Open Access Article
Open Access ArticleDirect observation of bicarbonate and water reduction on gold: understanding the potential dependent proton source during hydrogen evolution†
Gang-Hua
Deng
 a,
Quansong
Zhu
a,
Quansong
Zhu
 b,
Jaclyn
Rebstock
b,
Jaclyn
Rebstock
 b,
Tomaz
Neves-Garcia
b and
L. Robert
Baker
b,
Tomaz
Neves-Garcia
b and
L. Robert
Baker
 *b
*b
aState Key Laboratory of Information Photonic and Optical Communications and School of Science, Beijing University of Posts and Telecommunications (BUPT), Beijing 100876, P. R. China
bDepartment of Chemistry and Biochemistry, The Ohio State University, Columbus, Ohio 43210, USA. E-mail: baker.2364@osu.edu
First published on 24th March 2023
Abstract
The electrochemical conversion of CO2 represents a promising way to simultaneously reduce CO2 emissions and store chemical energy. However, the competition between CO2 reduction (CO2R) and the H2 evolution reaction (HER) hinders the efficient conversion of CO2 in aqueous solution. In water, CO2 is in dynamic equilibrium with H2CO3, HCO3−, and CO32−. While CO2 and its associated carbonate species represent carbon sources for CO2R, recent studies by Koper and co-workers indicate that H2CO3 and HCO3− also act as proton sources during HER (J. Am. Chem. Soc. 2020, 142, 4154–4161, ACS Catal. 2021, 11, 4936–4945, J. Catal. 2022, 405, 346–354), which can favorably compete with water at certain potentials. However, accurately distinguishing between competing reaction mechanisms as a function of potential requires direct observation of the non-equilibrium product distribution present at the electrode/electrolyte interface. In this study, we employ vibrational sum frequency generation (VSFG) spectroscopy to directly probe the interfacial species produced during competing HER/CO2R on Au electrodes. The vibrational spectra at the Ar-purged Na2SO4 solution/Au interface, where only HER occurs, show a strong peak around 3650 cm−1, which appears at the HER onset potential and is assigned to OH−. Notably, this species is absent for the CO2-purged Na2SO4 solution/gold interface; instead, a peak around 3400 cm−1 appears at catalytic potential, which is assigned to CO32− in the electrochemical double layer. These spectral reporters allow us to differentiate between HER mechanisms based on water reduction (OH− product) and HCO3− reduction (CO32− product). Monitoring the relative intensities of these features as a function of potential in NaHCO3 electrolyte reveals that the proton donor switches from HCO3− at low overpotential to H2O at higher overpotential. This work represents the first direct detection of OH− on a metal electrode produced during HER and provides important insights into the surface reactions that mediate selectivity between HER and CO2R in aqueous solution.
1 Introduction
The hydrogen evolution reaction (HER) is of great importance in electrocatalysis and sustainable energy production, and a large number of experimental and theoretical studies have sought to unravel the mechanisms of HER.1–3 Another important reaction for energy storage and greenhouse gas remediation is CO2 reduction (CO2R). However, during electrocatalytic CO2R in aqueous electrolyte, HER competes with CO2R limiting the faradaic efficiency of CO2 conversion. Therefore, to design systems to optimize the selectivity for CO2R, it is necessary to understand the mechanism of HER. Much effort has been devoted to control the catalyst surface properties that promote activity and selectivity for CO2R while suppressing HER.4–12 However, by comparison, less attention has been devoted to understand the role of the various interfacial species (H2O, CO2, H2CO3, HCO3− and OH−) on the competition between CO2 R and HER. One of the main questions is: among H2O, H2CO3 and HCO3−, which is the proton source for HER during CO2R? Recently, much effort has been dedicated by Koper and co-workers to study the mechanism of HER in bicarbonate solutions by electrokinetic measurements.13–16 They conclude that at increasingly negative potentials, the proton donor for HER changes from H2CO3 to HCO3− to H2O.15 However, because these species invariably equilibrate, direct spectroscopic evidence is still needed to confirm the actual species produced at the electrode/electrolyte interface in order to distinguish between these surface reactions.Vibrational spectra of interfacial water can be a sensitive indicator of structure and composition of the electrochemical double layer.17–24 Various methods for interface-specific vibrational spectroscopy, including VSFG, surface enhanced Raman spectroscopy (SERS), and shell-isolated nanoparticle enhanced Raman spectroscopy (SHINERS) has been shown to be a powerful technique to understand interfacial water structure at solid/liquid interfaces. Focusing here on electrochemical HER, it was demonstrated that the structure of the interfacial water molecule can vary with the potential on the electrode surfaces.20–23,25–27 A strong correlation between the interfacial water structure and the HER activity on a Pt surface was demonstrated by Shen et al. with in situ Raman spectroscopy.26 They find that the first layer of water molecules changes from proton acceptors to proton donors with increasing pH and the reactivity of the interfacial water varied its structure. In a more recent study, using SHINERS, Wang et al. also showed that structurally ordered interfacial water facilitated high efficiency electron transfer across the interface, resulting in increased HER rates.25 However, despite the importance of this electrochemical reaction, direct detection of the HER products at the electrode/electrolyte interface is still missing. Specifically, OH− is the direct product of water reduction, but to date no spectroscopic detection of this species on an electrode surface has been reported. In this study, we employ in situ VSFG to measure the interfacial water spectra during active HER in Na2SO4 and NaHCO3 solutions using a Au electrode. By directly observing the interfacial water spectrum as the system is pushed away from equilibrium under applied potential, we seek to differentiate between water and bicarbonate reduction and to understand the related effects of proton source and interfacial pH buffering on the kinetics of HER.
