Open Access Article
Open Access ArticleDistal meta-C–H functionalization of α-substituted cinnamates†
Manickam
Bakthadoss
 * and
Tadiparthi Thirupathi
Reddy
* and
Tadiparthi Thirupathi
Reddy
Department of Chemistry, Pondicherry University, Pondicherry, 605014, India. E-mail: bhakthadoss@yahoo.com
First published on 23rd February 2023
Abstract
Development of a novel strategy for the palladium-catalyzed selective meta-C–H activation of α-substituted cinnamates and their heterocyclic analogues with various alkenes using nitrile as a directing group (DG) has been described. Importantly, we introduced naphthoquinone, benzoquinones, maleimides and sulfolene as coupling partners in the meta-C–H activation reaction for the first time. Notably, allylation, acetoxylation and cyanation were also achieved through distal meta-C–H functionalization. This novel protocol also includes the coupling of various olefin-tethered bioactive molecules with high selectivity.
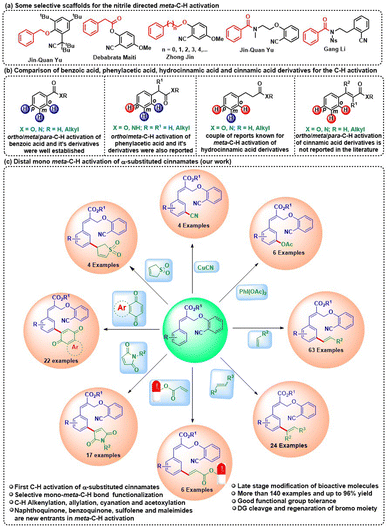
Over the last two decades, the transition metal-catalyzed functionalization of arene C–H bonds has been extensively studied using various directing groups. In particular, the ortho-C–H activation of arenes using various DGs is well established compared to meta and para-C–H bond activations.1 Among various DGs, the nitrile group is employed as the DG for the ortho, meta and para-C–H functionalization of various arene compounds.2 In the field of meta-C–H bond activation, various research groups such as Yu, Maiti, Jin, Tan, Li, Ackermann and others have developed a variety of scaffolds for the meta-C–H functionalization of aromatic compounds3 (Scheme 1a). In the C–H activation chemistry, benzoic acid derivatives have been utilized for selective ortho, meta and para-C–H functionalization while phenylacetic acid derivatives have been selectively utilized for ortho and meta-C–H functionalization. Similarly, hydrocinnamic acid derivatives are also utilized for meta-C–H functionalization. Though the C–H activation chemistry of benzoic acid, phenylacetic acid and hydrocinnamic acid derivatives was explored, the C–H activation of cinnamates was not reported in the literature (Scheme 1b). The C–H activation of the arene part of the cinnamates poses a remarkable challenge in C–H activation chemistry due to the unfavourable transoid geometry of the aryl moiety and ester group which can also act as a DG. To circumvent this problem, we hypothesized that a cinnamate substrate bearing a branch at the α-position that contains a directing group, which in turn will bring the arene group and DG group into close proximity, will enable the C–H bond activation of the arene group via metal chelation. It is important to note that having an activated double bond in the scaffold (α-substituted cinnamate) and activating a selective arene C–H bond poses additional challenges due to interfering reactions that are associated with the reactive olefinic site of the α, β-unsaturated ester.4
Moreover, the reactive double bond and DG-containing substrate for the C–H activation reaction have never been explored. Therefore, the functionalization of the arene C–H bond in cinnamates is highly challenging and a very useful endeavour in the field of organic synthesis.
Cinnamic acid derivatives are privileged scaffolds that are an integral part of many natural products and biologically active molecules. Cinnamic acid derivatives have gained a lot of interest from researchers due to their important biological activities such as antiproliferative, antiangiogenic, antitumorigenic, and antioxidant properties.5 Cinnamic acid derivatives such as sinapic acid, caffeic acid, ferulic acid, and isoferulic acid have been shown to have a variety of pharmacological properties, including anticancer, antioxidant, immunomodulation and anti-inflammation activities.6 Because of the pharmacological relevance of cinnamic acid derivatives, there has been constant interest in the synthesis and functionalization of cinnamates. Since cinnamates are not functionalised via the C–H activation reaction so far, we initiated our study towards a distal C–H activation of cinnamates through the DG strategy. Herein, we disclose our discovery of diversity-oriented C–H functionalization (alkenylation, allylation, acetoxylation and cyanation) of α-substituted cinnamate derivatives by using nitrile as a DG to produce a library of mono-meta-functionalized α-substituted cinnamates with excellent meta-selectivity for the first time (Scheme 1c).
