Open Access Article
Open Access ArticleA light-activated polymer with excellent serum tolerance for intracellular protein delivery†
Lanfang
Ren
a,
Li
Jiang
b,
Qianyi
Ren
a,
Jia
Lv
a,
Linyong
Zhu
b and
Yiyun
Cheng
 *a
*a
aShanghai Frontiers Science Center of Genome Editing and Cell Therapy, Shanghai Key Laboratory of Regulatory Biology, School of Life Sciences, East China Normal University, Shanghai, 200241, China. E-mail: yycheng@mail.ustc.edu.cn
bSchool of Biomedical Engineering, Shanghai Jiaotong University, Shanghai, 200240, China
First published on 21st January 2023
Abstract
The design of efficient materials for intracellular protein delivery has attracted great interest in recent years; however, most current materials for this purpose are limited by poor serum stability due to the early release of cargoes triggered by abundant serum proteins. Here, we propose a light-activated crosslinking (LAC) strategy to prepare efficient polymers with excellent serum tolerance for intracellular protein delivery. A cationic dendrimer engineered with photoactivatable O-nitrobenzene moieties co-assembles with cargo proteins via ionic interactions, followed by light activation to yield aldehyde groups on the dendrimer and the formation of imine bonds with cargo proteins. The light-activated complexes show high stability in buffer and serum solutions, but dis-assemble under low pH conditions. As a result, the polymer successfully delivers cargo proteins green fluorescent protein and β-galactosidase into cells with maintained bioactivity even in the presence of 50% serum. The LAC strategy proposed in this study provides a new insight to improve the serum stability of polymers for intracellular protein delivery.
Introduction
Protein therapeutics have attracted great interest in the pharmaceutical industry due to their advantages such as high potency and specificity, low adverse effects, limited immunogenicity, and faster approval compared to traditional small-molecule drugs.1 The sales of global therapeutic proteins have reached 98.1 billion dollars in 2021.2 However, protein therapeutics are hampered by two major limitations including susceptibility to enzymatic degradation and difficulty to cross biological barriers such as cell membranes.3–8 As a result, all current protein drugs have been developed based on extracellular targets.9,10Several strategies have been proposed to achieve intracellular protein delivery.11–14 Physical methods such as electroporation and nanospearing that could transiently disrupt cell membranes are used to deliver proteins into cell cytosol,15 but these methods cannot be translated for in vivo protein delivery. Conjugation of cargo proteins with cell membrane permeable ligands is another widely used method for intracellular protein delivery.16–20 Proteins modified with cell penetrant peptides, cationic polymers, and amphiphilic molecules could be internalized by cells via endocytosis, but the method was associated with complicated synthesis and possibility of altered protein bioactivity after modification. Intracellular protein delivery using carriers such as nanoparticles and polymers is the most promising strategy in recent years.21–30 Cargo proteins were loaded by cell-permeable carriers via non-covalent interactions