Open Access Article
Open Access ArticleTemplate-assisted synthesis of isomeric copper(I) clusters with tunable structures showing photophysical and electrochemical properties†
Jun-Jie
Fang
,
Zheng
Liu
,
Yang-Lin
Shen
,
Yun-Peng
Xie
 * and
Xing
Lu
* and
Xing
Lu
 *
*
State Key Laboratory of Materials Processing and Die & Mould Technology, School of Materials Science and Engineering, Huazhong University of Science and Technology, Wuhan 430074, China. E-mail: xieyp@hust.edu.cn; lux@hust.edu.cn
First published on 13th October 2023
Abstract
A comparative study of structure–property relationships in isomeric and isostructural atomically precise clusters is an ideal approach to unravel their fundamental properties. Herein, seven high-nuclearity copper(I) alkynyl clusters utilizing template-assisted strategies were synthesized. Spherical Cu36 and Cu56 clusters are formed with a [M@(V/PO4)6] (M: Cu2+, Na+, K+) skeleton motif, while peanut-shaped Cu56 clusters feature four separate PO4 templates. Experiments and theoretical calculations suggested that the photophysical properties of these clusters are dependent on both the inner templates and outer phosphonate ligands. Phenyl and 1-naphthyl phosphate-protected clusters exhibited enhanced emission features attributed to numerous well-arranged intermolecular C–H⋯π interactions between the ligands. Moreover, the electrocatalytic CO2 reduction properties suggested that internal PO4 templates and external naphthyl groups could promote an increase in C2 products (C2H4 and C2H5OH). Our research provides new insight into the design and synthesis of multifunctional copper(I) clusters, and highlights the significance of atomic-level comparative studies of structure–property relationships.
Introduction
During the past few decades, atomically precise metal nanoclusters have contributed significantly to the rapid development of nanomaterials by elucidating structure–property relationships.1–6 Alkynyl ligands have more abundant coordination modes than the commonly used thiolate and phosphine ligands, and therefore alkynyl-protected coinage metal clusters have remarkable structures.7–11 However, the reaction between alkynyl ligands and Cu+ often produces insoluble polymers, which increases the difficulty of synthesis and isolation.12,13 To address this issue, template agents have been introduced into the synthesis process to balance the charge distribution of the clusters and prevent or interrupt the formation of insoluble polymers resulting from the reaction between alkynyl and Cu+. As a result, the synthesis of high-nuclearity copper(I) alkynyl clusters with larger size and unique properties has been facilitated.14–17Polyoxometalates (POMs) have attracted considerable attention due to their structural diversity and desirable properties in materials science, medicinal chemistry, and catalysis.18–20 Previous studies have reported silver alkynyl clusters with richer structures and more unique properties constructed with POMs.21–24 Polyoxovanadates and polyoxomolybdates, both members of the POM family, constitute a fascinating family of polynuclear oxo-anions that exhibit variable coordination geometries, but have a strong tendency to exhibit mixed-valent states,25–27 which leads to the easy oxidation of Cu+. This oxidizability significantly increases the difficulty in synthesizing copper(I) alkynyl clusters constructed with POMs.
Metal phosphonates not only exhibit various structures ranging from discrete molecules to multi-dimensional coordination polymers that provide a bridge for the construction of high-nuclearity metal clusters,28–30 but also hold potential applications in biotechnology, water treatment, photochemistry, and magnetism.31–34 We previously reported the use of (nBu/tBu)PO3H2 as precursors to construct a series of silver clusters. (nBu/tBu)PO3H2 units serve as tripod pillars that have a variety of coordination modes with metals and can be used as structure-directing templates to form enlarged composite clusters.23,24,35–37 However, the reactions of polyoxometalates and phosphonates in this system are complicated, and V/P elements tend to exhibit multiple valent states that are prone to oxidize Cu+. Consequently, it is more difficult to synthesize copper(I) clusters with POM or phosphonate templates than silver(I) clusters. So far, only a few successfully synthesized copper(I) alkynyl clusters constructed with POM or phosphonate templates have been reported, including Mak's previously reported Cu33,38 Cu46,39 Cu47,39 Cu62 (ref. 38) and our reported Cu25.40
Recently, atomically precise copper clusters and their correlations between structures and properties have received a lot of attention, particularly with regard to photoluminescence41,42 and CO2 reduction reaction (CO2RR).43–45 For example, Jin et al. found that the phosphorescence quantum efficiency of M@Cu14 (M: Au, Cl) nanoclusters can be tuned by a single-atom kernel.46 Zang et al. observed that Au doping in the innermost shell of Au12(AgCu)38 significantly enhances near-infrared photoluminescence intensity.47 Bakr et al. reported that extended C–H⋯π and π⋯π intermolecular ligand interactions significantly enhance photoluminescence.48 Atomically precise clusters with well-defined architectures and chemical compositions have attracted attention in the field of heterogeneous catalysis.49–53 For instance, Liu et al. synthesized Cu32H20L12 (L: S2P(OiPr)2) exhibiting high selectivity (FEHCOOH = 89% at −0.3 V, 83% at −0.4 V) for the CO2RR.54 Zang et al. observed that ditetrahedron-shaped Cu8 exhibited a higher FEHCOOH (≈92%) at −1.0 V than the cube-shaped Cu8.55 Tang et al. reported that M15 (Au7Ag8, Ag9Cu6, and Au2Ag8Cu5) exhibits drastically different CO2RR performances in a wide voltage range.56 These atomically precise clusters facilitate systematic comparative studies of structure–property relationships.
In this study, we designed a synthesis strategy to address the aforementioned challenges by adding weak reducing agents and phosphonate ligands, successfully synthesizing seven copper(I) alkynyl clusters. Single crystal structure analysis revealed that the structural modulation of these clusters can be achieved through the bonding of metal cations to templates. One Cu2+, Na+, or K+ ion captured by six VO4 or PO4 tetrahedrons constructs a [M@(V/PO4)6] (M: Cu2+, Na+, K+) skeleton, which tends to form structurally similar spherical Cu36 and Cu56 clusters. Meanwhile, in the reaction system where Na+ and K+ ions are replaced with NH4+, peanut-shaped Cu56 clusters can be constructed from four PO4 tetrahedrons. The crystalline-state emissions of these clusters depend on the inner templates and the outer phosphonate ligands. The surfaces of PhOPO32− and 1-NaphOPO32− protected clusters are interconnected through numerous well-arranged intermolecular C–H⋯π interactions between the ligands of adjacent clusters, leading to enhanced emission. Electrocatalytic measurements of CO2 reduction reaction showed that PO4-encapsulated Cu56 possesses higher catalytic activity for the CO2RR than VO4-encapsulated Cu56, and the PO4 template and external naphthyl group can improve the yield of C2 products (C2H4 and C2H5OH).
