Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Design, synthesis and antiproliferative screening of newly synthesized coumarin-acrylamide hybrids as potential cytotoxic and apoptosis inducing agents†
Hany M. Abd El-Lateef*ab,
Lina M. A. Abdel Ghanyc,
Rasha Mohammed Saleemd,
Ali Hassan Ahmed Maghrabie,
Maryam Abdulrahman Yahya Alahdalf,
Eman Hussain Khalifa Alig,
Botros Y. Beshayh,
Islam Zaki *i and
Reham E. Masoudj
*i and
Reham E. Masoudj
aDepartment of Chemistry, College of Science, King Faisal University, Al-Ahsa 31982, Saudi Arabia. E-mail: hmahmed@kfu.edu.sa
bDepartment of Chemistry, Faculty of Science, Sohag University, Sohag 82524, Egypt
cPharmaceutical Chemistry Department, College of Pharmaceutical Sciences and Drug Manufacturing, Misr University for Science and Technology, 6th of October City, Egypt
dDepartment of Laboratory Medicine, Faculty of Applied Medical Sciences, Al-Baha University, Al-Baha 65431, Saudi Arabia
eDepartment of Biology, Faculty of Applied Science, Umm Al-Qura University, Makkah 24381, Saudi Arabia
fDepartment of Biology, Faculty of Applied Science, Umm Al-Qura University, Makkah 24381, Saudi Arabia
gDepartment of Laboratory Medicine, Faculty of Applied Medical Sciences, Albaha University, Saudi Arabia
hPharmaceutical Sciences (Pharmaceutical Chemistry) Department, College of Pharmacy, Arab Academy for Science, Technology and Maritime Transport, Alexandria, Egypt
iPharmaceutical Organic Chemistry Department, Faculty of Pharmacy, Port Said University, Port Said, Egypt. E-mail: Eslam.Zaki@pharm.psu.edu.eg
jClinical Pharmacology Department, Faculty of Medicine, Port Said University, Port Said 42526, Egypt
First published on 6th November 2023
Abstract
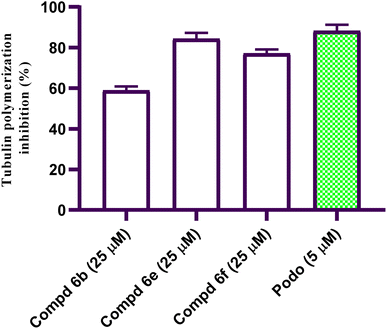
On the basis of the observed biological activity of coumarin and acrylamide derivatives, a new set of coumarin-acrylamide-CA-4 hybrids was designed and synthesized. These compounds were investigated for their cytotoxic activity against cancerous human liver cell line HepG2 cells using 5-fluorouracil (5-FU) as a reference drug. Compound 6e had promising antiproliferative activity with an IC50 value of 1.88 μM against HepG2 cells compared to 5-FU (IC50 = 7.18 μM). The results of β-tubulin polymerization inhibition indicated that coumarin-acrylamide derivative 6e was the most active, with a percentage inhibition value of 84.34% compared to podophyllotoxin (88.19% β-tubulin inhibition). Moreover, the active coumarin-acrylamide molecule 6e exerted cell cycle cession at the G2/M phase stage of HepG2 cells. In addition, this compound produced a 15.24-fold increase in apoptotic cell induction compared to no-treatment control. These observations were supported by histopathological studies of liver sections. The conducted docking studies illustrated that 6e is perfectly positioned within the tubulin colchicine binding site, indicating a significant interaction that may underlie its potent tubulin inhibitory activity. The main objective of the study was to develop new potent anticancer compounds that might be further optimized to prevent the progression of cancer disease.
1. Introduction
Cancer stands as the world's foremost contributor to untimely fatalities caused by non-communicable ailments.1 It represents an unbridled proliferation of irregular cells attributed to malfunctions within the apoptotic process.2–4 Hepatocellular carcinoma (HCC), an aggressive tumor, emerges as the predominant form of primary liver malignancy. HCC stands as the second primary cause of cancer-related deaths, characterized by its grim prognosis and formidable resistance to traditional chemotherapy treatments.5 As per the World Health Organization, liver cancer ranks as the most prevalent malignancy among the Egyptian population, boasting incidence and mortality rates of 19.7% and 29.4%, respectively.6Coumarins, characterized by their fundamental structural core of benzopyrone, have attracted significant attention in the scientific community due to their abundant sources, easy synthesis methods, and diverse pharmacological properties.7–9 Notably, coumarins exhibit impressive anti-tumor capabilities through a variety of mechanisms, including carbonic anhydrase inhibition, targeting of PI3K/Akt/mTOR signaling pathways, activation of cell apoptosis proteins, suppression of tumor multidrug resistance, interference with microtubule polymerization, regulation of reactive oxygen species, and inhibition of tumor angiogenesis.9,10 Whether obtained naturally from different plants or created through modifications to the core coumarin structure, both forms of coumarins have demonstrated their ability, in laboratory experiments, to effectively inhibit the growth and proliferation of tumor cells at low concentrations without harming normal cells.9 This underscores coumarins' potential as a promising class of highly selective anti-tumor drugs.
The acrylate component serves as a prevalent structural framework found in a wide array of both natural and artificially synthesized small molecules that exhibit a wide range of intriguing biological properties.11,12 It has been documented to manifest various biomedical effects, including its potential as an anticancer agent.13 The exploration of acrylate-based compounds has brought forth novel molecules with the capacity to act as anticancer agents, involving diverse mechanisms such as the inhibition of β-tubulin and protein kinases.14,15 These discoveries indicate that the acrylate pharmacophore presents a promising molecular foundation for further adaptations aimed at developing more potent candidates for anticancer drugs.16
Furthermore, combretastatin A-4 (CA-4) stands out as the most prevalent member within the combretastatin family, originally derived from the African Combretum caffrum tree.17–19 CA-4 displays potent antimitotic properties by binding to the colchicine binding site, with the dimethoxybenzene portion, along with other elements of the CA-4 ring, playing a pivotal role in enhancing binding affinity. CA-4 advanced to phase II and phase III stages during clinical trials.20,21 However, CA-4 is plagued by several pharmacokinetic drawbacks, including limited water solubility,22–24 as well as a short plasma half-life and susceptibility to isomerization from the active cis form to the inactive trans form under in vivo conditions.
