Sub- and supercritical water conversion of organic-rich shale with low-maturity for oil and gas generation: using Chang 7 shale as an example†
Qiuyang
Zhao
 *a,
Yu
Dong
a,
Lichen
Zheng
a,
Tian
Xie
a,
Baercheng
Bawaa
a,
Hui
Jin
ab and
Liejin
Guo
*a
*a,
Yu
Dong
a,
Lichen
Zheng
a,
Tian
Xie
a,
Baercheng
Bawaa
a,
Hui
Jin
ab and
Liejin
Guo
*a
aState Key Laboratory of Multiphase Flow in Power Engineering, Xi'an Jiaotong University, Xi'an 710049, PR China. E-mail: qyzhao@mail.xjtu.edu.cn; lj-guo@mail.xjtu.edu.cn
bXinjin Weihua Institute of Clean Energy Research, Foshan 528216, PR China
First published on 18th November 2022
Abstract
Organic-rich shale resources are large reserves with high hydrocarbon generation potential but are difficult to exploit due to their high solid kerogen content. Supercritical water conversion was proposed as an alternative method to convert kerogen into oil and gas because supercritical water has favorable solubility, dispersion, and reactivity. In this study, Chang 7 shale containing a high TOC content of 15.11%, type II kerogen, and low Ro of 0.36–0.38% in the Ordos basin was taken as a typical example of organic-rich shale with low maturity. A series of experiments at the temperatures of 300–650 °C and a pressure of 25 Mpa were carried out to test the feasibility, and the shale conversion performance was analyzed from three perspectives, hydrocarbon generation of kerogen, the effect of inorganic minerals, and shale pore evolution. The optimum oil and gas yields were, respectively, found to be 352.1 mg (g TOC)−1 (g TOC refers to total organic carbon mass in shale) at 380 °C and 852.0 mL (g TOC)−1 at 650 °C. Compared with pyrolysis in the aluminium retort, supercritical water conversion raised the oil yield at the same temperature (171.4 mg (g TOC)−1 at 380 °C) or reduced the temperature with the same yield (346.8 mg (g TOC)−1 at 520 °C). Chang 7 shale minerals as a whole increased the oil yield by 34.2% at 380 °C but had a negligible effect on gas generation. Among them, the carbonate (dolomite) promoted oil generation but inhibited gas generation, while the silicates (feldspar, quartz, and clay) did the opposite, and the pyrite favored both oil and gas generation. Additionally, the supercritical water conversion significantly increased the shale pore volume and specific surface area because the hydrocarbon generation of kerogen produced many nanopores with slit-like shapes and diameters of 50–5000 nm. This paper provides an in-depth understanding of sub- and supercritical water conversion of low-maturity shale for oil and gas production.
1. Introduction
As conventional oil and gas resources are dwindling, unconventional oil and gas resources become increasingly important over the decades. The success of the US shale revolution1–4 has further inspired more countries to target shale resources. The organic matters in the US marine shales with high maturity are mainly hydrocarbon oil and gas, which can be produced by fracturing the tight shale reservoirs.5,6 As the world's largest oil importer, China is struggling to exploit shale resources to boost oil and gas production due to its large shale reserves of about 100 billion tons.7,8 However, the lacustrine shales with low maturity dominate in China, accounting for about 60–80%, and the organic matter is mainly solid kerogen, which is insoluble in usual organic solvents.7,8 The key to exploiting organic-rich shale with low maturity is to accelerate hydrocarbon oil and gas generation from kerogen.Based on the temperature–time complementary effect of kerogen evolution, a series of heating methods have been proposed for the in situ conversion of oil shale, which are usually divided into three categories according to different heating modes, that is, conduction, convection, and radiation.9,10 The typical conductive heating technologies are Shell ICP (In Situ Conversion Process),11,12 and ExxonMobil Electrofrac™13 by direct electric heating. Shell ICP is considered feasible through several field tests in Green River oil shale reservoirs,14,15 but it usually takes more than one year of heating period to produce oil and gas because of the slow rate of conductive heating and poor thermal conductivity of shale. The typical radiative heating technology is Raytheon RF/CF16 by the radio frequency heating and convective heating and driving supercritical CO2, but the range of radiative heating is small