Open Access Article
Open Access ArticleWater sensitivity of heteroepitaxial Cu-MOF films: dissolution and re-crystallization of 3D-oriented MOF superstructures†
Lea A.
Brandner
 a,
Mercedes
Linares-Moreau
a,
Mercedes
Linares-Moreau
 a,
Guojun
Zhou
b,
Heinz
Amenitsch
a,
Guojun
Zhou
b,
Heinz
Amenitsch
 c,
Simone
Dal Zilio
c,
Simone
Dal Zilio
 d,
Zhehao
Huang
d,
Zhehao
Huang
 b,
Christian
Doonan
b,
Christian
Doonan
 *e and
Paolo
Falcaro
*e and
Paolo
Falcaro
 *a
*a
aInstitute of Physical and Theoretical Chemistry, Graz University of Technology, 8010 Graz, Austria. E-mail: paolo.falcaro@tugraz.at
bDepartment of Materials and Environmental Chemistry, Stockholm University, Stockholm SE-106 91, Sweden
cInstitute of Inorganic Chemistry, Graz University of Technology, 8010 Graz, Austria
dCNR-IOM – Istituto Officina dei Materiali, SS 14, km 163.5, Basovizza, Trieste, 34149, Italy
eDepartment of Chemistry, The University of Adelaide, Adelaide, South Australia 5005, Australia. E-mail: christian.doonan@adelaide.edu.au
First published on 17th October 2023
Abstract
3D-oriented metal–organic framework (MOF) films and patterns have recently emerged as promising platforms for sensing and photonic applications. These oriented polycrystalline materials are typically prepared by heteroepitaxial growth from aligned inorganic nanostructures and display anisotropic functional properties, such as guest molecule alignment and polarized fluorescence. However, to identify suitable conditions for the integration of these 3D-oriented MOF superstructures into functional devices, the effect of water (gaseous and liquid) on different frameworks should be determined. We note that the hydrolytic stability of these heteroepitaxially grown MOF films is currently unexplored. In this work, we present an in-depth analysis of the structural evolution of aligned 2D and 3D Cu-based MOFs grown from Cu(OH)2 coatings. Specifically, 3D-oriented Cu2L2 and Cu2L2DABCO films (L = 1,4-benzenedicarboxylate, BDC; biphenyl-4,4-dicarboxylate, BPDC; DABCO = 1,4-diazabicyclo[2.2.2]octane) were exposed to 50% relative humidity (RH), 80% RH and liquid water. The combined use of X-ray diffraction, infrared spectroscopy, and scanning electron microscopy shows that the sensitivity towards humid environments critically depends on the presence of the DABCO pillar ligand. While oriented films of 2D MOF layers stay intact upon exposure to all levels of humidity, hydrolysis of Cu2L2DABCO is observed. In addition, we report that in environments with high water content, 3D-oriented Cu2(BDC)2DABCO recrystallizes as 3D-oriented Cu2(BDC)2. The heteroepitaxial MOF-to-MOF transformation mechanism was studied with in situ synchrotron experiments, time-resolved AFM measurements, and electron diffraction. These findings provide valuable information on the stability of oriented MOF films for their application in functional devices and highlight the potential for the fabrication of 3D-oriented superstructures via MOF-to-MOF transformations.
Introduction
Metal–organic frameworks (MOFs) are a class of extended materials consisting of inorganic nodes and multitopic organic ligands.1,2 By carefully selecting their organic and inorganic components the physical and structural properties of MOFs can be customized.3 The control over chemical and structural properties enables the preparation of MOFs with relevant electric,4 magnetic5 and optical6 functionalities. Furthermore, advances in fabrication protocols have enabled the preparation of polycrystalline MOF thin films and patterns7–10 and the potential to exploit such MOF properties in devices.11–13 Recent progress in the fabrication of MOF devices focused on the control over directional functional properties (i.e. anisotropic properties); for this, fabrication protocols affording precise alignment of individual porous crystals are required.14,15 In this context, precisely oriented polycrystalline systems have been recently reported.16–18 In particular, 3D-oriented MOF films and patterns have been prepared from ceramic nanostructures with uniform crystallographic orientation.19–21 In this process, pre-aligned Cu(OH)2 nanobelts (NBs) are deposited on a solid support; in the presence of alcoholic solutions with suitable dicarboxylic linkers,22 the NBs act as source of Cu(II) and sacrificial substrate for the epitaxial growth of the MOF coating.23 The resulting 3D-oriented MOF films, and more recently 3D-oriented micropatterns, exhibit anisotropy-derived properties, such as guest molecule alignment, polarized fluorescence, or polarization-dependent plasmon-resonance, among others.19,21,24–26An aspect of MOF devices that requires further study is how the material structure changes under typical working conditions, e.g. humidity. We hypothesized that the exposure of heteroepitaxially oriented MOF films to humidity could result in chemical and structural changes. In the literature, several Cu-based MOFs were reported to show sensitivity towards water, resulting in degradation of the crystal structure and loss of functional properties.27–33 For archetypical HKUST-1 (Cu3(BTC)2, BTC = 1,3,5-benzene tricarboxylate),34 it has been reported that a relative humidity (RH) above 50% RH results in the decomposition of the framework.35 Interestingly, for other MOFs (e.g. MOF-177), the interaction with water molecules triggers the re-organization of the unit cell into new crystalline phases.36 Recently, Fu and co-workers reported the epitaxial recrystallization from a single-crystal MOF to an oriented MOF superstructure (i.e. an ordered polycrystalline structure).37 Inspired by these works, we decided to examine the effect of water on heteroepitaxially grown Cu-MOF films. We examined two aspects: (i) the hydrolytic stability of different heteroepitaxially grown Cu-MOF films and (ii) the potential rearrangement of 3D-oriented MOF films into different ordered polycrystalline structures. In particular, in this study, we assess the influence of water on four different oriented Cu-MOF films (Cu2L2, Cu2L2DABCO, (L = 1,4-benzenedicarboxylate, BDC; biphenyl-4,4-dicarboxylate, BPDC) exposed to varying levels of humidity (Fig. 1). Heteroepitaxial MOF films were grown from sacrificial Cu(OH)2 oriented nanostructures and exposed to 50% RH, 80% RH, and liquid water. Changes in crystallinity, chemical composition, and morphology were monitored over a period of 7 days, using X-ray diffraction (XRD), Fourier-transform infrared spectroscopy (FTIR), and scanning electron microscopy (SEM). While the 2D-frameworks Cu2(BDC)2 and Cu2(BPDC)2 maintained their physical and chemical properties in the presence of water, we observed the hydrolysis and concomitant transformation from Cu2(BDC)2DABCO films to Cu2BDC2, as well as structural degradation of Cu2(BPDC)2DABCO starting from 50% RH. To elucidate the timescale and mechanism of the MOF transformation process, we performed time-resolved grazing incidence wide-angle X-ray scattering (GIWAXS) experiments using synchrotron radiation. These results were also correlated to time-resolved atomic force microscopy (AFM) measurements to study the corresponding changes in morphology and electron diffraction for structural changes. The collected data reveal that, at room-temperature, the MOF transformation process starts with the dissolution of the MOF crystals, then the initial 3D-oriented polycrystalline film undergoes heteroepitaxial recrystallization into a distinct 3D-oriented polycrystalline superstructure. We posit that this study (i) will help the progress of oriented MOF films towards micro- and opto-electronic devices and (ii) will advance the knowledge on ordered polycrystalline MOF superstructures.