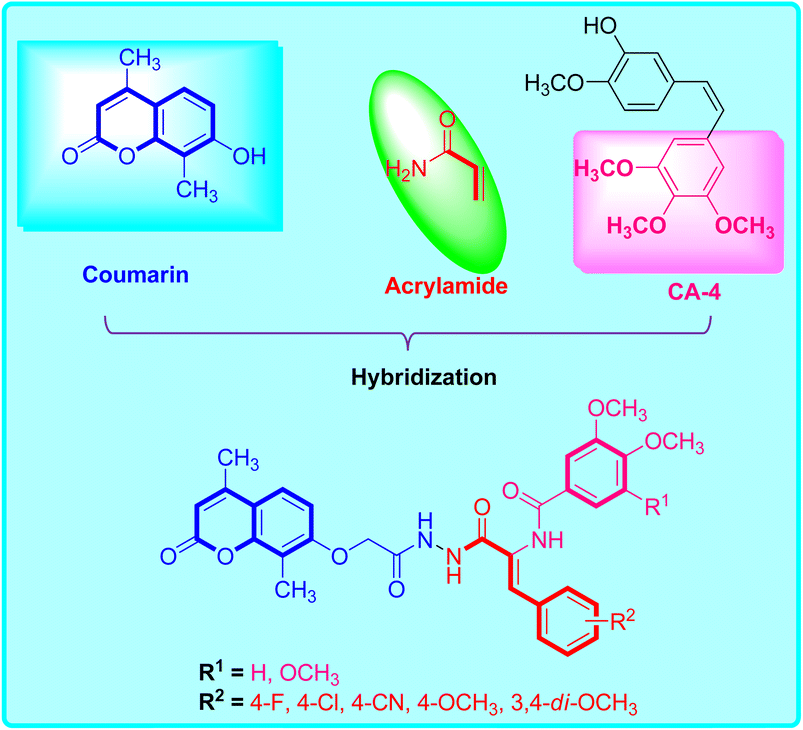
In light of these discoveries, our objective is to devise innovative antitubulin inhibitors, employing a hybrid structure-based approach as an effective strategy for creating novel drug candidates.8,25 This initiative aims to address the escalating prevalence of resistance to current therapeutic remedies. In this endeavor, we have amalgamated 7-substituted coumarin, the acrylate framework, and the trimethoxybenzene component from CA-4 (as depicted in Fig. 1) into a unified molecule with the goal of enhancing their capacity to inhibit microtubule polymerization. Furthermore, we have implemented various substitution patterns on both the central phenyl and the distally substituted trimethoxybenzene ring to investigate their influence on tubulin-binding and inhibitory activity akin to colchicine. The underlying purpose of this design is to probe the potential antiproliferative effects of these compounds on the HepG2 liver cancer cell line.
2. Results and discussion
2.1. Chemistry
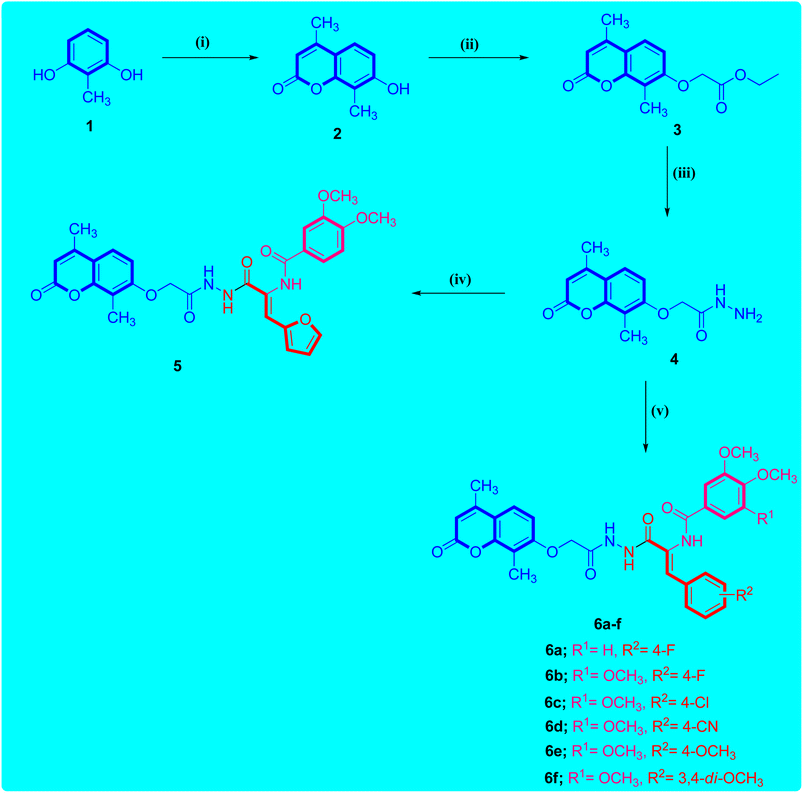
Scheme 1 illustrates the adopted synthetic pathway for the target coumarin-acrylamide compounds 5 and 6a–f. The key hydrazide intermediate 4 was synthesized by reported by O-alkylation of 4,8-dimethyl-7-hydroxycoumarin 2 with ethyl 2-chloroacetate in the presence of potassium carbonate to give coumarin ethyl acetate ester 3 that underwent hydrazinolysis with hydrazine hydrate in pure ethanol to afford the key coumarin acetohydrazide intermediate 4 in good yield.26The reaction of the key acetohydrazide intermediate 4 with the respective aryl acrylate ethyl ester in absolute ethanol and a catalytic amount of glacial acetic acid promotes the reaction to afford the target coumarin-aryl acrylamide hybrids 5 and 6a–f. The structures of the obtained coumarin-aryl acrylamide derivatives 5 and 6a–f were verified by spectroscopic methods and elemental microanalytical data. For instance, the 1H-NMR spectrum of coumarin-3-(4-chlorophenyl)acrylamide derivative 6c as an example showed one singlet (δ 10.26) and two doublets (δ 10.40 and 9.79) for the three amide protons. The protons of the 4,8-dimethylcoumarin moiety appeared as two doublets for one proton each at δ 7.73 and 7.02 ppm with a coupling constant of 8.3 Hz and singlet for one proton at δ 6.98 ppm. In addition, the protons of the 3-(4-chlorophenyl)acrylamide function appeared as two doublets for two protons each with a coupling constant of 8.1 Hz. Besides, the presence of a doublet (δ 7.38 ppm) with a coupling constant of 7.0 Hz was assigned to olefin proton of the acrylamide moiety. Additionally, the methoxy protons of the 3,4,5-trimethoxybenzamide group appeared as two singlets at δ 3.85 and 3.74 ppm for two methoxy and one methoxy groups, respectively. Furthermore, the signal observed at δ 4.80 ppm was assigned to methylene (OCH2) protons in the compound. Additionally, the two methyl protons appeared as two singlets at δ 2.37 and 2.08 ppm for three protons each.