and it is still in development without a field test. Convective heat transfer by high-temperature fluids flow can significantly increase the heating rate, and the representative technologies include Chevron Crush (natural gas, CO2), Amos's CCR (conduction, convection, reflux of hydrocarbon gas),17 TYUT MTI (superheated steam)18,19 and JLU NCW (near critical water).20,21 Zhao et al.10 conducted a comprehensive analysis of the convective heating technology with different heating fluids (steam, hydrocarbon gas, CO2, N2, and other fluids) and concluded that superheated steam is the best heat carrier/agent. It should be noted that these convective heating technologies are primarily designed for the in situ conversion of shallow oil shale. However, neither superheated steam nor near/subcritical water can be used to exploit organic-rich shale with low maturity because of its high reservoir pressure (about 20–25 MPa at the depth of above 1000 m). Above the critical temperature and pressure (374 °C, 22.1 MPa), water becomes supercritical, and it acts as not only a heat carrier but also an organic solvent.22–24 Supercritical water injection is proposed as an innovative technology for the in situ conversion of deep shale with low maturity, and the key to this technology is whether supercritical water can promote kerogen conversion to produce oil and gas.
Given its favourable solubility, dispersion, and reactivity, supercritical water has been widely used in organic matter conversion, including liquefaction and gasification of coal and biomass and upgrading of heavy oil and oil sands.25–28 Coal and biomass in supercritical water will be mainly converted into liquid fuels at relatively low temperatures of 350–500 °C29–32 and gaseous fuels (H2, CH4, CO2, CO, etc.) at higher temperatures of 500–700 °C.33–36 The conversion efficiency and products quality in supercritical water are higher than those in N2, steam, subcritical water, and other solvents such as toluene,37–39 because supercritical water can act as an organic solvent, reactant (e.g. hydrogen donor) and catalyst (acid or base catalyst).40–42 For the recovery and upgrading of heavy oil, supercritical water was found to be miscible with heavy oil and to convert asphaltene into maltene with little or no coking, thus significantly improving oil recovery and oil quality.43–48 As such, supercritical water is an excellent medium for organic matter conversion.
Thermal maturation of organic-rich shale has been extensively studied in the conditions of N2,49–65 steam,18,19,66–69 subcritical water,20,21,70–74 carbon dioxide, and other solvents.37,75–84 Kerogen undergoes two parallel paths in thermal conversion, that are, a direct decomposition to oil and gas, and a successive pathway with macromolecular bitumen or heavy oil as an intermediate.50,51 The product's yield and composition depend on feedstock properties including TOC (total organic carbon), maturity (vitrinite reflectance, Ro) and minerals, process parameters (temperature, pressure, and residence time), and the reactor type. Temperature is found to be a major factor, and oil production increases first and then decreases with temperature. The suggested optimal temperature for in situ conversion in literature is all in the range of 350–450 °C, and the corresponding Ro is 0.8–1.2%. In addition, shale minerals play an important role in organic matter conversion.20,60–62,85 It was found that the conversion reactions were catalyzed by clay minerals and carbonates but inhibited by silicates. The pore structure evolution of shale during thermal maturation is another concern because nanoscale pores in shale restrict the migration of generated oil and gas. There are mainly slit-shaped and ink-bottle nanopores in the shale according to N2 adsorption analysis (isotherm of type II or type IV, hysteresis loop of type H2 or H3). During the heating process, micropores (<2 nm) and mesopores (2–50 nm) are transformed into mesopores and macropores (>50 nm), resulting in an increase in the pore volume and permeability, and it is attributed to liquid and gas hydrocarbon generation from solid kerogen.59–62,66,70 From the above discussion, the hydrocarbon generation potential of kerogen, the effect of inorganic minerals, and the evolution of nanopores are the basis for an in-depth understanding of the thermal maturation of organic-rich shales and for evaluating the technical feasibility.
