrally surrounded by six hydroxide ions. The octahedral units form infinite two-dimensional layers by edge-sharing. A three-dimensional structure is formed by the stacking of the layers on top of each other. The positive charge nature of the layer arises from replacing the M2+ cation with the M3+ cation.17,18 The LDH general formula is [M1−x2+Mx3+ (OH)2]x+(Ax/nn−)·mH2O where (M2+ = Mg2+, Ca2+, Zn2+,…); (M3+ = Al3+, Cr3+, Co3+,…), and x is the molar ratio of M3+/(M2+ + M3+); 0.2 ≤ x ≤ 0.33.19 The counterpart interlayer anion (An−) could be inorganic (e.g., halides, nitrate, and carbonate) or organic (e.g., polymers, anionic drugs, and amino acids).17,18,20 The LDH materials have many potential applications in many fields, such as catalysis, adsorption, the chemical industry, and medical applications.20 Recently, LDH materials have been applied in energy conversion and storage21 and renewable energy production.22 It can be functionalized to be used as a sensor and light-emitting material.23 LDH materials are used as photocatalysts for CO2 or methane conversion.22,24–26
Due to the structural features of the LDH, which permit the good distribution of the metals within the layer structure, and due to the good catalytic properties of copper,27 we aimed to prepare LDH materials containing copper and aluminium (Cu–Al LDH) to be used as a photocatalyst for GHGs conversion. The Cu–Al LDH materials are precipitated homogeneously by the urea hydrolysis process. Urea is considered a precipitating agent because, when hydrolyzed, it produces hydroxide ions that raise the pH of the solution, consequently resulting in metal hydroxide precipitation. Its hydrolysis rate depends mainly on the temperature.20 We have used microwave (MW) irradiation as a green source of energy.28–30 By controlling the reaction conditions, urea-derived anions can be intercalated within the LDH structure, which could be considered in situ surface functionalization.31–34 It was reported that the insertion of heteroatoms (such as nitrogen) within the catalyst structure enhances its photocatalytic activity.15 The main target of this work is to measure the photocatalytic activity of the homogeneously precipitated Cu–Al LDH for the GHGs (CH4, CO2, and H2O) conversion. To the best of our knowledge, no data have been reported for the use of homogenously precipitated Cu–Al LDH materials for this reaction previously (Table S1†).
2 Materials and methods
2.1 Materials synthesis
The chemicals used were copper(II) nitrate trihydrate (Cu (NO3)2·3H2O, assay ≥98 wt%), aluminium nitrate nonahydrate (Al(NO3)3·9H2O, assay ≥98 wt%), and urea (assay ≥99.5 wt%). All raw materials were purchased from Sigma-Aldrich and used without any further purification. The water used in this work was deionized using a WaterPro system (Labconco Co., USA).In the typical synthesis, a solution of the metal salts (MII = Cu and MIII = Al) nitrate and urea (U) mixture was suited in a 1 L three-neck glass flask (synthesis reactor). The total metal (M) concentration was 0.05 mol L−1. The MII/MIII molar ratios and urea to total metal concentration molar ratios were varied as indicated in Table 1 and Section S1.† The synthesis reactor system was illustrated in our previous work.32 The mixture was subjected to different MW irradiation (Table 1) in the domestic MW oven, operating at a frequency of 2.45 GHz and a maximum energy of 900 watt; (Daewoo, South Korea) with continuous stirring by a mechanical stirrer (Stuart, UK). The synthesis reaction was performed under atmospheric pressure.
| Sample code | MW power | U/M | MII/MIII |
|---|---|---|---|
| Effect of MW power | |||
| C1 | 180 | 10 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| C2 | 270 | 10 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| C3 | 360 | 10 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Effect of U/M ratio | |||
| C4 | 270 | 7 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| C5 | 270 | 5 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| C6 | 270 | 4 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| C7 | 270 | 3 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Effect of MII/MIII ratio | |||
| C8 | 270 | 5 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| C9 | 270 | 3 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| C10 | 270 | 5 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
The synthesis time was fixed for all the prepared materials at 90 minutes. The temperature of the reaction was maintained at ∼95 °C, and the pH of the solution was periodically measured by a pH meter model pH-213 (Hanna, USA). A bluish-green precipitate (PPT) was distinguished. The reaction was stopped immediately after the reaction time by cold water. The formed PPT was then centrifuged (MPW-352; Poland), washed several times with deionized water, and dried at 80 °C. The sample abbreviations and synthesis conditions are listed in Table 1.
2.2 Characterization
The physicochemical properties of the synthesized materials were characterized by different techniques. The crystallinity and phase identification were determined using powder X-ray diffraction (XRD) (X'Pert PRO, PANalytical, Netherlands) by Cu Kα radiation at 40 kV with a 2θ angle range from 4° to 70°, a step size of 2θ = 0.02°, and a scanning step time of 0.4 s. The surface functional groups were determined by Fourier Transform infrared (FT-IR) analysis (Nicolet IS 50 FTIR Spectrometer (Thermo-Fisher, USA)). The elemental analysis was performed using energy-dispersive spectroscopy (EDS) (Zeiss SmartEDX detector). The optical properties of the prepared LDH samples were examined using diffuse reflectance UV/vis spectroscopy (DR-UV/vis), GBC Cintra-3030UV visible spectrophotometer. The materials' photoluminescence (PL) spectra were recorded by Cary Eclipse Fluorescence Spectrometer equipped with Xe-lamp (Agilent, USA). The excitation wavelength was 350 nm. The material morphologies were characterized using a field emission scanning electron microscope (FE-SEM), Carl Zeiss, Sigma VP 300 (Germany).2.3 Photocatalytic activity
The ability of the prepared catalysts towards CH4/CO2/H2O photoconversion under visible light in a dynamic flow system was tested. The source of visible light is an ozone-free Xenon arc lamp (150 watt), model OPS A-150 Newport Cooperation. The working power was 140 watt. In a gas-tight glass vessel reactor, 50 mg of the catalyst was mixed with 150 mL of deionized water under stirring. A flow of CH4/CO2 (85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 vol%) was allowed to pass through the reactor in the dark for 1 h. Then, the light is turned on while the gas flow (5 mL min−1) is passed. The gas concentration before and after the reaction was continuously measured by an online natural gas analyzer (Chromatec Crystal 9000, Russia). The instrument is a gas chromatograph equipped with two thermal conductivity detectors (TCD) and a flame ionization detector. The analysis columns used are Hp-1 60, MolSeive, and Carboxen 1000. To measure the product formed in the liquid phase, after the reaction time, the liquid portion was collected and transferred to the headspace vials. The liquid samples were heated in the headspace for 1 h, then the liberated gases were analyzed using headspace gas chromatography-mass spectroscopy (HS-GC-MS); Chromatec Corporation, Russia.
15 vol%) was allowed to pass through the reactor in the dark for 1 h. Then, the light is turned on while the gas flow (5 mL min−1) is passed. The gas concentration before and after the reaction was continuously measured by an online natural gas analyzer (Chromatec Crystal 9000, Russia). The instrument is a gas chromatograph equipped with two thermal conductivity detectors (TCD) and a flame ionization detector. The analysis columns used are Hp-1 60, MolSeive, and Carboxen 1000. To measure the product formed in the liquid phase, after the reaction time, the liquid portion was collected and transferred to the headspace vials. The liquid samples were heated in the headspace for 1 h, then the liberated gases were analyzed using headspace gas chromatography-mass spectroscopy (HS-GC-MS); Chromatec Corporation, Russia.
The light-off experiment was performed under the same conditions as the reaction but without both light illumination and catalyst. The gas mixture was continuously fed through the catalytic reactor, which held 150 mL of deionized water. The flow rate was 5 mL min−1. The change in gas mixture concentrations before and after the reactor was measured.