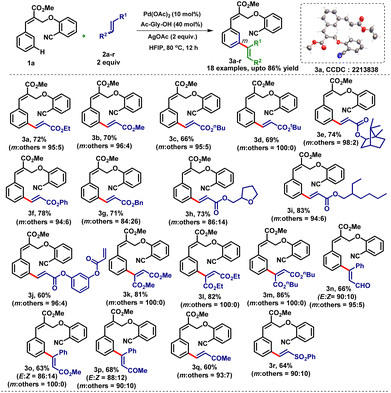
To execute our idea, we have synthesized the requisite model substrate (α-substituted cinnamate 1a) i.e. methyl (E)-2-((2-cyanophenoxy)methyl)-3-phenylacrylate from the bromo derivative of the Baylis–Hillman adduct for the meta-C–H activation reaction (see the ESI†). The treatment of α-substituted cinnamate scaffold 1a with ethyl acrylate 2a as a coupling partner in the presence of palladium acetate (10 mol%), mono protected amino acid as a ligand (Form-Gly-OH, 40 mol%) and silver acetate (2 equiv.) as an oxidant in HFIP solvent at 80 °C for 24 h provided the meta-coupled product 3a in low yield (20%) with high selectivity (m![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) others = 93
others = 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7). To improve the yield of the reaction, we screened various catalysts, ligands, oxidants and solvents (see the ESI†). The best result (72%, m
7). To improve the yield of the reaction, we screened various catalysts, ligands, oxidants and solvents (see the ESI†). The best result (72%, m![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) others = 95
others = 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) was obtained when we performed the reaction with Pd(OAc)2 (10 mol%), Ac-Gly-OH (40 mol%) and AgOAc (2 equiv.) in HFIP solvent at 80 °C for 12 h. Additionally, we also screened other directing groups having aldehyde, ketone and nitro functionalities for the meta-C–H activation reaction. Unfortunately, we did not obtain the desired product in any of the cases (see the ESI†). Therefore, we started our further studies with the nitrile group as the directing group which provided the desired meta-C–H activation product 3a in 72% yield. Encouraged by this result, we explored the scope of various alkene coupling partners with 1a under the optimized reaction conditions. Accordingly, first, we employed several acrylates 2a–j with 1a and obtained the corresponding mono-alkenylated products 3a–j in very good yields (60–83%) with high meta-selectivity (Table 1). The electron-withdrawing acrylates containing alkyl, aryl and benzyl groups (3e–j) of differing complexities were well tolerated. Even symmetrical and unsymmetrical internal alkenes such as maleates (2k–m), trans-cinnamaldehyde (2n), methyl cinnamate (2o) and benzylideneacetone (2p) also delivered the desired meta-C–H olefinated products (3k–p) in very good yields (63–86%) with excellent meta-selectivity. Notably, di-substituted trans-olefinic coupling partners (2n–p) provided the meta-C–H activation products 3n–p (Table 1) with E and Z isomers where the E isomer was found to be the major isomer. This selectivity clearly indicates that the selective E-double bond formation is thermodynamic in origin. Interestingly, olefins such as methyl vinyl ketone (2q) and phenyl vinyl sulfone (2r) also worked well and led to the corresponding meta-coupled products 3q and 3r in 60% and 64% yields respectively. The compound 3a was further confirmed by single-crystal X-ray diffraction analysis.
5) was obtained when we performed the reaction with Pd(OAc)2 (10 mol%), Ac-Gly-OH (40 mol%) and AgOAc (2 equiv.) in HFIP solvent at 80 °C for 12 h. Additionally, we also screened other directing groups having aldehyde, ketone and nitro functionalities for the meta-C–H activation reaction. Unfortunately, we did not obtain the desired product in any of the cases (see the ESI†). Therefore, we started our further studies with the nitrile group as the directing group which provided the desired meta-C–H activation product 3a in 72% yield. Encouraged by this result, we explored the scope of various alkene coupling partners with 1a under the optimized reaction conditions. Accordingly, first, we employed several acrylates 2a–j with 1a and obtained the corresponding mono-alkenylated products 3a–j in very good yields (60–83%) with high meta-selectivity (Table 1). The electron-withdrawing acrylates containing alkyl, aryl and benzyl groups (3e–j) of differing complexities were well tolerated. Even symmetrical and unsymmetrical internal alkenes such as maleates (2k–m), trans-cinnamaldehyde (2n), methyl cinnamate (2o) and benzylideneacetone (2p) also delivered the desired meta-C–H olefinated products (3k–p) in very good yields (63–86%) with excellent meta-selectivity. Notably, di-substituted trans-olefinic coupling partners (2n–p) provided the meta-C–H activation products 3n–p (Table 1) with E and Z isomers where the E isomer was found to be the major isomer. This selectivity clearly indicates that the selective E-double bond formation is thermodynamic in origin. Interestingly, olefins such as methyl vinyl ketone (2q) and phenyl vinyl sulfone (2r) also worked well and led to the corresponding meta-coupled products 3q and 3r in 60% and 64% yields respectively. The compound 3a was further confirmed by single-crystal X-ray diffraction analysis.
