Open Access Article
Open Access ArticleAn AND-gate bioluminescent probe for precise tumor imaging†
Chenchen
Wang
 a,
Yajian
Hong
a,
Ling
Dong
b,
Hu
Cheng
a,
Duo
Jin
a,
Ronghua
Zhao
c,
Zian
Yu
a and
Yue
Yuan
a,
Yajian
Hong
a,
Ling
Dong
b,
Hu
Cheng
a,
Duo
Jin
a,
Ronghua
Zhao
c,
Zian
Yu
a and
Yue
Yuan
 *a
*a
aDepartment of Chemistry, University of Science and Technology of China, 96 Jinzhai Road, Hefei, Anhui 230026, China. E-mail: yueyuan@ustc.edu.cn
bDepartment of Chemistry and Chemical Engineering, Hefei Normal University, Hefei, Anhui 230061, China
cCenter for Biomedical Imaging, University of Science and Technology of China, Hefei, Anhui 230026, China
First published on 3rd May 2023
Abstract
Sensitivity and specificity are two indispensable requirements to ensure diagnostic accuracy. Dual-locked probes with “AND-gate” logic theory have emerged as a powerful tool to enhance imaging specificity, avoid “false positive” results, and realize correlation analysis. In addition, bioluminescence imaging (BLI) is an excitation-free optical modality with high sensitivity and low background and can thus be combined with a dual-locked strategy for precise disease imaging. Here, we developed a novel AND-gate bioluminescent probe, FK-Luc-BH, which is capable of responding to two different tumor biomarkers (cathepsin L and ClO−). The good specificity of FK-Luc-BH was proven, as an obvious BL signal could only be observed in the solution containing both cathepsin L (CTSL) and ClO−. 4T1-fLuc cells and tumors treated with FK-Luc-BH exhibited significantly higher BL signals than those treated with unresponsive control compound Ac-Luc-EA or cotreated with FK-Luc-BH and a ClO− scavenger/cathepsin inhibitor, demonstrating the ability of FK-Luc-BH to precisely recognize tumors in which CTSL and ClO− coexist.
Introduction
Enhancement of diagnostic accuracy, including sensitivity and specificity, is of undoubted importance for clinical tumor imaging, especially early tumor diagnosis and imaging-guided surgery, to precisely delineate tumor margins in pre- and intra-operative realms and easily distinguish the tumor microenvironment from the surrounding normal tissues.1,2 To realize tumor-specific imaging, many single-locked probes have been reported to enhance or suppress imaging signals at tumor sites after binding to or reacting with a specific biomarker, such as metal ions, proteinases, nucleic acids, membrane receptors, reactive oxygen species (ROS), and many other possible targets.3–9 But sometimes, biomarkers that are overexpressed in tumors also exhibit considerable concentrations in other normal tissues, which may cause undesirable or even destructive “false-positive” diagnosis results.10Inspired by the molecular logic gate reported by A. P. de Silva 29 years ago, multi-locked probes were proposed to regulate the imaging signal only when the simultaneous coexistence of two or more stimuli occurs, such as the “AND-gate” that is composed of two or more inputs but only a single output.11 Compared with a single-locked probe, the requirement of one more tumor-specific stimulus at the same site, i.e. a dual-locked probe, is capable of raising imaging accuracy, diminishing the misdiagnosis rate, and reflecting the interrelationship between two biomarkers in tumor development.12,13 By changing the combination of biomarker-responsive sites, various dual-locked probes can be built for imaging different diseases or biological events.10 An ideal dual-locked probe requires orthogonal responsive sites so that each biomarker can process only its corresponding responsive site.14,15 Hence, employing a dual-locked probe to respond to two different types of biomarkers (such as one protease with one receptor or one ROS) was preferred to avoid cross reactions or their co-overexpression in normal tissues.
