Open Access Article
Open Access ArticleA series of caged fluorophores for calibrating light intensity†
Mrinal
Mandal
 a,
Hessam Sepasi
Tehrani
a,
Qianhua
Mai
a,
Emma
Simon
a,
Marie-Aude
Plamont
a,
Hessam Sepasi
Tehrani
a,
Qianhua
Mai
a,
Emma
Simon
a,
Marie-Aude
Plamont
 a,
Christine
Rampon
b,
Sophie
Vriz
b,
Isabelle
Aujard
*a,
Thomas
Le Saux
a,
Christine
Rampon
b,
Sophie
Vriz
b,
Isabelle
Aujard
*a,
Thomas
Le Saux
 *a and
Ludovic
Jullien
*a and
Ludovic
Jullien
 *a
*a
aPASTEUR, Département de chimie, École normale supérieure, PSL University, Sorbonne Université, CNRS, 24, rue Lhomond, 75005 Paris, France. E-mail: Isabelle.Aujard@ens.psl.eu; Thomas.Lesaux@ens.psl.eu; Ludovic.Jullien@ens.psl.eu
bLaboratoire des biomolécules (LBM), Département de chimie, École normale supérieure, PSL University, Sorbonne Université, CNRS, 24, rue Lhomond, 75005 Paris, France
First published on 10th November 2023
Abstract
Absolute measurement of light intensity is sought for in multiple areas of chemistry, biology, physics, and engineering. It can be achieved by using an actinometer from analyzing the time-course of its reaction extent on applying constant light. However, most reported actinometers exploit the absorbance observable for reporting the reaction extent, which is not very sensitive nor relevant in imaging systems. In this work, we report a series of hydrophobic and hydrophilic caged fluorophores that overcome the preceding limitations. Based on the robust pyranine backbone, they can easily be synthesized on a large scale in one to a few steps. Their brightness increases over illumination and their uncaging cross-sections have been thoroughly characterized upon one- and two-photon excitation. As a demonstration of their use, we calibrated light intensity in various chemical and biological samples, which have been observed with epifluorescence and confocal imaging systems.
Introduction
Light is a trigger and reactant, which is endowed with attractive features. Indeed, it is essentially non-invasive, can be delivered with exclusive temporal and spatial resolution, and be used as well for introducing a perturbation and reading out its impact. Moreover, many light sources are nowadays available to deliver various photon fluxes in different wavelength ranges. Light has accordingly found ubiquitous exploitation in many academic and applied fields of Chemistry (e.g. photocatalysis1,2), Biology (e.g. optogenetics3–5), Physics (e.g. fluorescence bioimaging6), and Engineering (e.g. decontamination,7 measurement of environmental radiation8–11).Despite its attractive features, light often suffers from the lack of characterization of its sources, which makes it difficult to compare the results obtained in various groups as well as to ensure reproducibility. Light meters in which a detector integrates the overall photon flux over an illuminated surface are not often available in the laboratories of Chemistry and Biology.12,13 Moreover, their proper use requires technical expertise and they do not give direct access to the photon flux density (irradiance). In contrast, actinometers can yield the photon flux density at a sample from analyzing the time dependence of the extent of their photoconversion.14,15 However, most of them rely on absorbance for reporting, which is not sensitive enough to measure light intensity in tiny samples (e.g. for calibrating the illumination of microscopes). Fluorescent actinometers are attractive to overcome this limitation.
Most photoactivatable fluorophores switch from a dark to a bright state under illumination. Obtained by grafting a caging group which acts as a fluorescence quencher16–18 or as a dark precursor in which fluorescence is restored upon light irradiation,19–24 they have been exploited in numerous fields (e.g. investigation of cell lineage,25,26 spatiotemporal interrogation of fluid flows,27 reconstruction of images with subdiffraction resolution28,29). However, they have only been sporadically characterized in order to yield quantitative information on the absolute value of the light intensity.23,30–34 Importantly, the task of measuring light intensity is not restricted to one-photon excitation. Indeed, two-photon excitation is currently used both in fluorescence imaging (where it benefits from intrinsic excellent spatial resolution) and in optogenetics (for local photoactivation). Since it relies on focusing light from a high laser power, it is crucial to get information on the light intensity at the sample to obtain the desired two-photon-driven excitation while avoiding any detrimental impact of illumination. Hence, in this manuscript, we report the design, the syntheses, and the characterization of a whole series of hydrophobic, hydrophilic, and cell-permeant caged fluorophores, which are subsequently implemented to calibrate light intensity with one- and two-photon excitation in various environments and under different geometries.
Results and discussion
Principle
We consider a caged fluorophore. It is first suddenly submitted to spatially homogeneous and time-constant illumination with one-photon excitation set at the level of light intensity I to be measured. Provided that the absorbance of its solution is lower than 0.15, the light intensity is then essentially constant along the optical path. When its photoactivation is rate-limiting, the time evolution of its fluorescence emission reporting its photoconversion extent can be reliably fitted with a monoexponential function. Under such a condition, the retrieved characteristic time τ gives access to the light intensity I which is given in eqn (1)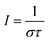 | (1) |
Accessing light intensity with two-photon excitation further requires to know the beam waist ωxy of the focused laser in order to get geometrical information. Then monoexponentially fitting the time evolution of the fluorescence signal from the illuminated solution of the caged fluorophore in a container (e.g. a microchamber or a cell) yields a characteristic time, which can be converted in the light power P at the sample with eqn (2).
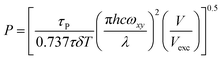 | (2) |
Design of the caged fluorophores
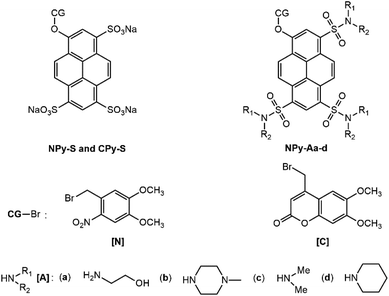
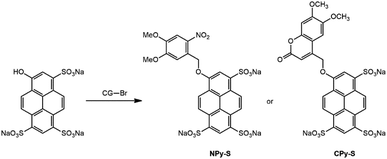
As a fluorophore scaffold, we chose 8-hydroxypyren-1,3,6-trisulfonic acid (HPTS, pyranine). This bright fluorophore is commercially available and highly water soluble, and it has found a wide range of applications as a tracer35,36 or as a pH indicator with a pKa equal to 7.3.37 After activation of the sulfonic groups, HPTS can be derivatized using nucleophiles to yield sulfonic esters and sulfonamides.38 In this work, we are interested to produce caged pyranine derivatives, which were anticipated to exhibit a range of solubility properties.HPTS itself has already been derivatized with a photolabile protecting group to produce caged fluorophores. The uncaging features of its 1-(2-nitrophenyl)ethyl ether30 have been established with one-30,31 and two-32 photon excitation. Its 4,5-dimethoxy-2-nitrobenzyloxy ether has also been reported in a patent.39 However, the reported syntheses of these caged ethers required tedious purification steps. In this manuscript, we first adopted the 4,5-dimethoxy-2-nitrobenzyl caging group, which benefits from a more red-shifted absorption than the 2-nitrobenzyl one so as to absorb light in a wavelength range useful for many applications. For the purpose of comparison, we also investigated the 6,7-dimethoxycoumarinyl-4-methyl caging group, which exhibits similar light absorption properties but a distinct mechanistic pathway for uncaging.17 The corresponding caged HPTS NPy-S and CPy-S (see Chart 1) have been produced with an efficient one-step synthetic protocol, which is compatible with the production of large amounts without any chromatography. Then NPy-S and CPy-S uncaging has been quantitatively characterized with one- and two-photon excitation and subsequently used for calibrating the light intensity of a confocal microscope.
