Open Access Article
Open Access ArticleCyclobutane-bearing restricted anchoring residues enabled geometry-specific hydrocarbon peptide stapling†
Baobao
Chen‡
a,
Chao
Liu‡
 a,
Wei
Cong
a,
Fei
Gao
a,
Wei
Cong
a,
Fei
Gao
 a,
Yan
Zou
a,
Yan
Zou
 b,
Li
Su
a,
Lei
Liu
b,
Li
Su
a,
Lei
Liu
 c,
Alexander
Hillisch
de,
Lutz
Lehmann
d,
Donald
Bierer
d,
Xiang
Li
*b and
Hong-Gang
Hu
c,
Alexander
Hillisch
de,
Lutz
Lehmann
d,
Donald
Bierer
d,
Xiang
Li
*b and
Hong-Gang
Hu
 *a
*a
aSchool of Medicine or Institute of Translational Medicine, Shanghai University, Shanghai 200444, China. E-mail: hhu66@shu.edu.cn
bSchool of Pharmacy, Second Military Medical University, Shanghai 200433, China. E-mail: xiangli@smmu.edu.cn
cDepartment of Chemistry, Tsinghua University, Beijing 100084, China
dBayer AG, Pharma Division, Drug Discovery Sciences, Aprather Weg 18A, Wuppertal 42096, Germany
eUCB BioSciences GmbH, Alfred-Nobel-Straße 10, 40789 Monheim am Rhein, Germany
First published on 29th September 2023
Abstract
Stapled peptides are regarded as the promising next-generation therapeutics because of their improved secondary structure, membrane permeability and metabolic stability as compared with the prototype linear peptides. Usually, stapled peptides are obtained by a hydrocarbon stapling technique, anchoring from paired olefin-terminated unnatural amino acids and the consequent ring-closing metathesis (RCM). To investigate the adaptability of the rigid cyclobutane structure in RCM and expand the chemical diversity of hydrocarbon peptide stapling, we herein described the rational design and efficient synthesis of cyclobutane-based conformationally constrained amino acids, termed (E)-1-amino-3-(but-3-en-1-yl)cyclobutane-1-carboxylic acid (E7) and (Z)-1-amino-3-(but-3-en-1-yl)cyclobutane-1-carboxylic acid (Z7). All four combinations including E7-E7, E7-Z7, Z7-Z7 and Z7-E7 were proven to be applicable in RCM-mediated peptide stapling to afford the corresponding geometry-specific stapled peptides. With the aid of the combined quantum and molecular mechanics, the E7-E7 combination was proven to be optimal in both the RCM reaction and helical stabilization. With the spike protein of SARS-CoV-2 as the target, a series of cyclobutane-bearing stapled peptides were obtained. Among them, E7-E7 geometry-specific stapled peptides indeed exhibit higher α-helicity and thus stronger biological activity than canonical hydrocarbon stapled peptides. We believe that this methodology possesses great potential to expand the scope of the existing peptide stapling strategy. These cyclobutane-bearing restricted anchoring residues served as effective supplements for the existing olefin-terminated unnatural amino acids and the resultant geometry-specific hydrocarbon peptide stapling provided more potential for peptide therapeutics.
Introduction
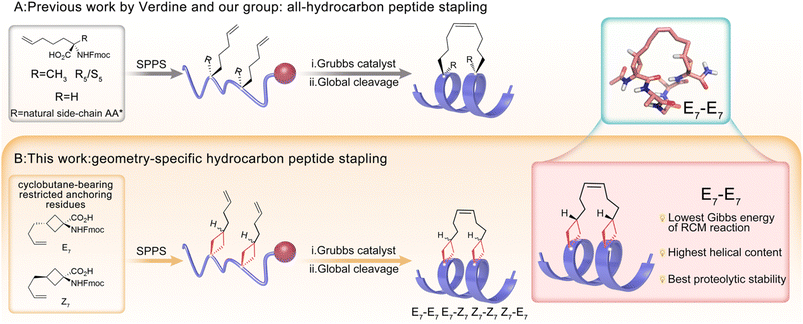
Protein–protein interactions (PPIs) regulate several signaling pathways and physiological processes,1–4 and dysfunction of these interactions is thought to be associated with many human diseases.5–7 Therefore, targeting specific protein–protein interactions to achieve the treatment of related diseases is currently a research hotspot in drug design.8–11 Benefiting from their unique physiochemical properties including the flexible backbone structure and a molecular size between those of small molecules and biomacromolecules (proteins and antibodies), peptides are regarded as suitable candidates for modulating or interfering with PPIs.12,13 However, linear peptides are rarely able to spontaneously form stable secondary structures (mostly helices) in solution, resulting in poor in vivo stability and membrane impermeability, which represent major stumbling blocks for peptide drug development.14–16Currently, the most straightforward and effective chemical modification to stabilize peptide helices is the peptide stapling strategy. It refers to the concept of peptide side-chain cross-linking via the peptidic or non-peptidic structure, such as disulfide surrogates,17–19 lactams,20,21 triazole,22–24 and other compounds,25,26 to preorganize a stable helical conformation with a reduced entropic penalty. Stapling strategies such as via dithiocarbamate27 (DTC), formaldehyde crosslinking,28 sulfonamide29 and other stapling strategies16,30–34 were recently developed, which successfully improved the physicochemical and pharmacological properties of linear peptides. Among these stapling strategies, the all-hydrocarbon stapling strategy developed by Verdine and co-workers35 is regarded as the most established one and has been applied in inhibiting diverse PPIs.36–38 The i, i + 4 position side-chain cross-linking was realized via the Grubbs reagent-catalyzed ring-closing metathesis (RCM) reaction between olefin-bearing amino acids S-2-(4-pentenyl)Ala-OH (S5) and/or R-2-(4-pentenyl)Ala-OH (R5). A notable example was ALRN-6924, an all-hydrocarbon stapled peptide antagonist of both MDM2 and MDMX, which reactivated the p53 pathway to kill tumor cells and has entered Phase 2 trials for advanced solid tumors and lymphomas.39 Another important case is the stapled peptide developed by Axel T Brunger's team, which was proven to be capable of treating the inability to control mucus in the respiratory tract.40 Peptide stapling has solved the druggability problem of peptide drugs to some extent. However, to expand the chemical diversity and continue investigating their potential, more stapling blocks with different chemical structures are still highly advantageous. For example, our research group has developed a series of new stapling amino acids containing natural amino acid side chains that can be used in the standard stapling chemistry of stable α-helical conformation peptides41 (Fig. 1A).