As a non-invasive biocompatible optical imaging modality, bioluminescence imaging (BLI) does not require an excitation source and thus has low background interference and an excellent signal-to-noise ratio (S/N).16 Unlike complex fluorescent dyes for fluorescence imaging or photoacoustic imaging, the commonly used BLI probe amino-D-luciferin (NH2-Luc) is a small water-soluble molecule that is easy to synthesize and modify. Over the past few decades, almost all developed BL probes have been single-locked by simply caging their hydroxyl (amino) or carboxyl groups with stimuli-responsive motifs to block their recognition with luciferase and subsequent light emission.17
Herein, combining a specific dual-locked strategy and sensitive BLI modality, an AND-gate BL probe FK-Luc-BH with cathepsins and hypochlorite (ClO−) as two inputs was constructed to realize accurate tumor imaging. Cathepsins belong to the papain subfamily of cysteine proteases and were reported as potential malignant tumor biomarkers; their overexpression is related to various types of cancers.18 Hypochlorite (ClO−) is a member of the ROS family that is overproduced in many disorders and diseases, including cancer and inflammation.19–22 The levels of both cathepsins and ClO− are significantly increased in the early stage of malignant tumors, which enables their corresponding biosensors to distinguish early-stage tumors and small metastases from surrounding normal tissues.23,24 After caging the amino group of NH2-Luc with a cathepsin-recognized substrate Phe-Lys (FK, mainly for cathepsin L in this work) and the carboxyl group of NH2-Luc with ClO− reactive motif benzoylhydrazine, a dual-locked BLI probe FK-Luc-BH was produced for the following tumor-targeted imaging (Fig. 1A). When the probe FK-Luc-BH enters tumor cells that co-express cathepsin L (CTSL) and ClO−, both peptide FK and benzoylhydrazine are removed to yield NH2-Luc, which reacts with firefly luciferase (fLuc), Mg2+, O2, and adenosine triphosphate (ATP) to emit a bright BL signal to precisely illuminate the tumor (Fig. 1B).
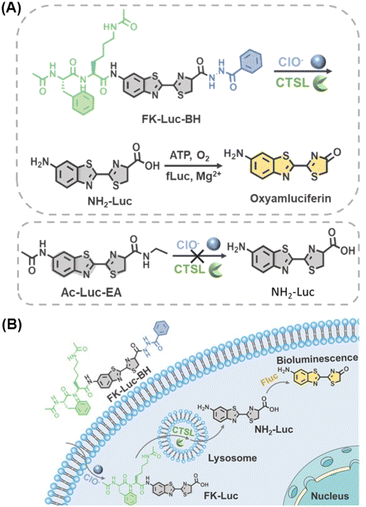 | ||
| Fig. 1 (A) Chemical structure and (B) schematic illustration of an AND-gate bioluminescent probe for simultaneous monitoring of cathepsins and ClO− in tumor cells. | ||
Results and discussion
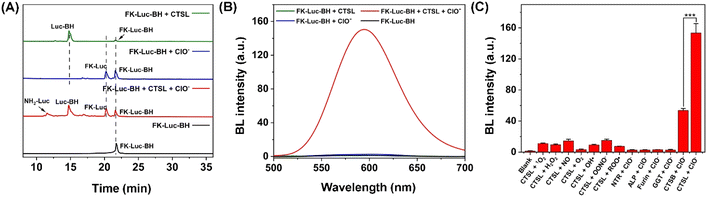
Before investigating the ability of FK-Luc-BH to react with cathepsins, FK-Luc was prepared to compare the enzymatic efficiencies of cathepsin B (CTSB) and CTSL, as both of them were reported to be capable of cleaving dipeptide FK (Scheme S1 and Fig. S1–S6†).2,25 Then a series of concentrations of CTSB (0.0–8.0 U/L) or CTSL (0.0–1.6 U/L) were incubated with 25 μM FK-Luc in working buffer at 37 °C for 2 h, and the BL intensities were measured after adding 0.1 mg mL−1 fLuc, 1 mM ATP, and 10 mM Mg2+ (Fig. S7A and S8A†). As shown in Fig. S7B and S8B,† a good linear relationship between the BL intensity at 595 nm and the concentration of CTSB (Y = 13.38 + 63.57X, R2 = 0.9950) or CTSL (Y = 10.01 + 483.2X, R2 = 0.9913) was obtained, and the limit of detection (LOD) of FK-Luc toward CTSB was calculated to be 0.03850 U/L (S/N = 3), which is about 7.0-fold higher than that for CTSL (0.005536 U/L, S/N = 3), indicating that the dipeptide FK was more sensitive to the CTSL response. Hence, the following in vitro experiments were mainly performed with CTSL. Furthermore, we synthesized a control probe Luc-BH with reactive motif benzoylhydrazine for ClO− detection (Scheme S2, Fig. S9 and S10†). After incubating different concentrations of ClO− with 25 μM of Luc-BH for 2 h at 37 °C, the BL intensity of Luc-BH at 595 nm was found to have a good linear relationship (Y = 0.8241X − 3.831, R2 = 0.9972) with the concentration of ClO− ranging from 10 to 80 μM (Fig. S11†), and the LOD of ClO− was calculated to be 0.8613 μM (S/N = 3). These results demonstrated the feasibility of the probe with a benzoylhydrazine structure for the ClO− response.The detailed synthetic procedures and characterization studies of FK-Luc-BH are shown in Scheme S3 and Fig. S12–S14,† including its 1H and 13C nuclear magnetic resonance (NMR) spectroscopy spectrum and mass spectrum (MS). After incubating 25 μM FK-Luc-BH with 2 U/L CTSL or 1 mM ClO− in CTSL working buffer at 37 °C for 4 h, the high-performance liquid chromatography (HPLC) assay combined with the MS results verified that Luc-BH (olive line in Fig. 2A, S15, S16, and Table S1†) was produced as a result of the enzymatic cleavage of peptide FK on probe FK-Luc-BH by CTSL, and FK-Luc was generated after oxidation of the benzoylhydrazine by ClO− (blue line in Fig. 2A, S15 and S17†). As predicted, FK-Luc-BH was able to convert to free NH2-Luc for BL imaging under the co-incubation of CTSL and ClO− (red line in Fig. 2A, S15 and S18†). Then we studied the BL properties of FK-Luc-BH after treatment with only CTSL, only ClO−, and both, respectively. As shown in Fig. 2B, when 25 μM FK-Luc-BH was co-incubated with both 2 U/L CTSL and 200 μM ClO− at 37 °C for 2 h, a clear BL signal could be observed after adding 0.1 mg mL−1 fLuc, 1 mM ATP, and 10 mM Mg2+, resulting from the generation of the fLuc substrate NH2-Luc (Fig. 1A). In contrast, in the absence of either CTSL or ClO−, their BL intensities after the addition of fLuc were as weak as that of untreated FK-Luc-BH (Fig. 2B), indicating that FK-Luc-BH could only be efficiently activated for BL imaging with the coexistence of both CTSL and ClO−. Then the reaction of FK-Luc-BH with ClO− was observed to follow second order kinetics with a rate constant of 0.3009 M−1 min−1 (Fig. S19†). The kinetic parameters of CTSB/CTSL toward probe FK-Luc-BH were investigated according to the Michaelis–Menten equation. As displayed in Fig. S20 and S21,† the calculated catalytic efficiency (kcat/Km) of CTSL (4.376 μM−1 min−1) is about 1 order of magnitude larger than that of CTSB (0.3412 μM−1 min−1), further suggesting that FK-Luc-BH was much more sensitive to CTSL than to CTSB.