3 Results and discussion
3.1 pH monitoring
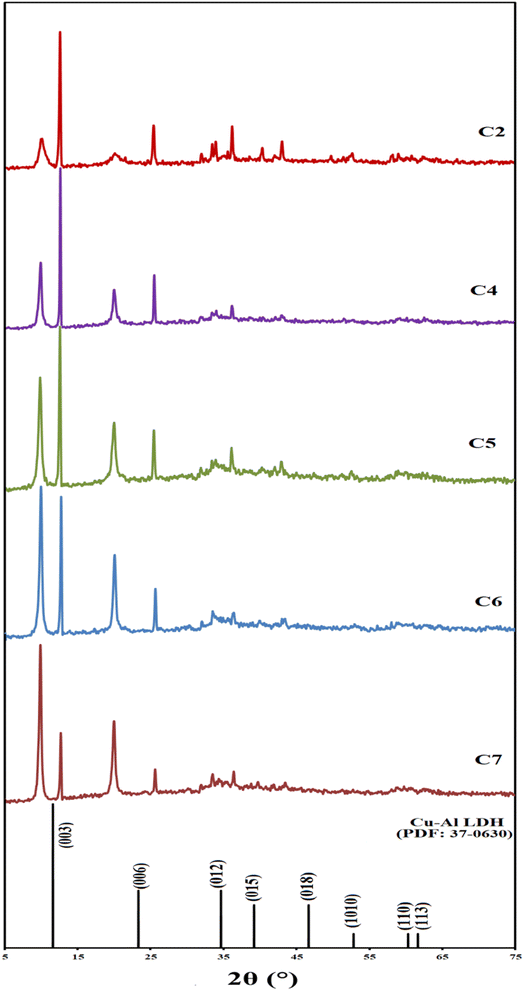
Monitoring the pH variation during the synthesis reaction gives important information about the hydrolysis process and, consequently, the formation of the formed phases. Three different factors are considered during the synthesis of the materials under investigation: the effect of the MW power, the effect of urea percentages, and the effect of the MII/MIII ratio (Table 1). The pH of the material synthesis solution is gradually raised by the action of the release of OH− due to urea hydrolysis.35 The detailed discussion of pH variation during the material synthesis reaction is listed in the ESI File in Section (S2), Fig. (S1–S4), and Tables (S2–S4).† The formation of PPT was observed when the pH exceeded the value of 4; its time depends on the reaction conditions. Thus, for the same reaction conditions, the PPT is formed earlier at a higher MW power, higher urea concentration, or high MIII ratio. Very low yield was noticed for samples C6, C7, and C9, with corresponding final pH values of 4.22, 4.06, and 3.92, respectively.![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m space group.36,37 Thus, assuming 3R packing of the layers, hkl diffraction plans (003), (006), and (012) are observed at 2θ = 12.6° (±0.1), 25.4° (±0.1), and 33.7° (±0.1) with the corresponding basal spaces of 7.0 (±0.1), 3.5 (±0.1), and 33.7 (±0.1) Å, respectively. Based on the layer thickness postulation of 4.8 Å, the interlayer gallery height can be calculated from the difference between the layer thickness and the d(003) spacing. The obtained interlayer gallery height was 2.2 (±0.1) Å, is suitable for carbonate intercalation and/or oriented anions.32,33,37,38
m space group.36,37 Thus, assuming 3R packing of the layers, hkl diffraction plans (003), (006), and (012) are observed at 2θ = 12.6° (±0.1), 25.4° (±0.1), and 33.7° (±0.1) with the corresponding basal spaces of 7.0 (±0.1), 3.5 (±0.1), and 33.7 (±0.1) Å, respectively. Based on the layer thickness postulation of 4.8 Å, the interlayer gallery height can be calculated from the difference between the layer thickness and the d(003) spacing. The obtained interlayer gallery height was 2.2 (±0.1) Å, is suitable for carbonate intercalation and/or oriented anions.32,33,37,38
The lattice parameters (a) and (C) for the LDH crystal structure are determined from d(011) and d(003), respectively. The (a) parameter corresponds to the cation–cation average distance within the brucite-like layer, thus a = 2d(110).18,36 The plane (011) is the first peak of the doublet that appeared at 2θ ∼ 60° and is characterized by a weak and broad peak. The (a) parameter depends mainly on the effective ionic radius of Cu2+ (0.74 Å) and Al3+ (0.54 Å) and ionic charge. Increasing aluminium content causes a decrease in (a) due to the increasing layer charge density.37 The weakening of the (110) plane is due to the presence of transition metal (Cu2+), that disrupts the octahedral cation coordination and, consequently, leads to poor long-range ordering (known as the Jahn–Teller distortion effect).27,39 It was observed that the intensity of the (110) plan decreased as the urea content decreased (Fig. 1), reflecting the role of urea percentage on the cation ordering within the layered structure. Furthermore, the (a) value decreases as the urea concentration increases, in agreement with that reported previously.40 This could be explained as follows: at the same synthesis reaction condition, increasing urea content results in a high hydrolysis rate, and further metal precipitation gives rise to the high LDH yield rate under the MW power, as indicated from the pH curves in Fig. S2 and Table S3.† The C parameter is the distance corresponding to one brucite and one interlayer distance. It could be calculated from the d-spacing of the (003) plane, where C = 3d(003) is directly affected by the interlayer anion size, orientation, the electrostatic charge between the negatively charged anion and the positively charged layers, and the degree of interlayer hydration.37,41–43
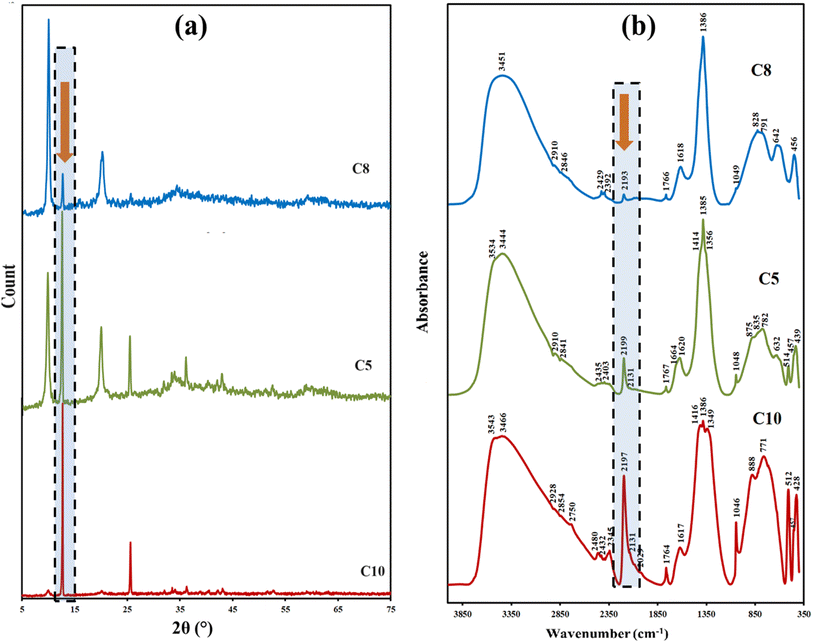
The previously obtained results were considered for LDH phase (I) because another LDH phase (II) was detected in all the as-prepared materials at lower 2θ close to 9.9, 19.9°, and 31.9°, corresponding to (003), (006), and (012) plans with d spaces of close to 8.8, and 2.8 Å. The LDH phase (II) is in agreement with that reported previously,44–46 and the presence of two LDH phases was reported by Peng and co-workers for the Cu–Al LDH prepared using the urea hydrolysis method (Table 2).46 The intensity of this phase varies according to the reaction conditions. The interlayer gallery heights are close to 4.1 Å, which could be due to the intercalation of larger-dimensional anions such as urea-derived anions.27,31 The intensity of LDH phase (II) relative to LDH phase (I) is increases as the urea percent decreases and as the Al percent increases (Table 2, Fig. 1 and 2a). For samples prepared at different MW powers, the gallery height of phase II increases as the MW power increases (Fig. S6†). This could be attributed to the increasing MW power that accelerated the urea hydrolysis and permitted the insertion of large amounts of the produced anions within the layers. This observation is in agreement with that observed in the plateau region (v) in the pH monitoring curve (Fig. S1†).
| Sample | Phase I | Phase II | Phase (II)/phase (I) (count%) | |||||
|---|---|---|---|---|---|---|---|---|
| d(003) (Å) | a (Å) | C (Å) | Gallery height (Å) | d(003) (Å) | C (Å) | Gallery height (Å) | ||
| Effect of MW power | ||||||||
| C1 | 7.072 | 3.048 | 21.215 | 2.272 | 8.274 | 24.823 | 3.474 | 11.393 |
| C2 | 7.018 | 3.050 | 21.054 | 2.218 | 8.805 | 26.414 | 4.005 | 17.556 |
| C3 | 7.018 | 3.047 | 21.055 | 2.218 | 8.889 | 26.667 | 4.089 | 12.194 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||
| Effect of U/M ratio | ||||||||
| C2 | 7.018 | 3.050 | 21.054 | 2.218 | 8.805 | 26.414 | 4.005 | 17.556 |
| C4 | 7.009 | 3.078 | 21.028 | 2.209 | 8.879 | 26.638 | 4.079 | 28.748 |
| C5 | 7.024 | 3.087 | 21.071 | 2.224 | 8.985 | 26.955 | 4.185 | 41.595 |
| C6 | 6.950 | 3.109 | 20.851 | 2.150 | 8.892 | 26.677 | 4.092 | 51.533 |
| C7 | 6.974 | 3.109 | 20.921 | 2.174 | 8.926 | 26.779 | 4.126 | 69.847 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||
| Effect of MII/MIII ratio | ||||||||
| C9 | 6.955 | — | 20.865 | 2.155 | 8.904 | 26.712 | 4.104 | 73.698 |
| C8 | 6.979 | 3.053 | 20.938 | 2.179 | 8.823 | 26.470 | 4.023 | 84.228 |
| C5 | 7.024 | 3.087 | 21.071 | 2.224 | 8.985 | 26.955 | 4.185 | 41.595 |
Sample C9 was prepared under low a U/M = 3 percent and a low MII/MIII = 2 ratio (Table 1). The corresponding C9 XRD pattern did not exhibit a peak at 2θ ∼ 60°, so its lattice parameter (a) could not be calculated (Table 2). In this regard, samples C6, C7, and C9 will not be considered for further investigation because of their very low ppt yield as indicated by the pH change curves (Fig. S2 and S4†) and the low intensity of the resulting LDH phases (Fig. 1 and S5†).
A broad and intense vibrational band in the region between 3000 and 4000 cm−1 is attributed to the hydrogen-bonded hydroxyl groups in the brucite-like layers with interlayer water molecules.37,46 It is characterized by two maxima with nearly the same intensities at ∼3540 cm−1 and ∼3440 cm−1. As the aluminium content increased, only one maximum was distinguished at ∼3455 cm−1 and became broader (Fig. 2b). This may suggest that the interaction between the layers, interlayer anions, and water molecules increases as the aluminium concentration increases.49 No isolated bands in this region are observed that are characteristics of copper hydroxide or aluminium hydroxide. This indicates that all hydroxyl groups are involved in the hydrogen bonding and confirms the results from the XRD for the purity of the material's LDH structure. The interlayer bending mode appears at ∼1620 cm−1.36,37
The asymmetric stretching vibrational ν3 mode for interlayer mono-dentate carbonate has appeared at the absorption band ∼1383 cm−1. The splitting of this peak, observed at ∼1350 cm−1, could be attributed to the lowering of the symmetry of carbonate anions. This indicates the different intercalation modes of bi-dentate carbonate. Another split shoulder is detected at ∼1410 cm−1, and it becomes more distinguished as the copper content increases. This band could be related to the ν3 mode of interlayer-free carbonate anions.31,50 The carbonate ν1 mode is detected as a small band at ∼1045 cm−1. This vibrational mode is IR inactive; however, the existence of the carbonate anions in the interlayer structure is affected by the electrostatic interaction with the positively charged layers and interlayer water molecules. This leads to the lowering of its symmetry; consequently, this mode becomes an IR active.36,37,42 The absorption band at ∼880 cm−1 is assigned to ν2 carbonate deformation mode.51 The small absorption bands at 2300 to 2480 cm−1 could be due to the physically adsorbed CO2 on different surface-accessible sites.
