Polyaniline/SWCNT composite films prepared via the solvent-induced strategy for flexible energy harvesting†
Penglu
Yu
a,
Ruili
Wu
a,
Chan
Liu
b,
Jinle
Lan
 *a,
Yuanhua
Lin
b and
Xiaoping
Yang
a
*a,
Yuanhua
Lin
b and
Xiaoping
Yang
a
aState Key Laboratory of Organic-Inorganic Composites, College of Materials Science and Engineering, Beijing University of Chemical Technology, Beijing 100029, P. R. China. E-mail: lanjl@mail.buct.edu.cn
bState Key Laboratory of New Ceramics and Fine Processing, School of Materials Science and Engineering, Tsinghua University, Beijing 100084, P. R. China
First published on 22nd November 2022
Abstract
Preparing high-performance, stable, and flexible thermoelectric materials via a simple method is significant for promoting practical applications of the thermoelectric system. Herein, a facile solvent-induced strategy has been developed to prepare a polyaniline (PANI)/single-walled carbon nanotube (SWCNT) flexible film without using any small-molecule dopants, which are relatively unstable. For the N,N-dimethylformamide (DMF) solvent-induced composite films, a high power factor of 207.3 μW m−1 K−2 has been obtained at room temperature when the content of SWCNTs is 90 wt% due to the induction effect of the SWCNTs and charge transfer effect between PANI and the SWCNTs. Furthermore, the effect of temperature on TE performance in the PANI-based nanocomposites has been investigated via in situ structural analysis. A prototype device with eight pieces of PANI/SWCNT films connected by silver wires has been fabricated and its maximum output power reaches 1628 μW at a temperature difference of 66.8 K, which corresponds to a normalized maximum output power density of 27.4 μW m−1 K−2. This work imparts a deeper understanding of PANI-based nanocomposites and has great significance in practical applications for wearable energy harvesting.
1. Instruction
Flexible thermoelectric (TE) materials, especially conducting polymers and their composites, possess high flexibility and considerable environmental stability. They can be processed into various forms, such as films and fabrics, and have promising potential in wearable thermoelectric generators (TEGs). TEGs have been considered semi-permanent energy suppliers, and are therefore significant for the Internet of Things (IoT) era and the new energy revolution.1–9 The efficiency of TE materials is evaluated using the dimensionless figure of merit, ZT = S2σTκ−1, where S is the Seebeck coefficient, σ is electrical conductivity, κ is thermal conductivity, and T is the absolute temperature. For conductive polymer materials with low thermal conductivity, the TE performance is mainly characterized using the power factor PF = S2σ.Polyaniline (PANI) is a promising material in the field of thermoelectric research. It has been extensively studied recently because of its ease of processing, tunable properties, and better stability compared to other intrinsically conducting polymers.10,11 So far, studies on optimization strategies to improve the thermoelectric performance of PANI have ranged from the synthesis of PANI derivatives to the preparation of PANI-based thermoelectric composites, from nanoparticle incorporation to molecular self-assembly, and from doping engineering to interface engineering.4,12–17 In brief, previous reports have revealed that higher PF can be obtained by properly optimizing the microstructure11,16 or by appropriately adding special nanomaterials. For instance, Lin et al.4 reported that Zr-MOF/PANI composite films possessed an exceptional Seebeck coefficient and outstanding PF of ∼664 μW m−1 K−2. Likewise, remarkable improvements were achieved in both electrical conductivity and the Seebeck coefficient by adding a small amount of NiO with PANI.12
Moreover, the combination of carbon nanotubes with PANI has attracted extensive attention.13 Incorporating carbon nanotubes (CNTs) in PANI to form hybrid composites could induce a highly ordered PANI microstructure.14,18–20 The electrically percolated CNT network would create perfect carrier transport pathways to enhance the electrical conductivity of the composites. The Seebeck coefficient is enhanced due to the interface effect.17,21 Previous studies have found that it is possible to modulate the thermoelectric performance of CNTs/PANI composites by doping engineering;15,22 protic acid served as dopants, such as small-molecule protic acid,23 polymer acid24 and the self-doped polymer protonic acid.25 However, most of the reports about doping engineering are focused on the types of protic acids, the doping mechanism of protic acids, and the doping level of protic acids.22,26 There are few reports to emphasize the importance of the interaction between PANI and CNTs and its influence on transport properties. Pristine carbon nanotubes are relatively good electron acceptors because of oxygen impurity.27 Therefore, strong interactions would be formed between the CNTs and amine groups, which have the electron-donating ability.28 The interaction between PANI and CNTs would result in charge transfer; thus the CNTs may compete with protic acid and have a dopant effect as suggested previously.29 The studies on polyaniline doping, heretofore, have focused more on various small-molecule protic acids30 without harsh environment tolerance. The samples would become partially de-doped in the alkaline or thermal environment31–33 which is in conflict with commercial applications in a complex environment.
