 Open Access Article
Open Access ArticleBioengineered multi-walled carbon nanotube (MWCNT) based biosensors and applications thereof
Sandeep
Kumar
 ab,
H. K.
Sidhu
ab,
H. K.
Sidhu
 a,
Ashok K.
Paul
a,
Ashok K.
Paul
 a,
Neha
Bhardwaj
a,
Neha
Bhardwaj
 c,
Neeraj S.
Thakur
c,
Neeraj S.
Thakur
 *d and
Akash
Deep
*d and
Akash
Deep
 *c
*c
aFaculty of Agriculture and Lifesciences, Desh Bhagat University, Mandi Gobindgarh, Punjab 147301, India
bCSIR - Central Scientific Instruments Organization, Sector 30 C, Chandigarh 160030, India
cEnergy and Environment unit, Institute of Nano Science and Technology, Sector 81, Mohali, Punjab 140306, India. E-mail: akashdeep@inst.ac.in
dDepartment of Pharmaceutical Sciences, College of Pharmacy, University of Oklahoma Health Sciences Center, 1110 N Stonewall Ave, Oklahoma City, OK 73117, USA. E-mail: neeraj-thakur@ouhsc.edu
First published on 6th October 2023
Abstract
The emergence of carbon nanotubes (CNTs) in the past decade has greatly promoted the development of biosensors, which provide a possible alternative to conventional detection systems. CNTs possess outstanding properties including good mechanical strength, photostability, better electrical conductivity, tunable photonic properties, ease of surface modification with functional groups, and the ability to conjugate with metal or organic components. The expanding family of CNTs, particularly multi-walled carbon nanotubes (MWCNTs), has been used for developing highly reliable and sensitive sensor systems. Bioengineered MWCNTs were fabricated by coupling with biological entities such as antibodies, aptamers, proteins, DNA, etc. as novel biosensing platforms. The present review aims to provide an overview of the recent developments in the field of bioengineered MWCNT-based biosensors. Recent research on CNT-based immunosensors, aptasensors, and enzymatic sensors is also discussed, along with some practical examples of such sensors. Finally, the applications of bioengineered gold nanoparticle–MWCNT (bio-AuNP–MWCNT) composites for the detection of analytes in food analysis, environmental monitoring, and clinical diagnosis have been discussed. The review concludes with a perspective on future developments in the field of bioengineered MWCNT-based sensors and their commercialization potential.
1. Introduction
Carbon nanotubes (CNTs) have been an intensively discussed topic of research for nearly more than two decades. They were first discovered by a Japanese scientist Sumio Iijima in the year 1991. The developments in the field of CNTs has come a long way as one of the highly investigated nanostructured materials with thousands of scientific research studies published every year.1–4 CNTs are structural allotropes of carbon in which the carbon atoms are arranged in an assembly of seamless hollow cylinders with one or more layers with open or closed ends. CNTs have been broadly classified into two groups: single-walled carbon nanotubes (SWCNTs) and multi-walled carbon nanotubes (MWCNTs). SWCNTs are cylindrical shells with a single atomic thickness and are considered the functional unit of material. Further, SWCNTs form the architecture of MWCNTs containing multiple co-axial cylinders of SWCNT constituents with increasing diameters around one axis.5,6 In short, CNTs can be perceived as graphene sheets with sp2 hybridized carbons rolled around themselves to produce robust long cylindrical structures. They are unique materials with diameters from a few nanometers and lengths of several nanometers to micrometers. They have been known to possess outstanding thermal, electrical, and mechanical properties. The functionalization of CNTs can improve their dispersibility in solvents and can enhance their interaction with other materials thereby increasing their scientific usage.7–9 Some production methods such as chemical vapor deposition (CVD), arc discharge, laser ablation, electrolysis, and hydrothermal synthesis have been developed for large-scale manufacturing of CNTs.8,10–12 The magnitude of properties and size of CNTs is usually dependent on the synthesis method, purification strategy, and post-synthesis functionalization. CNTs have been extensively researched worldwide for their applications in materials sciences,13,14 electrochemistry,15,16 electronics, superconductors,17–19 catalysis,20,21 energy storage,22 water and environmental remediation,23–26 textiles,27 biosensors,28–31 biomedicine and drug delivery,32–34etc.Biosensing is an emerging area in different fields of contemporary research. Biosensors are powerful analytical devices for the detection of analytes in a myriad of applications including environmental remediation, clinical diagnosis, biomedicine, healthcare, agriculture, food industry, pharmaceuticals, industrial processing, and monitoring.35–38 Biosensors are widely popular because of their amazing features such as high selectivity towards the target analyte, short detection time, ultralow detection limits, point-of-care testing, the potential to be miniaturized for portable use, and require less sample processing procedures as compared to conventional analytical techniques.39 The most significant trend in sensor research during the last decade is the application of nanomaterials in biosensor fabrication. Nanomaterials, such as carbon nanotubes, metallic nanoparticles, semiconductor quantum dots, nanocrystals, nanowires, and carbon-based nanomaterials including CNTs, graphene, and carbon dots, have been exploited for the construction of high throughput biosensors with practical utility.37,40–45
Among all the applications of carbon nanomaterials, CNT-assisted biosensing platforms are garnering significant attention for real-time applications in sensing. The chemical, physical, biological, and mechanical properties of CNTs make them one of the smart nanomaterials for the development of robust sensing systems for environmental monitoring and analytical chemistry. CNT materials show prominent use as biorecognition elements, reaction supporters, or carriers in different sensors.46,47 Different surface functionalities have been achieved by functionalizing the surface of CNTs through chemical adsorption. For instance, CNTs can serve as good platforms for conjugating other molecules via exohedral functionalization, while in the case of endohedral functionalization, the CNT's walls can be opened and filled with metals or organic materials.48 Non-covalent functionalization of CNTs can be attained either through sonication or π–π interactions. Functionalized CNTs are known to cross biological barriers including cell membranes and can enter the cells. This process of internalization and release of CNTs in the cells is of particular interest in biology and biosensor research.49 Moreover, several studies suggested that functionalized CNTs can resolve issues related to biocompatibility and toxicity.50–53
MWCNTs can be easily modified by hydroxyl, amino, and carboxyl groups for conjugation with biomolecules to develop immunosensors, aptasensors, and enzymatic sensors. MWCNTs have been explored as appropriate carriers for immobilizing antibodies and aptamers.54–58 Enzyme immobilization of MWCNTs can also be performed using physical adsorption, with the assistance of surfactants, or direct covalent linking.59 Recently, the combination of MWCNTs and gold nanoparticles (AuNPs) to create a hybrid composite (MWCNT–AuNP) has also been researched for applications in biosensing.60–64 AuNPs can be directly conjugated to MWCTNs by physical absorption without any chemical bonding between the two counterparts, or they can be chemically linked together to produce a stable structure through covalent bonding via π–π stacking and hydrophobic and electrostatic interactions.65 These materials have been particularly utilized in sensing applications for the detection of biomolecules, gases, food contaminants, and water pollutants.
The present review aims to document the recent development in the applications of MWCNT-based biosensors. Great efforts have been made in scientific research in which MWCNTs have been conjugated with antibodies, aptamers, and enzymes to develop immunosensors, aptasensors, and enzymatic sensors. Also, the exploration of novel advancements for developing functionalized AuNPs and MWCNTs with their utilization for biosensing is a dynamic research territory. Further, the review article focuses on developments of MWCNT–AuNP composites for biosensing applications in environmental remediation, food analysis, and others.
2. Significance of carbon nanotubes in biosensing applications
CNT-based biosensors are proving to be very beneficial in analytical sensor development. The construction of a simple CNT-based biosensor requires two components: the biorecognition element and the transducer. The CNT's surface is modified with bio-elements (such as proteins, antibodies, aptamers, enzymes, cells, etc.) working as the biological receptive part. The transducer converts the concentration of analyte used to a measurable physical signal including optical and electrochemical signals. The purification of CNTs after the synthesis process needs treatment with oxidizing acids that can create carboxyl moieties on the surface of CNTs. These carboxyl functionalities can further provide sites for possible covalent functionalization of CNTs to a bio-recognition element. CNTs are mostly used as working electrodes in biosensors due to their astonishing low detection limit. The successful performance of CNT-based biosensors requires the optimization of various physicochemical properties, along with their surface functionalization and immobilization.The use of MWCNTs in sensor development has its advantages and disadvantages as compared to the use of SWCNTs. MWCNTs are known to show better mechanical strength and stability than SWCNTs, which is an important point to be considered during sensor development.66–68 Since the performance of the sensing material is dependent on the material structures, such material should possess high mechanical and thermal stability.69 Hence, MWCNTs offer a greater opportunity to construct sensors with good performance and stability. Furthermore, MWCNTs are cheaper to produce in bulk synthesis without the use of any catalyst material. Also, they can be integrated with a suitable polymer or composite to enhance the mechanical, physical, and chemical properties. On the other hand, SWCNTs generally produced in the presence of catalysts are of poor purity. From the manufacturing point of view, MWCNTs are therefore preferred over SWCNT biosensors. Overall, it can be deduced that SWCNTs are more expensive and are often less pure than MWCNTs.70 Besides, it is also observed that MWCNTs show better performance in rogue and corrosive environments. They can be therefore well suited in complex and real-world samples without any effect on sensor sensitivity and performance. However, there are certain applications, where SWCNTs are more preferred over MWCNTs. For instance, SWCNTs are more efficient in drug delivery applications since they have an ultra-high surface area and good drug loading capacity than their multi-walled counterparts.71–73 Due to their amazing electronic properties, SWCNTs have gained great attention in electrochemical biosensors.
SWCNT-derived biosensors are known to have limited specific surface area to interact with larger bio-elements like mammalian cells, have an uncontrolled manufacturing process, and undergo chemical modification.74 Moreover, the insolubility of SWCNTs can largely restrict their importance in biological and biomedical sensors. To improve the solubility of SWCNTs in aqueous solutions, nanocomposites with biocompatible properties have been particularly designed.75 MWCNTs can be used as the modified scaffolds for electrodes. The electron transfer process in MWCNTs is greater with excellent conduction and electro-catalytic characteristics. Many of the enzymatic biosensors have incorporated MWCNT-modified electrodes as multifunctional scaffolds.76–78 The surface of MWCNTs can be chemically modified with –SH groups, –OH groups, and –COOH groups to improve the uniformity of film on the electrode surface. MWCNTs also provide a better platform for immobilizing different biomolecules that can exhibit complementary activities.47,74 Therefore, MWCNT biosensors have proved to be more promising in sensor fabrication.
There are some key advantages that CNTs have over graphene oxide (GO) and graphdiyne (GDY) for use in biosensors. One of the biggest advantages of CNTs is their high electrical conductivity. This makes them ideal for use in electrochemical biosensors, where they can be used to transfer electrons between the biomolecule and the electrode.15 GO and GDY are also electrically conductive, but not to the same extent as CNTs. This can lead to slower electron transfer rates and lower sensitivity in electrochemical biosensors.16,79 CNTs are very strong and lightweight. This makes them ideal for use in portable and wearable biosensors. CNTs have a high aspect ratio, which means they are long and narrow structures.80,81 This property allows for a large surface area and enhances their interaction with biomolecules, increasing the sensitivity of biosensors, as compared to GO- and GDY-based sensors.82 The tailored electronic properties and structural diversity of GDY hold promise for future biosensor development, although it is less explored compared to CNTs and GO.
There are still some challenges that need to be addressed before CNTs can be widely used in biosensors. The main challenge is that CNTs can aggregate in solution.83 This can make it difficult to disperse CNTs evenly in the sensing matrix and can lead to decreased sensitivity of the biosensor. They are also susceptible to oxidation reactions.83 This can lead to changes in their electrical properties and can reduce the sensor's performance and lifespan. The cost of CNTs can be a barrier to their widespread and large-scale use in biosensors. The fabrication of biosensors often requires CNTs of specific sizes and helicities. However, it is difficult to control the size of CNTs during manufacturing.84 Additionally, it is challenging to produce CNTs that are both cost-effective and high-purity at scale. As a result, the current market prices of CNTs are too high for most commercial applications. The reproducibility of CNT-based biosensors can be a challenge. This is because the properties of CNTs can vary depending on the manufacturing process.85 New manufacturing methods that can produce CNTs with more consistent properties should be developed. Despite these challenges, CNTs have the potential to be a valuable material for biosensing applications. The development of CNT-based biosensors have many different aspects which require cooperation between materials scientists and engineers who fabricate the biosensors.