The absorption band that appeared at ∼2198 cm−1 could be attributed to the intercalated n-bonded isocyanate group.29,51 This band decreased as the copper content decreased (Fig. 2b). This reflects the role of metal type in the urea hydrolysis reaction rate and, consequently, the type of the produced urea-derived anion. The very small shoulders at ∼2131 and 2090 cm−1 could be an indication of the different intercalated modes of the isocyanate groups.52,53 The small band at ∼630 cm−1 could be due to the bending vibration of NCO.53,54 This band is not distinguished in LDH samples with high aluminium content. The sharp and small band at ∼512 cm−1 is found to increase with copper content (Fig. 2b), which may be due to the bending vibration of the C–N bond.55 By comparing the results from the IR spectra and XRD patterns of samples prepared at different MII/MIII ratios (Fig. 2a and b; in the shaded region under the arrows), it was observed that the intensity of the absorption band related to the isocyanate group (at ∼2198 cm−1) is directly proportional to the intensity of the XRD peak at 2θ∼ 12.6° (d ∼7.0 Å). As the isocyanate IR absorption band increases, the intensity of the diffraction peak at 2θ ∼ 12.6° increases. According to the reference material data (PDF file no. 37-0630), this d spacing is for the presence of intercalated carbonate anions. From the above results, it could be concluded that the d spacing (∼7.0 Å) of the prepared Cu–Al LDH materials is suitable for the intercalation of carbonate anion and oriented isocyanate groups. The intercalated anions in the interlayer space could be oriented in order to maximize the interaction with the positively charged layers.56
The appearance of shoulders at ∼2900 and 2830 cm−1 may indicate the hydrogen-bonded bridging water molecule with carbonate and NH vibration.57 The small shoulder that appeared at ∼1666 cm−1 could be assigned to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group in the amide group (–CO–NH–).54,58,59 The presence of NHCOO– species is supported by the small vibrational band at ∼1764 cm−1. This band becomes sharper and more intense as the copper content increases.58,60 In addition, the band maximum is at ∼3444 cm−1 and shifts to a higher wavenumber as the Cu content increases.61,62 This could be assigned to the hydrogen-bonded stretching NH groups. These results are in agreement with the XRD data. Thus, the intercalation of bi-dentate carbonate anions and oriented isocyanate species inside the layered structure could be responsible for the d003 spacing of 7.0 (±0.1) Å. Moreover, the d003 spacing of ∼9.9 Å could be suitable for the intercalation of bigger urea-derived anions.31
O group in the amide group (–CO–NH–).54,58,59 The presence of NHCOO– species is supported by the small vibrational band at ∼1764 cm−1. This band becomes sharper and more intense as the copper content increases.58,60 In addition, the band maximum is at ∼3444 cm−1 and shifts to a higher wavenumber as the Cu content increases.61,62 This could be assigned to the hydrogen-bonded stretching NH groups. These results are in agreement with the XRD data. Thus, the intercalation of bi-dentate carbonate anions and oriented isocyanate species inside the layered structure could be responsible for the d003 spacing of 7.0 (±0.1) Å. Moreover, the d003 spacing of ∼9.9 Å could be suitable for the intercalation of bigger urea-derived anions.31
The EDX analysis also confirmed the presence of nitrogen-containing groups. Fig. (S9) and Table (S5)† represent the EDX analysis and elemental atom percent results of sample C4. This is in agreement with the pH change observation, XRD, and FTIR data. The results confirm the theoretical metals percentage ratio (Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al = 3
Al = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).
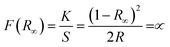 | (1) |
 , and ∝ is the absorption coefficient.
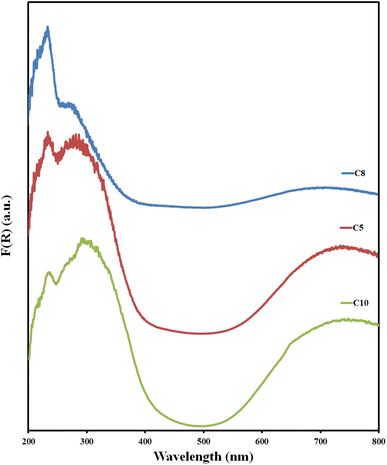
, and ∝ is the absorption coefficient.The characteristics of the DR UV-vis spectra analysis of the prepared Cu–Al LDH materials are represented in Fig. 3 and S10,† where a plot of Kubelka–Munk (K–M) function F(R∞) vs. the wavelength is shown. The spectra indicate the presence of three absorption edges in the regions 200–250 (I), 250–400 (II), and 600–800 nm (III) as follows:
1- In region I, absorption edges ∼233 nm (intense peak), its intensity increases as the MW power increases; decreases with aluminum content; and is slightly affected by the change in urea concentration. This band has not been detected previously in typical Cu–Al-LDH materials.42 This band could be due to the type of intercalated anion that contains a nitrogen group, where the presence of nitrogen in the catalyst structure enhances its optical properties.65–67
2- Region II is a broad shoulder with an absorption edge of ∼275 nm, whose intensity decreases with increasing MW power. It becomes more distinct with increasing copper content and is slightly shifted to a lower wavelength with increasing urea concentration. This band may be due to the O2− → Cu2− ligand-to-metal charge transfer transition.37,42,68
3- Region III, a very broad peak at ∼730 nm, has an intensity that decreases with increasing MW power and increasing urea concentration. This band could be attributed to the d–d transition 2Eg(D) → 2T2g(D) of the Cu2+(d9) distorted octahedron due to the Jahn–Teller effect.37,42,49 Regarding the MII/MIII ratio, the band gap is decreasing with increasing Cu (d9) content. This indicates a decrease in the d–d transition energy barrier.69
The energy band gap (Eg) was calculated using the Tauc method67,68,70 (Section S3†).
Multiple band gaps have been observed in the plot of the (K–M) function with the photon energy, with the main band gap (I) at ∼3.0, band gap (II) at ∼1.2, and band gap (III) at ∼4.00 (eV); Fig. (S11–S13).† The fit of their values to the Tauc model is listed in Table 3.71 The multi-band gaps were reported previously.51,72,73
| Material | Band (I) | Band (II) | Band (III) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Extrapolated Eg (eV) | Fit to (∝hν)1/n | Extrapolated Eg (eV) | Fit to (∝hν)1/n | Extrapolated Eg (eV) | Fit to (∝hν)1/n | ||||
| n = 2 | n = 1/2 | n = 2 | n = 1/2 | n = 2 | n = 1/2 | ||||
| Effect of MW power | |||||||||
| C1 | 2.7 | 2.9 | 3.3 | 1.2 | 1.2 | 1.4 | 4.2 | 4.4 | 4.75 |
| C2 | 3.1 | 3.2 | 3.5 | 1.2 | 1.3 | 1.5 | 4.15 | 4.3 | 4.6 |
| C3 | 2.6 | 2.9 | 3.1 | 1.1 | 1.2 | 1.4 | 4.5 | 4.6 | 4.9 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||||
| Effect of urea concentration | |||||||||
| C2 | 3.1 | 3.2 | 3.5 | 1.2 | 1.3 | 1.5 | 4.15 | 4.3 | 4.6 |
| C4 | 3 | 3.1 | 3.3 | 1.1 | 1.2 | 1.3 | 3.35 | 3.7 | 4.3 |
| C5 | 3.1 | 3.15 | 3.4 | 1.05 | 1.1 | 1.3 | 3.3 | 4.7 | 4.4 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||||
| Effect of MII/MIII ratio | |||||||||
| C8 | 3.2 | 3.2 | 3.7 | 1.2 | 1.2 | 1.4 | 4.4 | 4.5 | 4.7 |
| C5 | 3.1 | 3.15 | 3.4 | 1.05 | 1.1 | 1.3 | 3.3 | 4.7 | 4.4 |
| C10 | 2.9 | 2.95 | 3.2 | 1.05 | 1.1 | 1.3 | 3.3 | 3.6 | 4.4 |
The DR UV-vis results demonstrate that the Cu–Al LDH materials could be regarded as visible light photocatalysts69 when compared with the results reported previously.74 Additionally, it might have potential uses as semiconductors in photosystems (like solar cells) or photochemistry.51
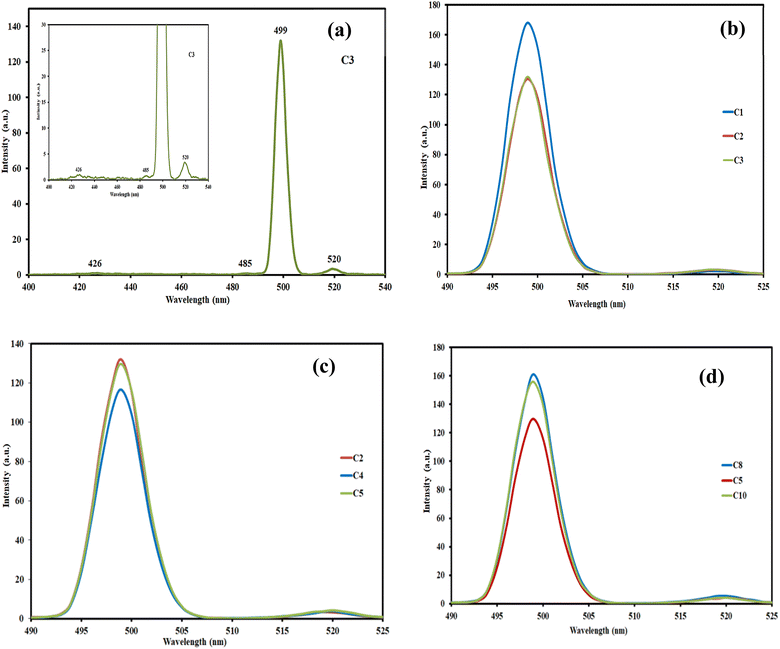
Fig. 4 shows the photoluminescence spectra of the prepared samples when excited at 350 nm. All samples exhibit four PL emission peaks in the visible region. Fig. 4a shows the detailed peaks taken from sample C3 as an example. The detected peaks were at ∼426 (small and broad peak), 483 (small shoulder), 499 (sharp and intense peak), and 520 nm (small and broad peak). The main cause of this activity is the presence of surface defects and surface-trapped charge carriers (excitons).77 This emission could be attributed to the charge-transfer transition of the electrons trapped in the oxygen vacancies that are considered photo-induced electron–hole (e−–H+) recombination centres.78 The most intense peak at 499 nm may be a result of emission generated by localized surface-trapped charge carriers recombination.79 These results reveal the presence of multiple energy levels responsible for Cu–Al LDH materials luminescence activity.80 The results also confirmed the data obtained from the DR-UV/vis analysis.