Additionally, few reports emphasize the role of solvents in the induction of composite microstructure during the fabrication and the effect on material properties. In our previous studies, we successfully prepared flexible PANI/SWCNT thermoelectric films with good performance. We studied the influence of the polymerization process, preparation method, and treatment of different solvents on thermoelectric properties.5,34 In this work, we prepared composites of SWCNTs and PANI using solvent-induced strategy in different solvents and explored the effect of solvent type on the microstructure of PANI-based composites. DMF solvent was able to induce the achievement of a uniform and orderly coating of PANI on SWCNTs. As a result, an optimized microstructure of the composites was formed, which improved the interface between them and enhanced the interfacial effect. Herein, we prepared flexible PANI/SWCNTs films with an enhanced thermoelectric performance by a facile solvent-induced strategy, without using any small-molecule dopants, but via the doping effect of carbon nanotubes. The PF of PANI/SWCNTs films could reach up to 207.3 μW m−1 K−2 at 300 K. An experimental investigation was also made to research the effect of temperature on the properties of the PANI/SWCNTs composites. The reversible changes in the oxidized forms of polyaniline with temperature were revealed via in situ infrared spectroscopy (in situ IR). Those changes might have a significant influence on the transport properties of polyaniline, which needs to be considered in practical applications. Finally, to verify the application potential of the PANI/SWCNTs in heat-to-electrical power conversion systems, a high-performance flexible thermoelectric generator module was achieved by an eight-leg flexible thermoelectric generator (TEG) assembled with the base film. The flexible TEG exhibited the maximum output power of 1628 nW and the highest normalized maximum power density of 27.4 μW m−1 K−2 at a temperature difference (ΔT) of 66.8 K.
2. Experimental
2.1 Materials and chemicals
All chemicals used in this study were of analytical grade. Aniline (ANI) was obtained from the Tianjin Guangfu Chemical Reagent Factory. Ammonium persulfate (APS) was purchased from Beijing BAILINGWEI Technology Co. Ltd. Hydrochloric acid (HCl) and ammonium hydroxide (NH3·H2O) were purchased from Sigma-Aldrich. Single-walled carbon nanotubes (SWCNTs) (diameter: 1–2 nm; length: 5–30 μm) with high purity (>95.0 wt%) and large specific surface area (>1075 m2 g−1) were received from Nanjing XFNANO Materials Technology Co. Ltd.2.2 Materials and chemicals
Synthesis of PANI: PANI powders were prepared following the method reported in the literature.5 A mixture of ANI, APS, and HCl was stirred in an ice bath for 12 h. After polymerization, the resulting product was collected by filtration and transferred into NH3·H2O solution with continuous stirring for 12 h at room temperature to de-dope the PANI. Subsequently, the suspension was filtered and rinsed with deionized water and ethanol to remove the residues, followed by vacuum drying in vials at 55 °C for 6 h.2.3 Preparation of PANI/SWCNTs flexible films
PANI/SWCNTs composite films were prepared in the following way. At first, charge de-doped PANI and SWCNTs at a certain weight ratio in DMF solvent to prepare dispersions, separately, then mix them, followed by stirring for one day. Finally, PANI/SWCNTs films were prepared by vacuum filtration and treated at a pressure of 10 MPa for 60 s after drying overnight. To study the effect of the content of SWCNTs on the properties of PANI/SWCNTs films, the weight fraction of SWCNTs was adjusted from 0.1 to 0.9 and the corresponding samples were named P-PANI-xCNT (where x indicates the weight fraction of CNTs and P-means the sample was cold pressed), respectively. The films prepared using DMF solvent were denoted P-DMF-xCNT films, while the P-EtOH-xCNT was prepared by a procedure similar to that mentioned above except that the DMF solvent was replaced by ethanol solvent.2.4 Characterization
The morphology of PANI and SWCNTs/PANI films was characterized by transmission electron microscopy (TEM, 2000 EX, JEOL, Japan) at an accelerating voltage of 100 kV. The surface construction of the pristine and composite films was observed by field emission scanning electron microscopy (SEM, Supra55, Carl Zeiss, Germany). In order to clarify the mechanism of internal electrical transport, conductive AFM (c-AFM, MFP-3D infinity AFM, Oxford) was also applied. Raman spectra were collected to identify the shift by using an InVia Reflex confocal Raman microscope (Renishaw, UK) within the wavenumber range of 1000–2000 cm−1 with a laser of 532 nm. The crystalline structures of the composites were examined by X-ray diffraction (XRD) using a Bruker D8 ADVANCE X-ray diffractometer with Cu Kα radiation (λ = 0.154 nm). X-ray photoelectron spectroscopy (XPS, ESCALAB 250, ThermoFisher Scientific, America) and Fourier Transform infrared spectroscopy (FTIR, Nicolet-380 and In situ IR, (Nicolet iS50)) were also used for composites structure analysis. The Hall coefficient (RH) was measured on a Hall system equipped with a 5 T magnetic field (Cryogenic Limited, U.K.). The carrier concentration (n) and carrier mobility (μ) were estimated from the measured RH and σ from the relation n = r/e|RH| (assuming the Hall factor r = 1.0) and μ = σ|RH|.The Seebeck coefficient (S) and electrical conductivity (σ) were measured simultaneously under a helium atmosphere on ZEM-3(ULVAC-RIKO, Japan).