The 13C-NMR spectrum of coumarin-3-(4-chlrophenyl)acrylamide compound 6c showed three peaks at δ 166.69–164.74 ppm attributed to the carbonyl carbon of triamide functions. The signal observed at δ 66.64 ppm was assigned to methylene (OCH2) carbon. In addition, the methoxy carbons of the 3,4,5-trimethoxybenzamide group appeared at δ 60.57 and 56.54 ppm for two methoxy carbons and one methoxy carbon, respectively. Moreover, the two methyl carbons resonated at δ 15.40 and 13.40 ppm. Finally, the remaining aromatic and olefinic carbons resonated at around δ 161.63–101.82 ppm.
2.2. Biology
| Compound | R1 | R2 | IC50 value (μM) | |
|---|---|---|---|---|
| HepG2 | HL-7702 | |||
| a NT: not tested. | ||||
| 5 | H | — | 13.18 ± 1.12 | NT |
| 6a | H | 4-F | 27.04 ± 1.43 | NT |
| 6b | OCH3 | 4-F | 6.77 ± 0.83 | NT |
| 6c | OCH3 | 4-Cl | 18.85 ± 1.27 | NT |
| 6d | OCH3 | 4-CN | 8.77 ± 0.99 | NT |
| 6e | OCH3 | 4-OCH3 | 1.88 ± 0.27 | 51.46 ± 0.38 |
| 6f | OCH3 | 3,4-di-OCH3 | 4.14 ± 0.62 | NT |
| 5-FU | — | — | 7.18 ± 0.14 | 35.11 ± 0.19 |
2.3. Molecular modeling study
Molecular docking investigations were carried out to elucidate the exceptional cytotoxicity and tubulin inhibitory potency of the most potent compound, 6e, in comparison to CA-4. Interestingly, both CA-4 and compound 6e were aligned and positioned precisely within the colchicine binding site of tubulin, exhibiting binding free energies of −6.2 and −5.9 kcal mol−1, respectively. Furthermore, CA-4 and compound 6e (as depicted in Fig. 6 and 7) both established H-bonds with the pivotal amino acid residue Cys241. Regarding compound 6e, the p-methoxyphenyl acrylamide moiety formed the crucial H-bond with Cys241 through its methoxy functionality. Besides, it established strong hydrophobic contacts with Leu248, Ala250, Leu255, Lys352, and Ala316, anchoring the methoxyphenyl acrylamide moiety in the tubulin pocket. Furthermore, the trimethoxy benzene ring flanked the polar pocket formed by Asn258, Met259, and Lys352. Moreover, the coumarin scaffold formed an H-bond with Asn258 and had a hydrophobic interaction with Lys354.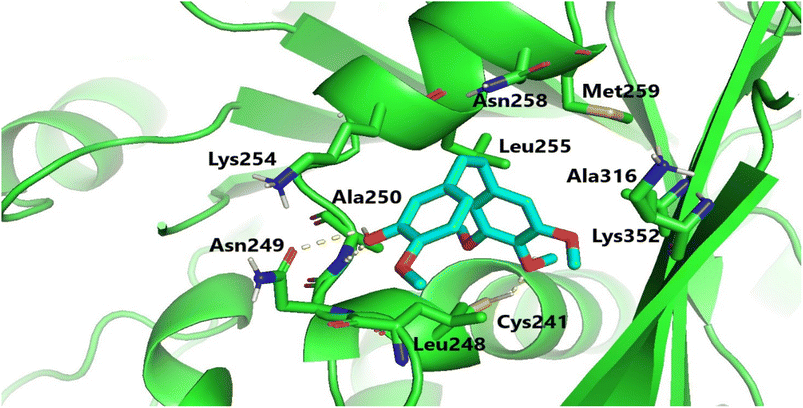 | ||
| Fig. 6 Binding configuration of CA-4 in tubulin's colchicine-binding pocket with highlighted hydrogen bonds. | ||
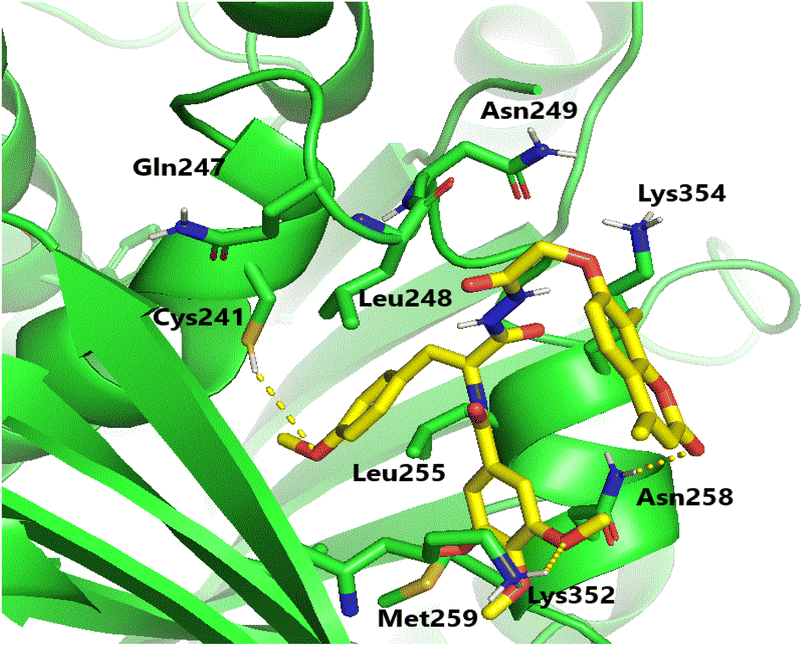 | ||
| Fig. 7 Binding configuration of active coumarin-acrylamide hybrid 6e in tubulin's colchicine-binding pocket with highlighted hydrogen bonds. | ||
It is worth noting that the formation of an H-bond with Cys241, along with the hydrophobic interactions involving the side chains of the aforementioned amino acid residues, as well as various polar interactions, significantly contributed to the protein-ligand binding process. These interactions may potentially explain the remarkable tubulin inhibitory activity observed for coumarin-acrylamide compound 6e.33–35
3. Conclusions
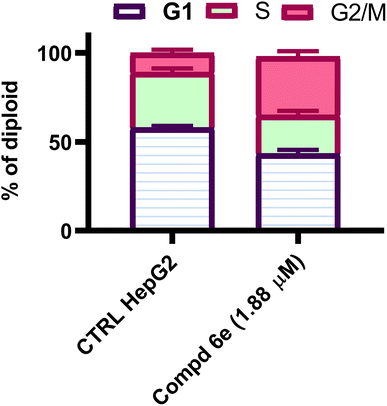
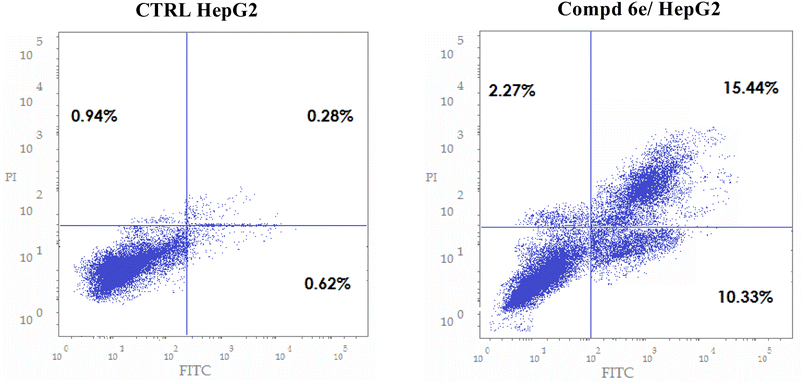
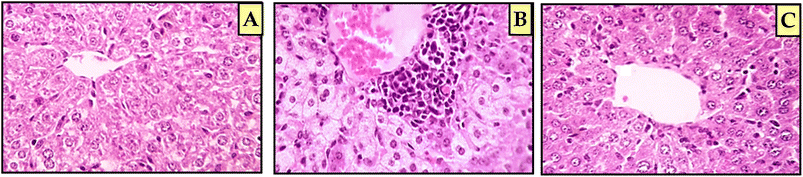
