 Open Access Article
Open Access ArticleRecent advances in MoS2-based nanomaterial sensors for room-temperature gas detection: a review
Xu
Tian
a,
Shanli
Wang
a,
Haoyu
Li
a,
Mengyao
Li
a,
Ting
Chen
*b,
Xuechun
Xiao
*a and
Yude
Wang
 *c
*c
aNational Center for International Research on Photoelectric and Energy Materials, School of Materials and Energy, Yunnan University, 650091 Kunming, People's Republic of China. E-mail: xchxiao@ynu.edu.cn; Fax: +86 871 65153832; Tel: +86 871 65035570
bInstitute of Materials Science & Devices, School of Materials Science and Engineering, Suzhou University of Science and Technology, Suzhou, 215009, People's Republic of China. E-mail: chenting@mail.usts.edu.cn
cKey Lab of Quantum Information of Yunnan Province, Yunnan University, 650091 Kunming, People's Republic of China. E-mail: ydwang@ynu.edu.cn
First published on 23rd December 2022
Abstract
The two-dimensional (2D) material, MoS2, has attracted great attention in the development of room-temperature gas sensors in recent years due to its large specific surface area, ultra-high carrier mobility, strong surface activity, and high adsorption coefficient. However, pristine MoS2 gas sensors still exhibit some drawbacks such as low sensing response, sluggish recovery process, and incomplete recovery, which are unfavorable for the application of gas sensors. Therefore, significant efforts have been devoted to the design of specific MoS2-based gas sensors with enhanced sensing properties. In this review, we aim to discuss the recent advances in MoS2-based nanomaterial sensors for room-temperature gas detection. Firstly, some strategies to improve the gas sensing performance of MoS2-based gas sensors are introduced, including designing morphologies, creating sulfur vacancies, decorating noble metals, doping elements, introducing light, and constructing composites. Secondly, the types of gases that can be detected by MoS2-based gas sensors are proposed and summarized, and their sensing mechanisms are also analyzed. Finally, an outlook is presented and the future research directions and challenges are discussed.
1. Introduction
The detection of toxic and harmful gases is important to ensure the safety of life and protect the environment. In the past few decades, semiconductor metal oxide (SMO) gas sensors have been the dominant tools for the detection of toxic gases such as volatile organic compounds (xylene, toluene, formaldehyde (HCHO), ammonia (NH3), acetone, ethanol, methanol, and isopropanol), flammable and explosive gases (methane (CH4), hydrogen (H2), propane (C3H8), carbon monoxide (CO), hydrogen sulfide (H2S)), nitrogen oxides (nitrogen monoxide (NO), nitrogen dioxide (NO2)), sulfur oxides (sulfur dioxide (SO2)), and carbon oxides (carbon dioxide (CO2)). To date, SMO gas sensors still occupy the central position in the field of gas detection due to their high sensing response, fast response/recovery time and excellent reproducibility. However, some deficiencies presented by SMO gas sensors include their poor selectivity and high operating temperature, which have not been addressed to date. In particular, their high operating temperature will be detrimental to energy saving and limit their application in some special fields. Therefore, it is necessary to develop low-power, high-sensing performance gas sensors.Recently, several reports have revealed that the emerging two-dimensional (2D) materials exhibit a sensing response to toxic gases at low/room temperature, which not only solves the problem of high power consumption of traditional gas sensors to a certain extent but also enable them to be applied in flexible wearable electronic devices to provide great convenience and achieve intelligent life. The 2D materials include reduced graphene oxide (rGO),1 transition metal dichalcogenides (TMDs),2 black phosphorus (BP),3 hexagonal boron nitride (h-BN),4 and transition metal carbides, nitrides and/or carbonitrides (MXenes),5 which can be considered as promising gas sensing materials owing to their unique single-atom layer structure. Specifically, they exhibit high specific surface area close to the theoretical extreme, excellent semiconductor performance, unique surface configurations with dangling bonds on their edge sites, and flexible basal planes.6–10 Among them, the layered TMDs with the composition of MX2 (M = Ti, Zr, Hf, V, Nb, Ta, Mo, W, Tc, Re, Pd, and Pt and X = S, Se, and Te)11 have gained intensive attention as gas sensing materials because of their strong spin–orbit coupling interaction, tunable electronic properties, and high interaction ability for the adsorption of gas molecules.12,13 Among the TMDs, the semiconductor MoS2 and WS2 with atomically thin-layered structures, lower bandgap, abundant edge active sites, and excellent electrical and/or chemical properties exhibit good gas sensing abilities at room temperature (RT).14–16 In particular, MoS2 has become the most ideal gas sensing material17,18 owing to its ultra-high carrier mobility, high adsorption coefficient, tunable bandgap (1.2–1.9 eV), and excellent field-effect transistor behavior.19–22 These parameters have a positive impact on the sensitivity and stability of gas sensors and the designability of novel sensing materials based on MoS2. MoS2 presents four crystal structures including 1H, 1T, 2H, and 3R, which are defined by the coordination relationship between the Mo and S atoms and the stacking order between their layers, as shown in Fig. 1. The numbers 1, 2, and 3 represent the number of S–Mo–S layers in each unit cell, while the letters T, H, and R represent triangle, hexagonal, and rhombohedral, respectively. The 1T-MoS2 phase shows metallic nature, whereas the 2H-MoS2 phase exhibits semiconductor characteristic (n-type). In terms of thermodynamics, besides the 2H phase, three other crystal phases of MoS2 possess a metastable structure, which can also be transformed under certain conditions.23 Therefore, the thermodynamically stable 2H-MoS2 structure dominates current applications.24
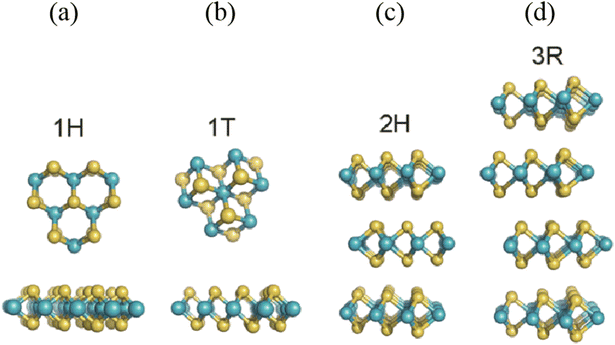 | ||
| Fig. 1 Different polymorphs or phases of MoS2: (a) 1H phase, (b) 1T phase, (c) 2H phase, and (d) 3R phase. Reprinted with permission from ref. 24. Copyright 2015, The Royal Society of Chemistry. | ||
Recently, several review papers highlighted 2D layered material-based resistive sensors.8,25,26 These works emphatically discussed the synthesis methods, gas sensing application of TMDs, and the sensing mechanisms of TMDs van der Waals nanocomposite junctions. Considering the advantages of MoS2 and its potential application in developing room-temperature gas sensors, herein, we mainly review the recent advances of MoS2 nanomaterial-based gas sensors for room temperature detection. Based on the existing review papers, we further present the development of MoS2 gas sensors and discuss them in detail. Initially, we discuss some strategies for improving the gas sensing properties of MoS2. Subsequently, we summarize the types of toxic gases that MoS2 can sense at RT. Moreover, the sensing mechanisms of MoS2-based gas sensors towards different gases are also discussed. Furthermore, we conclude this review with some perspectives and outlooks on this new trend in the field of gas sensing.
2. Strategies to improve the gas sensing performance of MoS2
Although MoS2 has shown great advantages in the development of room temperature gas sensors, it still faces some challenges, for instance, due to the stacking of the S–Mo–S layers, bulk MoS2 does not have sufficient contact with gas molecules and forms poor conductive network signals, which lead to a low response value and slow response recovery rate. Especially, the incomplete recovery at RT is a severe challenge for MoS2-based gas sensors. In this regard, more efforts have been devoted to designing specific MoS2-based RT gas sensors with enhanced sensing properties. The improvement strategies include designing morphologies, creating sulfur vacancies, decorating noble metals, doping elements, introducing light, and constructing composites. In this part, we summarize the above-mentioned strategies for improving the gas sensing performance of MoS2 materials.2.1 Morphology design
For sensing applications, the morphology of MoS2 plays a crucial role in enhancing the sensing performance by providing more reactive sites. A change in the morphology of MoS2 refers to its dimensions, which can be varied from zero, one, and two to three-dimensional nanostructures. MoS2 with different dimensions exhibit unique physical and optoelectronic properties, defects, exposed facets, porosity, atomic configuration,27 and thus its gas sensing properties will also be different. When MoS2 is compressed to zero-dimensional, completely special electronic and photophysical properties are generated due to the quantum confinement and edge effects,28 such as a higher direct bandgap of 3.96 eV,25 larger edge-to-volume ratio, and higher in-plane electron transport rate. Niu et al.29 synthesized MoS2 quantum dots (MQDs) via the combined high speed shear, sonication and solvothermal treatment of bulk MoS2 in N,N-dimethylformamide. Fig. 2a shows the HRTEM image of MQDs with an average size of 7.8 nm. NH3 and NO2 gases were recognized by the MQD sensor at RT. The dynamic sensing response of the MQD sensor towards various concentrations of NO2 (Fig. 2b) and NH3 (Fig. 2c) revealed that it had almost the same response value for both gases. However, the recovery was not complete due to the high-energy binding sites of the MQDs. This research team is working on how to balance the relationship between the selectivity and fast desorption in their further study.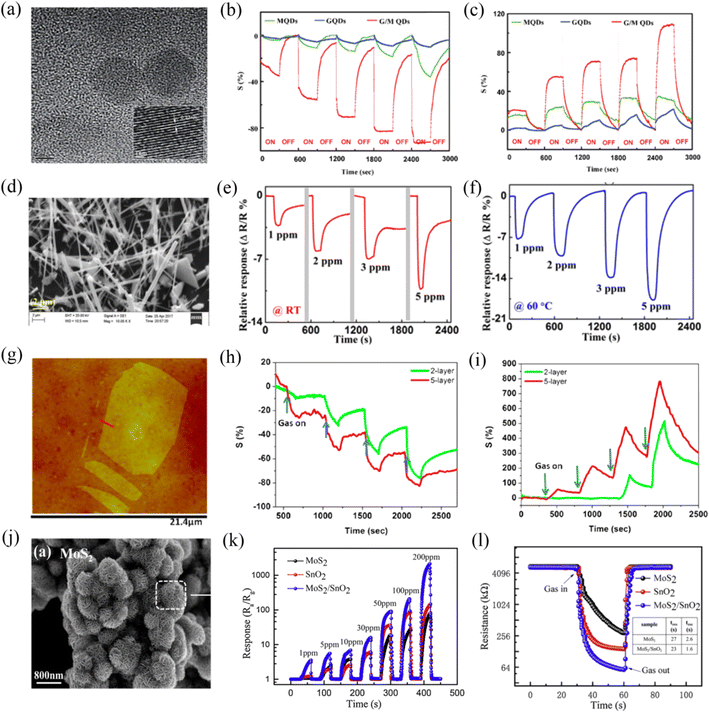 | ||
| Fig. 2 (a) HRTEM of MQDs. Dynamic response of the MQDs (green) upon exposure to increasing (b) NO2 and (c) NH3 concentrations. Reprinted with permission from ref. 29. Copyright 2016, The Royal Society of Chemistry. (d) SEM image of MoS2 nanowires. Transient response of the MoS2 nanowire sensor at (e) room temperature (RT) and (f) 60 °C. Reprinted with permission from ref. 30. Copyright 2018, AIP Publishing. (g) AFM image of single-layer MoS2 sheet. Comparative two- and five-layer MoS2 cyclic sensing performances with (h) NH3 and (i) NO2 (for 100, 200, 500, and 1000 ppm). Reprinted with permission from ref. 31. Copyright 2013, the American Chemical Society. (j) SEM images of MoS2 nanoflowers. (k) Responses curves of MoS2, SnO2, and SnO2/MoS2 sensors to various concentrations (1–200 ppm) of NH3. (l) Resistance curves of MoS2, SnO2, and SnO2/MoS2 to 50 ppm of NH3 at room temperature (the insert table indicates the response and recovery times). Reprinted with permission from ref. 33. Copyright 2020, Elsevier B.V. | ||
One-dimensional MoS2 nanostructures include nanowires and nanotubes. Their electronic properties also vary with a change in their diameter and chirality, for example, MoS2 nanotubes exhibit a larger bond length and smaller semiconducting bandgap than that of the bulk MoS2 nanosheets.25 Kumar et al.30 reported the fabrication of an NO2 sensor based on one-dimensional MoS2 nanowires (Fig. 2d), which were synthesized using chemical transport reaction through controlled turbulent vapor flow. The results showed that the MoS2 nanowire sensor displayed a high sensing response to NO2 gas; however, it still faced the problem of incomplete recovery at RT due to the strong binding between NO2 and the reactive sites of MoS2, as shown in Fig. 2e. Thus, to address its difficult recovery and low response at RT, this team investigated its sensing behavior at a high operating temperature (60 °C) (Fig. 2f). They proposed that the relatively quick adsorption and desorption of NO2 gas molecules from MoS2 at 60 °C were attributed to its high conductivity and the rapid interaction of gas molecules with the exposed edge sites of the nanowires. Also, they indicated that the oxygen and humidity occupy a large number of reactive sites in the MoS2 nanowires at RT, and thus there were less NO2 molecules to participate in the reaction, resulting in a weak response to NO2 at RT.
MoS2 with monolayer or few-layer two-dimensional nanostructures is currently the most studied in the field of gas sensing. Monolayer MoS2 shows a direct bandgap of 1.8 eV, while bulk MoS2 possesses an indirect bandgap of 1.2 eV. This transition endows monolayer MoS2 with superior semiconductor properties. Meanwhile, monolayer or few-layer MoS2 expose abundant edge sites and a high specific surface area, which may be beneficial for the absorption of gas molecules. In addition, it also exhibits high toughness and has potential to be applied on flexible substrates. Late et al.31 investigated whether the single-layer MoS2 is an ideal structure for enhancing the gas sensing performances. The AFM image of single-layer MoS2 is shown in Fig. 2g. They found that the single-layer MoS2 device was not stable over time. For clarity and brevity, they examined the gas sensing responses of two-layer and five-layer MoS2 to various concentrations of NH3 (Fig. 2h) and NO2 (Fig. 2i) gases at RT because they were the thinnest and the thickest, respectively. The results showed that five-layer MoS2 had better sensitivity compared to that of the two-layer MoS2, they agreed that this may be due to the different electronic structures with a variation in thickness (layering). However, this issue is complicated and needs further study. Li et al.32 prepared few-layer MoS2 nanosheets via mechanical exfoliation for the RT detection of NO2. This sensor achieved high responsivity and ultrafast recovery behavior to NO2. They proposed that the high sensitivity was caused by the thin thickness of MoS2, while the fast recovery time was attributed to the weak van der Waals force between NO2 and MoS2.