such as ionic and hydrophobic interactions for intracellular protein delivery.31–38 However, cargo proteins loaded by these carriers are easily released under physiological conditions due to competitive binding with abundant serum proteins. Strengthening the interactions with cargo proteins can improve the serum stability of carriers, but the delivery systems are usually associated with problems such as difficulty in intracellular protein release.39 It is highly desired to develop carriers that can deliver proteins inside cells in the presence of abundant serum proteins.40,41
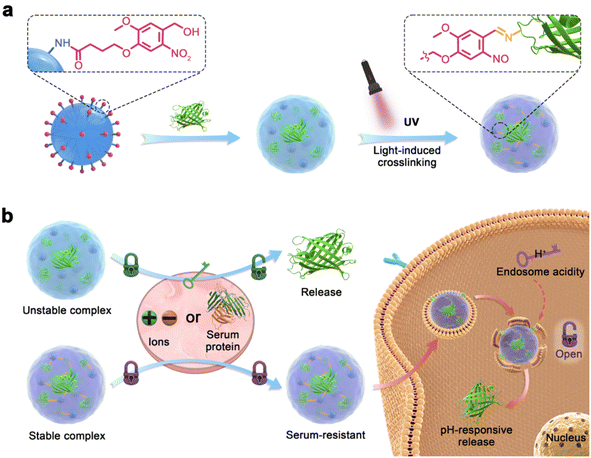
Here, we propose a light-activated crosslinking (LAC) strategy to prepare polymers with excellent serum tolerance for intracellular protein delivery (Fig. 1). O-Nitrobenzene (NB) and its derivatives were reported to be photo-activatable ligands that generate bioactive aldehyde groups under ultra-violet (UV) light irradiation,42–44 and the ligands were widely used for photo-activated ligation or bio-adhesion.45–50 The NB ligand (named NB-NHS) is modified on the surface of a cationic dendrimer to yield the protein-delivering material (abbreviated as ND, Fig. 1a). ND forms complexes with cargo proteins via non-covalent ionic and hydrophobic interactions, but the complexes are easily dis-assembled in the presence of salt ions and serum proteins due to competitive binding. After UV light (365 nm) irradiation, the ND complexes show strongly improved buffer and serum stabilities via the formation of dynamic covalent imine bonds between the polymer and cargo protein. After the complexes were endocytosed into cells, the imine bonds in the complexes will be cleaved by endolysosomal acidity, which triggers the release of bound proteins by ND (Fig. 1b). The study aims to provide a novel strategy to achieve efficient intracellular protein delivery in the presence of abundant serum proteins.
Results and discussion
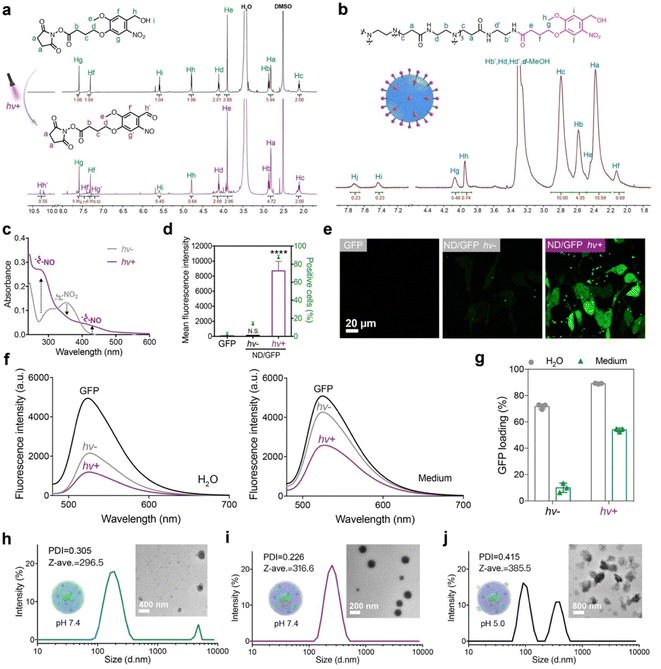
The photoreactive NB ligand was synthesized and characterized by 1H NMR (Fig. 2a), 13C NMR (Fig. S7†), and ESI-MS (Fig. S8†). After irradiation with 365 nm UV light at 10 mW cm−2 for 300 s, the proton signals Hg, Hf, Hh, and Hi on the NB ligand were greatly decreased, while new peaks Hg’, Hf’ and Hh’ appeared, suggesting that light irradiation activated the generation of aldehyde groups on NB-NHS. The NB ligand was then conjugated onto the surface of a generation 5 polyamidoamine dendrimer via amidation reactions, and the conjugate ND was characterized by 1H NMR (Fig. 2b). The average number of NB moieties modified on each dendrimer was measured to be about 11. The photoreactive properties of ND were further verified by UV-vis spectroscopy. After irradiation with UV light for 20 s, the absorbance peak for the NO2 group around 350 nm decreased, while peaks for the NO group at 280 nm and 430 nm greatly increased (Fig. 2c), which confirmed the photo-activatable properties of ND as proposed.Green fluorescent protein (GFP) was first used as a model protein to evaluate the performance of the ND polymer in cytosolic protein delivery. ND failed to efficiently deliver GFP into 143B cells before LAC; however, the polymer showed significantly improved delivery efficacy when ND/GFP complexes