Open Access Article
Open Access ArticleAn exfoliated redox active imide covalent organic framework for metal free hydrogen gas sensing†
Nany
Thokala
a,
Kiran
Vankayala
 b,
Asmita Dileep
Gaonkar
b,
Ganga
Periyasamy
b,
Asmita Dileep
Gaonkar
b,
Ganga
Periyasamy
 c,
Kashifa
Fazl-Ur-Rahman
c,
Krishnaveni
Valle
a,
Marilyn Esclance
DMello
d,
Keloth
Basavaiah
c,
Kashifa
Fazl-Ur-Rahman
c,
Krishnaveni
Valle
a,
Marilyn Esclance
DMello
d,
Keloth
Basavaiah
 a and
Suresh Babu
Kalidindi
a and
Suresh Babu
Kalidindi
 *e
*e
aDepartment of Chemistry, Andhra University, Visakhapatnam, 530003, India
bDepartment of Chemistry, Birla Institute of Technology and Science, Pilani, K. K. Birla Goa campus, Goa 403726, India
cDepartment of Chemistry, Bangalore University, Bangalore-56, India
dMaterials Science Division, Poornaprajna Institute of Scientific Research, Bidalur post, Devanahalli, Bengaluru, 562 110, India
eDepartment of Chemistry, Central Tribal University of Andhra Pradesh, AU PG Centre, Vizianagaram, 535003, India. E-mail: ksureshu@gmail.com; suresh.babu@ctuap.ac.in
First published on 15th June 2023
Abstract
A two dimensional (2D) redox active donor–acceptor COF made of triphenylamine (TPA) and naphthalenediimide (NDI) acted as an efficient hydrogen chemisresistor and performed better than traditional metal oxides. Calculations have shown that the charge transfer interaction between H2 and an NDI linker through a carbonyl functionality enables a change in the resistance of the material upon exposure to H2 gas.
Covalent organic frameworks (COFs) are a versatile class of crystalline porous organic polymers which have been drawing the interest of researchers from a wide range of areas since their discovery in 2005 by Yaghi et al.1 COFs are acclaimed for multiple applications like gas storage,2 supports for catalysts,3 adsorption and separation of gases,4 energy storage,5 opto-electronics6 and very recently in sensing.7 Two dimensional (2D) COFs in particular have gained much attention due to their interesting electronic and optical properties because of their extended in-plane π-conjugation. Uniform distribution of active functionalities over these extended frameworks increases the analyte interaction during sensing applications and ensures proper signal transduction.8 Although COFs have been widely studied as sensors based on fluorescence and absorption mechanisms,9,10 COF based chemiresistive sensors are few.
Sensors based on the measurement of change in the resistance upon exposure to analyte gas, i.e. chemiresistors, are much simpler, and miniaturization can be done quite easily.11 In this direction, Q. H. Wang et al. have reported for the first time the use of COFs for the detection of NH3 using covalent triazine framework (CTF)12a based COFs. Subsequently, COFs containing imine,12b heptazine12c and truxene12d linkages are used for sensing NH3, NO2, humidity, etc. Although these latest studies highlight the potential of COFs for chemisresitive gas sensing, looking at the diverse COFs available in the literature, the area is still in an early stage (only 8 studies so far, to the best of our knowledge).12e–h For instance, redox active COFs have not been explored in this direction. Also, neat COF based H2 sensors are yet to be developed. H2 is an emerging alternative source of clean energy and at the same time, it is highly flammable at concentrations above 4% in air.13 Pd based chemisresistive H2 sensors have been widely reported due to good affinity of Pd towards H2. Although most of the Pd based sensors exhibit quick response, the recovery times are not appreciable.14 Further, Pd is an expensive metal, which warrants the development of cost-effective H2 sensors that are devoid of expensive metals like Pd, Pt, etc.