2 Results and discussion
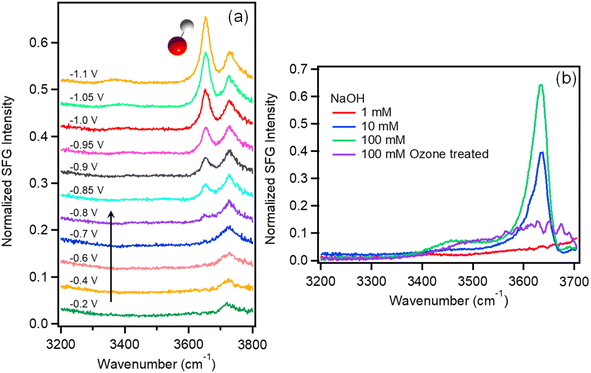
VSFG is a second order nonlinear technique with surface/interface specificity, which has been widely used to investigate molecular orientation, dynamics, and chemical reactions on surfaces.28–31 The details of the instrument, including the VSFG electrochemical cell, which provides in situ measurements of the Au/electrolyte interface at current densities exceeding 1 mA cm−2 in the absence of mass transport limitations,32,33 can be found in the ESI.† Using this instrument, we have quantified the in situ detection limit on a Au electrode to be better than 1% of a surface monolayer.32,33Fig. 1a shows the VSFG spectra of interfacial water for 0.05 M Na2SO4 saturated with Ar as a function of applied potential. Before the onset of HER at approximately −0.8 V vs. Ag/AgCl, there is only one sharp peak observed at 3730 cm−1. This peak corresponds to H2O that is not H-bonded with other water molecules and is assigned to free OH.34–37 Using VSFG, a number of groups have reported the free OH stretch of interfacial water at the aqueous dielectric interface,38,39 but only recently has this feature been observed at the Au/electrolyte interface under applied potential.37,40 This feature is best described as interfacial water with one non-H-bonded OH group pointing toward the Au electrode.37When the potential reaches −0.8 V near the onset of HER, another sharp peak appears around 3650 cm−1, which increases in intensity as the applied potential becomes more negative, eventually becoming the dominate feature in the spectrum at potentials below −1.0 V. This feature is similar to the free OH stretch, and due to its narrow line width, indicating that this feature is also from non-H-bonded OH. Based on the shift in peak position and fact that this feature only appears near the onset potential of HER, we assign this feature to the free OH stretch of surface hydroxide (OH−).
This is consistent with previous assignments for the free OH− stretch observed in the gas phase41 as well as in alkali hydroxide solutions.42–45 To confirm this assignment, VSFG spectra of NaOH solutions at varying concentration between 1 and 100 mM were also measured on Au under open circuit potential as shown in Fig. 1b. In addition to the free OH stretch of interfacial water around 3730 cm−1, these solutions show another sharp peak around 3635 cm−1 at increasing NaOH concentrations, which supports the assignment of this feature as interfacial OH−. Additional control experiments using isotope exchange, which further confirm this assignment, are described in the ESI Section 2 (Fig. S1–S2).†
Similar sharp peaks resulting from non-H-bonded/weakly coupled OH− have been observed by VSFG at the CaF2/liquid interface.46–48 Based on these observations, we hypothesize that the OH− anion measured here resides directly on the Au surface inside of the Stern layer with the H atom oriented toward the Au electrode and the O atom possibly coordinated to a Na+ cation. To test whether this species is directly interacting with the Au surface, we treated a Au electrode with UV-generated ozone to form a hydrophilic surface oxide layer. The purple curve in Fig. 1b shows the SFG spectrum of the electrode/electrolyte interface using this oxidized Au electrode. As shown, the peak at 3650 cm−1 disappears even for 100 mM NaOH solution. It should be noted that the open circuit potential (OCP) for ozone-treated Au surface is 210 mV which is higher than that of normal Au surface (−100 mV). However, the 300 mV difference of OCP should not account for the absence of the OH− peak. When a positive potential of 0.4 V was applied for a normal Au electrode, the OH− peak still shows in the spectrum (Fig. S3†). The peak begins to decrease substantially when the applied potential reaches 0.6 V. At this potential the gold electrode begins to oxidize and form hydrogen bonds with OH− causing the peak intensity to decrease. Thus, we attribute the loss of this free OH stretch to H-bonding of the interfacial OH− anion with the oxidized Au electrode, which cannot occur on a metallic Au surface. Note that the OH− anion should be repelled from the negatively charged electrode during HER, indicating that detection of OH− on Au at negative potential represents a transient, surface species formed in situ as a product of HER. The transient nature of this species is confirmed by a potential jump experiment (see Fig. S4†), where the intensity of the OH− peak immediately decreases to near zero once the potential is stepped more positive, and active HER stops. This finding is consistent with recent theoretical predictions by Li et al. of non-H-bonded OH− produced at the metal/electrolyte interface inside the outer Helmholtz plane during HER.49 To our knowledge, this is the first direct observation of OH− produced inside the Helmholtz plane during HER. Blue shift of the OH− peak around 3650 cm−1 is observed as shown in Table S1.† The shift is only 2 cm−1 from −0.85 V to −1.1 V, which is smaller than the spectral resolution of our instrument (∼10 cm−1). The absence of a significant Stark shift for this species is consistent with a previous study by Tong et al.37 The reason that OH− shows such a small Stark shift may be due to direct interactions of OH− with alkali cations in the Stern layer such that it probes local rather than average electric fields. However, confirming this hypothesis will require further investigation.
Since the OH− peak around 3650 cm−1 represents the local concentration of OH−, it is related to the local pH. In fact, it may be possible in the future to use this OH− signature as an indicator to quantitatively measure the local pH of the electrode surface. At present, as demonstrated above the surface OH− resides in the first molecular layer at the electrode surface. After the surface OH− diffuses away from the surface, it forms hydrogen bonds with the surrounding water molecules, and the feature around 3650 cm−1 disappears. A number of other techniques have been used to study the local pH near the electrode/electrolyte interface, such as SEIRAS,50 and RRDE based local pH measurements.51,52 Although each of these techniques measures related signals, they each sample different regions away from the actual interfaces (approximately 10 nm for SEIRAS and μm to mm scale for RRDE) and provide different but complementary information.