The caged HPTS derivatives contain three ionizable sulfonic acid groups. Hence, they are well suited for applications in aqueous solutions. In contrast, they cannot be used for biological applications requiring internalization into cells nor in organic solvents. To overcome this limitation, we produced trisulfonamide pyranines, which have been caged with the 4,5-dimethoxy-2-nitrobenzyl group (see Chart 1). To favor water solubility while enabling crossing of the cell bilayers, we adopted the ethanolamino and N-methylpiperazino sulfonamide substituents, which possess some organic characteristics but as well traits favoring water-solubility either mediated by hydrogen bonding or generation of positive charges by protonation. Hence, we targeted the corresponding caged pyranine derivatives NPy-Aa and NPy-Ab. To achieve solubility in organic solvents while generating various steric hindrances to modulate any possible aggregation of the pyrene backbone, we chose dimethylamino and piperidino sulfonamide substituents and targeted the caged trisulfonamide pyranine derivatives NPy-Ac and NPy-Ad. We herein report the syntheses of these caged pyranine derivatives, their uncaging properties, and their application for measuring light intensity in living cells and zebrafish embryos.
Syntheses
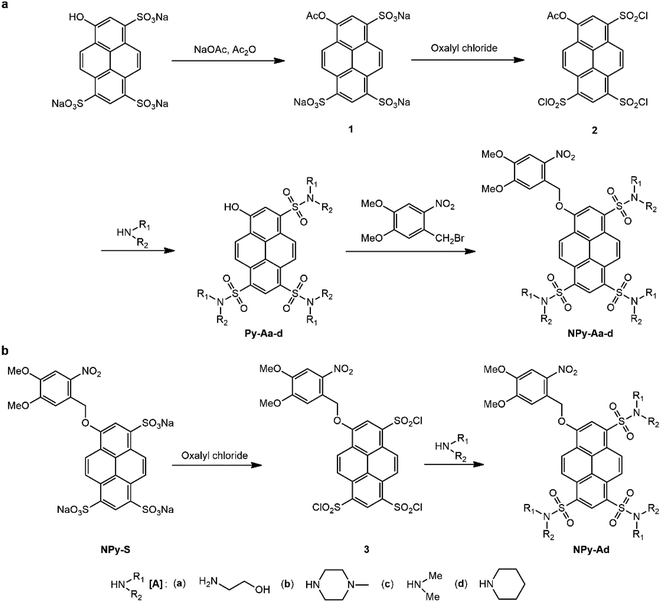
The caged HPTS NPy-S and CPy-S have been synthesized in one step with 90% and 74% respective yields upon etherifying the HPTS phenol with 4,5-dimethoxy-2-nitrobenzylbromide and 6,7-dimethoxy-4-bromomethyl coumarin (Scheme 1).The caged trisulfonamide pyranine derivatives were synthesized in four steps (Scheme 2a). In the first step, ester 1 was produced in 92% yield from acylating the HPTS phenol with acetic anhydride.35 In the next step, its sulfonic acid groups were converted to chlorosulfonyl groups by reacting with oxalyl chloride to form compound 2, which was used for the next step without any further purification.
The free trisulfonamide pyranine derivatives Py-Aa-d were then obtained by condensation of compound 2 with the amines in fair to good yields (50–71%). Then the caged trisulfonamide pyranine derivatives NPy-Aa-d were synthesized by reacting the free pyranine derivatives Py-Aa-d with 4,5-dimethoxy-2-nitrobenzylbromide in the presence of potassium carbonate in 54–80% yield. Alternatively, the caged trisulfonamide pyranine derivative NPy-Ad was obtained in 50% yield from converting the sulfonic acid groups of NPy-S to chlorosulfonyl groups with oxalyl chloride, followed by reaction with piperidine (Scheme 2b). It is noteworthy that none of these syntheses requires any dedicated separation nor tedious purification. In this respect, the pathway starting from the NPy-S caged HPTS enables the elimination of any residual free fluorophore Py-Ad in the final caged pyranine derivative NPy-Ad.
First investigations
All the free or caged pyranine derivatives are readily soluble in dimethyl sulfoxide to produce stock solutions. Hence, they were first dissolved in dimethyl sulfoxide and then diluted in their respective solvents for all experimental studies. The hydrophilic free and caged pyranine derivatives were diluted either in 10 mM pH = 7.4 PBS buffer or 10 mM pH = 8.4 Trizma buffer for NPy-Aa-b, NPy-S and CPy-S. At the corresponding pH, the phenol group of the free pyranine derivative is fully ionized (vide infra). This results in maximizing the fluorescence emission upon 488 nm wavelength excitation, which is widely available on commercially available optical setups. The hydrophobic free and caged pyranine derivatives were generally diluted in 1 mM pH = 8.4 Trizma buffer/acetonitrile 1/19 (v/v) for NPy-Ac-d. Here the organic solvent enables good solubility whereas the presence of water is required to liberate the free pyranine derivative17 and the basicity of the aqueous buffer causes phenol ionization of the free pyranine.At neutral pH, the free pyranine derivatives Py-Aa-d exhibit a strong absorption band in the 440–550 nm wavelength range peaking at 500 nm, which is red-shifted by 50 nm in comparison to HPTS (see Fig. S3†). Upon decreasing pH, this absorption band drops and a new absorption band appears at 420 nm as anticipated from the gradual protonation of the phenolate yielding the phenol (see Fig. S4†). The dependence of the absorbance at 488 nm and 420 nm on pH was utilized to extract the ground state pKa of the phenol function in the hydrophilic trisulfonamide pyranine derivatives Py-Aa-b. We found 6.0 ± 0.1 for Py-Aa, which is in reasonable agreement with reported values for pyranine trisulfonamides.38 For Py-Ab, the dependence of the absorbance on pH was better accounted for by taking into account two proton exchange constants. We found 4.7 ± 0.1 and 6.6 ± 0.1, which are interpreted as arising from ionization of the phenol and a protonated N-methyl nitrogen respectively. The pKa of Py-Aa is lower than the one of the parent HPTS (7.3 (ref. 37)) as anticipated from the absence of any destabilizing electrostatic interaction in the phenolate form. It is even lower in Py-Ab as expected from the stabilizing interaction between the negative charge of the phenolate and the positive charge of the ammonium. At neutral pH, the corresponding caged derivatives (NPy-Aa-d, NPy-S, and CPy-S) exhibit a maximum of absorption around 420 nm similar to the one observed for the phenol form of the free pyranine as expected from engaging its OH group in an ether linkage. All the spectroscopic parameters are reported in Table 1 (see also Table 3).