3. Bioconjugation of multi-walled carbon nanotubes and applications
Different categories of MWCNT-biosensors have been designed to date to attach MWCNTs with biological elements such as DNA, proteins, antibodies, aptamers, and enzymes (Fig. 1). The majority of these biosensors are based on electrochemical detection that comprises the reference electrode, working electrode, and counter electrode. To completely take advantage of the MWCNT materials in biosensing, the MWCNTs must be properly functionalized and immobilized for better solubility, biocompatibility, and functionality. In the following section, different sensors based on the bioconjugation of antibodies, aptamers, and enzymes with MWCNTs are discussed with a special focus on their sensing applications (Table 1).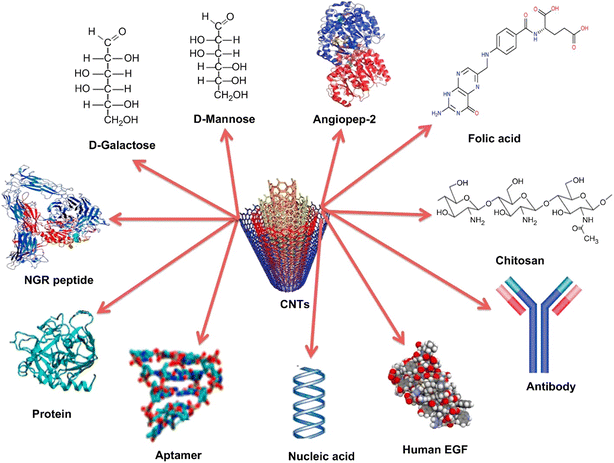 | ||
| Fig. 1 Various biological molecules that can be conjugated to CNTs in the construction of biosensors. Reprinted with permission from Mehra et al.86 Copyright© Elsevier. | ||
| Order | Sensor material | Types of biosensor | Analyte | LOD | Sensitivity (range) | Ref. |
|---|---|---|---|---|---|---|
| 1. | MWCNTs/Fe3O4 | Immunosensor | PSA | 0.39 ng mL−1 | 2.5 pg mL−1 to 100 ng mL−1 | 87 |
| 2. | MWCNTs/cobalt phosphide | Immunosensor | Carcinoembryonic antigen | 10 fg mL−1 | 10−4–100 ng mL−1 | 88 |
| 3. | MWCNTs/Si3N4 | Immunosensor | CYFRA21-1 | 2 fg mL−1 | 0.01–1 pg mL−1 | 89 |
| 4. | MWCNTs | Immunosensor | Alpha-feto protein | 1.1 ng mL−1 | 10 ng mL−1–50 μg mL−1 | 90 |
| 5. | MWCNTs/PDDA | Immunosensor | Aflatoxin B1 | 0.03 ng mL−1 | 0.05–25 ng mL−1 | 91 |
| 6. | MWCNTs/Fe3O4/chitosan | Immunosensor | Carbohydrate antigen | 0.163 pg mL−1 | 1 pg mL−1–100 ng mL−1 | 92 |
| 7. | MWCNTs/L-glutamic acid | Aptasensor | Tetracycline | 3.7 × 10−17 M | 10−16–10−6 M | 93 |
| 8. | MWCNTs/Ce-MOF | Aptasensor | Zearalenone | 10−5 ng mL−1 | 5 × 10−5–50 ng mL−1 | 94 |
| 9. | MWCNTs/Au nanoshell | Aptasensor | Profenofos | 0.052 ng mL−1 | 0.1–1 × 105 ng mL−1 | 95 |
| 10. | MWCNTs/Nafion | Aptasensor | Ochratoxin A | 1 pg mL−1 | 0.005–10 ng mL−1 | 96 |
| 11. | MWCNTs/Ni–Fe LDH/BiVO4 | Aptasensor | Ofloxacin | 0.03 nM | 0.1–16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 nM 000 nM |
97 |
| 12. | MWCNTs/MoS2 | Aptasensor | Kanamycin | 0.21 pg mL−1 | 10−3–103 ng mL−1 | 98 |
| 13. | MWCNTs/TiO2 | Aptasensor | Malachite green | 8.68 pg mL−1 | 0.01–1000 ng mL−1 | 99 |
| 14. | MWCNTs/chitosan | Aptasensor | Exosomes | 1 particle per mL | 10–2 × 1010 particles per mL | 100 |
| 15. | MWCNTs/polyvinyl alcohol | Enzymatic sensor | Glucose | 0.15 μM | 0.1–20 mM | 101 |
| 16. | MWCNTs/copolymer | Enzymatic sensor | Glucose | 0.36 μM | 1 μM–5 mM | 102 |
| 17. | MWCNTs/nickel–cobalt | Non-enzymatic sensor | Glucose | — | 0–1.25 mM | 103 |
| 18. | MWCNTs/WS2 | DNA biosensor | Hepatitis B viral genome | 2.5 fM | 10 fM–1 nM | 104 |
3.1 Immunosensor development
Immunosensors are analytical devices that utilize the immunochemical recognition between antigens and antibodies to selectively detect several biomolecules such as proteins, hormones, and drugs. The detection is based on ligand affinity where the antibody interacts with the antigen to produce different signals on the base material and this signal is measured by the transducer.105,106 Immunosensor technology is gaining high prominence in clinical medicine while diagnosis is also becoming an important area of research. An electrochemical immunosensor combines the immunological reaction between antibody–antigen and the electrochemical technology to produce high-performance sensors with fast detection time and high precision as compared to traditional immunoassays.107,108 MWCNTs are often coupled with antibodies to develop immunosensors. To improve the sensor sensitivity of MWCNT-based immunosensors, scientists have coupled MWCNTs with metal or organic materials to produce nanocomposites. The combination of metallic NPs, bimetallic NPs, or metal oxide NPs with MWCNT-modified electrodes can largely enhance detection sensitivity. Apart from that, MWCNTs can be modified with functional groups (amino, hydroxyl, etc.), magnetic nanoparticles (MNPs), ionic liquids, mesoporous silica, fullerenes, and graphene to develop highly sensitive immunosensors.109 Electrochemical immunosensors based on MWCNTs have been developed to recognize cancer biomarkers,88,89,110,111 biomolecules,90,112 clinical diagnosis,92,113 microbial pathogens and toxins,91,114,115 antibiotics,116 and environmental pollutants.117,118 Electrocatalytic labels (nanocatalysts) are also being utilized for fabricating sandwich-type electrochemical immunosensors due to the generation of high catalytic current to improve the sensor sensitivity. MWCNTs can be utilized as signal labels and can provide a large surface area for the immobilization of secondary antibodies in sandwich immunosensors. For instance, a sandwich-type immunosensor was proposed for the detection of prostate-specific antigen as shown in Fig. 2. Other sensors have also been fabricated to detect different classes of analytes using the same approach.88,119–121 The main challenges suffered by MWCNT immunosensors are miniaturization of biosensors and cost-effectiveness. In the near future, MWCNTs show a promising approach for fabrication of point-of-care devices for sensing purposes.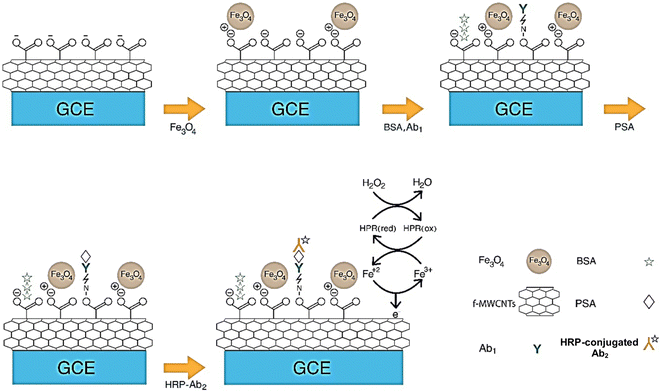 | ||
| Fig. 2 Development of a highly sensitive sandwich-type electrochemical immunosensor using MWCNTs and magnetic NPs for detection of prostate specific antigen (PSA). Reprinted with permission from Shamsazar et al.87 Copyright© 2021 Elsevier. | ||
3.2 Aptasensor development
Aptasensors have gained increasing attention in sensing applications. Aptamers are generally ssDNA molecules that bind to a specific complementary sequence. Aptamers can efficiently compete with antibodies as recognition elements. DNA aptamers are more stable and easier to synthesize with high selectivity. MWCNTs offer many opportunities for the development of electrochemical as well as optical aptasensors based on aptamer assembly.122,123 Aptamer immobilization on the surface of MWCNTs can provide a better sensitive signal and less noise. Aptamer immobilization is usually based on three mechanisms: physical adsorption, covalent bonding, and affinity binding.57,124 Recently, electrochemical aptasensors developed with MWCNT composites have been utilized in the detection of microbial pathogens and toxins,56,94,125–127 bisphenol A,128–130 pesticides,95,131 antibiotics,93,132–134 and biomolecules.135–139 MWCNT-based aptamers provide certain advantages such as good sensitivity, portable nature, rapid detection, simple instrumentation, and low fabrication costs, as compared to traditional immunoassays.3.3 Enzymatic sensor development
Enzymatic sensors based on MWCNTs are a popular class of biosensors in which the tubular structures of MWCNTs provide a large surface area that can be efficiently used to immobilize enzyme molecules. This can largely improve the response signal of biosensors. These sensors utilize the biomolecular conjugation and specificity of the enzyme to direct electrochemical reactions between the enzymes and bulk CNT materials.59,140,141 There have been several studies published on MWCNT-based enzymatic sensors developed with glucose oxidase (GO), horseradish peroxidase (HRP), tyrosinase, cholesterol oxidase (CO), cholesterol esterase (CE), alkaline phosphatase, acetylcholinesterase, urease, lactate oxidase, dihydrofolic acid reductase (DHFR), etc. A number of these enzymes catalyze substrate reactions including glucose, lactate, cholesterol, amino acids, urate, pyruvate, glutamate, alcohol, folic acid, and hydroxybutyrate to produce products such as NADH and hydrogen peroxide that can be detected electrochemically.142–145 An enzyme can be immobilized on MWCNTs via covalent bonding, physical adsorption, cross-linking, and even entrapment. Physical adsorption between the enzyme and MWCNTs can be achieved by one of the interactions such as hydrogen bonding, van der Waals, hydrophobic, hydrophilic, or ionic interactions. In this method, there are high chances of the release of the enzyme from the material support due to weaker bonds.146,147 In cross-linking, the enzyme is linked to support material (i.e. CNTs) via cross-linking agents (glutaraldehyde, glyoxal, epichlorohydrin, and 1-ethyl-3-(3-dimethyl aminopropyl) carbodiimide (EDC)) together with N-hydroxysuccinimide (NHS) to enhance attachment.148 The covalent bonding leads to a stronger attachment between the enzyme and the carrier.149 The majority of prior research has applied the application of MWCNT-enzymatic sensors in sensing glucose,101–103 cholesterol,62,150,151 urea,152,153 catechol,154etc.3.4 DNA biosensor development
DNA biosensors have been particularly used in clinical diagnosis, medical sciences, forensics, drug discovery, and testing of genetic and infectious diseases. In sensor development, the nucleic acid (dsDNA) can adsorb strongly on the MWCNT surface and hence can be used to construct MWCNT–DNA bio complex for sensing applications in many scientific areas.155,156 DNAs are ideal biorecognition elements. DNA biosensors are used for the detection of metal ions, small metabolites, organic dyes, peptides, proteins, cancer cells, and pathogenic microorganisms.157,158 The covalent attachment of MWCNTs and DNA in electrochemical biosensors leads to fast electron transfer. Moreover, MWCNTs can also be self-assembled or can be deposited vertically on gold (Au) substrates followed by adsorption of DNA molecules.155,159,160 A literature survey shows that MWCNT–DNA biosensors have been exploited for the detection of DNA methyltransferase and site-specific DNA sequences,161,162 viral genomic DNA,104 miRNA,163 gender determination in fish,164 pirazon,165 bacteria,166 and food contaminants.167,168 Recently, polypyrrole and hydroxyapatite nanoparticles with MWCNTs were used in the construction of an electrochemical DNA biosensor for Mycobacterium tuberculosis.169 The response surface methodology (RSM) operations were performed to optimize the best conditions for maximum performance of the biosensor (Fig. 3). It can be anticipated that the clinical diagnosis and pathology will be largely dependent on the successful development and implementation of DNA biosensors.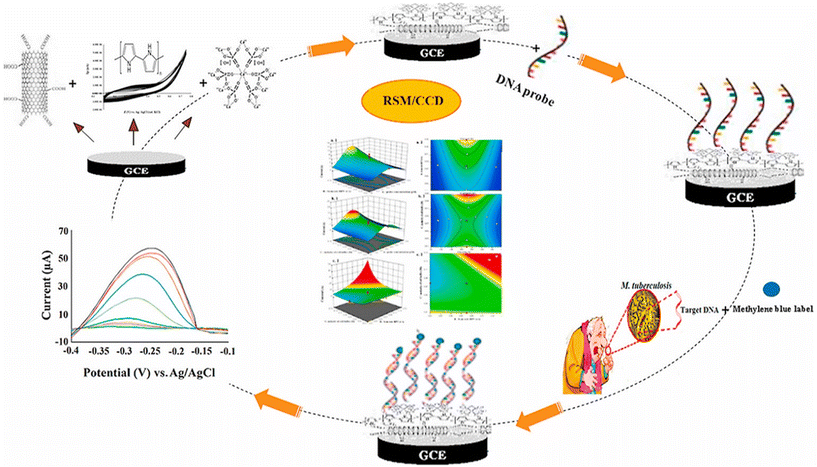 | ||
| Fig. 3 A representation of an electrochemical DNA biosensor for the detection of Mycobacterium tuberculosis, whose conditions are optimized using the RSM technique. Reprinted with permission from Rizi et al.169 Copyright© 2021 Elsevier. | ||
4. Multiwalled carbon nanotube–gold nanoparticle composites (MWCNT–AuNP composites)
Nanocomposites are described as hybrid materials consisting of a nanomaterial incorporated into a mixture with a suitable polymer, matrix, ceramics, inorganic materials, etc. to improve and enhance its properties. The composites of MWCNTs and AuNPs have attained significant attention for applications in biosensors (DNA, proteins, glucose), gas sensors (oxygen, water vapor), and heavy metal sensors.170,171 A detailed discussion on the synthesis and biosensing applications of MWCNT–AuNP composites is provided in the following sections.4.1 Synthesis of MWCNT–AuNP composites
The integration of MWCNTs and AuNPs to generate nanocomposites can generate novel properties not found earlier in their counterparts. The use of AuNPs in these composites provides the advantages of facile synthesis, excellent electrical conductivity, and good catalytic properties. Also, the AuNPs possess unique properties such as strong adsorption ability, good biocompatibility, and conductivity.172,173 Thus, the combination of MWCNTs and AuNPs can aid in the enhancement of immunosensor sensitivity. In addition, the MWCNT–AuNP nanocomposites exhibit huge surface areas to immobilize plentiful biomolecules along with an enhanced electron transfer process. Different methods exist for the synthesis of MWCNT–AuNP composites such as physical adsorption, in situ chemical deposition, and ex situ chemical deposition. The AuNPs can be directly decorated on the CNT support material by physical absorption. In the direct deposition of AuNPs on MWCNTs, the AuNPs are attached to modified or unmodified MWCNTs without any linking molecule. Surface functionalization of MWCNTs can be performed to generate new functional groups (–COOH) on their surface to facilitate the linking process. Physical deposition of AuNPs is generally performed in an ultrahigh vacuum environment and produces MWCNT–AuNP composites with good yield and high purity.174 The direct deposition process possesses some disadvantages including weak bonding between AuNPs and MWCNTs, high chances of detachment, less uniformity, and longer reaction times. In chemical deposition, the in situ procedure deposits AuNPs on CNTs during AuNP synthesis, whereas AuNPs are synthesized before the deposition in the ex situ method.175 Also, wet chemical deposition can be done which involves covalent/non-covalent bonding between the modified MWCNT surface and AuNPs due to hydrophobic, π–π stacking, and electrostatic interactions. Several studies have reported the chemical attachment of AuNPs onto MWCNTs.61,176,177 Furthermore, proteins can also mediate the formation of assembly between AuNPs and MWCNTs to produce various hybrids of MWCNTs.178 One novel approach for producing AuNP decorated MWCNTs was also reported recently using cysteaminium chloride via the formation of a zwitterionic acid–base bond (as shown in Fig. 4).65 Though several methods have been developed for the synthesis of MWCNT–AuNP composites, still there is a need for research on the controlled deposition of Au nanostructures on the CNT surface.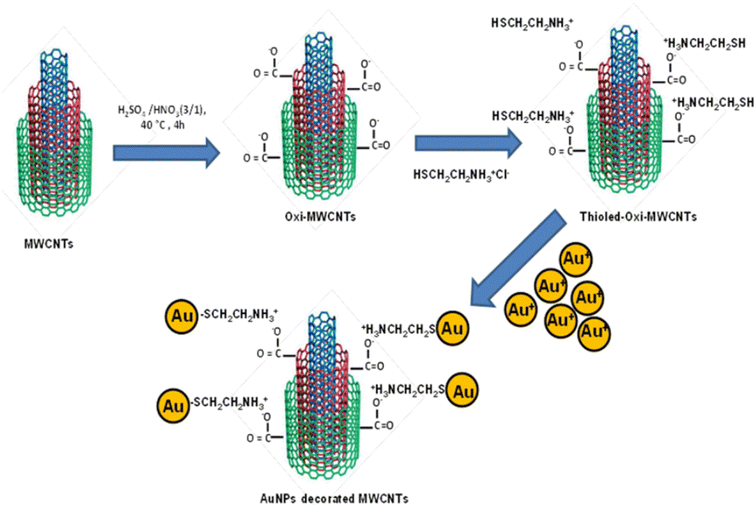 | ||