Three-dimensional nanoflower-like MoS2 (Fig. 2j) assembled by several nanosheets has also received great attention for gas sensing. MoS2 nanoflower is mainly synthesized via a hydrothermal process. Wang et al.33 prepared MoS2 nanoflowers via a simple hydrothermal method at 200 °C for 22 h. Fig. 2k shows the dynamic sensing response curves of MoS2, SnO2, and SnO2/MoS2 sensors towards different concentrations of NH3 at RT. It was observed that the nanoflower-structured MoS2 and its nanocomposite-based gas sensors exhibited high sensing response values. The resistance curves (Fig. 2l) of the MoS2, SnO2, and SnO2/MoS2 sensors exposed to 50 ppm NH3 revealed that they displayed a very fast response and recovery rate (27/2.6 s for MoS2 sensor), which seems to be very interesting. Thang et al.34 discussed the effect of the hydrothermal growth times of 24, 36, 48, and 60 h on the sensitivity of the obtained MoS2 nanoflowers and concluded that 48 h was the best growth time. The 48 h-MoS2 nanoflowers showed a high gas response of 67.4% and high selectivity to 10 ppm NO2 at RT. The superior sensing performance of the 48 h-MoS2 nanoflower was ascribed to its largest specific surface area, smallest crystallite size, and lowest activation energy among the prepared samples. The dynamic resistance characteristic revealed that the 48 h-MoS2 sensor exhibited complete response and recovery to NO2 gas at RT. The authors ascribed this result to the high specific surface area and defects of the 48 h-MoS2. They proposed that several factors such as high specific surface area, defective/strained surface, and weak van der Waals binding between the target gas and the MoS2 surface affected the gas adsorption and desorption behavior. However, the complete recovery mechanism of the MoS2 sensor is a complex case, and there are some disputes due to the combined effects of physi- and chemi-sorption, role of defects sites and transduction mechanism.35
2.2 Vacancy promotion
The lack of adsorption sites in MoS2 has become the main bottleneck in realizing a high sensing performance at RT. It has been theoretically and experimentally proven that the vacancies in MoS2 act as high-energy binding sites and play an important role in enhancement the gas sensing performance. The vacancies mainly refer to two types, i.e., Mo vacancy and S vacancies. However, the lower binding energy of S vacancy (2.12 eV) compared to Mo vacancy (6.20 eV) makes its construction more desirable, wherein the S vacancy is defined as the absence of one or two sulfur atoms per MoS2.36,37 The strategy of generating S vacancies in MoS2 aims to reduce the Gibbs free energy of gas adsorption,38 increase the amount of charge transfer,39 facilitate molecular adsorption and chemical functionalization,40 offer abundant active sites, and even cause the dissociation of gas molecules.41 At present, S vacancies can be achieved by microwave-hydrothermal treatment, liquid-phase ultrasonic exfoliation, metal quantum dot loading,42 electron irradiation and thermal annealing.36,43,44Xia et al.43 discussed the NO2 gas sensing performance of conventional MoS2 (C-MoS2) and sulfur-vacancy-enriched MoS2 (SV-MoS2) under dark and near-infrared (NIR) light conditions at RT, respectively. The researchers employed X-ray diffraction (XRD), electron paramagnetic resonance (EPR), and X-ray photoelectron spectroscopy (XPS) characterization techniques to prove the presence of sulfur vacancies, enriched S vacancy defects, and defect-related surface species in the MoS2 samples, as shown in Fig. 3a–c, respectively. The response in Fig. 3d reveals that the SV-MoS2 sensor showed a better gas sensing performance to 200 ppm NO2 than the C-MoS2 sensor in both the dark and under NIR illumination, which can be ascribed to the presence of more active centers and increased electron transfer introduced by the S vacancies. Moreover, the response value of the SV-MoS2 sensor under NIR light had a significant improvement compared to that in a dark environment, while a slight increase occurred in the C-MoS2 sensor, demonstrating that the S vacancy-induced photocurrent could effectively detect NO2 gas at RT.
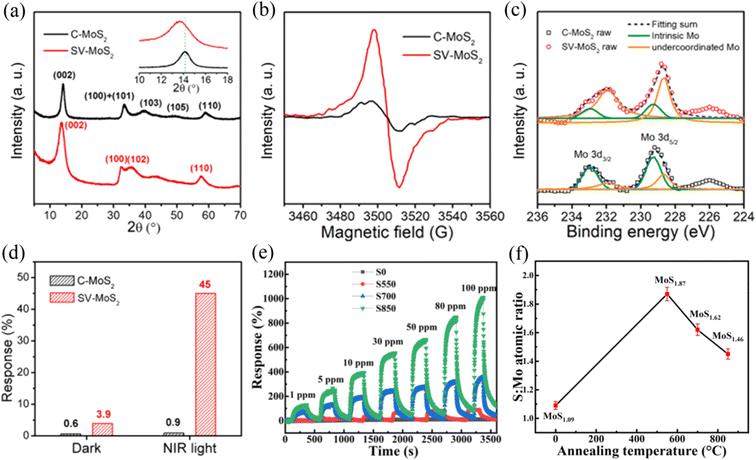 | ||
Fig. 3 (a) XRD, (b) EPR, (c) Mo 3d XPS spectra of C-MoS2 and SV-MoS2 samples. (d) Gas responses of C-MoS2 and SV-MoS2 sensors in the dark and under NIR illumination. Reprinted with permission from ref. 43. Copyright 2019, the American Chemical Society. (e) Dynamic response curves of the S0, S550, S700, and S850 sensors toward different concentrations of NO2 at room temperature. (f) Corresponding S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mo atomic ratio of S0, S550, S700, and S850. Reprinted with permission from ref. 45. Copyright 2022, Elsevier B.V. Mo atomic ratio of S0, S550, S700, and S850. Reprinted with permission from ref. 45. Copyright 2022, Elsevier B.V. | ||
Zhang et al.45 introduced S vacancies in 2D-in-3D architecture MoS2 by high temperature annealing in an argon atmosphere. They compared the sensing properties of different MoS2 samples obtained at various annealing temperatures of 0 °C, 550 °C, 700 °C, and 850 °C to NO2 at RT. The results showed that the hierarchical MoS2 annealed at 850 °C exhibited an extremely high gas sensing performance in terms of sensitivity (Fig. 3e), selectivity and stability. These excellent sensing properties can be attributed to the large number of S vacancies in MoS2, which were generated upon high temperature annealing and led to the strong interlayer coupling and spin–orbit coupling effects. The generation of S vacancies was confirmed by the decrease in the S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mo ratio (Fig. 3f) under high temperature annealing by XPS measurements. In this regard, S vacancies play an extremely important role in improving the gas sensing performance of MoS2 materials.
Mo ratio (Fig. 3f) under high temperature annealing by XPS measurements. In this regard, S vacancies play an extremely important role in improving the gas sensing performance of MoS2 materials.
In addition, density functional theory (DFT) calculations also revealed that MoS2 rich in S vacancies possessed a higher sensing performance to gases. Li et al.46 calculated the adsorption properties and charge transfer of NO molecules on monolayer MoS2 (MoS2-MLs), S vacancy-defective MoS2-MLs (S-vacancy), and vacancy complex of Mo and its nearby three sulfur vacancies (MoS3-vacancy) by density functional theory (DFT). The adsorption energy of an NO molecule on the most stable adsorption models of MoS2-MLs, S-vacancy, and MoS3-vacancy was 0.14 eV, 2.57 eV and 1.95 eV, respectively. The theoretical results demonstrated that the MoS3-vacancy and S-vacancy-defective MoS2-MLs showed stronger chemisorption and greater electron transfer effects than pure MoS2-ML, implying that S-vacancy defects can effectively improve the NO sensing performance of MoS2.
Although the vacancies on the surface of MoS2 acts as active sites for the adsorption of gas molecules, their high adsorption energy will also result in a slow response and recovery rate.35
2.3 Noble metal decoration
The decoration of noble metals (NMs) on MoS2 has also been reported as another effective strategy to improve its gas sensing properties. NMs such as Au, Ag, Pt, Pd, Rh, and Ru are usually used as effective catalysts to enhance the surface reactivity of sensing materials and accelerate the reaction between the adsorbed oxygen species and the gas molecules.47 Meanwhile, they can also change the electron accumulation and enhance the electron transfer due to the different work functions between the NMs and sensing materials. Moreover, NMs possess affinity for some specific gas molecules and assist in overcoming the problem of selectivity to a certain extent.48Jaiswal et al.49 reported the preparation of a vertically aligned edge-oriented MoS2 hybrid nanostructured thin film decorated with Pd nanoparticles (Pd/MoS2) on quartz and Si substrates using the DC magnetron sputtering technique. The 2D and 3D AFM micrographs of the Pd-functionalized vertically aligned MoS2 thin film are shown in Fig. 4a and b, respectively. The Pd/MoS2 hybrid film sensor exhibited an enhanced response of 33.7% and fast response/recovery rate (∼16/38 s) compared to the pristine MoS2 thin film sensor (1.2% response value and ∼29/158 s response/recovery time) to 500 ppm H2 gas at RT (Fig. 4c). The enhancement in the H2 gas sensing performance of the Pd/MoS2 hybrid film sensor can be attributed to three aspects. Firstly, the catalytic activity of the small Pd nanoparticles endowed the hydrogen molecules with efficient decomposition ability. Secondly, the unique porous nanostructure of the vertically aligned edge-enriched MoS2 possessed a higher specific surface area. Finally, the Schottky barrier at the junction between Pd and MoS2 increased the electrical resistance in air due to the barrier height, becoming more sensitive to a change in H2 resistance.
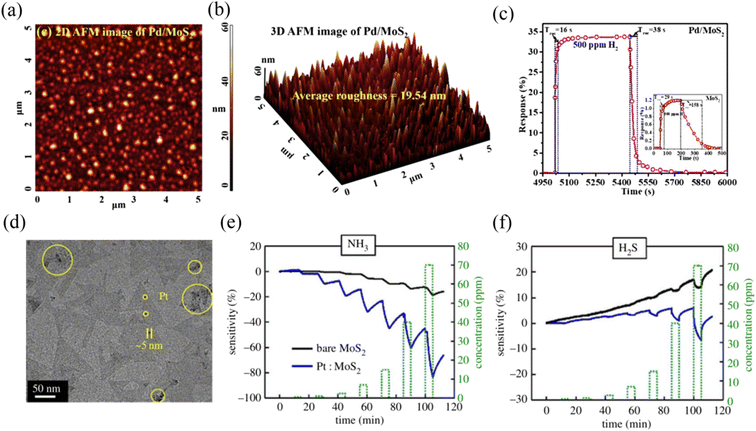 | ||
| Fig. 4 (a) 2D and (b) 3D AFM micrographs of Pd-functionalized vertically aligned MoS2 thin film. (c) Sensor response curve of the Pd/MoS2 hybrid and pristine MoS2. Reprinted with permission from ref. 49. Copyright 2020, Elsevier B.V. (d) TEM images of the Pt/MoS2. Gas-sensing characteristics of the MoS2 and Pt/MoS2 gas sensors for (e) NH3 and (f) H2S. Reprinted with permission from ref. 51. Copyright 2020, IEEE Xplore. | ||
Halvaee et al.50 synthesized Ag/MoS2 nanorods via the hydrothermal method. This sensor displayed a selective sensing response for methanol vapor at RT. Firstly, the researchers discussed the effect of different amounts of Ag nanoparticles on the response of the sensor. They found that the mass ratio of 2 wt% Ag nanoparticles loaded on MoS2 resulted in the best methanol sensing response. The improved gas sensing properties can be ascribed to the catalytic oxidation and chemical sensitization of Ag nanoparticles. Meanwhile, the selectivity of Ag/MoS2 to methanol was much better than that of pure MoS2. In addition to the small size of methanol, which could easily penetrate the layered MoS2, Ag had a better decoration effect to improve the selectivity.
Park et al.51 prepared two-dimensional MoS2via a metal organic chemical vapour deposition (MOCVD) method, and subsequently modified its surface with Pt particles (Fig. 4d). Pt particles have a double p-type doping effect compared to Au particles and possess good corrosion and oxidation resistance. Accordingly, this sensor recognized both NH3 and H2S gases at RT; however, the response for H2S was lower than that for NH3, as shown in Fig. 4e and f, respectively, confirming that there was less charge transfer between H2S and Pt/MoS2. Meanwhile, the response value of Pt/MoS2 for the target gases was higher than that of bare MoS2, demonstrating that the Pt particles made an excellent contribution to the improvement in gas sensing performance.
2.4 Element doping
Element doping refers to a change in lattice constant due to the incorporation of dopants in the lattice of MoS2 or replacement of the Mo, S lattice sites. In this process, the binding energy will be greatly enhanced and defects will be formed to become new active sites, and the electrical properties will also be changed due to the decrease in the electron–hole recombination rate.52 The doped elements can be divided into metal and nonmetal, where the metal dopants include Zn, W, Nb, Fe, Co, Ni, Cu, Ti, V, Ta, Al, and Ga,45,53–58 and nonmetal dopants include N, Si, B, N, P, and Cl.59–61 However, most doping strategies focus on theoretical calculations based on density functional theory (DFT),62–65 where theoretical results reveal that doped-MoS2 sensors exhibit a higher adsorption energy, stronger noncovalent interaction, greater carrier transport number, and faster conductivity rate to target gases.60,62,63 Therefore, more efforts should be devoted to the experimental exploration of doping MoS2. At present, some experimental studies have been reported.Wu et al.59 designed an N element-doped MoS2 gas sensor by controlling the solvothermal temperature to realize the conversion of MoS2 from n-type to p-type. The researchers proposed that doping could also address the challenge of sluggish sensing of MoS2 at RT owing to the adjustable active sites and electrical property. Fig. 5a displays the gas sensing response value of pristine MoS2 and optimal N-doped MoS2 (NMoS2-2) sensors to various concentrations of NO2 at RT. It was observed that the NMoS2-2 sensor showed obvious p-type semiconductor feature because the N atoms have one less valance electron than the S atoms in the MoS2 matrix. Meanwhile, the sensing response value of the NMoS2–2 sensor was not obviously improved compared to that of the pristine MoS2. However, the fast response/recovery rate (Fig. 5b and c) of the NMoS2-2 sensor revealed that there was a superior fast charge transfer character, as confirmed by the Hall effect. DFT calculations revealed that there was a favorable surface interaction between the N-doped MoS2 and NO2 molecules after N doping. Therefore, N-doping in MoS2 resulted in a significant improvement in NO2 sensing response/recovery ability.