We next proceeded to a first series of preliminary illumination experiments to establish the relevance of the design of the caged pyranine derivatives NPy-Aa-d, NPy-S, and CPy-S and to fix optimal conditions for their reliable use as fluorescent actinometers for measuring light intensity. More precisely, we followed the time evolution of the fluorescence signal upon applying time constant and spatially homogeneous light at 365 nm on solutions of the caged pyranine derivatives (NPy-Aa-d, NPy-S, and CPy-S) contained in 54 μL cuvettes (3 mm optical pathlength). Fig. S5–S12† display the results. Depending on the nature of the caged pyranine derivative and on the experimental conditions, we observed an increase of the fluorescence signal by a factor of 1.4 to 20, which mainly occurred at the 50–500 s time scale at (0.1–2.5) 10−8 E s−1 light intensity and (0.6–14) 10−4 E m−2 s−1 photon flux density. We further evaluated 17–72% as the range of the uncaging yield from comparing the fluorescence intensities of solutions of the free pyranine derivatives (Py-Aa-d and HPTS) with the ones of solutions of the caged pyranine derivatives (NPy-Aa-d, NPy-S, and CPy-S) at the same concentration after uncaging. In particular we selected optimal experimental conditions in which the time evolution of the fluorescence signal of the illuminated solution could be reliably fitted with a monoexponential fitting function to extract the characteristic time τ, which led us to retrieve first values of the uncaging cross-sections σ from the knowledge of the applied light intensity I by using eqn (1) (see Table 2). In parallel, we performed experiments with biological samples with the caged pyranine derivatives NPy-Aa-b. We noticed that we could not achieve any penetration of NPy-Aa in living cells even after a long incubation (see Fig. S18†), which led us to discard the latter caged pyranine derivative for further investigations.
| Pyranine derivatives | σ I (m2 mol−1) | σ II (m2 mol−1) | σ III (m2 mol−1) |
|---|---|---|---|
| NPy-Aa | 1.6 ± 0.2 | — | — |
| NPy-Ab | 6.8 ± 0.7 | 6.2 ± 0.6 | 8.2 ± 0.8 |
| NPy-Ac | 18.3 ± 2.0 | — | — |
| NPy-Ad | 15.6 ± 1.6 | 38.9 ± 3.9 | 25.2 ± 2.5 |
| NPy-S | 8.4 ± 0.7 | 16.3 ± 1.6 | 6.0 ± 0.6 |
| CPy-S | 560 ± 56 | 462 ± 46 | 695 ± 70 |
As an outcome of this first series of experiments, we selected four caged fluorophores for further characterization making them relevant for calibration of light intensity in various environments: (i) NPy-Ad for applications in organic solvents and hydrophobic media; (ii) NPy-S and CPy-S for applications in aqueous solutions; (iii) NPy-Ab for biological applications requiring permeation through cell bilayers.
Quantitative analysis of uncaging of NPy-Ab, NPy-Ad, NPy-S and CPy-S with one-photon excitation
Fig. 1A, C, E and G display the time evolution of the absorption and emission spectra of the caged pyranine derivatives (NPy-Ab, NPy-Ad, NPy-S and CPy-S) upon illumination in a 1 × 1 cm2 quartz cuvette with a LED emitting at 365 nm in 10 mM pH = 7.4 PBS, 1 mM pH = 8.4 Trizma buffer/acetonitrile 1/19 (v/v), and 10 mM pH = 8.4 Trizma buffer respectively.As anticipated from uncaging at the considered pH, one observes the gradual decay of the absorption band associated with the caged pyranine derivatives NPy-Ad, NPy-S and CPy-S and the rise of the absorption band associated with the anionic form of the free pyranine Py-Ad and HPTS. The presence of isosbestic points further suggests the absence of any observable intermediate in the conversion of the caged into the free pyranine at the considered time scale.
As expected from caging the phenolic OH group, all the caged pyranine derivatives are weakly fluorescent or non-fluorescent upon excitation at 450–480 nm. Under illumination at 365 nm, one observes the gradual rise of the fluorescence emission identical to the one of the free pyranine, which supports the photorelease occurrence. We next analyzed the kinetics of the time evolutions of the absorbance and fluorescence signals. As shown in Fig. 1B, D, F and H, they were satisfactorily fitted with monoexponential functions and the extracted characteristic times were found in the same range for both observables. Hence, 642 and 515, 173 and 134, 535 and 298, and 82 and 72 s were found with absorbance and fluorescence for NPy-Ab, NPy-Ad, NPy-S and CPy-S respectively.
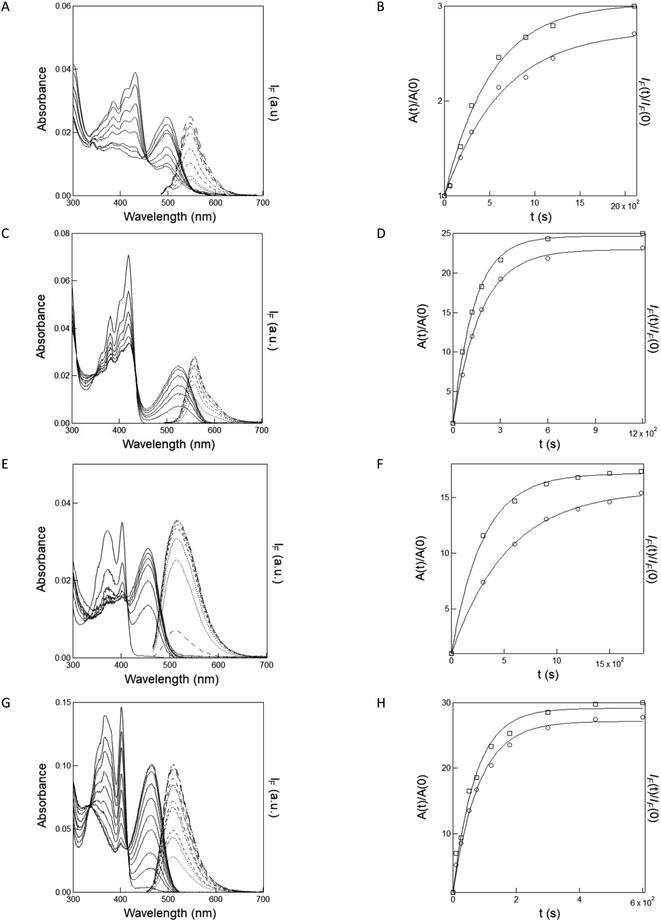 | ||
| Fig. 1 Quantitative analysis of NPy-Ab, NPy-Ad, NPy-S and CPy-S uncaging at 365 nm from absorption and fluorescence reporting. (A, C, E, G) Time evolution of the absorption (solid lines) and emission (dotted lines; λexc = 480 nm in (A, C) and 450 nm in (E, G)) spectra of solutions contained in a 1 × 1 cm2 quartz cuvette exposed to irradiation at 365 nm at constant light intensity I. (A) 2 μM NPy-Ab in 10 mM pH = 8.4 Trizma buffer, I = 7.2 × 10−8 E s−1. Time (s): 0, 60, 180, 300, 600, 900, 1200, 2100; C: 2 μM NPy-Ad in 1 mM pH = 8.4 Trizma buffer/acetonitrile 1/19 (v/v), I = 8.7 10−8 E s−1. Time (s): 0, 60, 120, 180, 300, 600, 1200; (E) 2 μM NPy-S in 10 mM pH = 8.4 Trizma buffer, I = 7.2 × 10−8 E s−1. Time (s): 0, 300, 600, 900, 1200, 1800; (G) 5 μM CPy-S in 10 mM pH = 8.4 Trizma buffer, I = 5.4 × 10−9 E s−1. Time (s): 0, 10, 25, 50, 75, 120, 180, 300, 450 and 600; (B, D, F, H): temporal evolution of the normalized absorbance (circles; λabs(nm): (B) 480; (D) 524; (F) 456; (H) 450) and normalized emission (squares; λexc (nm), λem (nm): (B) 480, 540; (D) 480, 556; (F), (H) 450,510). Markers: Experimental data from (A), (C), (E), (G), lines: monoexponential fit with eqn (S1).† Characteristic times have been retrieved from the absorbance and fluorescence data respectively: (B) 642 s and 515 s; (D) 173 s and 134 s; (F) 535 s and 298 s; (H) 82 s and 72 s. T = 293 K. | ||