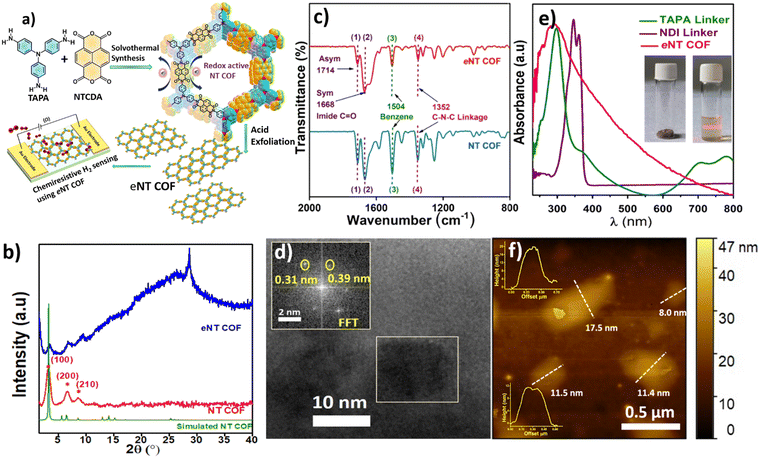
In the present study, a chemiresistive sensing device is fabricated using a colloidal dispersion of an exfoliated redox active COF constructed from naphthalene-1,4,5,8-tetracaboxylic dianhydride (NDA) and tris(4 aminophenyl)amine (TAPA), NDA–TAPA COF (NT COF) as a sensing element. The NT COF is a 2D crystalline porous polyarylimide well known for its high redox activity.15a The regular arrangement of electron donor units (triphenylamine, TPA) and electron acceptor units (naphthalenediimide, NDI) in the framework makes this material attractive for electronic applications.15b Notably, this COF with robust cyclic imide linkages and strong π–π conjugation has high thermal and chemical stability.15c Keeping its structural and intrinsic properties in mind, we reasoned that this COF could be a suitable candidate for the chemiresistive detection of reducing gases like H2.
The NT COF was synthesised by a solvothermal method where the imidization of precursors, namely naphthalene-1,4,5,8-tetracarboxylic dianhydride (0.15 mmol) and tris(4 aminophenyl)amine linker (0.10 mmol), was carried out under controlled conditions following the literature procedure16 (Fig. 1a). Bulk COF powders typically consist of insoluble microcrystalline aggregated particles that are difficult to disperse in a suitable solvent, leading to challenges in processability of bulk COFs, which in turn becomes a critical aspect in electronic device fabrication.17 Since COF layers are held together by weak forces, they can be brought into solvents by using apt exfoliation methods. Among various reported exfoliation methods,18 we employed the acid exfoliation method for delaminating the layers of the NT COF, to obtain the exfoliated NT COF hitherto named as eNT COF. The acid exfoliation procedure involves vigorous stirring of the NT COF in a mixture of anhydrous acetonitrile, anhydrous tetrahydrofuran and trifluoroacetic acid (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio). A similar methodology was previously used for the exfoliation of imine based COFs.19
2 ratio). A similar methodology was previously used for the exfoliation of imine based COFs.19
The PXRD pattern of the eNT COF suggests that the crystal structure of the bulk 2D COF is majorly intact even after exfoliation, suggesting insignificant structural rearrangements.20 However, a new reflection at 2θ = 28.5° corresponding to an interlayer distance of 0.31 nm is observed which probably appeared due to the restacking of layers during exfoliation21 (Fig. 1b). In the FT-IR spectra, the retention of peaks at 1714, 1668 and 1352 cm−1 confirms the intactness of the imide linkage in the eNT COF22 in comparison with the bulk NT COF (Fig. 1c). We also noticed the appearance of new peaks at 1640 and 3435 cm−1 which can be attributed to the conversion of some of the imide linkages in the NT COF to amic acid linkages during exfoliation (Fig. S6 in the ESI†).23 Nevertheless, Raman spectral bands observed in the case of NT COF remain unchanged in eNT COF, suggesting the intactness of the COF structure even after exfoliation (Fig. S7 in the ESI†). The eNT COF maintained an uninterrupted π-conjugation in the framework similar to the parent material. The UV-visible spectrum of eNT COF showed a broad absorption peak that extended up to 800 nm, similar to the bulk NT COF (Fig. S8 in the ESI†), whereas the absorption by the monomers is largely restricted to 250–400 nm range. This indicates the