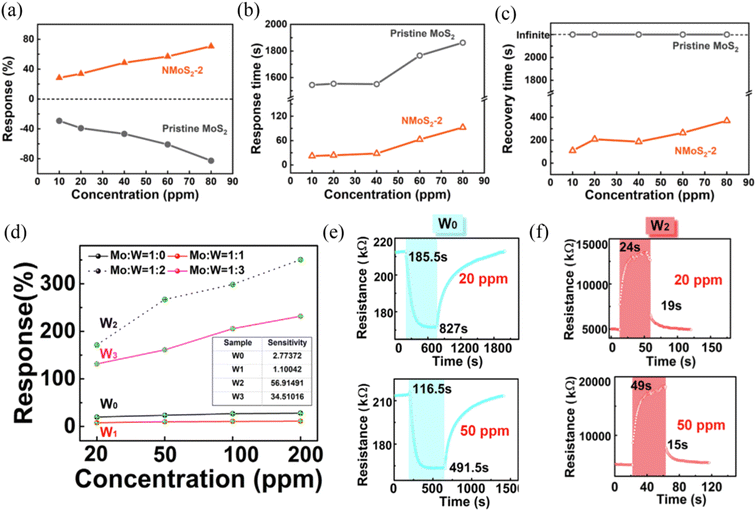 | ||
| Fig. 5 (a) Response, (b) response time, and (c) recovery time of NMoS2-2 and pristine MoS2 upon exposure to 10, 20, 40, 60, and 80 ppm NO2. Reprinted with permission from ref. 59. Copyright 2021, Elsevier B.V. (d) Response value versus NO2 concentration for W0–W3. (e) Transient response characteristic of (e) W0 and (f) W3 at 20 and 50 ppm NO2. Reprinted with permission from ref. 53. Copyright 2020, Elsevier B.V. | ||
Liu et al.53 synthesized W-doped MoS2 sensors with different W ratios via a hydrothermal method. The results showed that appropriate ratios between Mo and W were conducive to enhancing the NO2 sensing properties at RT. As shown in Fig. 5d, when the Mo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) W ratio was 1
W ratio was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (named W2), the sensing response was observed to be the best for various concentrations of NO2. Furthermore, the response/recovery times of the W-doped MoS2 (W2) sensor (Fig. 5f) was greatly improved compared to the undoped MoS2 sensor (Fig. 5e), which was mainly attributed to the effective suppression of defects by W doping.
2 (named W2), the sensing response was observed to be the best for various concentrations of NO2. Furthermore, the response/recovery times of the W-doped MoS2 (W2) sensor (Fig. 5f) was greatly improved compared to the undoped MoS2 sensor (Fig. 5e), which was mainly attributed to the effective suppression of defects by W doping.
Briefly, according to the current research results, the doping method can be regarded as an effective method to solve the slow response/recovery ability of MoS2.
2.5 Light assistance
Light assistance has shown promise for the activation of gas sensor materials. MoS2 possesses a tunable band gap and excellent photoelectrical properties, and thus it is also an effective way to improve its gas sensing performance by light activation. Light activation mainly assists the recovery rate of MoS2 gas sensors,66 and the photochemical reaction occurring between the light-generated electron/hole carriers in MoS2 and adsorbed gas molecules promotes the desorption process.67,68 At present, two light activation gas sensing mechanisms have been proposed, i.e., the “optoelectronic” and “photocatalytic” mechanisms. The optoelectronic mechanism refers to the generation of a photocurrent, which regulates the conductivity of the material and causes a large change in the resistance of the sensor upon gas exposure.15,69 The photocatalytic mechanism considers the process of photocatalytic oxidation of reducing gases into NOx, CO2 and H2O,70,71 thus accelerating the chemisorption reaction between the sensing material and target gases.Wang et al.72 proposed the visible-light photocatalytic enhancement gas sensing mechanism based on MoS2/rGO hybrids for the detection of formaldehyde (HCHO) at RT. The comparison of response/recovery times of the MoS2/rGO sensor to 10 ppm HCHO in the dark and under visible-light illumination, as shown in Fig. 6a, which revealed that the visible light accelerated the gas molecule adsorption/desorption process. In addition, the O2-TPD spectra of MoS2, as shown in Fig. 6b, demonstrated that visible light induced the adsorption of more oxygen species. Meanwhile, CO2 peaks at 1358 and 1572 cm−1 and broad H2O peak at around 3420 cm−1 were observed by in situ IR spectroscopy (Fig. 6c) when MoS2 was exposed to HCHO and illuminated by visible light, which suggests that the visible-light illumination triggered the photocatalytic oxidation of HCHO to CO2 and H2O on the surface of MoS2.
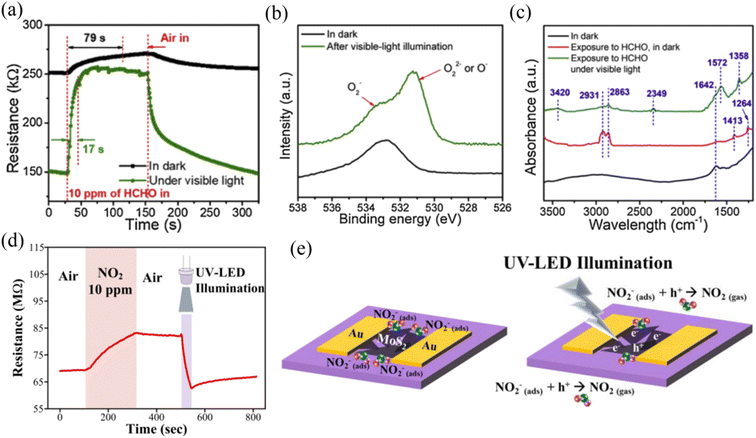 | ||
| Fig. 6 (a) Dynamic resistance variations of the MoS2/rGO sensor to 10 ppm HCHO in the dark and under visible-light illumination. (b) O 1s XPS spectra of MoS2 in the dark and after visible-light illumination for 5 min. (c) in situ IR spectra of the MoS2 sample under different conditions. Reprinted with permission from ref. 72. Copyright 2020 Elsevier B.V. (d) Transient sensor response upon exposure to 10 ppm NO2, and a UV-LED was turned on during the recovery process. (e) Schematic of the recovery mechanism for MoS2 under UV-LED illumination after NO2 exposure. Reprinted with permission from ref. 73. Copyright 2019, IOP Publishing Ltd Printed in the UK. | ||
Kang et al.73 reported that UV light-illuminated MoS2 could achieve the recovery of its initial resistance when NO2 gas was withdrawn at RT (Fig. 6d). They believed that excitons were generated in MoS2 under UV light illumination, which could be separated into electrons and holes when an in-plane electric field of 2 kV cm−1 was applied. The absorbed NO2− by capturing electrons from MoS2 previously would react with the photo-generated holes to result in the formation of NO2, which accelerated the desorption process (Fig. 6e). Meanwhile, the photo-generated electrons remaining in the conduction band of MoS2 would decrease the resistance. Thus, this explains why UV-light illumination caused a rapid return to the initial resistance of the platform after releasing NO2 gas.
2.6 Construction of composites
The construction of MoS2-based composite gas sensors has been demonstrated to be one of the most effective methods to improve the gas sensing properties. In comparison to pure MoS2, MoS2 nanocomposites with well-designed architectures are more desirable. The types of composites include binary and ternary structures, which can achieve an enhancement in gas sensing performance by making use of the merits of each component to generate synergistic effects and construct heterojunctions. The heterojunctions include n–n, n–p, and p–p types; however, MoS2 can exhibit either a p- or n-type gas sensing response to reductive vapor depending on its annealing temperature in air.74 The heterojunctions can effectively rectify the electron transfer at the contact surface of two materials and increase the interface barrier due to their different Fermi levels, which can significantly improve the gas sensitivity of composite sensing materials. Moreover, MoS2-based composites accelerate the response/recovery rate of the sensor to some extent. Therefore, constructing composites of MoS2 may be one of the most effective modification methods. Materials compounded with MoS2 can be classified into the following categories:(i) Metal oxide semiconductors: n-type CeO2,75 ZnO,76 SnO2,77 WO3,78 In2O3,79 TiO2,80 and MoO3 (ref. 81) and p-type CuO,82 Co3O4,83 NiO,84 Cu2O,85 PANI,86 and PPy.87
Bai et al.88 reported the preparation of a room-temperature NO2 gas sensor based on an MoS2/SnO2 p–n heterojunction. MoS2 exhibited p-type semiconductor behavior in this work, which was induced by the oxygen vacancies/defects. The MoS2 nanoflakes were vertically grown on the SnO2 nanotubes via electrospinning, and subsequent hydrothermal method, as shown in the SEM image in Fig. 7a. The optimal MoS2@SnO2-2 sensor (the mole ratio of Sn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mo was 1
Mo was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/2) exhibited the highest sensing response value compared to the other mole ratio sensors and pristine SnO2 sensor towards different concentrations of NO2 gas (Fig. 7b). Meanwhile, its response/recovery times (2.2/10.54 s) were also fast. The enhancement in the gas sensing properties could be attributed to the unique morphological structure, high specific surface area, large number of sulfur edge active sites, and p–n heterojunction created between MoS2 and SnO2. The sensing mechanism could be explained by the surface depletion layer model caused by oxygen adsorption, as shown in Fig. 7c. The ionized chemisorbed oxygen (O2−) produced on the surface of sensing material formed NO3− by introducing NO2 gas due to the oxidation reaction. This process caused a change in the carrier concentration, and especially after the formation of heterojunctions, this change would be greater.
1/2) exhibited the highest sensing response value compared to the other mole ratio sensors and pristine SnO2 sensor towards different concentrations of NO2 gas (Fig. 7b). Meanwhile, its response/recovery times (2.2/10.54 s) were also fast. The enhancement in the gas sensing properties could be attributed to the unique morphological structure, high specific surface area, large number of sulfur edge active sites, and p–n heterojunction created between MoS2 and SnO2. The sensing mechanism could be explained by the surface depletion layer model caused by oxygen adsorption, as shown in Fig. 7c. The ionized chemisorbed oxygen (O2−) produced on the surface of sensing material formed NO3− by introducing NO2 gas due to the oxidation reaction. This process caused a change in the carrier concentration, and especially after the formation of heterojunctions, this change would be greater.
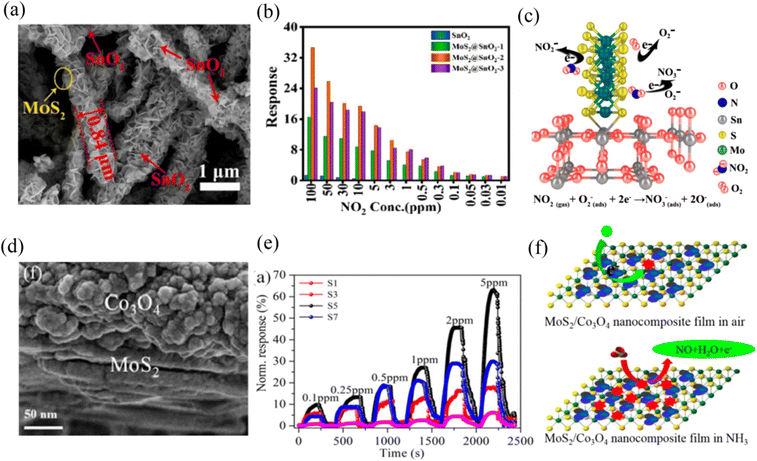 | ||
| Fig. 7 (a) SEM images of MoS2@SnO2-2 nanocomposite. (b) Responses of the prepared sensors to different concentrations of NO2. (c) Schematic of sensing mechanisms of MoS2@SnO2-2 nanocomposite. Reprinted with permission from ref. 88. Copyright 2021 Elsevier B.V. (d) SEM image of Co3O2/MoS2 sample. (e) NH3 gas-sensing properties of LbL self-assembled MoS2/Co3O4 nanocomposite sensors with different layers. (f) Schematic of the sensing mechanism of n-type MoS2/p-type Co3O4 hybrid in air and ammonia. Reprinted with permission from ref. 89. Copyright 2017, the American Chemical Society. | ||
Zhang et al.89 fabricated a Co3O4/MoS2 p–n heterojunction nanocomposite (Fig. 7d) sensor on interdigital electrodes via the layer-by-layer self-assembly route. Firstly, they discussed the effect of the number of layers on the composite assembled with one, three, five, and seven layers (S1, S3, S5, and S7) on the NH3 gas sensing performance at RT, respectively. The five-layered Co3O2/MoS2 sensor exhibited the best NH3 sensing response, as shown in Fig. 7e. The sensing mechanism could also be ascribed to the large change in the width of the depletion layer when exposed to an air and NH3 atmosphere, respectively, which was caused by the p-n heterojunction. NH3 reacted with the adsorbed O2− to produce NO gas and release electrons (Fig. 7f), which resulted in an increase in the resistance of the sensor.
(ii) Two-dimensional materials: transition metal dichalcogenides (TMDs) such as WS2,90,91 WSe2,92 and VS2;93 hexagonal boron nitride (h-BN);94 transition metal carbides, nitrides and/or carbonitrides such as Ti3C2Tx MXene;95 reduced graphene oxide (rGO);96–98 and graphene.99
The MoS2 composites with other TMDs can change the amplitude of variation in target gases to increase the response value. For example, Zheng et al.100 synthesized 2D van der Waals junctions by stacking n-type and p-type atomically thin MoS2 films via chemical vapor deposition (CVD) and soft-chemistry route, respectively. This idea was very interesting and meaningful. They employed the two different semiconductor characteristics of MoS2 to construct a p-n junction sensor. This sensor displayed outstanding sensitivity to NO2 at RT, which was much higher than that of pristine n-type and p-type MoS2. The enhanced sensing performance was ascribed to the built-in electric field generated at the p-n interface, which resulted in a huge change in resistance upon contact with NO2 molecules.
Ikram et al.91 reported the preparation of an MoS2@WS2 heterojunction sensor for the effective detection of NO2 at RT. When the sensor contacted with NO2 molecules, more electrons in the composite could be captured by NO2 compared to that of the single MoS2 or WS2 component due to the double-electron supply effect, which caused a higher change in resistance. In addition, Zhang et al.93 proposed that the combination of different TMDs with different geometrical and electronically energetic alignments exhibited unique features. Porous VS2 with intrinsic metallic and highly conductive characteristics was epitaxially grown on MoS2 nanosheets. They constructed an MoS2/VS2 quartz crystal microbalance sensor, which showed high sensitivity and selectivity to NH3. The metallic VS2 transferred electrons to MoS2, causing more electrons to accumulate on the side of MoS2, which contributed to the O2 acquiring a large number of electrons to form adsorbed oxygen and increased the initial resistance of the heterostructure in air. Therefore, it showed better sensitivity than the pure MoS2 and VS2.
Liu et al.94 designed an MoS2 gas sensor capped with a thin layer of h-BN. They found that the h-BN layer capped on the MoS2 layer improved the device stability, robustness and anti-fading capacity, while leaving the gas sensing capability unchanged due to the strong oxidation resistance of h-BN.