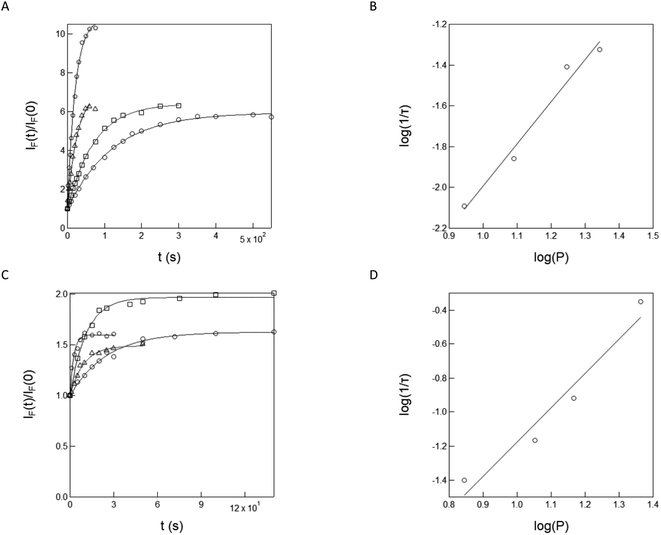
In a second series of experiments for quantitative characterization of the uncaging of NPy-Ab, NPy-Ad, NPy-S and CPy-S, we reproduced the experiments displayed in Fig. 1 upon irradiation at various light intensities delivered by a LED at 365 nm (see Fig. S13†). Then we applied the monoexponential fitting function given in eqn (S1)† to the temporal rises of the normalized fluorescence in order to retrieve the characteristic times of the photoconversion τ. As displayed in Fig. 2A–D, the inverse of the characteristic times of the photoconversion linearly depends on the light intensity. This behavior demonstrates that the rate limiting step of uncaging is photochemical at least up to 15.6 × 10−8 E s−1 light intensity. A linear fit was used to retrieve the values of the uncaging cross-sections from the slope: 6.2, 38.9, 16.3 and 462 m2 mol−1 were found for NPy-Ab, NPy-Ad, NPy-S and CPy-S respectively (see Table 2).
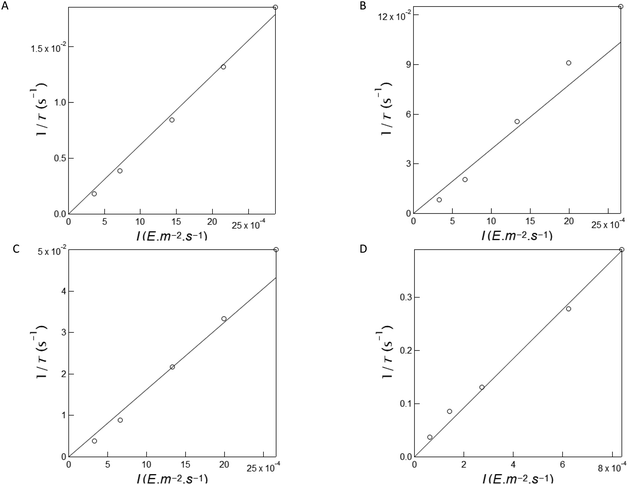 | ||
| Fig. 2 Measurement of the uncaging cross-section of NPy-Ab, NPy-Ad, NPy-S and CPy-S at 365 nm. Light intensity-dependence of the inverse of the characteristic time τ retrieved from the monoexponential fit of the fluorescence rise under 365 nm illumination for NPy-Ab (A), NPy-Ad (B), NPy-S (C) and CPy-S (D) in Fig. S11a–d.† Markers: Experimental data; solid line: linear fit. The extracted slopes are 6.2, 38.9, 16.3 and 462 m2 mol−1 for NPy-Ab, NPy-Ad, NPy-S and CPy-S respectively. T = 293 K. | ||
Equipped with the values of the uncaging cross-sections at 365 nm, we expanded the range of excitation wavelengths for uncaging by using a Xe lamp as a light source. Hence, we recorded the time evolution of the fluorescence signal from solutions of NPy-Ab, NPy-Ad, NPy-S and CPy-S upon applying illumination from 300 to 440 nm (see Fig. S14†). After monoexponential fitting, we retrieved the corresponding characteristic times, which were converted into values of the uncaging cross-section σ from knowledge of the light power measured with a power meter by further using the value of the uncaging cross-sections at 365 nm as a reference. The results are displayed in Table 3. Combined with the absorption spectra of NPy-Ab, NPy-Ad, NPy-S and CPy-S displayed in Fig. S3B, D and E,† they have been used to retrieve the uncaging quantum yields of NPy-Ab, NPy-Ad, NPy-S and CPy-S at several wavelengths (see Table 3).
| Caged pyranine derivatives | λ exc (nm) | ε(λ) (mM−1 cm−1) | σ(λ) (m2 mol−1) | 103 × φ(λ) |
|---|---|---|---|---|
| NPy-Ab | 300 | 24 | 2.6 ± 0.3 | 0.5 ± 0.1 |
| 365 | 14 | 6.2 ± 0.6 | 1.9 ± 0.2 | |
| 405 | 18 | 1.4 ± 0.1 | 0.3 ± 0.02 | |
| 440 | 18 | 2.7 ± 0.3 | 0.7 ± 0.1 | |
| NPy-Ad | 300 | 32 | 97.0 ± 9 | 13.2 ± 1.2 |
| 365 | 17 | 38.9 ± 3.9 | 9.9 ± 1.0 | |
| 405 | 27 | 43.0 ± 4.3 | 6.9 ± 0.7 | |
| 440 | 4 | 9.2 ± 1 | 10 ± 1.1 | |
| NPy-S | 300 | 6.5 | 29.1 ± 2.9 | 19.5 ± 1.9 |
| 365 | 16 | 16.3 ± 1.6 | 4.4 ± 0.4 | |
| 405 | 16 | 4.7 ± 0.5 | 1.3 ± 0.1 | |
| CPy-S | 300 | 10 | 544 ± 54 | 236 ± 23 |
| 365 | 25 | 462 ± 46 | 80 ± 8 | |
| 405 | 22 | 464 ± 46 | 92 ± 9 | |
| 440 | 0.6 | 14 ± 1 | 101 ± 10 |
The uncaging quantum yields of NPy-Ab, NPy-Ad, and NPy-S are in the expected range found for diverse substrates caged with the nitroveratryl group.40 In contrast, one notices the high value of the uncaging quantum yield of CPy-S. Interestingly, non-vanishing quantum yields of uncaging are found for NPy-Ab and NPy-Ad at 440 nm at which wavelength the caging group does not significantly absorb.40 This observation suggests that there is a coupling between the pyranine and the nitroveratryl chromophore for driving uncaging, which may also account for some variation of the extracted quantum yields with the excitation wavelength. This result is in line with previous observations in which coupling a chromophore with a caging nitrobenzyl group significantly altered the cross-section for uncaging of the latter with one-and two-photon excitation.30,33
In the last series of experiments for quantitative characterization of the uncaging of NPy-Ab, NPy-Ad, NPy-S and CPy-S, we measured their uncaging cross-section at 365 nm by using capillary electrophoresis or HPLC to quantitate both the disappearance of the caged pyranines and the formation of the photoreleased pyranine upon illumination at 365 nm. Fig. 3A–D display the results. As expected, the peak associated with the caged pyranine derivative gradually disappears and it tends towards zero. At the same rate, the peak from the free pyranine grows and saturates at concentrations indicating an uncaging yield in the (19–67)% range. After monoexponential fitting of the time concentration evolutions, we retrieved the characteristic times associated with the decay of the caged pyranine derivatives and the rise of the photoreleased pyranine derivatives and subsequently extracted the uncaging cross-sections: 8.2 ± 0.8, 28.9 ± 2.9 and 21.5 ± 2.1, 6.0 ± 0.6 and 6.0 ± 0.6, 606 ± 61 and 785 ± 78 m2 mol−1 were respectively found for NPy-Ab, NPy-Ad, NPy-S and CPy-S in fair agreement with the values retrieved from the preceding fluorescence experiments (see Table 2).