The lower PL intensity is an indication of longer charge separation and, consequently, a lower recombination rate of photogenerated electrons and holes that are formed during the excitation process.81,82 Gevers and co-workers have demonstrated that there are several reasons for the LDH luminescence emission broadening, such as:83
- Surface defects result from layer stacking disorder, edges and surface sites, oxygen, and cation vacancies.
- The unique LDH structure permits excitons to be generated within the interlayer and interlayer formations.
- Excitons that are stabilized by the Al3+ cation islands within the layers.
- The presence of interlayer anions assists the holes' stabilization, resulting in lowering the emission energy. This may cause the PL peak to broaden.
The insertion of nitrogen-containing groups (resulting from urea degradation or intercalated amino groups) may maximize the hole stabilization process, thus enhancing the LDH optical, as reported previously.78,84–86 Regarding the effect of urea concentration (Fig. 4c), the C4 sample, with U/M = 7, represents the lowest intensity of the main peak (at ∼499 nm). The MII/MIII ratio variation (Fig. 4d) revealed that C5, with MII/MIII = 3, had the lowest intensity. This observation is in agreement with that reported previously.87 For the samples prepared at different MW power, power at 270 and 360 watt (C2 and C3) shows a comparable intensity (Fig. 4b).
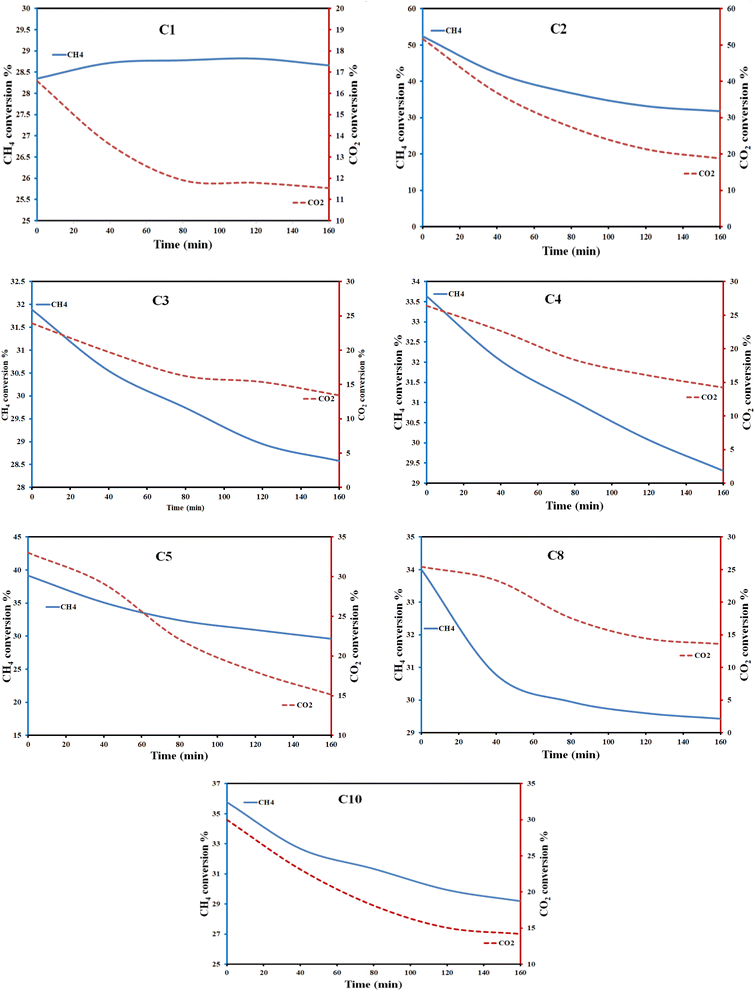 | ||
| Fig. 6 Variation of CH4 and CO2 conversion percent under the photo reaction conditions using the Cu–Al LDH catalysts. | ||
Fig. S16† represents the comparison between the conversion percentages of CH4 and CO2 for all the catalysts prepared at different conditions, respectively. It is obvious that, although both CH4 and CO2 are converted to some extent, both gases interact differently with the catalyst surface along the reaction time.
Fig. 7, S17, and S18† illustrate the cumulative converted amounts of both CH4 and CO2 over time under the reaction conditions as well as the selectivity of formaldehyde production. The remaining sample fractions were caused by the synthesis of more highly oxygenated hydrocarbons, and the formaldehyde selectivity was greater than 99%. It was observed that, regarding the samples prepared at different MW power, the C2 sample converted a higher amount of CH4 (7.47 mmol mL−1 g−1) and CO2 (1.02 mmol mL−1 g−1); however, the selectivity for formaldehyde is higher with the C3 sample (Fig. 6). Compared to samples prepared at different urea concentrations, the C2 sample with a higher urea ratio was observed to convert a higher amount of CH4 and CO2, Fig. S15.† For samples prepared at different MII/MIII ratios, sample C5, with MII/MIII = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 represents the highest conversion activity and the highest formaldehyde selectivity percent (Fig. S16†).
1 represents the highest conversion activity and the highest formaldehyde selectivity percent (Fig. S16†).
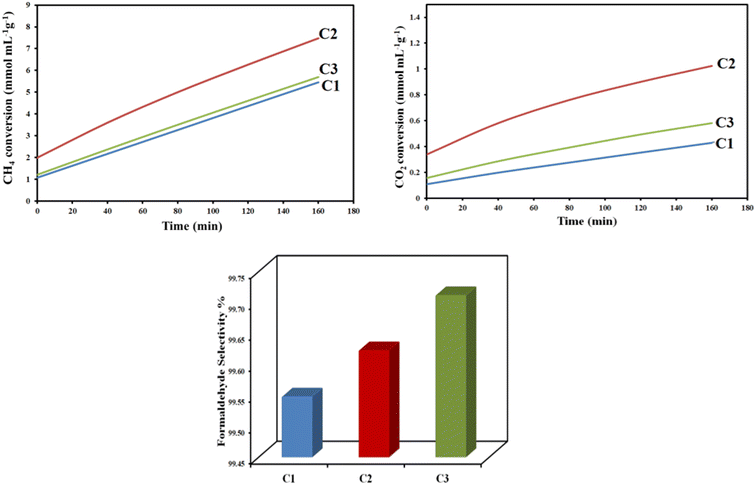 | ||
| Fig. 7 Accumulative converted amounts of CH4 and CO2 with time and corresponding formaldehyde selectivity% using the Cu–Al LDH catalysts prepared at different MW power. | ||
We selected sample C4 to study the effect of increasing the catalyst dose and the effect of cycling under repeated light on/off. Fig. 8 shows the variation of CH4 and CO2 conversion% with the catalyst dose (0.05, 0.1, and 0.5 g catalyst). The data shows that the higher dosage results in a low conversion rate. The behaviour of methane conversion is different at a low catalyst dose (0.05 g). For higher catalyst doses (>0.05 g), the conversion starts with a low percentage, increases with time, and decreases again. In addition, the selectivity percentage of formaldehyde was higher with the low catalyst dose of 0.05 g than with the higher doses of 0.1 and 0.5 g (Fig. S19†). Increasing the catalyst dose will result in particles crowding in the solution; thus, it could suffer from the light shielding effect. In this case, the particles cannot absorb light, resulting in less conversion and less selectivity. This behaviour indicates the essential role of the catalyst in the conversion process.88
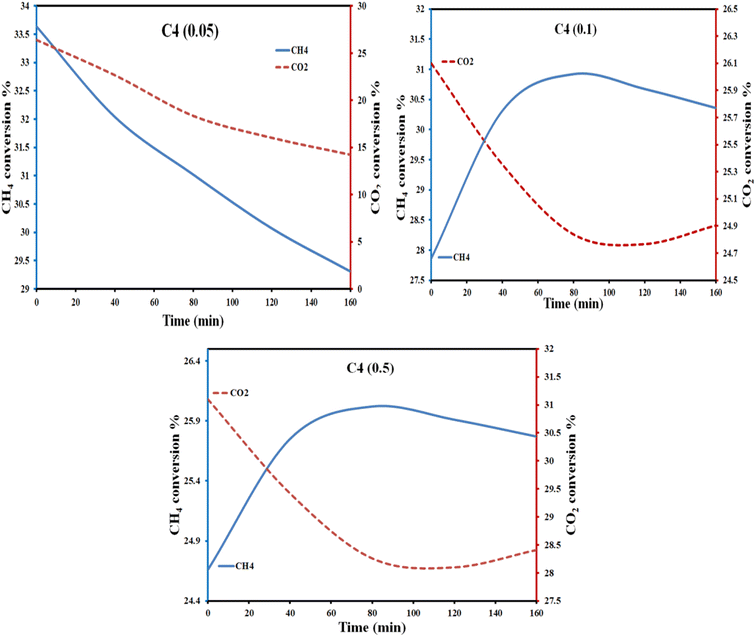 | ||
| Fig. 8 Effect of C4 catalyst dose (0.05, 0.1, and 0.5 g) on the variation of CH4 and CO2 conversion percent under the same photo reaction conditions. | ||
It was reported that the high H2O/CO2 ratio could lead to the formation of products such as formaldehyde, methanol, or formic acid in the liquid phase.89 Furthermore, regarding CH4 photoconversion, Wei et al. found that increasing water could be considered an effective strategy to enhance formaldehyde selectivity.90 The high amount of water promotes catalyst dilution and enhances light absorption.88,91 Moreover, the presence of the formed formaldehyde molecules on the catalyst surface will increase their chance of being oxidized to form CO2. However, the excessive water amount assists the formaldehyde desorption from the catalyst surface, consequently suppressing its oxidation.88,92 The detection of formaldehyde as a product is affected by its adsorption strength on the catalyst surface. If it is strongly bound to the surface, it is hard to detect.93
We tested the change in concentration of the gas mixture in the dark, where the gas flowed through the reactor system at the same conditions as the reaction but without using both light illumination and a catalyst (Fig. S20†). A small variation in the gas concentration is observed compared with the initial gas concentration value (before the catalytic reactor). This variation may be attributed to the diffusivity of both CH4 and CO2 in water.94