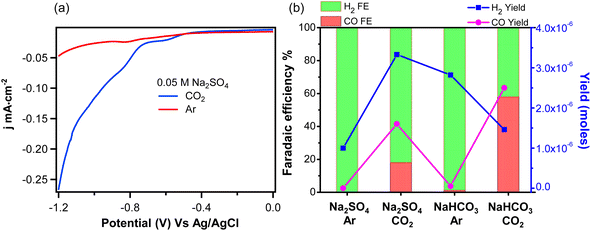
The experiments above represent direct observation of HER in the absence of CO2R. We now consider the interfacial water spectrum measured in CO2-purged Na2SO4 electrolyte where HER is in competition with CO2R, and H2CO3 and HCO3− represent additional proton donors, which are not present in the Ar-purged electrolyte. VSFG spectra of 0.05 M Na2SO4 solution saturated with CO2 are shown in Fig. 2a as a function of potential. At potentials more positive than −0.6 V, we observe two main features: free OH at 3730 cm−1 and a broad peak around 3630 cm−1, which is assigned to a weakly hydrogen-bonded or singly hydrogen-bonded water.36,40 The absence of peaks at lower frequency, which are usually observed at air/liquid34,36,53 or solid/liquid interface54–56 indicates a relatively disordered water structure at the Au electrode surface.18 As more negative potential is applied, an additional peak around 3400 cm−1 appears that increases with negative potential. This peak can be assigned either to liquid-like water34 or to a H-bonding water network with a net lower coordination compared to tetrahedral water around 3200 cm−1.53,55,56 Overall, the spectra of Na2SO4 solution saturated with CO2 exhibit very different features compared to Ar-saturated electrolyte. Specifically, the sharp peak corresponding to surface OH− at 3650 cm−1 is not observed in CO2-saturated electrolyte, and the peak around 3400 cm−1, which is absent for Ar-purged electrolyte, becomes significant under catalytic potentials in CO2-saturated electrolyte.
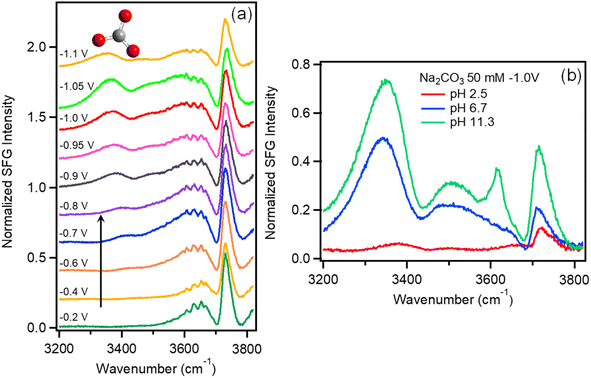 | ||
| Fig. 2 (a) VSFG spectra of Na2SO4 (0.05 M) purged with CO2 as a function of potential vs. Ag/AgCl. (b) VSFG spectra of 50 mM Na2CO3 solutions as a function of pH. | ||
The feature at 3400 cm−1 cannot be attributed solely to the influence of Na+ or SO42− on the interfacial water structure because these species are present in both Ar- and CO2-purged electrolyte. In addition to Na+ and SO42−, CO2-saturated electrolyte contains H2CO3, HCO3−, and CO32−, which is absent in Ar-purged electrolyte, and the equilibrium concentration of these species varies as a function of pH. To identify the origin of this feature, we explored the effects of various electrolyte compositions on the interfacial water spectrum. Results show that this peak is weak in Ar-purged NaCl, but is much more prominent in CO2-purged NaCl as well as Ar- or CO2-purged NaHCO3 electrolytes (see Fig. S5†), confirming a correlation between this feature and the presence of carbonate species at the interface. Additionally, we show that at fixed potential the intensity of this feature tracks closely with the NaHCO3 electrolyte concentration (see Fig. S6†). Based on these observations, we attribute this feature to water structure induced by carbonate species present in the electrochemical double layer.
To evaluate the relative contributions of H2CO3, HCO3−, and CO32− to this feature, Fig. 2b plots the VSFG spectra in 0.05 M Na2CO3 electrolyte at −1.0 V as a function of pH. The spectrum with pH = 11.3 (green curve) represents pure 0.05 M Na2CO3 solution. To prepare the solution with pH = 6.7 (blue curve), this same solution is purged with CO2, which converts the majority of CO32− anions into HCO3− (see ESI Fig. S7†). To prepare the pH = 2.5 solution (red curve), HCl is added to 0.05 M Na2CO3 solution. At pH = 2.5, CO32− is almost completely converted to H2CO3 as show in Fig. S7.†Fig. 2b shows that the spectrum obtained at pH = 11.3 includes a strong feature at 3400 cm−1, as well as a peak at 3640 cm−1 corresponding to free OH−. As pH is decreased to 6.7, we observe that the OH− peak disappears, and the 3400 cm−1 feature decreases in intensity. At pH = 2.5 the peak at 3400 cm−1 entirely disappears, suggesting that this feature can be attributed to CO32− with perhaps a minor contribution from HCO3−, but that neutral H2CO3 does not contribute. While additional work is required to understand the actual water structure associated with these specific ion effects, here we use this feature as a spectral indicator of HCO3− and CO32− at the Au/electrolyte interface. It is important to note that, although negatively charged, HCO3− and CO32− can approach the Au surface under negative potential due to ion pairing interactions with the Na+ cations comprising the Stern layer.57–60
Before further discussion, we consider the χ(3) bulk contribution in the spectra measured here. As demonstrated by several previous studies,23,61–64 the χ(3) bulk contribution in interfacial water spectra is common at charged interfaces: χeff(2) = χs(2) + χB(3)Φ, in which χeff(2) is the total SFG response, χs(2) is the surface contribution, χB(3) is the bulk contribution induced by the interfacial electric field and Φ is the surface potential. As shown in Fig. S8,† the χB(3) bulk contribution in our spectra is less than 20% due to the 467 fs delay time of IR and 800 nm beams (see the details in ESI Section 6†). The absence of interference effects between bulk contribution and free-OH peaks also suggest that the χB(3) contribution does not significantly influence the shape or intensity of the free OH stretch reported here (Fig. S9†). Lastly, the peak around 3200 cm−1 which is very pronounced in χB(3) bulk contribution61,62 is not seen for all the spectra in this work (Fig. S9 & S10†). This further indicates that the χB(3) contribution is small. Although the χB(3) bulk contribution in interfacial water spectra presented in this work is small, disentangling the χB(3) bulk contribution is very important to gain a detailed physical picture of the Stern layer, and further work is required for a quantitative analysis.