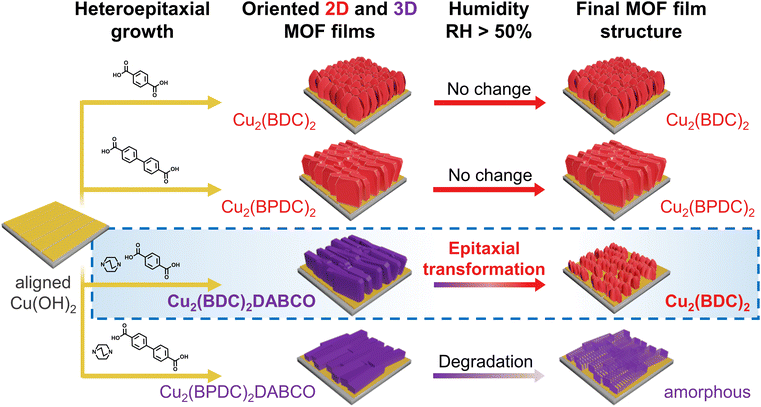 | ||
| Fig. 1 Schematic illustration of the structural evolution of heteroepitaxially grown, oriented MOF films under humid conditions. | ||
Experimental
Materials
1,4-Benzenedicarboxylic acid (H2BDC), 1,4-diazabicyclo[2.2.2]octane (DABCO), absolute ethanol (EtOH, 99.7%), methanol (MeOH), dichloromethane (DCM), and acetic acid (99%) were purchased from Sigma Aldrich. Biphenyl-4,4-dicarboxylic acid (H2BPDC) was bought from Tokyo Chemical Industry Co. Ltd. Cu(OH)2 nanobelts (NBs) were prepared and deposited on Si pieces (1.5 × 1.5 cm2) according to previously reported procedures.19,23 All reagents were used without further purification.Characterization
Powder X-ray diffraction patterns were obtained using a Rigaku Smart Lab instrument with CuKα radiation (λ = 0.154 nm). To assess the crystalline orientation of the MOFs films, intensity profiles of selected reflections were recorded by azimuthal angle scans in the in-plane XRD configuration. FTIR spectra were recorded in transmission mode using a Bruker ALPHA spectrometer in the 4000–400 cm−1 range (average over 32 scans, 4 cm−1 spectral resolution). Sample morphologies were imaged by using a ZEISS Sigma 300 FEG scanning electron microscope, equipped with standard SE2 and INLENS detectors. Time-resolved grazing incidence wide-angle X-ray scattering (GIWAXS) measurements were performed at the Austrian SAXS beamline at Elettra Sincrotrone, Trieste, Italy.38 An X-ray beam with a wavelength of 0.077 nm and a photon energy of 16 keV was used. 2D GIWAXS patterns were recorded with a Pilatus3 1 M detector at a grazing angle of 1°. Silver behenate was used to calibrate the angular scale of the detector. The intensity of the scattered photons was recorded and diffraction patterns parallel (qy, in-plane cut) and perpendicular (qz, out-of-plane cut) to the sample surface considered for data analysis. Samples were measured in a q-range of 0.83 < q < 31 nm−1. The relative humidity (RH) during the SAXS experiments was controlled with a cylindrical metal chamber, equipped with two Kapton windows for passage of the incident and scattered beam (Fig. S1†).39 RH values were set by mixing different proportions of dry (<3% RH) and humid (>95% RH) air. A humidity sensor combined with a proportional integral derivative (PID) controller was used to tune the humidity inside the chamber. For the experiments performed in H2O, a customized 3D-printed chemical cell, equipped with two Kapton windows and solvent inlet was used (Fig. S1†).40 For azimuthal angle scans, samples were mounted on a motorized stage and rotated along the axis normal to the surface. All GIWAXS measurements were conducted at a constant temperature of 25 ± 1 °C. More experimental details are given in the ESI.† Time-resolved AFM measurements were performed using an Oxford Instruments Asylum Cypher ES in blueDrive tapping mode, equipped with BudgetSensors Tap300 silicon cantilevers. The measurements were performed inside a closed environmental control cell. Initially, a flow of dry Ar was used for calibration of the measurement parameters. Then, the dry Ar flow was exchanged with a humid Ar flow (80% RH measured at the exit of the chamber). Scans were collected continuously at a scan rate of 9 Hz. When comparing the GIWAXS and the AFM setups, it is worth noting that the different setups (e.g. different fluxes and volume of humid air in the reaction chambers) resulted in slightly different kinetics. Transmission electron microscope (TEM) data was obtained on a JEOL JEM2100 microscope, which was operated at 200 kV (Cs 1.0 mm, point resolution 0.23 nm). Images were recorded with a Gatan Orius 833 CCD camera (resolution 2048 × 2048 pixels, pixel size 7.4 μm). Electron diffraction patterns were recorded with a Timepix pixel detector QTPX-262k (512 × 512 pixels, pixel size 55 μm, Amsterdam Sci. Ins.).Results and discussion
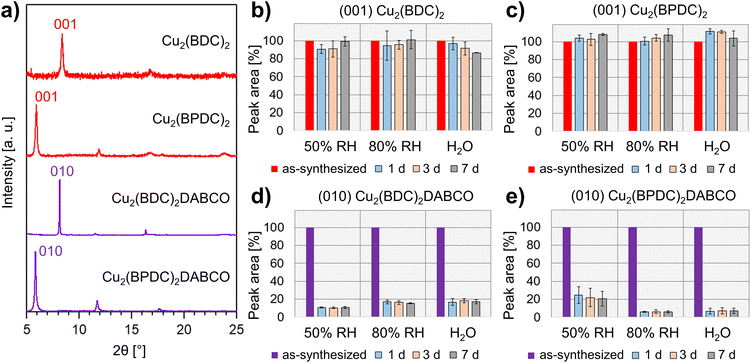
Understanding structural changes in 3D-oriented MOF films under various environmental conditions, particularly different RH, is crucial for the successful implementation of these materials into functional devices. For example, optical components that are susceptible to change of properties because of humidity require encasing. To evaluate potential humidity values for our initial tests, we note that RH = 50% aligns with the upper limit of relative humidity recommended by the FDA for laboratory settings, and falls within the optimal range of 30–60% RH, which is considered ideal for human health in indoor environments.41 Therefore, we chose 50% RH as a reasonable environmental condition to simulate the handling of these components and their indoor use. In order to further understand the interaction between water and the heteroepitaxially grown MOF films, including potential degradation processes, we also explored higher humidity values including 80% RH and liquid water as extreme testing conditions. The oriented MOF films were prepared from aligned Cu(OH)2 NBs on Si substrates via previously reported literature protocols and outlined as follows.19–21 For the synthesis of Cu2(BDC)2 and Cu2(BPDC)2 films, oriented Cu(OH)2 NBs substrates were immersed in ethanol/water mixtures, containing H2BDC or H2BPDC, and reacted for 30 min (H2BDC) and 1 h (H2BPDC) at room temperature. Cu2(BDC)2DABCO and Cu2(BPDC)2DABCO films were grown from Cu(OH)2 templates in methanolic solutions of the corresponding organic linker and DABCO for 1 h (70 °C, H2BDC, DABCO) and 1.5 h (60 °C, H2BPDC, DABCO), respectively. For each type of heteroepitaxially grown MOF film, a set of triplicate samples was placed in humidity-controlled chambers set at 50% RH and 80% RH, and immersed in DI water. To assess changes in crystallinity, chemical composition and morphology, we examined the MOF films by using X-ray diffraction (XRD), Fourier-transform infrared spectroscopy (FTIR), and scanning electron microscopy (SEM). After characterization of the as-synthesized samples, the films were analysed after 1, 3, and 7 days. The recorded diffraction patterns were baseline-corrected and the dominant MOF reflection integrated. To capture the structural change for each film and minimize fluctuations related to variations in the mass of crystalline material across samples, each sample was compared to itself: the peak area of the as-synthesized sample was set to 100% and the change in signal monitored over the 7 d. Average and standard deviation of the recorded peak areas in % were calculated for each condition from the set of triplicate samples.For the as-synthesized MOF films, diffraction plots (Fig. 2a) were consistent with those reported in the literature. Specifically, we observed diffraction peaks at different angles: 8.38°, assigned to the (001) plane of Cu2(BDC)2;19 5.93°, assigned to the (001) plane of Cu2(BPDC)2;19 8.16°, assigned to the (010) plane of Cu2(BDC)2DABCO;20,21 and 5.85°, assigned to the (010) plane of Cu2(BPDC)2DABCO.20 Azimuthal intensity in-plane XRD-scans confirmed the successful growth of the oriented MOF films (Fig. S2†). Reflections of residual Cu(OH)2 at 16.74° and 23.83°, assigned to the (020) and (021) planes, were also observed for Cu2(BDC)2, Cu2(BPDC)2, and Cu2(BDC)2DABCO in the out-of-plane XRD patterns. For Cu2(BPDC)2DABCO, these signals were absent in the diffraction pattern, indicating full consumption of the sacrificial Cu(OH)2 NBs. Next, we analysed the diffraction patterns of the films exposed to the different RH values. For the heteroepitaxially grown 2D frameworks Cu2(BDC)2 and Cu2(BDPC)2, the peak areas associated with the respective (001) reflections changed within 10–15% of the original signals under all tested conditions (Fig. 2b, c and S3†). It is noteworthy that the error bars overlap for all data points; this excludes significant changes in crystallinity with increasing RH and exposure time. In contrast, we found a significant decrease in signal intensity for Cu2(BDC)2DABCO and Cu2(BPDC)2DABCO films after exposure to the different humid environments (Fig. 2d and e). For Cu2(BDC)2DABCO, the peak areas assigned to the (010) reflection decreased by 90% after exposure to 50% RH for 1 d. In addition, the peak position shifts from 8.16° to 8.27° (Fig. S4†). Interestingly, at 80% RH and in water, the peak area stabilized at approximately 15–18% of the original value, but displayed an even larger shift to 8.37° (Fig. S5†), which is consistent with the (001) reflection of Cu2(BDC)2. It was also observed that for Cu2(BDC)2DABCO films, the diffraction signals associated with the (021) plane of the remaining Cu(OH)2, exhibited similar intensity at 50% RH, but decreased slightly at 80% RH and in water (Fig. S5†). For oriented Cu2(BPDC)2DABCO films, the peak areas attributed to the signal of the (010) reflection dropped by 75% after the first day under 50% RH, followed by another intensity decrease down to 20% of the original peak area after 7 d (Fig. S6†). At 80% RH and in water the integrated peak areas dropped by over 90% within 24 h, indicating almost complete loss of crystallinity (Fig. S6†).