presence of delocalized π-conjugation in eNT COF (Fig. 1e). The fast Fourier transform (FFT) image generated from the HRTEM data shows diffraction spots corresponding to lattice spacings of 0.39 and 0.31 nm. The 0.39 nm lattice spacing matches with d001 of NT COF, whereas 0.31 nm (2θ = 28.5) is assigned to a new interlayer distance that has been created due to acid exfoliation (Fig. 1d). Atomic force microscopy (AFM) images reveal the thickness of nanosheets in the range of 17 to 20 nm which supports the exfoliation of the bulk COF to few-layer-thick 2D sheets (∼40–50 layers) (insets of Fig. 1f show the height profiles of a few grains). Further, FESEM analysis of the drop-cast colloidal dispersions of eNT COF revealed interconnected flattened flakes (Fig. S5 in the ESI†). The solid state CP-MAS 13C NMR spectrum of eNT COF exhibited a characteristic resonance at 163 ppm corresponding to the carbonyl carbon of the imide ring and additional signals observed at 110 to 150 ppm represent the aromatic carbon backbone (Fig. S9 in the ESI†). The 13C NMR spectrum of eNT COF was found to be consistent with those in earlier reports.22
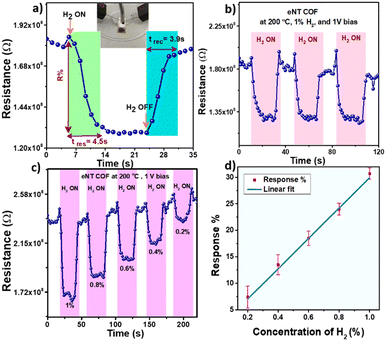
To assess H2 sensing functionalities of the COF materials, interdigitated Au electrodes (IDEs) having 10 pairs of fingers with a 100 μm spacing were used. The sensing experiments were carried out using a home built setup described in Scheme S1.† The bulk NT COF was suspended in ethanol and drop-cast on an IDE at 60 °C under a N2 flow (500 sccm) which is heated to 80 °C in order to obtain an active layer/film. Initially, the bulk NT COF was exposed to 1% H2 at 200 °C and 1 V bias was applied across the electrodes. The bulk COF did not respond to H2 gas, rather it exhibited a noisy baseline (Fig. S16 in the ESI†). Bulk COF powders possess voids or huge grain boundaries between the particles. This can block electron mobility and hinder signal transduction.17 Under similar conditions, IDE devices consisting of eNT COF as the active component (Fig. 2a, inset) exhibit a clear response to H2 gas with a sensing response of 30.7 ± 0.26% with 4.5 ± 0.6 s and 3.9 ± 0.3 s response and recovery times respectively (Fig. 2a). The exfoliation provides good contact between COF particles and easy access to the active sites which facilitates the interaction of analyte gas with the COF in a favourable manner.24Fig. 2b shows the response–recovery cycle profile of the eNT COF-based sensor recorded at 200 °C and 1 V bias upon exposure to 1% H2 gas. The sensor is found to be recyclable at this temperature with >99% recovery (Fig. S15, Table S4 in the ESI†). Further, to evaluate the characteristics of the present sensor, studies are carried out at temperatures ranging from 80 °C to 250 °C. At 80 °C, the percentage response (R%) is measured to be 60.0 with response and recovery times 4.5 ± 0.6 s and 3.9 ± 0.3 s respectively (Fig. S17 in the ESI†). With the increase in temperature, we noticed less noise and more stable baselines owing to the increase of electron density in the conduction band. Later, the temperature is increased further from 100 °C to 250 °C with an interval of 50 °C. The sensing data obtained at 100 °C and 150 °C are given in Fig. S18 and S19, and Table S5 and S6 in the ESI† respectively. At 200 °C, the SNR improved by 7 fold as evident from Fig. 3a. However, a further increase in temperature to 250 °C (Fig. S21 in the ESI†) resulted in the reduction of both R% and SNR values. Thus, 200 °C is