Having identified these spectral signatures, we now consider the influence of CO2-purging on the kinetics of HER and CO2R at the Au/electrolyte interface. The linear sweep voltammograms of Ar- and CO2- saturated Na2SO4 solution are shown in Fig. 3a. The current densities agree with ones extracted during SFG measurement as shown in Fig. S11.† The onset potential for HER in Ar saturated Na2SO4 solution is around −0.8 V, but changes to −0.6 V for CO2-saturated Na2SO4 solution. Fig. 3a is replotted vs. RHE as shown in Fig. S12† given that the pH of Ar- and CO2-saturated Na2SO4 solution are 7 and 5.6 respectively. The potential difference vs. RHE due to this change in electrolyte pH is only 0.08 V as shown in Fig. S12.† Apparently, the lower onset potential observed in CO2-saturated electrolyte cannot be explained solely by the change in bulk pH. Additionally, the current density is much higher for CO2-saturated Na2SO4 solution at higher overpotentials indicating an increased rate of HER. Alternatively, CO2R, which can occur only in CO2-saturated Na2SO4 solution, could lower the onset potential and increase the current density by creating an additional reaction channel for CO2R. In order to distinguish between currents generated by HER and CO2R, faradaic efficiencies (FE) for both reactions are measured in various Ar- and CO2-saturated electrolytes at an applied potential of −1.2 V vs. Ag/AgCl. As shown in Fig. 3b, the measured FE of CO for Ar- and CO2-saturated Na2SO4 solutions are only 0% and 18.1% respectively. This indicates that the ∼5-fold increase in current density observed for CO2-saturated Na2SO4 electrolyte cannot be solely attributed to CO2R. Rather, CO2 purging significantly enhances the rate of HER as measured here by direct detection of H2.
These measurements demonstrate that the presence of HCO3− and CO32− formed from CO2 purging significantly improves HER kinetics. This enhancement cannot be simply explained by the change in bulk pH. Instead, there are two possible mechanisms for HER enhancement by CO2 purging: the first is interfacial pH buffering, and the second is the direct reduction of H2CO3/HCO3− to produce H2. Considering the first mechanism, as shown by eqn (1)–(4), when water serves as the primary proton donor, the resulting OH− anion produced at the Au electrode diffuses out of the Stern layer and reacts with H2CO3/HCO3−/CO2. Consequently, the presence of H2CO3/HCO3−/CO2 may help avoid the accumulation of OH− by serving as a buffer of the interfacial pH during active HER. Considering the second mechanism, as shown by eqn (5)–(6), H2CO3 and HCO3− can also directly serve as the proton donor for HER. This mechanism does not require pH buffering since OH− is not directly generated as an intermediate in this reaction. However, OH− can be indirectly produced during this process by subsequent equilibration of HCO3− and CO32− with water (i.e., reverse reaction of eqn. (2)–(4)). Because these two pathways will eventually achieve the same equilibrium distribution of interfacial species regardless of the actual proton source for HER, these mechanisms can only be distinguished by monitoring surface speciation as the electrochemical double layer is pushed out of equilibrium during active HER.
| 2H2O + 2e− ⇌ H2 + 2OH− | (1) |
| HCO3− + OH− ⇌ H2O + CO32− | (2) |
| H2CO3 + OH− ⇌ H2O + HCO3− | (3) |
| CO2 + OH− ⇌ HCO3− | (4) |
| 2H2CO3 + 2e− ⇌ H2 + 2HCO3− | (5) |
| 2HCO−3 + 2e− ⇌ H2 + 2CO32− | (6) |
Above we showed the interfacial water spectrum as a function of potential in Ar- and CO2-purged Na2SO4 electrolyte. In Ar-purged Na2SO4 electrolyte, we observe the production of surface OH− resulting from direct water reduction (Fig. 1). In contrast, OH− is not observed in CO2-purged Na2SO4 electrolyte; instead, we detect the presence of CO32− in the electrochemical double layer during HER (Fig. 2). In addition to the loss of surface OH−, CO2 purging results in an approximately 5-fold increase in the rate of HER (Fig. 3). It is not initially clear, whether the loss of OH− and the associated rate increase for HER is the result of pH buffering by H2CO3/HCO3−/CO2 (eqn (2)–(4)) or if H2CO3/HCO3− serve as the primary proton source for H2 production (eqn (5) and (6)). We now consider HER in 0.1 M NaHCO3 electrolyte, which is commonly used for CO2R. Understanding the mechanism of H2 production in this electrolyte is important, where HER is in direct competition with the FE for CO2R. Below we show that in this solution HER is governed by two potential-dependent regimes based on direct reduction of HCO3− and water, respectively. Fig. 3b shows the kinetics of HER and CO2R in Ar- and CO2-purged 0.1 M NaHCO3. In the case of Ar-purged electrolyte, we observe almost unity FE for H2 indicating a negligible rate of CO2R, and this finding is also consistent with a recent study by Dunwell et al.59 With CO2-purging the yield of CO increases and the FE for CO2R reaches approximately 60%. Focusing on Ar-purged NaHCO3 electrolyte, where only HER occurs, we find that the H2 yield is almost 3-fold higher compared to Ar-purged Na2SO4 electrolyte. Again, this enhancement can be either the result of interfacial pH buffering by the much higher concentration of HCO3− or the result of direct reduction of HCO3− to produce H2. Below we show that the actual enhancement is a potential-dependent combination of these two effects.