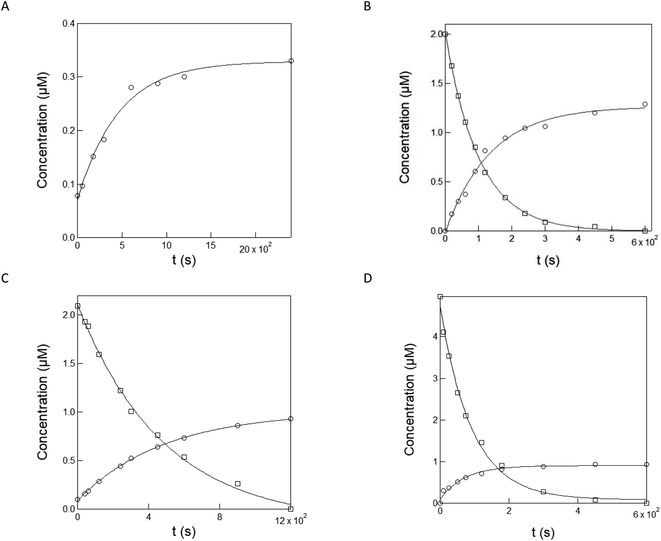 | ||
| Fig. 3 Evaluation of the kinetics and yield associated with uncaging of NPy-Ab (by capillary electrophoresis), NPy-Ad, NPy-S and CPy-S (by HPLC) upon illumination at 365 nm LED. Time evolution of the composition of solutions of NPy-Ab ((A) 1 μM in 10 mM pH = 8.4 Trizma buffer), NPy-Ad ((B) 2 μM in 1 mM pH = 7.4 PBS buffer/acetonitrile 1/19 v/v), NPy-S ((C) 2 μM in10 mM pH = 8.4 Trizma buffer), and CPy-S ((D) 5 μM in 10 mM pH = 8.4 Trizma buffer) upon illumination at 365 nm at 1.0 × 10−7 E s−1 (for NPy-Ab, NPy-Ad and NPy-S) and 5.4 × 10−9 E s−1 (for CPy-S). Markers: concentrations of the caged compounds NPy-Ad, NPy-S, and CPy-S (squares) and photoreleased compounds Py-Ab, Py-Ad and HPTS (circles) as extracted from the peak areas in the electropherogram (for NPy-Ab) and HPLC chromatograms (NPy-Ad, NPy-S and CPy-S); solid lines: monoexponential fit with eqn (S1);† the characteristic times 101, 484, 92 s for NPy-Ad, NPy-S and CPy-S and 464, 136, 486, and 71 s for Py-Ab, Py-Ad and HPTS have been retrieved from (A), (B), (C) and (D) respectively. T = 293 K. | ||
We eventually ended up this series of uncaging experiments in cuvettes by evaluating the kinetics of photobleaching upon submitting 2 μM solutions of the free pyranine derivatives Py-Ab, Py-Ad and HPTS to LED illumination at 365 nm (see Fig. S15†). We could extract an estimate of their photobleaching cross-section: 0.7, 2.7, and 1.4 m2.mol−1 were retrieved for Py-Ab, Py-Ad and HPTS respectively, which suggests that no significant photobleaching should occur upon using the caged pyranine derivatives for measuring light intensity.
Quantitative analysis of uncaging of NPy-S and CPy-S with two-photon excitation
We also performed a series of experiments on NPy-S and CPy-S to measure their cross-section for uncaging with two-photon excitation. For designing the measurement protocol, we took advantage of the increase of the fluorescence signal upon uncaging. Fig. 4A and C respectively display the temporal evolution of the fluorescence emission from 3 μM solution of NPy-S and CPy-S in 10 mM pH = 8.4 Trizma buffer upon irradiation at 750 nm at (7–23) mW laser powers. One observes a continuous increase, which is in line with the realization of the uncaging process observed above. We retrieved the characteristic time τ from applying a monoexponential fitting function and we verified that the dependence of the inverse of τ with the applied laser power was quadratic as expected from eqn (2). More precisely, we found 2.1 and 2.0 for the power exponent for NPy-S and CPy-S respectively. Hence, we could conclude that the observed uncaging behavior did result from two-photon absorption. We could also extract the associated uncaging cross-sections with two-photon excitation. We retrieved 4.2 × 10−3 and 4.2 × 10−2 GM for NPy-S and CPy-S respectively. In particular, the uncaging cross-section with two-photon excitation found for NPy-S compares well with the 4 × 10−3 GM value, which we previously found for another nitroveratryl caged phenol.41Applications
As a first application, we implemented the caged pyranine derivative NPy-Ab for measuring light intensity in cultured cells. Living HeLa and HEK cells were conditioned for 30 min with 0.5 or 1 μM solutions of NPy-Ab in DMEM medium without generating any significant cell death (Fig. S16†). Their external medium was subsequently washed prior to illumination to avoid any possible interference, which could originate from photoreleasing the free fluorophore in the incubating solution. Then the HeLa and HEK cells were imaged with an epifluorescence by using 405 nm LED illumination. Fig. 5 displays the results. As shown in Fig. 5A and C, both cells conditioned with DMEM medium only did not yield any rise of the fluorescence emission upon illumination with a 405 nm LED. In contrast, we evidenced a rise of the fluorescence emission within 0.5 (Fig. 5B and D) or 1 (Fig. S17†) μM NPy-Ab-conditioned cells, which significantly departed from the corresponding behavior observed in the background and was consistent with the photorelease of free Py-Ab (see also Fig. S18† for a similar observation with 365 nm LED illumination). Moreover, we observed that the characteristic times retrieved from processing the fluorescence increase with a monoexponential function from illuminated cells under identical illumination conditions were essentially similar (0.21 and 0.23 s for HeLa and HEK cells respectively) and independent of the concentration of the NPy-Ab solution (0.21 and 0.28 s (in HeLa cells); 0.23 and 0.21 s (in HEK cells) for 0.5 and 1 μM respectively) (Fig. 5; see also Fig. S17†). Hence, we could adopt the uncaging cross-section of NPy-Ab in 10 mM pH = 8.4 Trizma buffer to extract the value of the photon flux density applied on both the cells and found 0.48 and 0.44 E m−2 s−1 for HeLa and HEK cells respectively under a 405 nm LED (and 0.1 E m−2 s−1 for HeLa cells under a 365 nm LED in Fig. S18†).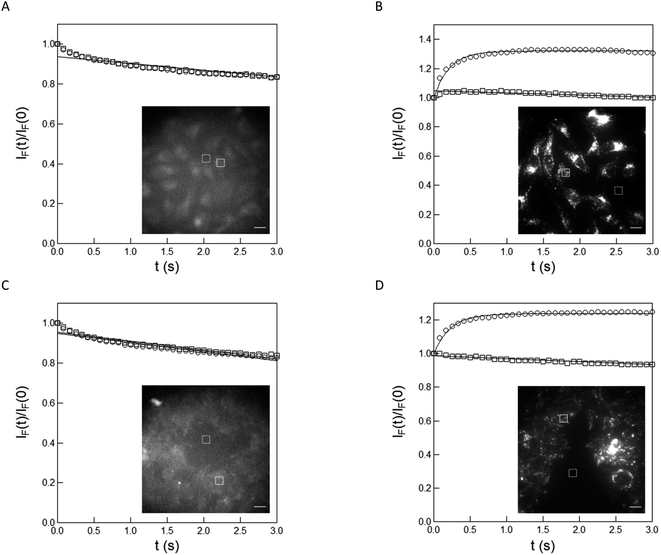 | ||