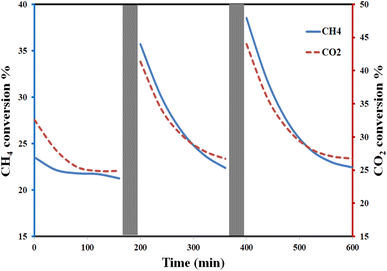
The durability of the Cu–Al LDH catalyst is shown in Fig. (9). Before each cycle, the light is turned off, and the gas flow is passed through the system in the dark for about 1 h before the light is turned on again. The conversion rate is enhanced after the first cycle. The minimum conversion percentage for both CH4 and CO2 is nearly constant in the repeated cycles. This behaviour could be related to the slow recombination rate of photogenerated electrons and holes during the excitation process by the incident light, as confirmed by the PL results. These results reflect the stability of Cu–Al LDH as the preferred photocatalyst for GHGs conversion. In addition, the presence of water molecules could play an important role in activating the active sites, preventing coke formation on the catalyst surface, and increasing the stability of the catalyst over time.95–97
3.1.6.1 Reaction mechanism. On the excitation of LDH by visible light, photons are absorbed with energy near the LDH band gap. The proposed reaction mechanism is illustrated in Scheme 1. The holes (h+) and photon-generated electrons (e−) are produced as a result of the movement of the excited electrons from the valence band to the conduction band.74
| LDH + hν → h+ + e− | (4) |
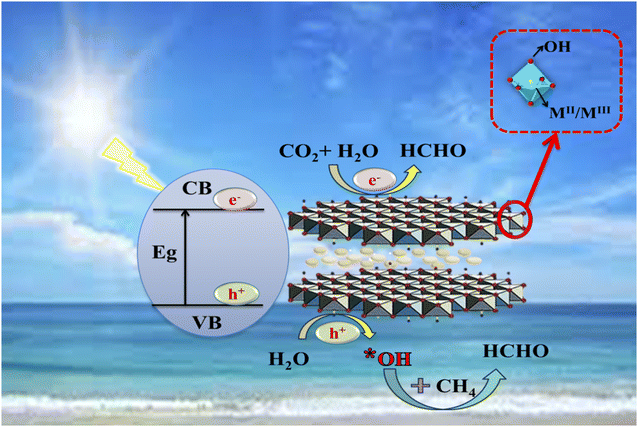 | ||
| Scheme 1 Proposed reaction mechanism of photoreforming greenhouse gases by Cu–Al LDH under visible light illumination. | ||
The O atoms in the hydroxyl group function as the focal point of photogenerated holes in the LDH photocatalysts (formed in the (003) phase surface (*)). The holes facilitate the oxidation of water molecules that are hydrogen bonded to the hydroxyl groups of the layers.69
| H2O + * → *OH + H+ + e− | (5) |
| *OH + H2O → *H2O2+ H+ + e− | (6) |
| *H2O2 → *OOH + H+ + e− | (7) |
| *OOH → * + O2 +H+ + e− | (8) |
The main product in this work was formaldehyde (as obtained from the GC-MS analysis), which could be formed as a result of CO2 and CH4 conversion. The mechanism of the reaction is uncertain; however, it could be as follows:
a- During CO2 reduction, CO2 is first introduced to water in the dark before light illumination, thus forming the carbonic acid specie. Then, upon illumination, CO2 could be reduced to formaldehyde:74
| (Dark) CO2+ H2O → HCO3− + H+ | (9) |
| (Light) HCO3− + 2H+ + 2e− → HCO2− + H2O | (10) |
| CO2 + e− → ˙CO2− | (11) |
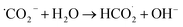 | (12) |
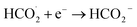 | (13) |
| HCO2− + 2H+ + 2e− → HCHO + OH− | (14) |
b- During methane oxidation, methane is not soluble in water,98 so it is not affected by the surrounding water in the dark. The mechanism could be predicted as follows:
• The superoxide (˙O2) that is generated from the absorption of the photogenerated electron by the oxygen on the layer's surface subsequently forms the HOO* radical (eqn (7)), then the hydroxyl radical (OH˙). Furthermore, the OH˙ radical can be produced from the reaction of photogenerated holes with water molecules. The presence of such species (*OH or *O2−) could accelerate CH4 activation by breaking the ˙C–H bond and forming the CH3 radical.68,99,100 It was stated that, due to the low reactivity of methane, the mobile OH˙ radicals could be more favourable for CH4 activation than the trapped radicals in the photogenerated holes.99 The produced ˙CH3 could be stabilized by the presence of nitrogen-containing groups on the catalyst surface.101
• The OH radical, considered an active oxidant, reacts with ˙CH3 once formed, and one of the products may be formaldehyde.102–104 It could be produced through the formation of the methoxy group (–OCH3) which is further oxidized by the active O− species to methanediol (HOCH2OH), then dehydrated to formaldehyde (HCHO).88,90,105,106
• The ˙CH3 radicals could be the source of the coupling products where higher hydrocarbons may be formed.101
In this work, a small amount of carbon monoxide (CO) was detected in the gas phase by GC-MS. This is in agreement with that reported previously, where the presence of excess water has a positive impact on the reduction of CO2 to CO.107 No formic acid or methanol was detected during the photoreaction in the gas or liquid phases. Moreover, higher hydrocarbon oxygenates such as ethanediol, glycolaldehyde, and acetaldehyde have been detected as small products in the liquid phase by GC-MS. This confirms the presence of a coupling reaction and may suggest the glyoxal mechanism, where the CHO radicle could be formed during the photo-excitation process.93
The introduction of nitrogen-containing groups within the catalyst structure increases its photocatalytic activity.108–110 The presence of urea-derived anions in the prepared Cu–Al LDH is believed to assist in the stabilization of the CO2 within the Cu–Al LDH structure. Thus, it allows a higher chance for conversion reaction during the catalyst excitation through the formed active species (trapped electrons and formed hole) (PL discussion).111
To the best of our knowledge, the conversion of GHGs (CH4, CO2, and H2O) using Cu–Al LDH papered by a homogenous precipitation process was not reported previously. Also, this work represents the direct formation of formaldehyde with high selectivity using Cu–Al LDH without using a scavenger substance under normal temperature and pressure conditions, which has not been reported previously. It is worth noting that the photoconversion of GHGs to fuel without using sacrificial scavengers is considered an approach to “green chemistry”.15,16,112 Table 4 illustrates the previously reported date for using the LDH photocatalyst in CO2 and/or CH4 conversion in comparison with the current work.