In this study, a new set of coumarin-acrylamide hybrids was designed and synthesized and their biological activities were evaluated as potent anti-tubulin activities. The results showed promising cytotoxic activity against the HepG2 cancerous human liver cell line. Among them, coumarin-3-(4-methoxyphenyl)acrylamide 6e (IC50 = 1.88 μM) and coumarin-3-(3,4-dimethoxyphenyl)acrylamide 6f (IC50 = 4.14 μM) were the most active against HepG2 cells, compared to 5-FU (IC50 = 7.18 μM). The results indicated that compound 6e showed good β-tubulin polymerization inhibitory potency with a percentage inhibition value of 84.34% compared to podophyllotoxin (88.19% polymerization inhibition). In addition, compound 6f showed moderate activity with a percent inhibition of 77.15%. Moreover, compound 6e exerted a significant increase in the level of HepG2 cells at the G2/M phase from 10.86% to 34.92%. Additionally, compound 6e demonstrated a significant increase in apoptotic cells in the late stage, from 0.28% to 15.44%. Furthermore, histopathological study of the EAC cell-bearing mice group treated with compound 6e revealed a mild improvement in histopathological characteristics. The outcomes of the docking studies imply that the anti-tubulin polymerization activity of the hybrid compound 6e, which combines coumarin and acrylamide moieties, may underlie its cytotoxic effects against the HepG2 liver cancer cell line. Notably, 6e with CA-4 exhibited precise alignment within the colchicine binding site of tubulin. Compound 6e formed a crucial H-bond with Cys241 and established strong hydrophobic contacts with several key amino acid residues, effectively anchoring itself within the tubulin pocket. These interactions, along with polar interactions, likely contribute to the outstanding tubulin inhibitory activity observed for compound 6e. Finally, coumarin-acrylamide molecule 6e has been identified as a new molecular scaffold that might be further modified to produce an intriguing chemotherapeutic candidate with potent β-tubulin polymerization inhibitory activity.4. Experimental
4.1. Chemistry
Yield: 78%; m.p.: 290–292 °C; 1H-NMR (400 MHz, DMSO-d6, ppm) δ: 10.30 (s, 1H, NH), 7.71 (d, J = 8.8 Hz, 1H, coumarin H-5), 7.01 (d, J = 8.9 Hz, 1H, coumarin H-6), 6.96 (s, 1H, coumarin H-3), 4.76 (s, 2H, OCH2), 2.36 (s, 3H, CH3), 2.07 (s, 3H, CH3). 13C-NMR (DMSO, 101 MHz, ppm) δ: 166.70, 161.61, 160.01, 153.23, 147.21, 126.64, 118.72, 114.66, 112.89, 101.85, 66.71, 15.40, 13.40. Analysis for C13H14N2O4 (262.26): calcd, C, 59.54; H, 5.38; N, 10.68; found, C, 59.58; H, 5.43; N, 10.57.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, the reaction mixture was left to cool to room temperature. The separated solid was filtered, washed with ether and crystallized from ethanol (70%) to afford pure coumarin-acrylamide derivatives 5 and 6a–f.
4, the reaction mixture was left to cool to room temperature. The separated solid was filtered, washed with ether and crystallized from ethanol (70%) to afford pure coumarin-acrylamide derivatives 5 and 6a–f.