were irradiated with 365 nm UV light at 10 mW cm−2 for 20 s (Fig. 2d and e). Further increase in irradiation time did not further increase the protein delivery efficacy (Fig. S9a and b†), indicating the fast transition of the benzyl alcohol moiety on NB into the benzyl aldehyde group after UV illumination. The irradiation time for LAC was fixed at 20 s for later studies. The yielding aldehyde groups on ND could react with lysine residues on GFP via the formation of dynamic covalent imine bonds, and improve the stability of ND/GFP complexes. We then measured the ND/GFP complexes in different solutions before and after LAC by fluorescence spectroscopy. In aqueous solution, ND interacts with GFP via ionic interactions and forms nanocomplexes in water, resulting in quenched GFP fluorescence. After light irradiation, the photoactivated aldehyde groups strengthened the interactions between ND and GFP via imine bonds, and thereby more efficient GFP fluorescence quenching was observed. In culture medium or PBS buffer, the ND/GFP complex is not stable due to the presence of highly abundant salt ions, but the complex still showed efficient fluorescence quenching after LAC (Fig. 2f and S9c†). The GFP loading efficiencies of the ND polymer were then quantitatively measured. As shown in Fig. 2g and S3d,† LAC treatment efficiently improved the GFP loading efficiency of ND, especially in culture medium and PBS buffer. The ND/GFP complexes before and after LAC were further measured by dynamic light scattering (DLS) and transmission electron microscopy (TEM). The results showed that LAC treatment facilitated the formation of uniform ND/GFP nanoparticles (Fig. 2h–j). The formed nanoparticles were unstable under pH 5.0, which is due to the acid-labile properties of imine bonds between ND and GFP. This behavior is beneficial for intracellular release of cargo proteins after localization in endo-lysosomes. These results together proved the beneficial role of LAC in improving the protein loading, complex stability, and intracellular delivery efficiency of ND/GFP complexes.
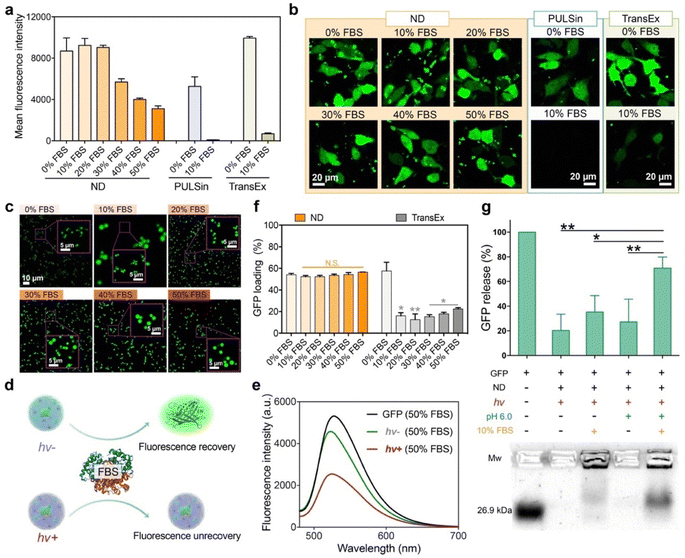
We further tested the possibility of the LAC-treated ND polymer in cytosolic protein delivery in the presence of serum proteins. As shown in Fig. 3a and b, the ND polymer after LAC efficiently delivered GFP into the cytosol of 143B cells in media containing 10–50% fetal bovine serum (FBS). The protein delivery efficacies of ND in media containing 10% and 20% FBS were scarcely changed compared to that in serum-free medium. Even in medium containing 50% FBS, the polymer still maintained relatively high delivery efficacy. In comparison, the commercial reagents for intracellular protein delivery such as PULSin and TransEx showed more than a 90% efficacy decrease in the presence of 10% FBS, suggesting the superior performance of the ND polymer under serum-containing conditions. We further tested the stability of the ND/GFP complex after LAC in serum-containing culture medium. As shown by confocal images in Fig. 3c, the LAC-treated complexes showed very similar structures and fluorescence signals in media containing 10–50% FBS compared to those in serum-free medium, suggesting that the bound GFP molecules in ND/GFP complexes were not replaced by