| Fig. 4 Decoration of MWCNTs with AuNPs using cysteaminium chloride functionalization. Reprinted with permission from Chinh et al.65 Copyright© 2019 Springer Nature. | ||
4.2 Applications of MWCNT–AuNP composites in biosensors
MWCNT–AuNP nanocomposites have been reported for several sensing applications for environmental monitoring and food analysis as discussed in detail in the following sub-sections and summarized in Table 2.| Order | MWCNT- and AuNP-based material | Analyte | LOD | Sensitivity (range) | Ref. |
|---|---|---|---|---|---|
| 19. | MWCNT/AuNC composite | miRNA-155 | 35 pM | 100 pM–2 nM | 179 |
| 20. | Ni-MOF/MWCNTs/AuNPs | HIV DNA | 0.13 nM | 10 nM–1 μM | 180 |
| 21. | MWCNTs/rGONRs-CdS: Eu NCs and Cu@AuNPs | Insulin | 0.04 pg mL−1 | 0.5 pg ml−1–50 ng mL−1 | 181 |
| 22. | Au-MWCNTs-PPy network | Cholesterol | 0.1 mM | 2–8 mM | 62 |
| 23. | MWCNTs–AuNPs/chitosan | Choline | 0.6 μM | 3–120 μM | 182 |
| 24. | MWCNT–EDAS–AuNPs | Dopamine | 0.07 μM | 0.1–9 μM | 183 |
| Ascorbic acid | 0.08 μM | 0.1–8 μM | |||
| 25. | Graphene foam/MWCNTs/AuNPs | Dopamine | 1.36 nM | 0.1–48 μM | 184 |
| Uric acid | 33.03 nM | 0.50–60 μM | |||
| 26. | AuNP decorated MWCNTs | Dopamine | 35 nM | — | 185 |
| 27. | COF/MWCNTs/AuNPs | Dopamine | 0.21 μM | 0.7–108 μM | 186 |
| Uric acid | 0.29 μM | 0.97–200 μM | |||
| 28. | MIP/AuNPs/MWCNTs/GO nanoribbons | 3-Nitrotyrosine | 50 nM | 0.2–50.0 μM | 187 |
| 29. | MWCNT–AuNP/ITO electrode | DJ-1/Park7 | 0.5 fg mL−1 | 4.7–4700 fg mL−1 | 188 |
| 30. | AuNPs/MWCNTs/6-hexanedithiol | Human serum albumin | 15.4 ng mL−1 | 0.1–100 μg mL−1 | 189 |
| 31. | AuNPs/MWCNTs/thiol-modified ceramic gel | Uric acid | 50 nM | — | 190 |
| Xanthine | 63 nM | ||||
| Caffeine | 354 nM | ||||
| 32. | AuNPs/Zr-MOF/MWCNTs | Adenine | 0.09 μM | 0.8–60 μM | 191 |
| Guanine | 0.08 μM | 0.8–60 μM | |||
| 33. | Magnetic MWCNT/AuNP/antibody | α-Fetoprotein | 3.33 fg mL−1 | 10 fg mL−1–100 ng mL−1 | 192 |
| 34. | MWCNT decorated AuNPs | TP53 | 10−17 M | 193 | |
| 35. | MWCNTs and AuNPs/HER 2 antibody | HER2 | 7.4 ng mL−1 | 10–110 ng mL−1 | 194 |
| 36. | MWCNTs, AuNPs/calixarenes | Paracetamol | 0.2 μM | 1–150 μM | 195 |
| 37. | Carboxylated MWCNTs/AuNPs | Cyproterone acetate | 1.66 × 10−8 M | 9.9 × 10−8 M −1.15 × 10−5 M | 196 |
| 38. | MIP/MWCNT–AuNP composites | Velpatasvir | 0.21 ng mL−1 | 0.649–80.0 ng mL−1 | 197 |
| 39. | AuNPs/MWCNTs/chitosan nanocomposite | Chlorpyrifos | 0.06 × 10−6 mg mL−1 | 0.1–40 × 10−6 mg mL−1 | 198 |
| 40. | Polyaniline/MWCNTs/AuNPs | Zn2+ | 0.039 μg L−1 | — | 199 |
| Pb2+ | 0.037 μg L−1 | ||||
| Cu2+ | 0.017 μg L−1 | ||||
| 41. | PANI/MWCNTs/AuNPs | Hg2+ | 0.08 ppm | 0.01–10 ppm | 200 |
| 42. | Chitosan/MWCNTs@GONRs/GCE | Dibutyl phthalate | 7 ng mL−1 | — | 201 |
| 43. | AuNPs/MWCNTs/hydrogel | 4-Nitrophenol | — | 1 × 10−8–5 × 10−5 M | 202 |
| 44. | Graphene/MWCNTs/AuNPs | Nitrite | 0.9 μM | 10–140 μM | 203 |
| 45. | MWCNT/copper–polyaniline/AuNPs | Nitrate | 0.09 μM | 0.8–30 μM | 204 |
| 46. | AuNPs–MWCNTs–chitosan nanocomposite | Salmonella typhimurium | 5 × 102 CFU mL−1 | 103–107 CFU mL−1 | 205 |
| 47. | MWCNTs, AuNPs/MIP | Diethylstilbestrol | 24.3 fg mL−1 | 10−10 to 10−6 mg mL−1 | 206 |
| 48. | MWCNTs/AuNPs/anti-ZEA antibody | Zearalenone | 0.15 pg mL−1 | 10−4–10−1 ng mL−1 | 207 |
| 49. | Polyethyleneimine/MWCNTs/AuNPs | Kidney bean | 0.023 μg mL−1 | 0.05 to 100 μg mL−1 | 208 |
| Lectins | |||||
| 50. | p-Aminothiophenol/MWCNTs/AuNPs | Quercetin | 3.3 × 10−10 M | — | 209 |
| Rutin | |||||
| 51. | AuNPs/MWCNTs | Tetracycline | 42 ppb | 0.2–0.6 ppm | 210 |
| 52. | MWCNTs, AuNPs, chitosan, and GO | Sunset yellow | 0.032 mg mL−1 | 10–90 mg mL−1 | 211 |
| 53. | MWCNTs/graphene/AuNPs | Antioxidants | — | — | 212 |
| Glucose | |||||
| Alcohol | |||||
| 54. | Prussian blue/MWCNTs/chitosan–AuNPs cryogel | Histamine | 1.81 μM | 2.50–125.0 μM | 213 |
| 55. | Fe3O4/MWCNT-COOH/AuNP-AFP antibodies | AFP | 1.09 pg mL−1 | 1 pg mL−1–10 μg mL−1 | 214 |
| 56. | AuNPs/MWCNT nanocomposites | Cell viability and drug evaluation (exocytosis dopamine sensing) | — | — | 215 |
| 57. | MWCNTs/AuNPs-acetaminophen | E. coli | 3.02 CFU mL−1 | — | 216 |
Detection of certain biomolecules is significant for early disease diagnosis and therapy (like insulin, cholesterol, and uric acid) has also been reported using the MWCNT–AuNP nanocomposite. For instance, an electro-chemiluminescent sensor for insulin was designed using MWCNTs/rGONRs-CdS: Eu NCs as the ECL signal generators and copper-modified AuNPs as the quenchers.181 The intensity of the ECL signal reduced with a corresponding increase in insulin concentration within the linear range of 0.5 to 50 ng ml−1 and a detection limit of 0.04 pg mL−1. Another study reported a voltammetric enzymatic biosensor using cholesterol oxidase enzyme immobilized MWCNTs as shown in Fig. 5.62 The sensor presented a sensitivity of 10.12 μA mM−1 cm−2 and a LOD of 0.1 mM. In another work, the detection of choline was reported for monitoring brain-related disorders. The amperometric sensor was developed through the deposition of MWCNTs and AuNPs on an electrode.182 Chitosan polymer was used to disperse MWCNTs followed by immobilization of the choline oxidase enzyme via glutaraldehyde linking. The sensor showed a LOD of 0.6 μM and a linear detection range from 3 to 120 μM. Also, silicate molecule-capped AuNPs and functionalized MWCNT (MWCNT–EDAS–AuNP) network nanocomposite was used for the simultaneous detection of dopamine and ascorbic acid.183 Another study developed an electrochemical sensor using graphene foam/MWCNT/AuNP composite-modified electrodes.184 The developed sensor was employed for the detection of dopamine in brain tissue and uric acid in human urine with high sensitivities. Similarly, a dopamine sensor was designed using AuNP decorated MWCNT composites as the modifier for GCE, with a detection limit of 35 nM.185 Recently, Guan et al. demonstrated an electrochemical sensor comprising a covalent organic framework, amino-modified MWCNTs, and AuNPs modified on GCE.186 The practical application of this sensor was demonstrated in the detection of dopamine and uric acid with LOD of 0.21 and 0.29 μM, respectively. Moreover, a screen-printed electrode biosensor based on AuNP/MWCNT nanocomposites has also been reported for exocytosis dopamine detection. The applicability of this system was also demonstrated as an evaluation tool for cell viability and drug activity evaluation.215
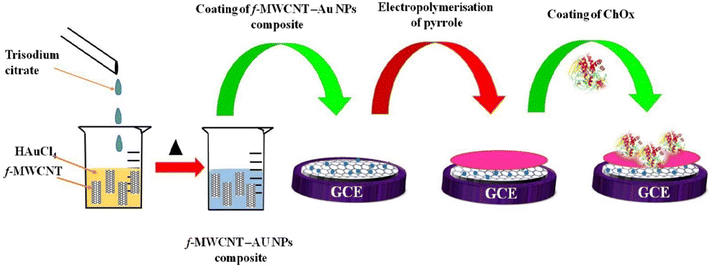 | ||
| Fig. 5 A cholesterol biosensor synthesized from immobilization of cholesterol oxidase enzyme (ChOx) on AuNP modified MWCNT-PPy electrode. Reprinted with permission from Alagappan et al.62 Copyright© 2020 Elsevier. | ||
3-Nitrotyrosine is a crucial protein related to the pathology of many diseases such as Alzheimer's disease, Parkinson's disease, and other cardiovascular diseases. A highly sensitive electrochemical sensor for 3-nitrotyrosine was constructed using electropolymerized pyrrole MIP doped with AuNPs modified on a GCE modified with MWCNTs and graphene oxide nanoribbons.187 The sensor produced a linear detection range from 0.2 to 50.0 μM with an LOD of 50 nM. Recently, an electrochemical neuro biosensor for the detection of protein DJ-1/Park7 was constructed using MWCNT–AuNP composite doped indium tin oxide electrode.188 The sensor was successfully checked for the presence of DJ-1/Park7 in cerebrospinal fluid and saliva.
Detection of human serum albumin (HSA), the most abundant protein in human serum, is necessary for clinical diagnosis and pre-treatment of various diseases. An impedimetric immunosensor was designed using the electrodeposition of AuNPs on an MWCNT-ionic liquid electrode using a 6-hexane dithiol (HDT) monolayer as a cross-linker.189 The detection range and LOD were found to be 0.1–100 μg mL−1 and 15.4 ng mL−1, respectively.
Determination of purines and their derivatives in body fluids can assess the pathological conditions in humans. In a study, a ceramic carbon electrode was fabricated by incorporating AuNPs and MWCNTs in a thiol-modified ceramic gel.190 This sol–gel sensor was able to detect purine-based compounds such as uric acid (UA), xanthine (XA), and caffeine (CA) because the gel matrix provided a good platform for encapsulating these materials for sensing purposes. The detection limits were determined to be 50, 63, and 354 nM for UA, XA, and CA, respectively. In another study based on AuNP-modified Zr-MOF and carboxyl-functionalized MWCNTs, a voltammetric sensor (Fig. 6) was designed for the detection of purines such as adenine and guanine in DNA.191 The sensor achieved a LOD of 0.09 and 0.08 μM for guanine and adenine, respectively.
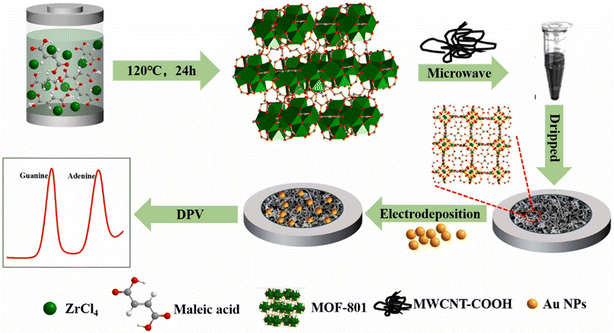 | ||
| Fig. 6 A schematic representation of the fabrication of guanine and adenine sensor using Zr-MOF, carboxylated MWCNTs, and AuNPs. Reprinted with permission from Guo et al.191 Copyright© 2021 Elsevier. | ||
Cancer biomarkers are the indicators of the progression of diseases such as pancreatic, breast, liver, and ovarian cancer. Biomarkers have possible applications in oncology, disease diagnosis, prediction of response to disease, and disease monitoring. One of the important biomarkers is α-fetoprotein found in the serum of patients suffering from liver cancer. The AuNP/CNT hybrid material was developed for amperometric sensing of α-fetoprotein (AFP) with a low detection limit of 0.6 ng mL−1.234 Similarly, a sandwich-type electrochemical immunosensor was fabricated for AFP detection.192 Recently, an advanced Fe3O4/MWCNT-COOH/AuNP-based electrochemical sensor was developed for the detection of trace liver cancer biomarker AFP in the picogram range (LOD 1.09 pg mL−1).214 Magnetic MWCNTs modified with AuNPs were produced and loaded with Pb2+ ions and secondary antibodies. The generated hybrid composite Pb2+@Au@MWCNTs-Fe3O4 produced a linear detection range of 10 fg mL−1 to 100 ng mL−1 and a LOD of 3.33 fg mL−1. TP53 is an important early diagnostic cancer marker. In a study, AuNPs grown on aligned MWCNTs served as a label-free DNA biosensor for the detection of mutations in the TP53 gene.193 The improved performance of the sensor was attributed to highly synergistic interactions between the MWCNT array and AuNPs. The sensor showed a good response to target DNA related to TP53 mutation detection with a low LOD of 10−17 M. The HER2 gene levels in the serum of patients can be beneficially used in the early detection of breast cancer. In this regard, an impedimetric immunosensor was fabricated by using MWCNTs and AuNPs decorated on an ionic liquid electrode.194 The monoclonal HER2 antibody was immobilized on AuNPs/MWCNTs and the antigen–antibody interactions were measured through impedance response. The sensor produced a low LOD of 7.4 ng mL−1 for HER2 detection.
There has been a continuous need for the analysis of pharmaceutical compounds and drugs. Monitoring paracetamol (PCM) levels in biological matrices is important for its regulation. In this context, an electrochemical sensor was reported based on GCE modified with MWCNTs, AuNPs, and calixarenes.195 The electrode showed good electrocatalytic activity and high electrochemical response toward PCM with a detection limit of 0.2 μM. In another study, the detection of cyproterone acetate (CPA) drug in pharmaceutical and body fluids was studied with a voltammetric sensor based on carboxylated MWCNT and AuNP modified carbon paste electrode.196 The sensor produced a linear response with CPA concentration in the range from 9.9 × 10−8 to 1.15 × 10−5 M with an LOD of 1.66 × 10−8 M. Another report successfully synthesized a novel MIP and MWCNT–AuNP composite-based structure for the analysis of velpatasvir drug in body fluids.197 The developed material showed a 3D starfish-like hollow skeleton that could detect VELPR with an LOD of 0.21 ng mL−1. As already discussed, studies on applications of MWCNT–AuNP composite in biomolecules and protein detection are well documented.