| a Isolated yields. The isomeric ratio was determined by 1H-NMR. |
|---|
|
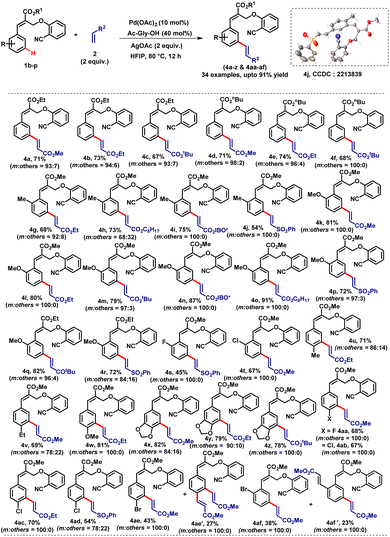
To study the substrate scope, we prepared various α-substituted cinnamates (1b–p) and treated them with different types of coupling partners (2) under the optimized reaction conditions which smoothly afforded the desired distal meta-coupled products (4a–z & 4aa–af) in very good yields with high meta-selectivity (Table 2). We observed that the electron-donating substituents on arene worked well and smoothly led to the mono meta-olefinated products. The halogen containing substrates also provided the mono meta-olefinated products with good yields. Notably, ortho-substituted cinnamates provided meta-coupled products (4g–t) with excellent regioselectivity by reacting at the less hindered position. The ortho and para bromo cinnamate derivatives afforded the meta-olefinated products 4ae and 4af in 43% and 38% yields along with the interesting diolefinated products 4ae' and 4af' in 27% and 23% yields respectively via sequential C–H activation and Heck coupling reactions. The structure of compound 4j was further confirmed by single-crystal X-ray diffraction analysis.
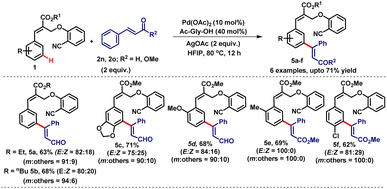
Furthermore, we employed less utilized α, β-unsaturated alkenes such as cinnamaldehyde (2n) and methyl cinnamate (2o) for the meta-selective C–H olefination reaction. The treatment of cinnamates (1) with cinnamaldehyde (2n)/methyl cinnamate (2o) under the optimized reaction conditions led to the anticipated meta-coupled products (5a–f) in good yields (Table 3). We observed the formation of E and Z isomers in the products where the thermodynamically controlled E-isomer was found to be the major isomer.
| a Isolated yields. The isomeric ratio was determined by 1H-NMR. |
|---|
|
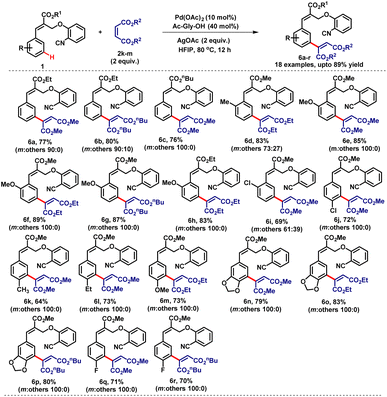
Similarly, various α-substituted cinnamate derivatives 1 with symmetrical olefin coupling partners such as maleates (2k–m) were investigated. The desired mono-olefinated products (6a–r) were formed in very good yields (64–89%) with excellent meta-selectivity (Table 4). The ortho-substituted substrates provided the meta-coupled products (6d–i) with high regioselectivity (coupled selectivly at a less hindered position). The cis-olefinic coupling partner such as maleates (2k–m) provided the E-isomers exclusively, which indicates that the stereoselectivity in the double bond is thermodynamic in origin.
| a Isolated yields. The isomeric ratio was determined by 1H-NMR. |
|---|
|
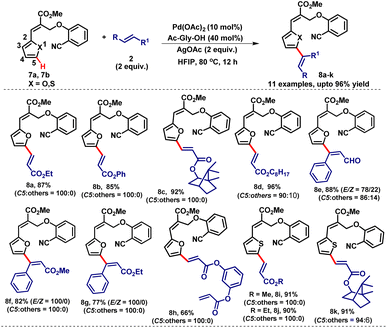
The versatility of this reaction was further demonstrated by subjecting the heterocyclic substrates having furan 7a and thiofuran 7b moieties for meta-C–H olefination with a variety of mono and di-substituted alkenes. The heteroaromatic scaffolds 7a and 7b were found to be compatible to furnish the corresponding C5-olefinated products 8a–k in very good yields with excellent regioselectivity (Table 5). Among the C5-olefinated products 8f and 8g were formed with excellent E-selectivity with excellent C5-selectivity.
| a Isolated yields. The isomeric ratio was determined by 1H-NMR. |
|---|
|
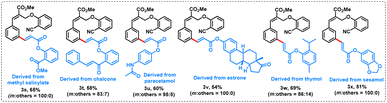
To exemplify the efficacy of our new protocol, the α-substituted cinnamate 1a was coupled with various bioactive molecule appended acrylates (2s–x) such as methyl salicylate (2s, treatment for minor aches and pain), chalcone (2t, treatment for viral disorders and stomach cancer and used in food additives and cosmetic formulation ingredients), paracetamol (2u, treatment for fever and aches), estrone (2v, hormone therapy), thymol (2w, antiseptic) and sesamol (2x, antioxidant) as coupling partners. Regardless of the steric bias of the bioactive coupling partners, excellent mono meta-selective olefinated products (3s–x) were obtained in moderate to good yields (51–69%) with excellent selectivity (Table 6).