A few attempts have been made to replace traditional oil shale retorting with supercritical water extraction in the last two decades.80–82,85–88 In sub- and supercritical water, hydrocarbon generation occurred in the temperature range of 300–500 °C, among which the optimal temperature for oil production is about 375–400 °C. The maximum oil yield in supercritical water is about 1.5 times that in N2 retorting and the temperature is reduced by about 100–150 °C.80,86 At higher temperatures, kerogen and generated bitumen or oil undergo secondary cracking, thus producing hydrocarbon gas with coke formation.89 Comparative analysis on different media shows that the relative solvating strength and pyrolysis potential were ranked as supercritical water > supercritical CO2 > CO2 retorting > N2 retorting and N2 retorting > CO2 retorting > supercritical water > supercritical CO2.81 The superiority of supercritical water is attributed to the fact that it can simultaneously act as a heat carrier, organic solvent, and reactant. However, as only sporadic results of shale sample conversion in supercritical water have been reported, the effect of temperature and the primary factors on hydrocarbon generation is still not clear. In addition, the shale pore evolution and the effect of inorganic minerals in supercritical water conversion are barely reported. This study explored the conversion fundamentals of organic-rich shale with low maturity in supercritical water. The shale of the seventh member in the Triassic Yanchang Formation (abbreviated as Chang 7 shale) is taken as an example for this study. It has huge reserves of about 4–4.5 billion tons90 and typical lacustrine shale, containing high TOC (14.32% on average) and Type-II kerogen. The hydrocarbon generation experiments were first conducted in a batch reactor over a large range of 300–650 °C. Then, the effect of inorganic minerals was investigated by continuous pickling. Finally, the shale pore structure evolution was measured by N2 adsorption/desorption and mercury intrusion.
2. Materials and methods
The sample is Chang 7 shale outcrops collected from in the south Ordos Basin from Tongchuan city in China. It is a typical lacustrine shale with a TOC of 15.11 wt% and vitrinite reflectance (Ro) of about 0.36–0.38%. The Rock-Eval pyrolysis under N2 show that the amounts of free hydrocarbons (S1) and hydrocarbons generation potential (S2) are, respectively, 4.31 mg g−1 and 76.35 mg g−1, and based on this, hydrogen index (HI) was calculated to be 505.29 mg (g TOC)−1. These basic properties of the Chang 7 shale sample are listed in Table 1. The test of low-temperature distillation of shale using the aluminum retort was carried out in an N2 atmosphere (pyrolysis in an N2 atmosphere) in accordance with the China National Standard GB/T 480-2010. The tar oil production is 25.9 mg g−1 at 380 °C and 52.4 mg g−1 at 520 °C, as shown in Table S4 in the ESI.†| Shale sample | Elemental analysis | TOC (wt%) | Rock pyrolysis (Rock Eval) | Vitrinite reflectance Ro (%) | Tar production | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C (wt%) | H (wt%) | N (wt%) | S (wt%) | S1 (mg g−1) | S2 (mg g−1) | T max (oC) | HI (mg g−1) | 380 °C (mg g−1) | 520 °C (mg g−1) | |||