Recently, cyclobutane-based conformationally constrained amino acids have been successfully used in solid-state NMR spectroscopy studies to determine the structure, alignment, and dynamics of membrane-bound peptides under quasi-native conditions.42–44 These unnatural amino acids are similar to the natural amino acids in steric size, polarity, ionizability, and conformational propensities, which may not perturb the native structure or function of peptides. Additionally, they are highly compatible with the standard procedures of peptide synthesis. Most importantly, the cyclobutane-based conformationally constrained structure ensures that the 19F reporter group is in a geometrically well-defined position relative to the peptide backbone.45 Because one of the important goals of peptide stapling is to restrict peptide backbone conformations, it remains interesting to explore whether these conformationally constrained amino acids may provide more rigid structures in stapled peptide development, resulting in better pharmacological activities.
As presented in Fig. 1B, we described herein the rational design and synthesis of novel cyclobutane-bearing restricted anchoring residues, termed (E)-1-amino-3-(but-3-en-1-yl)cyclobutane-1-carboxylic acid (E7) and (Z)-1-amino-3-(but-3-en-1-yl)cyclobutane-1-carboxylic acid (Z7). Both and either E7 and Z7 could be used in RCM-mediated hydrocarbon peptide stapling. Four geometry-specific stapled peptides involving E7-E7, E7-Z7, Z7-Z7 and Z7-E7 combinations were obtained, of which the E7-E7 combination was proven to be optimal in the RCM reaction according to both in silico and on-resin experiments. Therefore, combining quantum and molecular mechanics, we demonstrated that the E7-E7 combination exhibited the highest helical content in secondary structural stabilization. A series of cyclobutane-bearing stapled peptides, with the spike protein of SARS-CoV-2 as the target, were obtained and E7-E7 geometry-specific stapled peptides were proven to display the highest α-helicity and best biological activities among varying isomers.
Results and discussion
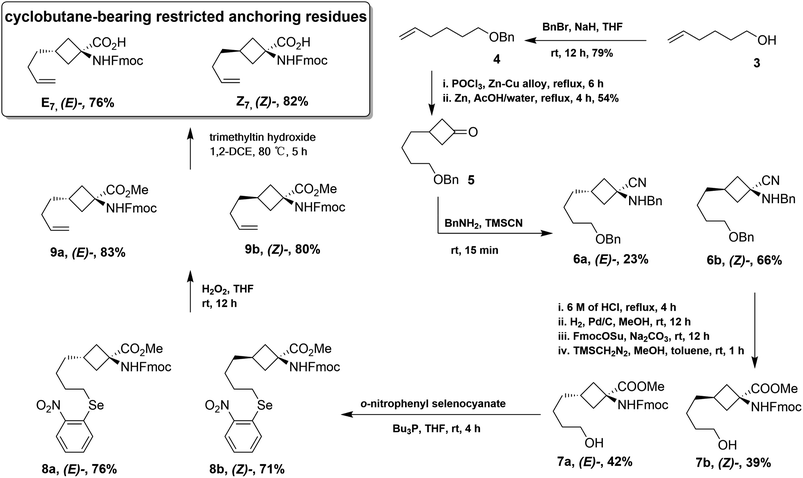
As presented in Scheme 1, the chemical synthesis of E7 and Z7 was completed using the commercially available hex-5-en-1-ol (3) as the starting material. In general, benzyl protection was easily implemented on hydroxyl group 3 with the treatment of NaH and BnBr to provide compound 4. Ketone 5 was obtained from compound 4via a [2 + 2] cycloaddition with dichloroketene generated in situ from trichloroacetyl chloride and a copper–zinc alloy and following a hydrogenation reaction catalyzed by zinc powder in acetic acid. The following Strecker reaction with benzylamine and trimethylsilyl cyanide (TMSCN) provided aminonitrile 6 as a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of stereoisomers that were easily separated by column chromatography on silica gel. 6a and 6b were then subjected to acidic hydrolysis with 6 M hydrochloric acid. The following Pd-catalyzed hydrogenolysis of the benzyl protection, N-Fmoc protection with Fmoc-OSu, and treatment with (trimethylsilyl)diazomethane provided compounds 7a and 7b. To perform the Grieco–Sharpless olefination reaction, 7a and 7b were reacted with o-nitrophenyl selenocyanate and tributylphosphine to yield 8a and 8b, and the subsequent elimination transformed 8a and 8b into the olefinic intermediates 9a and 9b, respectively, via the oxidation of hydrogen peroxide. Finally, selective deprotection of methyl esters was realized with the treatment of trimethyltin hydroxide to provide target amino acids E7 and Z7 whose absolute geometries were confirmed by following NOESY experiments (Fig. S1†).