2.5 Assembly and measurement of planar TEG
A TEG with eight legs was assembled with the P-DMF-0.9CNT film on polyethylene glycol terephthalate (PET). Each TE leg measured 5 mm in width, 25 mm in length, and 10 μm in thickness. The planar TEG was placed on a temperature-controlled heater and a commercial TE cooler was placed on the top side. The Keysight 34972A Data Acquisition/Data Logger was used to measure its output performance as well as the hot end and cold end temperatures.3. Results and discussion
By the fabrication process demonstrated in the experimental section, PANI/SWCNTs composite films were prepared with two different solvents. Five different sets of PANI/SWCNTs hybrid films with different CNT weight fractions (10, 30, 50, 70, and 90 wt%) were prepared.Fig. 1 shows the microstructure of the composites prepared using ethanol and DMF solvents. It is obvious that CNTs and PANI have different morphologies when induced by different solvents. As shown in SEM images (Fig. 1(a) and (b)) of the DMF-0.1CNT films, it was confirmed that the carbon nanotubes were well dispersed in the composites and a continuous network was formed in the composite films. In addition, a thin layer of PANI component was uniformly coated on the surface of the CNTs due to the induction of DMF solvent. PANI as the interlayer spacer would prevent the aggregation of CNTs to some extent. Meanwhile, a great number of nanointerfaces were formed, which benefit the composite interaction between CNTs and PANI. As the content of CNTs increased, the coating layers of PANI gradually became thinner and smoother (Fig. S2(a)–(d)†). The TEM images (Fig. 1(c) and (d)) further demonstrate that the CNTs were coated with a thin layer of polyaniline. Some adjacent PANI/SWCNT bundles were connected by PANI. In this case, PANI played a dual role. It improved the conductive network to improve the electrical transport properties of composite materials. Simultaneously, heterogeneous structures were formed to scatter low-energy carriers, thus increasing the Seebeck coefficient. In contrast, a large number of polyaniline agglomerated particles were observed to be unevenly dispersed around the surface of the CNTs in the composites prepared using ethanol solvent (Fig. 1(e) and (f)). The SEM image (Fig. S1(e)†) also shows that isolated and agglomerated PANI particles were deposited in the network of CNT bundles. In this case, PANI had a certain role as an interfacial binder and filler in the composite films. But the microstructure was not as well developed as in the composites prepared using DMF solvent. It was more like a physical mixing between the PANI particles and CNT bundles.
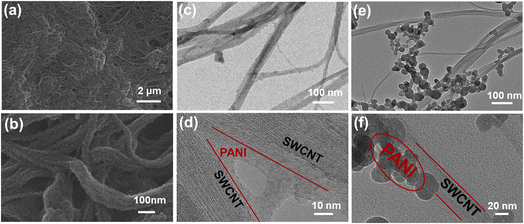 | ||
| Fig. 1 (a) Low- and (b) high-magnification SEM images of the DMF-0.1CNT film. Typical TEM images of (c and d) DMF-0.1CNT and (e and f) EtOH-0.1CNT. | ||
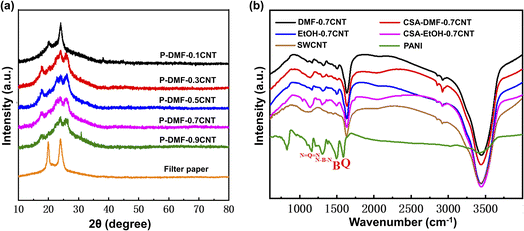
The structure and morphology of PANI-0.7CNT films prepared using different solvents were investigated by X-ray diffraction (XRD) patterns (Fig. S2†). The pure PANI films exhibited three broad peaks at 2θ = 15°, 20°, and 25°, which were caused by the repeat units of the polyaniline chain, the periodicity parallel to the polymer chain, and the periodicity perpendicular to the polymer chain. For the pure SWCNTs, a broad diffraction peak at 2θ = 25.6° was assigned to the (002) graphite plane of the SWNTs.17 Compared with a broad bulge of 2θ = 20° to 30° observed in the XRD pattern of the samples before cold pressing, clear diffraction peaks were observed after cold pressing. This indicated that the PANI particles and CNTs were more compact and orderly after the cold pressing treatment, which was more conducive to the synergistic interaction between them. In addition, the diffraction peaks of films prepared using the DMF solvent were more intense after the cold pressing treatment compared to the films prepared using the ethanol solvent. In the XRD pattern of P-DMF-0.7CNT composite films, the peak at 2θ = 15° was weakened compared to that in the pure polyaniline film, which was found in the PANI doped with methylene blue.35 The other characteristic peaks of the composite films became more distinct and sharper, suggesting that the crystallinity increased and the molecular arrangement of the PANI became more orderly, which may be ascribed to the conformation transition caused by the charge transfer36 and inductive effect of SWCNTs on PANI.18 The XRD patterns of P-DMF-xCNT composite films with different CNT contents are shown in Fig. 2(a). All samples exhibited similar characteristic peaks to the P-DMF-0.7CNT. With the increase in the CNTs content, the characteristic peak of polyaniline became gradually weaker. Among them, the P-DMF-0.1CNT film was highly influenced by the filter paper in the test because it was too thin in thickness.
Fig. 2(b) shows the FTIR spectra of the PANI, SWCNTs, and PANI-0.7CNT composite films. The FTIR spectrum of raw SWCNTs exhibited a band near 1636 cm−1 that was attributed to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching of the polyaromatic backbone of the SWCNTs.37 The peak at 3421 cm−1 was attributed to N–H stretching vibration in PANI and the peak at 826 cm−1 was attributed to C–C and C–H stretches for benzenoid units of PANI.38 FTIR peaks of PANI located at 1587, 1495, 1306 and 1162 cm−1 were characteristic of the quinonoid structure, benzenoid structure, C–N aromatic amine, and –N
C stretching of the polyaromatic backbone of the SWCNTs.37 The peak at 3421 cm−1 was attributed to N–H stretching vibration in PANI and the peak at 826 cm−1 was attributed to C–C and C–H stretches for benzenoid units of PANI.38 FTIR peaks of PANI located at 1587, 1495, 1306 and 1162 cm−1 were characteristic of the quinonoid structure, benzenoid structure, C–N aromatic amine, and –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) quinoid
quinoid![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– (electron-like band) stretching modes of PANI.39 A striking difference between PANI and DMF-0.7CNT composites was found in that these peaks shifted to 1596, 1498, 1319, and 1166 cm−1. The blue shifts of the absorption bands were attributed to the strong interaction between macromolecule chains and SWCNTs, such as π–π stacking and the charge transfer effect. The shifts were also observed in the EtOH-0.7CNT composites, but the trend was not as pronounced as in DMF-0.7CNT composites. Meanwhile, the composites prepared from camphorsulfonic acid-doped PANI (CSA-doped PANI) were also investigated using FIIR spectra analysis. It was found that a slight blue shift occurred in the peaks at 1587 and 1495 cm−1, while the peak at 1162 cm−1 showed a redshift. In addition, a characteristic broad band extending from 1700 to 4000 cm−1 was observed in the FTIR spectra of PANI-0.7CNT composites, which was due to the free-charge carrier transfer in the PANI.32 These various changes indicated the interaction between PANI and SWCNTs that may have resulted in charge transfer.