4.1.3.1. (Z)-N-(3-(2-(2-(4,8-Dimethyl-2-oxo-2H-chromen-7-yloxy)acetyl)hydrazinyl)-1-(furan-2-yl)-3-oxoprop-1-en-2-yl)-3,4-dimethoxybenzamide (5). Yield: 71%; m.p.: 219–221 °C; 1H-NMR (400 MHz, DMSO-d6, ppm) δ: 10.33 (d, J = 31.4 Hz, 1H, NH), 10.08 (d, J = 72.4 Hz, 1H, NH), 9.65 (d, J = 69.5 Hz, 1H, NH), 7.80 (d, J = 21.6 Hz, 1H, coumarin H-5), 7.73–7.60 (m, 3H, furan CH and Ar–H), 7.17 (s, 1H, olefinic CH), 7.11–7.05 (m, 1H, Ar–H), 7.01 (d, J = 8.8 Hz, 1H, coumarin H-6), 6.96 (s, 1H, coumarin H-3), 6.78 (dd, J = 37.2, 3.1 Hz, 1H, furan CH), 6.61 (d, J = 15.2 Hz, 1H, furan CH), 4.77 (d, J = 25.0 Hz, 2H, OCH2), 3.84 (s, 6H, 2OCH3), 2.36 (s, 3H, CH3), 2.07 (s, 3H, CH3). 13C-NMR (DMSO, 101 MHz, ppm) δ: 170.67, 166.20, 165.30, 163.89, 161.17, 159.69, 152.77, 151.66, 149.59, 148.17, 146.76, 144.69, 126.18, 126.13, 126.06, 121.44, 118.20, 118.10, 114.23, 114.13, 112.42, 111.33, 110.88, 101.35, 66.19, 55.67, 55.62, 14.92, 12.92. Analysis for C29H27N3O9 (561.54): calcd, C, 62.03; H, 4.85; N, 7.48; found, C, 61.88; H, 4.94; N, 7.56.
4.1.3.2. (Z)-N-(3-(2-(2-(4,8-Dimethyl-2-oxo-2H-chromen-7-yloxy)acetyl)hydrazinyl)-1-(4-fluorophenyl)-3-oxoprop-1-en-2-yl)-3,4-dimethoxybenzamide (6a). Yield: 66%; m.p.: 236–238 °C; 1H-NMR (400 MHz, DMSO-d6, ppm) δ: 10.21 (d, J = 44.6 Hz, 2H, 2NH), 9.73 (d, J = 109.2 Hz, 1H, NH), 7.72 (d, J = 9.0 Hz, 1H, coumarin H-5), 7.69–7.58 (m, 3H, Ar–H), 7.26 (d, J = 9.8 Hz, 2H, Ar–H), 7.22 (d, J = 8.8 Hz, 1H, Ar–H), 7.13–7.08 (m, 1H, olefinic CH), 7.08–6.99 (m, 2H, Ar–H and coumarin H-6), 6.97 (d, J = 2.2 Hz, 1H, coumarin H-3), 4.80 (d, J = 35.2 Hz, 2H, OCH2), 3.84 (s, 3H, OCH3), 3.83 (s, 3H, OCH3), 2.37 (s, 3H, CH3), 2.08 (s, 3H, CH3). 13C-NMR (DMSO, 101 MHz, ppm) δ: 166.65 (d, JC-F = 138.2 Hz), 165.98, 164.93, 163.69, 161.62, 160.16, 153.24, 152.20, 148.64, 147.22, 132.15, 132.06 (d, JC-F = 8.4 Hz), 131.09, 131.06, 129.25, 128.98 (d, JC-F = 5.4 Hz), 126.60, 126.24, 121.92, 118.66, 116.13, 115.91 (d, JC-F = 21.6 Hz), 114.59, 112.88, 111.79, 111.34, 66.65, 56.14, 56.07, 15.38, 13.39. Analysis for C31H28FN3O8 (589.57): calcd, C, 63.15; H, 4.79; N, 7.13; found, C, 63.22; H, 4.83; N, 7.03.
4.1.3.3. (Z)-N-(3-(2-(2-(4,8-Dimethyl-2-oxo-2H-chromen-7-yloxy)acetyl)hydrazinyl)-1-(4-fluorophenyl)-3-oxoprop-1-en-2-yl)-3,4,5-trimethoxybenzamide (6b). Yield: 74%; m.p.: 238–240 °C; 1H-NMR (400 MHz, DMSO-d6, ppm) δ: 10.29 (s, 2H, 2NH), 8.38 (dd, J = 8.6, 5.8 Hz, 2H, Ar–H), 7.72 (d, J = 8.9 Hz, 1H, coumarin H-5), 7.40 (d, J = 8.9 Hz, 1H, Ar–H), 7.37 (s, 1H, olefinic CH), 7.36 (d, J = 5.0 Hz, 1H, Ar–H), 7.26 (d, J = 10.3 Hz, 1H, coumarin H-6), 7.02 (dd, J = 8.8, 2.5 Hz, 2H, Ar–H), 6.97 (s, J = 2.4 Hz, 1H, coumarin H-3), 4.76 (s, 2H, OCH2), 3.92 (s, 6H, 2OCH3), 3.81 (s, 3H, OCH3), 2.37 (s, 3H, CH3), 2.08 (s, 3H, CH3). 13C-NMR (DMSO, 101 MHz, ppm) δ: 167.34 (d, JC–F = 164.8 Hz), 166.70, 165.20, 163.26, 162.70, 161.60, 160.00, 153.75, 153.23, 147.20, 142.86, 135.31 (d, JC–F = 8.9 Hz), 133.17, 130.59 (d, JC–F = 27.2 Hz), 129.57, 126.63, 120.44, 118.72, 116.79 (d, JC–F = 21.9 Hz), 116.57, 114.65, 112.88, 105.83, 101.85, 66.70, 60.84, 56.71, 15.39, 13.39. Analysis for C32H30FN3O9 (619.59): calcd, C, 62.03; H, 4.88; N, 6.78; found, C, 62.11; H, 5.02; N, 6.64.
4.1.3.4. (Z)-N-(1-(4-Chlorophenyl)-3-(2-(2-(4,8-dimethyl-2-oxo-2H-chromen-7-yloxy)acetyl)hydrazinyl)-3-oxoprop-1-en-2-yl)-3,4,5-trimethoxybenzamide (6c). Yield: 68%; m.p.: 247–249 °C; 1H-NMR (400 MHz, DMSO-d6, ppm) δ: 10.40 (d, J = 15.6 Hz, 1H, NH), 10.26 (s, 1H, NH), 9.79 (d, J = 14.4 Hz, 1H, NH), 7.73 (d, J = 8.3 Hz, 1H, coumarin H-5), 7.68 (d, J = 8.1 Hz, 2H, Ar–H), 7.48 (d, J = 8.1 Hz, 2H, Ar–H), 7.38 (d, J = 7.0 Hz, 1H, olefinic CH), 7.35 (s, 2H, Ar–H), 7.02 (d, J = 8.3 Hz, 1H, coumarin H-6), 6.98 (s, 1H, coumarin H-3), 4.80 (s, 2H, OCH2), 3.85 (s, 6H, 2OCH3), 3.74 (s, 3H, OCH3), 2.37 (s, 3H, CH3), 2.08 (s, 3H, CH3). 13C-NMR (DMSO, 101 MHz, ppm) δ: 166.69, 165.81, 164.74, 161.63, 160.16, 153.24, 153.02, 147.23, 140.93, 134.26, 133.76, 133.37, 131.58, 129.97, 129.13, 128.68, 126.62, 118.68, 114.60, 112.89, 106.09, 101.82, 66.64, 60.57, 56.54, 15.40, 13.40. Analysis for C32H30ClN3O9 (636.05): calcd, C, 60.43; H, 4.75; N, 6.61; found, C, 60.29; H, 4.58; N, 6.69.