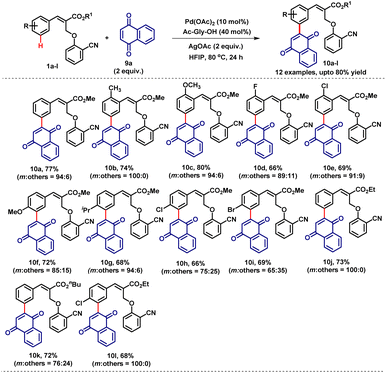
Then we turned our focus on quinones as coupling partners for the meta-C–H activation reaction. Quinones and their analogs are found in various pharmaceuticals and natural products and these units are versatile building blocks in the synthesis of various organic scaffolds.7a,b There have been no studies in the literature to date on the insertion of naphthoquinone and benzoquinone as coupling partners in meta-C–H bond activation chemistry. Moreover, utilizing naphthoquinone and benzoquinone as coupling partners in the C–H activation reaction with α-substituted cinnamate 1a is more challenging due to more reactive sites as well as because the carbonyl groups of naphthoquinone and benzoquinone can also act as DGs which may lead to competitive reactions at multiple reactive sites and may produce multiple products.7c,d To examine the robustness of our novel strategy for meta-selective C–H bond olefination, we employed naphthoquinone 9a and benzoquinone 9b as coupling partners. Accordingly, the reaction of α-substituted cinnamate 1a with naphthoquinone 9a under the optimized reaction conditions smoothly provided the desired mono-meta-naphthoquinone coupled product 10a in 77% yield with very good selectivity (m![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) others = 94
others = 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6). We also investigated the scope of various α-substituted cinnamates (1) with naphthoquinone (9a) which resulted the desired meta-naphthoquinone coupled products (10b–l) in very good yields (65–74%) (Table 7). Notably, high regioselectivity (less hindered position) was observed for ortho substituted substrates that produce the meta-coupled products (10b–e). Then, we focused our attention towards the reaction of 1a with benzoquinone 9b as the coupling partner under the optimized reaction conditions. However, we did not observe the formation of the expected meta-coupled product. It is important to note that benzoquinone is more reactive and known for undergoing cyclisation with aromatic substrates. It is very challenging to retain the benzoquinone moiety unaltered after the reaction. To circumvent this problem, we performed the reaction at room temperature instead of 80 °C. Pleasingly, the reaction smoothly furnished the expected mono-meta-benzoquinone coupled product 10m in good yield (66%).
6). We also investigated the scope of various α-substituted cinnamates (1) with naphthoquinone (9a) which resulted the desired meta-naphthoquinone coupled products (10b–l) in very good yields (65–74%) (Table 7). Notably, high regioselectivity (less hindered position) was observed for ortho substituted substrates that produce the meta-coupled products (10b–e). Then, we focused our attention towards the reaction of 1a with benzoquinone 9b as the coupling partner under the optimized reaction conditions. However, we did not observe the formation of the expected meta-coupled product. It is important to note that benzoquinone is more reactive and known for undergoing cyclisation with aromatic substrates. It is very challenging to retain the benzoquinone moiety unaltered after the reaction. To circumvent this problem, we performed the reaction at room temperature instead of 80 °C. Pleasingly, the reaction smoothly furnished the expected mono-meta-benzoquinone coupled product 10m in good yield (66%).
| a Isolated yields. The isomeric ratio was determined by 1H-NMR. |
|---|
|
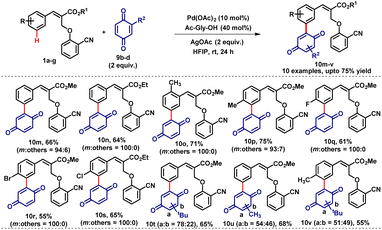
Furthermore, various cinnamates (1b–g) were subjected to benzoquinone 9b and afforded the corresponding meta-coupled products (10n–s) in moderate to very good yields (55–80%) with high meta-selectivity (Table 8).
| a Isolated yields. The isomeric ratio was determined by 1H-NMR. |
|---|
|
Additionally, substituted benzoquinones (9c & 9d) were also treated with cinnamates 1 which resulted in a mixture of regioisomeric products 10t–v in good yields. It is important to note that in both cases (naphthoquinone & benzoquinone) exclusively meta-coupled products have been observed even though various reactive sites and various functional groups are present with comparable reactivities.