found to be the optimum temperature for eNT COF that would exhibit better performance. The sensor was exposed to different concentrations of H2 gas ranging from 1 to 0.2% at 200 °C (Fig. 2c). A good linear relationship was obtained between R% and concentration of H2 with R2 = 0.97 (Fig. 2d). The R% increases from 7.4 ± 0.25 to 30.7 ± 0.26 with the increase in concentration of H2 from 0.2 to 1%. It can be noted that there is only a marginal increase in the response time from 3.2 ± 0.8 s to 4.5 ± 0.6 s and recovery time from 3.7± 1.5 s to 3.9 ± 0.3 s with the increase in temperature. The limit of detection (LOD) of eNT COF is found to be 0.14% (section S8 in the ESI†). The overall sensing data are given at 200 °C in Fig. S20 and Table S7 in the ESI.† The performance of the eNT COF chemiresistor for H2 sensing is superior to those of many traditional metal oxide (SnO2, ZnO, WO3, etc.)25 based sensors as shown in Table S2 in ESI.† Further, PXRD, FT-IR, and Raman spectra, and UV-visible spectral data for eNT COF after H2 sensing were collected (Fig. S10 in the ESI†). These data ascertain insignificant changes in the structure of eNT COF after H2 sensing, suggesting the robustness of eNT-COF to realize durable sensors.
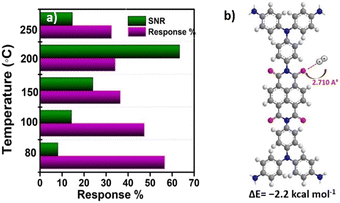 | ||
| Fig. 3 a) Comparison of response % versus signal-to-noise ratio of the eNT COF gas sensor at different temperatures. b) Molecular electrostatic potential plot of the optimized binding site. | ||
To obtain an insight into the sensing mechanism of eNT COF, DFT calculations are carried out by considering the structural models of the monomer using the Gaussian 09 package (computational details are given in the ESI†). The HOMO is localised on the TPA unit (donor) and LUMO on the NDI unit (acceptor).16 Further, the calculations suggested electrostatic interactions primarily between H2 and the carbonyl oxygen atoms (with a distance of 2.710 Å) of the NDI linker with an associated binding energy of −2.2 kcal mol−1 (Fig. 3b). The computed partial Mulliken charge shows the charge transfer from an H2 molecule to an oxygen atom of NDI (Table S3 in the ESI†). Notably, when the model system was subjected to a uniform electric field of 0.001 V Å−1, the relative negative charge on the carbonyl oxygen atom of NDI increased. As a result, the electrostatic interaction between oxygen and H2 increased, by ∼0.5 kcal mol−1. Nevertheless, the interactions between the framework and H2 are reversible, and the charge transfer direction supports the observed negative sigmoidal response.
Overall, the first COF based H2 sensor with attractive gas sensing characteristics has been successfully developed using a structurally rich redox active NT COF. The bulk NT COF powder has been converted into a colloidal suspension using an acid exfoliation method in order to increase the processability for device fabrication. Although eNT COF could sense H2 at 80 °C, a good trade-off between the signal-to-noise ratio and response (R%: 30.7 ± 0.26, SNR = 65) was achieved at 200 °C. The performance noted for eNT COF is superior to those for several widely studied metal oxides. DFT calculations have suggested that a carbonyl oxygen of the NDI linker interacts reversibly with H2 through a charge transfer from H2 to the carbonyl oxygen of NDI. This was also supported by the Mulliken charge analysis. Overall, the study demonstrates the first COF based H2 sensor and expands the scope of applicability of COFs for chemiresistive sensing.