Next, we analysed the chemical changes in the different MOF films by monitoring characteristic vibrational modes over time at the different RH values. We focused on the asymmetric and symmetric carboxylate stretching modes and compared to the pristine samples and previously reported spectra23,24,42 to determine the coordination and protonation state of the carboxylic ligands. For the as-synthesized Cu2(BDC)2 films, the asymmetric and symmetric carboxylate stretching modes were observed at 1579 cm−1 and 1398 cm−1, respectively. For pristine Cu2(BPDC)2, the corresponding vibrational bands appeared at 1583 cm−1 and 1423 cm−1. In the case of Cu2(BDC)2DABCO, we recorded absorption bands at 1630 cm−1, assigned to νasymm(COO−), and overlapping signals at 1433 cm−1 and 1394 cm−1, associated with the νsymm(COO−) vibration and a benzene ring mode.27 As-synthesized Cu2(BPDC)2DABCO films exhibited an asymmetric carboxylate stretching mode at 1623 cm−1 and a broad band at 1406 cm−1, assigned to νsymm(COO−).
During the exposure period of 7 days, the vibrational modes of Cu2(BDC)2 and Cu2(BPDC)2 preserved both intensity and position; no additional bands appeared in the spectra for all tested conditions (Fig. S7†). These data indicate the negligible influence of water on the chemical composition of the samples. For the Cu2(BDC)2DABCO films, the IR spectra revealed several changes induced by humid conditions. After exposure to 50% RH for 1 d, the carboxylate modes lost approximately half their intensity, indicating a substantial degradation of the sample. Additionally, a new vibrational band appeared at 1579 cm−1; this mode could be ascribed to asymmetric carboxylate stretching of Cu2(BDC)2 (Fig. S8†). Vibrational modes attributed to the DABCO pillar ligand in the region at 2960 cm−1 (in-phase νasymm(CH2)), at 1318 cm−1 (in-phase γtwist(CH2), in-phase νasymm(NC3)) and at 1060 cm−1 (in-phase νasymm(NC3), νasymm(C–C), γtwist(CH2)) were also observed43 and showed only a minimal change in intensity when exposed to 50% RH over time (Fig. S9†). Similar changes in the spectra were observed for Cu2(BDC)2DABCO films exposed to 80% RH and water; however, in these samples, the initial asymmetric COO− band disappeared completely, resulting in carboxylate modes consistent with pure Cu2(BDC)2 MOF (Fig. S8†). Additionally, the vibrational bands attributed to DABCO disappeared in water, indicating the removal of the pillar ligand from the framework (Fig. S9†). At 80% RH, the intensity of the DABCO-related band at 1060 cm−1 decreased over time and Raman maps suggest that DABCO molecules rearrange and aggregate in different regions of the film surface (Fig. S10†). For Cu2(BPDC)2DABCO films, a similar drop in intensity of the asymmetric carboxylate stretching vibration was observed within the first 24 h at 50% RH. At the same time, new bands at 1586 cm−1 and 1540 cm−1 emerged, matching the vibrational modes of Cu2(BPDC)2 (Fig. S11†). At 80% RH and in water, the asymmetric COO− mode of the initial framework disappeared completely and only the vibrational signals similar to Cu2(BPDC)2 remained (Fig. S11†). While the in-phase νasymm(CH2) mode of the DABCO shows little intensity for as-synthesized and exposed films, the vibrational bands at 1318 cm−1 and 1060 cm−1 are stable at 50% RH, but disappear at high water loading, similar to the observations for Cu2(BDC)2DABCO (Fig. S12†).
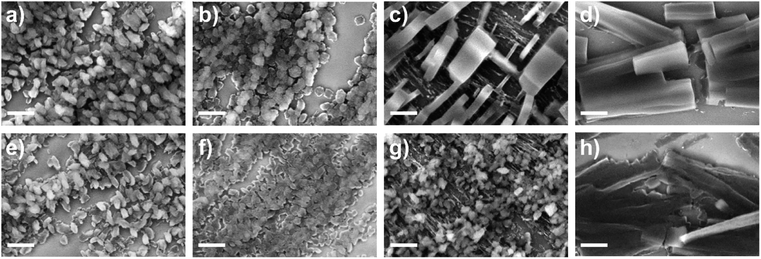
To correlate the changes observed in the XRD patterns and FTIR spectra with changes of the films' morphologies, we examined the samples as-synthesized and after exposure to the different environments, using scanning electron microscopy (SEM). Pristine films of the 2D frameworks Cu2(BDC)2 and Cu2(BPDC)2 showed small, plate-like crystals with diameters of approximately 150 nm (Fig. 3a and b). In contrast to this, micrographs of Cu2(BDC)2DABCO films revealed partially intergrown, cuboid crystals, with an average length of 600 nm and width in the 150–400 nm range (Fig. 3c). Pristine Cu2(BPDC)2DABCO films also exhibited a cuboid morphology with crystal lengths up to 2.2 μm and an average width of 500 nm (Fig. 3d). These morphologies are comparable with previous reports of heteroepitaxially grown Cu-MOF films from Cu(OH)2 substrates.19–21
When compared to the micrographs of as-synthesized samples, the morphologies of Cu2(BDC)2 and Cu2(BPDC)2 exposed to different RH values remain unchanged (Fig. 3e, f, S13 and S14†) indicating minimal impact of humidity and water on the 2D-layered MOF crystal shapes. However, significant changes were observed in the case of Cu2(BDC)2DABCO. After 7 days at 50% RH, the original large cuboid crystals are replaced by small plate-like crystals (Fig. 3g and S15†). We note that the new crystalline morphology is similar to the one of Cu2(BDC)2 MOFs (Fig. 3a and e). At higher water loadings, the transformation from a cuboid to a plate-like morphology occurs at a faster rate (e.g. < 1 d at 80% RH and in H2O). These observations suggest the transformation from Cu2(BDC)2DABCO to Cu2(BDC)2.
For Cu2(BPDC)2DABCO, the overall cuboid crystal shapes remained prominent after the 7 d exposure to 50% RH, but less defined crystal edges and increased surface roughness were observed (Fig. 3h). Under high humidity conditions, individual crystals became gradually more difficult to distinguish and micrographs show holes covering the surface of the remaining material (Fig. S16†).