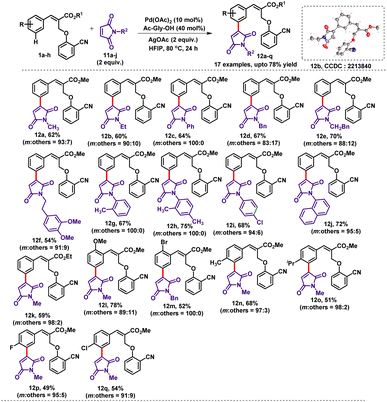
Next to check the generality and applicability of the reaction, we concentrated on utilizing maleimides as coupling partners for the meta-C–H activation reaction. Maleimide derivatives are an integral part of various natural products and pharmaceuticals. Maleimide derivatives have been used as coupling partners in ortho-C–H activation and are not employed for meta-C–H activation chemistry.8 Therefore, we focused our attention towards the incorporation of N-substituted maleimides as coupling partners at the meta-position of the substrate 1. Accordingly, the reaction of 1a with N-methyl maleimide 11a under the standard reaction conditions delivered the desired meta coupled product 12a in 62% yield with excellent selectivity (m![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) others = 93
others = 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7). Furthermore, α-substituted cinnamate (1a) was treated with different types of N-alkyl and aryl maleimides 11b–j which produced the expected mono-meta-olefinated products 12a–j in moderate to very good yields (54–72%) with excellent selectivity (Table 9). Furthermore, various α-substituted cinnamates (1) were treated with N-substituted maleimides (11a & 11d) under the optimised reaction conditions which provided the anticipated meta-coupled products 12k–q in good yields with high selectivity.
7). Furthermore, α-substituted cinnamate (1a) was treated with different types of N-alkyl and aryl maleimides 11b–j which produced the expected mono-meta-olefinated products 12a–j in moderate to very good yields (54–72%) with excellent selectivity (Table 9). Furthermore, various α-substituted cinnamates (1) were treated with N-substituted maleimides (11a & 11d) under the optimised reaction conditions which provided the anticipated meta-coupled products 12k–q in good yields with high selectivity.
| a Isolated yields. The isomeric ratio was determined by 1H-NMR. |
|---|
|
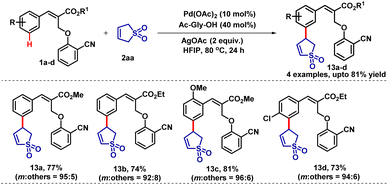
The ortho-substituted cinnamates regioselectively produced the meta-coupled products (coupled selectively at less hindered positions) 12l and 12m in good yields. The compound 12b was further confirmed by single-crystal X-ray diffraction analysis. Sulfolene and its derivatives are important building blocks in organic synthesis and also an integral part of many biologically active molecules.9 In the C–H activation chemistry, sulfolene has never been used as a coupling partner. Therefore, we envisaged that sulfolene could be utilised as a coupling partner in the C–H activation reactions. Accordingly, we treated sulfolene (2aa) with substrate 1 under the standard reaction conditions. To our delight, the desired meta-selective 4-aryl 2-sulfolene derivatives (13a–d) have been formed in excellent yields (73–81%) with high selectivity (Table 10). The formation of the 4-aryl 2-sulfolene derivatives could be explained by the regioselective elimination of more acidic β-Ha rather than β-Hb elimination.
| a Isolated yields. The isomeric ratio was determined by 1H-NMR. |
|---|
|
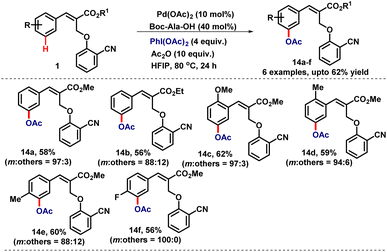
To expand the robustness of the α-substituted cinnamates for other C–H functionalization reactions, we planned to carry out acetoxylation and cyanation reactions to generate the corresponding meta-coupled products utilising appropriate reagents.10 Accordingly, the α-substituted cinnamates (1) were treated with diacetoxyiodobenzene under the standard reaction conditions which produced the meta-coupled product in 49% yield with good selectivity (m![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) others = 86
others = 86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14). However, when we replaced Ac-Gly-OH with Boc-Ala-OH the yield of the reaction increased from 49% to 58% with excellent selectivity (m
14). However, when we replaced Ac-Gly-OH with Boc-Ala-OH the yield of the reaction increased from 49% to 58% with excellent selectivity (m![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) others = 97
others = 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3).
3).