| Chang 7 | 15.84 | 2.33 | 2.71 | 3.80 | 15.11 | 4.31 | 76.35 | 443 | 505.29 | 0.36–0.38 | 25.9 | 52.4 |
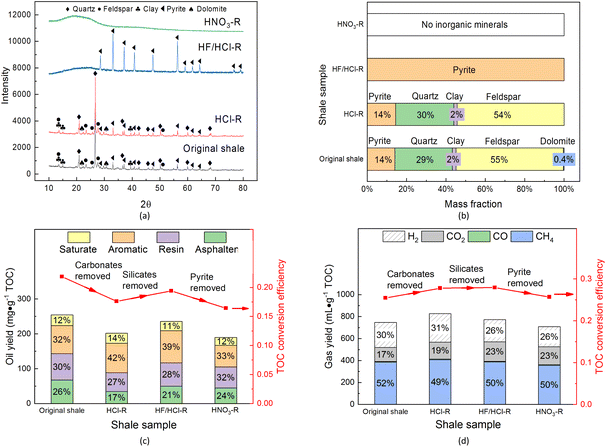
The shale sample was first extracted using a Soxhlet extractor with carbon disulfide, and then pickled with hydrochloric acid (HCl), a mixed solution of hydrofluoric acid and hydrochloric acid (HF/HCl) and nitric acid (HNO3) in turn removes carbonates, aluminosilicates (clay, feldspar, quartz, etc.), and pyrites, and the final residual solid is approximated as kerogen. The XRD patterns of the residual solids are shown in Fig. 3a. The range of shale particle diameters is 75–106 μm for analyzing the hydrocarbon generation characteristics in Section 3.1 and the effects of minerals in Section 3.2, and it is 250–380 μm and 6–10 mm for measuring pore evolution by N2 adsorption/desorption and mercury intrusion porosimetry respectively in Section 3.3. Before the pore structure measurements, free oil in the shale was removed by the Soxhlet extraction method.
The experiments of supercritical water conversion were carried out in a batch reactor with a volume of 80 mL (an inner diameter of 40 mm and a height of 65 mm), as shown in Fig. 1. The reaction temperature and pressure were, respectively, measured using a K-type thermocouple with a precision of ±0.5 °C and a capacitive pressure sensor with a precision of ±0.05 MPa. The shale particles or small blocks and water were heated using an electromagnetic heater. The reaction was terminated by quenching the reactor into cold water. A gas sample was collected using a gas bag and quantified using a wet flowmeter. The generated oil was washed off from the spent shale using carbon disulfide and then the solvent was evaporated at 46 °C, which inevitably lead to the loss of light hydrocarbon with a boiling point below 46 °C in the generated oil. The experiments were performed at a pressure of 25 MPa, a temperature of 300–650 °C, and a duration of 4 h. All experiments were performed at the same pressure by adjusting the mass of the shale sample and water for different reaction temperatures. The rate of mass balance before and after conversion ranged from 90% to 95%, and the detailed data for each experiment are shown in Table S2 and Fig. S1 in the ESI.† The generated gas was characterized by gas chromatography (Agilent 7890a with thermal conductivity detector and flame ionization detector). The generated oil was characterized using an elemental analyzer (Elementar Vario Macro cube on CHNS mode) and thin-layer chromatography (IATROSCAN MK-6). The shale pore structure was measured by N2 adsorption/desorption (Micromeritics ASAP 2020) and mercury intrusion porosimetry (Quantachrome Poremaster 60).