To confirm the specificity of FK-Luc-BH for imaging tumor cells co-overexpressing both CTSL and ClO−, the selectivity of FK-Luc-BH was assessed over a series of biological species that widely occur in living cells. After the addition of fLuc, ATP, and Mg2+, no obvious BL signals were noticed when FK-Luc-BH was incubated with different types of ROS and nitrogen species (RNS), e.g., nitric oxide (NO), hydrogen peroxide (H2O2), hydroxyl radical (˙OH), peroxynitrite (ONOO−), peroxyl radical (ROO˙), superoxide anion (O2−), singlet oxygen (1O2), and ClO−, as well as intracellular abundant reduced glutathione (GSH) and common cancer cell-overexpressing enzymes including nitroreductase (NTR), alkaline phosphatase (ALP), furin, γ-glutamyltranspeptidase (GGT), and CTSB, respectively (37 °C, 2 h).26–29 Remarkable enhancements in BL intensity were only observed when incubating FK-Luc-BH simultaneously with 200 μM ClO− and 2 U/L CTSL/CTSB, and CTSL triggered significantly higher intensity than CTSB (Fig. S22†). To further confirm the specificity of FK-Luc-BH toward cathepsins with ClO−, the selectivity of FK-Luc-BH against various potential interfering stimulus pairs was tested respectively. As displayed in Fig. 2C, the BL intensity changes were evident only in the copresence of CTSB/CTSL with ClO−, indicating that FK-Luc-BH shows rather better selectivity towards cathepsins + ClO− compared with other potential intracellular interfering stimulus pairs and is thus able to accurately identify cathepsin and ClO− co-abundant environments.
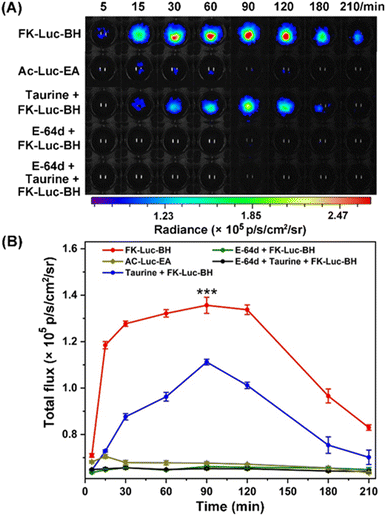
To prove that the BL signal of FK-Luc-BH was indeed mediated by cathepsins and ClO−, a control compound Ac-Luc-EA was constructed with an acetyl group in lieu of the CTSL substrate FK and benzoylhydrazine was replaced by ethylamine; hence, it cannot be cleaved by CTSL and oxidized by ClO− to expose NH2-Luc for BLI (Fig. S23–S26†). The cytotoxicities of FK-Luc-BH and the control compound Ac-Luc-EA were studied on CTSL-overexpressed 4T1 mouse mammary tumor cells by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Fig. S27 and S28†). High survival rates of the 4T1 cells were obtained after treatment with 12.5 μM, 25 μM, and 50 μM FK-Luc-BH or Ac-Luc-EA for 48 h, respectively, suggesting their good biocompatibility (Fig. S29†).30 Then the BL signals were dynamically monitored after incubating fLuc-transfected 4T1 cells (4T1-fLuc) with 25 μM FK-Luc-BH or 25 μM control compound Ac-Luc-EA. The signal intensity of FK-Luc-BH treated cells gradually increased to their maximum at 90 min after an initial rapid increase from 5 min to 30 min and could still be detected at 210 min post-incubation, demonstrating that endogenous ClO− and CTSL could trigger FK-Luc-BH to yield free NH2-Luc for BL “on”. The Ac-Luc-EA incubated cells, by contrast, exhibited very weak BL signals all through 5 min to 210 min, because the fLuc substrate was incapable of being activated in 4T1 cells (Fig. 3). To further confirm that the activation of FK-Luc-BH was induced by ClO− and CTSL, 4T1-fLuc cells were preincubated with 250 mM ClO− scavenger taurine for 5 min, 500 μM E-64d for 30 min, and 500 μM E-64d for 25 min together with 250 mM taurine for another 5 min (the incubation time of E-64d is 30 min in total), respectively. The time courses of the BL signal after incubation with 25 μM FK-Luc-BH showed that all their BL signal intensities were very weak from 5 min to 210 min and significantly lower than those of FK-Luc-BH treated fLuc-transfected 4T1 cells, which further proves that FK-Luc-BH is indeed a dual-locked AND-gate BL probe for tumor cell imaging, as its illumination specifically requires both ClO− and CTSL (Fig. 3 and S30†).