Results and discussion
Synthesis and general characterization
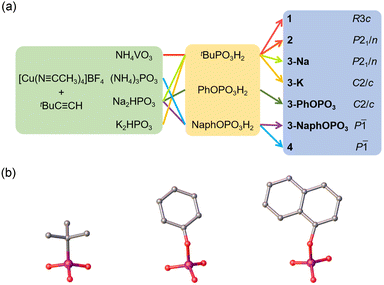
The synthesis of clusters, [CuII(VO4)6@CuI36(tBuPO3)6(V3O3OH)(V3O6OH)(tBuC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C)18] (1), [CuII(VO4)6@CuI56(tBuPO3)6(tBuC
C)18] (1), [CuII(VO4)6@CuI56(tBuPO3)6(tBuC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C)26]F2 (2), [NaI(PO4)6@CuI56(tBuPO3)6(tBuC
C)26]F2 (2), [NaI(PO4)6@CuI56(tBuPO3)6(tBuC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C)26]F (3-Na), [KI(PO4)6@CuI56(tBuPO3)6(tBuC
C)26]F (3-Na), [KI(PO4)6@CuI56(tBuPO3)6(tBuC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C)26]F (3-K), [NaI(PO4)6@CuI56(PhOPO3)6(tBuC
C)26]F (3-K), [NaI(PO4)6@CuI56(PhOPO3)6(tBuC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C)26]F (3-PhOPO3, PhOPO3 = phenyl phosphate), [NaI(PO4)6@CuI56(1-NaphOPO3)6(tBuC
C)26]F (3-PhOPO3, PhOPO3 = phenyl phosphate), [NaI(PO4)6@CuI56(1-NaphOPO3)6(tBuC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C)26]F (3-NaphOPO3, 1-NaphOPO3 = 1-naphthyl phosphate), [(PO4)4@CuI56(1-NaphOPO3)6(tBuC
C)26]F (3-NaphOPO3, 1-NaphOPO3 = 1-naphthyl phosphate), [(PO4)4@CuI56(1-NaphOPO3)6(tBuC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C)32] (4), requires meticulous control over various phosphonates and vanadates.
C)32] (4), requires meticulous control over various phosphonates and vanadates.
To achieve this, we employed three different phosphonate ligands with varying steric hindrances to regulate their structures (Fig. 1). Reaction conditions were optimized to obtain the crystalline products, including the ratio of template precursors and (tBu/PhO/1-NaphO)PO3H2 ligands, time of ultrasonic conditions, amount of Et3N used, amount of PhMe2SiH added, and solvothermal temperature. Adjusting the addition ratio of NH4VO3 and tBuPO3H2 allowed us to obtain single crystals of 1 and 2. It should be noted that precise control of the amount of PhMe2SiH added was crucial to overcome the oxidizability of template precursors. Under natural light, the appearance of single crystals of 1–4 is dark red blocky and yellow blocky, respectively (Fig. S1†).
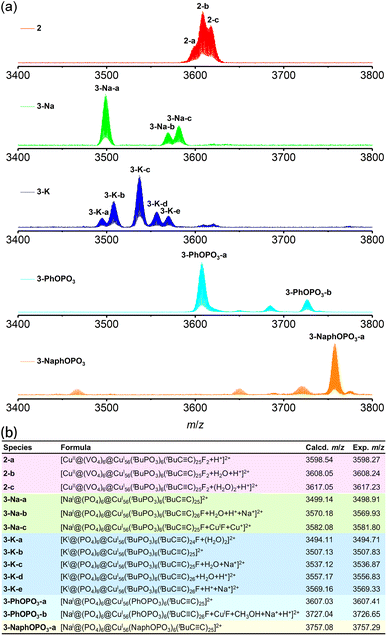
We elucidated the molecular structures of 1–4 through single crystal X-ray diffraction (SCXRD), and we have listed their crystal data, structure refinement, and selected bond lengths in ESI Tables 1, 2 and 5–12.† SCXRD analysis revealed that clusters 1 and 4 are both neutral clusters. While crystals of 1 and 4 are insoluble in common solvents, other crystals dissolve easily in CH2Cl2, CH3OH, and CH3CH2OH, providing opportunities to examine their solution stabilities and ionic valences using electrospray ionization mass spectrometry (ESI-MS). For example, in the positive ion mode ESI-MS of 2 dissolved in CH3OH and CH2Cl2, the two-charge prominent peaks centered at m/z = 3598.27 (2-a), 3608.24 (2-b), and 3617.23 (2-c) are assigned formulas of [CuII@(VO4)6@CuI56(tBuPO3)6(tBuC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C)25F2 + H+]2+ (calcd m/z = 3598.54), [CuII@(VO4)6@CuI56(tBuPO3)6(tBuC
C)25F2 + H+]2+ (calcd m/z = 3598.54), [CuII@(VO4)6@CuI56(tBuPO3)6(tBuC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C)25F2 + H2O + H+]2+ (calcd m/z = 3608.05), and [CuII@(VO4)6@CuI56(tBuPO3)6(tBuC
C)25F2 + H2O + H+]2+ (calcd m/z = 3608.05), and [CuII@(VO4)6@CuI56(tBuPO3)6(tBuC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C)25F2 + (H2O)2 + H+]2+ (calcd m/z = 3617.05), respectively (Fig. 2). We accurately identified other species produced by ionization of one or two tBuC
C)25F2 + (H2O)2 + H+]2+ (calcd m/z = 3617.05), respectively (Fig. 2). We accurately identified other species produced by ionization of one or two tBuC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C− ligands from the cations of the pristine clusters or by further addition of H+, Na+, or H2O, indicating that these clusters are well stabilized in solution (Fig. S10–S15†).