Furthermore, the scope of the reaction was checked with various cinnamates having different substitutions and producing the desired meta-coupled products (14a–f) in 56–62% yields with high meta-selectivity (Table 11). We observed that the meta-coupled products 14c and 14d were formed with high regioselectivity (coupled selectively at a less hindered position).
| a Isolated yields. The isomeric ratio was determined by 1H-NMR. |
|---|
|
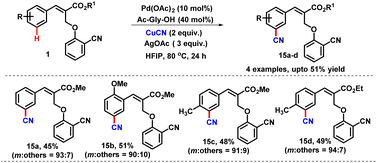
Similarly, we also applied the same strategy for the meta-cyanation reaction. Accordingly, the treatment of cinnamate 1a with CuCN led to the meta-cyanated product 15a in 45% yield with excellent selectivity (m![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) others = 93
others = 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7). The scope of the reaction was further checked under the same reaction conditions which provided the meta-cyanated products (15b–d) in moderate yields with high selectivity as depicted in Table 12. We observed that the meta-coupled product 15b formed with high regioselectivity (coupled selectively at a less hindered position).
7). The scope of the reaction was further checked under the same reaction conditions which provided the meta-cyanated products (15b–d) in moderate yields with high selectivity as depicted in Table 12. We observed that the meta-coupled product 15b formed with high regioselectivity (coupled selectively at a less hindered position).
| a Isolated yields. The isomeric ratio was determined by 1H-NMR. |
|---|
|
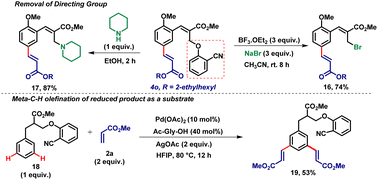
Finally, the DG i.e. the 2-hydroxybenzonitrile group was easily removed and produced the meta-olefinated product 16 in 74% yield using NaBr and BF3OEt2 by the regeneration of the initially utilized bromo moiety of the Bailys–Hillman adduct (Scheme 2). On the other hand, the directing group was replaced with the piperidine moiety which produced the corresponding allylamine product 17 with 87% yield (Scheme 2). In order to confirm the importance of the olefinic double bond present in the cinnamate scaffold, we have synthesized the corresponding reduced product 18 and subjected it to the C–H olefination reaction with methyl acrylate 2a under the optimized reaction conditions. In this case, a di-meta-olefinated product 19 was obtained in 53% yield (Scheme 2) which indicates that the double bond facilitates the formation of mono-meta-coupled products.
Finally, a representative catalytic cycle is proposed for the distal mono meta-C–H olefination where the palladium complex was confirmed through HRMS (see the ESI†).
Conclusions
In conclusion, we have developed a novel strategy for the distal meta-C–H olefination of α-substituted cinnamates with different kinds of coupling partners with high meta-selectivity for the first time. The new entrants such as naphthoquinone, benzoquinones, maleimides and sulfolene have been shown to be coupling partners in the meta-C–H activation reaction. The meta-C–H functionalization has been further extended to allylation, acetoxylation and cyanation reactions. Importantly, several bioactive molecules tethered to olefins such as estrone, paracetamol, methyl salicylate, chalcone, thymol and sesamol were also utilised as coupling partners. The present diversity-oriented methodology is novel and general with a wide range of substrate scope (more than 140 examples) and tolerates diverse functional groups. This novel strategy also opens a new avenue for making a library of a wide variety of meta-functionalized cinnamate scaffolds for biological screening.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank DST-SERB (ref no.: CRG/2019/006323) for financial support. T. T. R. thanks CSIR, New Delhi, for a Direct-Senior Research Fellowship (D-SRF). We thank Pondicherry University for the NMR and ESI-HRMS facilities.Notes and references