Pesticide residues are a huge threat to human health causing acute and chronic toxicity. Detection of pesticide residues in the environment is a serious concern for environmental protection. Chlorpyrifos is a common organophosphate pesticide extensively used in agricultural practices but harmful to human health. Detecting chlorpyrifos is therefore important for promoting environmental safety. In this context, an electrochemical multilayered immunosensor was designed with AuNPs and MWCNTs/chitosan nanocomposite for chlorpyrifos sensing.198 AuNPs were used as a platform for the immobilization of chlorpyrifos antibody. Under optimal conditions, the immunosensor displayed a wide linear range from 0.1 to 40 × 10−6 mg mL−1 with a detection limit of 0.06 × 10−6 mg mL−1. Detection of heavy metals in environmental samples has also been reported using screen-printed carbon electrodes modified with polyaniline/MWCNT/AuNP composite.199 The sensor was further utilized for simultaneous sensing of Zn2+, Pb2+, and Cu2+ ions using anodic stripping voltammetry with defection limits of 0.039, 0.037, and 0.017 μg L−1, respectively, as shown in Fig. 7. Also, a recent study reported the synthesis of PANI/MWCNT/AuNP composites for electrochemical detection of mercury in cosmetic products using methylene blue as a redox indicator.200
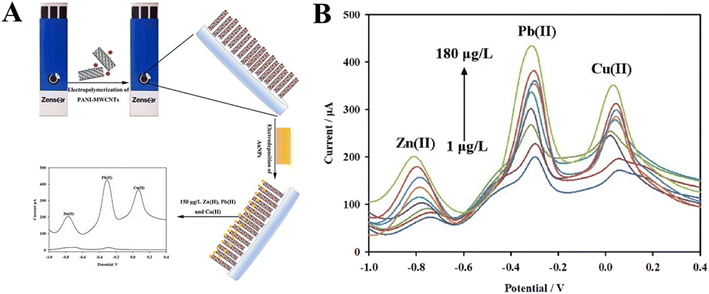 | ||
| Fig. 7 Electrochemical detection of heavy metal ions (Zn2+, Pb2+, and Cu2+) using AuNP/polyaniline–MWCNT modified carbon electrode. (A) Construction of the sensing platform. (B) Stripping voltammograms for various concentrations of Zn2+, Pb2+, and Cu2+. Reprinted with permission from Shao et al.199 Copyright© 2021 Elsevier. | ||
Furthermore, there has been a certain focus on the analysis of emerging contaminants in the environment. For example, dibutyl phthalate (DBP) is present in the environment as a toxic compound found in trace levels. It is produced by the excessive use of plastics and food packaging materials. A study reported a novel impedimetric immunosensor for DBP detection by utilizing chitosan/MWCNTs@GONRs/GCE-induced signal amplification with a good detection limit of 7 ng ml−1.201 Also, a hydrogel nanocomposite mixed with AuNPs and MWCNTs was prepared for the electrochemical sensor in the detection of 4-nitrophenol (4-NP) with good sensitivity.202 Similarly, nitrite and nitrate are prominent environmental contaminants mainly produced due to the excessive use of nitrogen-containing fertilizers. Recently, a graphene-based electrode modified with MWCNTs and AuNP films was examined for the detection of nitrite in water samples ranging from 10 to 140 μM with a detection limit of 0.9 μM.203 The sensor electrode oxidized nitrite at low potential and higher currents, thereby making it insensitive to the presence of common interfering ions. Also, a nanocomposite based on electrosynthesis of AuNPs on MWCNT/copper–polyaniline (Cu–PANI) was effectively examined for the presence of nitrate ions in environmental samples with a good LOD of 0.09 μM.204 All these studies have shown that the MWCNT–AuNP composites have great potential in the screening of environmental pollution.
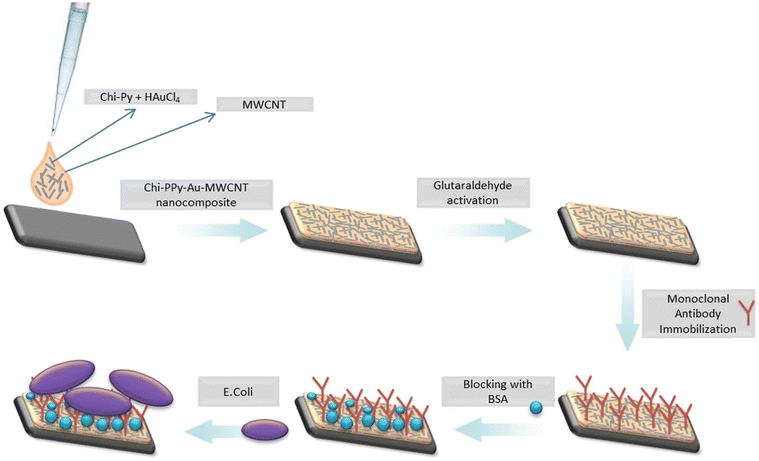 | ||
| Fig. 8 Fabrication of an electrochemical immunosensor for sensing E. coli by using a nanocomposite made of PPy/AuNP/MWCNT/chitosan. Reprinted with permission from Güner et al.115 Copyright© 2017 Elsevier. | ||
Diethylstilbestrol (DES) is a synthetic estrogen that is generally administered to meat and milk-producing animals. Analysis of DES residues is important for preventing its toxicity. In a study, a glassy carbon electrode was developed by decorating MWCNTs and AuNPs, electroplated by sol–gel MIP.206 The sensor produced differential pulse voltammetric response with the addition of DES in the detection range of 10−10 to 10−6 mg mL−1. The sensor produced highly selective and sensitive results for the detection of DES in milk.
Zearalenone (ZEA) contamination can occur in food products including cereals, mainly maize, and manufactured foods. A study was conducted to determine ZEA by using carbon screen-printed electrodes modified with AuNPs (attached with anti-ZEA antibody), and MWCNT dispersions.207 The sensor was used in the amperometric detection of ZEA at an applied potential of −0.3 V. The developed sensor showed a LOD of 0.15 pg mL−1.
Kidney bean lectins (KBLs) are carbohydrate-binding proteins, whose quantification is important for monitoring the allergic activity of KBL in foods. A study developed an immunosensor for highly sensitive detection of KBL activity.208 The composite of polyethyleneimine functionalized MWCNTs and AuNPs coated on a GCE was immobilized with KBL antibodies. Under optimum conditions, the immunosensor extended a good linear response with KBL from 0.05 to 100 μg mL−1 and LOD of 0.023 μg mL−1.
Detection of polyphenolic compounds such as flavonoids in food products is important for assessing food quality and safety. AuNPs functionalized on p-aminothiophenol modified MWCNTs were coated on a glassy carbon electrode (GCE) for simultaneous detection of quercetin and rutin in fruit samples.209 Besides phenolic compounds, monitoring the levels of antibiotics in food products is important due to the adverse effects of antibiotics on humans. Palisoc et al. fabricated a highly sensitive tetracycline sensor by modification of GCE by AuNPs and MWCNTs via electrodeposition technique.210 This electrode was employed as a working electrode in DPV to analyze tetracycline residues in organic and non-organic chicken with an LOD of 42 ppb.
Food colorant dyes have been widely used in the food industry to impart color and texture to food products. However, they may cause allergies, anxiety, diarrhea, and even cancer. Detection of food colorants is necessary to prevent their hazardous effects. A biosensor based on the modification of electrodes with MWCNTs, AuNPs, chitosan, and GO was synthesized for sensing sunset yellow (SY) in foodstuffs.211 The sensor employed a differential pulse voltammetric method for the detection of SY with an LOD of 0.032 mg mL−1 and was successfully validated with candy and soft drink samples.
Bioenzymatic sensors can be developed using different enzymes and can be applied in food analysis. For instance, a study reported the application of ionic liquids as media for electrode materials for the development of disposable amperometric biosensors.212 The electrodes modified with MWCNTs, graphene, and AuNPs were modified with lipase, glucose oxidase, or alcohol dehydrogenase for sensing antioxidants, glucose, and alcohol in foodstuffs. Similarly, a study reported that the histamine levels in seafood can be detected by an enzymatic biosensor comprising a screen-printed carbon electrode (Fig. 9) modified with Prussian blue electrodeposited on MWCNTs covered with chitosan–AuNP cryogel.213 It achieved a good LOD of 1.81 μM and could detect histamine in fish and shrimps.
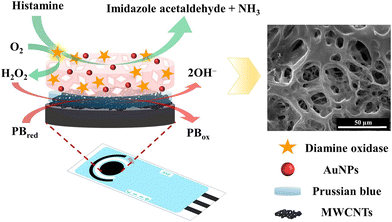 | ||
| Fig. 9 Development of histamine biosensor using screen-printed carbon electrode modified with chitosan–AuNPs-cryogel on Prussian blue-coated MWCNTs. Reprinted with permission from Nontipichet et al.213 Copyright© 2021 Elsevier. | ||
5. Conclusion and future perspective
The present review provides a comprehensive discussion regarding the biosensing applications of MWCNT-based nanocomposites for the detection of several analytes. The combination of two materials MWCNTs and AuNPs in nanocomposites imparts many benefits such as large surface area, tuneability, rich surface functionality, and chemical stability, that can be effectively introduced into the biosensor systems for detecting many analytes including biomolecules, proteins, environmental pollutants, food toxins, etc. Different methods such as direct attachment of AuNPs on MWCNTs, or wet chemical deposition for fabricating MWCNT–AuNP composites have been developed, but still there should be research on the controlled deposition of AuNPs on the MWCNT surface and a uniform and efficient distribution of AuNPs on the MWCNTs. Furthermore, there should be enough room for modification and advancements of these composites so that several other nanomaterials can be attached to them to produce a better synergic effect. There should be a focus on the synthesis of multifunctional AuNPs and MWCNTs with diverse sizes for sensing multiple analytes with high selectivity and sensitivity. Additional applications need to be explored based on the characteristics of these two nanomaterials and the nanocomposites.Author contributions
Sandeep Kumar: conceptualization, review roadmap, investigation, writing – original draft, review & editing. H. K. Sidhu: review roadmap, critical investigation, writing, review & editing. Ashok K. Paul: review roadmap, critical investigation, writing, review & editing. Neha Bhardwaj: conceptualization, review roadmap, critical investigation, supervision, writing, review & editing. Neeraj S. Thakur: conceptualization, review roadmap, supervision, project administration, writing, review & editing. Akash Deep: conceptualization, review roadmap, writing, review & editing, supervision, project administration.Conflicts of interest
There is no conflict of interest.Acknowledgements
Neha Bhardwaj acknowledges DST (Department of Science and Technology), New Delhi, for the INSPIRE Faculty grant (Reg. no. IFA18-LSPA 127). Neeraj S. Thakur would like to thank Dr. Vibhuti Agrahari and the University of Oklahoma Health Sciences Center, Oklahoma City, for providing resources and a postdoctoral fellowship.References
- S. Iijima, Carbon nanotubes: past, present, and future, Phys. B, 2002, 323, 1–5 CrossRef CAS.
- S. Iijima, Synthesis of carbon nanotubes, Nature, 1991, 354, 56–58 CrossRef CAS.
- P. K. Babele, M. K. Verma and R. K. Bhatia, Carbon nanotubes: A review on risks assessment, mechanism of toxicity and future directives to prevent health implication, Biocell, 2021, 45, 267 CAS.
- W. Dai and D. Wang, Cutting Methods and Perspectives of Carbon Nanotubes, J. Phys. Chem. C, 2021, 125, 9593–9617 CrossRef CAS.
- N. Gupta, S. M. Gupta and S. Sharma, Synthesis, characterization and dispersion stability of water-based Cu–CNT hybrid nanofluid without surfactant, Microfluid. Nanofluid., 2021, 25, 1–14 CrossRef.
- N. Mohd Nurazzi, M. Muhammad Asyraf, A. Khalina, N. Abdullah, F. A. Sabaruddin, S. H. Kamarudin, S. Ahmad, A. M. Mahat, C. L. Lee and H. Aisyah, Fabrication, Functionalization, and Application of Carbon Nanotube-Reinforced Polymer Composite: An Overview, Polymer, 2021, 13, 1047 Search PubMed.
- M. Alavi, E. Jabari and E. Jabbari, Functionalized carbon-based nanomaterials and quantum dots with antibacterial activity: a review, Expert Rev. Anti-infect. Ther., 2021, 19, 35–44 CrossRef CAS PubMed.
- Z. Abousalman-Rezvani, P. Eskandari, H. Roghani-Mamaqani and M. Salami-Kalajahi, Functionalization of carbon nanotubes by combination of controlled radical polymerization and “grafting to” method, Adv. Colloid Interface Sci., 2020, 278, 102126 CrossRef CAS PubMed.
- B. Verma and C. Balomajumder, Surface modification of one-dimensional Carbon Nanotubes: A review for the management of heavy metals in wastewater, Environ. Technol. Innovation, 2020, 17, 100596 CrossRef CAS.
- D. Janas, From Bio to Nano: A Review of Sustainable Methods of Synthesis of Carbon Nanotubes, Sustainability, 2020, 12, 4115 CrossRef CAS.
- N. Anzar, R. Hasan, M. Tyagi, N. Yadav and J. Narang, Carbon nanotube-A review on Synthesis, Properties and plethora of applications in the field of biomedical science, Sens. Int., 2020, 1, 100003 CrossRef.
- M. V. Kharlamova and D. Eder, Carbon Nanotubes: Synthesis, Properties, and New Developments in Research, Synthesis and Applications of Nanocarbons, 2020, pp. 107–147 Search PubMed.
- A. Loiseau, P. Launois, P. Petit, S. Roche and J.-P. Salvetat, Understanding carbon nanotubes, Lect. Notes Phys., 2006, 677, 495–543 Search PubMed.
- O. Zhou, H. Shimoda, B. Gao, S. Oh, L. Fleming and G. Yue, Materials science of carbon nanotubes: Fabrication, integration, and properties of macroscopic structures of carbon nanotubes, Acc. Chem. Res., 2002, 35, 1045–1053 CrossRef CAS PubMed.
- S. Alim, J. Vejayan, M. M. Yusoff and A. Kafi, Recent uses of carbon nanotubes & gold nanoparticles in electrochemistry with application in biosensing: a review, Biosens. Bioelectron., 2018, 121, 125–136 CrossRef CAS.
- L. Wang and M. Pumera, Electrochemical catalysis at low dimensional carbons: graphene, carbon nanotubes and beyond–a review, Appl. Mater. Today, 2016, 5, 134–141 CrossRef.
- T. Niu, Carbon nanotubes advance next-generation electronics, Nano Today, 2020, 35, 100992 CrossRef CAS.
- M. Li, Z. Xiong, S. Shao, L. Shao, S.-T. Han, H. Wang and J. Zhao, Multimodal optoelectronic neuromorphic electronics based on lead-free perovskite-mixed carbon nanotubes, Carbon, 2021, 176, 592–601 CrossRef CAS.
- A. P. Leggiero, S. D. Driess, E. D. Loughran, D. J. McIntyre, R. K. Hailstone, C. D. Cress, I. Puchades and B. J. Landi, Platinum nanometal interconnection of copper–carbon nanotube hybrid electrical conductors, Carbon, 2020, 168, 290–301 CrossRef CAS.
- D. Vairavapandian, P. Vichchulada and M. D. Lay, Preparation and modification of carbon nanotubes: Review of recent advances and applications in catalysis and sensing, Anal. Chim. Acta, 2008, 626, 119–129 CrossRef CAS PubMed.
- P. Serp, M. Corrias and P. Kalck, Carbon nanotubes and nanofibers in catalysis, Appl. Catal., A, 2003, 253, 337–358 CrossRef CAS.
- F. Tao, Y. Liu, X. Ren, A. Jiang, H. Wei, X. Zhai, F. Wang, H.-R. Stock, S. Wen and F. Ren, Carbon Nanotube-based Nanomaterials for High-performance Sodium-ion batteries: Recent advances and perspectives, J. Alloys Compd., 2021, 159742 CrossRef CAS.
- Y. Tang, S. Zhang, Y. Su, D. Wu, Y. Zhao and B. Xie, Removal of microplastics from aqueous solutions by magnetic carbon nanotubes, Chem. Eng. J., 2021, 406, 126804 CrossRef CAS.
