Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Repurposing a long-wavelength fluorescent boronate probe for the detection of reactive oxygen species (ROS) in bacteria†
Kai-Cheng
Yan
 ad,
Naing
Thet
ad,
Naing
Thet
 a,
Rachel A.
Heylen
a,
Rachel A.
Heylen
 a,
Adam C.
Sedgwick
a,
Adam C.
Sedgwick
 *b,
Tony D.
James
*b,
Tony D.
James
 *ac,
A. Toby A.
Jenkins
*ac,
A. Toby A.
Jenkins
 *a and
Xiao-Peng
He
*a and
Xiao-Peng
He
 *de
*de
aDepartment of Chemistry, University of Bath, Bath, BA2 7AY, UK. E-mail: t.d.james@bath.ac.uk; a.t.a.jenkins@bath.ac.uk
bChemistry Research Laboratory, University of Oxford, Mansfield Road, OX1 3TA, UK. E-mail: adam.sedgwick@chem.ox.ac.uk
cSchool of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang 453007, China
dKey Laboratory for Advanced Materials and Joint International Research Laboratory of Precision Chemistry and Molecular Engineering, Feringa Nobel Prize Scientist Joint Research Center, School of Chemistry and Molecular Engineering, East China University of Science and Technology, 130 Meilong Rd, Shanghai 200237, China. E-mail: xphe@ecust.edu.cn
eThe International Cooperation Laboratory on Signal Transduction, Eastern Hepatobiliary Surgery Hospital, National Center for Liver Cancer, Shanghai 200438, China
First published on 13th July 2023
Abstract
Fluorescent probes are extensively used with ever-increasing functions for biological and medical applications. To further solve problems associated with tissue damage, long-wavelength fluorophores have been proposed for the construction of sensors with decreased energy requirements for activation and increased biocompatibility. As such we have repurposed an effective and biologically appliable probe TCF-Bpin combining boronate activating groups and a TCF-OH fluorophore, for monitoring the production of peroxynitrite (ONOO−) by bacteria upon treatment with antibiotics. In addition, externally added ONOO− was intracellularly visualized, which makes not only the detection of oxidative stress in bacteria possible but also increases the understanding of the mechanisms.
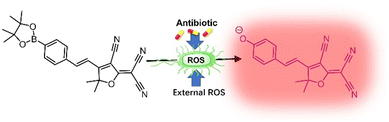
Since 2015, 2-(3-cyano-4,5,5-trimethylfuran-2(5H)-ylidene)malononitrile (TCF) has been reported as a long-wavelength fluorophore which exhibits low-toxicity towards tissues and bright fluorescence.1 Moreover, the internal charge transfer (ICT) donor–π–acceptor (D–π–A) system imparts several advantages including signal stability, deep tissue penetration, and detection sensitivity; which are superior to systems such as dicyanomethylene-4H-pyran (DCM), another red-emitting fluorophore.2–5 This has made TCF-based fluorophores or imaging agents potentially clinically useful and brought them into consideration for many fundamental research applications. It is well-known that TCF-OH is strongly fluorescent and can be significantly quenched upon locking the phenolic hydroxyl group with protecting groups, leading to the emergence of many “turn-on” fluorescence probes for different sensing targets based on the different locking groups.6–8 Within the currently-developed TCF-based fluorescence probes, TCF-Bpin, is locked by a boronate group and can be released to TCF-OH under the action of reactive oxygen species (ROS) which are markers for many human diseases. This probe has been used for the solutional sensing of hypochlorite (ClO−),1 while more recent research has indicated that it was more reactive and sensitive towards the more clinically indicative diseases (inflammation, cancer, ischemia–reperfusion, neurodegenerative, etc.) and peroxynitrite (ONOO−). Therefore, the sensitive fluorescence in situ imaging of intracellular ONOO− was realized without toxicity towards cells.6 These previous reports encouraged us to explore the use of the TCF-Bpin probe with bacteria.