C− ligands from the cations of the pristine clusters or by further addition of H+, Na+, or H2O, indicating that these clusters are well stabilized in solution (Fig. S10–S15†).
Powder X-ray diffraction (PXRD) patterns of them versus the calculated patterns from single-crystal X-ray diffraction revealed their high phase purity, as shown in Fig. S16.† Their good thermal stability under the crystalline-state below 220 °C is exhibited in their thermogravimetric analysis (TGA) diagrams (Fig. S17†). Taking sample 2 as an example, the major weight loss (56.9%) in the range of 300–440 °C corresponds to a large proportion of ligand (tBuC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C− and tBuPO32−) decomposition, with a second weight loss (1.5%) between 440 and 800 °C that may be attributed to a fraction of ligand (tBuC
C− and tBuPO32−) decomposition, with a second weight loss (1.5%) between 440 and 800 °C that may be attributed to a fraction of ligand (tBuC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C− and tBuPO32−), CuI, and [VO4]3− volatilization. The Fourier transform infrared (FT-IR) spectra confirmed the existence of alkynyl (2090 cm−1) in these clusters (Fig. S18†).
C− and tBuPO32−), CuI, and [VO4]3− volatilization. The Fourier transform infrared (FT-IR) spectra confirmed the existence of alkynyl (2090 cm−1) in these clusters (Fig. S18†).
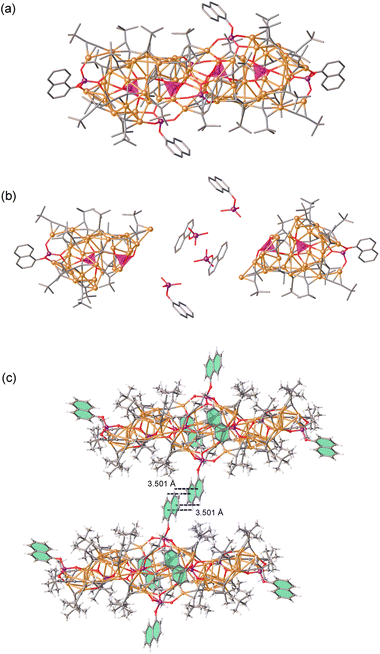
Crystal structures of 1–4
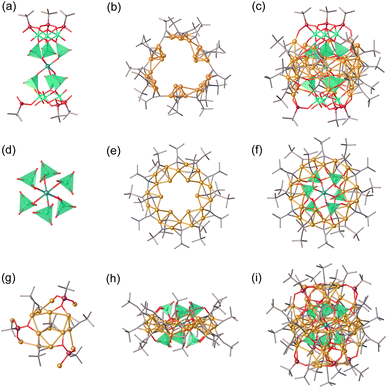
Metal clusters can be synthesized using a variety of building blocks, and as previously reported, cap-shaped [VO2(OH)(tBuPO3)]2−, [(VO2)(tBuPO3)2]3−, [V3O6(OH)(tBuPO3)3]4−, and [V4O8(tBuPO3)4]4− units have been found to be versatile for the anion-templated synthesis of high-nuclearity silver(I) clusters.24,37 Herein we report the successful formation of copper clusters using [VO4]3−/[PO4]3− tetrahedrons and [(tBu/PhO/1-NaphO)PO3]2− ligands as demonstrated in Fig. 3. The resulting cluster 1 crystallizes in the R3c space group, being assembled from one penetrating [CuII(VO4)6@(tBuPO3)6(V3O3OH)(V3O6OH)]18− skeleton and one [CuI36(tBuC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C)18]18+ ring at the waist with argentophilic Cu⋯Cu bond distances in the range 2.511–3.016 Å. The [CuI36(tBuC
C)18]18+ ring at the waist with argentophilic Cu⋯Cu bond distances in the range 2.511–3.016 Å. The [CuI36(tBuC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C)18]18+ ring has a van der Waals diameter of approximately 19.8 Å (Fig. S2†). The oxidizability of vanadate in situ generated a Cu2+ cation,39 which is bridged by six [VO4]3− tetrahedrons, forming an elongated octahedral coordination geometry CuIIO6.
C)18]18+ ring has a van der Waals diameter of approximately 19.8 Å (Fig. S2†). The oxidizability of vanadate in situ generated a Cu2+ cation,39 which is bridged by six [VO4]3− tetrahedrons, forming an elongated octahedral coordination geometry CuIIO6.
By reducing the addition of vanadates and reaction time, cluster 2 was successfully synthesized. Furthermore, clusters 3-Na, 3-K, 3-PhOPO3, and 3-NaphOPO3 were obtained by replacing vanadates with phosphonates resulting in crystal structures similar to cluster 2. For instance, SCXRD analysis and ESI-MS revealed that the overall charge balance of cluster 2 against the cationic cluster is provided by two F− counteranions. As shown in Fig. 3, spherical cluster 2 has a multishelled core architecture of CuII@(VO4)6@CuI56(tBuPO3)6(tBuC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C)26, with argentophilic Cu⋯Cu bond distances in the range 2.423–3.055 Å. Furthermore, the in situ generated Cu2+ cation is encapsulated in six [VO4]3− templates. The wreath-shaped [Cu24(tBuC
C)26, with argentophilic Cu⋯Cu bond distances in the range 2.423–3.055 Å. Furthermore, the in situ generated Cu2+ cation is encapsulated in six [VO4]3− templates. The wreath-shaped [Cu24(tBuC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C)18]6+ ring has a hexagonal inner Cu12 unit, which is connected with the [CuII@(VO4)6]16− polyoxoanion template to form the wheel-shaped [CuII@(VO4)6@Cu24(tBuC
C)18]6+ ring has a hexagonal inner Cu12 unit, which is connected with the [CuII@(VO4)6]16− polyoxoanion template to form the wheel-shaped [CuII@(VO4)6@Cu24(tBuC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C)18]10− unit with a van der Waals diameter of about 20.4 Å (Fig. S2†). Cluster 2 is formed by fusing two cap-like [Cu16(tBuC
C)18]10− unit with a van der Waals diameter of about 20.4 Å (Fig. S2†). Cluster 2 is formed by fusing two cap-like [Cu16(tBuC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C)4(tBuPO3)3]6+ peripheral structural units staggered exactly by 60° on the upper and lower sides of the wheel-shaped unit. The packing pattern of crystal 2 shows that these molecules are well-arranged through intermolecular C–H⋯F interactions (4.079, 4.119, and 4.244 Å) (Fig. S3†).