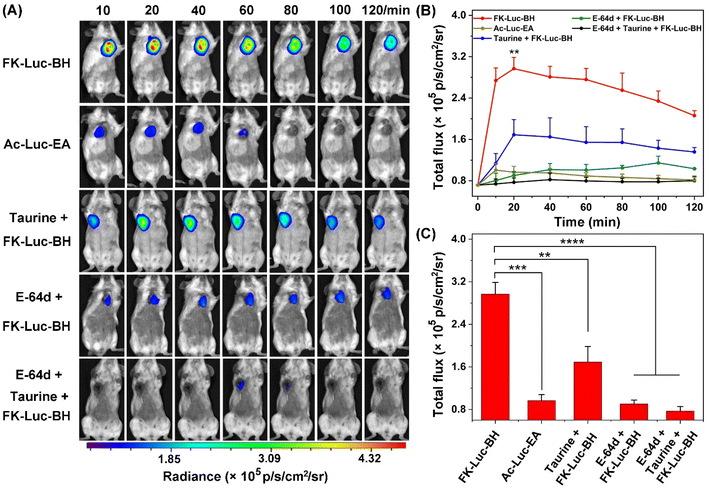
Finally, the capability of FK-Luc-BH to detect the coexistence of cathepsins and ClO−in vivo for precise tumor diagnosis was evaluated. 3 × 105 4T1-fLuc cells were implanted into the mammary fat pads of 7-week-old Balb/c mice to generate an orthotopic mouse model of breast cancer. After 7 days, the tumor-bearing Balb/c mice were randomly divided into five groups (n = 3 for each group): mice in the experimental group FK-Luc-BH or the control group Ac-Luc-EA were injected intraperitoneally (i.p.) with FK-Luc-BH or Ac-Luc-EA (12.5 μmol kg−1); mice in the control groups taurine + FK-Luc-BH, E-64d + FK-Luc-BH, and taurine + E-64d + FK-Luc-BH were pre-injected i.p. with 3 mmol kg−1 taurine for 5 min, 0.05 mmol kg−1 E-64d for 1 h, and 0.05 mmol kg−1 E-64d for 55 min together with 3 mmol kg−1 taurine for another 5 min, respectively, followed by the intraperitoneal administration of FK-Luc-BH (12.5 μmol kg−1). For each group, time-course BL images of the breast tumor-bearing Balb/c mice were acquired from 10 to 120 min after the injection of FK-Luc-BH. As shown in Fig. 4, the BL intensity from the 4T1-fLuc tumors in the FK-Luc-BH group (the top row) obviously increased post-injection and reached its peak at 20 min; over 59% of the BL signal remained even at 120 min after injection, suggesting that FK-Luc-BH could quickly respond to ClO− and CTSL, accompanied by producing NH2-Luc for BL generation. In comparison, the BL signal from the control group Ac-Luc-EA was much lower at 20 min and difficult to observe at 80 min post-injection, resulting from its unresponsive molecular structure. For the other three control groups taurine + FK-Luc-BH, E-64d + FK-Luc-BH, and taurine + E-64d + FK-Luc-BH, the expression level of either ClO− or CTSL was reduced, and the BL signals of the tumors were significantly lower than those of the FK-Luc-BH group during the observation period. The quantitative results showed that the BL intensities of the taurine + FK-Luc-BH group, E-64d + FK-Luc-BH group, and E-64d + taurine + FK-Luc-BH group were 1.76-, 3.29-, and 3.86-fold lower than that of the FK-Luc-BH group at 20 min, respectively. These results demonstrated that FK-Luc-BH could be employed for sensitively and efficiently imaging tumor cells co-expressing ClO− and CTSL. Similar to the above cell experiment results, among all the control groups, the relatively higher BL signal in the control group taurine + FK-Luc-BH was probably caused by the incomplete consumption of ClO− by taurine in tumor cells. In addition, the systemic toxicities of BL probes were evaluated for each group after the last time point for imaging (120 min after the injection of FK-Luc-BH or Ac-Luc-EA). The mice were sacrificed before blood was collected from the eyeballs, then the major organs (heart, liver, spleen, lung, and kidney) and tumors were selected for hematoxylin–eosin (HE) staining. In contrast with the mice in the sham-operated group, which were only i.p. injected with PBS, the HE staining results revealed that no pathological changes were detected in all tissues mentioned above (Fig. S31†), indicating the good biocompatibility of the AND-gate BL probe FK-Luc-BH.