| Catalyst | Reactor | Light source | Reaction conditions | Reactant | Analysis method | Product | References |
|---|---|---|---|---|---|---|---|
| Cu–Al LDH intercalated with urea derived anions | Homemade gas-tight glass flow reactor | Solar simulator (150 W xenon lamp) | Dynamic flow system at room temperature | CH4/CO2/H2O | • Online-gas chromatography (FID/TCD) | Formaldehyde (direct formation) | This work |
| • Headspace gas chromatography-mass spectroscopy (HS-GC-MS) | |||||||
| TiO2/Co–Al LDH | Cylindrical glass reactor | 300 W xenon lamp | 15 °C | CO2 (99.9%) bubbled in water | Gas chromatography | CH3OH/CH4 | 113 |
| Mg–Al LDH | Stainless steel cylindrical reactor with a quartz window at the top | Solar simulator (150 W xenon lamp) | 0.05 mL min−1 CO2 for 30 min | Gas chromatography (TCD/FID) | CO/CH4 | 89 | |
| Noble metal (Pt, Pd, Au) loaded Zn–Cr LDH | Top-irradiation-type quartz glass vessel | UV irradiation from 200 W Hg–Xe lamps | Room temperature | CO2 gas (99.995%) with water vapor | Gas chromatography (FID) | CO | 114 |
| Zn–Cu–Al/Ga LDH | Flat bottom of the quartz reactor | UV-visible light from the 500 W xenon arc lamp | 32–40 °C | (2.3 kPa) CO2/(21.7 kPa) H2 | Online-gas chromatography (TCD) | Methanol | 71 and 115 |
| Zn–Cu–Al/Ga LDH | Pyrex glass plate | UV-visible light from the 500 W xenon arc lamp | High pressure (0.4 MPa) | CO2/H2 (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 v/v) 7 v/v) |
Gas chromatography (TCD) | Methanol | 116 |
| Mg–In LDH | Closed circulating system with a quartz glass ceiling for illumination | UV irradiation from 200 W Hg–Xe lamp | CO2/H2O | • Gas chromatography (TCD) | CO/O2 | 117 | |
| • Mass spectroscopy | |||||||
| Fluorinated Mg–Al LDH and Ni–Al LDH | Quartz inner-irradiation type reaction vessel | UV irradiation from 400 W high-pressure Hg lamp | NaCl solution scavenger | CO2/H2O | • Gas chromatography (TCD) | CO | 118 |
| • Gas chromatography-mass spectroscopy (GC-MS) | |||||||
| Calcined Mg–Co LDH | Quartz vessel | UV irradiation from 300 W Xe lamp | Under vacuum | 0.13 MPa CH4/H2O | • Gas chromatography (TCD) | Coupling product (C2H6) | 119 |
| • Gas chromatography-mass spectroscopy (GC-MS) | |||||||
| Ni–Al LDH intercalated with Cl anion | Quartz glass reactor | 400 W high-pressure Hg lamp | Contentious flow system/35 °C | CO2/NaCl solution | Barrier discharge ionization detector gas chromatography (BID-GC) | CO | 97 |
| Cu–Al based LDH (Cu–Mg–Al LDH, Cu–Zn–Al LDH and Cu–Ni–Al LDH) | Gas-tight system | Visible light irradiation from 500 W Xe lamp | 25 °C/aA gas pump is used to accelerate gas diffusion | CO2/H2O | Gas chromatography (TCD/FID) | Methanol through the intermediated product (formaldehyde & formic acid) | 74 |
| ZnO@Cu–Zn–Al LDH | Quartz glass reactor | UV-visible irradiation from 450 W Xe lamp | 25 °C. After 60 min the reaction vessel was heated up to 200 °C | CO2 (30 mL min−1)/H2O | Gas chromatography (FID) | CH4 | 120 |
Industrially, formaldehyde is a value-added product. It has many potential applications, such as being the raw material for the synthesis of oxymethylene ether (OME) compounds (used for combustion fuels), fuel additives, or alternative liquid fuels. Also, it can be used as a liquid organic hydrogen carrier (LOHC) in hydrogen fuel cells.121–123 In this work, GHGs are converted to value-added products by utilizing visible light energy (as a green chemistry approach) using a low-cost, environmentally friendly photocatalyst.
4 Conclusion
Anthropogenic activity results in the accumulation of GHGs in the atmosphere, consequently causing global warming phenomena that have a detrimental effect on the environment and health systems. Utilization of GHGs (especially CO2, CH4, and H2O) to produce value-added material is considered a way to reduce their accumulation in the atmosphere. In this work, we aimed to convert these gases using a photo-efficient catalyst under visible light.Due to their distinctive structural characteristics, layered double hydroxide (LDH) materials have inspired us. So, we have successfully homogenously precipitated Cu–Al LDH by urea hydrolysis under MW irradiation. The effects of MW power, urea concentration, and aluminium content percent were studied. Controlling the reaction conditions allows the intercalation of the urea-derived anions within the layer structure. These nitrogen-containing anions enhanced the optical properties of the prepared nano Cu–Al LDH materials.
This work illustrates, for the first time, the use of Cu–Al LDH intercalated urea-derived anions for the direct production of formaldehyde with high selectivity (>99%) from the GHGs. No sacrificial scavenger material was used in this work. The reaction was performed under visible light, at room temperature, and under atmospheric pressure. The highest activity was for material prepared at U/M = 10, MW = 270 wt, and MII/MIII = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Under the reaction conditions, the converted amounts were 7.48 and 1.02 mmol mL−1 g−1 for CH4 and CO2, respectively. The presence of excess water enhances the stability and durability of the LDH catalysts over several reaction cycles. Increasing the LDH catalyst dosage resulted in a decrease in conversion percent and formaldehyde selectivity. Thus, this work provides a green chemistry approach to utilizing visible light energy, GHGs, and low-cost environmentally friendly photocatalysts to produce valuable products.
1. Under the reaction conditions, the converted amounts were 7.48 and 1.02 mmol mL−1 g−1 for CH4 and CO2, respectively. The presence of excess water enhances the stability and durability of the LDH catalysts over several reaction cycles. Increasing the LDH catalyst dosage resulted in a decrease in conversion percent and formaldehyde selectivity. Thus, this work provides a green chemistry approach to utilizing visible light energy, GHGs, and low-cost environmentally friendly photocatalysts to produce valuable products.
Ethics approval and consent to participate
The authors have approved and participated in the manuscript.Consent for publication
Publication has been approved by the authors.Data availability
All related data and materials are within the manuscript.Author contributions
Ayat A.-E. Sakr: conceptualization, methodology, validation, investigation, resources, writing – original draft, writing – review & editing, visualization, project administration, funding acquisition, supervision. Dalia R. Abd El-Hafiz: methodology, validation, investigation, resources, supervision, project administration. Osama Elgabry: methodology, Eman S. Abdulla: methodology. Mohamed A. Ebiad: methodology, validation. Tamer Zaki: supervision.Conflicts of interest
The authors declare no competing interests.Acknowledgements
The authors wish to thank Science, Technology & Innovation Funding Authority (STDF) (Project ID: STDF 33496) for their financial support of this work. Also, the authors would like to greatly thank the teams of EPRI Central Labs for their support during this research.References
- S. Sonwani and P. Saxena, Greenhouse Gases: Sources, Sinks and Mitigation, Springer Nature Singapore Pte Ltd, 2022 Search PubMed.
- H.-O. Pörtner, D. C. Roberts, E. S. Poloczanska, K. Mintenbeck, M. Tignor, A. Alegría, M. Craig, S. Langsdorf, S. Löschke, V. Möller and A. Okem, in Climate Change 2022: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change, ed. H.-O. Pörtner, D. C. Roberts, M. Tignor, E. S. Poloczanska, K. Mintenbeck, A. Alegría, M. Craig, S. Langsdorf, S. Löschke, V. Möller, A. Okem and B. Rama, Cambridge University Press, Cambridge, UK, 2022 Search PubMed.
- UNFCCC, in CONFERENCE OF THE PARTIES. Third session Kyoto, 1-10 December 1997 Agenda item 5, United Nations Framework Convention on Climate Change, 1997, pp. 1–24 Search PubMed.
- G. Myhre, D. Shindell, F.-M. Bréon, W. Collins, J. Fuglestvedt, J. Huang, D. Koch, J.-F. Lamarque, D. Lee, B. Mendoza, T. Nakajima, A. Robock, G. Stephens, T. Takemura and H. Zhang, in Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, ed. T. F. Stocker, D. Qin, G.-K. Plattner, M. Tignor, S. K. Allen, J. Boschung, A. Nauels, Y. Xia, V. Bex and P. M. Midgley, Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, 2013 Search PubMed.
- IPCC, Climate Change 2022: Mitigation of Climate Change. Contribution of Working Group III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change, Cambridge University Press, Cambridge, UK and New York, NY, USA, 2022 Search PubMed.
- IEA, World Energy Outlook 2022, International Energy Agency (IEA), 2022, https://www.iea.org/ Search PubMed.
- BP, BP Energy Outlook: 2022 edition, BP p.l.c, 2022 Search PubMed.
- UNEP/CCAC, Global Methane Assessment: 2030 Baseline Report, Climate and Clean Air Coalition (CCAC) convened by United Nations Environment Programme (UNEP), Nairobi, 2022 Search PubMed.
- UNEP/CCAC, Global Methane Assessment: 2030 Baseline Report. Summary for Policymakers, Climate and Clean Air Coalition (CCAC) convened by United Nations Environment Programme (UNEP), Nairobi, 2022 Search PubMed.
- L. Jeffry, M. Y. Ong, S. Nomanbhay, M. Mofijur, M. Mubashir and P. L. Show, Fuel, 2021, 301, 121017 CrossRef CAS.
- S. Zhao, H. Liang, X. Hu, S. Li and K. Daasbjerg, Angew. Chem., Int. Ed., 2022, 61, e202204008 CrossRef CAS PubMed.
- Q. Li, Y. Ouyang, H. Li, L. Wang and J. Zeng, Angew. Chem., Int. Ed., 2022, 61, e202108069 CrossRef CAS PubMed.
- M. Aresta, Carbon Dioxide as a Direct Chemical Feedstock, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim, 2010 Search PubMed.
- D. M. D'Alessandro, B. Smit and J. R. Long, Angew. Chem., Int. Ed., 2010, 49, 6058–6082 CrossRef PubMed.
- C. Y. Toe, C. Tsounis, J. Zhang, H. Masood, D. Gunawan, J. Scott and R. Amal, Energy Environ. Sci., 2021, 14, 1140–1175 RSC.
- Y. H. Park, G. Murali, J. K. R. Modigunta, I. In and S. Il In, Front. Chem., 2021, 9, 1–7 Search PubMed.
- S. Petit and J. Madejova, Handbook of Clay Science, 2013, vol. 5 Search PubMed.