| Fig. 5 Measurement of the photon flux density in living HeLa and HEK cells at 405 nm in epifluorescence microscopy. Time evolution of the fluorescence signal from living HeLa (A), (B) and HEK (C), (D) cells (inset; scale bar: 20 μm) conditioned with DMEM ((A), (C); control experiment) and 0.5 μM NPy-Ab (B), (D) under illumination at 405 nm. Markers: the circles and squares are associated with the frames delimited by the solid and dashed lines in the inset images respectively. Fluorescence signal as extracted from integrating the fluorescence level over the ROIs; solid lines: monoexponential fit with eqn (S1);† the characteristic times 0.21 and 0.23 s have been retrieved from processing the fluorescence signal over the frames delimited by the solid line in (B) and (D) respectively. T = 298 K. | ||
The preceding series of experiments have been performed at photon flux densities reaching at most 10−1 E m−2 s−1. Such values are significantly lower than the ones currently exploited in high performance optical microscopies. Hence, we utilized the caged pyranine derivative NPy-Ab, NPy-S and CPy-S in order to explore the possibility of measuring the light intensity of a confocal microscope, which exploits photon flux densities up to 104 E m−2 s−1. They were conditioned as liquid thin films sandwiched between two glass slides and submitted to dual illumination at 405 nm (for uncaging) and 488 nm (inactive for uncaging but favorable for imaging the photoreleased fluorophore). As displayed in Fig. 6A–C, the illumination governs an increase of the fluorescence intensity, which is in line with photoreleasing the free pyranine derivatives. We also retrieved the characteristic time τ from the time evolution of the fluorescence signals, from which we could extract 9.8, 4.3, and 444 m2 mol−1 for the uncaging cross-section at 405 nm of NPy-Ab, NPy-S and CPy-S respectively from further exploiting the applied light power (independently measured with a power meter) and the beam waist of the 405 nm laser in the focal plane (see ESI†). The latter values of the uncaging cross-sections are in the range measured from the previous cuvette experiments (see Table 3). Hence the reasonable agreement between the values of the uncaging cross-sections over a wide range of photon flux densities suggests that the three caged pyranines NPy-Ab, NPy-S and CPy-S can be implemented to extract the photon flux density in a variety of optical setups. Specifically, we subsequently used the 9.8 m2 mol−1 uncaging cross-section for NPy-Ab measured in confocal microscopy together with eqn (1) to retrieve the light intensity I in epifluorescence and confocal microscopy in biological systems.
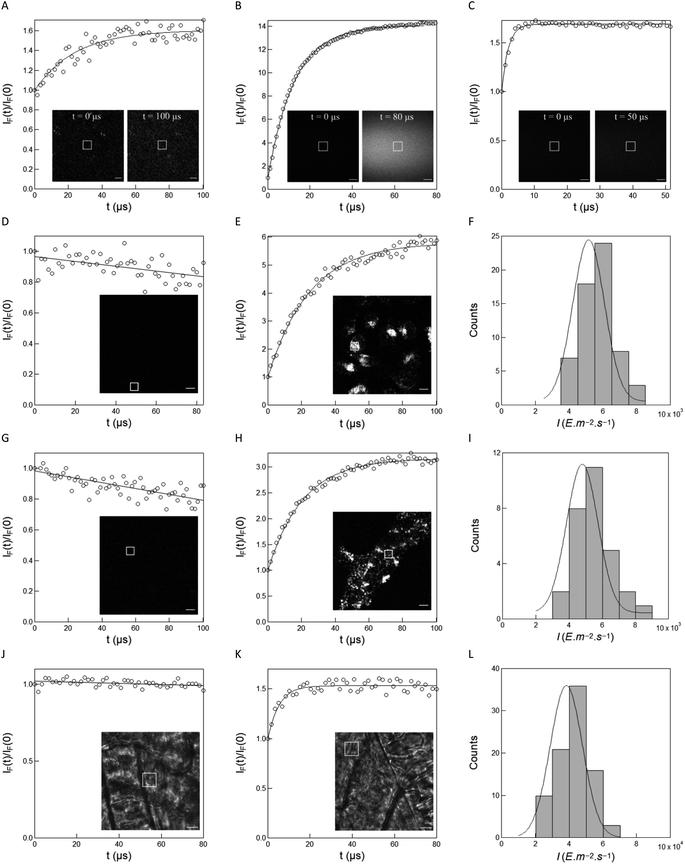 | ||
Fig. 6 Measurements of the photon flux density delivered at 405 nm in confocal microscopy. (A–C) Time evolution of the fluorescence signal from a liquid film (inset; scale bar: 10 μm for (A), and 50 μm for (B) and (C)) of 1 μM NPy-Ab in 10 mM pH = 8.4 trizma buffer (laser power: 2% for 405 nm; frame: 256 × 256 pixel2; zoom factor: 4; dwell time: 1.7 μs) (A), 1 μM NPy-S in 10 mM pH = 8.4 Trizma buffer (laser power: 20% for 405 nm and 100% for 488 nm; frame: 1024 × 1024 pixel pixel2; zoom factor: 1; dwell time: 1.03 μs) (B) and 2 μM CPy-S in 10 mM pH = 8.4 Trizma buffer (laser power: 1% for 405 nm and 100% for 488 nm; frame: 1024 × 1024 pixel pixel2; zoom factor: 1; dwell time: 1.03 μs) (C) and of living HeLa (D, E) and HEK (G, H) (laser power: 2%; frame: 256 × 256 pixel pixel2; zoom factor: 4; dwell time: 1.7 μs) and fluorescence image (inset; scale bar: 10 μm) cells conditioned with DMEM (D, G; control experiment) and 1 μM NPy-Ab (E, H; together with 2 μM DMSO originating from the NPy-Ab stock solution) in DMEM, and of living zebrafish embryos (J, K) (laser power: 10%; frame: 256 × 256 pixel2; zoom factor: 5; dwell time: 1.7 μs) and bright field image (inset; scale bar: 10 μm) conditioned with 2 μM DMSO (J; control experiment) and 1 μM NPy-Ab (K; together with 2 μM DMSO originating from the NPy-Ab stock solution) in water. Markers: Fluorescence signal as extracted from integrating the fluorescence level over the ROI (white frame in the inset image); solid line: monoexponential fit with eqn (S1);† the characteristic time (μs) has been retrieved from processing the fluorescence signal over the ROI. A: 24; B: 13; C: 2; D: 25; H: 21; K: 5.3; F, I, L: distribution of light intensity I in living HeLa (F) and HEK (I) cells, and in living zebrafish embryos (L) conditioned with 1 μM NPy-Ab in DMEM (F, I) and water (L). Bars: Frequency of light intensity extracted from different ROIs (F: sample size: 60 ROIs, minimum/maximum bin: 3.0 × 103/8.0 × 103 E m−2 s−1 and bin size: 1.0 × 103 E m−2 s−1; I: sample size: 29 ROIs, minimum/maximum bin: 2.5 × 103/8.0 × 103 E m−2 s−1 and bin size: 1.0 × 103 E m−2 s−1; L: sample size: 88 ROIs from 10 embryos, minimum/maximum bin: 1.5 × 104/7.0 × 104 E m−2 s−1 and bin size: 1.0 × 104 E m−2 s−1); Solid lines: Fit with Eq. 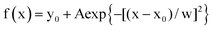 . T = 293 K. . T = 293 K. | ||
As a demanded application in the context of optogenetics, we then exploited the caged pyranine derivative NPy-Ab for measuring light intensity delivered at 405 nm from a confocal microscope in living HeLa, HEK and zebrafish embryos.