- R. Wang, D. Chen, Q. Wang, Y. Ying, W. Gao and L. Xie, Recent advances in applications of carbon nanotubes for desalination: A review, Nanomaterials, 2020, 10, 1203 CrossRef CAS.
- M. Selvaraj, A. Hai, F. Banat and M. A. Haija, Application and prospects of carbon nanostructured materials in water treatment: A review, J. Water Process Eng., 2020, 33, 100996 CrossRef.
- D. Kumar, H. Singh, A. Jain, V. Sharma, N. Bhardwaj, S. Puri and M. Khatri, Response surface methodology for removal of copper (II) ions from aqueous solutions by DMSA@ SiO2@ Fe3O4 nanocomposite, Chem. Pap., 2023, 77, 1907–1920 CrossRef CAS.
- R. Kumar, K. K. Kar and K. Dasgupta, Enhanced electrical, mechanical, and viscoelastic properties of carbon-carbon composites using carbon nanotubes coated carbon textile as reinforcement, J. Compos. Mater., 2020, 55, 1733–1748 CrossRef.
- C. I. Justino, T. A. Rocha-Santos and A. C. Duarte, Advances in point-of-care technologies with biosensors based on carbon nanotubes, TrAC, Trends Anal. Chem., 2013, 45, 24–36 CrossRef CAS.
- T.-T. Tran and A. Mulchandani, Carbon nanotubes and graphene nano field-effect transistor-based biosensors, TrAC, Trends Anal. Chem., 2016, 79, 222–232 CrossRef CAS.
- L. Yi-Wei, C. Yan, M. Yao-Hong, S. Jian-Guo, W. Yuan-Xiu, Q. Cui-Hua and L. Qiu-Shun, Recent advances in the dehydrogenase biosensors based on carbon nanotube modified electrodes, Chin. J. Anal. Chem., 2014, 42, 759–765 Search PubMed.
- J. Lee, Carbon Nanotube-Based Biosensors Using Fusion Technologies with Biologicals & Chemicals for Food Assessment, Biosensors, 2023, 13, 183 CrossRef CAS PubMed.
- R. Jha, A. Singh, P. Sharma and N. K. Fuloria, Smart carbon nanotubes for drug delivery system: A comprehensive study, J. Drug Delivery Sci. Technol., 2020, 101811 CrossRef CAS.
- A. R. Kiran, G. K. Kumari and P. T. Krishnamurthy, Carbon nanotubes in drug delivery: Focus on anticancer therapies, J. Drug Delivery Sci. Technol., 2020, 101892 CrossRef.
- N. Jain and S. Tiwari, Biomedical application of carbon nanotubes (CNTs) in vulnerable parts of the body and its toxicity study: A state-of-the-art-review, Mater. Today: Proc., 2021, 46, 7608–7617 CAS.
- P. D'Orazio, Biosensors in clinical chemistry—2011 update, Clin. Chim. Acta, 2011, 412, 1749–1761 CrossRef PubMed.
- B. Pérez-López and A. Merkoçi, Nanomaterials based biosensors for food analysis applications, Trends Food Sci. Technol., 2011, 22, 625–639 CrossRef.
- H. Singh, A. Bamrah, S. K. Bhardwaj, A. Deep, M. Khatri, K.-H. Kim and N. Bhardwaj, Nanomaterial-based fluorescent sensors for the detection of lead ions, J. Hazard. Mater., 2020, 124379 Search PubMed.
- Z. Kotsiri, J. Vidic and A. Vantarakis, Applications of biosensors for bacteria and virus detection in food and water–A systematic review, J. Environ. Sci., 2022, 111, 367–379 CrossRef PubMed.
- A. Haleem, M. Javaid, R. P. Singh, R. Suman and S. Rab, Biosensors applications in medical field: A brief review, Sens. Int., 2021, 100100 CrossRef.
- M. Lv, Y. Liu, J. Geng, X. Kou, Z. Xin and D. Yang, Engineering nanomaterials-based biosensors for food safety detection, Biosens. Bioelectron., 2018, 106, 122–128 CrossRef CAS PubMed.
- B. D. Malhotra and M. A. Ali, Nanomaterials in biosensors: fundamentals and applications, Nanomaterials for Biosensors, 2018, p. 1 Search PubMed.
- R. Gupta, N. Raza, S. K. Bhardwaj, K. Vikrant, K.-H. Kim and N. Bhardwaj, Advances in nanomaterial-based electrochemical biosensors for the detection of microbial toxins, pathogenic bacteria in food matrices, J. Hazard. Mater., 2021, 401, 123379 CrossRef CAS PubMed.
- A. Joshi and K.-H. Kim, Recent advances in nanomaterial-based electrochemical detection of antibiotics: challenges and future perspectives, Biosens. Bioelectron., 2020, 153, 112046 CrossRef CAS PubMed.
- A. Hashem, M. M. Hossain, M. Al Mamun, K. Simarani and M. R. Johan, Nanomaterials based electrochemical nucleic acid biosensors for environmental monitoring: A review, Appl. Surf. Sci. Adv., 2021, 4, 100064 CrossRef.
- H. Singh, S. Singh, S. K. Bhardwaj, G. Kaur, M. Khatri, A. Deep and N. Bhardwaj, Development of carbon quantum dot-based lateral flow immunoassay for sensitive detection of aflatoxin M1 in milk, Food Chem., 2022, 393, 133374 CrossRef CAS PubMed.
- G. A. Rivas, M. C. Rodriguez, M. D. Rubianes, F. A. Gutierrez, M. Eguilaz, P. R. Dalmasso, E. N. Primo, C. Tettamanti, M. L. Ramirez and A. Montemerlo, Carbon nanotubes-based electrochemical (bio) sensors for biomarkers, Appl. Mater. Today, 2017, 9, 566–588 CrossRef.
- P. Gallay, M. Eguílaz and G. Rivas, Designing electrochemical interfaces based on nanohybrids of avidin functionalized-carbon nanotubes and ruthenium nanoparticles as peroxidase-like nanozyme with supramolecular recognition properties for site-specific anchoring of biotinylated residues, Biosens. Bioelectron., 2020, 148, 111764 CrossRef CAS PubMed.
- G. Tuci, J. Filippi, A. Rossin, L. Luconi, C. Pham-Huu, D. Yakhvarov, F. Vizza and G. Giambastiani, CO2 Electrochemical Reduction by Exohedral N-Pyridine Decorated Metal-Free Carbon Nanotubes, Energies, 2020, 13, 2703 CrossRef CAS.
- C.-M. Tîlmaciu and M. C. Morris, Carbon nanotube biosensors, Front. Chem., 2015, 3, 59 Search PubMed.
- A. Erdely, M. Dahm, B. T. Chen, P. C. Zeidler-Erdely, J. E. Fernback, M. E. Birch, D. E. Evans, M. L. Kashon, J. A. Deddens and T. Hulderman, Carbon nanotube dosimetry: from workplace exposure assessment to inhalation toxicology, Part. Fibre Toxicol., 2013, 10, 1–14 CrossRef PubMed.
- S. Smart, A. Cassady, G. Lu and D. Martin, The biocompatibility of carbon nanotubes, Carbon, 2006, 44, 1034–1047 CrossRef CAS.
- S. Wu, B. Duan, A. Lu, Y. Wang, Q. Ye and L. Zhang, Biocompatible chitin/carbon nanotubes composite hydrogels as neuronal growth substrates, Carbohydr. Polym., 2017, 174, 830–840 CrossRef CAS PubMed.
- J. Kaur, H. Singh and M. Khatri, Regulatory Considerations for Safety of Nanomaterials, Nanomedicine for Bioactives: Healthcare applications, 2020, pp. 431–450 Search PubMed.
- S. Singal, A. K. Srivastava and B. Gahtori, Immunoassay for troponin I using a glassy carbon electrode modified with a hybrid film consisting of graphene and multiwalled carbon nanotubes and decorated with platinum nanoparticles, Microchim. Acta, 2016, 183, 1375–1384 CrossRef CAS.
- B. Rezaei, A. M. Shoushtari, M. Rabiee, L. Uzun, W. C. Mak and A. P. Turner, An electrochemical immunosensor for cardiac Troponin I using electrospun carboxylated multi-walled carbon nanotube-whiskered nanofibres, Talanta, 2018, 182, 178–186 CrossRef CAS PubMed.
- M. R. Hasan, T. Pulingam, J. N. Appaturi, A. N. Zifruddin, S. J. Teh, T. W. Lim, F. Ibrahim, B. F. Leo and K. L. Thong, Carbon nanotube-based aptasensor for sensitive electrochemical detection of whole-cell Salmonella, Anal. Biochem., 2018, 554, 34–43 CrossRef CAS PubMed.
- G. Evtugyn, A. Porfireva, R. Shamagsumova and T. Hianik, Advances in Electrochemical Aptasensors Based on Carbon Nanomaterials, Chemosensors, 2020, 8, 96 CrossRef CAS.
- J. N. Appaturi, T. Pulingam, K. L. Thong, S. Muniandy, N. Ahmad and B. F. Leo, Rapid and sensitive detection of Salmonella with reduced graphene oxide-carbon nanotube based electrochemical aptasensor, Anal. Biochem., 2020, 589, 113489 CrossRef CAS PubMed.
- W. Feng and P. Ji, Enzymes immobilized on carbon nanotubes, Biotechnol. Adv., 2011, 29, 889–895 CrossRef CAS PubMed.
- T. Sainsbury, J. Stolarczyk and D. Fitzmaurice, An experimental and theoretical study of the self-assembly of gold nanoparticles at the surface of functionalized multiwalled carbon nanotubes, J. Phys. Chem. B, 2005, 109, 16310–16325 CrossRef CAS PubMed.
- A. Fási, I. Pálinkó, J. W. Seo, Z. Kónya, K. Hernádi and I. Kiricsi, Sonication assisted gold deposition on multiwall carbon nanotubes, Chem. Phys. Lett., 2003, 372, 848–852 CrossRef.
- M. Alagappan, S. Immanuel, R. Sivasubramanian and A. Kandaswamy, Development of cholesterol biosensor using Au nanoparticles decorated f-MWCNT covered with polypyrrole network, Arabian J. Chem., 2020, 13, 2001–2010 CrossRef CAS.
- B. Mutharani, P. Ranganathan, S.-M. Chen, T.-W. Chen, G. E. Eldesoky, M. A. Ali and S. M. Wabaidur, Temperature-enabled reversible “On/Off” switch-like hazardous herbicide picloram voltammetric sensor in agricultural and environmental samples based on thermo-responsive PVCL-tethered MWCNT@ Au catalyst, J. Hazard. Mater., 2021, 402, 123672 CrossRef CAS PubMed.
- M. Moghaddari, F. Yousefi, M. Ghaedi and K. Dashtian, A simple approach for the sonochemical loading of Au, Ag and Pd nanoparticle on functionalized MWCNT and subsequent dispersion studies for removal of organic dyes: Artificial neural network and response surface methodology studies, Ultrason. Sonochem., 2018, 42, 422–433 CrossRef CAS PubMed.
- V. D. Chinh, G. Speranza, C. Migliaresi, N. Van Chuc, V. M. Tan and N.-T. Phuong, Synthesis of gold nanoparticles decorated with multiwalled carbon nanotubes (Au-MWCNTs) via cysteaminium chloride functionalization, Sci. Rep., 2019, 9, 1–9 CrossRef PubMed.
- M.-F. Yu, O. Lourie, M. J. Dyer, K. Moloni, T. F. Kelly and R. S. Ruoff, Strength and breaking mechanism of multiwalled carbon nanotubes under tensile load, Science, 2000, 287, 637–640 CrossRef CAS PubMed.
- S. Manafi, M. Ebrahimi, F. S. Bidabadi and I. Mobasherpour, Structural properties and mechanical behavior of SWCNTs and MWCNTs reinforced Al2O3 fabricated by spark plasma sintering, Ceram. Int., 2019, 45, 15928–15933 CrossRef CAS.
- T. Zhou, G. C. Tsui, J. Liang, S. Zou, C. Y. Tang and V. Mišković-Stanković, Thermal properties and thermal stability of PP/MWCNT composites, Composites, Part B, 2016, 90, 107–114 CrossRef CAS.
- Z. Wang, W. Zeng and Z. Li, Advanced Nanomaterials for Sensing Applications, Frontiers Media SA, 2019 Search PubMed.
- N. Saifuddin, A. Raziah and A. Junizah, Carbon nanotubes: a review on structure and their interaction with proteins, J. Chem., 2013, 2013, 1–18 CrossRef.
- M. J. Kiani, M. Razak, F. Che Harun, M. Ahmadi and M. Rahmani, SWCNT-based biosensor modelling for pH detection, J. Nanomater., 2015, 2015, 1–7 CrossRef.
- M. Heidarian, A. Khazaei and J. Saien, Grafting drugs to functionalized single-wall carbon nanotubes as a potential method for drug delivery, Phys. Chem. Res., 2021, 9, 57–68 CAS.
- X. Liu, D. Xu, C. Liao, Y. Fang and B. Guo, Development of a promising drug delivery for formononetin: Cyclodextrin-modified single-walled carbon nanotubes, J. Drug Delivery Sci. Technol., 2018, 43, 461–468 CrossRef CAS.
- I.-H. Cho, D. H. Kim and S. Park, Electrochemical biosensors: Perspective on functional nanomaterials for on-site analysis, Biomater. Res., 2020, 24, 1–12 CrossRef PubMed.
- J. Pan, F. Li and J. H. Choi, Single-walled carbon nanotubes as optical probes for bio-sensing and imaging, J. Mater. Chem. B, 2017, 5, 6511–6522 RSC.
- M. Bilal, T. A. Nguyen and H. M. Iqbal, Multifunctional carbon nanotubes and their derived nano-constructs for enzyme immobilization–A paradigm shift in biocatalyst design, Coord. Chem. Rev., 2020, 422, 213475 CrossRef CAS.
- M. Arugula and A. Simonian, Nanocarbon-Based Multi-Functional Biointerfaces: Design and Applications, ECS J. Solid State Sci. Technol., 2016, 5, M3045 CrossRef CAS.
- P. Pinyou, V. Blay, L. M. Muresan and T. Noguer, Enzyme-modified electrodes for biosensors and biofuel cells, Mater. Horiz., 2019, 6, 1336–1358 RSC.
- M. Inagaki and F. Kang, Graphene derivatives: graphane, fluorographene, graphene oxide, graphyne and graphdiyne, J. Mater. Chem. A, 2014, 2, 13193–13206 RSC.
- D. C. Ferrier and K. C. Honeychurch, Carbon nanotube (CNT)-based biosensors, Biosensors, 2021, 11, 486 CrossRef CAS PubMed.
- H. Meskher, C. M. Hussain, A. Thakur, R. Sathyamurthy, I. Lynch, P. Singh, K. Tan and R. Saidur, Recent Trends in Carbon Nanotube (CNT) based biosensors for fast and sensitive detection of human viruses: A critical review, Nanoscale Adv., 2022, 5, 992–1010 RSC.
- X. Dang and H. Zhao, Graphdiyne: A promising 2D all-carbon nanomaterial for sensing and biosensing, TrAC, Trends Anal. Chem., 2021, 137, 116194 CrossRef CAS.
- M. M. Falinski, M. A. Garland, S. M. Hashmi, R. L. Tanguay and J. B. Zimmerman, Establishing structure-property-hazard relationships for multi-walled carbon nanotubes: The role of aggregation, surface charge, and oxidative stress on embryonic zebrafish mortality, Carbon, 2019, 155, 587–600 CrossRef CAS PubMed.