1 mixture of stereoisomers that were easily separated by column chromatography on silica gel. 6a and 6b were then subjected to acidic hydrolysis with 6 M hydrochloric acid. The following Pd-catalyzed hydrogenolysis of the benzyl protection, N-Fmoc protection with Fmoc-OSu, and treatment with (trimethylsilyl)diazomethane provided compounds 7a and 7b. To perform the Grieco–Sharpless olefination reaction, 7a and 7b were reacted with o-nitrophenyl selenocyanate and tributylphosphine to yield 8a and 8b, and the subsequent elimination transformed 8a and 8b into the olefinic intermediates 9a and 9b, respectively, via the oxidation of hydrogen peroxide. Finally, selective deprotection of methyl esters was realized with the treatment of trimethyltin hydroxide to provide target amino acids E7 and Z7 whose absolute geometries were confirmed by following NOESY experiments (Fig. S1†).
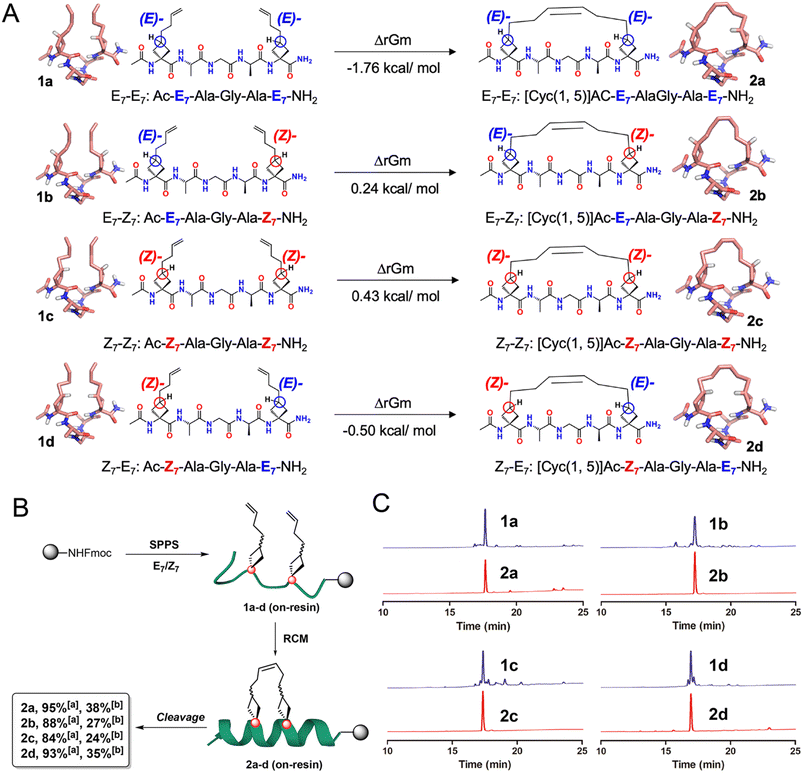
With E7 and Z7 in hand, our next plan was to perform the RCM-mediated peptide stapling on resin. Either E7 or Z7 could be introduced at i, i + 4 space, representing four varying combinations. Density functional theory (DFT)-based theoretical calculation was first performed to explore the RCM reaction involving different combinations of E7 and Z7, starting from Ac-E7/Z7-Ala-Gly-Ala-E7/Z7-NH2 peptide sequences. As presented in Fig. 2A, the Gibbs free energies (ΔrGm) of the four combinations of RCM reactions were −1.76, 0.24, 0.43 and −0.50 kcal mol−1, respectively. According to the thermodynamic principle, the more negative the ΔrGm, the easier it was to perform the reaction, resulting in more stable products. Therefore, it could be predicted that, compared with those of E7-Z7 and Z7-Z7 combinations, the RCM reactions of E7-E7 and Z7-E7 combinations were easier to perform and the former combination serves as the optimal one.