In the case of Ti3C2Tx MXene, it has high conductivity and active termination groups of Tx = –F, –OH, and –O. Yan et al.101 analyzed the NO2 sensing reinforcement of the MoS2/Ti3C2Tx MXene composite sensor, where they considered that the excellent electrical property of MXene will make up for the deficiency of MoS2 in this respect. A large number of carriers was transferred from MXene to MoS2 to create a similar Fermi energy level. The role of MXene was similar to the above-mentioned metallic VS2. In addition, the surface active groups would be more conducive to adsorbing the NO2 oxidizing gas.
Graphene and rGO with a large surface area and high charge carrier mobility, which have been considered as alternative sensing material candidates or gas sensing performance modification materials. Graphene can be used to detect individual molecules, causing the ultimate sensitivity.102 Sangeetha et al.103 reported that the enhanced gas sensing properties of an MoS2/graphene sensor towards NO2 including outstanding sensitivity and rapid response/recovery times (22/35 s) were attributed to the synergistic effect of the two materials. The MoS2 nanoparticles connected with graphene promoted the absorption of more gas molecules in the presence of evanescent wave light. Compared with graphene, rGO is rich in surface vacancies and oxygen functional groups.104,105 Chen et al.96 constructed 3D MoS2/rGO composites via a low temperature self-assembly method as a low-temperature NO2 gas sensor. They believed that the improvement in the gas sensing performance of MoS2/rGO compared to pure MoS2 and rGO in addition to the contribution of heterojunction between the rGO nanosheet and MoS2 nanoflowers, was attributed to the chemically active sites, large surface area, and van der Waals forces of rGO, which are also advantageous for gas adsorption.
(iii) Other functional materials: multi-walled carbon nanotubes (MWCNT),106 poly(3-hexylthiophene) (P3HT),107 C3N4,108 PbS,109 GaN,110 CdTe,111 ZnS,112 SnS2,113etc.
MoS2 composites with other functional materials also combine the merits of these materials such as high electrical conductivity, unique electronic transfer channels, similar sensitivity and selectivity, and high specific surface area to comprehensively improve the gas sensing performance or use the synergistic effect between these materials and MoS2 to achieve the goal of gas sensing. Chen et al.112 synthesized 2D/0D MoS2/ZnS heterostructures, which achieved the highly sensitive and recoverable detection of NO2 at RT. The recovery time of the composite sensor to 5 ppm NO2 was 4.6 min, which was much shorter than that of bare MoS2. The p–n heterojunction created between MoS2 and ZnS could act as a charge transfer bridge during NO2 adsorption and desorption. Besides, the enriched active sites of MoS2, the synergistic effects between the two components promoted an enhancement in sensing properties.
Jaiswal et al.111 employed CdTe quantum dots with high sensitivity to NO2 gas at RT to decorate MoS2 nanoworms. The composite sensor could efficiently achieve spill-over effects and change the electronic structure. Furthermore, the p–n heterojunction, synergistic effect, defective intersurfaces, and unique morphology with large specific surface area jointly facilitated the high and fast adsorption of NO2 molecules.
Besides the above-mentioned MoS2-based binary-structured composite gas sensors, MoS2-based ternary-structured composites have also been designed to achieve ideal gas sensing performances due to their unique/novel muti-level hierarchical heterostructures and multiple synergistic effects.
In our previous work,114 a novel two-dimensional Ti3C2Tx MXene@TiO2/MoS2 heterostructure was synthesized for the efficient and selective detection of NH3 at RT. Its morphology is shown in Fig. 8a, where MoS2 nanosheets grew on the surface of MXene and rectangular TiO2 particles were derived from MXene during the high-temperature hydrothermal process. It could be seen that the composite sensors (MTM) exhibited a higher NH3 gas sensing response value compared to that of pristine MXene and MoS2, as shown in Fig. 8b, and outstanding selectivity was exhibited by the MTM-2 composite sensor, as shown in Fig. 8c. Finally, we concluded that the enhancement in the gas sensing performance was ascribed to the unique morphology and p–n heterojunction of the ternary MXene@TiO2/MoS2 composite. Moreover, the insertion of TiO2 expanded the interlayer spacing of the Ti3C2Tx MXene and provided more reactive sites for NH3 adsorption.
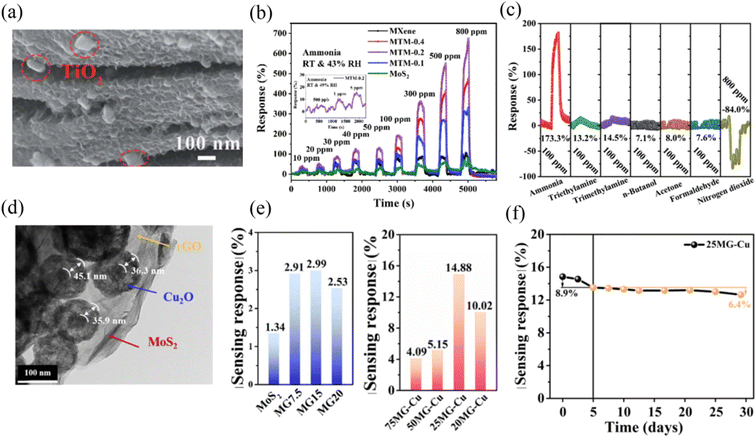 | ||
| Fig. 8 (a) SEM images of Ti3C2Tx MXene@TiO2/MoS2 (MTM-0.2). (b) Dynamic sensing characteristics of the prepared sensors to ammonia vapor at RT of 27 °C and RH of 43%. (c) Gas sensing responses of the Ti3C2Tx MXene@TiO2/MoS2 (MTM-0.2)-based gas sensor for a concentration of 100 ppm of various gases at RT of 25 °C and RH of 41%. Reprinted with permission from ref. 114. Copyright 2022, The Royal Society of Chemistry. (d) TEM image of MoS2–rGO–Cu2O (MG–Cu) ternary composite. (e) Sensing response of MG and MG–Cu with different amounts of graphene to 500 ppb NO2 at room temperature. (f) Stability of 25 MG–Cu sensor to 500 ppb NO2 at room temperature. Reprinted with permission from ref. 115. Copyright 2021, Elsevier B.V. | ||
Ding et al.115 constructed an MoS2–rGO–Cu2O (MG–Cu) ternary composite for the efficient detection of NO2 at RT. The hollow Cu2O nanospheres were anchored on the surface of MoS2–rGO, and the TEM image of this composite is shown in Fig. 8d. The sensor exhibited 11- and 5-times higher sensing response values to 500 ppb NO2 compared to pure MoS2 and binary MoS2–rGO, respectively (Fig. 8e). Besides, it also displayed excellent long-term stability (Fig. 8f). The superior sensing properties of this ternary composite sensor were mainly ascribed to the porous Cu2O, which acted as a gas molecule permeation diffusion channel, while MoS2–rGO acted as the bridge for electron transport. Meanwhile, the synergy of the shell-structure and heterojunction constructions among the three components contributed to the enhanced performance.
3. Categories of gas detected by MoS2-based sensors
According to the discussion in the previous section, it can be seen that MoS2-based gas sensors mainly show excellent recognition for NO2 and NH3 gases at RT. Alternatively, a few other gases can also be detected at RT such as nitric oxide (NO), hydrogen (H2), ethanol, methanol, formaldehyde (HCHO), carbon monoxide (CO), sulfur dioxide (SO2), benzene, acetone, and triethylamine (TEA), but the relevant reports are relatively scarce. In this case, it is worth exploring why MoS2-based gas sensors can identify these gases, especially for NO2 and NH3 detection. In this section, we will classify the different gases detected by MoS2-based gas sensors at RT and discuss their sensing mechanisms.3.1 NO2, NO, CO, and SO2
NO2 possesses high electrophilicity as an electron acceptor,112 which means that it can easily trap electrons from the conduction band of sensing materials without high energies, causing an increase in the hole concentration of MoS2 and a large change in the resistance of the sensor. Moreover, MoS2 has more adsorption sites for NO2 molecules. Regarding this, some theoretical calculation studies have verified the stronger affinity of MoS2 for NO2. Yue et al.116 employed first-principles calculations to investigate the adsorption energy and charge transfer of various gas molecules such as H2, O2, H2O, NH3, NO, NO2, and CO on monolayer MoS2. They concluded that all the calculated gas molecules were physically adsorbed on the surface of MoS2. However, regardless of the adsorption sites on MoS2 including H site (top of the MoS2 hexagon), TS (top of S atoms) site, and B site (top of Mo–S bonds), NO2 exhibited the highest adsorption energy and more charge transfer than other gases. Meanwhile, the H site was the most favorable adsorption site for H2O, NH3, and NO2 molecules, resulting in adsorption energies of −234, −250, and −276 meV, respectively. Jiang et al.117 also carried out the first-principles calculations to verify that perfect-layered MoS2 (without vacancy) exhibited higher adsorption energies for N-based gas molecules such as NO and NO2 compared with other gases. Meanwhile, this team also calculated the adsorption energies of NH3, NO, and NO2 adsorbed on defective MoS2 with Mo vacancy and S vacancy. They found that the adsorption energies of NO and NO2 on defective MoS2 with Mo vacancy increased remarkably compared with perfect MoS2. The electron localization function indicated that O–S and N–S covalent bonds were formed between NO and defective MoS2, NO2 and defective MoS2, respectively, demonstrating that there was chemical adsorption between them.Besides theoretical studies, experimental studies have also confirmed that there is strong interaction between NO2 molecules and MoS2. Ikram et al.108 reported the preparation of a highly sensitive RT NO2 sensor based on MoS2/C3N4 hybrid material. They confirmed the presence of the Mo–N bond based on the high-resolution N 1s spectra of the MoS2/C3N4 hybrid after absorbing NO2, illustrating that Mo was a strong adsorption site for N-based gases.
The gas sensing mechanism of MoS2-based gas sensors towards NO2 at RT is mainly based on the Langmuir–Hinshelwood (adsorption–desorption) model.118,119 Specifically, in an air atmosphere, the O2 molecules surround the surface of MoS2-based nanomaterials and extract free electrons from the conduction band of MoS2 to form adsorbed oxygen species such as O2−, O−, and O2−. The equations describing this reaction are as follows:
| O2(gas) → O2(ads) | (1) |
| O2(ads) + e− → O2ads− | (2) |
| O2ads− + e− → 2Oads− | (3) |
| Oads− + e− → Oads2− | (4) |
However, the oxygen ion O2− is predominant at low temperature (RT∼150 °C).120 The formation of O2− results in a high baseline resistance for n-type MoS2 or low baseline resistance for p-type MoS2. When introducing NO2 on the surface of MoS2, the oxidising gas further captures electrons from MoS2 to form NO2−, and more holes accumulate in the conduction band of MoS2, causing a higher resistance for n-type MoS2 or lower resistance for p-type MoS2. Meanwhile, the NO2 gas will also react with O2− to generate NO3−. When an MoS2-based sensor is put into an air atmosphere again, NO2− and NO3− would desorb and the released electrons come back to MoS2, and thus the resistance will decrease for n-type MoS2 or increase for p-type MoS2 again. The reaction is as follows:
| NO2(gas) + e− → NO2ads− | (5) |
| 2NO2(gas) + O2ads− + e− → 2NO3ads− | (6) |
| NO2ads− + 2NO3ads− → 3NO2(gas) + O2(gas) + e− | (7) |
In the case of NO gas, it is also an electron acceptor and easily oxidized into NO2 gas in air. Although some theoretical studies show that the adsorption interaction of MoS2 for NO is weaker than that of NO2, there is also chemical adsorption and significant charge transfer between it and MoS2, as confirmed by density of states analysis.46 To date, NO room-temperature gas sensors based on MoS2 have also been reported, and the sensing mechanism is according to the following equations:
| NO(gas) + e− → NOads− | (8) |
| NOads− + O2ads− + e− → NO(gas) + O2ads− | (9) |
When MoS2-based gas sensors are exposed to NO gas, the reduction reaction of NO occurred, as shown in eqn (8), which leads to an increase in resistance for n-type MoS2. Once the NO gas is withdrawn, the electrons return from NOads− to MoS2, resulting in a decrease in the resistance of MoS2.
In addition to NO2 and NO gases, CO and SO2 can also be detected by MoS2-based gas sensors at RT, but there are not many reports in this regard. Their sensing mechanisms are the same as NO2 and NO on the surface of MoS2, which is based on the interaction between absorbing oxygen and gas molecules to release electrons, leading to a change in the resistance of MoS2. Zhang et al.121 reported the preparation of a highly sensitive Ag-loaded ZnO/MoS2 ternary nanocomposite room-temperature CO sensor. They described the sensing reaction by eqn (10), as follows:
| CO + Oads− → CO2 + e− | (10) |
When the sensor was exposed to CO, its resistance decreased due to the release of electrons. The presence of noble metal Ag with catalytic activity accelerated the reaction.
Zhang et al.122 demonstrated that Ni-doped MoS2-based gas sensors exhibited an excellent SO2 sensing performance at RT. The Ni-doped MoS2 system had strong electrochemical activity due to the overlap of the conduction band and valence band, where the flow of electrons was easier from the valence band to conduction band. When the SO2 and Ni-doped MoS2 system interacted, the bond length values of the SO2 molecules and the electronic structure of the Ni-doped MoS2 system changed significantly, as verified by DFT calculation.