Structural analysis of the 3D-oriented MOF films after exposure to humid environments
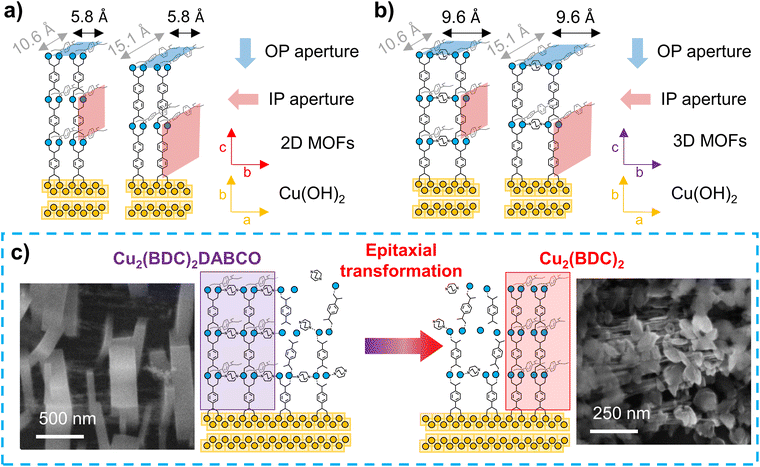
It has been shown in the literature that exposure of Cu2(BDC)2DABCO to 50% RH resulted in reduced Cu–Cu interaction, the formation of a Cu–OH moiety and protonation of the carboxylate linkers.27 An effective hydrolysis of the coordination bonds requires the accumulation of a certain amount of water in the pores, similar to capillary condensation.44 Consequently, for MOFs with larger voids, a higher partial water pressure is required to facilitate the displacement of the organic linkers. Based on this premise, the MOF films containing BDC with pore channels of 7.5 Å are expected to be more sensitive towards water than their BPDC counterparts, which exhibit wider pores of 10.8 Å.21,45 Additionally, the access to the MOF voids through suitable pore apertures must also be considered. In the case of heteroepitaxially grown Cu2(BDC)2 and Cu2(BPDC)2 films, the 2D MOF layers are closely spaced by 5.8 Å,19 resulting in small hydrophobic pore openings perpendicular to the substrate surface (Fig. 4a). In contrast, for the pillar-layered 3D MOF films, Cu2(BDC)2DABCO and Cu2(BPDC)2DABCO, the stacking distance is increased to 9.63 Å (Fig. 4b).19,20 Studies by Fischer and co-workers showed that for layer-by-layer grown SURMOFs, adsorption rates are significantly higher when the MOF voids could be accessed through apertures perpendicular to the substrate surface, while in-plane pore channels with limited out-of-plane access points resulted in lower adsorption rates, especially in densely packed films.46 Based on these studies, we expected a lower hydrolytic sensitivity for heteroepitaxial 2D MOF films and higher sensitivity for heteroepitaxial 3D MOF films (i.e. those containing DABCO).Our experimental results show that humidity has negligible effects on the crystallinity, chemical composition and morphology of heteroepitaxially grown Cu2(BDC)2 and Cu2(BPDC)2 films. Thus, these oriented 2D Cu-MOF frameworks are structurally stable even in highly humid environments. The similar behaviour observed for both linkers (BDC and BPDC) suggests that for these MOFs, the small hydrophobic pore windows prevent an effective mass transfer of water molecules into the larger in-plane pores. Thus, the MOF crystal orientation could have a stabilizing contribution. We note that the effect of hydrophobic pore windows was also previously reported for hydrophobic frameworks, which showed a significantly higher affinity towards non-polar molecules over polar guests.47
In contrast, in the exposed 3D-oriented Cu2(BDC)2DABCO film, reduced crystallinity and decreased intensity of the carboxylate vibrations in the IR spectra are ascribed to the hydrolysis of the framework. In addition, the appearance of new vibrational bands and a plate-like morphology indicate the formation of Cu2(BDC)2. Although partial decomposition of powdery Cu2(BDC)2DABCO at 50% RH has been previously observed,27 the transformation from 3D-oriented Cu2(BDC)2DABCO films to Cu2(BDC)2 coatings has not been reported to date. In the PXRD patterns recorded of Cu2(BDC)2DABCO exposed to 50% RH, the shift of the (010) reflection to higher 2θ values indicates the partial hydrolysis of the framework as previously described.27 However, the co-presence of vibrational bands that can be ascribed to Cu2(BDC)2DABCO and Cu2(BDC)2, indicates that at 50% RH the MOF-to-MOF transformation is incomplete. In contrast, when Cu2(BDC)2DABCO is exposed to 80% RH or liquid water, the final diffraction patterns and IR spectra match those of pure Cu2(BDC)2, suggesting a complete transformation from the pillar-layered MOF to the 2D framework. We note that there is an emerging research in the synthesis of MOF superstructures;16,17,37 for example, it has been demonstrated that a Zn2(BDC)2DABCO MOF single crystal can evolve in an iso-oriented MOF superstructure.48 While in single-crystal transformations, the crystal shape is typically preserved,49 our SEM data indicates that the initial Cu2(BDC)2DABCO crystals are replaced by Cu2(BDC)2 crystals with different morphology (see RH = 80% Fig. S15†). This morphological change could indicate that the formation of the 2D MOF occurs via recrystallization of building units that were initially part of the Cu2(BDC)2DABCO framework (Fig. 4c). Considering that DABCO has a water solubility of 61 g/100 g H2O,50 whereas H2BDC is rather insoluble,51 it is expected to have a high concentration of BDC, originated from the dissolution of the original framework, available for recrystallization. On the other hand, after hydrolysis of the coordination bonds, DABCO is expected to be easily removed from the framework. This hypothesis is confirmed by the absence of the DABCO vibrational bands from Cu2(BDC)2DABCO films after exposure to liquid water. We further note that Fischer and co-workers reported that Cu2(BDC)2DABCO does not form in the presence of water (40 °C), but the synthesis yields Cu2(BDC)2 crystals instead.52 This suggests that, under the investigated humid conditions, the recrystallization of Cu2(BDC)2DABCO at room temperature is unlikely while the formation of Cu2(BDC)2 is favoured.
We hypothesize a second contribution to the growth of Cu2(BDC)2: by SEM we noted that in the Cu2(BDC)2DABCO films, we could observe regions exposing uncoated Cu(OH)2 NBs (Fig. S15†). The presence of these residual NBs in pristine Cu2(BDC)2DABCO films was also confirmed by XRD, showing the (021) reflection of Cu(OH)2 in the diffraction pattern. When immersing Cu2(BDC)2DABCO films in liquid water, we then observed the formation of plate-like Cu2(BDC)2 crystallites in these regions of exposed NBs. X-ray diffraction analyses of these films showed that the appearance of Cu2(BDC)2 was concomitant with a slight decrease of the (021) Cu(OH)2 intensity. This suggests that after degradation of the original framework, Cu2(BDC)2 could be partially formed from unreacted Cu(OH)2 directly on the NBs.
Overall the data suggest that immersion of Cu2(BDC)2DABCO films in water triggers both, a heteroepitaxial transformation into Cu2(BDC)2 and a fresh heteroepitaxial growth from the exposed Cu(OH)2.
In order to assess how the transformation process influences the overall crystalline orientation of the MOF film, we investigated the azimuthal angle dependence of the intensity of the respective (100) reflections of Cu2(BDC)2DABCO and Cu2(BDC)2 before and after exposure to 80% RH and liquid water. The intensity profile of the as prepared 3D-oriented Cu2(BDC)2DABCO film showed two intensity maxima of the (100) reflection at approx. 60° and 240° (Fig. 5a). The (001) planes in the same sample have their maxima shifted by 90°, complying with the tetragonal MOF unit cell53 and confirming the in-plane order in the as-synthesized film. After exposure to 80% RH, the intensity profile of the (100) reflection ascribed to Cu2(BDC)2 shows also two intensity maxima shifted by 180° (Fig. 5b); the profile of the (001) reflection confirms the decomposition of Cu2(BDC)2DABCO crystals. Similar intensity profiles were observed when Cu2(BDC)2DABCO films were immersed in water; however, in this case, a broader distribution of crystalline orientations was recorded (Fig. S17†). Notably, the position of the maxima of the new Cu2(BDC)2 phase coincides with the maxima of the pristine oriented Cu2(BDC)2DABCO. This indicates that the (100) planes of Cu2(BDC)2DABCO might take an active role in the recrystallization of the 2D MOF layers by directing the growth of Cu2(BDC)2 during the MOF-to-MOF transformation process. The mechanism could be explained by the lattice match between the two frameworks, which was also recently reported for the epitaxial recrystallization of oriented MOF nanostructures from a labile Cu-MOF single crystal.37 This epitaxial recrystallization from 3D-oriented Cu2(BDC)2DABCO films to Cu2(BDC)2 coatings, here supported by chemical, structural and morphological investigations, is the first evidence that an oriented MOF superstructure could be transformed into an iso-oriented, chemically and structurally distinct MOF superstructure. To further examine the kinetics and morphological changes of this process, we performed additional in situ experiments (vide infra).