In the absence of any control of the internal concentration in NPy-Ab within these systems, we preliminarily studied its significance in the uncaging kinetics. Fig. S7† shows that the characteristic times extracted for NPy-Ab uncaging at the same 15.6 × 10−9 E s−1 light intensity were similar at different concentrations spanning the 0.5–2 μM range thereby suggesting the NPy-Ab uncaging cross-section to be not dependent on its concentration within the investigated range.
We first examined the measurement of light intensity in living HeLa and HEK cells. Living cells conditioned with DMEM medium did not promote any fluorescence emission rise upon illumination at 405 nm (Fig. 6D and G). In contrast, a fluorescence rise satisfactorily fitted with a monoexponential function was observed in both HeLa and HEK cells incubated for 30 min with 1 μM NPy-Ab solution in DMEM medium and then washed with DMEM medium to avoid uncaging any external NPy-Ab (Fig. 6E and H; see also Fig. S19†). The latter observation was in line with the photo-release without any significant leakage through the cell membrane of the free pyranine derivative Py-Ab (see Fig. S20†). Using 9.8 m2 mol−1 for the uncaging cross-section of NPy-Ab, we converted the distribution of characteristic times τ into the distribution of light intensity with 5.1 × 103 and 4.8 × 103 E m−2 s−1 average for living HeLa and HEK cells respectively (Fig. 6F and I).
These results allow us to measure the light intensity within the living cultured cells or zebrafish embryos without interference of diffusion of free Py-Ab.
We then proceeded with the measurement of light intensity in 5 hpf zebrafish embryos conditioned overnight with 1 μM NPy-Ab in water and then washed with water to remove any external NPy-Ab (see also Fig. S21†). They were placed in an agarose mold prior to light scanning with a confocal microscope under illumination at 405 nm. In the control experiment shown in Fig. 6J, illumination of NPy-Ab-free embryos did not promote any rise of the fluorescence emission. In contrast, the fluorescence signal rose upon illumination for the embryos conditioned with 1 μM NPy-Ab (Fig. 6K), which suggested NPy-Ab uncaging to occur with release of the free pyranine derivative Py-Ab (similar observations were made for embryos conditioned with 0.1 or 2 μM NPy-Ab; see Fig. S21†). We retrieved the characteristic times τ from applying a monoexponential function on the time evolutions of the fluorescence signal. Exploiting the 9.8 m2 mol−1 uncaging cross-section of NPy-Ab measured in confocal microscopy and eqn (1), we then extracted the distribution of light intensity I in living zebrafishes peaking at 3.8 × 104 E m−2 s−1 average value (Fig. 6L).
Conclusions
This report introduces and quantitatively characterizes the photoactivation properties of easily accessible caged fluorophores, which could find applications in multiple communities of end-users. We first set up syntheses of both hydrophilic and hydrophobic caged pyranine derivatives, which can be performed on a large scale and do not require any dedicated separation nor tedious purification. The resulting dark caged pyranine derivatives have been shown to liberate the corresponding fluorescent pyranines in a diversity of media upon illumination. Thus, a solution of caged pyranine absorbing constant light delivers a fluorescence signal, the time evolution of which can be robustly fitted with a monoexponential function. From extracting the characteristic time at known light intensities, we retrieved the associated uncaging cross-sections over a wide window of wavelengths and a broad range of light intensities. Equipped with this information, the present caged pyranine derivatives can be conversely harnessed for measuring light intensity with UV light in the 300–450 nm and 750 nm wavelength range with one and two-photon excitation respectively. In particular, we demonstrated their relevance for applications in aqueous solutions, hydrophobic solvents, and biological samples.Data availability
Data for this paper, including the absorption spectra of the caged pyranine derivatives are available at Zenodo at https://zenodo.org/doi/10.5281/zenodo.10137354.Author contributions
Conceptualization: I. A., T. L. S., L. J.; formal analysis: M. M., H. S. T., Q. M., E. S., I. A., T. L. S., L. J.; funding acquisition: I. A., T. L. S., L. J.; investigation: M. M., H. S. T., Q. M., E. S., M.-A. P., I. A., T. L. S., C. R., S. V.; methodology: M. M., I. A., T. L. S., L. J.; project administration: L. J.; resources: M. M., H. S. T., Q. M., E. S., M.-A. P., I. A., T. L. S., C. R., S. V.; supervision: I. A., T. L. S., L. J.; validation: M. M., H. S. T., Q. M., I. A.; visualization: M. M., H. S. T., I. A., L. J.; writing (original draft): M. M., H. S. T., I. A., L. J.; writing (review & editing): all authors.Conflicts of interest
The authors have a patent related to the content of this paper.Acknowledgements
This work was supported by the ANR (France BioImaging – ANR-10-INBS-04, Morphoscope2 – ANR-11-EQPX-0029, IPGG – ANR-10-IDEX-0001-02 PSL, ANR-10-LABX-31, and ANR-19-CE11-0005), the ITMO Cancer of Aviesan within the framework of the 2021–2030 Cancer Control Strategy on funds administered by Inserm, and the EIC Pathfinder Open DREAM (GA number 101046451).Notes and references
- D. Cambié, C. Bottecchia, N. J. W. Straathof, V. Hessel and T. Noël, Chem. Rev., 2016, 116, 10276–10341 CrossRef.
- D. Ravelli, D. Dondi, M. Fagnoni and A. Albini, Chem. Soc. Rev., 2009, 38, 1999–2011 RSC.
- G. Miesenböck, Science, 2009, 326, 395–399 CrossRef.