The sensing mechanism of MoS2-based gas sensors towards SO2 is based on eqn (11),123 as follows:
| SO2 + O2ads− + e− → SO3 + e− | (11) |
Table 1 summarizes the MoS2 nanomaterial-based gas sensors for the detection of NO2, NO, CO, and SO2 gases at RT in recent years. It can be seen that there are more reports focused on the detection of NO2 rather than NO, CO, and SO2, illustrating that MoS2 has a strong interaction for N-based gases. In addition, it is difficult for pristine MoS2 NO2 sensors to recovery completely, and thus several modification strategies have greatly improved their response and recovery rate to a certain extent.
| Materials | Gases | Concentration (ppm) | Response (Ra/Rg, Rg/Ra) or [(ΔR/R) × 100%] | Response/recovery time (s) | Ref./year |
|---|---|---|---|---|---|
| MoS2 nanowires | NO2 | 5 | ∼10.5% | Incomplete recovery | 30/2018 |
| Vertically aligned MoS2 on SiO2 nanorod | NO2 | 50 | 390% | Incomplete recovery | 124/2018 |
| MoS2 monolayer | NO2 | 0.02 | 20% | ∼/12 h | 125/2014 |
| MoS2 bilayer film | NO2 | 100 | 26.4% | 11.3/5.3 min | 126/2017 |
| MoS2 nanosheets | NO2 | 5 | 88% | 85/1420 | 127/2021 |
| MoS2 vertically aligned layers | NO2 | 100 | 10% | Not recovered | 128/2015 |
| Vertically aligned MoS2 flake | NO2 | 50 | ∼48.32% | 98/not recovered | 129/2018 |
| 1 | ∼3.4% | 68/not recovered | |||
| MoS2 nanoflowers | NO2 | 5 | ∼59% | 125/485 | 34/2020 |
| MoS2 flakes (UV light-activated) | NO2 | 100 | 27.92% | 29/350 | 130/2017 |
| MoS2 nanosheets (UV light-activated) | NO2 | 5 | ∼1.15 | Complete recovery | 73/2019 |
| Au/MoS2 (visible light-enhanced) | NO2 | 1 | 8.1 | ∼/27 | 131/2021 |
| La/MoS2 | NO2 | 10 | 45.34% | 89.1/95.4 | 132/2020 |
| Co/MoS2 | NO2 | 100 | 51.08% | 10/600 | 58/2022 |
| Ni/MoS2 | NO2 | 200 | 45.2% | 28/250 | 133/2022 |
| WO3/MoS2 | NO2 | 10 | 1.17 | Complete recovery | 78/2019 |
| SnO2/MoS2 | NO2 | 5 | 18.7 | 74/complete recovery | 77/2019 |
| ZnO/MoS2 | NO2 | 5 | 3050% | 40/300 | 118/2018 |
| In2O3/MoS2 | NO2 | 1 | 39.4 | 72/118 | 79/2022 |
| CuO/MoS2 (red light-activated) | NO2 | 10 | ∼8 | 33.9/55.6 | 134/2022 |
| MOF-In2O3/MoS2 | NO2 | 10 | 9.36 | 152/179 (20 ppm) | 135/2019 |
| MoS2@MoO2 | NO2 | 100 | ∼19 | 1.06/22.9 | 136/2019 |
| PbS/MoS2 | NO2 | 100 | 22.5% | 30/235 | 109/2019 |
| MoS2/ZnS | NO2 | 5 | 7.2 | ∼/4.6 min | 112/2021 |
| CdTe/MoS2 | NO2 | 10 | ∼40% | 16/114 | 111/2020 |
| SnS2/MoS2 | NO2 | 100 | ∼26 | 15.2/28.2 | 137/2020 |
| WS2/MoS2 | NO2 | 0.02 | 26.12 | 1.6/27.7 | 91/2019 |
| MoS2/Ti3C2Tx MXene | NO2 | 100 | 65.6% | About 750/not recovered | 101/2022 |
| Ti3C2/MoS2 | NO2 | 100 | 46.9 | Incomplete recovery | 95/2022 |
| CTAB-MoS2/rGO | NO2 | 8 | 37.64% | Incomplete recovery | 97/2022 |
| Mo2Ti3C2Tx/MoS2 | NO2 | 50 | 415.8% | 34.8/140.5 | 138/2022 |
| MoS2/C3N4 | NO2 | 30 | ∼49 | 2.3/30.5 | 108/2020 |
| MoS2–rGO–Cu2O | NO2 | 0.5 | 14.8% | Incomplete recovery | 115/2021 |
| rGO/MoS2 | NO2 | 40 | 25 | 160/3300 | 139/2018 |
| MoS2−xSex | NO | 3 | 48% | 410/340 | 140/2021 |
| 3D cone-shaped MoS2 (UV light-activated) | NO | 0.06 | 200% | 130/∼ | 68/2019 |
| 3D cone-shaped MoS2 (white light-activated) | NO | 0.06 | 75% | 150/∼ | 68/2019 |
| MoS2 monolayer (UV light-activated) | NO | 100 | 25.63% | About 250/600 | 141/2019 |
| CNFs/CoS2/MoS2 | NO | 50 | 19% | 60/260 min | 142/2020 |
| MoS2/Si nanowire array | NO | 50 | 3518% | 680/668 | 143/2017 |
| Pt–ZnO/MoS2 | CO | 5 | 5.08% | 45/60 | 121/2017 |
| Ni–MoS2 | SO2 | 5 | 7.4% | 50/56 | 122/2017 |
| SnO2/MoS2 (UV light-activated) | SO2 | 1 | 4.68 | 217/633 | 144/2021 |
3.2 NH3
In contrast to NO2, NH3 gas is a well-known electron donor owing to the fact that it contains a pair of lone electrons, which are not involved in bonding. Therefore, the electron concentration will increase for the n-type MoS2 sensing layer when exposed to NH3, resulting in a low resistance. The adsorption energies of CO, NO2, and NH3 on pristine MoS2 were analyzed by DFT calculation.145 The results showed that the most stable adsorption energies for CO, NO2, and NH3 were 0.008, −0.131, and −0.217 eV, respectively, implying that the high interaction between NH3 and MoS2. The low positive value of 0.008 indicated that CO on MoS2 was exothermic, unstable, and weakly adsorbed. Zhao et al.146 also employed DFT calculation to investigate the adsorption energies of O2, NO, NO2, and NH3 gas molecules on pristine MoS2. They found that the adsorption energies values of O2, NO, NO2, and NH3 gases on MoS2 were 0.013, 0.026, 0.037, and 0.041 eV, respectively. Although all these gases exhibited weak physical adsorption interaction on MoS2, obviously, NH3 had the highest.Sharma et al.147 and Singh et al.148 verified the high sensitivity of MoS2 to NH3 gas at RT via experimental measurements. Another important parameter involved is the response/recovery time, and these researchers observed that the pristine MoS2-based NH3 sensors showed a fast response/recovery time of 22/32 s towards 100 ppm NH3 and 75/130 s towards 50 ppm NH3, indicating that a fast and complete recovery can be achieved when NH3 gas was detected.
The gas sensing mechanism of the MoS2-based gas sensor towards NH3 at RT is also based on the adsorption–desorption theory. The following equations are used to describe the interaction between NH3 and the MoS2 sensing layer.
| 4NH3 + 5O2ads− → 4NO + 6H2O + 5e− | (12) |
When MoS2 sensors are exposed to the reducing NH3 gas, the NH3 molecules will react with O2ads− to form NO and H2O accompanied by the release of electrons; meanwhile, NH3 molecule itself contains lone pair electrons, which makes more electrons return to the conduction band of MoS2, causing a large change in resistance.
To further improve the gas sensing performance of MoS2-based gas sensors to NH3 at RT, several MoS2 nanocomposite NH3 gas sensors have been proposed in recent years. Table 2 presents a summary of MoS2 nanomaterial-based gas sensors for the detection of NH3 gas at RT.
| Materials | Concentration (ppm) | Response (Ra/Rg, Rg/Ra) or [(ΔR/R) × 100%] | Response/recovery time (s) | Ref./year |
|---|---|---|---|---|
| NiO/MoS2 | 10 | 63% | 160/117 (20 ppm) | 84/2019 |
| MoS2/CuO | 100 | ∼47% | 17/26 | 82/2018 |
| MoS2 nanostructure | 50 | 10% | 75/130 | 148 /2020 |
| MoS2 thin film | 100 | 2.2 | 22/32 | 137/2018 |
| MoS2/ZnO | 50 | 46.2% | 10/11 | 149/2017 |
| MoS2/Co3O4 | 5 | ∼65% | 98/100 | 89/2017 |
| MoS2/MWCNTs | 100 | ∼42% | 80/90 (50 ppm) | 106/2021 |
| SnO2/MoS2 | 50 | 91.26 | 23/1.6 | 33/2020 |
| MoS2/MWCNT | 150 | ∼26% | 65/70 | 150/2020 |
| Co3O4/MoS2 | 50 | 4.2 | 105/353 | 83/2022 |
| MoS2/SnO2 | 50 | 53% | Complete recovery | 151/2021 |
| PANI/MoS2/SnO2 | 100 | 10.9 | 21/130 | 152/2021 |
| MoS2 nanochains | 200 | 40% | 80/70 | 153/2022 |
| P3HT/MoS2 | 4 | 8% | 100/500 | 107/2016 |
| MoS2/MoO3 | 50 | ∼54% | 45/53 | 154/2021 |
| PANI/MWCNTs/MoS2 | 5 | 40.12% | 56/50 | 155/2018 |
| PANI/MoS2 | 5 | 10.94% | 98/57 | 155/2018 |
| Ti3C2Tx MXene@TiO2/MoS2 | 100 | 163.3% | 117/88 | 114/2022 |
3.3 H2
H2 as an abundant, green and renewable energy source has been used in various fields such as fuel cells, automobiles, and power plants.156 Moreover, it is also applied in the chemical industry, nuclear reactors, petroleum extraction, and semiconductor processing.157 However, H2 is also associated with many potential safety hazards duo to its explosive and flammable nature.158 Especially when its concentration is higher than 4% in the atmosphere, an explosion will occur. Therefore, the efficient detection of H2 is particularly important. Currently, although SMO H2 sensors exhibit high gas sensing response values, their high operating temperature also brings hidden dangers to a certain extent because the explosive limit of H2 is easily reached at a high temperature. Thus, the detection of H2 at low or room temperature will greatly improve the safety. To date, many low or room-temperature H2 sensors based on MoS2 have been reported. Theoretically, MoS2 is not sensitive to nonpolar molecules of H2.159 Bollinger et al.160 believed that the edges of MoS2 behave like metallic inter-connecting wires for the adsorption of H2 at RT. Dolui et al.161 and Gomez et al.162 also proposed that H2 behaves as an electron acceptor, which is favourable for absorption along the edges of MoS2 flakes. To date, the main approach employed to increase the sensitivity of MoS2 to H2 is its functionalization with noble metals including Au, Ag, Pt, and Pd. Zhang et al.163 investigated the effect of different noble metals (Cu, Au, Ag, Pt, and Pd) decorated on monolayer MoS2 on its hydrogen sensing performances by first principles. They concluded that the introduction of all the noble metals had a positive effect on H2 adsorption, which contributed to the hybridization of the noble metal d, S p, Mo d and H s orbitals. Especially Pt and Pd could enhance the adsorption interaction and increase the charge transfer between H2 molecules and monolayer MoS2. Some experimental studies are also consistent with the theoretical results. Baek et al.,164 Jaiswal et al.49 and Mai et al.165 used Pd to functionalize MoS2 and realize the detection of H2 at RT. The former research groups suggested that the mechanism of H2 sensing on Pd/MoS2 is ascribed to the electron transfer from MoS2 and Pd in air due to the lower work function of MoS2 than Pd. Alternatively, the formation of Pd-hydride (PdHx) on Pd surface when exposed to H2 resulted in electron transfer in the opposite direction from PdHx to MoS2, resulting in a change in sensor resistance. The latter research group concluded that the deposition of Pd nanoclusters on MoS2 caused p-type semiconductor behavior in the Pd/MoS2 composite. Meanwhile, the strong affinity of Pd provided more favorable adsorption sites for H2 molecules and initiated their chemical reactions.Besides the use of noble metals to trigger the sensitive response of MoS2 to H2 at RT, another strategy is to compound some potential materials that respond to H2, such as MoO3,166 graphene,167 and SnO2 (ref. 168) with MoS2 as suitable templates or supports. Table 3 displays the MoS2 nanomaterial-based gas sensors for H2 gas detection at RT. The sensing mechanism can be explained based on the interaction between H2 molecules and O2ads−. The whole reaction can be given by the following equations:
| H2(gas) → H2(ads) | (13) |
| H2(ads) → 2H(ads) | (14) |
| 2H(ads) + O2ads− → H2O + e− | (15) |
| Materials | Concentration (ppm) | Response (Ra/Rg, Rg/Ra) or [(ΔR/R) × 100%] | Response/recovery time (s) | Ref./year |
|---|---|---|---|---|
| MoS2/CsxWO3 | 500 | 50.6% | 60/120 | 169/2022 |
| UNCD/MoS2/ZnO | 100 | 50.3% | 8/12 | 170/2019 |
| Bulk-MoS2 | 100 | 14.2% | 28/42 | 171/2019 |
| Pd–MoS2/Si | 1% | ∼53.3% | ∼13.1/15.03 min | 164/2017 |
| RGO/MoS2 | 200 | ∼1.1% | ∼ | 172/2017 |
| Pd–SnO2/MoS2 | 5000 | 18% | 30/19 | 173/2017 |
| Pd/MoS2 (light-activated) | 140 | 17.45 ± 1.02% | 351/515 (120 ppm) | 165/2021 |
| Pd/MoS2 | 500 | 33.7% | 16/38 | 49/2020 |
| Vertically aligned MoS2/Si | 100 | 685.7% | 109/102 | 174/2016 |
| Edge-oriented MoS2 flake | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1% | 14.3/137 | 175/2017 |
| MoS2/GaN | 5% | ∼25% | ∼ | 110/2019 |
| Zn-doped MoO3/MoS2 | 500 | 28.91% | 24.6/18.5 | 176/2022 |
| MoS2/graphene | 1000 | 8.1% | 32/33 | 177/2022 |
| MoS2/ZnO | 500 | 51.5% | 14/19 | 178/2021 |
3.4 Other VOCs
The other VOC gases that can be detected by MoS2-based gas sensors at RT include ethanol, methanol, formaldehyde (HCHO), and benzene. VOCs gases, as reducing agents, present electron-donating characteristics similar to NH3. To date, there are a few reports on the detection of these gases at RT by MoS2-based sensing devices, which mainly consider the activity, electronic characteristics, molecular size of the gas itself, and the affinity of sensitive materials to gas molecules.Wu et al.179 prepared an Fe–TiO2/MoS2 composite film ethanol RT sensor. They proposed that Fe ion doping can optimize the electrical property of the sensing material. The sensor was sensitive to ethanol, which was attributed to the fact that the hydroxyl in the rotating ethanol molecule faced the Fe–TiO2 substrate and elongation of the C–O and H–O bonds on the adsorption surface of Fe–TiO2, which resulted in a shorter adsorption distance and higher adsorption strength. The density of states revealed that there was strong adsorption interaction between ethanol and Fe–TiO2 due to the large shift in the energy level of the Fe 3d and O 2p orbitals after adsorption. Finally, combined with the p–n heterojunctions generated at the interface of n-type Fe–TiO2 and p-type MoS2, the sensing response to ethanol was stronger.
Chakraborty et al.180 analyzed the highly selective methanol sensing mechanism of electrodeposited pristine MoS2 using first principle analysis. They found that although the electron-donating capability and charge transfer of 2-propanol and ethanol were higher than that of methanol, the smaller dimension of methanol, two favorable adsorption sites (Ori-A and Ori-B) of methanol on MoS2 surface, and approximately 20-times larger adsorption energy than that of ethanol and 2-propanol were the main reasons for the high sensitivity of MoS2 towards the detection of methanol.