3. Results and discussion
3.1. Hydrocarbon generation characteristics in sub- and supercritical water
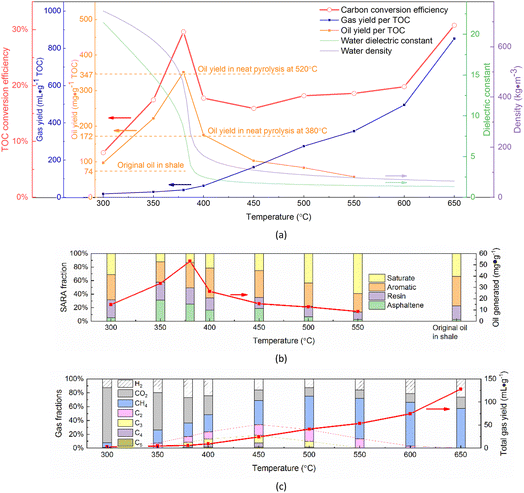
The hydrocarbon generation efficiency of shale in sub- and supercritical water is shown in Fig. 2a. As the temperature increased from 300 °C to 650 °C, the oil yield increased first and then decreased, reaching the peak of 352.1 mg (g TOC)−1 at 380 °C, while the gas yield continued to increase, especially above 400 °C and up to 852.0 mL (g TOC)−1 at 650 °C. As a result, the total organic carbon conversion efficiency showed a process of increasing first, then decreasing and finally increasing with temperature. The carbon conversion efficiency was large across the critical region of water where its density and dielectric constant changed dramatically. This indicated that oil and gas yields from the supercritical water conversion of shale could be regulated by temperature and the optimum temperatures for oil and gas generation were 380 °C and 650 °C, respectively. The tar oil yield by N2 pyrolysis in the aluminium retort was 171.4 mg (g TOC)−1 at 380 °C and 346.8 mg (g TOC)−1 at 520 °C. In contrast, the supercritical water conversion seemed to be a more efficient method for the hydrocarbon generation of shale, because it could improve the oil yield at the same temperature or reduce the temperature at the same yield. In addition, the N2 pyrolysis experiments in a batch reactor at the temperature of 350–450 °C was also performed for comparison. The oil yield reached its maximum of 172.5 mg (g TOC)−1 at 400 °C, as shown in Table S5,† which was significantly lower than the oil yield of 346.8 mg (g TOC)−1 at 520 °C in the aluminum retort and 352.1 mg (g TOC)−1 at 380 °C by supercritical water conversion.The SARA fractions (saturate, aromatic, resin, and asphaltene) of the generated oil are shown in Fig. 2b. The oil yield and composition at 300 °C are basically the same as that of the original oil in shale and only a tiny amount of gas was generated, suggesting that hydrocarbon generation of kerogen was almost negligible. With the temperature rising to 350 °C, the oil yield and the proportion of asphaltene ratio both increased significantly, which indicated that shale kerogen began to generate hydrocarbons and converted them into macromolecular asphaltene first. As the temperature continued to rise, the saturate proportion increased gradually while the proportions of asphaltene, resin, and aromatics decreased. It meant that secondary decomposition occurred and asphaltene was the intermediate of kerogen conversion to light oil and gas.
The generated syngas consisted mainly of H2, CO2, and hydrocarbon gases of CH4, C2, and C3+, and their variations with temperature are shown in Fig. 2c. The CH4 proportion increased with temperature from 7.0% at 300 °C to 63.4% at 600 °C. Meanwhile, the proportion of other hydrocarbon gases (C2 and C3+) showed a unimodal distribution, and it reached the maximum of 34.3% at 450 °C and almost disappeared at below 300 °C and above 650 °C. This was because the rising temperature promoted kerogen and oil decomposition (CxHyOz → H2 + CO2 + CH4 + C2 + C3+ + intermediates), resulting in an initial increase in all hydrocarbon gases (CH4, C2, and C3+) yields. As the temperature rose above 450 °C, the hydrocarbon generation potential of kerogen gradually exhausted and the hydrocarbon gases of C2 and C3+ were further decomposed into CH4. Besides, CH4 could also be formed by the methanation reaction (CO/CO2 + H2 → CH4 + H2O). For non-hydrocarbon gases of H2 and CO2, the CO2 proportion decreased with temperature in the range of 300–500 °C and the H2 proportion had a maximum of 26.9% at 380 °C. The CO production was ignored due to its low maximum amount of 0.4% at 650 °C. At a temperature above 500 °C, the CO2 proportion was kept unchanged but the H2 proportion increased with temperature. This was because, near the critical temperature, H2 was produced by macromolecular polymerization and CO2 mainly came from the decomposition of oxygen-containing functional groups20,86 (e.g. ester hydrolysis and carboxyl decarboxylation) because of little carbonate minerals in Chang 7 shale sample. At above 500 °C, the radical reactions of steam reforming (CxHyOz + H2O → CO + H2) and water-gas shift (CO + H2O → CO2 + H2) contributed to the non-hydrocarbon gases production.91,92 Obviously, supercritical water acted as a hydrogen donor, just as it was in supercritical water gasification of organic matter. The detailed supercritical water gasification mechanism of shale organic matter was analyzed in our previous work.89
Previous studies18,49–57,59–62,66–69,93 have reported that oil generated from shale kerogen in N2 or steam both followed a hydrocarbon generation mechanism with two parallel pathways, kerogen decomposition into oil and gas directly or through bitumen (or called pyrobitumen, thermobitumen) as an intermediate. A study94 reported that the activation energies of heavy fraction formation, light fractions formation, and gas formation during hydrocarbon generation of kerogen in pyrolysis in N2 atmosphere are 39–49 kcal mol−1, 56–59 kcal mol−1 and 57–74 kcal mol−1, respectively. Fig. 2a shows that only a small amount of gas was formed in the favourable temperature range of 350–400 °C for oil generation. Fig. 2b and c show that as the temperature increased, the generated oil became lighter and the gas yield increased. This indicated that the hydrocarbon generation of kerogen in supercritical water also obeyed the above parallel pathways and was dominated by the indirect decomposition with asphaltene as an intermediate.