- D. G. Evans and R. C. T. Slade, in Structure and Bonding, ed. D. M. P. Mingos, Springer-Verag Berlin Heidelberg, Germany, 2006, vol. 119, pp. 1–87 Search PubMed.
- F. Cavani, F. Trifiro and A. Vaccari, Catal. Today, 1991, 11, 173–301 CrossRef CAS.
- J. He, M. Wei, B. Li, Y. Kang, D. G. Evans and X. Duan, in Struct. Bond., ed. D. M. P. Mingos, © Springer-Verlag Berlin Heidelberg, 2006, vol. 119, pp. 89–119 Search PubMed.
- X. Long, Z. Wang, S. Xiao, Y. An and S. Yang, Biochem. Pharmacol., 2016, 19, 213–226 CAS.
- M. P. Jerome, F. A. Alahmad, M. T. Salem and M. Tahir, J. Environ. Chem. Eng., 2022, 10, 108151 CrossRef CAS.
- D. Yan, J. Lu, M. Wei, D. G. Evans and X. Duan, J. Mater. Chem., 2011, 21, 13128–13139 RSC.
- G. Zhang, X. Zhang, Y. Meng, G. Pan, Z. Ni and S. Xia, Chem. Eng. J., 2020, 392, 123684 CrossRef CAS.
- J. Wang, R. Li, D. Zeng, W. Wang, Y. Zhang, L. Zhang and W. Wang, Chem. Eng. J., 2023, 452, 139505 CrossRef CAS.
- N. Dewangan, W. M. Hui, S. Jayaprakash, A. Bawah, A. J. Poerjoto, T. Jie, A. Jangam, K. Hidajat and S. Kawi, Catal. Today, 2020, 356, 490–513 CrossRef CAS.
- J. Li, S. Zhang, Y. Chen, T. Liu, C. Liu, X. Zhang, M. Yi, Z. Chu and X. Han, RSC Adv., 2017, 7, 29051–29057 RSC.
- I. Bilecka and M. Niederberger, Nanoscale, 2010, 2, 1358–1374 RSC.
- P. Benito, F. M. Labajos and V. Rives, Pure Appl. Chem., 2009, 81, 1459–1471 CAS.
- M. Baghbanzadeh, L. Carbone, P. D. Cozzoli and C. O. Kappe, Angew. Chem., Int. Ed., 2011, 50, 11312–11359 CrossRef CAS PubMed.
- S. Faramawy, T. Zaki, A. A. E. Sakr, O. Saber, A. K. Aboul-Gheit and S. A. Hassan, J. Nat. Gas Sci. Eng., 2018, 54, 72–82 CrossRef CAS.
- A. A.-E. Sakr, T. Zaki, O. Elgabry, M. A. Ebiad, S. M. El-Sabagh and M. M. Emara, Adv. Powder Technol., 2021, 32, 4096–4109 CrossRef CAS.
- A. A.-E. Sakr, T. Zaki, O. Elgabry, M. A. Ebiad, S. M. El-Sabagh and M. M. Emara, Appl. Clay Sci., 2018, 160, 263–269 CrossRef CAS.
- A. A.-E. Sakr, T. Zaki, O. Saber, S. A. Hassan, A. K. Aboul-Gheit and S. Faramawy, J. Taiwan Inst. Chem. Eng., 2013, 44, 957–962 CrossRef CAS.
- R. J. M. J. Vogels, J. T. Kloprogge and J. W. Geus, J. Colloid Interface Sci., 2005, 285, 86–93 CrossRef CAS PubMed.
- R. Trujillano, M. J. Holgado, F. Pigazo and V. Rives, Phys. B, 2006, 373, 267–273 CrossRef CAS.
- V. Rives and S. Kannan, J. Mater. Chem., 2000, 10, 489–495 RSC.
- S. Velu and C. S. Swamy, Appl. Catal., A, 1996, 145, 141–153 CrossRef CAS.
- K. Jayanthi, P. Vishnu Kamath and G. Periyasamy, Eur. J. Inorg. Chem., 2017, 2017, 3675–3682 CrossRef CAS.
- M. R. Berber, I. H. Hafez, K. Minagawa, M. Katoh, T. Mori and M. Tanaka, J. Mol. Struct., 2013, 1033, 104–112 CrossRef CAS.
- L. D. S. Neto, C. G. Anchieta, J. L. S. Duarte, L. Meili and J. T. Freire, ACS Omega, 2021, 6, 21819–21829 CrossRef PubMed.
- M. Crivello, C. Pérez, E. Herrero, G. Ghione, S. Casuscelli and E. Rodríguez-Castellón, Catal. Today, 2005, 108, 215–222 CrossRef.
- X. Li, T. Würger, C. Feiler, R. H. Meißner, M. Serdechnova, C. Blawert and M. L. Zheludkevich, ACS Omega, 2022, 7, 12412–12423 CrossRef CAS PubMed.
- F. Zhao, L. Fan, K. Xu, D. Hua, G. Zhan and S. F. Zhou, J. CO2 Util., 2019, 33, 222–232 CrossRef CAS.
- N. Fu, S. Zhang, Y. Ma, Z. Yang and W. Liu, RSC Adv., 2020, 10, 9808–9813 RSC.
- X. Peng, M. Wang, F. Hu, F. Qiu, T. Zhang, H. Dai and Z. Cao, J. Environ. Manage., 2018, 220, 173–182 CrossRef CAS PubMed.
- P. Benito, F. M. Labajos, J. Rocha and V. Rives, Microporous Mesoporous Mater., 2006, 94, 148–158 CrossRef CAS.
- J. T. Kloprogge, L. Hickey and R. L. Frost, J. Solid State Chem., 2004, 177, 4047–4057 CrossRef CAS.
- V. Rives, A. Dubey and S. Kannan, Phys. Chem. Chem. Phys., 2001, 3, 4826–4836 RSC.
- O. Saber and H. Tagaya, J. Porous Mater., 2003, 10, 83–91 CrossRef CAS.
- J. D. Yong, R. Valdez, M. Á. Armenta, N. Arjona, G. Pina-Luis and A. Olivas, RSC Adv., 2022, 12, 16955–16965 RSC.
- J. M. Hu, Y. Li, Q. Li, Y. F. Zhang, W. Lin and G. X. Jia, J. Solid State Chem., 2004, 177, 2763–2771 CrossRef CAS.
- B. Mavis and M. Akinc, Chem. Mater., 2006, 18, 5317–5325 CrossRef CAS.
- Y. Qiu and L. Gao, J. Am. Ceram. Soc., 2004, 87, 352–357 CrossRef CAS.
- R. Keuleers, G. S. Papaefstathiou, C. P. Raptopoulou, S. P. Perlepes and H. O. Desseyn, J. Mol. Struct., 2000, 525, 173–183 CrossRef CAS.
- P. S. Braterman, Z. P. Xu and F. Yarberry, in Handbook of Layered Materials, ed. S. M. Auerbach, K. a. Carrado and P. K. Dutta, Marcel Dekker, Inc., 2004 Search PubMed.
- J. T. Kloprogge, L. Hickey and R. L. Frost, J. Raman Spectrosc., 2004, 35, 967–974 CrossRef CAS.
- J. J. Lin and T. Y. Juang, Polymer, 2004, 45, 7887–7893 CrossRef CAS.
- É. Makó, J. Kristóf, E. Horváth and V. Vágvölgyi, J. Colloid Interface Sci., 2009, 330, 367–373 CrossRef PubMed.
- J. T. Kloprogge, L. Hickey, R. Trujillano, M. J. Holgado, M. S. San Román, V. Rives, W. N. Martens and R. L. Frost, Cryst. Growth Des., 2006, 6, 1533–1536 CrossRef CAS.
- R. Keuleers, H. O. Desseyn, B. Rousseau and C. Van Alsenoy, J. Phys. Chem. A, 1999, 103, 4621–4630 CrossRef CAS.
- J. Grdadolnik and Y. Maréchal, J. Mol. Struct., 2002, 615, 177–189 CrossRef CAS.
- R. G. Gavinehroudi, A. Mahjoub, M. Karimi, S. Sadeghi, A. Heydari, H. Mohebali and S. Ghamami, Green Chem., 2022, 24, 6965–6979 RSC.
- P. R. Jubu, O. S. Obaseki, F. K. Yam, S. M. Stephen, A. A. Avaa, A. A. McAsule, Y. Yusof and D. A. Otor, J. Opt., 2023, 52(3), 1426–1435 CrossRef.
- R. Jaiswal, J. Bharambe, N. Patel, A. Dashora, D. C. Kothari and A. Miotello, Appl. Catal., B, 2015, 168–169, 333–341 CrossRef CAS.
- T. H. Kim, G. Go, H. Cho, Y. Song, C.-G. Lee and Y.-H. Choa, Front. Chem., 2018, 6, 458 CrossRef CAS PubMed.
- G. D. Gesesse, A. Gomis-berenguer, M. Barthe and C. O. Ania, J. Photochem. Photobiol., A, 2020, 398, 112622 CrossRef CAS.
- K. Parida, L. Mohapatra and N. Baliarsingh, J. Phys. Chem. C, 2012, 116, 22417–22424 CrossRef CAS.
- S. Xu, H. Yan and M. Wei, J. Phys. Chem. C, 2017, 121, 2683–2695 CrossRef CAS.
- S. Nayak and K. M. Parida, ACS Omega, 2018, 3, 7324–7343 CrossRef CAS PubMed.
- N. Ahmed, M. Morikawa and Y. Izumi, Catal. Today, 2012, 185, 263–269 CrossRef CAS.
- S. Vojkovic, J. Fernandez, S. Elgueta, F. E. Vega, S. D. Rojas, R. A. Wheatley, B. Seifert, S. Wallentowitz and A. L. Cabrera, SN Appl. Sci., 2019, 1, 1–10 CAS.