C)4(tBuPO3)3]6+ peripheral structural units staggered exactly by 60° on the upper and lower sides of the wheel-shaped unit. The packing pattern of crystal 2 shows that these molecules are well-arranged through intermolecular C–H⋯F interactions (4.079, 4.119, and 4.244 Å) (Fig. S3†).
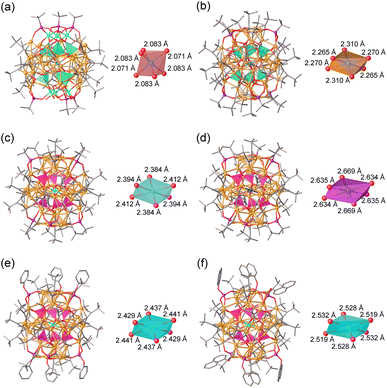
Both spherical copper clusters, Cu36 and Cu56, have similar internal distorted octahedron kernels, which are often observed in POMs.57 As shown in Fig. 4, internal elongated MO6 octahedron skeletons are regulated by external distinct cap-shaped units. Specifically, the CuIIO6 octahedron of cluster 1 has CuII⋯OV (OV: oxygen atom of the [VO4]3− templates) bond distances ranging between 2.071 and 2.083 Å, whereas the CuII⋯OV bond distances in cluster 2, capped by [Cu16(tBuC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C)4(tBuPO3)3] units, are longer and range between 2.265, 2.270 and 2.310 Å. Additionally, cluster 3-K has a K+ ion core with a larger ion radius than the Na+ ion, causing a larger KO6 octahedron (K⋯OP (OP: oxygen atom of the [PO4]3− templates) bond distances: 2.634, 2.635, and 2.669 Å) than the NaO6 octagonal. The steric hindrances of outer phosphonate ligands increase from tBuPO32−, PhOPO32− to 1-NaphOPO32−, resulting in a gradual increase of the internal NaO6 octagon size and the change of the space group from P21/n, C2/c, to P
C)4(tBuPO3)3] units, are longer and range between 2.265, 2.270 and 2.310 Å. Additionally, cluster 3-K has a K+ ion core with a larger ion radius than the Na+ ion, causing a larger KO6 octahedron (K⋯OP (OP: oxygen atom of the [PO4]3− templates) bond distances: 2.634, 2.635, and 2.669 Å) than the NaO6 octagonal. The steric hindrances of outer phosphonate ligands increase from tBuPO32−, PhOPO32− to 1-NaphOPO32−, resulting in a gradual increase of the internal NaO6 octagon size and the change of the space group from P21/n, C2/c, to P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) .
.
In the reaction system where Na2HPO3 and K2HPO3 are replaced by (NH4)3PO3, cluster 4 can be constructed from PO4 tetrahedrons without the presence of Na+ or K+ ions. As shown in Fig. 5, cluster 4 possesses a peanut-shaped structure that consists of four separate [PO4]3− tetrahedrons, thirty-two tBuC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C− ligands, six 1-NaphOPO32− ligands, and fifty-six Cu+ ions with argentophilic Cu⋯Cu bond distances ranging from 2.401 to 3.056 Å. The absence of metal cations inside the cluster allows for the aggregation of [PO4]3− tetrahedrons into the cluster. Cluster substructures [(PO4)2@CuI28(1-NaphOPO3)(tBuC
C− ligands, six 1-NaphOPO32− ligands, and fifty-six Cu+ ions with argentophilic Cu⋯Cu bond distances ranging from 2.401 to 3.056 Å. The absence of metal cations inside the cluster allows for the aggregation of [PO4]3− tetrahedrons into the cluster. Cluster substructures [(PO4)2@CuI28(1-NaphOPO3)(tBuC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C)16]4+ are bridged by four 1-NaphOPO32− ligands, with each oxygen atom coordinated to one copper atom.
C)16]4+ are bridged by four 1-NaphOPO32− ligands, with each oxygen atom coordinated to one copper atom.
It is noteworthy that the replacement of tBuPO32− ligands with PhOPO32− and 1-NaphOPO32− ligands leads to the formation of numerous strong intermolecular C–H⋯π interactions between adjacent clusters. Fig. S6–S8† demonstrate how nearby 1-NaphOPO32− and tBuC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C− ligands in cluster 4 form intermolecular C–H⋯π interactions at distances ranging from 2.862 to 3.626 Å. In cluster 3-PhOPO3, intermolecular C–H⋯π interactions (at distances of 3.479 and 3.754 Å) are formed by PhOPO32− ligands, while in cluster 3-NaphOPO3, intermolecular C–H⋯π interactions (at distances of 2.821, 3.342, and 3.598 Å) are formed by 1-NaphOPO32− ligands. These strong interactions between adjacent clusters are crucial to their luminous properties.
C− ligands in cluster 4 form intermolecular C–H⋯π interactions at distances ranging from 2.862 to 3.626 Å. In cluster 3-PhOPO3, intermolecular C–H⋯π interactions (at distances of 3.479 and 3.754 Å) are formed by PhOPO32− ligands, while in cluster 3-NaphOPO3, intermolecular C–H⋯π interactions (at distances of 2.821, 3.342, and 3.598 Å) are formed by 1-NaphOPO32− ligands. These strong interactions between adjacent clusters are crucial to their luminous properties.
The coordination modes of alkynyl and phosphonate ligands with Cu+ result in a diverse range of crystal structures, as demonstrated in Fig. S9.† Seven coordination modes, including μ2-ησ1, ηπ1; μ3-ησ1, ησ1, ηπ1; μ3-ησ1, ηπ1, ηπ1.; μ3-ησ1, ησ1, ησ1; μ4-ησ1, ησ1, ησ1, ησ1; μ4-ησ1, ησ1, ησ1, ηπ1; μ4-ησ1, ησ1, ηπ1, ηπ1, are observed. Overall, the introduction of multiple VO4 and PO4 tetrahedrons with abundant terminal oxygen atoms during the synthesis of copper(I) alkynyl clusters not only balances the positive charges on the Cu(I) shells, but also effectively prevents or interrupts insoluble polymers formed by the reaction of alkynyl with Cu+.