- (a) O. Daugulis, H. Q. Do and D. Shabashov, Acc. Chem. Res., 2009, 42, 1074 CrossRef CAS PubMed; (b) T. W. Lyons and M. S. Sanford, Chem. Rev., 2010, 110, 1147 CrossRef CAS PubMed; (c) K. M. Engle, T. S. Mei, M. Wasa and J. Q. Yu, Acc. Chem. Res., 2012, 45, 788 CrossRef CAS PubMed; (d) M. Zhang, Y. Zhang, X. Jie, H. Zhao, G. Li and W. Su, Org. Chem. Front., 2014, 1, 843 RSC; (e) C. Sambiagio, D. Schönbauer, R. Blieck, T. Dao-Huy, G. Pototschnig, P. Schaaf, T. Wiesinger, M. F. Zia, J. Wencel-Delord and T. A. Besset, Chem. Soc. Rev., 2018, 47, 6603 RSC; (f) X. Chen, K. M. Engle, D. H. Wang and J. Q. Yu, Angew. Chem., Int. Ed., 2009, 48, 5094 CrossRef CAS PubMed; (g) Z. Dong, Z. Ren, S. J. Thompson, Y. Xu and G. Dong, Chem. Rev., 2017, 117, 9333 CrossRef CAS PubMed; (h) M. T. Mihai, G. R. Genov and R. J. Phipps, Chem. Soc. Rev., 2018, 47, 149 RSC; (i) X. Xu and J. Luo, ChemSusChem, 2019, 12, 4601 CrossRef CAS PubMed; (j) K. X. Tang, C. M. Wang, T. H. Gao, L. Chen, L. Fan and L. P. Sun, Adv. Synth. Catal., 2019, 361, 26 CrossRef CAS; (k) S. Rej and N. Chatani, Angew. Chem., Int. Ed., 2019, 58, 8304 CrossRef CAS PubMed; (l) P. Gandeepan, T. Müller, D. Zell, G. Cera, S. Warratz and L. Ackermann, Chem. Rev., 2019, 119, 2192 CrossRef CAS PubMed; (m) S. Dutta, T. Bhattacharya, F. J. Geffers, M. Bürger, D. Maiti and D. B. Werz, Chem. Sci., 2022, 13, 2551 RSC; (n) Y. Ke, L. Mengjie, S. Hao and G. Haibo, Chem. Sci., 2020, 11, 12616 RSC; (o) M. Bakthadoss and P. V. Kumar, Adv. Synth. Catal., 2018, 360, 2650 CrossRef CAS; (p) M. Bakthadoss, T. T. Reddy, V. Agarwal and D. S. Sharada, Chem. Commun., 2022, 58, 1406 RSC; (q) M. Bakthadoss, P. V. Kumar, P. R. Kumar and V. Agarwal, Org. Biomol. Chem., 2019, 17, 4465 RSC; (r) M. Bakthadoss, T. T. Reddy and D. S. Sharada, RSC Adv, 2020, 10, 31570 RSC; (s) M. Bakthadoss, P. V. Kumar, R. Kumar and M. Surendar, New J. Chem., 2019, 43, 14190 RSC; (t) M. Bakthadoss, T. T. Reddy and D. S. Sharada, RSC Adv, 2020, 10, 31570 RSC; (u) M. Bakthadoss, P. V. Kumar and T. S. Reddy, Eur. J. Org. Chem., 2017, 4439 CrossRef CAS.
- (a) G. Meng, N. Y. S. Lam, E. L. Lucas, T. Gorden, S. Denis, P. Verma, N. Chekshin and J. Q. Yu, J. Am. Chem. Soc., 2020, 142, 10571 CrossRef CAS PubMed; (b) A. Dey, S. K. Sinha, T. K. Achar and D. Maiti, Angew. Chem., Int. Ed., 2020, 58, 10820 CrossRef PubMed.
- (a) D. S. Leow, G. Li, T. S. Mei and J. Q. Yu, Nature, 2012, 486, 518 CrossRef CAS PubMed; (b) Y. F. Yang, G. J. Cheng, P. Liu, D. Leow, T. Y. Sun, P. Chen, X. Zhang, J. Q. Yu, Y. D. Wu and K. N. Houk, J. Am. Chem. Soc., 2014, 136, 344 CrossRef CAS PubMed; (c) Y. Deng and J. Q. Yu, Angew. Chem., Int. Ed., 2015, 54, 888 CrossRef CAS PubMed; (d) R. Y. Tang, G. Li and J. Q. Yu, Nature, 2014, 507, 215 CrossRef CAS PubMed; (e) L. Fang, T. G. Saint-Denis, B. L. H. Taylor, S. Ahlquist, K. Hong, S. Liu, L. Han, K. N. Houk and J. Q. Yu, J. Am. Chem. Soc., 2017, 139, 10702 CrossRef CAS PubMed; (f) H. J. Xu, Y. Lu, M. E. Farmer, H. W. Wang, D. Zhao, Y. S. Kang, W. Y. Sun and J. Q. Yu, J. Am. Chem. Soc., 2017, 139, 2200 CrossRef CAS PubMed; (g) G. Yang, P. Lindovska, D. Zhu, J. Kim, P. Wang, R. Y. Tang, M. Movassaghi and J. Q. Yu, J. Am. Chem. Soc., 2014, 136, 10807 CrossRef CAS PubMed; (h) S. Lee, H. Lee and K. L. Tan, J. Am. Chem. Soc., 2013, 135, 18778 CrossRef CAS PubMed; (i) M. Bera, A. Modak, T. Patra, A. Maji and D. Maiti, Org. Lett., 2014, 16, 5760 CrossRef CAS PubMed; (j) M. Bera, S. Agasti, R. Chowdhury, R. Mondal, D. Pal and D. Maiti, Angew. Chem., Int. Ed., 2017, 56, 5272 CrossRef CAS PubMed; (k) M. Bera, S. K. Sahoo and D. Maiti, ACS Catal., 2016, 6, 3575 CrossRef CAS; (l) S. Bag, T. Patra, A. Modak, A. Deb, S. Maity, U. Dutta, A. Dey, R. Kancherla, A. Maji, A. Hazra, M. Bera and D. Maiti, J. Am. Chem. Soc., 2015, 137, 11888 CrossRef CAS PubMed; (m) T. Patra, S. Bag, R. Kancherla, A. Mondal, A. Dey, S. Pimparkar, S. Agasti, A. Modak and D. Maiti, Angew. Chem., Int. Ed., 2016, 55, 7751 CrossRef CAS PubMed; (n) A. Modak, A. Mondal, R. Watile, S. Mukherjee and D. Maiti, Chem. Commun., 2016, 52, 13916 RSC; (o) L. Zhang, C. Zhao, Y. Liu, J. Xu, X. Xu and Z. Jin, Angew. Chem., Int. Ed., 2017, 56, 12245 CrossRef CAS PubMed; (p) B. Wang, Y. Zhou, N. Xu, X. Xu, X. Xu and Z. Jin, Org. Lett., 2019, 21, 1885 CrossRef CAS PubMed; (q) L. Cai, H. Ji, L. Yang and G. Li, Nat. Commun., 2016, 7, 10443 CrossRef PubMed; (r) H. Wang, F. Fu, C. Zhou and G. Li, Chem. Sci., 2022, 13, 8686 RSC; (s) N. Hofmann and L. Ackermann, J. Am. Chem. Soc., 2013, 135, 5877 CrossRef CAS PubMed; (t) J. Li, S. Warratz, D. Zell, S. D. Sarkar, E. E. Ishikawa and L. Ackermann, J. Am. Chem. Soc., 2015, 137, 13894 CrossRef CAS PubMed.