Fig. 4a shows the water spectra taken in Ar-purged NaHCO3 solution as a function of potential. At potentials more positive than −0.8 V, these spectra are quite similar to those of CO2-saturated Na2SO4 solution shown in Fig. 2a. As more negative potential is applied, we observe the appearance of a strong feature at 3400 cm−1. Note that this peak is assigned to CO32− since this feature only appears at the onset potential for HER despite the high concentration of HCO3− in this electrolyte. When the applied potential reaches −0.9 V the OH− peak at 3650 cm−1 also appears. The interfacial product distribution has negligible contribution from CO2R because the FE of CO is only about 1.2% (Fig. 3b) in Ar-saturated NaHCO3 solution. From these observations we conclude that surface OH− is produced either by direct water reduction or by HCO3− reduction to CO32− followed by equilibration with water to produce OH− and HCO3− (eqn (2)). If the latter is true, we would also see the OH− peak at 3650 cm−1 for Na2CO3 solution (0.1 M) since the CO32− concentration in Na2CO3 solution is much higher than in NaHCO3 solution (0.1 M). As shown in Fig. S13,† there is no apparent peak for Na2CO3 solution. This indicates that the surface OH− observed in Fig. 4a is produced by direct water reduction. Consequently, the observation of the surface OH− in NaHCO3 solution provides strong evidence that the proton source of HER in CO2-saturated Na2SO4 solution is not water. As demonstrated above, during electrochemical water reduction, surface OH− is a transient, non-equilibrium species that resides at the very first layer of the electrode surface. Due to the repulsive Coulomb force, OH− is repelled by the negatively charged Au electrode and is expected to diffuse rapidly into the electric double layer. Based on our previous works, the electric field for 0.1 M NaHCO3 at the electrode surface at −1.0 V is as high as ∼4 × 107 V cm−1.40,65 The intense electric field represents a strong driving force for free OH− to desorb and diffuse away from the surface. Outside of the outer Helmholtz plane (OHP), OH− will form H-bond with surrounding water, and we can no longer observe the characteristic stretch of free OH− at 3650 cm−1. Consequently, we only detect free OH− generated by water reduction inside the OHP, while OH− produced indirectly viaeqn (2) and (3) occurs in the diffuse layer and beyond and will not be detected as free OH− as shown in Fig. S13.† Therefore, without sufficient hydroxide ions generated at the surface, the hydroxide peak around 3650 cm−1 is not observed for the CO2-saturated Na2SO4 solution. If buffering by the HCO3−/H2CO3 equilibrium (eqn (2) and (3)) for the CO2-saturated Na2SO4 solution is sufficient to consume all the surface OH− prior to desorption we would not be able to detect the free OH− peak for 0.1 M NaHCO3 solution (Fig. 4) since the HCO3− concentration is much higher than HCO3−/H2CO3 in Na2SO4 solution (Fig. 2). The concentrations of H2CO3 and HCO3− in CO2-saturated Na2SO4 solution are only ∼5.6 × 10−5 M, 4.9 × 10−6 M respectively. The actual concentration of HCO3− in Ar-purged 0.1 M NaHCO3 is close to the 0.1 M formal concentration, which is several orders higher than HCO3−/H2CO3 in Na2SO4 solution. As shown in Fig. 4a, when the applied potential reaches −0.9 V, the surface OH− peak around 3650 cm−1 appears and becomes more intense as increasingly negative potentials are applied. This suggests that even in 0.1 M NaHCO3 solution, HCO3− is not sufficient to consume all the surface OH− generated by water reduction, not to mention Na2SO4 solution with much lower HCO3−/H2CO3 concentrations. Thus, the complete disappearance of the hydroxide peak for the CO2-saturated Na2SO4 solution indicates that the proton source switched from water to H2CO3 and HCO3− otherwise the hydroxide peak should be observed. As pointed out in a recent work by Liu et al.52 the equilibrium between HCO3− and CO32− should be established within microseconds, while it takes on the order of 10 s to reach equilibrium for the CO2/HCO3− buffer at pH 5.35–7.35 due to the slow hydration of CO2. In our work, the relatively fast buffer by HCO3− is not sufficient to consume the surface OH− (Fig. 1a). The much slower equilibration for the CO2/HCO3− buffer certainly cannot be responsible for the complete disappearance of the surface OH− peak of the CO2-saturated Na2SO4 solution. All these observations suggest that in CO2-saturated Na2SO4 solution the proton source is HCO3−/H2CO3 rather than H2O.
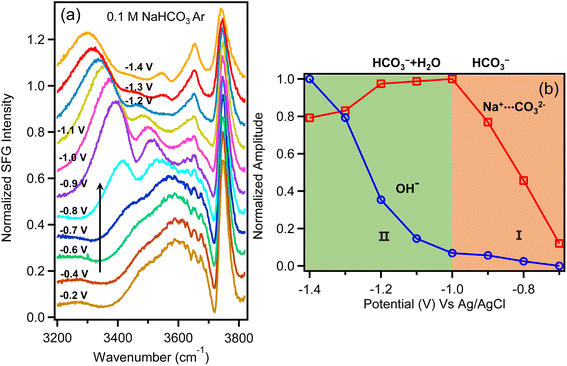 | ||
| Fig. 4 (a) VSFG spectra of NaHCO3 (0.1 M) purged with Ar as a function of potential vs. Ag/AgCl. (b). Relative amplitudes of CO32− feature at approximately 3400 cm−1 (red curve) and OH− feature at 3640 cm−1 (blue curve) as a function of potential. The amplitudes are extracted from fitting results of the curves (Table S2†). Amplitudes of both peaks are independently normalized to their maximum intensity observed in the potential range between −0.2 to −1.4 V. | ||
As for NaHCO3 solution, to resolve between the two above mentioned mechanisms, we plot the intensity of the features corresponding to OH− and CO32− as a function of potential (Fig. 4b), showing two regions with different trends. In the potential window between −0.8 V and −1.0 V, the intensity of OH− remains low while CO32− increases significantly with applied potential. In contrast, at higher overpotential beyond −1.0 V, the intensity of OH− increases rapidly while the intensity of CO32− decreases. The distinct behaviour in these two regions suggests that the mechanism of HER is potential dependent with −1.2 V as the approximate crossover point where the intensity of OH− becomes the dominate feature in the interfacial water spectrum.