Photophysical properties
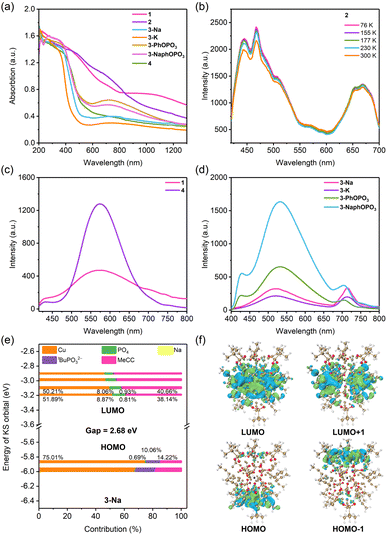
The optical properties of seven Cu36 and Cu56 clusters in their crystalline state were investigated through a series of spectroscopic analyses (Fig. 6a). The absorption features of clusters constructed by VO4 tetrahedrons were found to be similar. Similarly, the absorption features of clusters constructed by PO4 tetrahedrons had peaks located at 200–400 and 720 nm (except 4), indicating that the electronic structures of these clusters are similar and in agreement with the single crystal data. The analysis of the absorption spectra revealed that clusters constructed by PO4 tetrahedrons have an optical band gap in the range of 1.93–2.64 eV (Fig. S19†).Photoluminescence (PL) properties correlate with both the optical absorption behaviors and crystal structures.58,59 The PL spectra of cluster 2 in its crystalline state showed emission peaks at 444, 468, 507, 572, 654, and 670 nm, indicating a mechanism of multiple-exciton emission (Fig. 6b). As the temperature decreased, the luminescence intensity increased slightly, while the peak position remained unchanged. No luminescence thermochromism was observed, indicating that temperature has little effect on the tight packing and bond lengths in the crystal structure (argentophilic Cu⋯Cu bond average distances of 2.723 Å and 2.710 Å were observed in 2 and 2-100 K, respectively). Clusters 1 and 4 both exhibited yellow emission centered at 574 nm. Meanwhile, 2-Na, 2-K, 3-PhOPO3, and 3-NaphOPO3 exhibited a main emission peak at 430 nm and a shoulder peak at 710 nm.
The photoluminescence quantum yield (PLQY) of these clusters ranges from 1.05% to 3.65% (ESI Table 3†). Clusters protected by PhOPO32− and 1-NaphOPO32− ligands exhibited three times higher luminescence intensity than those protected by tBuPO32− ligands. There was a slightly enhanced quantum yield for clusters protected by PhOPO32− and 1-NaphOPO32− ligands. The strong intramolecular C–H⋯π interactions connecting adjacent clusters restrict the rotation and vibration of some tBuC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C−, PhOPO32−, and 1-NaphOPO32− ligands and effectively decrease nonradiative decay, accounting for the enhanced emission. The PL lifetime decay curves of each metal cluster were examined to determine the key characteristics of their excited state dynamics. For 2, decay curves of the 468 and 670 nm peaks were well fitted using a double exponential function, while other clusters required a triple exponential function for adequate fitting (Fig. S20–S22 and Table S3†). The exciton lifetimes τ1 and τ2 for the 670 nm peak of 2 at 300 K were determined to be 4.36 (97.72%) and 59.23 ns (2.28%), respectively, and were observed to remain relatively stable at different temperatures. In contrast, other clusters were found to have a contribution from microsecond lifetime processes; for example, in 3-Na, the exciton lifetimes τ1, τ2, and τ3 for the 670 nm peak were determined to be 38.20 (62.79%), 361.17 (21.31%), and 2315.16 ns (15.90%), respectively.
C−, PhOPO32−, and 1-NaphOPO32− ligands and effectively decrease nonradiative decay, accounting for the enhanced emission. The PL lifetime decay curves of each metal cluster were examined to determine the key characteristics of their excited state dynamics. For 2, decay curves of the 468 and 670 nm peaks were well fitted using a double exponential function, while other clusters required a triple exponential function for adequate fitting (Fig. S20–S22 and Table S3†). The exciton lifetimes τ1 and τ2 for the 670 nm peak of 2 at 300 K were determined to be 4.36 (97.72%) and 59.23 ns (2.28%), respectively, and were observed to remain relatively stable at different temperatures. In contrast, other clusters were found to have a contribution from microsecond lifetime processes; for example, in 3-Na, the exciton lifetimes τ1, τ2, and τ3 for the 670 nm peak were determined to be 38.20 (62.79%), 361.17 (21.31%), and 2315.16 ns (15.90%), respectively.