To prove the improvement of this novel AND-gate bioluminescent probe over the single-locked probes, 3 × 105 4T1-fLuc cells were implanted separately into the second and fourth mammary fat pads of the 7-week-old Balb/c mice. When the tumors reached about 200 mm3, the mice were randomly divided into seven groups (n = 3) for comparing the BL properties of FK-Luc-BH and single-locked probes Luc-BH (ClO−-responsive) and FK-Luc (CTSL-responsive). Mice in the experimental group FK-Luc-BH were administered i.p. with 12.5 μmol kg−1FK-Luc-BH, and the tumors around the second and fourth mammary fat pads are close to each other in the BL intensity (Fig. S32A and B†). However, for the control group taurine + FK-Luc-BH or the control group E-64d + FK-Luc-BH, when 12.5 μmol kg−1FK-Luc-BH was injected i.p. after the intratumoral injection of 3 mmol kg−1 taurine or 0.05 mmol kg−1 E-64d into either of the tumors of each mouse, the BL signal of the tumors that were preinjected with taurine or E-64d was significantly lower than that of the other unpretreated ones (Fig. S32A, C and D†). These results further demonstrate that the BL “turn-on” of FK-Luc-BH requires both ClO− and cathepsins, which will make this AND-gate probe more precise than the single-locked probes discussed below. To assess the BL properties of the single-locked probes Luc-BH and FK-Luc, after the intratumoral injection of 3 mmol kg−1 taurine or 0.05 mmol kg−1 E-64d into either of the tumors of each mouse, the mice were administered i.p. with Luc-BH or FK-Luc (12.5 μmol kg−1), respectively. As shown in Fig. S33,† the BL intensity of Luc-BH was largely influenced by the level of ClO− but not CTSL, hence making it unable to differentiate the tumors with other ClO−-related diseases, such as inflammation and neurodegenerative disorders.19,31–34 Similarly, the BL intensity of FK-Luc only depends on the concentration of CTSL (Fig. S34†), that may cause undesirable background signals and even false-positive results in other CTSL-sufficient tissues, for example, the lung and liver.35 To further validate the good specificity of FK-Luc-BH, the Balb/c mice were randomly divided into three groups (n = 3 for each group), and the mice in each group were intraperitoneally administered with 12.5 μmol kg−1FK-Luc-BH, FK-Luc, or Luc-BH. 40 min after injection, the Balb/c mice were sacrificed and their organs (hearts, lungs, livers, spleens, kidneys, and muscles) were harvested to prepare 15 wt% tissue homogenates. After the addition of 0.1 mg mL−1 fLuc, 1 mM ATP, and 10 mM Mg2+, the BL signal of tissue homogenates was measured using a small animal imaging system. As shown in Fig. S35,†FK-Luc-BH showed a lower background BL signal in the tissues than FK-Luc and Luc-BH. All of the above results suggest that the dual-locked BL probe FK-Luc-BH shows lower background interference and better specificity compared with the single-locked probes for the imaging of tumors co-expressing ClO− and CTSL.