With the in silico energetic analysis as the reference, we next synthesized four stapled peptides through solid-phase peptide synthesis (SPPS) anchoring from cyclobutane-bearing restricted E7 and Z7 (Fig. 2B). E7 and Z7 were introduced into the peptide backbone using O-(6-chloro-1-hydroxybenzotriazol-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate (HCTU)/N,N-diisopropylethylamine (DIPEA) coupling reagents to obtain on-resin linear peptides (on-resin 1a–d). Then first generation of the Grubbs reagent enabled the RCM reaction to provide on-resin intermediates (on-resin 2a–d). Finally, crude target peptides 2a–d with different combinations (E7-E7, E7-Z7, Z7-Z7 and Z7-E7) were successfully obtained after cleavage and global deprotection in the presence of trifluoroacetic acid (TFA)/H2O/triisopropylsilane (TIPs). Subsequent analysis and identification via high performance liquid chromatography (HPLC) and mass spectrometry (MS) demonstrated that all the macrocyclization processes were completed with satisfactory conversion rates (84–95%). After purification by semi-preparative reversed-phase HPLC, geometry-specific stapled peptides 2a–d were obtained in high isolated yields from 24% to 38% (Fig. 2C). Notably, 2a was obtained with the highest conversion (95%) and isolated yield (38%), consistent with the theoretical calculation results. The above results demonstrated that these cyclobutane-bearing restricted anchoring residues were efficient for generating the geometry-specific and hydrocarbon stapled peptides, and the involving RCM reactions were highly compatible with SPPS.
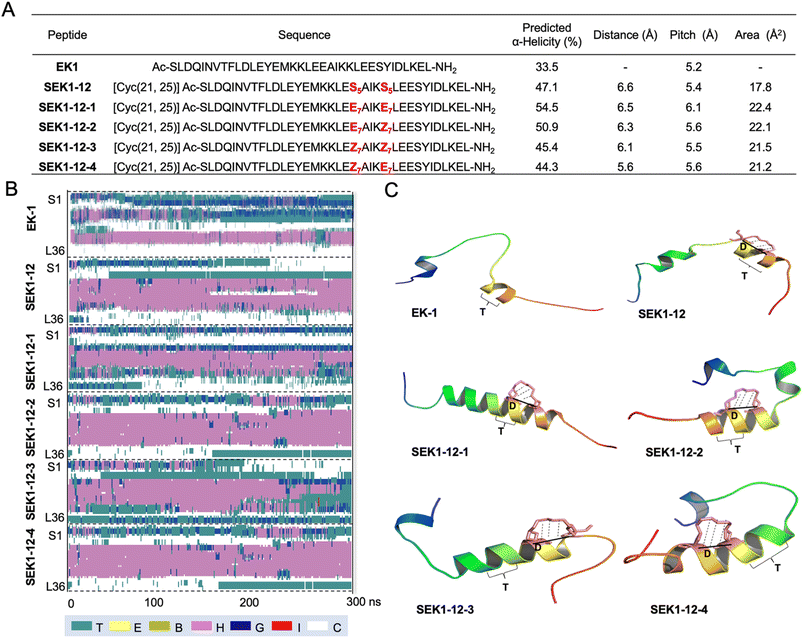
After verification of the operability of E7 and Z7 for RCM-mediated peptide stapling, the next step is to evaluate their ability to stabilize the secondary structure of these geometry-specific stapled peptides. SARS-CoV-2 is highly pathogenic and infectious. The acute respiratory disease pandemic that started in 2019 has seriously endangered human public safety.46,47 The formation of 6-HB (HR1–HR2 complex) during spike protein-mediated membrane fusion can be used as a conserved and potential target for the design of fusion inhibitors.48,49 Therefore, we selected our team's previously developed stapled SEK1-12 peptide (unpublished data), which targets the HR1 domain of the SARS-CoV-2 spike protein, as a template peptide. We constructed and efficiently obtained the stapled peptides SEK1-12-1, SEK1-12-2, SEK1-12-3 and SEK1-12-4 using cyclobutane-bearing restricted anchoring residues E7 and/or Z7 (Fig. 3A).
Based on reasonable stapled peptide structures, the helicity of the six peptides was analyzed in detail by using long time molecular dynamics simulations. Fig. 3B shows the change of the secondary structure of stapled peptides with simulation time after quantum mechanics optimization. There is a great difference in helicity distribution among the six systems, and the order from high to low is SEK1-12-1 > SEK1-12-2 > SEK1-12 > SEK1-12-3 > SEK1-12-4 > EK1. Specifically, SEK1-12-1 and SEK1-12-2 both can maintain the screw well: anchor distance D and pitch T both are distributed in an optimal range, and the abduction area S was the largest, which is more flexibility and conducive to promoting the stability of the screw (Fig. 3C). In terms of SEK1-12-3 and SEK1-12-4, only one of the D and T parameters is in the appropriate range, and the abduction area S is also relatively small, which results in unstable screw with low helicity. The abduction area S of SEK1-12 decreased significantly, but the spiral stability was actually higher than that of SEK1-12-3 and SEK1-12-4, which may be related to the replacement of cyclobutane at the anchor point. Compared with EK-1, the other five systems (SEK1-12, SEK1-12-1, SEK1-12-2, SEK1-12-3 and SEK1-12-4) all contain a stapled structure, and the helicity has been greatly improved as expected. To sum up, the helicity of stapled peptides is related to many factors, such as fatty linker and chirality, affecting the distance D between anchoring residues, the helix pitch T, and the abducting area S. These predictive results theoretically suggested that some of these geometry-specific hydrocarbon stapled peptides may provide better secondary structure stability than the classical all-hydrocarbon stapled peptides, which motivated us to perform a follow-up in-depth exploration.