3.2. Effect of inorganic minerals on oil and gas generation from kerogen
Inorganic minerals in shale may play a catalytic or inhibitory role in the hydrocarbon generation of kerogen in supercritical water, and the effect of inorganic minerals on the kerogen conversion in supercritical water was investigated. Herein the free oil in shale was first extracted with carbon disulfide by using a Soxhlet extractor, and then the inorganic minerals of carbonates, silicates including quartz and pyrite were removed by stepwise pickling with HCl, HCl/HF, and HNO3.60,95,96 The mineral amounts in Chang 7 shale samples after pickling are shown in Fig. 3a and b. In the XRD pattern of HNO3-R, there was only a broad hump at about 2θ = 20° representing organic matters, which indicated that minerals had been completely removed in the HNO3-R sample. The yields and compositions of the generated oil at 380 °C and generated gas at 650 °C are shown in Fig. 3c and d, respectively. As shown in Fig. 3c, the minerals increased oil yield by 34.2%, among which, dolomite and pyrite promoted oil generation with the increase in yields of Re/As, and Ar, respectively, while the silicates containing feldspar, quartz, and clay inhibited it. As shown in Fig. 3d, the minerals slightly affected gas yield, among which, the silicates and pyrite both promoted gas generation, while the carbonate inhibited it. It was found that the order of oil SARA fraction and gas component was aromatic > resin > asphaltene > saturate and CH4 > H2 > CO2 whether the minerals were removed or not, which indicated that the compositions of generated oil and gas mainly depended on the kerogen property.For oil generation in supercritical water, Al-Harahsheh et al.97 found that the pyrolysis activation energy of oil shale (70–83 kJ mol−1) was lower than that of kerogen (82–112 kJ mol−1) by TGA (Thermo Gravimetric Analysis). This indicated that inorganic minerals as a whole could catalyze kerogen decomposition in both supercritical water and N2 atmosphere. Ballice et al.98 reported that the carbonates, silicates, and pyrite played catalytic, inhibitive, and inactive roles, respectively, in net pyrolysis below 550 °C. This difference meant that pyrite was the catalyst in supercritical water rather than N2. Vakhin et al.85,99,100 reported that the clay, as a silicate, catalyzed the hydrothermal reaction of heavy oil at 300 °C, showing an increase in saturate content and a decrease in resin and asphaltene contents. This indicated that the clay inhibited kerogen decomposition to oil but promoted a heavy fraction (resin and asphaltene) cracking into a light fraction (saturate and aromatic). It could be found that different minerals had different effects on oil and gas generation. For the Chang 7 shale, the carbonate promoted oil generation but inhibited gas generation, while the silicate did the opposite. The pyrite benefited both oil and gas generation. These findings are probably applicable to other shales with kerogen type II and minerals containing feldspar, quartz, pyrite, clay, and dolomite, which of course needs further verification. It is worth noting that acid fracturing has been widely used to improve the porosity and permeability of tight reservoirs by dissolving inorganic minerals using HCl, HF, or organic acids. So, for in situ conversion of shale resources by supercritical water injection, different acids should be selected for fracturing according to the target of generating oil or gas. Overall, in the supercritical water conversion of shale, taking the Chang 7 sample, as an example, shale minerals could significantly enhance oil generation but not gas generation. Selecting an appropriate acid for fracturing is expected to promote hydrocarbon generation.