- K. Deisseroth, Nat. Methods, 2011, 8, 26–29 CrossRef CAS PubMed.
- Z. Feng, W. Zhang, J. Xu, C. Gauron, B. Ducos, S. Vriz, M. Volovitch, L. Jullien, S. Weiss and D. Bensimon, Rep. Prog. Phys., 2013, 76, 072601 CrossRef.
- D. L. Coutu and T. Schroeder, J. Cell Sci., 2013, 126, 3805–3815 CAS.
- A. Su, S. Grist, A. Geldert, A. Gopal and A. Herr, PLoS One, 2021, 16, e024355 Search PubMed.
- A. Bérces, A. Fekete, S. Gáspár, P. Gróf, P. Rettberg, G. Horneck and G. Rontó, J. Photochem. Photobiol., B, 1999, 53, 36–43 CrossRef.
- J. Longstreth, F. R. de Gruijl, M. L. Kripke, S. Abseck, F. Arnold, H. I. Slaper, G. Velders, Y. Takizawa and J. C. van der Leun, J. Photochem. Photobiol., B, 1998, 46, 20–39 CrossRef CAS PubMed.
- S. Aslam and K. Edgget, Wearable radiation detector, US Pat., US9024271B2, 2015 Search PubMed.
- P. Foller, I. Fritz, C. Olguin, S. Wrobel, C. Le Maitre, E. Kang and S. Tibbits, Sensing of solar ultraviolet radiation by wearable colorimetry, WO2018232387A1, 2018.
- U. Megerle, R. Lechner, B. König and E. Riedle, Photochem. Photobiol. Sci., 2010, 9, 1400–1406 CrossRef CAS.
- D. Grünwald, S. M. Shenoy, S. Burke and R. H. Singer, Nat. Protoc., 2008, 3, 1809–1814 CrossRef.
- H. J. Kuhn, S. E. Braslavsky and R. Schmidt, Pure Appl. Chem., 2004, 76, 2105–2146 CAS.
- M. Reinfelds, V. Hermanns, T. Halbritter, J. Wachtveitl, M. Braun, T. Slanina and A. Heckel, ChemPhotoChem, 2019, 3, 441–449 CrossRef CAS.
- G. C. R. Ellis-Davies, Nat. Methods, 2007, 4, 619–628 CrossRef CAS PubMed.
- P. Klán, T. Šolomek, C. G. Bochet, A. Blanc, R. Givens, M. Rubina, V. Popik, A. Kostikov and J. Wirz, Chem. Rev., 2013, 113, 119–191 CrossRef.
- J. B. Grimm, A. J. Sung, W. R. Legant, P. Hulamm, S. M. Matlosz, E. Betzig and L. D. Lavis, ACS Chem. Biol., 2013, 8, 1303–1310 CrossRef CAS PubMed.
- V. N. Belov, C. A. Wurm, V. P. Boyarskiy, S. Jakobs and S. W. Hell, Angew. Chem., Int. Ed., 2010, 49, 3520–3523 CrossRef CAS PubMed.
- A. Loredo, J. Tang, L. Wang, K.-L. Wu, Z. Peng and H. Xiao, Chem. Sci., 2020, 11, 4410–4415 RSC.
- J. Tang, M. A. Robichaux, K.-L. Wu, J. Pei, N. T. Nguyen, Y. Zhou, T. G. Wensel and H. Xiao, J. Am. Chem. Soc., 2019, 141, 14699–14706 CrossRef CAS PubMed.
- J. B. Grimm, B. P. English, H. Choi, A. K. Muthusamy, B. P. Mehl, P. Dong, T. A. Brown, J. Lippincott-Schwartz, Z. Liu, T. Lionnet and L. D. Lavis, Nat. Methods, 2016, 13, 985–988 CrossRef CAS PubMed.
- D. Warther, F. Bolze, J. Léonard, S. Gug, A. Specht, D. Puliti, X.-H. Sun, P. Kessler, Y. Lutz, J.-L. Vonesch, B. Winsor, J.-F. Nicoud and M. Goeldner, J. Am. Chem. Soc., 2010, 132, 2585–2590 CrossRef CAS PubMed.
- F. M. Raymo, Int. Scholarly Res. Not., 2012 DOI:10.5402/2012/619251.
- D. J. Kozlowski, T. Murakami, R. K. Ho and E. S. Weinberg, Biochem. Cell Biol., 1997, 75, 551–562 CrossRef CAS.
- J. A. Clanton, I. A. Shestopalov, J. K. Chen and J. T. Gamse, J. Vis. Exp., 2011, 50, 2672 Search PubMed.
- W. R. Lempert and S. R. Harris, Meas. Sci. Technol., 2000, 11, 1251–1258 CrossRef.
- P. Sengupta, S. B. Van Engelenburg and J. Lippincott-Schwartz, Chem. Rev., 2014, 114, 3189–3202 CrossRef CAS PubMed.
- M. Fernández-Suárez and A. Y. Ting, Nat. Rev. Mol. Cell Biol., 2008, 9, 929–943 CrossRef.
- R. Jasuja, J. Keyoung, G. P. Reid, D. R. Trentham and S. Khan, Biophys. J., 1999, 76, 1706–1719 CrossRef CAS PubMed.
- F. F. Trigo, J. E. Corrie and D. Ogden, J. Neurosci. Methods, 2009, 180, 9–21 CrossRef.
- N. I. Kiskin, R. Chillingworth, J. A. McCray, D. Piston and D. Ogden, Eur. Biophys. J., 2002, 30, 588–604 CrossRef CAS.
- Y. Zhao, Q. Zheng, K. Dakin, K. Xu, M. L. Martinez and W.-H. Li, J. Am. Chem. Soc., 2004, 126, 4653–4663 CrossRef CAS.
- L. Lilge, T. J. Flotte, I. E. Kochevar, S. L. Jacques and F. Hillenkamp, Photochem. Photobiol., 1993, 58, 37–44 CrossRef CAS.
- S. R. Bobe, A. M. Raynor, S. V. Bhosale and S. V. Bhosale, Aust. J. Chem., 2013, 67, 615–619 CrossRef.
- B. L. Bloodgood and B. L. Sabatini, Science, 2005, 310, 866–869 CrossRef CAS PubMed.
- O. S. Wolfbeis, E. Fürlinger, H. Kroneis and H. Marsoner, Fresenius' Z. Anal. Chem., 1983, 314, 119–124 CrossRef CAS.
- B. Finkler, C. Spies, M. Vester, F. Walte, K. Omlor, I. Riemann, M. Zimmer, F. Stracke, M. Gerhards and G. Jung, Photochem. Photobiol. Sci., 2014, 13, 548–562 CrossRef CAS PubMed.
- R. P. Haugland and K. R. Gee, Photocleavable derivatives of hydroxypyrenesulfonic acids, US Pat., US5514710A, 1996 Search PubMed.
- I. Aujard, C. Benbrahim, M. Gouget, O. Ruel, J. B. Baudin, P. Neveu and L. Jullien, Chem.–Eur. J., 2006, 12, 6865–6879 CrossRef CAS PubMed.
- D. K. Sinha, P. Neveu, N. Gagey, I. Aujard, C. Benbrahim-Bouzidi, T. Le Saux, C. Rampon, C. Gauron, B. Goetz and S. Dubruille, ChemBioChem, 2010, 11, 653–663 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3sc04183b |
| This journal is © The Royal Society of Chemistry 2023 |