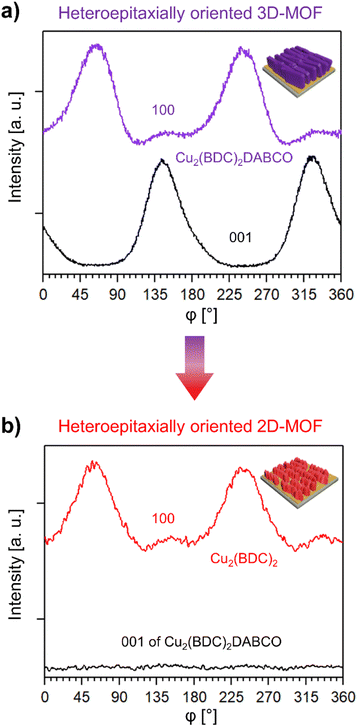 | ||
| Fig. 5 (a) Azimuthal intensity profile of the (100) and (001) reflection of Cu2(BDC)2DABCO before exposure to 80% RH, (b) azimuthal intensity profile of the (100) reflection of newly formed Cu2(BDC)2 after exposure to 80% RH and of the (001) reflection of Cu2(BDC)2DABCO, confirming the absence of the DABCO pillar ligand. The average intensity of the (001) reflection of Cu2(BDC)2DABCO in (b) is similar to the lowest intensity measured in the azimuthal scan of Cu2(BDC)2 (see Fig. S17† for plots with intensities). | ||
For the heteroepitaxially grown Cu2(BPDC)2DABCO MOF film exposed to 50% RH, the crystallinity also decreased significantly within the initial 24 h, suggesting the hydrolysis of the network. However, this drop is not as drastic as for Cu2(BDC)2DABCO under the same environmental condition (Cu2(BDC)2DABCO: 90% drop of the signal after 24 h at 50% RH), consistent with the hypothesis that higher relative humidity is needed to effectively displace the linkers in MOFs with larger pore sizes. Interestingly, the IR spectra of exposed Cu2(BPDC)2DABCO films show the appearance of vibrational bands that could be ascribed to the presence of Cu2(BPDC)2. This suggests that during the storage in humid environments, the crystalline Cu2(BPDC)2DABCO 3D framework tends to evolve into a Cu2(BPDC)2 2D network. A transformation mechanism similar to that of Cu2(BDC)2DABCO seems plausible due to the low water-solubility of the BPDC linker. However, unlike the previously described MOF-to-MOF transformation with BDC, the XRD diffraction patterns show minimal crystallinity with increasing water loading (Fig. S6†). This is consistent with the SEM micrographs, which show how Cu2(BPDC)2DABCO crystals progressively lose their sharp crystal edges and holes appear on the crystal surface (Fig. S16†); this morphological change is typically indicative of degradation by-products with low crystallinity.33,54
Time-resolved analysis of the heteroepitaxial MOF-to-MOF transformation
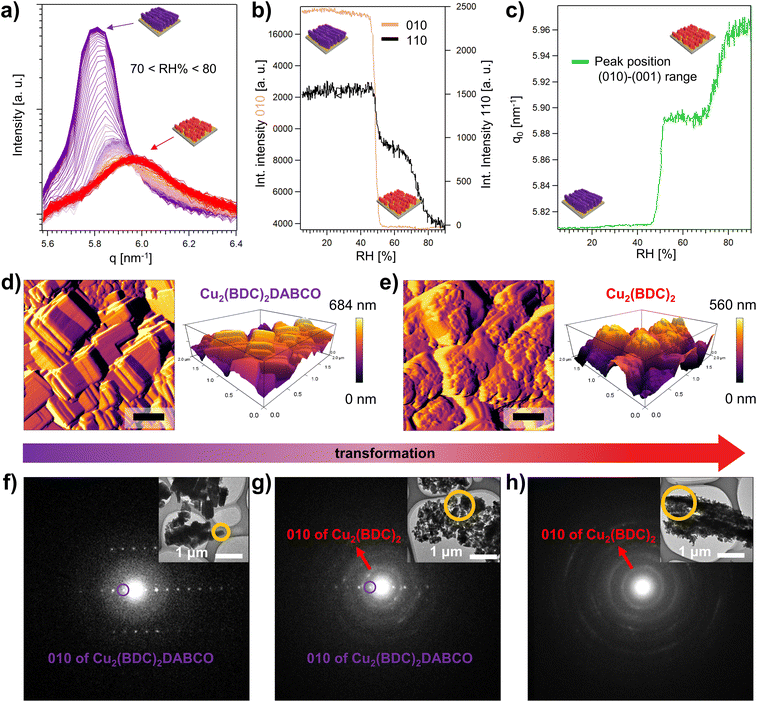
In situ methods are a potent tool to investigate the formation, growth, and decomposition of MOFs.55 For example, time-resolved XRD was used to understand defect formation in pillar-layered Zn2(BDC-TM)2DABCO under humid environments,56 and in situ single crystal XRD revealed mechanistic aspects of the water sorption of Y-shp-MOF-5.57 Using synchrotron scattering techniques, the structural changes in the framework can be observed with a time resolution as small as 100 ms, revealing important kinetic information about MOF growth or degradation.58–60 We note that small angle X-ray scattering (SAXS) was used to understand the decomposition of HKUST-1 under controlled humidity.61 Inspired by these works, we used grazing incidence wide angle X-ray scattering (GIWAXS) to perform time-resolved studies on Cu2(BDC)2DABCO films and elucidate the epitaxial recrystallization into Cu2(BDC)2.High-resolution GIWAXS patterns were recorded using a humidity chamber set at 50% RH, 80% RH, and increasing humidity from 5–90% RH. For these in situ studies, a time resolution of 60 s between the acquisition of the diffractograms was selected. Additionally, we monitored the transformation of Cu2(BDC)2DABCO films immersed in water, using a customized chemical cell at an increased time resolution of 1.1 s. For data analysis, we considered the out-of-plane component of the collected 2D images to monitor the (010) reflection of Cu2(BDC)2DABCO, and the (001) reflection of Cu2(BDC)2, and correlated the structural changes to the water loading during the in situ measurements. Since the d-spacing of the investigated reflections of the 3D and 2D framework is similar (1.08 nm for Cu2(BDC)2DABCO, and 1.05 nm for Cu2(BDC)2), the collected reflections partially overlap. To selectively follow the degradation of oriented Cu2(BDC)2DABCO crystals, we also monitored the (110) reflection (Fig. S4†). In addition, to examine the role of Cu(OH)2 in the transformation process, we monitored the (021) reflection ((021) does not overlap with second order reflections from Cu2(BDC)2DABCO or Cu2(BDC)2 in the region at 11.5 nm−1).