To further elucidate the PL mechanism, density functional theory (DFT) calculations were performed using the ORCA 5.0.3 program.60,61 Based on the single-crystal data, tBuC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C− was replaced with MeC
C− was replaced with MeC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C− to reduce the computational cost. In Fig. 6 and S23–S26,† the highest occupied molecular orbitals (HOMO and HOMO−1) of spherical Cu56 clusters were mainly located on the upper and lower cap-shaped structural units, while the lowest unoccupied molecular orbitals (LUMO and LUMO+1) were distributed on the wheel-shaped structural units at the waist. Comparative analysis of the contribution of different components to the LUMO and HOMO suggests that the PL mechanism arises from alkynyl → Cu(I) 3LMCT (ligand to metal charge transfer) excited states that are modified by encapsulated templates and outer phosphonate ligands within the crystal structure.62
C− to reduce the computational cost. In Fig. 6 and S23–S26,† the highest occupied molecular orbitals (HOMO and HOMO−1) of spherical Cu56 clusters were mainly located on the upper and lower cap-shaped structural units, while the lowest unoccupied molecular orbitals (LUMO and LUMO+1) were distributed on the wheel-shaped structural units at the waist. Comparative analysis of the contribution of different components to the LUMO and HOMO suggests that the PL mechanism arises from alkynyl → Cu(I) 3LMCT (ligand to metal charge transfer) excited states that are modified by encapsulated templates and outer phosphonate ligands within the crystal structure.62
Electrochemical properties
ESI-MS reveals that tBuC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C− ligands can be isolated from the as-prepared clusters in solution. Fig. S27† illustrates the space-filling model structures of the spherical Cu56 clusters with the removal of a tBuC
C− ligands can be isolated from the as-prepared clusters in solution. Fig. S27† illustrates the space-filling model structures of the spherical Cu56 clusters with the removal of a tBuC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C− ligand exposing several active sites formed by the Cu atoms. These spherical Cu56 clusters have similar configurations while differing in terms of their encapsulated templates and external phosphonate ligands, which could result in distinct catalytic behaviors for the CO2RR.55
C− ligand exposing several active sites formed by the Cu atoms. These spherical Cu56 clusters have similar configurations while differing in terms of their encapsulated templates and external phosphonate ligands, which could result in distinct catalytic behaviors for the CO2RR.55
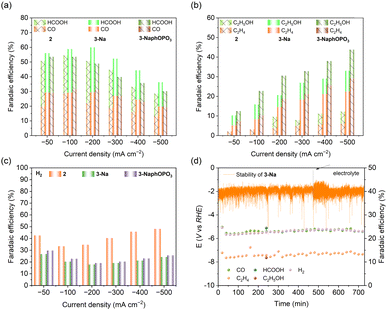
We conducted electrochemical experiments on 2, 3-Na, and 3-NaphOPO3 clusters for the CO2RR at different constant current densities (−50 to −500 mA cm−2) in a flow cell, analyzing the gas and liquid products by gas chromatography (GC) and 1H NMR, respectively. At low current densities HCOOH, CO, and H2 are the primary products, while at higher current densities, C2H4 and C2H5OH emerge in greater quantities (Fig. 7 and S28–S32†). Among these clusters, 3-Na and 3-NaphOPO3 display similar CO2RR properties. As the applied current density increases (−50 to −500 mA cm−2), the faradaic efficiency for C1 products initially rises and subsequently declines gradually, whereas the faradaic efficiency for C2 products (FEC2H4 + FEC2H5OH) generally increases over the same range of current densities. Notably, 2 exhibits higher faradaic efficiency for H2 than 3-Na and 3-NaphOPO3. At a current density of −200 mA cm−2, 3-Na demonstrates the highest faradaic efficiency for C1 products (FECO ∼ 30% + FEHCOOH ∼ 30%) and the lowest faradaic efficiency for H2 (∼17%). Under current densities ranging from −50 to −500 mA cm−2, 3-NaphOPO3 displays higher FEC2 products than the two other clusters, reaching up to ∼45% at −500 mA cm−2, with respective faradaic efficiencies for C2H4 and C2H5OH of ∼30% and ∼15%. Compared with the VO4 template, the internal PO4 template and external naphthyl groups can promote C–C coupling, resulting in an increase in C2 products. This observation indicates that CO2RR properties are not solely reliant on surface morphology but also influenced by the internal VO4/PO4 tetrahedral template.
Moreover, we selected 3-Na and 3-NaphOPO3 clusters with a higher FEC1 as examples to evaluate their stability using chronoamperometric measurements. The current densities of gaseous products remained stable for at least 720 minutes, as shown in Fig. 7d and S29.† Despite minor fluctuations in the faradaic efficiencies of CO, C2H4, and H2 at the start of the measurement, the overall current density remained relatively constant over the 720-minute period, indicating favorable catalytic sustainability. Finally, we compared these three clusters with atomically precise metal clusters exhibiting CO2RR properties previously reported. As summarized in ESI Table 4,† it is worth noting that the CO selectivity of these three clusters is lower than that of gold clusters,45 However, 3-NaphOPO3 exhibits better faradaic efficiency for C2 products, and copper, with its lower price, presents a more competitive alternative to gold.
Conclusions
In conclusion, we present template-assisted strategies for the customization of seven high-nuclearity copper(I) alkynyl clusters at the atomic level. Single-crystal structural analysis reveals the multishelled core architecture of spherical Cu36 and Cu56 clusters assembled by a [M@(V/PO4)6] (M: Cu2+, Na+, K+) skeleton, while the peanut-shaped Cu56 cluster is constructed using four separate PO4 templates. The crystalline-state emissions of these clusters are dependent on the inner templates and outer phosphonate ligands. The enhanced luminescence observed can be attributed to numerous well-arranged intermolecular C–H⋯π interactions between the ligands of phenyl and 1-naphthyl phosphate protected clusters. The electrocatalytic properties of CO2 reduction reaction are affected by both the surface morphology and the internal VO4/PO4 template. Specifically, internal PO4 templates and external naphthyl groups could promote an increase in C2 products. This study provides ingenious strategies for tailoring the structures of nanoclusters at an atomically precise level and highlights the significance of atomic-level comparative studies of structure–property relationships.Data availability
All experimental and computational data are included in the ESI.† Crystallographic data for the structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre under deposition numbers CCDC 2219441, 2219447, 2116536, 2219449, 2219459, 2219463, 2219464, and 2285409.†Author contributions
Y.-P. X. and X. L. conceived the original idea. Y.-P. X. designed and directed the synthesis experiments. J.-J. F. performed the experiments and analyzed the data. J.-J. F., Z. L., and Y.-L. S. performed the structural characterization. J.-J. F. collected and analyzed the ESI-MS, PL, and CO2RR data. Y.-P. X. directed the DFT calculations. J.-J. F. conducted the DFT calculations. All authors have given approval to the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge financial support from the National Natural Science Foundation of China (No. 21771071, 22171094, 21925104, and 92261204) and the Hubei Provincial Natural Science Foundation of China (No. 2021CFA020). We thank the staff in the Analytical and Testing Center at the Huazhong University of Science and Technology for all related measurements. We thank the staff at the BL17B beamline of the National Center for Protein Sciences Shanghai (NCPSS) at Shanghai Synchrotron Radiation Facility for the assistance with data collection.References
- R. Hamze, J. L. Peltier, D. Sylvinson, M. Jung, J. Cardenas, R. Haiges, M. Soleilhavoup, R. Jazzar, P. I. Djurovich, G. Bertrand and M. E. Thompson, Science, 2019, 363, 601–606 CrossRef CAS PubMed.