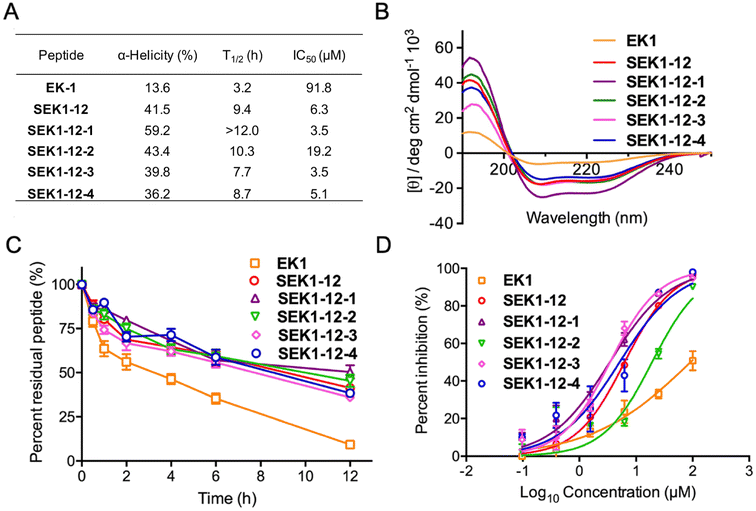
As such, all EK1-derived stapled peptides were successfully synthesized with efficient yields (26–32%). Then, their secondary structures were analyzed by circular dichroism (CD) experiments (Fig. 4A and B). The results indicated that the linear peptide EK1 exhibited a weak helical conformation with a helicity of 13.6%. It has been reported that the α-methyl group can enhance the helical stability of stapled peptides.50 For validating the role of the introduced α-methyl and cyclobutyl groups of the stapled peptides in helical stabilization, we synthesized another stapled peptide counterpart without the α-methyl group, termed SEK1-12-5, and determined its helical content through experimental and theoretical calculations (Fig. S2†). The helicity of SEK1-12-5 was 33.8%, which was lower than 41.5% of SEK1-12 and 59.2% of SEK1-12-1. Notably, the helical contents of the geometry-specific hydrocarbon stapled peptides exhibited a significant difference, ranging from 36.2% to 59.2%. Among them, SEK1-12-1 (E7-E7) exhibited the highest helicity, approximately 1.5-fold that of SEK-1-12. These results indicated that the geometry-specific hydrocarbon stapling strategy could improve the helicity of linear peptides and classic hydrocarbon stapled peptides. More importantly, consistent with the theoretical calculation results, this improvement effect can be more pronounced than classical all-hydrocarbon stapling when properly paired E7 and E7 residues participated. Subsequently, α-chymotrypsin was incubated with these stapled peptides to evaluate its protease stability (Fig. 4C). 95% of the linear peptide quickly degraded after approximately 12 h of exposure, with a half-life of 3 h (E7-E7). In sharp contrast, all the stapled peptides exhibited obviously enhanced proteolytic stability, of which more than half SEK1-12-1 remained intact even after 12 h of exposure. This remarkable proteolytic stability was similar to that of previously reported cyclobutene-bearing cyclopeptides.51
To verify the antiviral activity of these peptides, we tested their inhibitory activity against live SARS-CoV-2 in a Biosafety Level 3 (BSL-3) facility at the Second Military Medical University (Fig. 4D). It was demonstrated that all the peptides were capable of inhibiting the replication of SARS-CoV-2 in a dose-dependent manner and the stapled peptides exhibited improved inhibitory activity than the original peptide EK1. Of note, except for SEK1-12-2, geometry-specific hydrocarbon stapled peptides exhibit better inhibitory activity than classical stapled peptides, indicating another effective stapling structure in SARS-CoV-2 peptide inhibitor development.