The time-resolved data reveals that the transformation of Cu2(BDC)2DABCO to Cu2(BDC)2 is highly dependent on the applied water loadings. GIWAXS data show that up to 50% RH, diffraction patterns remain identical to the as-synthesized sample (Fig. S18†), indicating water-stability up to this humidity value. However, once 50% RH is reached, the intensities of the (010) and (110) Cu2(BDC)2DABCO reflections rapidly drop and shift to higher q values (Fig. S18 and S19†). The shift was previously observed by Chabal and co-workers with a laboratory X-ray diffractometer and attributed to the partial hydrolysis of Cu2(BDC)2DABCO powder exposed to 50% RH.27 In our GIWAXS investigation, from 50% to 70% RH, the intensity and the d-spacing of the diffraction signal further decreases, indicating enhanced sensitivity towards higher water contents after this intermediate stage (Fig. S19†). Between 70% and 80% RH, the (110) reflection of Cu2(BDC)2DABCO eventually disappears and only the diffraction pattern of Cu2(BDC)2 remains (Fig. 6a, S19, and Movie S1†), suggesting the end of the heteroepitaxial conversion from Cu2(BDC)2DABCO to Cu2(BDC)2. The integrated intensity of the (010)-(001) region and the (110) reflection of Cu2(BDC)2DABCO over the whole experiment time span reveals that the transformation is not a linear process, and it occurs in two steps (Fig. 6b). At 50% RH, an initial sharp decline can be ascribed to the partial hydrolysis of the MOF.27 However, the (110) plateaus until 70–80% RH, where a second abrupt decline of intensity evidences a significant degradation of the remaining 3D framework. Due to the overlapping signals of Cu2(BDC)2DABCO and Cu2(BDC)2 in the region between 5.6 and 6.4 nm−1, this second step could not be observed for the (010) reflection and it is notably difficult to assess when the formation of the 2D framework takes place. However, by plotting the peak position maxima of the (010)-(001) reflection range versus the applied humidity, the two-step mechanism is also evident (Fig. 6c). In accordance with the integrated intensity profile of the (110) reflection of Cu2(BDC)2DABCO, the first step takes place at 50% RH, showing a shift from 5.81 nm−1 to 5.89 nm−1, which is consistent with the previously reported increase of the (010) interlayer distance caused by the partial hydrolysis of the 3D-framework.27 The second shift in the q-value at 70–80% RH can then be attributed to the recrystallization of the Cu2(BDC)2 film upon dissolution of the DABCO ligand. When RH is increased by 10% min−1 and held at a target value of 80%, the transformation to Cu2(BDC)2 is complete within 20 min (Fig. S20†). Under immersion in water, the kinetics of the process is faster and it takes only a few seconds (Fig. S21†). This suggests that, under these conditions, the coordination bonds of Cu2(BDC)2DABCO are almost instantly broken. When examining the Cu(OH)2 patterns, we note that the signal assigned to the (021) reflection of Cu(OH)2 remains unchanged up to 50% RH, and shows only a slight decrease in intensity for higher RH values including liquid water (Fig. S18–20†). This slight consumption of the residual metal hydroxide concomitant to the formation of Cu2(BDC)2 suggests a partial heteroepitaxial growth process on aligned Cu(OH)2 nanobelts.
High magnification SEM micrographs recorded after the in situ experiment at 50% RH show crystals of Cu2(BDC)2DABCO with rounded corners and soft edges, which is in accordance with the reduced crystallinity observed for this intermediate stage of the transformation process (Fig. S22†). In contrast, after exposure to 80% RH for 30 min, the previously described regions of concentrated polycrystalline Cu2(BDC)2 were observed while the surrounding Cu(OH)2 nanostructures seemed mostly unaffected (Fig. S23†). In case of exposure of Cu2(BDC)2DABCO to liquid water, the images show densely populated regions surrounded by single Cu2(BDC)2 particles on the previously unreacted Cu(OH)2 NBs (Fig. S24†). These results are consistent with the previously recorded SEM micrographs (vide supra). SEM-EDX analysis, comparing pristine Cu2(BDC)2DABCO and Cu2(BDC)2 after transformation at 80% RH, shows that nitrogen is effectively removed in the transformed film. This indicates that DABCO is indeed released from the framework and its sublimation62 is enhanced under high-vacuum conditions during the SEM-EDX measurement (Fig. S25†).
To further examine the morphological changes during the heteroepitaxial transformation from Cu2(BDC)2DABCO to Cu2(BDC)2, time-resolved AFM measurements were performed in a small environmental chamber under humid Ar flow (80% RH at exit of the chamber). The in situ morphological analysis revealed that within a few minutes in humid conditions, terrace-like steps and rounded crystal corners appear, indicating a degradation of the original cuboid morphology for the Cu2(BDC)2DABCO film. Over time (Fig. 6d, e, S26, and Movie S2†), we observed the formation of the plate-like morphology of Cu2(BDC)2, which preferentially grows in an ordered fashion, spreading from Cu2(BDC)2DABCO crystal edges towards the centre of the crystal faces. The higher reactivity of crystal edges was previously noticed and ascribed to a higher concentration of defects.63 During the transformation, the crystallite size changed from approx. 530 × 290 nm (initial Cu2(BDC)2DABCO) to 110 × 80 nm (transformed Cu2(BDC)2) (Table S1†). In addition to this, 3D AFM topography images show that the maximum height of 648 nm of the Cu2(BDC)2DABCO film is reduced to 560 nm after exposure to 80% RH for 100 min (Fig. 6d, e). This decrease in the film thickness can be attributed to partial material loss or vertical contraction as the initial framework progressively transforms from the surface towards underlying layers. Similar changes in film thickness were estimated from SEM micrographs recorded at a 45° tilting angle (Fig. S27†).
To validate the phase transition from Cu2(BDC)2DABCO to Cu2(BDC)2, selected area electron diffraction (SAED) was used to analyse the nanocrystals scratched from the Si substrates. For a single pristine Cu2(BDC)2DABCO crystallite, the obtained electron diffraction pattern revealed sharp spots (Fig. 6f) with a calculated d-value of d010 = 1.08 nm, which agrees well with our PXRD data (d = 1.08 nm) and the reported crystal structure of the MOF.20,53 Then, to access the intermediate stage of the transformation, a Cu2(BDC)2DABCO film was exposed to 80% RH for 10 min, solvent-exchanged to dichloromethane, evacuated, and stored under inert conditions (Ar) until measurement. The electron diffraction of this sample shows the previously described diffraction spots of the Cu2(BDC)2DABCO phase (d010 = 1.08 nm) as well as broad diffraction rings attributed to Cu2(BDC)2, indicating the co-existence of both MOF phases (Fig. 6g). The diffraction rings consist of overlapping diffraction spots, which can be attributed to the electron diffraction from multiple Cu2(BDC)2 crystallites being included in the selected area. The calculated d-value of d010 = 0.59 nm corresponds to that of reported Cu2(BDC)2.19 For the film exposed to 80% RH for 3 h, the electron diffraction pattern shows only diffraction rings of Cu2(BDC)2 (d010 = 0.59 nm), confirming the complete MOF-to-MOF transformation in the crystal phases (Fig. 6h).
In order to determine how the MOF-to-MOF transformation influences the porosity of the different frameworks, powdery Cu2(BDC)2DABCO was exposed to H2O and the formation of the 2D framework confirmed by PXRD (Fig. S28†). N2 adsorption isotherms then revealed that the transformed Cu2(BDC)2 shows similar properties as pristine Cu2(BDC)2, with BET surface areas of 160 m2 g−1 and 145 m2 g−1, respectively (Fig. S28†).
Overall, these changes observed in morphology, in situ X-ray scattering, and electron diffraction demonstrate that at 80% RH the transformation from Cu2(BDC)2DABCO films to Cu2(BDC)2 occurs preferentially via a dissolution and recrystallization pathway. The time-resolved experiments further demonstrate that a 3D-ordered MOF superstructure can undergo a heteroepitaxial recrystallization mechanism.
Conclusions
In this study, we investigated the structural stability of heteroepitaxially grown MOF films in environments ranging from 50% RH to liquid H2O. We found that the 2D frameworks Cu2(BDC)2 and Cu2(BPDC)2, organized as 3D-oriented polycrystalline MOF films, maintain their crystallinity, chemical composition and morphology at all water loadings. This stability in environmental conditions is desirable for applications of oriented MOF films in functional devices. For the pillar-layered 3D frameworks, Cu2(BDC)2DABCO and Cu2(BPDC)2DABCO, the related oriented MOF films undergo significant degradation and morphological changes even in milder environments of 50% RH. We ascribed this water sensitivity to the increased water transfer via the MOF voids that have larger out-of-plane pore apertures. Thus, for practical applications of these 3D-ordered films, protection from humidity is required. Preservation strategies64,65 could involve the design of MOFs with enhanced hydrophobic properties,66,67 the deposition of hydrophobic coatings,68 or the utilization of protective vacuum cases.69While the degradation product of Cu2(BPDC)2DABCO was found to be amorphous, we observed conditions (i.e. RH > 70%) in which an ordered film of Cu2(BDC)2DABCO transforms into an ordered film of Cu2(BDC)2. By combining time-resolved synchrotron experiments and in situ AFM measurements, we identified salient aspects of the transformation mechanism, including the hydrolysis of the original framework and the recrystallization of the building blocks as an oriented 2D MOF coating. The recrystallization was demonstrated to follow a heteroepitaxial pathway. These results highlight that in conditions that allow a MOF-to-MOF transformation, an original organization of crystals in a 3D-oriented superstructure could lead to oriented, but distinct polycrystalline MOF systems. Our results suggest that the conversion among 3D-oriented superstructures could be a valuable alternative synthetic approach for the fabrication of oriented MOF films.