the highly abundant serum proteins in media. TEM images also confirmed the excellent stability of LAC-treated ND/GFP nanoparticles in media containing FBS (Fig. S10a†). We further used fluorescence spectroscopy to reveal the stability of ND/GFP complexes in serum. As shown in Fig. 3d–e and S10b–c,† the fluorescence intensities of ND/GFP complexes without LAC were almost recovered when 50% FBS was added to DI water or culture medium, which is caused by GFP release from the complex due to competitive binding of serum proteins to ND. However, LAC treatment strongly improved the serum resistance of ND/GFP complexes as revealed by the slightly recovered GFP fluorescence intensity even in 50% FBS. In addition, the ND polymer after LAC treatment showed very similar GFP loading efficiencies in medium with or without FBS, while the commercial reagent TransEx showed greatly decreased protein loading in the presence of FBS (Fig. 3f). We next investigated the release responsiveness of cargo proteins from ND/GFP complexes via an agarose gel electrophoresis assay. As shown in Fig. 3g, LAC treated complexes showed minimal GFP release in the presence of 10% FBS at pH 7.4, while the released proteins were significantly increased when the pH was decreased to 6.0, suggesting pH-responsive release behavior of the delivery system. These results together confirmed the great potential of the LAC strategy in the improvement of protein delivery efficacy in serum-containing media and intracellular responsive release of cargo proteins.
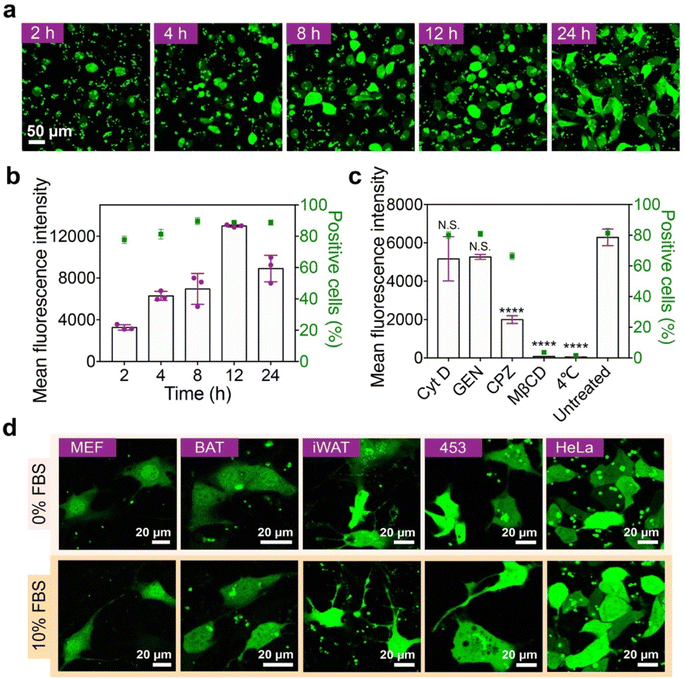
The effects of ND dose and incubation time on the GFP delivery efficacy were further investigated. The delivery efficacy of the ND polymer increases with increasing polymer dose, but a high polymer dose may lead to cytotoxicity in the treated cells (Fig. S11a–c†). According to the results, the ND polymer at 32 μg mL−1 with relatively high delivery efficacy and low cytotoxicity was chosen for later studies. The delivery efficacy of ND also increases along with the incubation time, and the highest efficacy is achieved at 12 h after incubation (Fig. 4a and b). Though the GFP fluorescence intensity of treated cells at 24 h was relatively lower, the delivered GFP molecules were more well distributed in the cells compared to those at 12 h. The internalization of ND/GFP complexes by 143B cells was efficiently inhibited at 4 °C or by pretreatment with methyl-β-cyclodextrin (MβCD) and chlorpromazine (CPZ) but scarcely affected by cytochalasin D (Cyt D) and genestein (GEN) (Fig. 4c), suggesting the involvement of lipid-raft and clathrin-dependent pathways during endocytosis of the nanoparticles. After treatment, the delivered ND/GFP nanoparticles were generally not co-localized with endolysosomes stained with Lysotracker red, suggesting efficient endosomal escape after 24 h incubation (Fig. S12†). Besides 143B cells, the LAC-treated ND polymer also efficiently delivered GFP into mouse embryonic fibroblast cells (MEF cells), mouse brown adipose tissue cells (BAT cells), mouse inguinal white adipose tissue cells (iWAT cells), human breast cancer cells (MDA-MB-453 cells), and HeLa cells in the presence of 10% serum (Fig. 4d), confirming the robustness of this material in cytosolic protein delivery.