4.1.3.5. (Z)-N-(1-(4-Cyanophenyl)-3-(2-(2-(4,8-dimethyl-2-oxo-2H-chromen-7-yloxy)acetyl)hydrazinyl)-3-oxoprop-1-en-2-yl)-3,4,5-trimethoxybenzamide (6d). Yield: 63%; m.p.: 246–248 °C; 1H-NMR (400 MHz, DMSO-d6, ppm) δ: 10.36 (s, 2H, 2NH), 9.86 (d, J = 175.5 Hz, 1H, NH), 7.86 (dt, J = 14.6, 7.4 Hz, 2H, Ar–H and coumarin H-5), 7.77 (t, J = 13.2 Hz, 2H, Ar–H), 7.71 (d, J = 8.9 Hz, 1H, Ar–H), 7.34 (d, J = 10.6 Hz, 2H, Ar–H), 7.22 (s, 1H, olefinic CH), 7.01 (d, J = 6.9 Hz, 1H, coumarin H-6), 6.97 (s, 1H, coumarin H-3), 4.81 (d, J = 37.5 Hz, 2H, OCH2), 3.84 (s, 6H, 2OCH3), 3.73 (s, 3H, OCH3), 2.36 (s, 3H, CH3), 2.07 (s, 3H, CH3). 13C-NMR (DMSO, 101 MHz, ppm) δ: 166.23, 165.37, 164.06, 161.15, 159.68, 152.77, 152.57, 146.74, 140.58, 138.89, 132.43, 131.63, 129.96, 128.33, 127.13, 126.13, 118.66, 118.21, 114.13, 112.42, 110.71, 105.66, 101.34, 66.16, 60.11, 56.08, 14.92, 12.92. Analysis for C33H30N4O9 (626.61): calcd, C, 63.25; H, 4.83; N, 8.94; found, C, 63.33; H, 4.95; N, 8.83.
4.1.3.6. (Z)-N-(3-(2-(2-(4,8-Dimethyl-2-oxo-2H-chromen-7-yloxy)acetyl)hydrazinyl)-1-(4-methoxyphenyl)-3-oxoprop-1-en-2-yl)-3,4,5-trimethoxybenzamide (6e). Yield: 69%; m.p.: 232–234 °C; 1H-NMR (400 MHz, DMSO-d6, ppm) δ: 10.30 (s, 2H, 2NH), 9.87 (d, J = 17.5 Hz, 1H, NH), 7.69 (d, J = 1.9 Hz, 1H, coumarin H-5), 7.63–7.55 (m, 2H, Ar–H), 7.38 (s, 2H, olefinic CH), 7.28 (d, J = 11.7 Hz, 1H, Ar–H), 7.01 (d, J = 6.6 Hz, 2H, coumarin H-6 and H-3), 6.97 (s, 2H, Ar–H), 4.76 (s, 2H, OCH2), 3.86 (s, 6H, 2OCH3), 3.76 (s, 3H, OCH3), 3.74 (s, 3H, OCH3), 2.36 (s, 3H, CH3), 2.08 (s, 3H, CH3). 13C-NMR (DMSO, 101 MHz, ppm) δ: 166.67, 165.78, 165.00, 161.61, 160.26, 160.01, 153.23, 152.98, 147.21, 131.76, 130.57, 130.38, 129.41, 127.22, 126.95, 126.63, 118.71, 114.65, 114.58, 112.89, 106.05, 101.84, 66.72, 60.57, 56.52, 55.69, 15.39, 13.40. Analysis for C33H33N3O10 (631.63): calcd, C, 62.75; H, 5.27; N, 6.65; found, C, 62.55; H, 5.16; N, 6.77.
4.1.3.7. (Z)-N-(1-(3,4-Dimethoxyphenyl)-3-(2-(2-(4,8-dimethyl-2-oxo-2H-chromen-7-yloxy)acetyl)hydrazinyl)-3-oxoprop-1-en-2-yl)-3,4,5-trimethoxybenzamide (6f). Yield: 59%; m.p.: 218–220 °C; 1H-NMR (400 MHz, DMSO-d6, ppm) δ: 10.34 (d, J = 41.6 Hz, 1H, NH), 10.16 (d, J = 20.4 Hz, 1H, NH), 9.72 (d, J = 97.9 Hz, 1H, NH), 7.75 (s, 2H, Ar–H), 7.69 (d, J = 1.9 Hz, 1H, coumarin H-5), 7.57 (d, J = 1.8 Hz, 1H, Ar–H), 7.20 (s, 1H, olefinic CH), 7.07 (d, J = 8.5 Hz, 1H, Ar–H), 7.04 (d, J = 3.5 Hz, 1H, coumarin H-6), 6.98 (d, J = 4.8 Hz, 2H, coumarin H-3 and Ar–H), 4.78 (d, J = 25.6 Hz, 2H, OCH2), 3.88 (s, 6H, 2OCH3), 3.86 (s, 3H, OCH3), 3.77 (s, 3H, OCH3), 3.62 (s, 3H, OCH3), 2.37 (s, 3H, CH3), 2.08 (s, 3H, CH3). 13C-NMR (DMSO, 101 MHz, ppm) δ: 167.03, 166.13, 162.34, 159.70, 153.48, 152.78, 151.67, 148.97, 148.09, 140.11, 132.14, 129.41, 128.97, 127.99, 126.13, 122.14, 118.19, 116.98, 114.12, 112.44, 111.90, 109.60, 107.17, 101.34, 66.23, 60.24, 60.04, 55.73, 55.59, 14.92, 12.92. Analysis for C34H35N3O11 (661.66): calcd, C, 61.72; H, 5.33; N, 6.35; found, C, 61.87; H, 5.52; N, 6.18.