Considering the relative intensities of these two features, we evaluate the two possible mechanisms for enhanced HER in NaHCO3 electrolyte. First we consider the pH buffering mechanism. As long as the consumption of OH−viaeqn (2) and (3) are faster than OH− production, no experimental technique is expected to unequivocally prove the presence of direct reduction of bicarbonate to carbonate. But as we demonstrated above, the consumption of OH−viaeqn (2) and (3) is slower than OH− production, otherwise the OH− peak should be observed in Fig. 2a in CO2-saturated Na2SO4 solution and the OH− peak should not be observed for Ar-saturated 0.1 M NaHCO3 solution in Fig. 4a. If we assume that enhanced HER is the result of interfacial pH buffering, then as the potential is increased and the buffering kinetics of HCO3− can no longer keep up with the rate of water reduction, we expect to see CO32− and OH− features grow in intensity together and saturate as the interface becomes increasingly basic. However, this is not what we observe. Instead, we observe a crossover at approximately −1.2 V where CO32− has decreased in intensity as the surface-bound OH− feature has increased significantly. This cannot be explained by an equilibrium reaction where the concentration of OH− and CO32− would trend in the same direction. Fig. 4b shows a measurable decrease in the intensity of the carbonate peak. If this peak is the result of buffering viaeqn (2) and (3) this peak should increase as potential becomes more negative, and eventually remain constant at the point where all interfacial bicarbonate has been converted to carbonate. However, the decrease of this peak cannot be a result of CO32− production viaeqn (2), where this equilibrium should cause the concentration of CO32− to increase monotonically and then saturate. Rather the decrease in intensity of this feature at potentials beyond −1.1 V is best explained as a crossover from bicarbonate reduction at more positive applied potential to primarily water reduction at more negative applied potential. Thus, Fig. 4b indicates a shift in the non-equilibrium interfacial product distribution as the proton source for HER switches from primarily HCO3− at low overpotential (CO32− product) to H2O at higher overpotential (OH− product). This picture is also consistent with a recent study by Koper and co-workers where kinetic measurements with a rotating ring disk electrode indicate that at increasing overpotential, the primary proton donor for HER in CO2 saturated NaHCO3 electrolyte switches from H2CO3 to HCO3− and finally to water at increasing overpotential.15 They predicted the crossover from HCO3− to water occurs at approximately −0.5 V vs. RHE, which closely matches the crossover point shown in Fig. 4b (note that −0.5 V vs. RHE is equivalent to −1.1 V vs. Ag/AgCl for Ar-saturated NaHCO3 electrolyte where pH is 8.3). Based on these direct measurements of the Au/electrolyte interface, we conclude that the significantly enhanced rate of HER in NaHCO3 electrolyte is the result of HCO3− reduction for potentials between −0.8 V to −1.0 V. A transition occurs between −1.0 V and −1.2 V where direct water reduction begins to occur. At potentials beyond −1.2 V HER kinetics appear to be dominated by direct water reduction, and any enhancement by HCO3− on the rate of HER is assumed to be the result of interfacial pH buffering. However, as shown by the crossover in Fig. 4b, pH buffering by HCO3− (eqn (2)) is unable to keep up with the kinetics of water reduction as the overpotential increases. This finding is also consistent with previous studies by Wuttig et al. who have shown that that the CO2/HCO3−/H2CO3 equilibrium suffers from slow kinetics, making it a sluggish buffer to maintain the surface pH.66
Overall, these observations reflect a competition between the acidity of the various potential proton donors and their potential dependent interfacial concentrations. Specifically, the pKa values of HCO3− and H2CO3 are 10.32 and 6.37, respectively, while water has a much higher pKa of 14. Although water (55 M) is 550 times more concentrated than HCO3− in 0.1 M NaHCO3 electrolyte, HCO3− is approximately 4 orders of magnitude more acidic. Thus HCO3− is observed to be the major proton donor at low overpotential where the electric field is not so strong as to completely repel negatively charged HCO3− anions from the interface. However, as overpotential increases, direct reduction of water becomes facile, and the increasingly negative electric field repels anions HCO3− from the interface. As this happens, the availability of HCO3− cannot keep up with the increasing rate of HER, and water eventually replaces HCO3− as the primary proton donor. The OH− produced by water reduction subsequently diffuses out of the Helmholtz plane where it is partially buffered by reaction with HCO3− and/or H2CO3. A similar effect also controls the kinetics for CO2-purged Na2SO4 (Fig. 2), although in this case the bulk solution pH is more acidic (pH = 5.6) compared to Ar-purged NaHCO3 (pH = 8.3). Due to the decrease in pH, the HCO3−/H2CO3 equilibrium is shifted toward H2CO3. Because H2CO3 is more acidic than HCO3− and does not carry a negative charge, the direct reduction of H2CO3 may preclude water reduction out to even more negative potential. We hypothesize that this explains why we do not observe OH− in CO2-purged Na2SO4 electrolyte out to potentials as negative as −1.1 V (see Fig. 2a).
3 Conclusions
In summary, in situ VSFG measurements provide direct observation of the Au/electrolyte interface during HER in various electrolytes. The presence of transient surface hydroxide ions (OH−) formed at electrode surface as the product of water reduction within the outer Helmholtz plane is reported for the first time. This species is indicated by the sharp hydroxide peak at 3650 cm−1. Notably this peak is absent for the CO2-saturated solution, where instead a peak around 3400 cm−1 is observed and is assigned to the presence of CO32− in the electrochemical double layer. By directly observing the response of these features as the system is pushed away from equilibrium during active HER, we are able to differentiate between water and HCO3− reduction and to understand the related effects of proton source and interfacial pH buffering on the kinetics of HER. The results provide important insights regarding how to suppress HER during CO2R. Specifically, for electrocatalysts with high activity at low overpotential, it is not necessary to reduce interfacial water concentration since water is not the proton donor for HER. Instead, reducing H2CO3 and HCO3− while maintaining a high interfacial concentration of CO2 is most important for suppressing HER. Alternatively, for electrocatalysts, which require high overpotential for CO2R, it is critical to reduce the concentration of interfacial water to suppress HER as water serves as the primary proton source below −1.2 V.Author contributions
L. R. B. and G.-H. D. conceived the experiments. G.-H. D. conducted SFG and LSV measurements. Q. Z. helped with the SFG experiments. T. N. conducted the FE measurements. G.-H. D., Q. Z., J. R., and L. R. B. analysed the results. G.-H. D. wrote the original draft. G.-H. D., Q. Z. and L. R. B. revised the manuscript. All of the authors reviewed the manuscript.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was supported by Chemical Sciences, Geosciences and Biosciences. Division, Office of Basic Energy Sciences, Office of Science, U.S. Department of Energy under DOE Grant No. DESC0020977. Film deposition was performed at the OSU Nanotech West laboratory. We thank Prof. Heather C. Allen, Prof. Phillip L. Geissler, Prof. David T. Limmer, and Dr Amro Dodin for helpful discussions.Notes and references
- G. Zhao, K. Rui, S. X. Dou and W. Sun, Adv. Funct. Mater., 2018, 28, 1803291 CrossRef.
- N. Dubouis and A. Grimaud, Chem. Sci., 2019, 10, 9165–9181 RSC.