- M. Cao, R. Pang, Q. Wang, Z. Han, Z. Wang, X. Dong, S. Li, S. Zang and T. C. W. Mak, J. Am. Chem. Soc., 2019, 141, 14505–14509 CrossRef CAS PubMed.
- S. Li, X. Du, B. Li, J. Wang, G. Li, G. Gao and S. Zang, J. Am. Chem. Soc., 2018, 140, 594–597 CrossRef CAS PubMed.
- R. Huang, Y. Wei, X. Dong, X. Wu, C. Du, S. Zang and T. C. W. Mak, Nat. Chem., 2017, 9, 689–697 CrossRef CAS PubMed.
- O. Fuhr, S. Dehnen and D. Fenske, Chem. Soc. Rev., 2013, 42, 1871–1906 RSC.
- H. Schmidbaur and A. Schier, Chem. Soc. Rev., 2012, 41, 370–412 RSC.
- Y. Sun, E. Wang, Y. Ren, K. Xiao, X. Liu, D. Yang, Y. Gao, W. Ding and Y. Zhu, Adv. Funct. Mater., 2019, 29, 1904242 CrossRef.
- Z. Wang, M. Wang, Y. Li, P. Luo, T. Jia, R. Huang, S. Zang and T. C. W. Mak, J. Am. Chem. Soc., 2018, 140, 1069–1076 CrossRef CAS PubMed.
- P. Yuan, R. Chen, X. Zhang, F. Chen, J. Yan, C. Sun, D. Ou, J. Peng, S. Lin, Z. Tang, B. K. Teo, L. S. Zheng and N. Zheng, Angew. Chem., Int. Ed., 2019, 58, 835–839 CrossRef CAS PubMed.
- R. W. Huang, X. Y. Dong, B. J. Yan, X. S. Du, D. H. Wei, S. Q. Zang and T. C. W. Mak, Angew. Chem., Int. Ed., 2018, 57, 8560–8566 CrossRef CAS PubMed.
- L. L. Zhang and T. C. W. Mak, Angew. Chem., Int. Ed., 2017, 56, 16228–16232 CrossRef CAS PubMed.
- X. Chang, K. Low, J. Wang, J. Huang and C. Che, Angew. Chem., Int. Ed., 2016, 55, 10312–10316 CrossRef CAS PubMed.
- S. S. Y. Chui, M. F. Y. Ng and C. Che, Chem.–Eur. J., 2005, 11, 1739–1749 CrossRef CAS PubMed.
- R. P. Brocha Silalahi, G. Huang, J. Liao, T. Chiu, K. K. Chakrahari, X. Wang, J. Cartron, S. Kahlal, J. Saillard and C. W. Liu, Inorg. Chem., 2020, 59, 2536–2547 CrossRef CAS PubMed.
- Y. Jin, S. Li, Z. Han, B. J. Yan, H. Y. Li, X. Y. Dong and S. Q. Zang, Angew. Chem., Int. Ed., 2019, 58, 12143–12148 CrossRef CAS PubMed.
- K. K. Chakrahari, R. P. B. Silalahi, T. H. Chiu, X. Wang, N. Azrou, S. Kahlal, Y. C. Liu, M. H. Chiang, J. Y. Saillard and C. W. Liu, Angew. Chem., Int. Ed., 2019, 58, 4943–4947 CrossRef CAS PubMed.
- T. C. Higgs, P. J. Bailey, S. Parsons and P. A. Tasker, Angew. Chem., Int. Ed., 2002, 41, 3038–3041 CrossRef CAS PubMed.
- P. Putaj and F. Lefebvre, Coord. Chem. Rev., 2011, 255, 1642–1685 CrossRef CAS.
- H. Lv, Y. V. Geletii, C. Zhao, J. W. Vickers, G. Zhu, Z. Luo, J. Song, T. Lian, D. G. Musaev and C. L. Hill, Chem. Soc. Rev., 2012, 41, 7572–7589 RSC.
- J. Zhang, Y. Huang, G. Li and Y. Wei, Coord. Chem. Rev., 2019, 378, 395–414 CrossRef CAS.
- J. Liu, L. Feng, H. Su, Z. Wang, Q. Zhao, X. Wang, C. Tung, D. Sun and L. Zheng, J. Am. Chem. Soc., 2018, 140, 1600–1603 CrossRef CAS PubMed.
- Z. Wang, H. Su, M. Kurmoo, C. Tung, D. Sun and L. Zheng, Nat. Commun., 2018, 9, 2094 CrossRef PubMed.
- Y. Xie, J. Jin, X. Lu and T. C. W. Mak, Angew. Chem., Int. Ed., 2015, 54, 15176–15180 CrossRef CAS PubMed.
- Y. Xie and T. C. W. Mak, J. Am. Chem. Soc., 2011, 133, 3760–3763 CrossRef CAS PubMed.
- G. Gao, P. Cheng and T. C. W. Mak, J. Am. Chem. Soc., 2009, 131, 18257–18259 CrossRef CAS PubMed.
- L. Chen, F. Jiang, Z. Lin, Y. Zhou, C. Yue and M. Hong, J. Am. Chem. Soc., 2005, 127, 8588–8589 CrossRef CAS PubMed.
- S. Wang and G. Yang, Chem. Rev., 2015, 115, 4893–4962 CrossRef CAS PubMed.
- F. A. Paz, J. Klinowski, S. M. Vilela, J. P. Tome, J. A. Cavaleiro and J. Rocha, Chem. Soc. Rev., 2012, 41, 1088–1110 RSC.
- K. J. Gagnon, H. P. Perry and A. Clearfield, Chem. Rev., 2012, 112, 1034–1054 CrossRef CAS PubMed.
- J. Goura and V. Chandrasekhar, Chem. Rev., 2015, 115, 6854–6965 CrossRef CAS PubMed.
- L. Zhang, R. Clérac, P. Heijboer and W. Schmitt, Angew. Chem., Int. Ed., 2012, 51, 3007–3011 CrossRef CAS PubMed.