Conclusions
In this study, we have designed and efficiently obtained two unique cyclobutane-bearing conformationally restricted and olefin-terminated amino acids, termed E7 and Z7. Using E7 and/or Z7 as a paired combination, four kinds of geometry-specific stapled peptides could be generated with the aid of the RCM reaction by in silico and on-resin experiments, of which the E7-E7 combination initiated stapling reaction was proven to be optimal. Targeting the spike protein HR1 domain of SARS-CoV-2 as a template peptide, we designed four geometry-specific hydrocarbon stapled peptides and also proved that SEK1-12-1 with E7-E7 combination exhibited the highest helical content using the combined quantum and molecular mechanics. Furthermore, SEK1-12-1 indeed exhibited the most stable helical conformation, the best proteolytic stability and thus the best anti-SARS-CoV-2 HR1 inhibitory activities. To sum up, our developed cyclobutane-bearing conformationally restricted anchoring residues with novel structure diversity were highly compatible with SPPS and RCM-mediated stapling chemistry. The generated geometry-specific hydrocarbon stapled peptide triggered from the E7-E7 combination was proven to be advantageous to the classic stapled counterpart in helical stabilization. In this regard, this methodology possesses great potential to expand the scope and structural diversity of the existing peptide stapling strategy. And thorough applications of this novel method into biologically active peptides are underway in our laboratory.Data availability
All supporting data are provided in the ESI.†Author contributions
BBC, HGH, XL and CL conceived and designed the study. BBC, CL, WC, FG, YZ, LL and DB performed the experiments. HGH, AH and LL helped with study design, and edited the manuscript. XL, BBC and CL wrote the paper. All authors read and approved the manuscript.Conflicts of interest
The authors claim that the researchers in this study have no conflicts of interest.Acknowledgements
This work was supported by the National Key R&D Program of China no. 2021YFC2100201 (to HH), the National Nature Science Foundation of China no. 22077078 (to HH), and the Shanghai Rising-Star Program (to XL).Notes and references
- J. F. Rual, K. Venkatesan, T. Hao, T. Hirozane-Kishikawa, A. Dricot, N. Li, G. F. Berriz, F. D. Gibbons, M. Dreze, N. Ayivi-Guedehoussou, N. Klitgord, C. Simon, M. Boxem, S. Milstein, J. Rosenberg, D. S. Goldberg, L. V. Zhang, S. L. Wong, G. Franklin, S. Li, J. S. Albala, J. Lim, C. Fraughton, E. Llamosas, S. Cevik, C. Bex, P. Lamesch, R. S. Sikorski, J. Vandenhaute, H. Y. Zoghbi, A. Smolyar, S. Bosak, R. Sequerra, L. Doucette-Stamm, M. E. Cusick, D. E. Hill, F. P. Roth and M. Vidal, Nature, 2005, 437, 1173–1178 CrossRef CAS PubMed.
- T. L. Nero, C. J. Morton, J. K. Holien, J. Wielens and M. W. Parker, Nat. Rev. Cancer, 2014, 14, 248–262 CrossRef CAS PubMed.
- V. Azzarito, K. Long, N. S. Murphy and A. J. Wilson, Nat. Chem., 2013, 5, 161–173 CrossRef CAS PubMed.
- L. D. Walensky and G. H. Bird, J. Med. Chem., 2014, 57, 6275–6288 CrossRef CAS PubMed.
- J. L. Nishikawa, A. Boeszoermenyi, L. A. Vale-Silva, R. Torelli, B. Posteraro, Y. J. Sohn, F. Ji, V. Gelev, D. Sanglard, M. Sanguinetti, R. I. Sadreyev, G. Mukherjee, J. Bhyravabhotla, S. J. Buhrlage, N. S. Gray, G. Wagner, A. M. Naar and H. Arthanari, Nature, 2016, 530, 485–489 CrossRef CAS PubMed.
- K. H. Khoo, C. S. Verma and D. P. Lane, Nat. Rev. Drug Discovery, 2014, 13, 217–236 CrossRef CAS PubMed.
- M. Wade, Y. C. Li and G. M. Wahl, Nat. Rev. Cancer, 2013, 13, 83–96 CrossRef CAS PubMed.
- D. E. Scott, A. R. Bayly, C. Abell and J. Skidmore, Nat. Rev. Drug Discovery, 2016, 15, 533–550 CrossRef CAS PubMed.
- L. G. Milroy, T. N. Grossmann, S. Hennig, L. Brunsveld and C. Ottmann, Chem. Rev., 2014, 114, 4695–4748 CrossRef CAS PubMed.
- I. Petta, S. Lievens, C. Libert, J. Tavernier and K. De Bosscher, Mol. Ther., 2016, 24, 707–718 CrossRef CAS PubMed.
- M. Rosell and J. Fernandez-Recio, Expert Opin. Drug Discovery, 2018, 13, 327–338 CrossRef CAS PubMed.
- G. J. B. Philippe, D. J. Craik and S. T. Henriques, Drug Discovery Today, 2021, 26, 1521–1531 CrossRef CAS PubMed.
- L. Wang, N. Wang, W. Zhang, X. Cheng, Z. Yan, G. Shao, X. Wang, R. Wang and C. Fu, Signal Transduction Targeted Ther., 2022, 7, 48 CrossRef CAS PubMed.
- K. Fosgerau and T. Hoffmann, Drug Discovery Today, 2015, 20, 122–128 CrossRef CAS PubMed.
- S. K. Dubey, S. Parab, N. Dabholkar, M. Agrawal, G. Singhvi, A. Alexander, R. A. Bapat and P. Kesharwani, Drug Discovery Today, 2021, 26, 931–950 CrossRef CAS PubMed.
- X. Li, S. Chen, W. D. Zhang and H. G. Hu, Chem. Rev., 2020, 120, 10079–10144 CrossRef CAS PubMed.