4.2. Biological studies
4.3. Molecular docking study
See ESI.†Conflicts of interest
The authors report no conflicts of interest.Acknowledgements
This work was supported by the Deanship of Scientific Research, Vice Presidency for Graduate Studies and Scientific Research, King Faisal University, Saudi Arabia [grant 4539].References
- C. P. Wild, E. Weiderpass and B. W. Stewart, World Cancer Report: Cancer Research for Cancer Prevention, International Agency for Research on Cancer WHO, Lyon, France, 2020, available online, https://publications.iarc.fr/586, accessed on 18 July 2022 Search PubMed.
- G. A. Çiftçi, U. Yıldırım Ş, M. D. Altıntop and Z. A. Kaplancıklı, Induction of apoptosis in lung adenocarcinoma and glioma cells by some oxadiazole derivatives, Med. Chem. Res., 2014, 23, 3353–3362 CrossRef.
- J. Wei, Y. Yang, Z. Wang, Z. Wang, C. Fu, J. Zhu, J. Shan, Y. Huang, B. Tang and D. Jiang, A potential anticancer ability of 1,2-di(quinazolin-4-yl)diselane against gastric cancer cells through ROS signaling pathway, Med. Chem. Res., 2017, 26, 841–848 CrossRef CAS.
- V. Singh, A. Khurana, U. Navik, P. Allawadhi, K. K. Bharani and R. Weiskirchen, Apoptosis and Pharmacological Therapies for Targeting Thereof for Cancer Therapeutics, Sci, 2022, 4, 15–27 CrossRef CAS.
- M. Dimri and A. Satyanarayana, Molecular Signaling Pathways and Therapeutic Targets in Hepatocellular Carcinoma, Cancers, 2020, 12, 491–502 CrossRef CAS PubMed.
- S. Riaz, M. Iqbal, R. Ullah, R. Zahra, G. A. Chotana, A. Faisal and R. S. Z. Saleem, Synthesis and evaluation of novel α-substituted chalcones with potent anti-cancer activities and ability to overcome multidrug resistance, Bioorg. Chem., 2019, 87, 123–135 CrossRef CAS PubMed.
- K. N. Venugopala, V. Rashmi and B. Odhav, Review on Natural Coumarin Lead Compounds for Their Pharmacological Activity, BioMed Res. Int., 2013, 2013, 963248–963262 CAS.
- L. M. A. A. Ghany, B. Y. Beshay, A. M. Youssef Moustafa, A. H. A. Maghrabi, E. H. K. Ali, R. M. Saleem, I. Zaki and N. Ryad, Design, synthesis, anti-inflammatory evaluation, and molecular modelling of new coumarin-based analogs combined curcumin and other heterocycles as potential TNF-α production inhibitors via upregulating Nrf2/HO-1, downregulating AKT/mTOR signalling pathways and downregulating NF-κB in LPS induced macrophages, J. Enzyme Inhib. Med. Chem., 2023, 38, 2243551–2243564 CrossRef.
- Y. Wu, J. Xu, Y. Liu, Y. Zeng and G. Wu, A Review on Anti-Tumor Mechanisms of Coumarins, Front. Oncol., 2020, 10, 1–11 CrossRef PubMed.
- A. Rawat and A. Vijaya Bhaskar Reddy, Recent advances on anticancer activity of coumarin derivatives, Eur. J. Med. Chem. Rep., 2022, 5, 100038–100057 CAS.
- E. Veignie, C. Ceballos, C. Len and C. Rafin, Design of New Antifungal Dithiocarbamic Esters Having Bio-Based Acrylate Moiety, ACS Omega, 2019, 4, 4779–4784 CrossRef CAS.
- G. Verma, G. Chashoo, A. Ali, M. F. Khan, W. Akhtar, I. Ali, M. Akhtar, M. M. Alam and M. Shaquiquzzaman, Synthesis of pyrazole acrylic acid based oxadiazole and amide derivatives as antimalarial and anticancer agents, Bioorg. Chem., 2018, 77, 106–124 CrossRef CAS PubMed.
- I. Zaki, S. A. Eid, M. S. Elghareb, A.-S. M. Abas, G. Mersal and F. Z. Mohammed, In Vitro Antitumor Evaluation of Acrylic Acid Derivatives Bearing Quinolinone Moiety as Novel Anticancer Agents, Anti-Cancer Agents Med. Chem., 2022, 22, 1634–1642 CrossRef CAS PubMed.
- K. O. Mohamed, I. Zaki, I. M. El-Deen and M. K. Abdelhameid, A new class of diamide scaffold: design, synthesis and biological evaluation as potent antimitotic agents, tubulin polymerization inhibition and apoptosis inducing activity studies, Bioorg. Chem., 2019, 84, 399–409 CrossRef CAS PubMed.
- N. E. A. A. El-Sattar, S. E. S. A. El-Hddad, M. M. Ghobashy, A. A. Zaher and K. El-Adl, Nanogel-mediated drug delivery system for anticancer agent: pH stimuli responsive poly(ethylene glycol/acrylic acid) nanogel prepared by gamma irradiation, Bioorg. Chem., 2022, 127, 105972–105986 CrossRef CAS PubMed.
- M. Hao, Y. Guo, Z. Zhang, H. Zhou, Q. Gu and J. Xu, 6-acrylic phenethyl ester-2-pyranone derivative induces apoptosis and G2/M arrest by targeting GRP94 in colorectal cancer, Bioorg. Chem., 2022, 123, 105802–105814 CrossRef CAS PubMed.
- C. D. Ley, M. R. Horsman and P. E. G. Kristjansen, Early Effects of Combretastatin-A4 Disodium Phosphate on Tumor Perfusion and Interstitial Fluid Pressure, Neoplasia, 2007, 9, 108–112 CrossRef CAS PubMed.
- S. Torijano-Gutiérrez, S. Díaz-Oltra, E. Falomir, J. Murga, M. Carda and J. A. Marco, Synthesis of combretastatin A-4 O-alkyl derivatives and evaluation of their cytotoxic, antiangiogenic and antitelomerase activity, Bioorg. Med. Chem., 2013, 21, 7267–7274 CrossRef.
- S. N. Baytas, N. Inceler, A. Yılmaz, A. Olgac, S. Menevse, E. Banoglu, E. Hamel, R. Bortolozzi and G. Viola, Synthesis, biological evaluation and molecular docking studies of trans-indole-3-acrylamide derivatives, a new class of tubulin polymerization inhibitors, Bioorg. Med. Chem., 2014, 22, 3096–3104 CrossRef CAS PubMed.