- M. Haga, K. Kobayashi and K. Terada, Coord. Chem. Rev., 2007, 251, 2688–2701 CrossRef CAS.
- S. Bao and L. Zheng, Coord. Chem. Rev., 2016, 319, 63–85 CrossRef CAS.
- X. W. Lv, C. C. Weng, Y. P. Zhu and Z. Y. Yuan, Small, 2021, 17, 2005304 CrossRef CAS PubMed.
- Y. Shen, J. Jin, J. Fang, Z. Liu, J. Shi, Y. Xie and X. Lu, Inorg. Chem., 2021, 60, 6276–6282 CrossRef CAS PubMed.
- J. Jin, Y. Xie, H. Cui, G. Duan, X. Lu and T. C. W. Mak, Inorg. Chem., 2017, 56, 10412–10417 CrossRef CAS PubMed.
- Y. Xie and T. C. W. Mak, Angew. Chem., Int. Ed., 2012, 51, 8783–8786 CrossRef CAS PubMed.
- L. Zhang and T. C. W. Mak, J. Am. Chem. Soc., 2016, 138, 2909–2912 CrossRef CAS PubMed.
- L. L. Zhang, G. Zhou, G. Zhou, H. Lee, N. Zhao, O. V. Prezhdo and T. C. W. Mak, Chem. Sci., 2019, 10, 10122–10128 RSC.
- J. Fang, Y. Shen, Z. Liu, C. Liu, Y. Xie and X. Lu, Inorg. Chem., 2021, 60, 13493–13499 CrossRef CAS PubMed.
- V. W. W. Yam and K. K. W. Lo, Chem. Soc. Rev., 1999, 28, 323–334 RSC.
- Z. N. Chen, N. Zhao, Y. Fan and J. Na, Coord. Chem. Rev., 2009, 253, 1–20 CrossRef CAS.
- H. Xu, D. Rebollar, H. He, L. Chong, Y. Liu, C. Liu, C. J. Sun, T. Li, J. V. Muntean, R. E. Winans, D. J. Liu and T. Xu, Nat. Energy, 2020, 5, 623–632 CrossRef CAS.
- S. Nitopi, E. Bertheussen, S. B. Scott, X. Liu, A. K. Engstfeld, S. Horch, B. Seger, I. Stephens, K. Chan, C. Hahn, J. K. Norskov, T. F. Jaramillo and I. Chorkendorff, Chem. Rev., 2019, 119, 7610–7672 CrossRef CAS PubMed.
- D. Raciti and C. Wang, ACS Energy Lett., 2018, 3, 1545–1556 CrossRef CAS.
- Y. Song, Y. Li, M. Zhou, X. Liu, H. Li, H. Wang, Y. Shen, M. Zhu and R. Jin, Sci. Adv., 2021, 7, eabd2091 CrossRef CAS PubMed.
- X. Ma, J. Jia, P. Luo, Z. Wang, S. Zang and T. C. W. Mak, Nano Res., 2022, 15, 5569–5574 CrossRef CAS.
- S. Nematulloev, R. W. Huang, J. Yin, A. Shkurenko, C. Dong, A. Ghosh, B. Alamer, R. Naphade, M. N. Hedhili, P. Maity, M. Eddaoudi, O. F. Mohammed and O. M. Bakr, Small, 2021, 17, 2006839 CrossRef CAS PubMed.
- J. Wang, F. Xu, Z. Y. Wang, S. Q. Zang and T. C. W. Mak, Angew. Chem., Int. Ed., 2022, 61, e202207492 CrossRef CAS PubMed.
- Z. J. Guan, J. J. Li, F. Hu and Q. M. Wang, Angew. Chem., Int. Ed., 2022, 61, e202209725 CrossRef CAS PubMed.
- H. Seong, V. Efremov, G. Park, H. Kim, J. S. Yoo and D. Lee, Angew. Chem., Int. Ed., 2021, 133, 14684–14691 CrossRef.
- S. Verma, Y. Hamasaki, C. Kim, W. Huang, S. Lu, H. M. Jhong, A. A. Gewirth, T. Fujigaya, N. Nakashima and P. J. A. Kenis, ACS Energy Lett., 2018, 3, 193–198 CrossRef CAS.
- X. Wan, J. Wang, Z. Nan and Q. Wang, Sci. Adv., 2017, 3, e1701823 CrossRef PubMed.
- Q. Tang, Y. Lee, D. Li, W. Choi, C. W. Liu, D. Lee and D. Jiang, J. Am. Chem. Soc., 2017, 139, 9728–9736 CrossRef CAS PubMed.
- L. J. Liu, Z. Y. Wang, Z. Y. Wang, R. Wang, S. Q. Zang and T. C. W. Mak, Angew. Chem., Int. Ed., 2022, 61, e202205626 CrossRef CAS PubMed.
- X. Ma, F. Sun, L. Qin, Y. Liu, X. Kang, L. Wang, D. Jiang, Q. Tang and Z. Tang, Chem. Sci., 2022, 13, 10149–10158 RSC.
- P. Yang and U. Kortz, Acc. Chem. Res., 2018, 51, 1599–1608 CrossRef CAS PubMed.
- S. Peng, H. Yang, D. Luo, M. Xie, W. Tang, G. Ning and D. Li, Inorg. Chem. Front., 2022, 9, 5327–5334 RSC.
- Y. Xie, Y. Shen, G. Duan, J. Han, L. Zhang and X. Lu, Mater. Chem. Front., 2020, 4, 2205–2222 RSC.
- T. Lu and F. Chen, J. Comput. Chem., 2012, 33, 580–592 CrossRef CAS PubMed.
- F. Neese, F. Wennmohs, U. Becker and C. Riplinger, J. Chem. Phys., 2020, 152, 224108 CrossRef CAS PubMed.
- G. F. Manbeck, W. W. Brennessel, R. A. Stockland and R. Eisenberg, J. Am. Chem. Soc., 2010, 132, 12307–12318 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2219441, 2116536, 2285409, 2219449, 2219459, 2219463, 2219464 and 2219447. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3sc04682f |
| This journal is © The Royal Society of Chemistry 2023 |