N– (electron-like band) stretching modes of PANI.39 A striking difference between PANI and DMF-0.7CNT composites was found in that these peaks shifted to 1596, 1498, 1319, and 1166 cm−1. The blue shifts of the absorption bands were attributed to the strong interaction between macromolecule chains and SWCNTs, such as π–π stacking and the charge transfer effect. The shifts were also observed in the EtOH-0.7CNT composites, but the trend was not as pronounced as in DMF-0.7CNT composites. Meanwhile, the composites prepared from camphorsulfonic acid-doped PANI (CSA-doped PANI) were also investigated using FIIR spectra analysis. It was found that a slight blue shift occurred in the peaks at 1587 and 1495 cm−1, while the peak at 1162 cm−1 showed a redshift. In addition, a characteristic broad band extending from 1700 to 4000 cm−1 was observed in the FTIR spectra of PANI-0.7CNT composites, which was due to the free-charge carrier transfer in the PANI.32 These various changes indicated the interaction between PANI and SWCNTs that may have resulted in charge transfer.
Fig. 3(a) presents the Raman spectra of the surface of PANI-0.7CNT films prepared using EtOH and DMF solvents. The characteristic peaks at around 1161, 1330, 1490, and 1591 cm−1 were successively assigned to the C–H bending vibrations of the quinoid/benzenoid ring, C–N+ vibration of the quinoid ring, N–H stretching vibration, and C–C stretching vibration of the benzenoid ring.40 Compared with pure PANI films (the peaks are located at 1171, 1337, 1501, and 1596 cm−1),5 all of these peaks underwent a certain change in redshift, which indicated that strong interactions existed between PANI and SWCNTs. Moreover, to investigate the homogeneity of the structure in the composite, Raman mapping analyses were performed on the P-DMF-0.7CNT composite film, the yellow dashed area in Fig. 3(b) shows the mapping points. The homogeneity of the structural distribution of the composite samples was demonstrated from the uniform distribution of the peak area at 1591 cm−1 in Fig. 3(c). It was observed from Fig. 3(d) that the characteristic diffraction peaks of the C–H bending vibration of the quinoid/benzenoid ring were mainly distributed between 1158 and 1161 cm−1, which proved the uniformity of the interaction strength between PANI and CNTs in the composite system. Fig. S3† shows the Raman spectra of P-DMF-xCNT composite films. As the content of CNTs increased, the peak at around 1591 cm−1 was enhanced and the intensities of the peaks at around 1490, 1218, and 1161 cm−1 were decreased. However, there was an abnormal change in the peak (1D peak) at around 1330 cm−1, which did not increase proportionally with the increase in second-order related harmonic G′-peak (around 1593 cm−1) and the 2D peak (around 2660 cm−1) of SWCNTs.41 If the characteristic peak of the linear deformation of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N+ bond was taken into account,42 this phenomenon would be easily explained and indirectly demonstrated the doping effect of SWCNTs. With the addition of SWCNTs, the C
N+ bond was taken into account,42 this phenomenon would be easily explained and indirectly demonstrated the doping effect of SWCNTs. With the addition of SWCNTs, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N+ bonds were formed and reached a high quantity, the peak at 1330 cm−1 reached a ceiling value when the CNT content was 70%. Then, the peak remained almost unchanged, as the decrease in C
N+ bonds were formed and reached a high quantity, the peak at 1330 cm−1 reached a ceiling value when the CNT content was 70%. Then, the peak remained almost unchanged, as the decrease in C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N+ bonds was nearly offset by the increase in D band with the increase in SWCNTs and the reduction of PANI. The P-DMF-0.7CNT films were examined by X-ray photoelectron spectroscopy (XPS), in order to further verify the structural changes of PANI in PANI/SWCNT composites. The XPS N 1s peaks for the PANI/SWCNTs composite films are shown in Fig. S4,† the major peak line was decomposed into three peak lines; the highest energy line was centered at ≈402.7 eV, which was assigned to the C
N+ bonds was nearly offset by the increase in D band with the increase in SWCNTs and the reduction of PANI. The P-DMF-0.7CNT films were examined by X-ray photoelectron spectroscopy (XPS), in order to further verify the structural changes of PANI in PANI/SWCNT composites. The XPS N 1s peaks for the PANI/SWCNTs composite films are shown in Fig. S4,† the major peak line was decomposed into three peak lines; the highest energy line was centered at ≈402.7 eV, which was assigned to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N+,43 proved that the charge transfer effect may positively charge the nitrogen atom. The other two lower energy lines were centered at 399.8 and 398.3 eV, which were successively assigned to the neutral amine (–NH–) and imine (–N