- M. Gao, D. Zhang, Q. Jin, C. Jiang, C. Wang, J. Li, F. Peng, D. Huang, J. Zhang and S. Song, Combretastatin-A4 phosphate improves the distribution and antitumor efficacy of albumin-bound paclitaxel in W256 breast carcinoma model, Oncotarget, 2016, 7, 58133–58141 CrossRef PubMed.
- P. Nathan, M. Zweifel, A. R. Padhani, D.-M. Koh, M. Ng, D. J. Collins, A. Harris, C. Carden, J. Smythe and N. Fisher, Phase I trial of combretastatin A4 phosphate (CA4P) in combination with bevacizumab in patients with advanced cancer, Clin. Cancer Res., 2012, 18, 3428–3439 CrossRef CAS PubMed.
- D. Simoni, R. Romagnoli, R. Baruchello, R. Rondanin, M. Rizzi, M. G. Pavani, D. Alloatti, G. Giannini, M. Marcellini, T. Riccioni, M. Castorina, M. B. Guglielmi, F. Bucci, P. Carminati and C. Pisano, Novel Combretastatin Analogues Endowed with Antitumor Activity, J. Med. Chem., 2006, 49, 3143–3152 CrossRef CAS PubMed.
- J. P. Stevenson, M. Rosen, W. Sun, M. Gallagher, D. G. Haller, D. Vaughn, B. Giantonio, R. Zimmer, W. P. Petros and M. Stratford, Phase I trial of the antivascular agent combretastatin A4 phosphate on a 5-day schedule to patients with cancer: magnetic resonance imaging evidence for altered tumor blood flow, J. Clin. Oncol., 2003, 21, 4428–4438 CrossRef CAS PubMed.
- M. Hawash, N. Jaradat, M. Abualhasan, M. Qneibi, H. Rifai, T. Saqfelhait, Y. Shqirat, A. Nazal, S. Omarya and T. Ibrahim, Evaluation of cytotoxic, COX inhibitory, and antimicrobial activities of novel isoxazole-carboxamide derivatives, Lett. Drug Des. Discovery, 2023, 20, 1994–2002 CrossRef CAS.
- M. Thomas, A. Bender and C. de Graaf, Integrating structure-based approaches in generative molecular design, Curr. Opin. Struct. Biol., 2023, 79, 102559 CrossRef CAS PubMed.
- N. Duangdee, W. Mahavorasirikul and S. Prateeptongkum, Design synthesis and anti-proliferative activity of some new coumarin substituted hydrazide–hydrazone derivatives, J. Chem. Sci., 2020, 132, 66–77 CrossRef CAS.
- Z. Hou, J. Liu, Z. Jin, G. Qiu, Q. Xie, S. Mi and J. Huang, Use of chemotherapy to treat hepatocellular carcinoma, BioSci. Trends, 2022, 16, 31–45 CrossRef CAS.
- M. K. Abdelhameid, I. Zaki, M. R. Mohammed and K. O. Mohamed, Design, synthesis, and cytotoxic screening of novel azole derivatives on hepatocellular carcinoma (HepG2 Cells), Bioorg. Chem., 2020, 101, 103995–104014 CrossRef CAS.
- H. M. A. El-Lateef, R. M. Saleem, M. A. Bazuhair, A. H. A. Maghrabi, E. H. K. Ali, I. Zaki and R. E. Masoud, Design, synthesis and tubulin polymerization inhibition activity of newly synthesized hydrazone-linked to combretastatin analogues as potential anticancer agents, J. Mol. Struct., 2023, 1292, 136190–136199 CrossRef CAS.
- T. Al-Warhi, L. S. Alqahtani, G. Alsharif, M. Abualnaja, O. A. Abu Ali, S. H. Qahl, H. A. E. Althagafi, F. Alharthi, I. Jafri, F. G. Elsaid, A. A. Shati, S. Aloufi, E. Fayad, I. Zaki and M. M. Morcoss, Design, Synthesis, and Investigation of Cytotoxic Activity of cis-Vinylamide-Linked Combretastatin Analogues as Potential Anticancer Agents, Symmetry, 2022, 14, 2088–2097 CrossRef CAS.
- D. S. Alshaya, R. M. Tawakul, I. Zaki, A. H. A. Almaaty, E. Fayad and Y. M. Abd El-Aziz, Design, synthesis and antiproliferative screening of newly synthesized acrylate derivatives as potential anticancer agents, RSC Adv., 2023, 13, 23538–23546 RSC.
- E. Fayad, S. A. Altalhi, M. M. Abualnaja, A. H. Alrohaimi, F. G. Elsaid, A. H. Abu Almaaty, R. M. Saleem, M. A. Bazuhair, A. H. Ahmed Maghrabi, B. Y. Beshay and I. Zaki, Novel Acrylate-Based Derivatives: Design, Synthesis, Antiproliferative Screening, and Docking Study as Potential Combretastatin Analogues, ACS Omega, 2023, 8, 38394–38405 CrossRef CAS PubMed.
- W. Li, H. Sun, S. Xu, Z. Zhu and J. Xu, Tubulin inhibitors targeting the colchicine binding site: a perspective of privileged structures, Future Med. Chem., 2017, 9, 1765–1794 CrossRef CAS PubMed.
- H. M. Abd El-Lateef, A. A. Elmaaty, L. M. A. Abdel Ghany, M. S. Abdel-Aziz, I. Zaki and N. Ryad, Design and Synthesis of 2-(4-Bromophenyl)Quinoline-4-Carbohydrazide Derivatives via Molecular Hybridization as Novel Microbial DNA-Gyrase Inhibitors, ACS Omega, 2023, 8, 17948–17965 CrossRef CAS PubMed.
- I. Zaki, A. M. Y. Moustafa, B. Y. Beshay, R. E. Masoud, M. A. I. Elbastawesy, M. A. S. Abourehab and M. Y. Zakaria, Design and synthesis of new trimethoxylphenyl-linked combretastatin analogues loaded on diamond nanoparticles as a panel for ameliorated solubility and antiproliferative activity, J. Enzyme Inhib. Med. Chem., 2022, 37, 2679–2701 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra06644d |
| This journal is © The Royal Society of Chemistry 2023 |