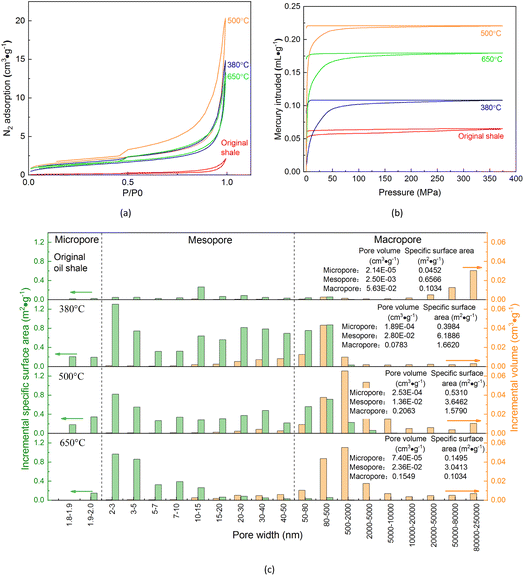
3.3. Evolution of shale pores during supercritical water conversion
Artificial maturation by supercritical water conversion would change the shale pore structures, thus affecting the reservoir permeability and fluid flow capacity. Considering that shale pore sizes range from nanometer to millimeter,20,59–62,66,70,83 the pore structure evolution in supercritical water conversion was characterized by combining the analysis of N2 adsorption and mercury intrusion porosimetry (MIP). The curves of mercury injection/ejection and N2 adsorption/desorption are shown in Fig. 4a and b, respectively. According to the IUPAC classification,101 the N2 adsorption isotherms of the original shale and spent shale were all type II with the hysteresis loops of type H3, indicating multi-layer adsorption and slit-like pore shape. At low-pressure region (P/P0 < 0.1), the low intercept on the vertical axis implied that a small number of micropores (<2 nm) were produced by supercritical water conversion. At the high-pressure region (P/P0 > 0.9), the steep rise in the adsorption quantity was due to capillary condensation, indicating that there were many mesopores (2–50 nm) and macropores (>50 nm) in shale samples. A large amount of mercury intrusion in relatively low pressure also meant that the pores in the original shale and spent shale were mainly mesopores and macropores rather than micropores, and the low efficiency in mercury ejection implied poor pore connectivity and seepage capacity. Compared with the original shale, the spent shale exhibited a larger maximum adsorption quantity and mercury dosage, which meant that the supercritical water conversion could significantly increase the shale pore volume.The distributions of pore volume and specific surface area of original shale and spent shales within the pore size range of 1.8 nm to 250 μm are shown in Fig. 4c. The pore volume and specific surface area were jointly characterized by N2 adsorption and mercury injection with the pore diameters below and above 10 nm.102 After the supercritical water conversion at 380 °C, the shale pore volume increased by 79.7% from 0.059 cm3 g−1 to 0.106 cm3 g−1 and the shale-specific surface area increased tenfold from 0.805 m2 g−1 to 8.249 m2 g−1. As the conversion temperature increased, the pore volume of the spent shale first increased to 0.220 cm3 g−1 at 500 °C and then decreased to 0.179 cm3 g−1 at 650 °C, while the specific surface area monotonically decreased to 3.294 m2 g−1 at 650 °C by 60.1%. It was also found that newly formed pores were mainly macropores with diameters ranging from 50 to 5000 nm. Study103 has reported that the pore structure evolution during shale thermal maturation is attributed to hydrocarbon generation or oxidation of organic matter and inorganic mineral decomposition (e.g. carbonates and clay dehydration). Considering that the Chang 7 shale sample used in this study had few carbonates and supercritical water was reductive but not oxidizing, hydrocarbon generation seemed to be the primary cause of the pore volume increase. It is worth noting that the decrease in the pore volume above 500 °C in the supercritical water was different from the increase in pore volume with temperature in TGA.59–62 This was because kerogen coking in supercritical water would block some pores while kerogen oxidation would generate new pores. The decreased specific surface area and pore volume at elevated temperatures would reduce gas adsorption and promote its expulsion. Overall, artificial maturation by supercritical water conversion could significantly increase the shale pore volume and specific surface area by producing many slit-like nanopores with a diameter of 50–5000 nm due to the hydrocarbon generation of kerogen.