- J. Zhu, L. Hu, P. Zhao, L. Y. S. Lee and K.-Y. Wong, Chem. Rev., 2020, 120, 851–918 CrossRef CAS PubMed.
- Y. Chen, C. W. Li and M. W. Kanan, J. Am. Chem. Soc., 2012, 134, 19969–19972 CrossRef CAS PubMed.
- Y. Hori, I. Takahashi, O. Koga and N. Hoshi, J. Mol. Catal. A: Chem., 2003, 199, 39–47 CrossRef CAS.
- X. Feng, K. Jiang, S. Fan and M. W. Kanan, J. Am. Chem. Soc., 2015, 137, 4606–4609 CrossRef CAS PubMed.
- J. Rosen, G. S. Hutchings, Q. Lu, S. Rivera, Y. Zhou, D. G. Vlachos and F. Jiao, ACS Catal., 2015, 5, 4293–4299 CrossRef CAS.
- S. Zhao, R. Jin and R. Jin, ACS Energy Lett., 2018, 3, 452–462 CrossRef CAS.
- X.-G. Zhang, X. Jin, D.-Y. Wu and Z.-Q. Tian, J. Phys. Chem. C, 2018, 122, 25447–25455 CrossRef CAS.
- N. Todoroki, H. Tei, H. Tsurumaki, T. Miyakawa, T. Inoue and T. Wadayama, ACS Catal., 2019, 9, 1383–1388 CrossRef CAS.
- S. Jeong, M.-H. Choi, G. S. Jagdale, Y. Zhong, N. P. Siepser, Y. Wang, X. Zhan, L. A. Baker and X. Ye, J. Am. Chem. Soc., 2022, 144, 12673–12680 CrossRef CAS PubMed.
- Y. J. Lim, D. Seo, S. A. Abbas, H. Jung, A. Ma, K.-S. Lee, G. Lee, H. Lee and K. M. Nam, Adv. Sci., 2022, 9, 2201491 CrossRef CAS PubMed.
- H. Ooka, M. C. Figueiredo and M. T. M. Koper, Langmuir, 2017, 33, 9307–9313 CrossRef CAS PubMed.
- A. Goyal, G. Marcandalli, V. A. Mints and M. T. M. Koper, J. Am. Chem. Soc., 2020, 142, 4154–4161 CrossRef CAS PubMed.
- G. Marcandalli, A. Goyal and M. T. M. Koper, ACS Catal., 2021, 11, 4936–4945 CrossRef CAS PubMed.
- G. Marcandalli, K. Boterman and M. T. M. Koper, J. Catal., 2022, 405, 346–354 CrossRef CAS.
- T. Iwasita and F. C. Nart, Prog. Surf. Sci., 1997, 55, 271–340 CrossRef CAS.
- S. Nihonyanagi, S. Ye, K. Uosaki, L. Dreesen, C. Humbert, P. Thiry and A. Peremans, Surf. Sci., 2004, 573, 11–16 CrossRef CAS.
- K.-i. Ataka, T. Yotsuyanagi and M. Osawa, J. Phys. Chem., 1996, 100, 10664–10672 CrossRef CAS.
- Z.-Q. Tian, B. Ren, Y.-X. Chen, S.-Z. Zou and B.-W. Mao, J. Chem. Soc., Faraday Trans., 1996, 92, 3829–3838 RSC.
- Y. X. Chen, S. Z. Zou, K. Q. Huang and Z. Q. Tian, J. Raman Spectrosc., 1998, 29, 749–756 CrossRef CAS.
- C.-Y. Li, J.-B. Le, Y.-H. Wang, S. Chen, Z.-L. Yang, J.-F. Li, J. Cheng and Z.-Q. Tian, Nat. Mater., 2019, 18, 697–701 CrossRef CAS PubMed.
- A. Montenegro, C. Dutta, M. Mammetkuliev, H. Shi, B. Hou, D. Bhattacharyya, B. Zhao, S. B. Cronin and A. V. Benderskii, Nature, 2021, 594, 62–65 CrossRef CAS PubMed.
- C.-Y. Li, M. Chen, S. Liu, X. Lu, J. Meng, J. Yan, H. D. Abruña, G. Feng and T. Lian, Nat. Commun., 2022, 13, 5330 CrossRef CAS PubMed.
- Y.-H. Wang, S. Zheng, W.-M. Yang, R.-Y. Zhou, Q.-F. He, P. Radjenovic, J.-C. Dong, S. Li, J. Zheng, Z.-L. Yang, G. Attard, F. Pan, Z.-Q. Tian and J.-F. Li, Nature, 2021, 600, 81–85 CrossRef CAS PubMed.
- L.-f. Shen, B.-a. Lu, Y.-y. Li, J. Liu, Z.-c. Huang-fu, H. Peng, J.-y. Ye, X.-m. Qu, J.-m. Zhang, G. Li, W.-b. Cai, Y.-x. Jiang and S.-g. Sun, Angew. Chem., Int. Ed., 2020, 59, 22397–22402 CrossRef CAS PubMed.
- X. Yang, J. Nash, N. Oliveira, Y. Yan and B. Xu, Angew. Chem., Int. Ed., 2019, 58, 17718–17723 CrossRef CAS PubMed.
- Y. R. Shen and V. Ostroverkhov, Chem. Rev., 2006, 106, 1140–1154 CrossRef CAS PubMed.
- H. Arnolds and M. Bonn, Surf. Sci. Rep., 2010, 65, 45–66 CrossRef CAS.
- C. S. Tian and Y. R. Shen, Surf. Sci. Rep., 2014, 69, 105–131 CrossRef CAS.
- D. Bhattacharyya, P. E. Videla, M. Cattaneo, V. S. Batista, T. Lian and C. P. Kubiak, Chem. Sci., 2021, 12, 10131–10149 RSC.
- S. Wallentine, S. Bandaranayake, S. Biswas and L. R. Baker, J. Phys. Chem. C, 2020, 124, 8057–8064 CrossRef CAS PubMed.
- S. Wallentine, S. Bandaranayake, S. Biswas and L. R. Baker, J. Phys. Chem. Lett., 2020, 11, 8307–8313 CrossRef CAS PubMed.
- Q. Du, R. Superfine, E. Freysz and Y. Shen, Phys. Rev. Lett., 1993, 70, 2313 CrossRef CAS PubMed.