Given that bacterial infections are a serious health concern, effective methods to track treatment process are in urgent demand (in particular methods to track oxidative stress which relates very closely with bacterial mortality), with this research we aimed to explore the possibility and potential of the probe to be repurposed for bacterial analysis (Scheme 1). ROS was monitored in bacterial cells, using TCF-Bpin, the cells had ROS externally added and stressed using chloramphenicol, tetracycline, and ciprofloxacin.9,10 It is worth mentioning that ClO− has not been reported as present in bacterial cells. While ONOO− can readily exist under stress at therapeutic levels, as it is generally formed between superoxide and nitrile oxide (NO).11–18 This aroused our interest and therefore we decided to explore if any sensor response can be observed from bacteria upon treatment with antibiotics.
In our previous work to detect ONOO−, we found that TCF-Bpin could react with hydrogen peroxide (H2O2), although the response is restricted. During initial solution tests, 10 μM of ONOO− could generate TCF-Bpin with a significant response (Fig. S1†), while the response towards both ClO− and H2O2 were minimal (Fig. S2 and S3†), even with concentrations 10-fold greater than ONOO−. While it is known that DMSO might to some extent quench available ROS.19 We observed similar responses in pure PBS solutions (data not shown). As such our results indicate that the probe could be used to monitor ONOO− expression in microbial pathogens. A sensitivity assay confirmed this observation, where TCF-Bpin was found to react with ONOO− at low concentrations (Fig. S4†). While upon the addition of glutathione (GSH), a biological antioxidant, the signal partly rebounds (Fig. S5†).
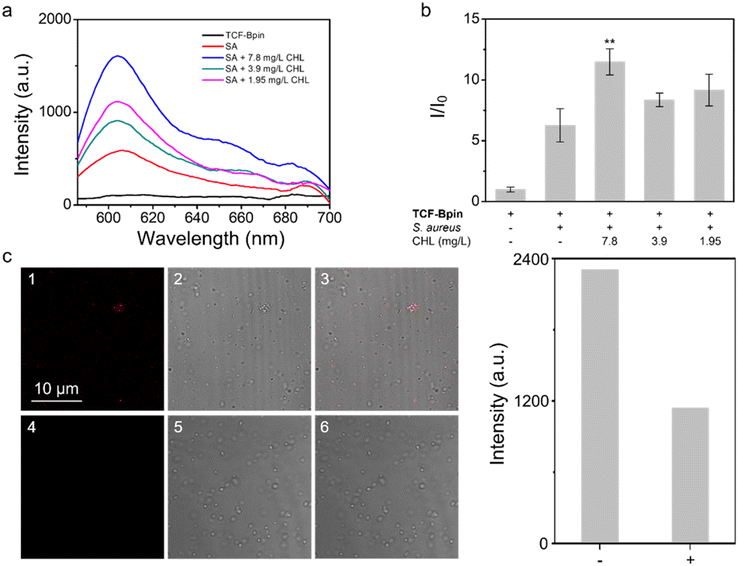
When it comes to the detection of antibiotic-induced stress, we sought to use the probe with drug-stressed bacterial cells to see if signals can be detected. Then, specific inhibitors were used to confirm that the ROS species responsible for the response was ONOO−. Using standard minimum inhibitory concentration (MIC) assays which determine the minimum concentration of an antibiotic required to completely inhibit bacterial growth (Fig. S6–S8†), we selected Pseudomonas aeruginosa (P. aeruginosa PAO1, Gram negative) and Staphylococcus aureus (S. aureus NCTC 10788, Gram positive) as model bacteria and screened three common broad-spectrum antibiotics, chloramphenicol, tetracycline, and ciprofloxacin. Then, the probe was added to bacteria incubated with sub-MIC levels of antibiotics (with antibiotic concentrations just below the full-inhibitory concentration). It was found that S. aureus generated detectable signals upon treatment with chloramphenicol (Fig. 1a and b), while the response for tetracycline and ciprofloxacin, and that among P. aeruginosa were generally negligible (Fig. S9–S13†). Further, we used two multidrug-resistant (MDR) strains, S. aureus Mu50 and P. aeruginosa ATCC BAA-2110, as controls. However, we still observed similar responses (Fig. S14–S19†), indicating that oxidative stress was not completely associated with drug resistance for these strains. Since, the drugs are still causing stress, suggestive of enhanced antioxidant levels existing in MDR strains to support survival. Using an ONOO− inhibitor uric acid, the signal decreased (Fig. S20†), thus confirming that the existence of ONOO− was due to antibiotic stresses. Moreover, no stress was detected among the original strains without external drug treatments (Fig. S21†), showing that the initial level of ROS in the bacteria can be ignored. Such changes in ROS are also occurring intracellularly. TCF-Bpin-stained chloramphenicol sub-MIC S. aureus exhibited fluorescence, which could be quenched by uric acid (Fig. 1c). For other antibiotics, although the intensity changed with or without inhibition, no clear images could be obtained (Fig. S22 and S23†).