Author contributions
Conceptualization: SBK and NT. Methodology: SBK, NT, KV, GP, KFUR, ADG, VK, KB and MED. Writing original draft: SBK, NT, KV and GP. Visualization: SBK. Supervision: SBK. Funding acquisition: SBK. The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
SBK acknowledges Science and Engineering Research Board (SERB)-Department of Science and Technology (DST), Govt. of India for funding under core research grant (CRG/2019/003925). Central sophisticated instrumentation facility (CSIF), BITS Pilani, KK Birla Goa campus is acknowledged for research facilities (SEM, XRD, Raman).Notes and references
-
(a) A. P. Côté, A. I. Benin, N. W. Ockwig, M. O'Keeffe, A. J. Matzger and O. M. Yaghi, Science, 2005, 310, 1166–1170 CrossRef PubMed
; (b) H. L. Nguyen, Chem. Sci., 2021, 12, 8632–8647 RSC
; (c) Y. Li, W. Chen, G. Xing, D. Jiang and L. Chen, Chem. Soc. Rev., 2020, 49, 2852–2868 RSC
.
- H. Furukawa and O. M. Yaghi, J. Am. Chem. Soc., 2009, 131, 8875–8883 CrossRef CAS PubMed
.
- H. Xu, J. Gao and D. Jiang, Nat. Chem., 2015, 7, 905–912 CrossRef CAS PubMed
.
- C. X. Yang, C. Liu, Y. M. Cao and X. P. Yan, Chem. Commun., 2015, 51, 12254–12257 RSC
.
- D. G. Wang, T. Qiu, W. Guo, Z. Liang, H. Tabassum, D. Xia and R. Zou, Energy Environ. Sci., 2021, 14, 688–728 RSC
.
- D. A. Vazquez-Molina, G. S. Mohammad-Pour, C. Lee, M. W. Logan, X. Duan, J. K. Harper and F. J. Uribe-Romo, J. Am. Chem. Soc., 2016, 138, 9767–9770 CrossRef CAS PubMed
.
- C. Zhao, L. Zhang, Q. Wang, L. Zhang, P. Zhu, J. Yu and Y. Zhang, ACS Appl. Mater. Interfaces, 2021, 13, 20397–20404 CrossRef CAS PubMed
.
- D. Jiang, Chem, 2020, 6, 2461–2483 CAS
.
- S. Y. Ding, M. Dong, Y. W. Wang, Y. T. Chen, H. Z. Wang, C. Y. Su and W. Wang, J. Am. Chem. Soc., 2016, 138, 3031–3037 CrossRef CAS PubMed
.
- Z. Li, Y. Zhang, H. Xia, Y. Mu and X. Liu, Chem. Commun., 2016, 52, 6613–6616 RSC
.
- M. E. DMello, N. G. Sundaram, A. Singh, A. K. Singh and S. B. Kalidindi, Chem. Commun., 2019, 55, 349–352 RSC
.
-
(a) L. M. Tao, F. Niu, D. Zhang, T. M. Wang and Q. H. Wang, New J. Chem., 2014, 38, 2774–2777 RSC
; (b) F. Niu, Z. W. Shao, J. L. Zhu, L. M. Tao and Y. Ding, J. Mater. Chem. C, 2021, 9, 8562–8569 RSC
; (c) N. Sharma, N. Sharma, P. Srinivasan, S. Kumar, J. B. Balaguru Rayappan and K. Kailasam, J. Mater. Chem. A, 2018, 6, 18389–18395 RSC
; (d) H. Singh, V. K. Tomer, N. Jena, I. Bala, N. Sharma, D. Nepak, A. De Sarkar, K. Kailasam and S. K. Pal, J. Mater. Chem. A, 2017, 5, 21820–21827 RSC
; (e) W. C. Ko, M. S. Kim, Y. J. Kwon, J. Jeong, W. R. Kim, H. Choi, J. K. Park and Y. K. Jeong, J. Mater. Chem. A, 2020, 8, 19246–19253 RSC
; (f) F. Niu, Z. W. Shao, L. M. Tao and Y. Ding, Sens. Actuators, B, 2020, 321, 128513 CrossRef CAS
; (g) K. Yang, W. Yuan, Z. Hua, Y. Tang, F. Yin and D. Xia, ACS Appl. Mater. Interfaces, 2020, 12, 3919–3927 CrossRef CAS PubMed
; (h) Z. Meng, R. M. Stolz and K. A. Mirica, J. Am. Chem. Soc., 2019, 141, 11929–11937 CrossRef CAS PubMed
.