N+,43 proved that the charge transfer effect may positively charge the nitrogen atom. The other two lower energy lines were centered at 399.8 and 398.3 eV, which were successively assigned to the neutral amine (–NH–) and imine (–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ).44
).44
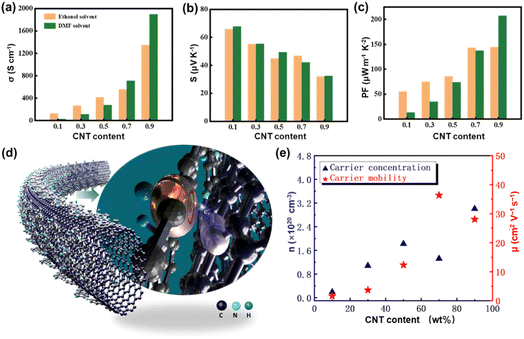
The TE properties of the PANI-xCNT films prepared using EtOH and DMF solvents at room temperature (RT) are shown in Fig. 4(a)–(c). All the samples exhibited excellent stability with almost complete retention of their original properties after treatment with water (Fig. S5†). It was interesting to observe that the electrical conductivity value of the P-DMF-xCNT film was lower than that of the P-EtOH-xCNT film at low CNT content and significantly higher than that of the P-EtOH-xCNT film at a high CNT content (Fig. 4(a)). The value of the Seebeck coefficient of the P-DMF-xCNT film was always approximately equal to that of the P-EtOH-xCNT film (Fig. 4(c)). The PF of the P-DMF-xCNTs composite was enhanced greatly by increasing the weight fraction of CNTs and reached a maximum of 207.3 μW m−1 K−2 at RT with 90 wt% CNT content, which was higher than that (144 μW m−1 K−2) of the P-EtOH-xCNTs.
Fig. S6† shows the increased RT electrical transport properties of the P-DMF-xCNT composites as an increasing function of CNT content. The electrical conductivity of composites reached up to 1895 S cm−1 at RT with 90 wt% SWCNT content, which was higher than that of the pure SWCNT film (470.1 S cm−1 at RT),6 indicating that this enhancement did not follow the simple mixing model. There was a significant increase in the electrical conductivity with every one percent SWCNTs content change at first and the normalized rate of increase (the rate of increase divided by the percentage change in SWCNTs content) of the electrical conductivity can reach 15%, which was likely due to the inductive effect of SWCNTs on PANI and the charge transfer effect mentioned previously. Then, it decreased to ∼7% and remained stable because of the gain saturation effect. Fig. 4(d) is the schematic diagram of the charge transfer effect between PANI and SWCNT. In addition, the carrier concentration and carrier mobility were investigated by a Hall measurement system. It showed that the carrier mobility of the composites enhanced significantly with increasing the weight fraction of CNTs, which was attributed to the fact that the addition of CNTs can improve the arrangement and orientation of the PANI, thereby increasing the carrier mobility.18,19 Meanwhile, the carrier concentration also increased significantly at first, which explained the higher increase rate of the electrical conductivity at the beginning.
In order to further identify the influence of CNT addition to PANI on electrical transport behaviors, the conductivity and topography mappings (Fig. 5) were obtained using conductive atomic force microscopy (C-AFM). The topography (Fig. 5(a)) and conductivity (Fig. 5(c)) of the surface of the pure PANI film were found to be uniform. The bright circular patterns in Fig. 5(a) may come from the aggregated particles of PANI. Some cluttered lines were observed in Fig. 5(c), which represented the CNT bundles. The conductivity mappings in Fig. 5(d) show that the CNT bundles and the PANI distributed around the CNT bundles exhibited much higher conductivity, as compared to other parts of the film. The higher electrical conduction of PANI distributed around the CNT bundles implicated the interactions between PANI and SWCNTs, which likely correlated with the inductive effect of SWCNTs on PANI and the charge transfer effect between PANI and SWCNTs, as discussed in previous XRD and FTIR sections. In conclusion, our c-AFM results provide proof for the existence of the interactions between PANI and SWCNTs in the composites, which greatly improved the electrical properties of the PANI layer.
 | ||
| Fig. 5 AFM topography (left) and corresponding c-AFM (right) of (a) and (b) pure PANI and (c) and (d) the P-DMF-0.7CNT film. | ||
Fig. 6a–c exhibit the TE properties of the P-DMF-xCNT films from 300 to 410 K. As the temperature increased, the electrical conductivity showed a decreased tendency to suggest a metallic or heavily doped semiconductor characteristic, while the Seebeck coefficients of the films increased initially and decreased afterward (the inflection point was around 373 K, for the film when the weight ratio of SWCNTs was greater than 10%). The results of the combination of the Seebeck coefficient and electrical conductivity, the PF of the P-DMF-0.9CNT reached a maximum value of 402 μW m−1 K−2 at 358 K, which was much higher than the maximum value of 329 μW m−1 K−2 for the P-EtOH-0.9CNT at 358 K (Fig. S7(c)†). The TE properties of the P-DMF-CNT composite film were very competitive when compared with the previously reported PANI/CNTs TE materials, as shown in Table 1.