- K. Balasubramanian and M. Burghard, Biosensors based on carbon nanotubes, Anal. Bioanal. Chem., 2006, 385, 452–468 CrossRef CAS PubMed.
- M. Sireesha, V. Jagadeesh Babu, A. S. Kranthi Kiran and S. Ramakrishna, A review on carbon nanotubes in biosensor devices and their applications in medicine, Nanocomposites, 2018, 4, 36–57 CrossRef CAS.
- N. K. Mehra, V. Mishra and N. Jain, A review of ligand tethered surface engineered carbon nanotubes, Biomaterials, 2014, 35, 1267–1283 CrossRef CAS PubMed.
- A. Shamsazar, A. Asadi, D. Seifzadeh and M. Mahdavi, A novel and highly sensitive sandwich-type immunosensor for prostate-specific antigen detection based on MWCNTs-Fe3O4 nanocomposite, Sens. Actuators, B, 2021, 346, 130459 CrossRef CAS.
- L. Cao, Y. Tan, W. Deng and Q. Xie, MWCNTs-CoP hybrids for dual-signal electrochemical immunosensing of carcinoembryonic antigen based on overall water splitting, Talanta, 2021, 233, 122521 CrossRef CAS PubMed.
- M. L. Yola, N. Atar and N. Özcan, A novel electrochemical lung cancer biomarker cytokeratin 19 fragment antigen 21-1 immunosensor based on Si 3 N 4/MoS 2 incorporated MWCNTs and core–shell type magnetic nanoparticles, Nanoscale, 2021, 13, 4660–4669 RSC.
- K. Ganbat, D. Pan, K. Chen, Z. Ning, L. Xing, Y. Zhang and Y. Shen, One-pot electrografting preparation of bifunctionalized carbon nanotubes for sensitive electrochemical immunosensing, J. Electroanal. Chem., 2020, 860, 113906 CrossRef CAS.
- S. Zhang, Y. Shen, G. Shen, S. Wang, G. Shen and R. Yu, Electrochemical immunosensor based on Pd–Au nanoparticles supported on functionalized PDDA-MWCNT nanocomposites for aflatoxin B1 detection, Anal. Biochem., 2016, 494, 10–15 CrossRef CAS PubMed.
- T. Kalyani, A. Sangili, A. Nanda, S. Prakash, A. Kaushik and S. K. Jana, Bio-nanocomposite based highly sensitive and label-free electrochemical immunosensor for endometriosis diagnostics application, Bioelectrochemistry, 2021, 139, 107740 CrossRef CAS PubMed.
- A. Benvidi, S. Yazdanparast, M. Rezaeinasab, M. D. Tezerjani and S. Abbasi, Designing and fabrication of a novel sensitive electrochemical aptasensor based on poly (L-glutamic acid)/MWCNTs modified glassy carbon electrode for determination of tetracycline, J. Electroanal. Chem., 2018, 808, 311–320 CrossRef CAS.
- H. Lai, P. Ming, M. Wu, S. Wang, D. Sun and H. Zhai, An electrochemical aptasensor based on P-Ce-MOF@ MWCNTs as signal amplification strategy for highly sensitive detection of zearalenone, Food Chem., 2023, 423, 136331 CrossRef CAS PubMed.
- H. Zhang, J. Sun, S. Cheng, H. Liu, F. Li, Y. Guo and X. Sun, A Dual-Amplification electrochemical aptasensor for profenofos detection, J. Electrochem. Soc., 2020, 167, 027515 CrossRef CAS.
- Y. Hou, Q. Xu, Y. Li, N. Long, P. Li, J. Wang, L. Zhou, P. Sheng and W. Kong, Ultrasensitive electrochemical aptasensor with Nafion-stabilized f-MWCNTs as signal enhancers for OTA detection, Bioelectrochemistry, 2023, 151, 108399 CrossRef CAS PubMed.
- X. Qi and S. Tao, MWCNT modified Ni–Fe LDH/BiVO 4 heterojunction: boosted visible-light-driven photoelectrochemical aptasensor for ofloxacin detection, RSC Adv., 2022, 12, 24269–24277 RSC.
- S. Cheng, R. Xu, F. Yang, J. Huang, X. Sun, X. Huang, H. Li, F. Li, Y. Guo and M. Hasanzadeh, Novel sandwich-type electrochemiluminescence aptasensor based on luminol functionalized aptamer as signal probe for kanamycin detection, Bioelectrochemistry, 2022, 147, 108174 CrossRef CAS PubMed.
- Z. Chen, H. Li, M. Xie, F. Zhao and S. Han, Label-Free Electrochemical Aptasensor for Sensitive Detection of Malachite Green Based on AuNPs/MWCNTs@ TiO2 Nanocomposites, Int. J. Mol. Sci., 2023, 24, 10594 CrossRef CAS PubMed.
- A. B. Hashkavayi, B. S. Cha, E. S. Lee and K. S. Park, Dual rolling circle amplification-enabled ultrasensitive multiplex detection of exosome biomarkers using electrochemical aptasensors, Anal. Chim. Acta, 2022, 1205, 339762 CrossRef CAS PubMed.
- S. Gupta, C. R. Prabha and C. Murthy, Functionalized multi-walled carbon nanotubes/polyvinyl alcohol membrane coated glassy carbon electrode for efficient enzyme immobilization and glucose sensing, J. Environ. Chem. Eng., 2016, 4, 3734–3740 CrossRef CAS.
- S. Xu, Y. Zhang, Y. Zhu, J. Wu, K. Li, G. Lin, X. Li, R. Liu, X. Liu and C.-P. Wong, Facile one-step fabrication of glucose oxidase loaded polymeric nanoparticles decorating MWCNTs for constructing glucose biosensing platform: Structure matters, Biosens. Bioelectron., 2019, 135, 153–159 CrossRef CAS PubMed.
- T. H. Ko, J.-G. Seong, S. Radhakrishnan, C.-S. Kwak, M.-S. Khil, H.-Y. Kim and B.-S. Kim, Dual functional nickel cobalt/MWCNT composite electrode-based electrochemical capacitor and enzymeless glucose biosensor applications: Influence of Ni/Co molar ratio, J. Ind. Eng. Chem., 2019, 73, 1–7 CrossRef CAS.
- X. Liu, H.-L. Shuai, Y.-J. Liu and K.-J. Huang, An electrochemical biosensor for DNA detection based on tungsten disulfide/multi-walled carbon nanotube composites and hybridization chain reaction amplification, Sens. Actuators, B, 2016, 235, 603–613 CrossRef CAS.
- M. Aizawa, Immunosensors, Biosensor Principles and Applications, 2019, pp. 249–266 Search PubMed.
- F. Mollarasouli, S. Kurbanoglu and S. A. Ozkan, The role of electrochemical immunosensors in clinical analysis, Biosensors, 2019, 9, 86 CrossRef CAS PubMed.
- F. Ricci, G. Adornetto and G. Palleschi, A review of experimental aspects of electrochemical immunosensors, Electrochim. Acta, 2012, 84, 74–83 CrossRef CAS.
- G. Duffy and E. Moore, Electrochemical immunosensors for food analysis: A review of recent developments, Anal. Lett., 2017, 50, 1–32 CrossRef CAS.
- H. Yang, W. Xu, X. Liang, Y. Yang and Y. Zhou, Carbon nanotubes in electrochemical, colorimetric, and fluorimetric immunosensors and immunoassays: a review, Microchim. Acta, 2020, 187, 1–18 CrossRef PubMed.
- M. Ghanavati, F. Tadayon and H. Bagheri, A novel label-free impedimetric immunosensor for sensitive detection of prostate specific antigen using Au nanoparticles/MWCNTs-graphene quantum dots nanocomposite, Microchem. J., 2020, 159, 105301 CrossRef CAS.
- T. Feng, Y. Wang and X. Qiao, Recent advances of carbon nanotubes-based electrochemical immunosensors for the detection of protein cancer biomarkers, Electroanalysis, 2017, 29, 662–675 CrossRef CAS.
- S. Guerrero, L. Agüí, P. Yáñez-Sedeño and J. Pingarrón, Design of electrochemical immunosensors using electro-click chemistry. Application to the detection of IL-1β cytokine in saliva, Bioelectrochemistry, 2020, 133, 107484 CrossRef CAS PubMed.
- K. Mahato, S. Kumar, A. Srivastava, P. K. Maurya, R. Singh and P. Chandra, Electrochemical immunosensors: fundamentals and applications in clinical diagnostics, Handbook of immunoassay technologies, Elsevier, 2018, pp. 359–414 Search PubMed.
- S. Muniandy, S. J. Teh, K. L. Thong, A. Thiha, I. J. Dinshaw, C. W. Lai, F. Ibrahim and B. F. Leo, Carbon nanomaterial-based electrochemical biosensors for foodborne bacterial detection, Crit. Rev. Anal. Chem., 2019, 49, 510–533 CrossRef CAS PubMed.
- A. Güner, E. Çevik, M. Şenel and L. Alpsoy, An electrochemical immunosensor for sensitive detection of Escherichia coli O157: H7 by using chitosan, MWCNT, polypyrrole with gold nanoparticles hybrid sensing platform, Food Chem., 2017, 229, 358–365 CrossRef PubMed.
- F. Long, Z. Zhang, Z. Yang, J. Zeng and Y. Jiang, Imprinted electrochemical sensor based on magnetic multi-walled carbon nanotube for sensitive determination of kanamycin, J. Electroanal. Chem., 2015, 755, 7–14 CrossRef CAS.
- W. Wu, M. Jia, Z. Zhang, X. Chen, Q. Zhang, W. Zhang, P. Li and L. Chen, Sensitive, selective and simultaneous electrochemical detection of multiple heavy metals in environment and food using a lowcost Fe3O4 nanoparticles/fluorinated multi-walled carbon nanotubes sensor, Ecotoxicol. Environ. Saf., 2019, 175, 243–250 CrossRef CAS PubMed.
- X. Dai, S. Wu and S. Li, Progress on electrochemical sensors for the determination of heavy metal ions from contaminated water, J. Chin. Adv. Mater. Soc., 2018, 6, 91–111 CrossRef CAS.
- J. Tang, D. Tang, B. Su, J. Huang, B. Qiu and G. Chen, Enzyme-free electrochemical immunoassay with catalytic reduction of p-nitrophenol and recycling of p-aminophenol using gold nanoparticles-coated carbon nanotubes as nanocatalysts, Biosens. Bioelectron., 2011, 26, 3219–3226 CrossRef CAS PubMed.
- X. Pei, B. Zhang, J. Tang, B. Liu, W. Lai and D. Tang, Sandwich-type immunosensors and immunoassays exploiting nanostructure labels: A review, Anal. Chim. Acta, 2013, 758, 1–18 CrossRef CAS PubMed.
- L. Liu, D. Deng, W. Sun, X. Yang, S. Yang and S. He, Electrochemical Biosensors with Electrocatalysts Based on Metallic Nanomaterials as Signal Labels, Int. J. Electrochem. Sci., 2018, 13, 10496–10513 CrossRef CAS.
- Z. Wang, J. Yu, R. Gui, H. Jin and Y. Xia, Carbon nanomaterials-based electrochemical aptasensors, Biosens. Bioelectron., 2016, 79, 136–149 CrossRef CAS PubMed.
- A. Sassolas, L. J. Blum and B. D. Leca-Bouvier, Electrochemical aptasensors, Electroanalysis, 2009, 21, 1237–1250 CrossRef CAS.
- H.-M. So, K. Won, Y. H. Kim, B.-K. Kim, B. H. Ryu, P. S. Na, H. Kim and J.-O. Lee, Single-walled carbon nanotube biosensors using aptamers as molecular recognition elements, J. Am. Chem. Soc., 2005, 127, 11906–11907 CrossRef CAS PubMed.
- S. S. Baghbaderani and A. Noorbakhsh, Novel chitosan-Nafion composite for fabrication of highly sensitive impedimetric and colorimetric As (III) aptasensor, Biosens. Bioelectron., 2019, 131, 1–8 CrossRef CAS PubMed.
- G. A. Zelada-Guillén, P. Blondeau, F. X. Rius and J. Riu, Carbon nanotube-based aptasensors for the rapid and ultrasensitive detection of bacteria, Methods, 2013, 63, 233–238 CrossRef PubMed.
- H. Yu, H. Yang, W. Liu, L. Jin, B. Jin and M. Wu, Novel electrochemiluminescence biosensor of fumonisin B1 detection using MWCNTs-PDMS flexible bipolar electrode, Talanta, 2023, 257, 124379 CrossRef CAS PubMed.
- M. Nazari, S. Kashanian, R. Rafipour and K. Omidfar, Biosensor design using an electroactive label-based aptamer to detect bisphenol A in serum samples, J. Biosci., 2019, 44, 1–10 CrossRef CAS.
- M. Baghayeri, R. Ansari, M. Nodehi, I. Razavipanah and H. Veisi, Label-free Electrochemical Bisphenol A Aptasensor Based on Designing and Fabrication of a Magnetic Gold Nanocomposite, Electroanalysis, 2018, 30, 2160–2166 CrossRef CAS.
- A. Azadbakht, M. Roushani, A. R. Abbasi and Z. Derikvand, A novel impedimetric aptasensor, based on functionalized carbon nanotubes and prussian blue as labels, Anal. Biochem., 2016, 512, 58–69 CrossRef CAS PubMed.
- T. Shiravand and A. Azadbakht, Impedimetric biosensor based on bimetallic AgPt nanoparticle-decorated carbon nanotubes as highly conductive film surface, J. Solid State Electrochem., 2017, 21, 1699–1711 CrossRef CAS.
- B.-S. He and S.-S. Yan, Electrochemical aptasensor based on aptamer-complimentary strand conjugate and thionine for sensitive detection of tetracycline with multi-walled carbon nanotubes and gold nanoparticles amplification, Anal. Methods, 2018, 10, 783–790 RSC.
- R. Aghajari and A. Azadbakht, Amplified detection of streptomycin using aptamer-conjugated palladium nanoparticles decorated on chitosan-carbon nanotube, Anal. Biochem., 2018, 547, 57–65 CrossRef CAS PubMed.
- B. He, L. Wang, X. Dong, X. Yan, M. Li, S. Yan and D. Yan, Aptamer-based thin film gold electrode modified with gold nanoparticles and carboxylated multi-walled carbon nanotubes for detecting oxytetracycline in chicken samples, Food Chem., 2019, 300, 125179 CrossRef CAS PubMed.
- S. Yarahmadi, A. Azadbakht and R. M. Derikvand, Hybrid synthetic receptor composed of molecularly imprinted polydopamine and aptamers for impedimetric biosensing of urea, Microchim. Acta, 2019, 186, 71 CrossRef PubMed.
- A. Azadbakht, M. Roushani, A. R. Abbasi and Z. Derikvand, Design and characterization of electrochemical dopamine–aptamer as convenient and integrated sensing platform, Anal. Biochem., 2016, 507, 47–57 CrossRef CAS PubMed.
- T. Ming, Y. Wang, J. Luo, J. Liu, S. Sun, Y. Xing, G. Xiao, H. Jin and X. Cai, Folding paper-based aptasensor platform coated with novel nanoassemblies for instant and highly sensitive detection of 17β-estradiol, ACS Sens., 2019, 4, 3186–3194 CrossRef CAS.
- A. Azadbakht and M. B. Gholivand, Polyethyleneimine wrapped carbon nanotubes in situ formed gold nanoparticles decorated with DNA and NAD+ as a novel bioeletrochemical sensing platform, Electrochim. Acta, 2014, 133, 82–92 CrossRef CAS.