- R. Zhao, P. Shi, J. B. Cui, C. Shi, X. X. Wei, J. Luo, Z. Xia, W. W. Shi, Y. Zhou, J. Tang, C. Tian, M. Meininghaus, D. Bierer, J. Shi, Y. M. Li and L. Liu, Angew. Chem., Int. Ed., 2023, 62, e202216365 CrossRef CAS PubMed.
- Q. Qu, S. Gao, F. Wu, M. G. Zhang, Y. Li, L. H. Zhang, D. Bierer, C. L. Tian, J. S. Zheng and L. Liu, Angew. Chem., Int. Ed., 2020, 59, 6037–6045 CrossRef CAS PubMed.
- H. K. Cui, Y. Guo, Y. He, F. L. Wang, H. N. Chang, Y. J. Wang, F. M. Wu, C. L. Tian and L. Liu, Angew. Chem., Int. Ed., 2013, 52, 9558–9562 CrossRef CAS PubMed.
- R. S. Harrison, N. E. Shepherd, H. N. Hoang, G. Ruiz-Gomez, T. A. Hill, R. W. Driver, V. S. Desai, P. R. Young, G. Abbenante and D. P. Fairlie, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 11686–11691 CrossRef CAS PubMed.
- A. d. D. Araujo, J. Lim, K. C. Wu, Y. Xiang, A. C. Good, R. Skerlj and D. P. Fairlie, J. Med. Chem., 2018, 61, 2962–2972 CrossRef PubMed.
- S. A. Kawamoto, A. Coleska, X. Ran, H. Yi, C. Y. Yang and S. Wang, J. Med. Chem., 2012, 55, 1137–1146 CrossRef CAS PubMed.
- N. J. Agard, J. A. Prescher and C. R. Bertozzi, J. Am. Chem. Soc., 2004, 126, 15046–15047 CrossRef CAS PubMed.
- Y. H. Lau, Y. Wu, M. Rossmann, B. X. Tan, P. de Andrade, Y. S. Tan, C. Verma, G. J. McKenzie, A. R. Venkitaraman, M. Hyvçnen and D. R. Spring, Angew. Chem., Int. Ed., 2015, 54, 15410–15413 CrossRef CAS PubMed.
- S. Learte-Aymami, C. Vidal, A. Gutierrez-Gonzalez and J. L. Mascarenas, Angew. Chem., Int. Ed., 2020, 59, 9149–9154 CrossRef CAS PubMed.
- A. A. Vinogradov, Z. N. Choo, K. A. Totaro and B. L. Pentelute, Org. Lett., 2016, 18, 1226–1229 CrossRef CAS PubMed.
- X. Li, W. D. Tolbert, H. G. Hu, N. Gohain, Y. Zou, F. Niu, W. X. He, W. Yuan, J. C. Su, M. Pazgier and W. Lu, Chem. Sci., 2019, 10, 1522–1530 RSC.
- B. Li, Z. Wan, H. Zheng, S. Cai, H. W. Tian, H. Tang, X. Chu, G. He, D. S. Guo, X. S. Xue and G. Chen, J. Am. Chem. Soc., 2022, 144, 10080–10090 CrossRef CAS PubMed.
- N. Georgakopoulos, S. Talapatra, D. Dikovskaya, S. Dayalan Naidu, M. Higgins, J. Gatliff, A. Ayhan, R. Nikoloudaki, M. Schaap, K. Valko, F. Javid, A. T. Dinkova-Kostova, F. Kozielski and G. Wells, J. Med. Chem., 2022, 65, 7380–7398 CrossRef CAS PubMed.
- H. Jo, N. Meinhardt, Y. Wu, S. Kulkarni, X. Hu, K. E. Low, P. L. Davies, W. F. DeGrado and D. C. Greenbaum, J. Am. Chem. Soc., 2012, 134, 17704–17713 CrossRef CAS PubMed.
- F. Zhang, O. Sadovski, S. J. Xin and G. A. Woolley, J. Am. Chem. Soc., 2007, 129, 14154–14155 CrossRef CAS PubMed.
- X. Zheng, Z. Li, W. Gao, X. Meng, X. Li, L. Y. P. Luk, Y. Zhao, Y. H. Tsai and C. Wu, J. Am. Chem. Soc., 2020, 142, 5097–5103 CrossRef CAS PubMed.
- Z. Bai, C. Cai, W. Sheng, Y. Ren and H. Wang, Angew. Chem., Int. Ed., 2020, 59, 14686–14692 CrossRef CAS PubMed.
- X. Li, Y. Zou and H. G. Hu, Chinese Chem. Lett., 2018, 29, 1088–1092 CrossRef CAS.
- C. E. Schafmeister, J. Po and G. L. Verdine, J. Am. Chem. Soc., 2000, 122, 5891–5892 CrossRef CAS.
- M. Feng, J. Q. Jin, L. Xia, T. Xiao, S. Mei, X. Wang, X. Huang, J. Chen, M. Liu, C. Chen, S. Rafi, A. X. Zhu, Y. X. Feng and D. Zhu, Sci. Adv., 2019, 5, eaau5240 CrossRef CAS.