Author contributions
L. A. B.: design of methodology, MOF synthesis, curation, writing – original draft; M. L.-M.: design of methodology, AFM and Raman measurements, writing – review & editing; G. Z. and Z. H.: TEM measurements, editing; H. A.: supervision GIWAXS measurements, technical support and analysis; S. D.-Z.: SEM and EDX measurements; C. D.: validation, writing – review & editing; P. F.: design of methodology, supervision, validation, writing – review & editing, funding acquisition.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are thankful for the support of M. de J. Velásquez-Hernández and V. Lipic during the synchrotron beam time (GIWAXS measurements) and M. de J. Velásquez-Hernández for her help with N2 adsorption measurements. The authors thank Behnaz Abbasgholi_NA for her support in SEM imaging. The authors acknowledge support from the European Research Council under the European Union's Horizon 2020 Programme (FP/2014-2020)/ERC Grant Agreement No. 771834—POPCRYSTAL and the TU Graz for the Lead Project (No. LP-03). G. Z. and Z. H. acknowledge support from the Swedish research council FORMAS (2020-00831) and the Swedish research council VR (2022-02939). The authors acknowledge the CERIC-ERIC Consortium for the access to the Austrian SAXS beamline experimental facilities at Elettra Synchrotron and financial support. The authors thank Masahide Takahashi and Kenji Okada for fruitful discussions.Notes and references
- H. Furukawa, K. E. Cordova, M. O'Keeffe and O. M. Yaghi, Science, 2013, 341, 1230444 CrossRef PubMed
.
- S. Kitagawa, R. Kitaura and S. Noro, Angew. Chem., Int. Ed., 2004, 43, 2334–2375 CrossRef CAS PubMed
.
- X. Zhang, Z. Chen, X. Liu, S. L. Hanna, X. Wang, R. Taheri-Ledari, A. Maleki, P. Li and O. K. Farha, Chem. Soc. Rev., 2020, 49, 7406–7427 RSC
.
- L. S. Xie, G. Skorupskii and M. Dincă, Chem. Rev., 2020, 120, 8536–8580 CrossRef CAS PubMed
.
- G. M. Espallargas and E. Coronado, Chem. Soc. Rev., 2018, 47, 533–557 RSC
.
- C. Cong and H. Ma, Adv. Opt. Mater., 2021, 9, 2100733 CrossRef CAS
.
- P. Falcaro, R. Ricco, C. M. Doherty, K. Liang, A. J. Hill and M. J. Styles, Chem. Soc. Rev., 2014, 43, 5513–5560 RSC
.
- J. Liu and C. Wöll, Chem. Soc. Rev., 2017, 46, 5730–5770 RSC
.
- L. Pilz, C. Natzeck, J. Wohlgemuth, N. Scheuermann, P. G. Weidler, I. Wagner, C. Wöll and M. Tsotsalas, Adv. Mater. Interfaces, 2023, 10, 2201771 CrossRef CAS
.
- O. Dalstein, E. Gkaniatsou, C. Sicard, O. Sel, H. Perrot, C. Serre, C. Boissière and M. Faustini, Angew. Chem., Int. Ed., 2017, 56, 14011–14015 CrossRef CAS PubMed
.
- O. Dalstein, D. R. Ceratti, C. Boissière, D. Grosso, A. Cattoni and M. Faustini, Adv. Funct. Mater., 2016, 26, 81–90 CrossRef CAS
.
- M. Krishtab, I. Stassen, T. Stassin, A. J. Cruz, O. O. Okudur, S. Armini, C. Wilson, S. De Gendt and R. Ameloot, Nat. Commun., 2019, 10, 3729 CrossRef PubMed
.
- W. Zhan, Q. Kuang, J. Zhou, X. Kong, Z. Xie and L. Zheng, J. Am. Chem. Soc., 2013, 135, 1926–1933 CrossRef CAS PubMed
.
- I. E. Khalil, J. Fonseca, M. R. Reithofer, T. Eder and J. M. Chin, Coord. Chem. Rev., 2023, 481, 215043 CrossRef CAS
.
- J. Fonseca, L. Meng, I. Imaz and D. Maspoch, Chem. Soc. Rev., 2023, 52, 2528–2543 RSC
.
- C. Avci, I. Imaz, A. Carné-Sánchez, J. A. Pariente, N. Tasios, J. Pérez-Carvajal, M. I. Alonso, A. Blanco, M. Dijkstra, C. López and D. Maspoch, Nat. Chem., 2018, 10, 78–84 CrossRef CAS PubMed
.
- F. Cheng, A. J. Young, J.-S. G. Bouillard, N. T. Kemp, R. Guillet-Nicolas, C. H. Hall, D. Roberts, A. H. Jaafar, A. M. Adawi, F. Kleitz, A. Imhof, M. R. Reithofer and J. M. Chin, J. Am. Chem. Soc., 2019, 141, 12989–12993 CrossRef CAS PubMed
.
- P. I. Scheurle, A. Mähringer, A. Biewald, A. Hartschuh, T. Bein and D. D. Medina, Chem. Mater., 2021, 33, 5896–5904 CrossRef CAS
.
- P. Falcaro, K. Okada, T. Hara, K. Ikigaki, Y. Tokudome, A. W. Thornton, A. J. Hill, T. Williams, C. Doonan and M. Takahashi, Nat. Mater., 2017, 16, 342–348 CrossRef CAS PubMed
.
- K. Okada, M. Nakanishi, K. Ikigaki, Y. Tokudome, P. Falcaro, C. J. Doonan and M. Takahashi, Chem. Sci., 2020, 11, 8005–8012 RSC
.
- M. de J. Velásquez-Hernández, M. Linares-Moreau, L. A. Brandner, B. Marmiroli, M. Barella, G. P. Acuna, S. D. Zilio, M. F. K. Verstreken, D. E. Kravchenko, O. M. Linder-Patton, J. D. Evans, H. Wiltsche, F. Carraro, H. Wolinski, R. Ameloot, C. Doonan and P. Falcaro, Adv. Mater., 2023, 35, 2211478 CrossRef PubMed
.
- A. Tarzia, M. Takahashi, P. Falcaro, A. W. Thornton, C. J. Doonan and D. M. Huang, ACS Appl. Mater. Interfaces, 2018, 10, 40938–40950 CrossRef CAS PubMed
.
- M. Linares-Moreau, L. A. Brandner, T. Kamencek, S. Klokic, F. Carraro, K. Okada, M. Takahashi, E. Zojer, C. J. Doonan and P. Falcaro, Adv. Mater. Interfaces, 2021, 8, 2101039 CrossRef CAS
.
- B. Baumgartner, R. Mashita, A. Fukatsu, K. Okada and M. Takahashi, Angew. Chem., Int. Ed., 2022, 61, e202201725 CrossRef CAS PubMed
.
- K. Okada, R. Mashita, A. Fukatsu and M. Takahashi, Nanoscale Adv., 2023, 5, 1795–1801 RSC
.
- S. Klokic, D. Naumenko, B. Marmiroli, F. Carraro, M. Linares-Moreau, S. D. Zilio, G. Birarda, R. Kargl, P. Falcaro and H. Amenitsch, Chem. Sci., 2022, 13, 11869–11877 RSC
.
- K. Tan, N. Nijem, P. Canepa, Q. Gong, J. Li, T. Thonhauser and Y. J. Chabal, Chem. Mater., 2012, 24, 3153–3167 CrossRef CAS
.
- N. C. Burtch, H. Jasuja and K. S. Walton, Chem. Rev., 2014, 114, 10575–10612 CrossRef CAS PubMed
.
- X. Liu, X. Wang and F. Kapteijn, Chem. Rev., 2020, 120, 8303–8377 CrossRef CAS PubMed
.
- M. E. A. Safy, M. Amin, R. R. Haikal, B. Elshazly, J. Wang, Y. Wang, C. Wöll and M. H. Alkordi, Chem. – Eur. J., 2020, 26, 7109–7117 CrossRef CAS PubMed
.