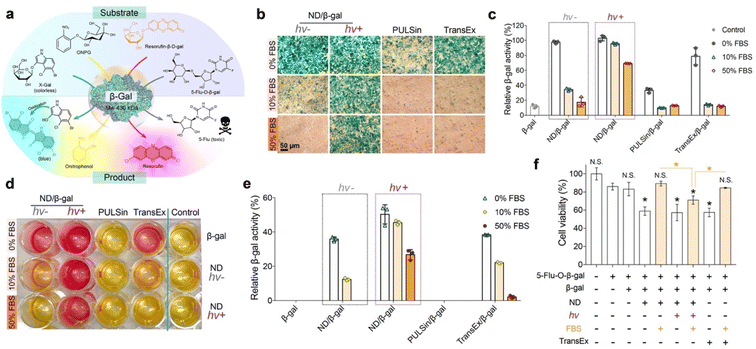
β-Galactosidase (β-gal) is capable of hydrolyzing the galactose residues from different substrates (Fig. 5a). As shown in Fig. S13,† the in vitro enzymatic activity of β-gal that complexed with the ND carrier after LAC would be partially inhibited in fresh medium (pH 7.4 or 6.0) or serum-containing medium (pH 7.4), while fully recovered in acidic serum-containing medium (pH 6.0). Hence, we presume that the cargo proteins can be released from the ND carrier and remain active after entering into cells. The efficacy of the ND polymer in the delivery of bioactive β-gal into 143B cells was next evaluated. The cells were first treated with ND/β-gal complexes with or without LAC for 24 h, and then with the substrates to analyze the delivery efficacy. First, a colorless substrate 5-bromo-4-chloro-3-indolyl-β-D-galactoside (X-gal) was used to stain the treated cells. As shown in Fig. 5b, the cells treated with ND/β-gal complexes (with or without LAC) and TransEx/β-gal complexes in serum-free media all showed obvious blue-colored products after X-gal staining; however, the number of blue-colored products was greatly decreased for ND/β-gal complexes without LAC and TransEx/β-gal complexes in serum-containing media. Similarly, the quantitative analysis via another substrate O-nitrophenyl-β-D-galactopyranoside (ONPG) showed superior β-gal delivery of the ND/β-gal complexes with LAC (Fig. 5c). Resorufin-β-D-galactopyranoside (resorufin-β-D-gal) can be hydrolyzed from non-fluorescent orange material into fluorescent red product by β-gal. After ordered treatment of the ND/β-gal complexes with LAC and resorufin-β-D-gal, the cells cultured with 0–50% serum were efficiently stained into red fluorescence (Fig. 5d). The relative quantitative result of resorufin-β-D-gal assay also demonstrated the maintained high β-gal activity for the ND/β-gal complexes with LAC in serum-containing media (Fig. 5e). 5-Fluorouridine-5′-O-β-D-galactopyranoside (5-flu-O-β-gal) is a low toxic compound, but it generates an anticancer drug 5-fluorouridine in the presence of β-gal. 5-Flu-O-β-gal was further used as the substrate to evaluate the amount of delivered β-gal into 143B cells. As shown in Fig. 5f, the cells treated with all the complexes in a serum-free medium showed obvious cytotoxicity after the addition of 5-flu-O-β-gal. However, the activities of ND/β-gal without LAC and TransEx/β-gal complexes were much decreased in serum-containing medium, while the activity for ND/β-gal complexes with LAC was maintained. These results together proved the important role of LAC treatment in improving the serum resistance of ND during intracellular protein delivery.