- E. A. Raymond, T. L. Tarbuck, M. G. Brown and G. L. Richmond, J. Phys. Chem. C, 2003, 107, 546–556 CrossRef CAS.
- W. Gan, D. Wu, Z. Zhang, R.-r. Feng and H.-f. Wang, J. Chem. Phys., 2006, 124, 114705 CrossRef PubMed.
- Y. Tong, F. Lapointe, M. Thämer, M. Wolf and R. K. Campen, Angew. Chem., Int. Ed., 2017, 56, 4211–4214 CrossRef CAS PubMed.
- S. Gopalakrishnan, D. Liu, H. C. Allen, M. Kuo and M. J. Shultz, Chem. Rev., 2006, 106, 1155–1175 CrossRef CAS PubMed.
- Y. R. Shen and V. Ostroverkhov, Chem. Rev., 2006, 106, 1140–1154 CrossRef CAS PubMed.
- Q. Zhu, S. K. Wallentine, G.-H. Deng, J. A. Rebstock and L. R. Baker, JACS Au, 2022, 2, 472–482 CrossRef CAS PubMed.
- W. H. Robertson, E. G. Diken, E. A. Price, J.-W. Shin and M. A. Johnson, Science, 2003, 299, 1367–1372 CrossRef CAS PubMed.
- K. R. Fega, D. S. Wilcox and D. O. R. Ben-Amotz, Appl. Spectrosc., 2012, 66, 282–288 CrossRef CAS PubMed.
- D. M. de Oliveira, A. J. Bredt, T. C. Miller, S. A. Corcelli and D. Ben-Amotz, J. Phys. Chem. C, 2021, 125, 1439–1446 CrossRef CAS PubMed.
- C. I. Drexler, T. C. Miller, B. A. Rogers, Y. C. Li, C. A. Daly, T. Yang, S. A. Corcelli and P. S. Cremer, J. Am. Chem. Soc., 2019, 141, 6930–6936 CrossRef CAS PubMed.
- J. Stefanski, C. Schmidt and S. Jahn, Phys. Chem. Chem. Phys., 2018, 20, 21629–21639 RSC.
- K. A. Becraft and G. L. Richmond, Langmuir, 2001, 17, 7721–7724 CrossRef CAS.
- R. Khatib, E. H. G. Backus, M. Bonn, M.-J. Perez-Haro, M.-P. Gaigeot and M. Sulpizi, Sci. Rep., 2016, 6, 24287 CrossRef CAS PubMed.
- Y. Wang, T. Seki, X. Liu, X. Yu, C.-C. Yu, K. F. Domke, J. Hunger, M. T. M. Koper, Y. Chen, Y. Nagata and M. Bonn, Angew. Chem., Int. Ed., 2023, e202216604 CAS.
- P. Li, Y. Jiang, Y. Hu, Y. Men, Y. Liu, W. Cai and S. Chen, Nat. Catal., 2022, 5, 900–911 CrossRef CAS.
- O. Ayemoba and A. Cuesta, ACS Appl. Mater. Interfaces, 2017, 9, 27377–27382 CrossRef CAS PubMed.
- M. C. O. Monteiro, X. Liu, B. J. L. Hagedoorn, D. D. Snabilié and M. T. M. Koper, ChemElectroChem, 2022, 9, e202101223 CAS.
- X. Liu, M. C. O. Monteiro and M. T. M. Koper, Phys. Chem. Chem. Phys., 2023, 25, 2897–2906 RSC.
- D. Liu, G. Ma, L. M. Levering and H. C. Allen, J. Phys. Chem. C, 2004, 108, 2252–2260 CrossRef CAS.
- L. Dalstein, E. Potapova and E. Tyrode, Phys. Chem. Chem. Phys., 2017, 19, 10343–10349 RSC.
- K. C. Jena and D. K. Hore, J. Phys. Chem. C, 2009, 113, 15364–15372 CrossRef CAS.
- J. Kim and P. S. Cremer, J. Am. Chem. Soc., 2000, 122, 12371–12372 CrossRef CAS.
- K. Arihara, F. Kitamura, T. Ohsaka and K. Tokuda, J. Electroanal. Chem., 2001, 510, 128–135 CrossRef CAS.
- M. Dunwell, X. Yang, B. P. Setzler, J. Anibal, Y. Yan and B. Xu, ACS Catal., 2018, 8, 3999–4008 CrossRef CAS.
- M. Dunwell, Q. Lu, J. M. Heyes, J. Rosen, J. G. Chen, Y. Yan, F. Jiao and B. Xu, J. Am. Chem. Soc., 2017, 139, 3774–3783 CrossRef CAS PubMed.
- W. Deng, T. Yuan, S. Chen, H. Li, C. Hu, H. Dong, B. Wu, T. Wang, J. Li, G. A. Ozin and J. Gong, Fundam. Res., 2021, 1, 432–438 CrossRef CAS.
- Y.-C. Wen, S. Zha, X. Liu, S. Yang, P. Guo, G. Shi, H. Fang, Y. R. Shen and C. Tian, Phys. Rev. Lett., 2016, 116, 016101 CrossRef PubMed.
- P. E. Ohno, H.-f. Wang and F. M. Geiger, Nat. Commun., 2017, 8, 1032 CrossRef PubMed.
- P. E. Ohno, H.-f. Wang, F. Paesani, J. L. Skinner and F. M. Geiger, J. Phys. Chem. C, 2018, 122, 4457–4464 CrossRef CAS PubMed.
- K. C. Jena, P. A. Covert and D. K. Hore, J. Phys. Chem. Lett., 2011, 2, 1056–1061 CrossRef CAS.
- J. A. Rebstock, Q. Zhu and L. R. Baker, Chem. Sci., 2022, 13, 7634–7643 RSC.
- A. Wuttig, Y. Yoon, J. Ryu and Y. Surendranath, J. Am. Chem. Soc., 2017, 139, 17109–17113 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: (1) Sum frequency generation setup, electrochemical and Faradaic efficiency measurements. (2)–(4). Control experiments (5) relative concentrations of H2CO3, HCO3− and CO32− as a function of pH (6) LSV curve vs. RHE. (7) Fitting results. See DOI: https://doi.org/10.1039/d3sc00897e |
| This journal is © The Royal Society of Chemistry 2023 |