- T. Beiki, G. Najafpour-Darzi, M. Mohammadi, M. Shakeri and R. Boukherroub, Fabrication of a novel electrochemical biosensor based on a molecular imprinted polymer-aptamer hybrid receptor for lysozyme determination, Anal. Bioanal. Chem., 2023, 415, 899–911 CrossRef CAS PubMed.
- V. Schroeder, S. Savagatrup, M. He, S. Lin and T. M. Swager, Carbon nanotube chemical sensors, Chem. Rev., 2018, 119, 599–663 CrossRef PubMed.
- J. Wang, Carbon-nanotube based electrochemical biosensors: A review, Electroanalysis: An International Journal Devoted to Fundamental and Practical Aspects of, Electroanalysis, 2005, 17, 7–14 CrossRef CAS.
- C. Zhu, G. Yang, H. Li, D. Du and Y. Lin, Electrochemical sensors and biosensors based on nanomaterials and nanostructures, Anal. Chem., 2015, 87, 230–249 CrossRef CAS PubMed.
- G. Luka, S. Ahmad, N. Falcone and H.-B. Kraatz, Advances in enzyme-based electrochemical sensors: current trends, benefits, and constraints, Bioelectronics and Medical Devices, Elsevier, 2019, pp. 555–590 Search PubMed.
- A. R. Jalalvand, An intelligent and novel electrochemical biosensor for simultaneous enzymatic biosensing of cholesterol and glucose in the presence of uric acid based on first-and second-order calibration methods, Microchem. J., 2023, 191, 108824 CrossRef CAS.
- B. Batra, S. Yadav, V. Kalra, M. Sharma and J. Rana, An electrochemical biosensor for the determination of folic acid in pregnant women based on DHFR/c-MWCNTs/TiO2NPs modified gold electrode, Sens. Int., 2023, 4, 100235 CrossRef.
- S. Gupta, C. Murthy and C. R. Prabha, Recent advances in carbon nanotube based electrochemical biosensors, Int. J. Biol. Macromol., 2018, 108, 687–703 CrossRef CAS.
- R. O. Cristóvão, M. R. Almeida, M. A. Barros, J. C. Nunes, R. A. Boaventura, J. M. Loureiro, J. L. Faria, M. C. Neves, M. G. Freire and V. C. Ebinuma-Santos, Development and characterization of a novel l-asparaginase/MWCNT nanobioconjugate, RSC Adv., 2020, 10, 31205–31213 RSC.
- R. Pauliukaite, M. E. Ghica, O. Fatibello-Filho and C. M. Brett, Electrochemical impedance studies of chitosan-modified electrodes for application in electrochemical sensors and biosensors, Electrochim. Acta, 2010, 55, 6239–6247 CrossRef CAS.
- S. Sen and P. Sarkar, A novel third-generation xanthine biosensor with enzyme modified glassy carbon electrode using electrodeposited MWCNT and nanogold polymer composite film, RSC Adv., 2015, 5, 95911–95925 RSC.
- V. Gautam, K. P. Singh and V. L. Yadav, Polyaniline/MWCNTs/starch modified carbon paste electrode for non-enzymatic detection of cholesterol: application to real sample (cow milk), Anal. Bioanal. Chem., 2018, 410, 2173–2181 CrossRef CAS PubMed.
- D. G. A. Kaariz, E. Darabi and S. M. Elahi, Fabrication of Au/ZnO/MWCNTs electrode and its characterization for electrochemical cholesterol biosensor, J. Theor. Appl. Phys., 2020, 14, 339–348 CrossRef.
- M. Dervisevic, E. Dervisevic and M. Şenel, Design of amperometric urea biosensor based on self-assembled monolayer of cystamine/PAMAM-grafted MWCNT/Urease, Sens. Actuators, B, 2018, 254, 93–101 CrossRef CAS.
- E. Dervisevic, M. Dervisevic, J. N. Nyangwebah and M. Şenel, Development of novel amperometric urea biosensor based on Fc-PAMAM and MWCNT bio-nanocomposite film, Sens. Actuators, B, 2017, 246, 920–926 CrossRef CAS.
- S. Kurbanoglu and S. A. Ozkan, A novel enzymatic biosensor for the detection of catechol using multi-walled carbon nanotubes and gold nanowires, Electrocatalysis, 2018, 9, 252–257 CrossRef CAS.
- S. Wang, R. Wang, P. Sellin and Q. Zhang, DNA biosensors based on self-assembled carbon nanotubes, Biochem. Biophys. Res. Commun., 2004, 325, 1433–1437 CrossRef CAS PubMed.
- P. He, Y. Xu and Y. Fang, Applications of carbon nanotubes in electrochemical DNA biosensors, Microchim. Acta, 2006, 152, 175 CrossRef CAS.
- B. Rafique, M. Iqbal, T. Mehmood and M. A. Shaheen, Electrochemical DNA biosensors: A review, Sens. Rev., 2019, 39, 34–50 CrossRef.
- C. Kokkinos, Electrochemical DNA biosensors based on labeling with nanoparticles, Nanomaterials, 2019, 9, 1361 CrossRef CAS PubMed.
- M. Guo, J. Chen, J. Li, L. Nie and S. Yao, Carbon nanotubes-based amperometric cholesterol biosensor fabricated through layer-by-layer technique, Electroanalysis, 2004, 16, 1992–1998 CrossRef CAS.
- S. Chawla, R. Rawal, S. Sharma and C. S. Pundir, An amperometric biosensor based on laccase immobilized onto nickel nanoparticles/carboxylated multiwalled carbon nanotubes/polyaniline modified gold electrode for determination of phenolic content in fruit juices, Biochem. Eng. J., 2012, 68, 76–84 CrossRef CAS.
- X. Dong, X. Lu, K. Zhang and Y. Zhang, Chronocoulometric DNA biosensor based on a glassy carbon electrode modified with gold nanoparticles, poly (dopamine) and carbon nanotubes, Microchim. Acta, 2013, 180, 101–108 CrossRef CAS.
- Y. Huang, M. Shi, L. Zhao, S. Zhao, K. Hu, Z.-F. Chen, J. Chen and H. Liang, Carbon nanotube signal amplification for ultrasensitive fluorescence polarization detection of DNA methyltransferase activity and inhibition, Biosens. Bioelectron., 2014, 54, 285–291 CrossRef CAS PubMed.
- M. Chen, C. Hou, D. Huo, M. Yang and H. Fa, An ultrasensitive electrochemical DNA biosensor based on a copper oxide nanowires/single-walled carbon nanotubes nanocomposite, Appl. Surf. Sci., 2016, 364, 703–709 CrossRef CAS.
- K. Saeedfar, L. Y. Heng and C. P. Chiang, A DNA biosensor based on gold nanoparticle decorated on carboxylated multi-walled carbon nanotubes for gender determination of Arowana fish, Bioelectrochemistry, 2017, 118, 106–113 CrossRef CAS PubMed.
- H. Pyman, H. Roshanfekr and S. Ansari, DNA-based electrochemical biosensor using chitosan–carbon nanotubes composite film for biodetection of Pirazon, Eurasian, Chem. Commun., 2020, 2, 213–225 Search PubMed.
- Y. Chen, S. Guo, M. Zhao, P. Zhang, Z. Xin, J. Tao and L. Bai, Amperometric DNA biosensor for Mycobacterium tuberculosis detection using flower-like carbon nanotubes-polyaniline nanohybrid and enzyme-assisted signal amplification strategy, Biosens. Bioelectron., 2018, 119, 215–220 CrossRef CAS PubMed.
- S. Leonardo, A. Toldrà and M. Campàs, Biosensors based on isothermal DNA amplification for bacterial detection in food safety and environmental monitoring, Sensors, 2021, 21, 602 CrossRef CAS PubMed.
- H. Kharrati-Koopaee, V. Rezaei and A. Esmailizadeh, DNA Biosensors Techniques and Their Applications in Food Safety, Environmental Protection and Biomedical Research: A mini-review, Journal of Cell and Developmental Biology, 2020, 3, 28–35 Search PubMed.
- K. S. Rizi, B. Hatamluyi, M. Rezayi, Z. Meshkat, M. Sankian, K. Ghazvini, H. Farsiani and E. Aryan, Response surface methodology optimized electrochemical DNA biosensor based on HAPNPTs/PPY/MWCNTs nanocomposite for detecting Mycobacterium tuberculosis, Talanta, 2021, 226, 122099 CrossRef CAS PubMed.
- R. Y. Zhang and H. Olin, Gold-carbon nanotube nanocomposites: synthesis and applications, Int. J. Biomed. Nanosci. Nanotechnol., 2011, 2, 112–135 CrossRef CAS.
- S. Rahmati, W. Doherty, A. Amani Babadi, M. S. Akmal Che, N. M. Julkapli, V. Hessel and K. K. Ostrikov, Gold–Carbon Nanocomposites for Environmental Contaminant Sensing, Micromachines, 2021, 12, 719 CrossRef PubMed.
- H. Singh, A. Bamrah, S. K. Bhardwaj, A. Deep, M. Khatri, R. J. Brown, N. Bhardwaj and K.-H. Kim, Recent advances in the application of noble metal nanoparticles in colorimetric sensors for lead ions, Environ. Sci.: Nano, 2021, 8, 863–889 RSC.
- L. Qin, G. Zeng, C. Lai, D. Huang, P. Xu, C. Zhang, M. Cheng, X. Liu, S. Liu and B. Li, “Gold rush” in modern science: fabrication strategies and typical advanced applications of gold nanoparticles in sensing, Coord. Chem. Rev., 2018, 359, 1–31 CrossRef CAS.
- R. Zhang, Q. Wang, L. Zhang, S. Yang, Z. Yang and B. Ding, The growth of uncoated gold nanoparticles on multiwalled carbon nanotubes, Colloids Surf., A, 2008, 312, 136–141 CrossRef CAS.
- Y. Hao, F. Xiao, C. Xiao-Xia, Q. Jin-Li, G. Xiao-Ling, X. Na and G. Lou-Jun, Electrochemical determination of bisphenol A on a glassy carbon electrode modified with gold nanoparticles loaded on reduced graphene oxide-multi walled carbon nanotubes composite, Chin. J. Anal. Chem., 2017, 45, 713–720 Search PubMed.
- K. LeeáTan, Growth of Pd, Pt, Ag and Au nanoparticles on carbon nanotubes, J. Mater. Chem., 2001, 11, 2378–2381 RSC.
- B. Kim and W. M. Sigmund, Functionalized multiwall carbon nanotube/gold nanoparticle composites, Langmuir, 2004, 20, 8239–8242 CrossRef CAS PubMed.
- G. Wei, C. Pan, J. Reichert and K. D. Jandt, Controlled assembly of protein-protected gold nanoparticles on noncovalent functionalized carbon nanotubes, Carbon, 2010, 48, 645–653 CrossRef CAS.
- H. Ma, N. Xue, Z. Li, K. Xing and X. Miao, Ultrasensitive detection of miRNA-155 using multi-walled carbon nanotube-gold nanocomposites as a novel fluorescence quenching platform, Sens. Actuators, B, 2018, 266, 221–227 CrossRef CAS.
- Q. Lu, T. Su, Z. Shang, D. Jin, Y. Shu, Q. Xu and X. Hu, Flexible paper-based Ni-MOF composite/AuNPs/CNTs film electrode for HIV DNA detection, Biosens. Bioelectron., 2021, 184, 113229 CrossRef CAS PubMed.
- B. Xing, T. Zhang, Q. Han, Q. Wei and D. Wu, Electrochemiluminescent immunoassay for insulin by using a quencher pair consisting of CdS: Eu nanoclusters loaded with multiwalled carbon nanotubes on reduced graphene oxide nanoribbons and gold nanoparticle-loaded octahedral Cu 2 O, Microchim. Acta, 2019, 186, 1–7 CrossRef CAS.
- H. S. Magar, M. E. Ghica, M. N. Abbas and C. M. Brett, A novel sensitive amperometric choline biosensor based on multiwalled carbon nanotubes and gold nanoparticles, Talanta, 2017, 167, 462–469 CrossRef CAS PubMed.
- V. Vinoth, J. J. Wu, A. M. Asiri and S. Anandan, Simultaneous detection of dopamine and ascorbic acid using silicate network interlinked gold nanoparticles and multi-walled carbon nanotubes, Sens. Actuators, B, 2015, 210, 731–741 CrossRef CAS.
- B. Huang, J. Liu, L. Lai, F. Yu, X. Ying, B.-C. Ye and Y. Li, A free-standing electrochemical sensor based on graphene foam-carbon nanotube composite coupled with gold nanoparticles and its sensing application for electrochemical determination of dopamine and uric acid, J. Electroanal. Chem., 2017, 801, 129–134 CrossRef CAS.
- D. C. Poudyal, A. Satpati, S. Kumar and S. K. Haram, High sensitive determination of dopamine through catalytic oxidation and preconcentration over gold-multiwall carbon nanotubes composite modified electrode, Mater. Sci. Eng., C, 2019, 103, 109788 CrossRef CAS PubMed.
- Q. Guan, H. Guo, R. Xue, M. Wang, X. Zhao, T. Fan, W. Yang, M. Xu and W. Yang, Electrochemical sensor based on covalent organic frameworks-MWCNT-NH2/AuNPs for simultaneous detection of dopamine and uric acid, J. Electroanal. Chem., 2021, 880, 114932 CrossRef CAS.
- S. Wang, G. Sun, Z. Chen, Y. Liang, Q. Zhou, Y. Pan and H. Zhai, Constructing a novel composite of molecularly imprinted polymer-coated AuNPs electrochemical sensor for the determination of 3-nitrotyrosine, Electrochim. Acta, 2018, 259, 893–902 CrossRef CAS.
- M. N. S. Karaboğa and M. K. Sezgintürk, A nano-composite based regenerative neuro biosensor sensitive to Parkinsonism-associated protein DJ-1/Park7 in cerebrospinal fluid and saliva, Bioelectrochemistry, 2021, 138, 107734 CrossRef PubMed.
- E. Arkan, R. Saber, Z. Karimi, A. Mostafaie and M. Shamsipur, Multiwall carbon nanotube-ionic liquid electrode modified with gold nanoparticles as a base for preparation of a novel impedimetric immunosensor for low level detection of human serum albumin in biological fluids, J. Pharm. Biomed. Anal., 2014, 92, 74–81 CrossRef CAS PubMed.
- C. Ferrag, M. Noroozifar and K. Kerman, Thiol functionalized carbon ceramic electrode modified with multi-walled carbon nanotubes and gold nanoparticles for simultaneous determination of purine derivatives, Mater. Sci. Eng., C, 2020, 110, 110568 CrossRef CAS PubMed.
- H. Guo, T. Zhang, M. Wang, L. Sun, J. Zhang, M. Yang, F. Yang, N. Wu and W. Yang, Electrochemical behavior of MOF-801/MWCNT-COOH/AuNPs: A highly selective electrochemical sensor for determination of guanine and adenine, Colloids Surf., A, 2021, 627, 127195 CrossRef CAS.
- F. Li, J. Han, L. Jiang, Y. Wang, Y. Li, Y. Dong and Q. Wei, An ultrasensitive sandwich-type electrochemical immunosensor based on signal amplification strategy of gold nanoparticles functionalized magnetic multi-walled carbon nanotubes loaded with lead ions, Biosens. Bioelectron., 2015, 68, 626–632 CrossRef CAS PubMed.