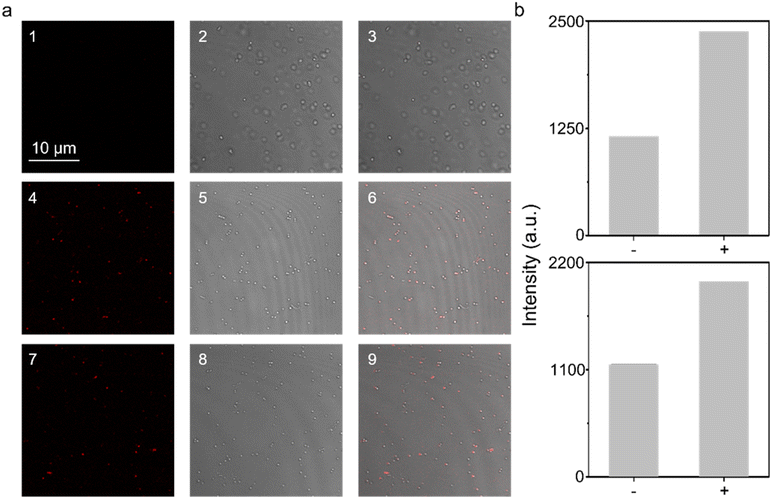
Subsequently, we explored the feasibility of the probe to detect ROS by external treatment of ROS. However, highly oxidative ROS are very toxic to bacteria, especially H2O2 and HClO which are used as household disinfectant sprays. Consequently, significant mortality was observed among the cells when treating them with ROS directly (Fig. S24†), where H2O2 and ClO− could completely kill the bacteria at 15 μM (Fig. S24†). As a result, it is not possible to allow the concentration to exceed 10 μM for detecting intracellular ROS, and hence signals from both H2O2 and ClO− can be ignored (since only a minimal signal can be seen when the concentration reaches 100 μM as shown in Fig. S2 and S3†). This hypothesis was verified under imaging conditions, where clear fluorescence staining was observed among ONOO−-treated cells (Fig. 2), while H2O2 and ClO− treated cells remained dark (Fig. S25 and S26†).
Moreover, SIN-1 (3-morpholi-nosydnonimine), a commercially available ONOO− generator was also used with S. aureus under imaging conditions. This resulted in detectable staining comparable to direct treatment with ONOO− (Fig. 2). In our previous study, we confirmed that the probe TCF-Bpin exhibits excellent biocompatibility, showing no damage to a range of cell lines including A549, HeLa, Hep-G2, and RAW264.7.6 Herein, the low-toxicity towards bacterial cell lines has further been verified, demonstrating that the probe would not induce any damage to bacteria by interrupting cellular processes (Fig. S27†).