- C. Li, A. Chandresh, Z. Zhang, S. Moulai and L. Heinke, Adv. Mater. Interfaces, 2022, 9, 2101947 CrossRef CAS
.
- M. Todaro, G. Buscarino, L. Sciortino, A. Alessi, F. Messina, M. Taddei, M. Ranocchiari, M. Cannas and F. M. Gelardi, J. Phys. Chem. C, 2016, 120, 12879–12889 CrossRef CAS
.
- M. P. Singh, N. R. Dhumal, H. J. Kim, J. Kiefer and J. A. Anderson, J. Phys. Chem. C, 2016, 120, 17323–17333 CrossRef CAS
.
- S. S.-Y. Chui, S. M.-F. Lo, J. P. H. Charmant, A. G. Orpen and I. D. Williams, Science, 1999, 283, 1148–1150 CrossRef CAS PubMed
.
- C. Huang, J. Dong, W. Sun, Z. Xue, J. Ma, L. Zheng, C. Liu, X. Li, K. Zhou, X. Qiao, Q. Song, W. Ma, L. Zhang, Z. Lin and T. Wang, Nat. Commun., 2019, 10, 2779 CrossRef PubMed
.
- D. Saha and S. Deng, J. Phys. Chem. Lett., 2010, 1, 73–78 CrossRef CAS
.
- L. Shao, F. Meng, J. Chen and Y. Fu, J. Mater. Chem. A, 2023, 11, 5027–5036 RSC
.
- H. Amenitsch, M. Rappolt, M. Kriechbaum, H. Mio, P. Laggner and S. Bernstorff, J. Synchrotron Radiat., 1998, 5, 506–508 CrossRef CAS PubMed
.
- B. Marmiroli, B. Sartori, A. R. Kyvik, I. Ratera and H. Amenitsch, Front. Mater., 2021, 8, 686353 CrossRef
.
- M. Bogar, I. Khalakhan, A. Gambitta, Y. Yakovlev and H. Amenitsch, J. Power Sources, 2020, 477, 229030 CrossRef CAS
.
- J. Razjouyan, H. Lee, B. Gilligan, C. Lindberg, H. Nguyen, K. Canada, A. Burton, A. Sharafkhaneh, K. Srinivasan, F. Currim, S. Ram, M. R. Mehl, N. Goebel, M. Lunden, S. Bhangar, J. Heerwagen, K. Kampschroer, E. M. Sternberg and B. Najafi, Indoor Air, 2020, 30, 167–179 CrossRef PubMed
.
- T. Truong, K. D. Nguyen, S. H. Doan and N. T. S. Phan, Appl. Catal., A, 2016, 510, 27–33 CrossRef CAS
.
- V. I. Kovalenko, A. A. Akhmadiyarov, A. E. Vandyukov and A. R. Khamatgalimov, J. Mol. Struct., 2012, 1028, 134–140 CrossRef CAS
.
- P. Guo, D. Dutta, A. G. Wong-Foy, D. W. Gidley and A. J. Matzger, J. Am. Chem. Soc., 2015, 137, 2651–2657 CrossRef CAS PubMed
.
- K. Seki and W. Mori, J. Phys. Chem. B, 2002, 106, 1380–1385 CrossRef CAS
.
- B. Liu, M. Tu and R. A. Fischer, Angew. Chem., Int. Ed., 2013, 52, 3402–3405 CrossRef CAS PubMed
.
- A. Bétard, S. Wannapaiboon and R. A. Fischer, Chem. Commun., 2012, 48, 10493–10495 RSC
.
- Z. Chen, S. Xiang, D. Zhao and B. Chen, Cryst. Growth Des., 2009, 9, 5293–5296 CrossRef CAS
.
- X.-J. Bai, X. Zhai, L.-Y. Zhang, Y. Fu and W. Qi, Matter, 2021, 4, 2919–2935 CrossRef CAS
.
- A. Farkas, G. A. Mills, W. E. Erner and J. B. Maerker, J. Chem. Eng. Data, 1959, 4, 334–335 CrossRef CAS
.
- N. Han, L. Zhu, L. Wang and R. Fu, Sep. Purif. Technol., 1999, 16, 175–180 CrossRef CAS
.
- Z. Wang, K. Rodewald, R. Medishetty, B. Rieger and R. A. Fischer, Cryst. Growth Des., 2018, 18, 7451–7459 CrossRef CAS
.
- Y. Kim, R. Haldar, H. Kim, J. Koo and K. Kim, Dalton Trans., 2016, 45, 4187–4192 RSC
.
- J.-S. Choi, W.-J. Son, J. Kim and W.-S. Ahn, Microporous Mesoporous Mater., 2008, 116, 727–731 CrossRef CAS
.
- M. J. Van Vleet, T. Weng, X. Li and J. R. Schmidt, Chem. Rev., 2018, 118, 3681–3721 CrossRef CAS PubMed
.
- N. C. Burtch, I. M. Walton, J. T. Hungerford, C. R. Morelock, Y. Jiao, J. Heinen, Y.-S. Chen, A. A. Yakovenko, W. Xu, D. Dubbeldam and K. S. Walton, Nat. Chem., 2020, 12, 186–192 CrossRef CAS PubMed
.
- R. G. AbdulHalim, P. M. Bhatt, Y. Belmabkhout, A. Shkurenko, K. Adil, L. J. Barbour and M. Eddaoudi, J. Am. Chem. Soc., 2017, 139, 10715–10722 CrossRef CAS PubMed
.
- F. Carraro, J. D. Williams, M. Linares-Moreau, C. Parise, W. Liang, H. Amenitsch, C. Doonan, C. O. Kappe and P. Falcaro, Angew. Chem., Int. Ed., 2020, 59, 8123–8127 CrossRef CAS PubMed
.
- M. de J. Velásquez-Hernández, R. Ricco, F. Carraro, F. T. Limpoco, M. Linares-Moreau, E. Leitner, H. Wiltsche, J. Rattenberger, H. Schröttner, P. Frühwirt, E. M. Stadler, G. Gescheidt, H. Amenitsch, C. J. Doonan and P. Falcaro, CrystEngComm, 2019, 21, 4538–4544 RSC
.
- E. Zanchetta, L. Malfatti, R. Ricco, M. J. Styles, F. Lisi, C. J. Coghlan, C. J. Doonan, A. J. Hill, G. Brusatin and P. Falcaro, Chem. Mater., 2015, 27, 690–699 CrossRef CAS
.
- N. Al-Janabi, A. Alfutimie, F. R. Siperstein and X. Fan, Front. Chem. Sci. Eng., 2016, 10, 103–107 CrossRef CAS
.
- D. I. Bugaenko, A. V. Karchava and M. A. Yurovskaya, Chem. Heterocycl. Compd., 2020, 56, 128–144 CrossRef CAS
.
- Z. Gu, W. Zhang, T. Pan, Y. Shen, P. Qin, P. Zhang, X. Li, L. Liu, L. Li, Y. Fu, W. Zhang and F. Huo, Research, 2021, 2021, 854946 Search PubMed
.
- M. Ding, X. Cai and H.-L. Jiang, Chem. Sci., 2019, 10, 10209–10230 RSC
.
- K. Jayaramulu, F. Geyer, A. Schneemann, Š. Kment, M. Otyepka, R. Zboril, D. Vollmer and R. A. Fischer, Adv. Mater., 2019, 31, 1900820 CrossRef PubMed
.
- J. Yang, A. Grzech, F. M. Mulder and T. J. Dingemans, Chem. Commun., 2011, 47, 5244–5246 RSC
.
- J. M. Taylor, R. Vaidhyanathan, S. S. Iremonger and G. K. H. Shimizu, J. Am. Chem. Soc., 2012, 134, 14338–14340 CrossRef CAS PubMed
.
- J. Castells-Gil, F. Novio, N. M. Padial, S. Tatay, D. Ruíz-Molina and C. Martí-Gastaldo, ACS Appl. Mater. Interfaces, 2017, 9, 44641–44648 CrossRef CAS PubMed
.
- Q. Zhao, F. Carrascoso, P. Gant, T. Wang, R. Frisenda and A. Castellanos-Gomez, J. Phys.: Mater., 2020, 3, 036001 CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3sc04135b |
| This journal is © The Royal Society of Chemistry 2023 |