Actually, pristine MoS2 does not have good sensitivity to formaldehyde, although it is a small molecule. Deng et al.181 employed DFT to investigate the adsorption of formaldehyde on Ni-, Pt-, Ti- and Pd-doped monolayer MoS2, respectively. They found that Ti–MoS2 was the dominant one in terms of adsorption energy. Moreover, the projected density of states (PDOS) and charge transfer indicate that the interaction between the formaldehyde molecule and Ti dopant was chemisorption via the Ti–O bond, illustrating that Ti–MoS2 may be suitable for the detection of formaldehyde. In addition, some compounds based on MoS2 can also be sensitive to formaldehyde, but the mechanism of their sensitivity has not been clearly defined.
Zhang et al.182 reported that a Pd–TiO2/MoS2 composite sensor showed selectivity and sensitivity towards benzene at RT. The sensing mechanism could be ascribed to the fact that Pd in TiO2/MoS2 has catalytic interaction toward benzene with a C–H bond and the synergistic effect of the ternary nanostructures, which can facilitate effective charge transport.
The following equations describe the reactions between the oxygen ion O2− created on the surface of MoS2-based sensing materials and ethanol, methanol, formaldehyde, and benzene molecules, respectively.183,184
| C2H5OH(ads) + 3O2ads− → 2CO2 + 3H2O + 6e− | (16) |
| 2CH3OH(ads) + 3O2ads− → 2CO2 + 4H2O + 3e− | (17) |
| HCHO(ads) + O2ads− → CO2 + H2O + e− | (18) |
| C6H6(ads) + 15O2ads− → 12CO2 + 6H2O + 15e− | (19) |
Table 4 presents a summary of the MoS2 nanomaterial-based gas sensors for the detection of ethanol, methanol, formaldehyde, and benzene gases at RT.
| Materials | Gases | Concentration (ppm) | Response (Ra/Rg, Rg/Ra) or [(ΔR/R) × 100%] | Response/recovery time (s) | Ref./year |
|---|---|---|---|---|---|
| CeO2/MoS2 | Ethanol | 50 | 7.78 | 7/5 | 75/2021 |
| α-Fe2O3/MoS2 | Ethanol | 100 | 88.9% | 6/5 (30 ppm) | 183/2018 |
| Fe–TiO2/MoS2 | Ethanol | 5 | 150% | 62/49 (1 ppm) | 179/2018 |
| Ag/MoS2 | Methanol | 100 | 21.6% | 240/1100 | 50/2021 |
| In2O3/MoS2 | Formaldehyde | 50 | 75.2% | 14/22 | 185/2018 |
| rGO/MoS2 | Formaldehyde | 10 | ∼2.7% | 73/∼ | 184/2017 |
| rGO/MoS2 | Formaldehyde | 10 | 4.8% | ∼ | 186/2017 |
| rGO/MoS2 (visible-light activated) | Formaldehyde | 10 | 64% | 79/17 | 72/2021 |
| Pd–TiO2/MoS2 | Benzene | 50 | 64% | 13/10 | 182/2018 |
4. Conclusions and outlook
Obviously, MoS2 exhibits great capabilities in the field of gas sensing, especially for room-temperature gas detection. In this review, firstly, the strategies for improving the gas sensing performance of MoS2 were introduced. Subsequently, the different types of gases that can be detected by MoS2-based gas sensors at room temperature were proposed and classified. Meanwhile, the sensing mechanisms of MoS2-based gas sensors towards different gases were also analyzed.Pristine MoS2 gas sensors exhibit low gas sensing response values and incomplete recovery problems at room temperature, which are unfavorable for gas detection. Consequently, various strategies have been developed for improving the gas sensing performance of MoS2 based gas sensors including morphology design, creating sulfur vacancies, decorating with noble metals, doping elements, light assistance, and construction of composites. Although the morphology design of MoS2 involves multiple patterns such as quantum dots, nanowires, nanosheets, and nanoflowers, each morphology exhibits unique physical and chemical properties and gas sensing performance characteristics, and the key issue of incomplete recovery has not been well solved. The vacancies in MoS2 belong to high energy binding sites, especially S vacancies as active sites to enhance the gas molecules adsorption. However, this high adsorption capacity will also result in a slow response and recovery rate. The decoration of the surface of MoS2 with noble metals can assist in overcoming the problem of selectivity to a certain extent due to the fact that noble metals possess affinity for some specific gas molecules. Element doping can address the challenge of sluggish sensing of MoS2 at room temperature owing to the adjustable active sites and electrical property. To date, doping strategies focus on theoretical calculations based on density functional theory, while experimental studies are rare. The light-assisted strategies include UV-light and visible-light activation. The power of these two lights is different, resulting in optoelectronic and photocatalytic gas sensing mechanisms, respectively, which accelerates the chemisorption reaction and causes a large change in the resistance of the sensor upon exposure to gases. Room-temperature MoS2 nanocomposite gas sensors are the most studied at present. The construction of composites of MoS2 (binary or ternary) can be considered one of the most effective modification methods to address the low gas sensing response and delayed recovery time of pristine MoS2 gas sensors. The heterojunctions and synergistic effects created by the different components are conducive to improve their comprehensive gas sensing performance. Especially the high electrical conductivity, unique electronic transfer channels, and similar sensitive selectivity are observed in nanocomposites.
According to the reports on the detection of several gases by MoS2-based gas sensors at room temperature such as NO2, NO, SO2, CO, NH3, H2, ethanol, methanol, formaldehyde, and benzene, MoS2 seems show strong adsorption interaction for N-based gases such as NO2 and NH3. NO2 as an electron acceptor exhibits high electrophilicity, which can easily trap electrons from the conduction band of MoS2. In contrast to NO2, NH3 acts as an electron donor with a pair of lone electrons that can give more electrons to MoS2, and thus the resistance of MoS2 sensors change greatly. Besides NO2 and NH3, H2 can also be detected by MoS2-based gas sensors at room temperature. Several researchers have proposed that H2 in nature favor absorption along the edges of MoS2, which behave like metallic inter-connecting wires to attract H2 at RT. The detection of other VOC gases such as ethanol, methanol, formaldehyde, and benzene by MoS2 nanocomposite gas sensors has also been reported, which is mainly related to the strong force on these gases at one of the special adsorption sites in the composites. To date, the sensing mechanisms of MoS2-based gas sensors for the above-mentioned gases are mainly based on the adsorption/desorption theories. The target gases react with the adsorbed oxygen ions O2ads− and release electrons to the conduction band of MoS2, resulting in a change in resistance and sensitive response.
Although the above-mentioned strategies have made great progress to improve the gas sensing properties of MoS2-based gas sensors at room temperature, there are still some interesting research directions and challenges that deserve to be explored.
Firstly, besides the strong interaction between MoS2 and gas molecules, the deeper reasons for the slow or incomplete recovery of MoS2 sensors to gases need to be investigated. The transduction mechanism, intrinsic characteristics, and desorption reaction seem to affect the recovery rate. In addition, NH3 is more easily desorbed from the surface of MoS2 than NO2 in the case of the same N-based gases, which is worth further discussion. Secondly, the gas sensing response, selectivity, and long-term stability of MoS2-based gas sensors are still unsatisfactory. Therefore, novel MoS2-based room temperature gas sensors should receive more attention. Some strategies such as adjusting the active sites of MoS2 from basal plane to edges, constructing advanced structured MoS2 nanocomposites, and optimizing the fabrication process of devices may be interesting points. Finally, the gas sensing mechanisms of MoS2 materials not only depend on the theories of adsorption–desorption and charge carrier transport, where the whole reactive process is complicated, and thus more crucial interactions between MoS2 and gas molecules need to be further studied.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 41876055 and 61761047), the Yunnan Provincial Department of Science and Technology through the Key Project for the Science and Technology (Grant No. 2017FA025), Program for Innovative Research Team (in Science and Technology) in University of Yunnan Province, and Project of the Department of Education of Yunnan Province (2022Y003).References
- Y. Zhou, X. Li, Y. J. Wang, H. L. Tai and Y. C. Guo, Anal. Chem., 2019, 91, 3311–3318 CrossRef CAS PubMed.
- T. Jarvinen, G. S. Lorite, J. Perantie, G. Toth, S. Saarakkala, V. K. Virtanen and K. Kordas, Nanotechnology, 2019, 30, 405501 CrossRef CAS.
- Y. J. Wang, Y. Zhou, Y. H. Wang, R. J. Zhang, J. Li, X. Li and Z. G. Zang, Sens. Actuators, B, 2021, 349, 130770 CrossRef CAS.
- M. Sajjad and P. Feng, Mater. Res. Bull., 2014, 49, 35–38 CrossRef CAS.
- Y. Zhou, Y. H. Wang, Y. J. Wang, H. C. Yu, R. J. Zhang, J. Li, Z. G. Zang and X. Li, ACS Appl. Mater. Interfaces, 2021, 13, 56485–56497 CrossRef CAS.
- M. Barzegar and B. Tudu, Surf. Innovations, 2018, 6, 205–230 CrossRef.
- Q. Li, J. P. Meng and Z. Li, J. Mater. Chem. A, 2022, 10, 8107–8128 RSC.
- X. H. Liu, T. T. Ma, N. Pinna and J. Zhang, Adv. Funct. Mater., 2017, 27, 1702168 CrossRef.
- T. H. Kim, Y. H. Kim, S. Y. Park and H. W. Jang, Chemosensors, 2017, 5, 15 CrossRef.
- G. Neri, Chemosensors, 2017, 5, 21 CrossRef.
- R. Kumar, N. Goel, M. Hojamberdiev and M. Kumar, Sens. Actuators, A, 2020, 303, 111875 CrossRef CAS.
- K. F. Mak, C. Lee, J. Hone, J. Shan and T. F. Heinz, Phys. Rev. Lett., 2010, 105, 136805 CrossRef PubMed.
- G. C. Lu, X. H. Liu, W. Zheng, J. Y. Xie, Z. S. Li, C. M. Lou, G. L. Lei and J. Zhang, Rare Met., 2022, 41(5), 1520–1528 CrossRef CAS.
- Y. S. Xu, J. Y. Xie, Y. F. Zhang, F. H. Tian, C. Yang, W. Zheng, X. H. Liu, J. Zhang and N. Pinna, J. Hazard. Mater., 2021, 411, 125120 CrossRef CAS.
- A. V. Agrawal, R. Kumar, S. Venkatesan, A. Zakhidov, G. Yang, J. M. Bao, M. Kumar and M. Kumar, ACS Sens., 2018, 3, 998–1004 CrossRef CAS PubMed.
- C. Yang, J. Y. Xie, C. M. Lou, W. Zheng, X. H. Liu and J. Zhang, Sens. Actuators, B, 2021, 333, 129571 CrossRef CAS.
- M. Kumar, A. V. Agrawal, M. Moradi and R. Yousefi, Nanomater. Air Rem., 2020, 107–130, DOI:10.1016/B978-0-12-818821-7.00006-3.
- A. V. Agrawal, N. Kumar and M. Kumar, Nano-Micro Lett., 2021, 13, 305–362 Search PubMed.
- Q. Y. He, Z. Y. Zeng, Z. Y. Yin, H. Li, S. X. Wu, X. Huang and H. Zhang, Small, 2012, 8, 2994–2999 CrossRef CAS.
- S. M. Cui, Z. H. Wen, X. K. Huang, J. B. Chang and J. H. Chen, Small, 2015, 11, 2305–2313 CrossRef CAS PubMed.
- B. Radisavljevic, A. Radenovic, J. Brivio, V. Giacometti and A. Kis, Nat. Nanotechnol., 2011, 6, 147–150 CrossRef CAS.
- K. Y. Ko, J. G. Song, Y. Kim, T. Choi, S. Shin and C. W. Lee, ACS Nano, 2016, 10, 9287–9296 CrossRef CAS PubMed.
- M. Kan, J. Y. Wang, X. W. Li, S. H. Zhang, Y. W. Li, Y. Kawazoe, Q. Sun and P. Jena, J. Phys. Chem. C, 2014, 118, 1515–5122 CrossRef CAS.
- D. Voiry, A. Mohiteb and M. Chhowalla, Chem. Soc. Rev., 2015, 44, 2702–2712 RSC.
- R. Kumar, W. Zheng, X. H. Liu, J. Zhang and M. Kumar, Adv. Mater. Technol., 2020, 5, 1901062 CrossRef CAS.
- W. Zheng, X. H. Liu, J. Y. Xie, G. C. Lu and J. Zhang, Coord. Chem. Rev., 2021, 447, 214151 CrossRef CAS.
- A. Hermawan, N. L. W. Septiani, A. Taufik, B. Yuliarto, Suyatman and S. Yin, Nano-Micro Lett., 2021, 13, 207 CrossRef CAS PubMed.
- Z. X. Gan, L. Z. Liu, H. Y. Wu, Y. L. Hao, Y. Shan, X. L. Wu and P. K. Chu, Appl. Phys. Lett., 2015, 106, 233113 CrossRef.
- Y. Niu, W. C. Jiao, R. G. Wang, G. M. Ding and Y. F. Huang, J. Mater. Chem. A, 2016, 4, 8198–8203 RSC.
- R. Kumar, N. Goel and M. Kumar, Appl. Phys. Lett., 2018, 112, 053502 CrossRef.
- D. J. Late, Y. K. Huang, B. Liu, J. Acharya, S. N. Shirodkar, J. J. Luo, A. M. Yan, D. Charles, U. V. Waghmare and V. P. Dravid, ACS Nano, 2013, 7, 4879–4891 CrossRef CAS PubMed.
- W. L. Li, Y. Zhang, X. Long, J. X. Cao, X. Xin, X. X. Guan, J. F. Peng and X. J. Zheng, Sensors, 2019, 19, 2123 CrossRef CAS.
- W. X. Wang, Y. H. Zhen, J. Y. Zhang, Y. D. Li, H. Zhong, Z. L. Jia, Y. Xiong, Q. Z. Xue, Y. G. Yan and N. S. Alharbi, Sens. Actuators, B, 2020, 321, 128471 CrossRef CAS.
- N. T. Thang, L. T. Hong, T. H. Nguyen, C. M. Hung, N. V. Duy, N. V. Hieu and N. D. Hoa, RSC Adv., 2020, 10, 12759–12771 RSC.
- H. Long, A. Harley-Trochimczyk, T. Pham, Z. Tang, T. Shi, A. Zettl, C. Carraro, M. A. Worsley and R. Maboudian, Adv. Funct. Mater., 2016, 26, 5158–5165 CrossRef CAS.
- J. H. Hong, Z. X. Hu, M. Probert, K. Li, D. H. Lv, X. N. Yang, L. Gu, N. N. Mao, Q. L. Feng and L. M. Xie, Nat. Commun., 2015, 6, 6293 CrossRef CAS.
- H. Y. Nan, Z. L. Wang, W. H. Wang, Z. Liang, Y. Lu, Q. Chen, D. W. He, P. H. Tan, F. Miao and X. R. Wang, ACS Nano, 2014, 8, 5738–5745 CrossRef CAS PubMed.