In summary, a TCF-based long-wavelength boronate probe TCF-Bpin has been repurposed for bacterial applications and used to detect the possible production of ONOO− due to oxidative stress caused by antibiotic treatment, which has not been previously reported. Meanwhile, externally added ONOO− has been visualized intracellularly using the probe. The inhibition-based “double-check” assay confirmed the production of ONOO− upon bacterial treatment with the antibiotic chloramphenicol, emphasizing not only the potential use of TCF-Bpin for detecting oxidative stress in bacteria but also the possibility of understanding the mechanism of oxidative stress.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
XPH wishes to thank the Natural National Science Foundation of China (No. 92253306), Open Funding Project of the State Key Laboratory of Bioreactor Engineering of East China University of Science and Technology, the Fundamental Research Funds for the Central Universities (222201717003) and the Programme of Introducing Talents of Discipline to Universities (B16017) for financial support. ATAJ would like to acknowledge funding from the EPSRC, grant reference: EP/V00462X/1. TDJ wishes to thank the University of Bath and the Open Research Fund of the School of Chemistry and Chemical Engineering, Henan Normal University (2020ZD01) for support. RAH thanks the Annette Trust and EPSRC for funding. ACS would like to thank the Glasstone Research fellowship (University of Oxford) and the major research grant from Jesus College Oxford for financial support.Notes and references
- W. Shu, L. Yan, Z. Wang, J. Liu, S. Zhang, C. Liu and B. Zhu, A novel visual and far-red fluorescent dual-channel probe for the rapid and sensitive detection of hypochlorite in aqueous solution and living cells, Sens. Actuators, B, 2015, 221, 1130–1136 CrossRef CAS.
- X. Wu, A. Shao, S. Zhu, Z. Guo and W. Zhu, A novel colorimetric and ratiometric NIR fluorescent sensor for glutathione based on dicyanomethylene-4H-pyran in living cells, Sci. China: Chem., 2016, 59, 62–69 CrossRef CAS.
- B.-B. Wang, Y. Wang, W.-N. Wu, Z.-H. Xu, X.-L. Zhao, Z.-Q. Xu and Y.-C. Fan, A near-infrared colorimetric and fluorescent dual-channel probe for cyanide detection based on dicyanomethylene-4H-pyran, Inorg. Chem. Commun., 2020, 122, 108245–108251 CrossRef CAS.
- J. Yang, M. Li and W.-H. Zhu, Dicyanomethylene-4H-pyran-based NIR fluorescent ratiometric chemosensor for pH measurement, Res. Chem. Intermed., 2018, 44, 3959–3969 CrossRef CAS.
- Y. Yang, L. Wang, M. Xu, J. Chen and Y. Qu, Triphenyl phosphate end-capped dicyanomethylene-4H-pyran as a near infrared fluorescent sensor for lysozyme in urine sample, Sens. Actuators, B, 2019, 284, 553–561 CrossRef CAS.
- A. C. Sedgwick, H.-H. Han, J. E. Gardiner, S. D. Bull, X.-P. He and T. D. James, Long-wavelength fluorescent boronate probes for the detection and intracellular imaging of peroxynitrite, Chem. Commun., 2017, 53, 12822–12825 RSC.
- B. Zhu, H. Kan, J. Liu, H. Liu, Q. Wei and B. Du, A highly selective ratiometric visual and red-emitting fluorescent dual-channel probe for imaging fluoride anions in living cells, Biosens. Bioelectron., 2014, 52, 298–303 CrossRef CAS PubMed.
- B. Zhu, W. Wang, L. Liu, H. Jiang, B. Du and Q. Wei, A highly selective colorimetric and long-wavelength fluorescent probe for Hg2+, Sens. Actuators, B, 2014, 191, 605–611 CrossRef CAS.
- I. Albesa, M. C. Becerra, P. C. Battan and P. L. Paez, Oxidative stress involved in the antibacterial action of different antibiotics, Biochem. Biophys. Res. Commun., 2004, 317, 605 CrossRef CAS PubMed.