- Y. S. Chang, B. Graves, V. Guerlavais, C. Tovar, K. Packman, K. H. To, K. A. Olson, K. Kesavan, P. Gangurde, A. Mukherjee, T. Baker, K. Darlak, C. Elkin, Z. Filipovic, F. Z. Qureshi, H. Cai, P. Berry, E. Feyfant, X. E. Shi, J. Horstick, D. A. Annis, A. M. Manning, N. Fotouhi, H. Nash, L. T. Vassilev and T. K. Sawyer, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 3445–3454 Search PubMed.
- M. L. Stewart, E. Fire, A. E. Keating and L. D. Walensky, Nat. Chem. Biol., 2010, 6, 595–601 CrossRef CAS PubMed.
- Y. S. Chang, B. Graves, V. Guerlavais, C. Tovar, K. Packman, K. H. To, K. A. Olson, K. Kesavan, P. Gangurde, A. Mukherjee, T. Baker, K. Darlak, C. Elkin, Z. Filipovic, F. Z. Qureshi, H. Cai, P. Berry, E. Feyfant, X. E. Shi, J. Horstick, D. A. Annis, A. M. Manning, N. Fotouhi, H. Nash, L. T. Vassilev and T. K. Sawyer, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 3445–3454 Search PubMed.
- Y. Lai, G. Fois, J. R. Flores, M. J. Tuvim, Q. Zhou, K. Yang, J. Leitz, J. Peters, Y. Zhang, R. A. Pfuetzner, L. Esquivies, P. Jones, M. Frick, B. F. Dickey and A. T. Brunger, Nature, 2022, 603, 949–956 CrossRef CAS PubMed.
- Y. Wu, Y. H. Li, X. Li, Y. Zou, H. L. Liao, L. Liu, Y. G. Chen, D. Bierer and H. G. Hu, Chem. Sci., 2017, 8, 7368–7373 RSC.
- A. N. Tkachenko, D. S. Radchenko, P. K. Mykhailiuk, S. Afonin, A. S. Ulrich and I. V. Komarov, Angew. Chem., Int. Ed., 2013, 52, 6504–6507 CrossRef CAS PubMed.
- O. M. Michurin, S. Afonin, M. Berditsch, C. G. Daniliuc, A. S. Ulrich, I. V. Komarov and D. S. Radchenko, Angew. Chem., Int. Ed., 2016, 55, 14595–14599 CrossRef CAS PubMed.
- O. M. Michurin, K. Tolmachova, S. Afonin, O. Babii, S. L. Grage, A. S. Ulrich, I. V. Komarov and D. S. Radchenko, Eur. J. Org. Chem., 2018, 2018, 3826–3833 CrossRef CAS.
- A. N. Tkachenko, P. K. Mykhailiuk, S. Afonin, D. S. Radchenko, V. S. Kubyshkin, A. S. Ulrich and I. V. Komarov, Angew. Chem., Int. Ed., 2013, 52, 1486–1489 CrossRef CAS PubMed.
- J. Li, Z. Zhang, J. Gu, R. Amini, A. G. Mansfield, J. Xia, D. White, H. D. Stacey, J. C. Ang, G. Panesar, A. Capretta, C. D. M. Filipe, K. Mossman, B. J. Salena, J. B. Gubbay, C. Balion, L. Soleymani, M. S. Miller, D. Yamamura, J. D. Brennan and Y. Li, J. Am. Chem. Soc., 2022, 144, 23465–23473 CrossRef CAS.
- B. Oberfeld, A. Achanta, K. Carpenter, P. Chen, N. M. Gilette, P. Langat, J. T. Said, A. E. Schiff, A. S. Zhou, A. K. Barczak and S. Pillai, Cell, 2020, 181, 954–954 CrossRef CAS.
- S. Xia, M. Liu, C. Wang, W. Xu, Q. Lan, S. Feng, F. Qi, L. Bao, L. Du, S. Liu, C. Qin, F. Sun, Z. Shi, Y. Zhu, S. Jiang and L. Lu, Cell Res., 2020, 30, 343–355 CrossRef CAS PubMed.
- S. Xia, L. Yan, W. Xu, A. S. Agrawal, A. Algaissi, C. K. Tseng, Q. Wang, L. Du, W. Tan, I. A. Wilson, S. Jiang, B. Yang and L. Lu, Sci. Adv., 2019, 5, eaav4580 CrossRef CAS PubMed.
- C. E. Schafmeister, J. Po and G. L. Verdine, J. Am. Chem. Soc., 2000, 122, 5891–5892 CrossRef CAS.
- T. Miura, T. R. Malla, C. D. Owen, A. Tumber, L. Brewitz, M. A. McDonough, E. Salah, N. Terasaka, T. Katoh, P. Lukacik, C. Strain-Damerell, H. Mikolajek, M. A. Walsh, A. Kawamura, C. J. Schofield and H. Suga, Nat. Chem., 2023, 15, 998–1005 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3sc04279k |
| ‡ These authors contributed to this work equally. |
| This journal is © The Royal Society of Chemistry 2023 |