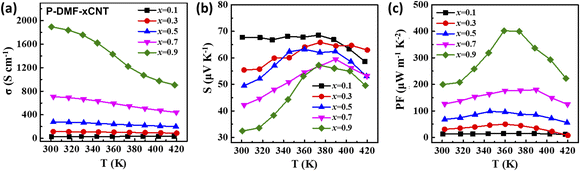 | ||
| Fig. 6 Temperature dependence of (a) electrical conductivity, (b) Seebeck coefficient, and (c) power factor for the P-DMF-xCNT composite films. | ||
| Synthetic method | Type of solvent used | PF (10−6 W m K−2) at room temperature | PF (10−6 W m K−2) at high temperature | Reference |
|---|---|---|---|---|
| In situ polymerization | m-Cresol | 217 | 20 | |
| Solution mixing | DMF | 114.4 | 402 | 34 |
| Solution mixing | m-Cresol | 56 | 100 | 5 |
| In situ polymerization | Water/ethanol (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
0.32 | 16 | |
| In situ polymerization | HCl solution | 20 | 18 | |
| In situ polymerization | Aqueous solution | 4.5 | 16.8 | 45 |
| In situ and dynamic interfacial polymerization | H2O, HCl | 0.74 | 1.21 | 46 |
| Solution mixing | m-Cresol | 51 | 47 | |
| Solution mixing | Ethanol | 114.6 | 329 | This work |
| Solution mixing | DMF | 207.3 | 402 | This work |
The structure transition of the P-DMF-0.7CNT film was investigated by means of in situ IR measurements over the temperature range 303 to 403 K, spectra are illustrated in Fig. 7. The film was then cooled to 288 K, reversible thermal behavior of the P-DMF-0.7CNT film was examined by comparing various vibration peaks of the obtained sample with the previous ones, which suggested that no degrading chemical reactions occur. In addition, IR was successfully applied to study H2O confined in the nanostructure. The spectral range used in the in situ IR enabled it to cover not only the OH stretching band and the bending mode of H2O, but also the librational and hindered translational band.48 The intense low-frequency components between 3284 and 3662 cm−1 were assigned to the bulk water in the composite and a decrease in the relative intensity with temperature could be determined as a reduction in water content, as low water content led to a decrease in the conductivity. The changes observed in the electrical conductivity of PANI induced by the water content were shown to be reversible to a high degree.49
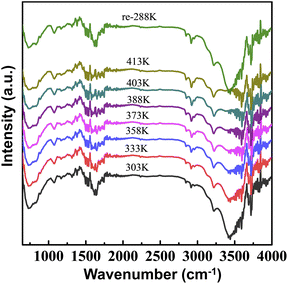 | ||
| Fig. 7 In situ infrared spectroscopy of the P-DMF-0.7CNT composite film. All spectra are normalized. | ||
With increasing operating temperature, the vibrational band characteristic of the quinonoid structure of the P-DMF-0.7CNT films formed at 1596 cm−1 was shifted to 1593 cm−1 and had a continuous enhancement of the relative intensity, indicating an increased quinonoid structure. Meanwhile, there was no significant shift of the characteristic absorption band at 1498 cm−1 but a fine weakening of the absorbance. The conductivity of PANI depended on two variables, namely, the degree of protonation of the material and the degree of oxidation. The reversible change of the FTIR peaks, which were characteristic of the quinonoid structure and the benzenoid structure, suggested the interconversion of the oxidation states of PANI. The oxidized form of polyaniline gradually changed from an emeraldine oxidation state to a nigraniline oxidation state with temperature and caused a reduction in electrical conductivity.50 Therefore, in situ IR measurements confirmed the change in water content and the interconversion of oxidized forms of PANI in the PANI/SWCNTs composite with temperature, and these variations effectively affected the total carrier transport behavior in the PANI-based nanocomposites.
As shown in Fig. 8(a), 8 pieces of P-DMF-0.9CNT films were series-connected by silver wires to form a planar thermoelectric generator (TEG). The length, width, and thickness of each leg were 25 mm, 5 mm, and 10 μm, respectively. There was a demonstration of the heat-to-electrical power conversion test using the TEG in Fig. 8(b) and (c). Fig. 8(d) and (e) illustrate the output voltage and power of the TEG at ΔT of 12.4 K, 39.3 K, 54.2 K, and 66.8 K, as a function of the current. The power density (PD) was obtained by dividing the power by the cross-sectional area and the number of legs. At the maximum ΔT of 66.8 K, a power output as high as 1628 nW was generated, which corresponded to a maximum output power density (PDmax) of 407 mW cm−2, indicating that it could be used to power smart wearable devices such as a wristwatch with low power consumption and was an excellent candidate for self-powered wearable systems. In addition, the normalized maximum power density (PDmax × l/ΔT2) was used for comparison, a value of 27.4 μW m−1 K−2 was calculated by dividing the product of maximum power density and length (l) of the TEG by the ΔT2,51 which demonstrated that the present TEG possessed an extremely competitive ability among the reported organic–inorganic flexible TEGs, as shown in Fig. 8(f).8,17,46,52–56
4. Conclusions
In this study, we successfully fabricated flexible PANI/SWCNTs nanocomposite films by a facile solvent-induced strategy without using any small-molecule dopants. In the DMF solvent-induced composite films, a significant enhancement in the thermoelectric performance was found, which could be explained by the inductive effect of SWCNTs on PANI and the charge transfer effect between PANI and SWCNTs. Based on this, a high power factor of 207.3 μW m−1 K−2 at room temperature was obtained. Furthermore, we explored the mechanisms of temperature influence on thermoelectric performance in PANI-based nanocomposites. The reversible changes in the oxidized forms of polyaniline and the water content with temperature were revealed by an in situ structural analysis, which had a significant influence on the transport properties of polyaniline. To a certain extent, the reversible partial functional failure of the PANI component caused by the changes may play a vital role in the influence of thermoelectric performance. In this sense, our work imparts a deeper understanding of the effects of temperature on thermoelectric performance in PANI-based nanocomposites. Moreover, a prototype device with eight pieces of PANI/SWCNTs films connected by a silver wire was fabricated. The maximum power density of the device reached up to 407 μW cm−2 at a ΔT of 66.8 K, which corresponded to a normalized maximum output power density of 27.4 μW m−1 K−2, and indicated a highly competitive advantage among reported organic–inorganic flexible TEGs. This work has great significance for the applications of the PANI-based nanocomposite film for wearable energy harvesting.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (No. 51772016) and Fundamental Research Funds for the Central Universities (XK1802-2).References
- K. Kanahashi, J. Pu and T. Takenobu, Adv. Energy Mater., 2020, 10, 1902842 CrossRef CAS.