- W. T. Koo, H. J. Cho, D. H. Kim, Y. H. Kim, H. Shin, R. M. Penner and I. D. Kim, ACS Nano, 2020, 14, 14284–14322 CrossRef CAS PubMed
.
- W. Zeng, T. Liu, D. Liu and E. Han, Sens. Actuators, B, 2011, 160, 455–462 CrossRef CAS
.
-
(a) G. Wang, N. Chandrasekhar, B. P. Biswal, D. Becker, S. Paasch, E. Brunner, M. Addicoat, M. Yu, R. Berger and X. Feng, Adv. Mater., 2019, 31, 1–6 Search PubMed
; (b) B. Sun, X. Li, T. Feng, S. Cai, T. Chen, C. Zhu, J. Zhang, D. Wang and Y. Liu, ACS Appl. Mater. Interfaces, 2020, 12, 51837–51845 CrossRef CAS PubMed
; (c) M. Yu, N. Chandrasekhar, R. K. M. Raghupathy, K. H. Ly, H. Zhang, E. Dmitrieva, C. Liang, X. Lu, T. D. Kühne, H. Mirhosseini, I. M. Weidinger and X. Feng, J. Am. Chem. Soc., 2020, 142, 19570–19578 CrossRef CAS PubMed
.
- J. Lv, Y. X. Tan, J. Xie, R. Yang, M. Yu, S. Sun, M. De Li, D. Yuan and Y. Wang, Angew. Chem., Int. Ed., 2018, 57, 12716–12720 CrossRef CAS PubMed
.
- X. Zhao, P. Pachfule and A. Thomas, Chem. Soc. Rev., 2021, 50, 6871–6913 RSC
.
- Y. Tao, W. Ji, X. Ding and B. H. Han, J. Mater. Chem. A, 2021, 9, 7336–7365 RSC
.
- D. W. Burke, C. Sun, I. Castano, N. C. Flanders, A. M. Evans, E. Vitaku, D. C. McLeod, R. H. Lambeth, L. X. Chen, N. C. Gianneschi and W. R. Dichtel, Angew. Chem., Int. Ed., 2020, 59, 5165–5171 CrossRef CAS PubMed
.
- L. Jiang, Y. Tian, T. Sun, Y. Zhu, H. Ren, X. Zou, Y. Ma, K. R. Meihaus, J. R. Long and G. Zhu, J. Am. Chem. Soc., 2018, 140, 15724–15730 CrossRef CAS PubMed
.
- C. Kang, Z. Zhang, V. Wee, A. K. Usadi, C. David, L. S. Baugh, S. Wang, Y. Wang and D. Zhao, J. Am. Chem. Soc., 2020, 142, 12995–13002 CrossRef CAS PubMed
.
- G. Li and Z. Wang, J. Phys. Chem. C, 2013, 117, 24428–24437 CrossRef CAS
.
- J.-W. Lee, G. Barin, G. W. Peterson, J. Xu, K. A. Colwell and J. R. Long, ACS Appl. Mater. Interfaces, 2017, 9, 33504–33510 CrossRef CAS PubMed
.
- Z. Wang, Y. Li, P. Liu, Q. Qi, F. Zhang, G. Lu, X. Zhao and X. Huang, Nanoscale, 2019, 11, 5330–5335 RSC
.
- H. Gu, Z. Wang and Y. Hu, Sensors, 2012, 12(5), 5517–5550 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3sd00097d |
| This journal is © The Royal Society of Chemistry 2023 |