Conclusions
In summary, we report a light-activated crosslinking strategy to achieve efficient intracellular protein delivery in serum-containing medium. The cationic polymer engineered with photoactivatable O-nitrobenzene moieties binds cargo proteins via ionic interactions to form nanoparticles. After light irradiation, the photoactivated aldehyde groups on the polymer further tag with amines on proteins via imine bonds to improve the complex stability. As a result, the polymer after light-activated crosslinking showed high efficacy in the delivery of cargo proteins into the cytosol of cells with maintained bioactivity. This study provides a versatile strategy to develop efficient polymers with high serum tolerance for intracellular protein delivery.Data availability
The datasets supporting this article have been uploaded as part of the ESI.†Author contributions
L. R. prepared and characterized the polymer, conducted the protein delivery experiments, and wrote the manuscript. L. J. synthesized and characterized the light-activatable NB ligand. Q. R. and J. L. performed part of the experiments on bioactive protein delivery and analyzed the mechanism and data. L. Z. and Y. C. designed and supervised the study, and wrote the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (22135002) and the Basic Research Program of Science and Technology Commission of Shanghai Municipality (21JC1401800), and the National Key R&D Program of China, Synthetic Biology Research (No. 2019YFA0904500).References
- B. Leader, Q. J. Baca and D. E. Golan, Nat. Rev. Drug Discovery, 2008, 7, 21–39 CrossRef CAS.
- C. Sánchez-Trasviña, M. Flores-Gatica, D. Enriquez-Ochoa, M. Rito-Palomares and K. Mayolo-Deloisa, Front. Bioeng. Biotechnol., 2021, 9, 717326 CrossRef.
- M. Chiper, K. Niederreither and G. Zuber, Adv. Healthcare Mater., 2018, 7, 1701040 CrossRef.
- D. C. Luther, T. Jeon, R. Goswami, H. Nagaraj, D. Kim, Y.-W. Lee and V. M. Rotello, Bioconjugate Chem., 2021, 32, 891–896 CrossRef CAS.
- X. Qin, C. Yu, J. Wei, L. Li, C. Zhang, Q. Wu, J. Liu, S. Q. Yao and W. Huang, Adv. Mater., 2019, 31, 1902791 CrossRef CAS.
- J. Lv, Q. Fan, H. Wang and Y. Cheng, Biomaterials, 2019, 218, 119358 CrossRef CAS PubMed.
- X. Liu, F. Wu, Y. Ji and L. Yin, Bioconjugate Chem., 2019, 30, 305–324 CrossRef PubMed.
- J. Xu, J. Lv, Q. Zhuang, Z. Yang, Z. Cao, L. Xu, P. Pei, C. Wang, H. Wu, Z. Dong, Y. Chao, C. Wang, K. Yang, R. Peng, Y. Cheng and Z. Liu, Nat. Nanotechnol., 2020, 15, 1043–1052 CrossRef CAS PubMed.
- J. Caravella and A. Lugovskoy, Curr. Opin. Chem. Biol., 2010, 14, 520–528 CrossRef CAS PubMed.
- R. Mo, T. Jiang, J. Di, W. Tai and Z. Gu, Chem. Soc. Rev., 2014, 43, 3595 RSC.
- W. Tai, P. Zhao and X. Gao, Sci. Adv., 2020, 6, eabb0310 CrossRef CAS PubMed.