- Y. Du, J. Xu, B. Paul and P. Eklund, Appl. Mater. Today, 2018, 12, 366–388 CrossRef.
- T. Sun, B. Zhou, Q. Zheng, L. Wang, W. Jiang and G. J. Snyder, Nat. Commun., 2020, 11, 1–10 CrossRef PubMed.
- C.-C. Lin, Y.-C. Huang, M. Usman, W.-H. Chao, W.-K. Lin, T.-T. Luo, W.-T. Whang, C.-H. Chen and K.-L. Lu, ACS Appl. Mater. Interfaces, 2019, 11, 3400–3406 CrossRef CAS PubMed.
- R. Wu, H. Yuan, C. Liu, J.-L. Lan, X. Yang and Y.-H. Lin, RSC Adv., 2018, 8, 26011–26019 RSC.
- C. Liu, D.-L. Shan, Z.-H. Shen, G.-K. Ren, Y. Wang, Z.-F. Zhou, J.-Y. Li, D. Yi, J.-L. Lan, L.-Q. Chen, G. J. Snyder, Y.-H. Lin and C.-W. Nan, Nano Energy, 2021, 89, 106380 CrossRef CAS.
- C.-J. Yao, H.-L. Zhang and Q. Zhang, Polymers, 2019, 11, 107 CrossRef PubMed.
- Y. Lu, Y. Qiu, Q. Jiang, K. Cai, Y. Du, H. Song, M. Gao, C. Huang, J. He and D. Hu, ACS Appl. Mater. Interfaces, 2018, 10, 42310–42319 CrossRef CAS PubMed.
- L. Feng, P. Yu, C. Liu, J. Lan, Y.-H. Lin and X. Yang, ACS Appl. Mater. Interfaces, 2022, 14, 23765–23774 CrossRef CAS PubMed.
- J. Wu, Y. Sun, W. Xu and Q. Zhang, Synth. Met., 2014, 189, 177–182 CrossRef CAS.
- Q. Yao, Q. Wang, L. Wang, Y. Wang, J. Sun, H. Zeng, Z. Jin, X. Huang and L. Chen, J. Mater. Chem. A, 2014, 2, 2634–2640 RSC.
- K. Sarkar, A. Debnath, K. Deb, A. Bera and B. Saha, Energy, 2019, 177, 203–210 CrossRef CAS.
- Y. Zhang, Q. Zhang and G. Chen, Carbon Energy, 2020, 2, 408–436 CrossRef CAS.
- Q. Wang, Q. Yao, J. Chang and L. Chen, Mater. Chem., 2012, 22, 17612–17618 RSC.
- H. Li, Y. Liang, S. Liu, F. Qiao, P. Li and C. He, Org. Electron., 2019, 69, 62–68 CrossRef CAS.
- J. Chen, L. Wang, X. Gui, Z. Lin, X. Ke, F. Hao, Y. Li, Y. Jiang, Y. Wu, X. Shi and L. Chen, Carbon, 2017, 114, 1–7 CrossRef CAS.
- L. Wang, Q. Yao, W. Shi, S. Qu and L. Chen, Mater. Chem. Front., 2017, 1, 741–748 RSC.
- Q. Yao, L. Chen, W. Zhang, S. Liufu and X. Chen, ACS Nano, 2010, 4, 2445–2451 CrossRef CAS PubMed.
- Y. Liao, C. Zhang, X. Wang, X.-G. Li, S. J. Ippolito, K. Kalantar-zadeh and R. B. Kaner, J. Phys. Chem. C, 2011, 115, 16187–16192 CrossRef CAS.
- L. Wang, Q. Yao, J. Xiao, K. Zeng, S. Qu, W. Shi, Q. Wang and L. Chen, Chem.–Asian J., 2016, 11, 1804–1810 CrossRef CAS PubMed.
- H. Wang, S. Yi and C. Yu, Polymer, 2016, 97, 487–495 CrossRef CAS.
- H. Li, S. Liu, P. Li, D. Yuan, X. Zhou, J. Sun, X. Lu and C. He, Carbon, 2018, 136, 292–298 CrossRef CAS.
- M. Gosh, A. Barman, A. K. Meikap, S. K. De and S. Chatterjee, Phys. Lett. A, 1999, 260, 138–148 CrossRef CAS.
- J. Tarver, J. E. Yoo, T. J. Dennes, J. Schwartz and Y.-L. Loo, Chem. Mater., 2009, 21, 280–286 CrossRef CAS.
- J. Yue and A. J. Epstein, J. Am. Chem. Soc., 1990, 112, 2800–2801 CrossRef CAS.
- H. Li, Y. Liang, Y. Liu, S. Liu, P. Li and C. He, Compos. Sci. Technol., 2021, 210, 108797 CrossRef CAS.