- H. Fayazfar, A. Afshar, M. Dolati and A. Dolati, DNA impedance biosensor for detection of cancer, TP53 gene mutation, based on gold nanoparticles/aligned carbon nanotubes modified electrode, Anal. Chim. Acta, 2014, 836, 34–44 CrossRef CAS PubMed.
- E. Arkan, R. Saber, Z. Karimi and M. Shamsipur, A novel antibody–antigen based impedimetric immunosensor for low level detection of HER2 in serum samples of breast cancer patients via modification of a gold nanoparticles decorated multiwall carbon nanotube-ionic liquid electrode, Anal. Chim. Acta, 2015, 874, 66–74 CrossRef CAS PubMed.
- Y. Chen, G. Zheng, Q. Shi, R. Zhao and M. Chen, Preparation of thiolated calix [8] arene/AuNPs/MWCNTs modified glassy carbon electrode and its electrocatalytic oxidation toward paracetamol, Sens. Actuators, B, 2018, 277, 289–296 CrossRef CAS.
- M. Ibrahim, H. Ibrahim, N. Almandil and A.-N. Kawde, Gold nanoparticles/f-MWCNT nanocomposites modified glassy carbon paste electrode as a novel voltammetric sensor for the determination of cyproterone acetate in pharmaceutical and human body fluids, Sens. Actuators, B, 2018, 274, 123–132 CrossRef CAS.
- M. M. El-Wekil, A. M. Mahmoud, A. A. Marzouk, S. A. Alkahtani and R. Ali, A novel molecularly imprinted sensing platform based on MWCNTs/AuNPs decorated 3D starfish like hollow nickel skeleton as a highly conductive nanocomposite for selective and ultrasensitive analysis of a novel pan-genotypic inhibitor velpatasvir in body fluids, J. Mol. Liq., 2018, 271, 105–111 CrossRef CAS.
- X. Sun, L. Qiao and X. Wang, A novel immunosensor based on Au nanoparticles and polyaniline/multiwall carbon nanotubes/chitosan nanocomposite film functionalized interface, Nano-Micro Lett., 2013, 5, 191–201 CrossRef CAS.
- Y. Shao, Y. Dong, L. Bin, L. Fan, L. Wang, X. Yuan, D. Li, X. Liu and S. Zhao, Application of gold nanoparticles/polyaniline-multi-walled carbon nanotubes modified screen-printed carbon electrode for electrochemical sensing of zinc, lead, and copper, Microchem. J., 2021, 106726 CrossRef CAS.
- N. A. Bohari, S. Siddiquee, S. Saallah, M. Misson and S. E. Arshad, Electrochemical Behaviour of Real-Time Sensor for Determination Mercury in Cosmetic Products Based on PANI/MWCNTs/AuNPs/ITO, Cosmetics, 2021, 8, 17 CrossRef CAS.
- Y.-R. Liang, Z.-M. Zhang, Z.-J. Liu, K. Wang, X.-Y. Wu, K. Zeng, H. Meng and Z. Zhang, A highly sensitive signal-amplified gold nanoparticle-based electrochemical immunosensor for dibutyl phthalate detection, Biosens. Bioelectron., 2017, 91, 199–202 CrossRef CAS PubMed.
- A. A. Al-Kahtani, T. Almuqati, N. Alhokbany, T. Ahamad, M. Naushad and S. M. Alshehri, A clean approach for the reduction of hazardous 4-nitrophenol using gold nanoparticles decorated multiwalled carbon nanotubes, J. Cleaner Prod., 2018, 191, 429–435 CrossRef CAS.
- S. Nasraoui, A. Al-Hamry, P. R. Teixeira, S. Ameur, L. G. Paterno, M. B. Ali and O. Kanoun, Electrochemical sensor for nitrite detection in water samples using flexible laser-induced graphene electrodes functionalized by CNT decorated by Au nanoparticles, J. Electroanal. Chem., 2021, 880, 114893 CrossRef CAS.
- M. H. Motaghedifard, S. M. Pourmortazavi, M. Alibolandi and S. Mirsadeghi, Au-modified organic/inorganic MWCNT/Cu/PANI hybrid nanocomposite electrode for electrochemical determination of nitrate ions, Microchim. Acta, 2021, 188, 1–12 CrossRef.
- J. Dong, H. Zhao, M. Xu, Q. Ma and S. Ai, A label-free electrochemical impedance immunosensor based on AuNPs/PAMAM-MWCNT-Chi nanocomposite modified glassy carbon electrode for detection of Salmonella typhimurium in milk, Food Chem., 2013, 141, 1980–1986 CrossRef CAS PubMed.
- J. Bai, X. Zhang, Y. Peng, X. Hong, Y. Liu, S. Jiang, B. Ning and Z. Gao, Ultrasensitive sensing of diethylstilbestrol based on AuNPs/MWCNTs-CS composites coupling with sol-gel molecularly imprinted polymer as a recognition element of an electrochemical sensor, Sens. Actuators, B, 2017, 238, 420–426 CrossRef CAS.
- W. I. Riberi, L. V. Tarditto, M. A. Zon, F. J. Arévalo and H. Fernández, Development of an electrochemical immunosensor to determine zearalenone in maize using carbon screen printed electrodes modified with multi-walled carbon nanotubes/polyethyleneimine dispersions, Sens. Actuators, B, 2018, 254, 1271–1277 CrossRef CAS.
- X. Sun, Y. Ye, S. He, Z. Wu, J. Yue, H. Sun and X. Cao, A novel oriented antibody immobilization based voltammetric immunosensor for allergenic activity detection of lectin in kidney bean by using AuNPs-PEI-MWCNTs modified electrode, Biosens. Bioelectron., 2019, 143, 111607 CrossRef CAS PubMed.
- M. L. Yola and N. Atar, A novel voltammetric sensor based on gold nanoparticles involved in p-aminothiophenol functionalized multi-walled carbon nanotubes: application to the simultaneous determination of quercetin and rutin, Electrochim. Acta, 2014, 119, 24–31 CrossRef CAS.
- S. Palisoc, P. G. De Leon, A. Alzona, L. Racines and M. Natividad, Highly sensitive determination of tetracycline in chicken meat and eggs using AuNP/MWCNT-modified glassy carbon electrodes, Heliyon, 2019, 5, e02147 CrossRef PubMed.
- K. Rovina, S. Siddiquee and S. M. Shaarani, Highly sensitive electrochemical determination of sunset yellow in commercial food products based on CHIT/GO/MWCNTs/AuNPs/GCE, Food Control, 2017, 82, 66–73 CrossRef CAS.
- D. Zappi, R. Caminiti, G. Ingo, C. Sadun, C. Tortolini and M. Antonelli, Biologically friendly room temperature ionic liquids and nanomaterials for the development of innovative enzymatic biosensors, Talanta, 2017, 175, 566–572 CrossRef CAS PubMed.
- N. Nontipichet, S. Khumngern, J. Choosang, P. Thavarungkul, P. Kanatharana and A. Numnuam, An enzymatic histamine biosensor based on a screen-printed carbon electrode modified with a chitosan–gold nanoparticles composite cryogel on Prussian blue-coated multi-walled carbon nanotubes, Food Chem., 2021, 130396 CrossRef CAS PubMed.
- H. Wu, G. Zhang and X. Yang, Electrochemical immunosensor based on Fe3O4/MWCNTs-COOH/AuNPs nanocomposites for trace liver cancer marker alpha-fetoprotein detection, Talanta, 2023, 259, 124492 CrossRef CAS PubMed.
- M.-M. Liu, F.-F. Zhang, H. Liu, M.-J. Wu, Z.-J. Liu and P.-F. Huang, Cell viability and drug evaluation biosensing system based on disposable AuNPs/MWCNT nanocomposite modified screen-printed electrode for exocytosis dopamine detection, Talanta, 2023, 254, 124118 CrossRef CAS PubMed.
- F. Behoftadeh, M. Faezi Ghasemi, A. Mojtahedi, K. Issazadeh, M. Golshekan and S. Alaei, Development of a newly designed biosensor using multi-walled carbon nanotubes (MWCNTs) with gold nanoparticles (AuNPs) in the presence of acetaminophen for detection of Escherichia coli, Arch. Microbiol., 2023, 205, 70 CrossRef CAS PubMed.
- B.-Y. Wu, S.-H. Hou, F. Yin, Z.-X. Zhao, Y.-Y. Wang, X.-S. Wang and Q. Chen, Amperometric glucose biosensor based on multilayer films via layer-by-layer self-assembly of multi-wall carbon nanotubes, gold nanoparticles and glucose oxidase on the Pt electrode, Biosens. Bioelectron., 2007, 22, 2854–2860 CrossRef CAS PubMed.
- Y. L. Yao and K. K. Shiu, Direct Electrochemistry of Glucose Oxidase at Carbon Nanotube-gold Colloid Modified Electrode with Poly (diallyldimethylammonium chloride) Coating, Electroanalysis, 2008, 20, 1542–1548 CrossRef CAS.
- J. Manso, M. Mena, P. Yanez-Sedeno and J. Pingarron, Electrochemical biosensors based on colloidal gold–carbon nanotubes composite electrodes, J. Electroanal. Chem., 2007, 603, 1–7 CrossRef CAS.
- Y. Yu, Z. Chen, S. He, B. Zhang, X. Li and M. Yao, Direct electron transfer of glucose oxidase and biosensing for glucose based on PDDA-capped gold nanoparticle modified graphene/multi-walled carbon nanotubes electrode, Biosens. Bioelectron., 2014, 52, 147–152 CrossRef CAS PubMed.
- M. Gougis, A. Tabet-Aoul, D. Ma and M. Mohamedi, Laser synthesis and tailor-design of nanosized gold onto carbon nanotubes for non-enzymatic electrochemical glucose sensor, Sens. Actuators, B, 2014, 193, 363–369 CrossRef CAS.
- L. Zhu, L. Xu, L. Tan, H. Tan, S. Yang and S. Yao, Direct electrochemistry of cholesterol oxidase immobilized on gold nanoparticles-decorated multiwalled carbon nanotubes and cholesterol sensing, Talanta, 2013, 106, 192–199 CrossRef CAS PubMed.
- N. Chauhan and C. S. Pundir, An amperometric uric acid biosensor based on multiwalled carbon nanotube–gold nanoparticle composite, Anal. Biochem., 2011, 413, 97–103 CrossRef CAS PubMed.
- D. Ragupathy, A. I. Gopalan and K.-P. Lee, Electrocatalytic oxidation and determination of ascorbic acid in the presence of dopamine at multiwalled carbon nanotube–silica network–gold nanoparticles based nanohybrid modified electrode, Sens. Actuators, B, 2010, 143, 696–703 CrossRef CAS.
- J. Wang, R. Yuan, Y. Chai, S. Cao, S. Guan, P. Fu and L. Min, A novel immunosensor based on gold nanoparticles and poly-(2, 6-pyridinediamine)/multiwall carbon nanotubes composite for immunoassay of human chorionic gonadotrophin, Biochem. Eng. J., 2010, 51, 95–101 CrossRef CAS.
- X.-L. Luo, J.-J. Xu, J.-L. Wang and H.-Y. Chen, Electrochemically deposited nanocomposite of chitosan and carbon nanotubes for biosensor application, Chem. Commun., 2005, 2169–2171 RSC.
- H. Yang, R. Yuan, Y. Chai, H. Su, Y. Zhuo, W. Jiang and Z. Song, Electrochemical immunosensor for human chorionic gonadotropin based on horseradish peroxidase–functionalized Prussian blue–carbon nanotubes/gold nanocomposites as labels for signal amplification, Electrochim. Acta, 2011, 56, 1973–1980 CrossRef CAS.
- A.-L. Sun, G.-R. Chen, Q.-L. Sheng and J.-B. Zheng, Sensitive label-free electrochemical immunoassay based on a redox matrix of gold nanoparticles/Azure I/multi-wall carbon nanotubes composite, Biochem. Eng. J., 2011, 57, 1–6 CrossRef CAS.
- Q. Li, D. Tang, J. Tang, B. Su, J. Huang and G. Chen, Carbon nanotube-based symbiotic coaxial nanocables with nanosilica and nanogold particles as labels for electrochemical immunoassay of carcinoembryonic antigen in biological fluids, Talanta, 2011, 84, 538–546 CrossRef CAS PubMed.
- S. Chen, R. Yuan, Y. Chai, L. Min, W. Li and Y. Xu, Electrochemical sensing platform based on tris (2, 2′-bipyridyl) cobalt (III) and multiwall carbon nanotubes–Nafion composite for immunoassay of carcinoma antigen-125, Electrochim. Acta, 2009, 54, 7242–7247 CrossRef CAS.
- D. Futra, L. Y. Heng, M. Z. Jaapar, A. Ulianas, K. Saeedfar and T. L. Ling, A novel electrochemical sensor for 17β-estradiol from molecularly imprinted polymeric microspheres and multi-walled carbon nanotubes grafted with gold nanoparticles, Anal. Methods, 2016, 8, 1381–1389 RSC.
- M. Moreno-Guzman, L. Agüí, A. Gonzalez-Cortes, P. Yanez-Sedeno and J. Pingarrón, Gold nanoparticles/carbon nanotubes/ionic liquid microsized paste electrode for the determination of cortisol and androsterone hormones, J. Solid State Electrochem., 2013, 17, 1591–1599 CrossRef CAS.
- Y. Zhang, J. Wang and M. Xu, A sensitive DNA biosensor fabricated with gold nanoparticles/ploy (p-aminobenzoic acid)/carbon nanotubes modified electrode, Colloids Surf., B, 2010, 75, 179–185 CrossRef CAS PubMed.
- J. Lin, C. He, L. Zhang and S. Zhang, Sensitive amperometric immunosensor for α-fetoprotein based on carbon nanotube/gold nanoparticle doped chitosan film, Anal. Biochem., 2009, 384, 130–135 CrossRef CAS.
- C. W. Tan, K. H. Tan, Y. T. Ong, A. R. Mohamed, S. H. S. Zein and S. H. Tan, Energy and environmental applications of carbon nanotubes, Environ. Chem. Lett., 2012, 10, 265–273 CrossRef CAS.
- P. Ramnani, N. M. Saucedo and A. Mulchandani, Carbon nanomaterial-based electrochemical biosensors for label-free sensing of environmental pollutants, Chemosphere, 2016, 143, 85–98 CrossRef CAS PubMed.
- J. Wang and Y. Lin, Functionalized carbon nanotubes and nanofibers for biosensing applications, TrAC, Trends Anal. Chem., 2008, 27, 619–626 CrossRef CAS PubMed.
- J. Chen, D. Liu, S. Li and D. Yao, Development of an amperometric enzyme electrode biosensor for sterigmatocystin detection, Enzyme Microb. Technol., 2010, 47, 119–126 CrossRef CAS.
- E. I. Maurer, K. K. Comfort, S. M. Hussain, J. J. Schlager and S. M. Mukhopadhyay, Novel platform development using an assembly of carbon nanotube, nanogold and immobilized RNA capture element towards rapid, selective sensing of bacteria, Sensors, 2012, 12, 8135–8144 CrossRef CAS PubMed.
- D. Wang, W. Dou, G. Zhao and Y. Chen, Immunosensor based on electrodeposition of gold-nanoparticles and ionic liquid composite for detection of Salmonella pullorum, J. Microbiol. Methods, 2014, 106, 110–118 CrossRef CAS PubMed.
- Y. Zhu, J. I. Son and Y.-B. Shim, Amplification strategy based on gold nanoparticle-decorated carbon nanotubes for neomycin immunosensors, Biosens. Bioelectron., 2010, 26, 1002–1008 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2023 |