- Z. Y. Qin, K. Xu, H. C. Yue, H. Wang, J. Zhang, C. Ouyang, C. S. Xie and D. W. Zeng, Sens. Actuators, B, 2018, 262, 771–779 CrossRef CAS.
- D. Burman, R. Ghosh, S. Santra, S. K. Ray and P. K. Guha, Nanotechnology, 2017, 28, 435502 CrossRef PubMed.
- R. Kumar, N. Goel, A. V. Agrawal, R. Raliya, S. Rajamani, G. Gupta, P. Biswas, M. Kumar and M. Kumar, IEEE Sens. J., 2020, 19, 10214–10220 Search PubMed.
- H. X. Li, M. Huang and G. Y. Cao, Phys. Chem. Chem. Phys., 2016, 21, 15110–15117 RSC.
- X. W. Chen, J. Shi, T. Wang, S. Y. Zheng, W. Lv, X. Y. Chen, J. H. Yang, M. Zeng, N. T. Hu and Y. J. Su, ACS Sens., 2022, 7, 816–826 CrossRef CAS PubMed.
- Y. Xia, C. Y. Hu, S. H. Guo, L. B. Zhang, M. J. Wang, J. H. Peng, L. Xu and J. Wang, ACS Appl. Nano Mater., 2020, 3, 665–673 CrossRef CAS.
- H. P. Komsa, J. Kotakoski, S. Kurasch, O. Lehtinen, U. Kaiser and A. V. Krasheninnikov, Phys. Rev. Lett., 2012, 109, 035503 CrossRef PubMed.
- L. C. Zhang, Y. Y. Liang, L. M. Yu, H. J. Wang and M. L. Yin, Sens. Actuators, B, 2022, 359, 131539 CrossRef CAS.
- F. F. Li and C. M. Shi, Appl. Surf. Sci., 2018, 434, 294–306 CrossRef CAS.
- X. Tian, X. X. Cui, T. R. Lai, J. R. Z. C. Yang, M. J. Xiao, B. S. Wang, X. C. Xiao and Y. D. Wang, Nano Mater. Sci., 2021, 3, 390–403 CrossRef CAS.
- N. Sakhuja, A. Gupta, R. Jha and N. Bhat, J. Alloys Compd., 2022, 899, 163166 CrossRef CAS.
- J. Jaiswal, P. Tiwari, P. Singh and R. Chandra, Sens. Actuators, B, 2020, 325, 128800 CrossRef CAS.
- P. Halvaee, S. Dehghani and M. Mohammadzadeh, IEEE Sens. J., 2021, 21, 4233–4240 CAS.
- J. Park, J. H. Mun, J. S. Shin and S. W. Kang, R. Soc. Open Sci., 2019, 5, 181462 CrossRef.
- H. Peng, J. Lu, C. X. Wu, Z. X. Yang, H. Chen, W. J. Song, P. Q. Li and H. Z. Yin, Appl. Surf. Sci., 2015, 353, 1003–1012 CrossRef CAS.
- C. Liu, Y. Zhang, J. Y. Hu, J. X. Ren, Y. Q. Song, J. F. Peng, M. Ma and J. J. Tan, Mater. Lett., 2020, 273, 127961 CrossRef CAS.
- S. G. Ramaraj, S. Nundy, P. Zhao, D. Elamaran, A. A. Tahir, Y. Hayakawa, M. Muruganathan, H. Mizuta and S. W. Kim, ACS Omega, 2022, 7, 10492–10501 CrossRef CAS.
- Z. Xiao, W. Wu, X. W. Wu and Y. F. Zhang, Chem. Phys. Lett., 2020, 755, 137768 CrossRef CAS.
- W. J. Hou, H. W. Mi, R. C. Peng, S. D. Peng, W. Zeng and Q. Zhou, Nanomaterials, 2021, 11, 314 CrossRef CAS PubMed.
- R. Y. Zhang, D. Fu, J. M. Ni, C. B. Sun and S. X. Song, Chem. Phys. Lett., 2019, 715, 273–277 CrossRef CAS.
- P. Bharathi, S. Harish, M. Shimomura, S. Ponnusamy, M. K. Mohan, J. Archana and M. Navaneethan, Sens. Actuators, B, 2022, 360, 131600 CrossRef CAS.
- R. Z. Wu, J. Y. Hao, S. L. Zheng, Q. Sun, T. T. Wang, D. Zhang, H. Zhang, Y. Wang and X. Zhou, Appl. Surf. Sci., 2021, 571, 151162 CrossRef.
- T. E. Gber, H. Louis, A. E. Owen, B. E. Etinwa, I. Benjamin, F. C. Asogwa, M. M. Orosun and E. A. Eno, RSC Adv., 2022, 12, 25992–26010 RSC.
- A. Kazemi, M. Rodner, M. R. Fadavieslam, P. D. Kaushik, I. G. Ivanov, J. Eriksson, M. Syvajarvi, R. Yakimova and G. R. Yazdi, Surf. Interfaces, 2021, 25, 101200 CrossRef CAS.
- J. Zhu, H. Zhang, Y. W. Tong, L. Zhao, Y. F. Zhang, Y. Z. Qiu and X. N. Lin, Appl. Surf. Sci., 2017, 419, 522–530 CrossRef CAS.
- E. Salih and A. I. Ayesh, Phys. E, 2021, 131, 114736 CrossRef CAS.
- K. N. Ding, Y. H. Lin and M. Y. Huang, Vacuum, 2016, 130, 146–153 CrossRef CAS.
- M. J. Szary, Appl. Surf. Sci., 2021, 547, 149026 CrossRef CAS.
- Y. Zhou, C. Zou, X. G. Lin and Y. C. Guo, Appl. Phys. Lett., 2018, 113, 082103 CrossRef.
- J. Guo, R. M. Wen, J. Y. Zhai and Z. L. Wang, Sci. Bull., 2019, 64, 128–135 CrossRef CAS.
- Y. Z. Chen, S. W. Wang, C. C. Yang, C. H. Chung, Y. C. Wang, S. W. H. Chen, C. W. Chen, T. Y. Su, H. N. Lin, H. C. Kuo and Y. L. Chueh, Nanoscale, 2019, 11, 10410–10419 RSC.
- T. Pham, G. Li, E. Bekyarova, M. E. Itkis and A. Mulchandani, ACS Nano, 2019, 13, 3196–3205 CrossRef CAS PubMed.
- P. C. Chen, S. Sukcharoenchoke, K. Ryu, L. G. Arco, A. Badmaev, C. Wang and C. Zhou, Adv. Mater., 2010, 22, 1900 CrossRef CAS PubMed.
- D. L. Wang, A. T. Chen and A. K. Y. Jen, Phys. Chem. Chem. Phys., 2013, 15, 5017–5021 RSC.
- J. Wang, H. Y. Deng, X. Li, C. Yang and Y. Xia, Sens. Actuators, B, 2019, 304, 127317 CrossRef.
- Y. Kang, S. Pyo, E. Jo and J. Kim, Nanotechnology, 2019, 30, 355504 CrossRef CAS.
- M. Donarelli, S. Prezioso, F. Perrozzi, F. Bisti, M. Nardone, L. Giancaterini, C. Cantalini and L. Ottaviano, Sens. Actuators, B, 2015, 207, 602–613 CrossRef CAS.
- J. H. Zhang, T. T. Li, J. Y. Guo, Y. Q. Hu and D. Z. Zhang, Appl. Surf. Sci., 2021, 568, 150942 CrossRef CAS.
- X. Chang, X. F. Li, X. R. Qiao, K. Li, Y. X. Li, T. C. Guo, L. Zhu and Q. Z. Xue, Sens. Actuators, B, 2019, 304, 127430 CrossRef.
- Y. T. Han, Y. J. Ma, Y. Liu, S. S. Xu, X. W. Chen, M. Zeng, N. T. Hu, Y. J. Su, Z. H. Zhou and Z. Yang, Appl. Surf. Sci., 2020, 493, 613–619 CrossRef.
- D. H. Baek, G. Choi, Y. Kwak, B. H. Cho and J. Kim, Proceedings of the IEEE International Conference on Micro Electro Mechanical Systems (MEMS), 2019 IEEE 32nd International Conference on Micro Electro Mechanical Systems, MEMS 2019, 2019, pp. 468–471 Search PubMed.
- Y. N. Liu, S. Li, S. Xiao and K. Du, Colloids Surf., A, 2022, 648, 129435 CrossRef CAS.
- P. X. Zhao, Y. Tang, J. Mao, Y. X. Chen, H. Song, J. W. Wang, Y. Song, Y. Q. Liang and X. M. Zhang, J. Alloys Compd., 2016, 674, 252–258 CrossRef CAS.
- R. Kumar, N. Goel, M. Mishra, G. Gupta, M. Fanetti, M. Valant and M. Kumar, Adv. Mater. Interfaces, 2018, 5, 1800071 CrossRef.
- S. Sharma, A. Kumar, N. Singh and D. Kaur, Sens. Actuators, B, 2018, 275, 499–507 CrossRef CAS.
- Y. Xiong, W. D. Liu, K. C. Wu, T. Liu, Y. M. Chen, X. Z. Wang and J. Tian, J. Alloys Compd., 2022, 927, 166962 CrossRef CAS.
- D. Z. Zhang, Y. B. Jin and Y. H. Cao, J. Mater. Sci.: Mater. Electron., 2019, 30, 573–581 CrossRef CAS.
- Y. Q. Ding, X. Z. Guo, B. S. Du, X. F. Hu, X. Yang, Y. He, Y. Zhou and Z. G. Zang, J. Mater. Chem. C, 2021, 9, 4838–4846 RSC.
- H. Yan, M. J. Zhong, Z. Lv and P. B. Wan, Small, 2017, 13, 1701697 CrossRef.
- S. Ahmad, I. Khan, A. Husain, A. Khan and A. M. Asiri, Polymer, 2021, 12, 3047 Search PubMed.
- X. Bai, H. Lv, Z. Liu, J. K. Chen, J. Wang, B. H. Sun, Y. Zhang, R. H. Wang and K. Y. Shi, J. Hazard. Mater., 2021, 416, 125830 CrossRef CAS.
- D. Z. Zhang, C. X. Jiang, P. Li and Y. E. Sun, ACS Appl. Mater. Interfaces, 2017, 9, 6462–6471 CrossRef CAS.
- J. Sun, N. Lin, H. Ren, C. Tang, L. T. Yang and X. Zhao, RSC Adv., 2016, 6, 17494–17503 RSC.
- M. Ikram, L. J. Liu, Y. Liu, L. F. Ma, H. Lv, M. Ullah, L. He, H. Y. Wu, R. H. Wang and K. Y. Shi, J. Mater. Chem. A, 2019, 7, 14602–14612 RSC.
- S. Dhara, H. Jawa, S. Ghosh, A. Varghese, D. Karmakar and S. Lodha, ACS Appl. Mater. Interfaces, 2021, 13, 30785–30796 CrossRef CAS.
- S. H. Zhang, J. Y. Wang, N. L. Torad, W. Xia, M. A. Aslam, Y. V. Kaneti, Z. F. Hou, Z. J. Ding, B. Da and A. Fatehmulla, Small, 2019, 16, 1901718 CrossRef.
- G. Liu, S. L. Rumyantsev, C. Jiang, M. S. Shur and A. A. Balandin, IEEE Electron Device Lett., 2015, 36, 1202–1204 CAS.
- V. Le, Y. Vasseghian, V. Doan, T. T. T. Nguyen, T. T. T. Vo, K. B. Vu, Q. H. Vu, T. D. Lam and V. A. Tran, Chemosphere, 2022, 291, 133025 CrossRef CAS PubMed.
- R. Zen, Y. B. Shi, T. M. Song, T. Wang, B. L. Tang, H. D. Niu and X. Y. Yu, Chemosphere, 2022, 9, 345 Search PubMed.
- W. B. Li, H. Li, R. Qian, S. J. Zhuo, P. F. Ju and Q. Chen, Nanomaterials, 2022, 12, 1300 CrossRef CAS.
- C. Yang, Y. Y. Wang, Z. K. Wu, Z. B. Zhang, N. T. Hu and C. S. Peng, Nanomaterials, 2022, 12, 901 CrossRef CAS PubMed.
- I. Jahangir, M. A. Uddin, A. K. Singh, M. V. S. Chandrashekhar and G. Koley, IEEE Sens. J., 2021, 21, 26549–26555 CAS.
- W. Zheng, Y. S. Xu, L. L. Zheng, C. Yang, N. Pinna, X. H. Liu and J. Zhang, Adv. Funct. Mater., 2020, 30, 2000435 CrossRef CAS.
- H. Yan, L. H. Chu, Z. Li, C. X. Sun, Y. X. Shi and J. Ma, Sensors and Actuators Reports, 2022, 4, 100103 CrossRef.
- F. Schedin, A. K. Geim, S. V. Morozov, E. W. Hill, P. Blake, M. I. Katsnelson and K. S. Novoselov, Nat. Mater., 2007, 6, 652–655 CrossRef CAS PubMed.
- M. Sangeetha and D. Madhan, Opt. Laser Technol., 2020, 127, 106193 CrossRef CAS.
- J. D. Fowler, M. J. Allen, V. C. Tung, Y. Yang, R. B. Kaner and B. H. Weiller, ACS Nano, 2009, 3, 301–306 CrossRef CAS PubMed.
- F. L. Meng, Z. Guo and X. J. Huang, TrAC, Trends Anal. Chem., 2015, 68, 37–47 CrossRef CAS.
- S. Singh and S. Sharma, Mater. Today, 2021, 45, 4910–4913 CAS.
- T. Xie, G. Z. Xie, Y. J. Su, H. F. Du, Z. B. Ye and Y. D. Jiang, Nanotechnology, 2016, 27, 065502 CrossRef PubMed.
- M. Ikram, H. Lv, Z. Liu, M. Khan, L. J. Liu, F. Raziq, X. Bai, M. Ullah, Y. Zhang and K. Y. Shi, Chem. Mater., 2020, 32, 7215–7225 CrossRef CAS.
- X. Xin, Y. Zhang, X. Guan, J. Cao, W. Li, X. Long and X. Tan, ACS Appl. Mater. Interfaces, 2019, 11, 9438–9447 CrossRef CAS.
- N. Goel, R. Kumar, S. K. Jain, S. Rajamani, B. Roul, G. Gupta, M. Kumar and S. B. Krupanidhi, Nanotechnology, 2019, 30, 314001 CrossRef CAS.
- J. Jaiswal, A. Sanger, P. Tiwari and R. Chandra, Sens. Actuators, B, 2020, 305, 127437 CrossRef CAS.
- C. Liu, X. W. Chen, H. Y. Luo, B. L. Li, J. Shi, C. Fan, J. H. Yang, M. Zeng, Z. H. Zhou and N. T. Hu, Sens. Actuators, B, 2021, 347, 130608 CrossRef CAS.