- M. Long, P. Hu, H. Wu, Y. Chen, B. Tana and W. Cai, J. Mater. Chem. A, 2015, 3, 5592–5598 RSC.
- M. Lv and H. Liu, J. Solid State Chem., 2015, 227, 232–238 CrossRef CAS.
- J. Liqiang, Q. Yichun, W. Baiqi, L. Shudan, J. Baojiang, Y. Libin, F. Wei, F. Honggang and S. Jiazhong, Sol. Energy Mater. Sol. Cells, 2006, 90, 1773–1787 CrossRef.
- K. Xu, Z. Zhang, G. Chen and J. Shen, RSC Adv., 2014, 4, 19218–19220 RSC.
- X. X. Guo, T. T. Hua, B. Menga, Y. Sunc and Y.-F. Han, Appl. Catal., B, 2020, 260, 118157 CrossRef CAS.
- Z. Zhai, X. Yang, L. Xu, C. Hu, L. Zhang, W. Hou and Y. Fan, Nanoscale, 2012, 4, 547–556 RSC.
- L. Mohapatra and K. M. Parida, Phys. Chem. Chem. Phys., 2014, 16, 20–22 RSC.
- F. Iskandar, A. Fajri, N. Nuraeni, E. Stavila, A. H. Aimon and B. W. Nuryadin, Mater. Res. Express, 2018, 5, 044003 CrossRef.
- D. Naveena, T. Logu, R. Dhanabal, K. Sethuraman and A. C. Bose, J. Mater. Sci.: Mater. Electron., 2019, 30, 561–572 CrossRef CAS.
- R. Gupta, N. K. Eswar, J. M. Modaka and G. Madras, RSC Adv., 2016, 6, 85675–85687 RSC.
- B. R. Gevers, E. Roduner and F. J. W. J. Labuschagné, Mater. Adv., 2022, 3, 962–977 RSC.
- M. F. Parveen, S. Umapathy, V. Dhanalakshmi and R. Anbarasan, J. Mater. Sci., 2009, 44, 5852–5860 CrossRef.
- J. Li, Z. Mei, L. Liu, H. Liang, A. Azarov, A. Kuznetsov, Y. Liu, A. Ji, Q. Meng and X. Du, Sci. Rep., 2014, 4, 7240 CrossRef CAS PubMed.
- Y. Cheng, X. Huang, X. Xi and H. Lin, Ceram. Int., 2018, 44, 5774–5779 CrossRef CAS.
- Z. Zhang, G. Chen and K. Xu, Ind. Eng. Chem. Res., 2013, 52, 11045–11049 CrossRef CAS.
- Y. Jiang, W. Zhao, S. Li, S. Wang, Y. Fan, F. Wang, X. Qiu, Y. Zhu, Y. Zhang, C. Long and Z. Tang, J. Am. Chem. Soc., 2022, 144, 15977–15987 CrossRef CAS PubMed.
- M. Flores-Flores, E. Luévano-hipólito, L. M. Torres, G. Morales-Mendoza and R. Gómez, J. Photochem. Photobiol., A, 2018, 363, 68–73 CrossRef CAS.
- S. Wei, X. Zhu, P. Zhang, Y. Fan, Z. Sun, X. Zhao, D. Han and L. Niu, Appl. Catal., B, 2021, 283, 119661 CrossRef CAS.
- H. Song, X. Meng, S. Wang, W. Zhou, X. Wang, T. Kako and J. Ye, J. Am. Chem. Soc., 2019, 141, 20507–20515 CrossRef CAS PubMed.
- L. Luo, X. Han, K. Wang, Y. Xu, L. Xiong, J. Ma, Z. Guo and J. Tang, Nat. Commun., 2023, 14, 2690 CrossRef CAS PubMed.
- Y. Wang, E. Chen and J. Tang, ACS Catal., 2022, 12, 7300–7316 CrossRef CAS PubMed.
- X. Zhao, H. Jin, Y. Chen and Z. Ge, Comput. Math. with Appl., 2021, 81, 759–771 CrossRef.
- B. Tahir, M. Tahir and N. A. S. Amin, Energy Convers. Manage., 2018, 159, 284–298 CrossRef CAS.
- B. Tahir, M. Tahir, M. A. C. Yunus, A. R. Mohamed, M. Siraj and A. Fatehmulla, Appl. Surf. Sci., 2020, 520, 146296 CrossRef CAS.
- Y. Tokudome, M. Fukui, S. Iguchi, Y. Hasegawa, K. Teramura, T. Tanaka, M. Takemoto, R. Katsura and M. Takahashi, J. Mater. Chem. A, 2018, 6, 9684–9690 RSC.
- J. Chanton, L. Chaser, P. Glasser and D. Siegel, in Stable Isotopes and Biosphere – Atmosphere Interactions. Processes and Biological Controls, ed. L. Flanagan, J. Ehleringer and D. Pataki, 2004, pp. 85–105 Search PubMed.
- L. K. Dhandole, S. H. Kim and G. Moon, J. Mater. Chem. A, 2022, 10, 19107–19128 RSC.
- Y. Jiang, S. Li, S. Wang, Y. Zhang, C. Long, J. Xie, X. Fan, W. Zhao, P. Xu, Y. Fan, C. Cui and Z. Tang, J. Am. Chem. Soc., 2023, 145, 2698–2707 CrossRef CAS PubMed.
- H. Kim, S. Lee, S. Jang, J. haeng Yu, J. S. Yoo and J. Oh, Appl. Catal., B, 2021, 292, 120161 CrossRef CAS.
- D. Ehhalt, M. Prather, F. Dentener, R. Derwent, E. Dlugokencky, E. Holland, I. Isaksen, J. Katima, V. Kirchhoff, P. Matson, P. Midgley and M. Wang, in Climate Change 2001: The Scientific Basis, Contribution of Working Group I to the Third Assessment Report of the Intergovernmental Panel on Climate Change (IPPC), 2001, pp. 239–287 Search PubMed.
- O. Boucher, P. Friedlingstein, B. Collins and K. P. Shine, Environ. Res. Lett., 2009, 4, 044007 CrossRef.
- IPCC, Climate Change 2013 – The Physical Science Basis, 2013 Search PubMed.
- S. V. L. Mahlaba, N. H. lal Mahomed, G. M. Leteba, A. Govender and E. van Steen, ACS Catal., 2023, 2023(13), 14770–14781 CrossRef.
- Y. Fan, P. Zhang, R. Lu, Y. Jiang, G. Pan, W. Wang, X. Zhu, S. Wei, D. Han and L. Niu, Catal. Commun., 2021, 161, 106365 CrossRef CAS.
- T. Kulandaivalu, A. R. Mohamed, K. A. Ali and M. Mohammadi, Renewable Sustainable Energy Rev., 2020, 134, 110363 CrossRef CAS.
- Y. Fu, D. Sun, Y. Chen, R. Huang, Z. Ding, X. Fu and Z. Li, Angew. Chem., Int. Ed., 2012, 51, 3364–3367 CrossRef CAS PubMed.
- Y. Tokudome, T. Morimoto, N. Tarutani, P. D. Vaz, C. D. Nunes, V. Prevot, G. B. G. Stenning and M. Takahashi, ACS Nano, 2016, 10, 5550–5559 CrossRef CAS PubMed.
- A. Kumar and R. Ananthakrishnan, Green Chem., 2020, 22, 1650–1661 RSC.
- Y. Liao, S.-W. Cao, Y. Yuan, Q. Gu, Z. Zhang and C. Xue, Chem. – Eur. J., 2014, 20, 10220–10222 CrossRef CAS PubMed.
- N. M. Dimitrijevic, B. K. Vijayan, O. G. Poluektov, T. Rajh, K. A. Gray, H. He and P. Zapol, J. Am. Chem. Soc., 2011, 133, 3964–3971 CrossRef CAS PubMed.
- A. Ziarati, A. Badiei, R. Grillo and T. Burgi, ACS Appl. Mater. Interfaces, 2019, 11, 5903–5910 CrossRef CAS PubMed.
- K. Katsumata, K. Sakai, K. Ikeda, G. Carja, N. Matsushita and K. Okada, Mater. Lett., 2013, 107, 138–140 CrossRef CAS.
- N. Ahmed, Y. Shibata, T. Taniguchi and Y. Izumi, J. Catal., 2011, 279, 123–135 CrossRef CAS.
- M. Miyano, H. Zhang, M. Yoshiba and Y. Izumi, Energy Technol., 2017, 5, 892–900 CrossRef CAS.
- K. Teramura, S. Iguchi, Y. Mizuno, T. Shishido and T. Tanaka, Angew. Chem., 2012, 124, 8132–8135 CrossRef.
- S. Iguchi, K. Teramura, S. Hosokawa and T. Tanaka, Appl. Catal., A, 2016, 521, 160–167 CrossRef CAS.
- Y. Xu, X. Sun, X. Wang, L. He, M. T. Wharmby, X. Hua, Y. Zhao and Y.-F. Song, Chem. – Eur. J., 2021, 27, 13211–13220 CrossRef CAS PubMed.
- Q. Guo, Q. Zhang, H. Wang, Z. Liu and Z. Zhao, Catal. Commun., 2016, 77, 118–122 CrossRef CAS.
- L. E. Heim, H. Konnerth and M. H. G. Prechtl, Green Chem., 2017, 19, 2347–2355 RSC.
- B. Niethammer, S. Wodarz, M. Betz, P. Haltenort, D. Oestreich, K. Hackbarth, U. Arnold, T. Otto and J. Sauer, Chem. Ing. Tech., 2018, 90, 99–112 CrossRef CAS.
- J. H. Lunsford, Catal. Today, 2000, 63, 165–174 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra06190f |
| This journal is © The Royal Society of Chemistry 2023 |