- (a) Y. Wang, D. Wei and M. Tang, J. Org. Chem., 2017, 82, 13043 CrossRef CAS PubMed; (b) C. Romano, D. Fiorito and C. Mazet, J. Am. Chem. Soc., 2019, 141, 16983 CrossRef CAS PubMed.
- (a) C. S. Kong, C. H. Jeong, J. S. Choi, K. J. Kim and J. W. Jeong, Phytother. Res., 2013, 27, 317 CrossRef CAS PubMed; (b) F. A. R. Barbosa, M. P. Rode, R. F. S. Canto, A. H. Silva, T. B. Creczynski-Pasa and A. L. Braga, ChemistrySelect, 2022, 27, 274 Search PubMed; (c) P. Su, Y. Shi, J. Wang, X. Shen and J. Zhang, Med. Chem., 2015, 15, 980 CAS; (d) F. Natella, M. Nardini, M. D. Felice and C. Scaccini, J. Agric. Food Chem., 1999, 47, 1453 CrossRef CAS PubMed.
- (a) N. Nićiforović and H. Abramovic, Compr. Rev. Food Sci. Food Saf., 2014, 13, 34 CrossRef PubMed; (b) F. Khan, N. I. Bamunuarachchi, N. Tabassum and Y. M. Kim, J. Agric. Food Chem., 2021, 69, 2979 CrossRef CAS PubMed; (c) Y. Zhang, J. Zhou, N. Zhang, L. Zhao, W. Wu, L. Zhang and F. Zhou, ACS Food Sci. Technol., 2022, 2, 1114 CrossRef CAS; (d) K. Wang, J. Tian, Y. Li, M. Liu, Y. Chao, Y. Cai, G. Zheng and Y. Fang, ACS Omega, 2021, 6, 17045 CrossRef CAS PubMed.
- (a) Y. Kumagai, Y. Shinkai, T. Miura and A. K. Cho, Annu. Rev. Pharmacol. Toxicol., 2012, 52, 221 CrossRef CAS PubMed; (b) J. L. Bolton and T. Dunlap, Chem. Res. Toxicol., 2017, 30, 13 Search PubMed; (c) A. Ilangovan and T. P. A. Krishna, in Organic Synthesis – A Nascent Relook, ed. B. P. Nandeshwarappa, IntechOpen, London, 2020, DOI:10.5772/intechopen.90930; (d) J. M. Wood, R. L. de Carvalho and E. N. S. Júnior, Chem. Rec., 2021, 21, 2604 CrossRef CAS PubMed.
- (a) C. Peifer, T. Stoiber, E. Unger, F. Totzke, C. Schächtele, D. Marmé, R. Brenk, G. Klebe, D. Schollmeyer and G. Dannhardt, J. Med. Chem., 2006, 49, 1271 CrossRef CAS PubMed; (b) R. Manoharan and M. Jeganmohan, Asian J. Org. Chem., 2019, 8, 1949 CrossRef CAS; (c) S. L. Liu, Y. Shi, C. Xue, L. Zhang, L. Zhou and M. P. Song, Eur. J. Org. Chem., 2021, 5862 CrossRef CAS.
- M. G. Brant and J. E Wulff, Synthesis, 2016, 48, 1 CAS.
- (a) A. Maji, B. Bhaskararao, S. Singha, R. B. Sunoj and D. Maiti, Chem. Sci., 2016, 7, 3147 RSC; (b) S. Pimparkar, T. Bhattacharya, A. Maji, A. Saha, R. Jayarajan, U. Dutta, G. Lu, D. W. Lupton and D. Maiti, Chem. – Eur. J., 2020, 26, 11558 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2213838, 2213839 and 2213840. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2sc06206b |
| This journal is © The Royal Society of Chemistry 2023 |