- J. Xu, Z. Li, Q. Fan, J. Lv, Y. Li and Y. Cheng, Adv. Mater., 2021, 2104355 CrossRef CAS PubMed.
- Y. Cheng, Chin. J. Chem., 2021, 39, 1443–1449 CrossRef CAS.
- J. Lv and Y. Cheng, Chem. Soc. Rev., 2021, 50, 5435–5467 RSC.
- Y. Cao, E. Ma, S. Cestellos-Blanco, B. Zhang, R. Qiu, Y. Su, J. A. Doudna and P. Yang, Proc. Natl. Acad. Sci. U. S. A., 2019, 116, 7899 CrossRef CAS PubMed.
- J. Fu, C. Yu, L. Li and S. Q. Yao, J. Am. Chem. Soc., 2015, 137, 12153–12160 CrossRef CAS PubMed.
- L. Qian, J. Fu, P. Yuan, S. Du, W. Huang, L. Li and S. Q. Yao, Angew. Chem., Int. Ed., 2018, 57, 1532–1536 CrossRef CAS PubMed.
- K. Maier and E. Wagner, J. Am. Chem. Soc., 2012, 134, 10169–10173 CrossRef CAS PubMed.
- D. Y. W. Ng, M. Arzt, Y. Wu, S. L. Kuan, M. Lamla and T. Weil, Angew. Chem., Int. Ed., 2014, 53, 324–328 CrossRef CAS PubMed.
- K. A. Mix, J. E. Lomax and R. T. Raines, J. Am. Chem. Soc., 2017, 139, 14396–14398 CrossRef CAS PubMed.
- J. A. Kretzmann, D. C. Luther, C. W. Evans, T. Jeon, W. Jerome, S. Gopalakrishnan, Y.-W. Lee, M. Norret, K. S. Iyer and V. M. Rotello, J. Am. Chem. Soc., 2021, 143, 4758–4765 CrossRef CAS PubMed.
- Y.-W. Lee, D. C. Luther, R. Goswami, T. Jeon, V. Clark, J. Elia, S. Gopalakrishnan and V. M. Rotello, J. Am. Chem. Soc., 2020, 142, 4349–4355 CrossRef CAS.
- K. Dutta, D. Hu, B. Zhao, A. E. Ribbe, J. Zhuang and S. Thayumanavan, J. Am. Chem. Soc., 2017, 139, 5676–5679 CrossRef CAS PubMed.
- B. Liu, M. Ianosi-Irimie and S. Thayumanavan, ACS Nano, 2019, 13, 9408–9420 CrossRef CAS PubMed.
- Y. Jiang, J. Zhang, F. Meng and Z. Zhong, ACS Nano, 2018, 12, 11070–11079 CrossRef CAS PubMed.
- Y. Jiang, W. Yang, J. Zhang, F. Meng and Z. Zhong, Adv. Mater., 2018, 30, 1800316 CrossRef PubMed.
- G. Li, S. Yuan, D. Deng, T. Ou, Y. Li, R. Sun, Q. Lei, X. Wang, W. Shen, Y. Cheng, Z. Liu and S. Wu, Adv. Funct. Mater., 2019, 29, 1901932 CrossRef.
- J. Lv, H. Wang, G. Rong and Y. Cheng, Acc. Chem. Res., 2022, 55, 722–733 CrossRef CAS PubMed.
- Z. Zhang, X. Gao, Y. Li, J. Lv, H. Wang and Y. Cheng, CCS Chem., 2022 DOI:10.31635/ccschem.022.202202098.
- L. Ren, Y. Gao and Y. Cheng, Bioact. Mater., 2022, 9, 44–53 CrossRef CAS PubMed.
- R. Tang, M. Wang, M. Ray, Y. Jiang, Z. Jiang, Q. Xu and V. M. Rotello, J. Am. Chem. Soc., 2017, 139, 8547–8551 CrossRef CAS PubMed.