Conclusion
In conclusion, by caging the amino group of NH2-Luc with the CTSL-recognizing substrate FK and the carboxyl group of NH2-Luc with the ClO− reactive motif benzoylhydrazine, an AND-gate BL probe FK-Luc-BH was designed for precise imaging of tumors where CTSL and ClO− co-localized. In vitro experiments indicated that FK-Luc-BH was able to efficiently convert to free NH2-Luc when simultaneously incubated with CTSL and ClO− and hence exhibited a bright BL signal after catalyzing by fLuc. The selectivity of FK-Luc-BH towards CTSL and ClO− was evidenced by monitoring the BL responses of FK-Luc-BH to various biological species in solution. Cell experiments and animal experiments demonstrated that the coordination of CTSL and ClO− enabled the BL signal of FK-Luc-BH to be significantly enhanced in 4T1-fLuc cells or 4T1-fLuc tumor sites, when compared with that of the unresponsive control compound Ac-Luc-EA group and the 4T1-fLuc cells or tumors that were co-treated with FK-Luc-BH with ClO− scavenger taurine or/and broad-spectrum cathepsin inhibitor E-64d, suggesting the good specificity and accuracy of FK-Luc-BH for tumor imaging. By replacing the caging motifs for NH2-Luc (i.e. FK and benzoylhydrazine in this work) with other responsive sites, this AND-gate platform can be employed for specifically imaging other diseases.Data availability
The ESI† includes all experimental details. General methods; syntheses and characterization studies of FK-Luc-BH, Luc-BH and Ac-Luc-EA.Author contributions
C. Wang performed syntheses, characterization, in vitro and in vivo experiments. Y. Hong, L. Dong, H. Chen and Z. Yu helped with characterization and cell experiments. D. Jin and R. Zhao helped with the construction of a 4T1-fLuc tumor bearing mouse model and in vivo experiments, respectively. Y. Yuan designed this project and wrote the paper. All the authors provided suggestions and comments on the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Key R&D Program of China (2022YFA1305100), the National Natural Science Foundation of China (22175168), the Collaborative Innovation Program of Hefei Science Center, CAS (2021HSC-CIP012), and the Fundamental Research Funds for the Central Universities (WK2060000020). For animal imaging, all animals received care according to the guidelines outlined in the Guide for the Care and Use of Laboratory Animals. The procedures were approved by the University of Science and Technology of China Animal Care and Use Committee (Permit Number: USTCACUC23010122026).References
- J. C. Widen, M. Tholen, J. J. Yim, A. Antaris, K. M. Casey, S. Rogalla, A. Klaassen, J. Sorger and M. Bogyo, Nat. Biomed. Eng., 2021, 5, 264–277 CrossRef CAS PubMed.
- X. B. Zhao, J. Y. Kang and Y. P. Shi, Anal. Chem., 2022, 94, 6574–6581 CrossRef CAS PubMed.
- J. Chan, S. C. Dodani and C. J. Chang, Nat. Chem., 2012, 4, 973–984 CrossRef CAS PubMed.
- X. Wu, R. Wang, N. Kwon, H. Ma and J. Yoon, Chem. Soc. Rev., 2022, 51, 450–463 RSC.
- Z. Qing, J. Xu, J. Hu, J. Zheng, L. He, Z. Zou, S. Yang, W. Tan and R. Yang, Angew. Chem., Int. Ed., 2019, 58, 11574–11585 CrossRef CAS PubMed.
- X. Zhao, Q. Han, N. Na and J. Ouyang, Anal. Chem., 2021, 93, 14514–14520 CrossRef CAS PubMed.
- L. Wu, A. C. Sedgwick, X. Sun, S. D. Bull, X. P. He and T. D. James, Acc. Chem. Res., 2019, 52, 2582–2597 CrossRef CAS PubMed.
- M. Yang, J. Fan, J. Du and X. Peng, Chem. Sci., 2020, 11, 5127–5141 RSC.
- Q. Miao and K. Pu, Adv. Mater., 2018, 30, 1801778 CrossRef PubMed.
- L. Wu, J. Huang, K. Pu and T. D. James, Nat. Rev. Chem., 2021, 5, 406–421 CrossRef CAS.
- A. P. de Sliva, N. H. Q. Gunaratne and C. P. McCoy, Nature, 1993, 364, 42–44 CrossRef.
- Y. Liu, L. Teng, C. Xu, H. W. Liu, S. Xu, H. Guo, L. Yuan and X. B. Zhang, Chem. Sci., 2019, 10, 10931–10936 RSC.
- S. Chen, M. Chen, J. Yang, X. Zeng, Y. Zhou, S. Yang, R. Yang, Q. Yuan and J. Zheng, Small, 2021, 17, 2100243 CrossRef CAS PubMed.
- L. Wu, H. H. Han, L. Liu, J. E. Gardiner, A. C. Sedgwick, C. Huang, S. D. Bull, X. P. He and T. D. James, Chem. Commun., 2018, 54, 11336–11339 RSC.
- J. L. Kolanowski, F. Liu and E. J. New, Chem. Soc. Rev., 2018, 47, 195–208 RSC.