- J. B. Liu, J. Y. Hu, C. Liu, Y. M. Tan, X. Peng and Y. Zhang, Rare Met., 2020, 40, 1536–1544 CrossRef.
- X. Tian, L. J. X. X. Cui, R. J. Zhao, T. Chen, X. C. Xiao and Y. D. Wang, J. Mater. Chem. A, 2022, 10, 5505–5519 RSC.
- Y. Q. Ding, X. Z. Guo, D. L. Kuang, X. F. Hu, Y. Zhou, Y. He and Z. G. Zang, J. Hazard. Mater., 2021, 416, 126218 CrossRef CAS PubMed.
- Q. Yue, Z. Z. Shao, S. L. Chang and J. B. Li, Nanoscale Res. Lett., 2013, 8, 425 CrossRef.
- W. F. Jiang, K. F. Chen, J. W. Wang, D. Geng, N. A. D. Lu and L. Li, Mater. Res. Express, 2021, 8, 055010 CrossRef CAS.
- Y. T. Han, D. Huang, Y. G. Ma, G. L. He, J. Hu, J. Zhang, N. T. Hu, Y. J. Su, Z. H. Zhou, Y. F. Zhang and Z. Yang, ACS Appl. Mater. Interfaces, 2018, 10, 22640–22649 CrossRef CAS PubMed.
- A. P. Lee and B. J. Reedy, Sens. Actuators, B, 1999, 60, 35–42 CrossRef CAS.
- N. Barsan and U. Weimar, J. Electroceram., 2001, 7, 143–167 CrossRef CAS.
- D. Z. Zhang, Y. E. Sun, C. X. Jiang, Y. Yao, D. Y. Wang and Y. Zhang, Sens. Actuators, B, 2017, 253, 1120–1128 CrossRef CAS.
- D. Z. Zhang, J. F. Wu, P. Li and Y. H. Cao, J. Mater. Chem. A, 2017, 5, 20666–20677 RSC.
- Y. Fu, J. Z. Li and H. Y. Xu, Mater. Sci. Semicond. Process., 2020, 114, 105073 CrossRef CAS.
- Y. S. Shim, K. C. Kwon, J. M. Suh, K. S. Choi, Y. G. Song, W. Sohn, S. Choi, K. Hong, J. M. Jeon and S. P. Hong, ACS Appl. Mater. Interfaces, 2018, 10, 31594–31602 CrossRef CAS.
- B. L. Liu, L. Chen, G. Liu, A. N. Abbas, M. Fathi and C. W. Zhou, ACS Nano, 2014, 8, 5304–5314 CrossRef CAS.
- T. T. Xu, Y. Y. Pei, Y. Y. Liu, D. Wu, Z. F. Shi, J. M. Xu, Y. T. Tian and X. J. Li, J. Alloys Compd., 2-17, 725, 253–259 Search PubMed.
- H. H. Hau, T. T. H. Duong, N. K. Man, T. T. V. Nga, C. T. Xuan, D. T. T. Le, N. V. Toan, C. M. Hung, N. V. Duy, N. V. Hieu and N. D. Hoa, Sens. Actuators, A, 2021, 332, 113137 CrossRef CAS.
- S. Y. Cho, S. J. Kim, Y. Lee, J.-S. Kim, W. B. Jung, H. W. Yoo, J. Kim and H. Jung, ACS Nano, 2015, 9, 9314 CrossRef.
- R. Kumar, P. K. Kulriya and M. Kumar, Nanotechnology, 2018, 29, 464001 CrossRef.
- R. Kumar, N. Goel and M. Kumar, ACS Sens., 2017, 2, 1744–1752 CrossRef CAS.
- P. Chen, J. Y. Hu and Y. Zhang, ACS Appl. Mater. Interfaces, 2021, 4, 5981–5991 CrossRef CAS.
- K. Rathi, A. N. Kumar and K. Pal, Nanotechnology, 2020, 31, 395504 CrossRef CAS PubMed.
- P. Bharathi, S. Harish, G. Mathankumar, M. K. Mohan, J. Archana, S. Kamalakannan, M. Prakash, M. Shimomura and M. Navaneethan, Appl. Surf. Sci., 2022, 600, 154086 CrossRef CAS.
- H. E. Bai, H. Guo, C. Feng, J. Wang, B. Liu, Z. L. Xie, F. Q. Guo, D. J. Chen, R. Zhang and Y. D. Zheng, Sens. Actuators, B, 2022, 368, 132131 CrossRef CAS.
- Z. M. Yang, D. Z. Zhang and H. N. Chen, Sens. Actuators, B, 2019, 300, 127037 CrossRef CAS.
- M. Ikram, L. Liu, Y. Liu, M. Ullah, L. Ma and S. U. H. Bakhtiar, Nanoscale, 2019, 11, 8554–8564 RSC.
- L. J. Liu, M. Ikram, L. F. Ma, X. Y. Zhang, H. Lv, M. Ullah, M. Khan, H. T. Yu and K. Y. Shi, J. Hazard. Mater., 2020, 393, 122325 CrossRef CAS PubMed.
- Q. N. Zhao, W. Z. Zhou, M. X. Zhang, Y. Wang, Z. H. Duan, C. L. Tan, B. H. Liu, F. P. Ouyang, Z. Yuan and H. L. Tai, Adv. Funct. Mater., 2022, 32, 2203528 CrossRef CAS.
- N. Kanaujiya, A. K. Golimar, P. C. Pandey and J. G. D. Varma, AIP Conf. Proc., 2018, 1953, 030142, DOI:10.1063/1.5032477.
- A. Taufik, Y. Asakura, T. Hasegawa and S. Yin, ACS Appl. Nano Mater., 2021, 4, 6861–6871 CrossRef CAS.
- S. Ramu, T. Chandrakalavathi, G. Murali, K. S. Kumar, A. Sudharani, M. Ramanadha, K. R. Peta, R. Jeyalakshmi and R. P. Vijayalakshmi, Mater. Res. Express, 2019, 6, 085075 CrossRef CAS.
- S. Y. Hou, R. Pang, S. L. Chang, L. Ye, J. Xu, X. C. Wang, Y. J. Zhang, Y. Y. Shang and A. Y. Cao, ACS Appl. Mater. Interfaces, 2020, 12, 29778–29786 CAS.
- D. Wu, Z. H. Lou, Y. G. Wang, T. T. Xu, Z. F. Shi, J. M. Xu, Y. T. Tian and X. J. Li, Nanotechnology, 2017, 28, 435503 CrossRef.
- X. X. He, Z. H. Ying, F. Wen, L. L. Li, X. L. Zheng, P. Zheng and G. F. Wang, Mater. Sci. Semicond. Process., 2021, 134, 105997 CrossRef CAS.
- Y. P. Miao, H. W. Bao, W. Fan, Y. Li and F. Ma, Surf. Interfaces, 2021, 27, 101580 CrossRef CAS.
- B. Zhao, C. Y. Li, L. L. Liu, B. Zhou, Q. K. Zhang, Z. Q. Chen and Z. Tang, Appl. Surf. Sci., 2016, 382, 280–287 CrossRef CAS.
- S. Sharma, A. Kumar and D. Kaur, AIP Conf. Proc., 2018, 1953, 030261 CrossRef.
- S. Singh and S. Sharma, AIP Conf. Proc., 2021, 2265, 030690 CrossRef.
- D. Z. Zhang, C. X. Jiang and Y. E. Sun, J. Alloys Compd., 2017, 698, 476–483 CrossRef CAS.
- S. Singh, S. Sharma, R. C. Singh and S. Sharma, Appl. Surf. Sci., 2020, 532, 147373 CrossRef CAS.
- S. Singh, R. M. Sattigeri, S. Kumar, P. K. Jha and S. Sharma, ACS Omega, 2021, 6, 11602–11613 CrossRef CAS PubMed.
- A. Liu, S. Y. Lv, L. Jiang, F. M. Liu, L. J. Zhao, J. Wang, X. L. Hu, Z. J. Yang, J. M. He, C. G. Wang and G. Y. Lu, Sens. Actuators, B, 2021, 332, 129444 CrossRef CAS.
- A. Q. Jian, J. H. Wang, H. Y. Lin, S. Q. Xu, D. Han, Z. Y. Yuan and K. Zhuo, ACS Omega, 2022, 7, 11664–11670 CrossRef CAS PubMed.
- S. Singh, J. Deb, U. Sarkar and S. Sharma, ACS Sustainable Chem. Eng., 2021, 9, 7328–7340 CrossRef CAS.
- D. Z. Zhang, Z. L. Wu, P. Li, X. Q. Zong, G. K. Dong and Y. Zhang, Sens. Actuators, B, 2018, 258, 895–905 CrossRef CAS.
- K. J. Yoon, S. I. Lee, H. An, J. Kim, J. W. Son, J. H. Lee, H. J. Je, H. W. Lee and B. K. Kim, Int. J. Hydrogen Energy, 2014, 39, 3868–3878 CrossRef CAS.
- S. E. Hosseini and M. A. Wahid, Renewable Sustainable Energy Rev., 2016, 57, 850–866 CrossRef CAS.
- M. M. Y. A. Alsaif, S. Balendhran, M. R. Field, K. Latham, W. Wlodarski, J. Z. Ou and K. K. Zadeh, Sens. Actuators, B, 2014, 192, 196–204 CrossRef CAS.
- F. K. Perkins, A. L. Friedman, E. Cobas, P. M. Campbell, G. G. Jernigan and B. T. Jonker, Nano Lett., 2013, 13, 668–673 CrossRef CAS.
- M. V. Bollinger, J. V. Lauritsen, K. W. Jacobsen, J. K. Nørskov, S. Helveg and F. Besenbacher, Phys. Rev. Lett., 2001, 87, 196803 CrossRef CAS PubMed.
- K. Dolui, I. Rungger and S. Sanvito, Phys. Rev. B: Condens. Matter Mater. Phys., 2013, 87, 165402 CrossRef.
- A. Castellanos-Gomez, N. Agrait and G. Rubio-Bollinger, Appl. Phys. Lett., 2010, 96, 213116 CrossRef.
- Z. Zhang, K. Chen, Q. Zhao, M. Huang and X. P. Ouyang, Mater. Res. Express, 2020, 7, 015501 CrossRef CAS.
- D. H. Baek and J. Kim, Sens. Actuators, B, 2017, 250, 686–691 CrossRef CAS.
- H. D. Mai, S. Jeong, T. K. Nguyen, J. S. Youn, S. Ahn, C. M. Park and K. J. Jeon, ACS Appl. Mater. Interfaces, 2012, 13, 14657–14665 Search PubMed.
- S. L. Yang, G. Lei, Z. G. Lan, W. Xie, B. P. Yang, H. X. Xu, Z. Wang and H. S. Gu, Int. J. Hydrogen Energy, 2019, 44, 7725–7733 CrossRef CAS.
- W. Wu, Z. Liu, L. A. Jauregui, Q. Yu, R. Pillai, H. Cao, J. Bao, Y. P. Chen and S. S. Pei, Sens. Actuators, B, 2010, 150, 296–300 CrossRef CAS.
- C. Pijolat, G. Tournier, P. Breuil, D. Matarin and P. Nivet, Sens. Actuators, B, 2002, 82, 166–175 CrossRef CAS.
- C. M. Wu, K. G. Motora, G. Y. Chen, D. H. Kuo and N. S. Gultom, Front. Mater. Sci., 2022, 9, 831725 CrossRef.
- A. Saravanan, B. R. Huang, J. P. Chu, A. Prasannan and H. C. Tsai, Sens. Actuators, B, 2019, 292, 70–79 CrossRef CAS.
- D. Kathiravan, B. R. Huang, A. Saravanan, A. Prasannan and P. D. Hong, Sens. Actuators, B, 2019, 279, 138–147 CrossRef CAS.
- (a) A. Venkatesan, S. Rathi, I. Y. Lee, J. Park, D. Lim, M. Kang, H. I. Joh, G. H. Kim and E. S. Kannan, Nanotechnology, 2017, 28, 365501 CrossRef CAS.
- D. Z. Zhang, Y. E. Sun, C. X. Jiang and Y. Zhang, Sens. Actuators, B, 2017, 242, 15–24 CrossRef CAS.
- L. Z. Hao, Y. J. Liu, W. Gao, Y. M. Liu, Z. D. Han, L. Q. Yu, Q. Z. Xue and J. Zhu, J. Alloys Compd., 2016, 682, 29–34 CrossRef CAS.
- A. V. Agrawal, R. Kumar, S. Venkatesan, A. Zakhidov, Z. Zhu, J. M. Bao and M. Kumar, Appl. Phys. Lett., 2017, 111, 093102 CrossRef.
- S. L. Yang, Z. Chen, Z. Wang, G. Lei, J. Xiong, H. X. Xu and H. S. Gu, Sens. Actuators, B, 2022, 367, 132026 CrossRef CAS.
- V. Munusami, K. Arutselvan, S. Vadivel and S. Govindasamy, Ceram. Int., 2022, 48, 29322–29331 CrossRef CAS.
- A. K. Vivekanandan, B. R. Huang, D. Kathiravan, A. Saravanan, A. Prasannan, H. C. Tsai and S. H. Chen, J. Alloys Compd., 2021, 854, 157102 CrossRef CAS.
- J. F. Wu, D. Z. Zhang and Y. H. Cao, J. Colloid Interface Sci., 2018, 529, 556–567 CrossRef CAS PubMed.
- B. Chakraborty, I. Maity, P. Chung, M. Ho and P. Bhattacharyya, IEEE Sens. J., 2021, 21, 16484–16491 CAS.
- X. X. Deng, X. Y. Liang, S. P. Ng and C. M. L. Wu, Appl. Surf. Sci., 2019, 484, 1244–1252 CrossRef CAS.
- D. Z. Zhang, C. X. Jiang and X. Y. Zhou, Talanta, 2018, 182, 324–332 CrossRef CAS PubMed.
- D. Z. Zhang, X. Fan, A. J. Yang and X. Q. Zong, J. Colloid Interface Sci., 2018, 523, 217–225 CrossRef CAS.
- X. Li, J. Wang, D. Xie, J. L. Xu, Y. Xia, L. Xiang and S. Komarneni, Mater. Lett., 2017, 189, 42–45 CrossRef CAS.
- D. Z. Zhang, C. X. Jiang and J. F. Wu, Sens. Actuators, B, 2018, 273, 176–184 CrossRef CAS.
- X. Li, J. Wang, D. Xie, J. L. Xu, Y. Xia, W. W. Li, L. Xiang, Z. M. Li, S. W. Xu and S. Komarneni, Nanotechnology, 2017, 28, 325501 CrossRef.
| This journal is © The Royal Society of Chemistry 2023 |