- R. Mout, M. Ray, G. Yesilbag Tonga, Y.-W. Lee, T. Tay, K. Sasaki and V. M. Rotello, ACS Nano, 2017, 11, 2452–2458 CrossRef CAS PubMed.
- N. D. Posey, C. R. Hango, L. M. Minter and G. N. Tew, Bioconjugate Chem., 2018, 29, 2679–2690 CrossRef CAS PubMed.
- F. Sgolastra, C. M. Backlund, E. Ilker Ozay, B. M. deRonde, L. M. Minter and G. N. Tew, J. Controlled Release, 2017, 254, 131–136 CrossRef CAS PubMed.
- X. Liu, Z. Zhao, F. Wu, Y. Chen and L. Yin, Adv. Mater., 2022, 34, 2108116 CrossRef CAS.
- C. Liu, T. Wan, H. Wang, S. Zhang, Y. Ping and Y. Cheng, Sci. Adv., 2019, 5, eaaw8922 CrossRef CAS PubMed.
- L. Ren, J. Lv, H. Wang and Y. Cheng, Angew. Chem., Int. Ed., 2020, 59, 4711–4719 CrossRef CAS PubMed.
- Z. Zhang, W. Shen, J. Ling, Y. Yan, J. Hu and Y. Cheng, Nat. Commun., 2018, 9, 1377 CrossRef PubMed.
- S. Zhang, J. Lv, P. Gao, Q. Feng, H. Wang and Y. Cheng, Nano Lett., 2021, 21, 7855–7861 CrossRef CAS PubMed.
- A. Barrios, M. Estrada and J. H. Moon, Angew. Chem., Int. Ed., 2022, 61, e202116722 CrossRef CAS PubMed.
- S. Zhang, E. Tan, R. Wang, P. Gao, H. Wang and Y. Cheng, Nano Lett., 2022, 22, 8233–8240 CrossRef CAS.
- W. Zhang, B. Bao, F. Jiang, Y. Zhang, R. Zhou, Y. Lu, S. Lin, Q. Lin, X. Jiang and L. Zhu, Adv. Mater., 2021, 33, 2105667 CrossRef CAS.
- Z. Ming, J. Fan, C. Bao, Y. Xue, Q. Lin and L. Zhu, Adv. Funct. Mater., 2018, 28, 1706918 CrossRef.
- Y. Hua, H. Xia, L. Jia, J. Zhao, D. Zhao, X. Yan, Y. Zhang, S. Tang, G. Zhou, L. Zhu and Q. Lin, Sci. Adv., 2021, 7, eabg0628 CrossRef CAS PubMed.
- Y. Zhou, H. Yang, C. Wang, Y. Xue, X. Wang, C. Bao and L. Zhu, Chem. Sci., 2021, 12, 3627–3632 RSC.
- J. Sun, W. Birnbaum, J. Anderski, M.-T. Picker, D. Mulac, K. Langer and D. Kuckling, Biomacromolecules, 2018, 19, 4677–4690 CrossRef CAS PubMed.
- K. Peng, I. Tomatsu, B. van den Broek, C. Cui, A. V. Korobko, J. van Noort, A. H. Meijer, H. P. Spaink and A. Kros, Soft Matter, 2011, 7, 4881 RSC.
- X. Tan, B. B. Li, X. Lu, F. Jia, C. Santori, P. Menon, H. Li, B. Zhang, J. J. Zhao and K. Zhang, J. Am. Chem. Soc., 2015, 137, 6112–6115 CrossRef CAS PubMed.
- S. S. Agasti, A. Chompoosor, C.-C. You, P. Ghosh, C. K. Kim and V. M. Rotello, J. Am. Chem. Soc., 2009, 131, 5728–5729 CrossRef CAS.
- P. Klán, T. Šolomek, C. G. Bochet, A. Blanc, R. Givens, M. Rubina, V. Popik, A. Kostikov and J. Wirz, Chem. Rev., 2013, 113, 119–191 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2sc05848k |
| This journal is © The Royal Society of Chemistry 2023 |