- A. C. Love and J. A. Prescher, Cell Chem. Biol., 2020, 27, 904–920 CrossRef CAS PubMed.
- A. J. Syed and J. C. Anderson, Chem. Soc. Rev., 2021, 50, 5668–5705 RSC.
- M. Verdoes, K. Oresic Bender, E. Segal, W. A. van der Linden, S. Syed, N. P. Withana, L. E. Sanman and M. Bogyo, J. Am. Chem. Soc., 2013, 135, 14726–14730 CrossRef CAS PubMed.
- Y. W. Yap, M. Whiteman and N. S. Cheung, Cell. Signalling, 2007, 19, 219–228 CrossRef CAS PubMed.
- A. Daugherty, J. L. Dunn, D. L. Rateri and J. W. Heinecke, J. Clin. Invest., 1994, 94, 437–444 CrossRef CAS PubMed.
- S. M. Wu and S. V. Pizzo, Arch. Biochem. Biophys., 2001, 391, 119–126 CrossRef CAS PubMed.
- S. Weitzman and L. Gordon, Blood, 1990, 76, 655–663 CrossRef CAS PubMed.
- S. R. Mullins, M. Sameni, G. Blum, M. Bogyo, B. F. Sloane and K. Moin, Biol. Chem., 2012, 393, 1405–1416 CrossRef CAS PubMed.
- M. Assi, Am. J. Physiol.: Regul., Integr. Comp. Physiol., 2017, 313, 646–653 CrossRef CAS PubMed.
- J. Huang, X. Chen, Y. Jiang, C. Zhang, S. He, H. Wang and K. Pu, Nat. Mater., 2022, 21, 598–607 CrossRef CAS PubMed.
- B. Hu, N. Song, Y. Cao, M. Li, X. Liu, Z. Zhou, L. Shi and Z. Yu, J. Am. Chem. Soc., 2021, 143, 13854–13864 CrossRef CAS PubMed.
- H. Li, Q. Yao, F. Xu, Y. Li, D. Kim, J. Chung, G. Baek, X. Wu, P. Hillman, E. Lee, H. Ge, J. Fan, J. Wang, S. Nam, X. Peng and J. Yoon, Angew. Chem., Int. Ed., 2020, 59, 10186–10195 CrossRef CAS PubMed.
- L. Zhu, H. W. Liu, Y. Yang, X. X. Hu, K. Li, S. Xu, J. B. Li, G. Ke and X. B. Zhang, Anal. Chem., 2019, 91, 9682–9689 CrossRef CAS PubMed.
- J. Liu, S. Zhang, B. Zhao, C. Shen, X. Zhang and G. Yang, Biosens. Bioelectron., 2019, 142, 111497 CrossRef CAS PubMed.
- D. Ma, Q. Zong, Y. Du, F. Yu, X. Xiao, R. Sun, Y. Guo, X. Wei and Y. Yuan, Acta Biomater., 2021, 135, 628–637 CrossRef CAS PubMed.
- H. Feng, Z. Zhang, Q. Meng, H. Jia, Y. Wang and R. Zhang, Adv. Sci., 2018, 5, 1800397 CrossRef PubMed.
- C. Ma, S. Hou, X. Zhou, Z. Wang and J. Yoon, Anal. Chem., 2021, 93, 9640–9646 CrossRef CAS PubMed.
- T. M. Jeitner, M. Kalogiannis, B. F. Krasnikov, I. Gomolin, M. R. Peltier and G. R. Moran, Toxicol. Sci., 2016, 151, 388–402 CrossRef CAS PubMed.
- C. Yin, H. Zhu, C. Xie, L. Zhang, P. Chen, Q. Fan, W. Huang and K. Pu, Adv. Funct. Mater., 2017, 27, 1700493 CrossRef.
- Y. Huang, S. Li, S. Huang, J. Tu, X. Chen, L. Xiao, B. Liu and X. Yuan, Front. Bioeng. Biotechnol., 2022, 10, 780751 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3sc00556a |
| This journal is © The Royal Society of Chemistry 2023 |