- M. Marcen, V. Ruiz, J. Serrano, S. Condon and P. Manas, Oxidative stress in E. coli cells upon exposure to heat treatments, Int. J. Food Microbiol., 2017, 241, 198 CrossRef CAS.
- M. K. Lee, J. Williams, R. J. Twieg, J. Rao and W. E. Moerner, Enzymatic activation of nitro-aryl fluorogens in live bacterial cells for enzymatic turnover-activated localization microscopy, Chem. Sci., 2013, 4, 220–225 RSC.
- L. Wu, H.-H. Han, L. Liu, J. E. Gardiner, A. C. Sedgwick, C. Huang, S. D. Bull, X.-P. He and T. D. James, ESIPT-based fluorescence probe for the rapid detection of peroxynitrite ‘AND’ biological thiols, Chem. Commun., 2018, 54, 11336–11339 RSC.
- A. C. Sedgwick, H.-H. Han, J. E. Gardiner, S. D. Bull, X.-P. He and T. D. James, The development of a novel AND logic based fluorescence probe for the detection of peroxynitrite and GSH, Chem. Sci., 2018, 9, 3672–3676 RSC.
- L. Wu, X. Tian, H.-H. Han, J. Wang, R. R. Groleau, P. Tosuwan, B. Wannalerse, A. C. Sedgwick, S. D. Bull, X.-P. He and T. D. James, A Simple Near-Infrared Fluorescent Probe for the Detection of Peroxynitrite, ChemistryOpen, 2019, 8, 1407–1409 CrossRef CAS PubMed.
- L. Dong, M. Fu, L. Liu, H.-H. Han, Y. Zang, G.-R. Chen, J. Li, X.-P. He and S. Vidal, Supramolecular assembly of TPE-based glycoclusters with dicyanomethylene-4H-pyran (DM) fluorescent probes improve their properties for peroxynitrite sensing and cell imaging, Chem. – Eur. J., 2020, 26, 1–9 CrossRef.
- L. Wu, X. Tian, R. R. Groleau, J. Wang, H.-H. Han, S. B. Reeksting, A. C. Sedgwick, X.-P. He, S. D. Bull and T. D. James, Coumarin-based fluorescent probe for the rapid detection of peroxynitrite ‘AND’ biological thiols, RSC Adv., 2020, 10, 13496–13499 RSC.
- W.-T. Dou, H.-H. Han, A. C. Sedgwick, G.-B. Zhu, Y. Zang, X.-R. Yang, J. Yoon, T. D. James, J. Li and X.-P. He, Fluorescent probes for the detection of disease-associated biomarkers, Sci. Bull., 2022, 67, 853–878 CrossRef CAS PubMed.
- L. Wu, J. Liu, X. Tian, R. R. Groleau, B. Feng, Y. Yang, A. C. Sedgwick, H.-H. Han, Y. Wang, H.-M. Wang, F. Huang, S. D. Bull, H. Zhang, C. Huang, Y. Zang, J. Li, X.-P. He, P. Li, B. Tang, T. D. James and J. L. Sessler, Dual-Channel Fluorescent Probe for the Simultaneous Monitoring of Peroxynitrite and Adenosine-5′-triphosphate in Cellular Applications, J. Am. Chem. Soc., 2022, 144, 174–183 CrossRef CAS PubMed.
- K. Pierzchała, M. Pięta, M. Rola, M. Świerczyńska, A. Artelska, K. Dębowska, R. Podsiadły, J. Pięta, J. Zielonka, A. Sikora, A. Marcinek and R. Michalski, Fluorescent probes for monitoring myeloperoxidase-derived hypochlorous acid: a comparative study, Sci. Rep., 2022, 12, 9314 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Additional figures and experimental section. See DOI: https://doi.org/10.1039/d3sd00049d |
| This journal is © The Royal Society of Chemistry 2023 |