- Y. Nonoguchi, K. Ohashi, R. Kanazawa, K. Ashiba, K. Hata, T. Nakagawa, C. Adachi, T. Tanase and T. Kawai, Sci. Rep., 2013, 3, 3344 CrossRef PubMed.
- M. Shim, A. Javey, N. W. Shi Kam and H. Dai, J. Am. Chem. Soc., 2001, 123, 11512–11513 CrossRef CAS PubMed.
- H. Zengin, W. Zhou, J. Jin, R. Czerw, D. w. Smith Jr, L. Echegoyen, D. l. Carroll, S. h. Foulger and J. Ballato, Adv. Mater., 2002, 14, 1480–1483 CrossRef CAS.
- M. Wan and J. Yang, J. Appl. Polym. Sci., 1995, 55, 399–405 CrossRef CAS.
- R. Ansari, W. E. Price and G. G. Wallace, Polymer, 1996, 37, 917–923 CrossRef CAS.
- Y. Wang and M. F. Rubner, Synth. Met., 1992, 47, 255–266 CrossRef CAS.
- P. Rannou, D. Rouchon, Y. F. Nicolau, M. Nechtschein and A. Ermolieff, Synth. Met., 1999, 101, 823–824 CrossRef CAS.
- L. Feng, R. Wu, C. Liu, J. Lan, Y.-H. Lin and X. Yang, ACS Appl. Energy Mater., 2021, 4, 4081–4089 CrossRef CAS.
- H. K. Chaudhari and D. S. Kelkar, Polym. Int., 1997, 42, 380–384 CrossRef CAS.
- J. P. Pouget, M. E. Jozefowicz, A. Epstein, X. Tang and A. G. Macdiarmid, Macromolecules, 1991, 24, 779–789 CrossRef CAS.
- Y. Liao, D.-G. Yu, X. Wang, W. Chain, X.-G. Li, E. M. V. Hoek and R. B. Kaner, Nanoscale, 2013, 5, 3856–3862 RSC.
- M. Amrithesh, S. Aravind, S. Jayalekshmi and R. S. Jayasree, J. Alloys Compd., 2008, 449, 176–179 CrossRef CAS.
- Y. Liao, C. Zhang, Y. Zhang, V. Strong, J. Tang, X.-G. Li, K. Kalantar-zadeh, E. M. V. Hoek, K. L. Wang and R. B. Kaner, Nano Lett., 2011, 11, 954–959 CrossRef CAS PubMed.
- J. Laska, R. Girault, S. Quillard, G. Louarn, A. Proń and S. Lefrant, Synth. Met., 1995, 75, 69–74 CrossRef CAS.
- S. Costa, E. Borowiak-Palen, M. Kruszyńska, A. Bachmatiuk and R. J. Kaleńczuk, Mater. Sci.-Pol., 2008, 26, 433–441 CAS.
- M. Cochet, G. Louarn, S. Quillard, J. P. Buisson and S. Lefrant, J. Raman Spectrosc., 2000, 31, 1041–1049 CrossRef CAS.
- S. N. Kumar, F. Gaillard, G. Bouyssoux and A. Sartre, Synth. Met., 1990, 36, 111–127 CrossRef CAS.
- B. C. Beard and P. Spellane, Chem. Mater., 1997, 9, 1949–1953 CrossRef CAS.
- M. J. Chatterjee, D. Banerjee and K. Chatterjee, Mater. Res. Express, 2016, 3, 085009 CrossRef.
- A. P. Sobha and S. K. Narayanankutty, IEEE Trans. Nanotechnol., 2014, 13, 835–841 CAS.
- L. Wang, Q. Yao, S. Qu, W. Shi and L. Chen, Org. Electron., 2016, 39, 146–152 CrossRef CAS.
- S. Dalla Bernardina, E. Paineau, J.-B. Brubach, P. Judeinstein, S. Rouzière, P. Launois and P. Roy, J. Am. Chem. Soc., 2016, 138, 10437–10443 CrossRef CAS PubMed.
- T. Taka, Synth. Met., 1993, 57, 5014–5019 CrossRef CAS.
- J.-C. Chiang and A. G. MacDiarmid, Synth. Met., 1986, 13, 193–205 CrossRef CAS.
- C. Jiang, Y. Ding, K. Cai, L. Tong, Y. Lu, W. Zhao and P. Wei, ACS Appl. Mater. Interfaces, 2020, 12, 9646–9655 CrossRef CAS PubMed.
- Q. Xu, S. Qu, C. Ming, P. Qiu, Q. Yao, C. Zhu, T.-R. Wei, J. He, X. Shi and L. Chen, Energy Environ. Sci., 2020, 13, 511–518 RSC.
- G. Wu, C. Gao, G. Chen, X. Wang and H. Wang, J. Mater. Chem. A, 2016, 4, 14187–14193 RSC.
- X. Liu, Y. Du, Q. Meng, Y. Dou, M. Jin, J. Xu and S. Z. Shen, Adv. Eng. Mater., 2020, 22, 2000605 CrossRef CAS.
- D. Liu, Z. Yan, Y. Zhao, Z. Zhang, B. Zhang, P. Shi and C. Xue, Results Phys., 2021, 31, 105061 CrossRef.
- D. Liu, Z. Yan, Y. Zhao, Z. Zhang, Y. Zhen, B. Zhang, P. Shi and C. Xue, J. Mater. Res. Technol., 2021, 15, 4452–4460 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2se01295b |
| This journal is © The Royal Society of Chemistry 2023 |