4. Conclusions
Low-maturity shale has large reserves but is difficult to exploit due to its high kerogen content. Supercritical water conversion was proposed as an alternative method to accelerate oil and gas generation from kerogen. Taking Chang 7 shale sample as an example, a series of experiments were performed at the temperature of 300–650 °C and the pressure of 25 MPa to test the feasibility of this method from three aspects, hydrocarbon generation of kerogen, effect of inorganic minerals and shale pore structures evolution. The results showed that when raising the temperature, the oil yield increased first and then decreased with a peak value of 352.1 mg (g TOC)−1 at 380 °C. Compared with pyrolysis in the aluminium retort, supercritical water conversion could raise the oil yield at the same temperature or reduce the temperature with the same yield. The gas yield quickly increased above 400 °C and reached 852.0 mL (g TOC)−1 at 650 °C, and the selectivities of CH4 and H2 also increased. The inorganic minerals as a whole appeared to enhance the oil yield by 34.2% at 380 °C but had little effect on the gas generation. For type II kerogen conversion in sub- and supercritical water, the carbonate (dolomite) promoted oil generation but inhibited gas generation, while the silicates (feldspar, quartz, and clay) did the opposite. The pyrite benefited both oil and gas generation. Supercritical water could produce many nanopores with a slit-like shape and diameter of 50–5000 nm by converting kerogen into oil and gas, resulting in a significant increase in the shale pore volume and specific surface area. The above results proved that sub- and supercritical water conversion is an effective and promising method for exploiting Chang 7 shale resource, and it seemed to apply to other low-maturity shales with high TOC content and type II kerogen.Author contributions
Qiuyang Zhao: conceptualization, methodology, funding acquisition, writing – original draft. Yu Dong: methodology, investigation, data curation. Lichen Zheng: investigation, data curation, formal analysis. Tian Xie: investigation, formal analysis. Baercheng Bawaa: investigation, data curation. Hui Jin: writing – review and editing. Liejin Guo: conceptualization, funding acquisition, writing – review and editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The work is supported by the National Nature Science Foundation of China (No. 52006171), the China Postdoctoral Science Foundation Funded Project (No. 2020M683476), and the Fundamental Research Funds for the Central Universities of China.Notes and references
- EIA, How much shale (tight) oil is produced in the United States?, https://www.eia.gov/tools/faqs/faq.php?id=847%26t=6, accessed June 2022.
- J. D. Hughes, Nature, 2013, 494, 307–308 CrossRef CAS.
- D. J. Soeder, J. Pet. Sci. Eng., 2018, 163, 399–420 CrossRef CAS.
- V. Salygin, I. Guliev, N. Chernysheva, E. Sokolova, N. Toropova and L. Egorova, Sustainability, 2019, 11, 1627 CrossRef.
- Q. Lei, Y. Xu, B. Cai, B. Guan, X. Wang, G. Bi, H. Li, S. Li, B. Ding, H. Fu, Z. Tong, T. Li and H. Zhang, Pet. Explor. Dev., 2022, 49, 191–199 CrossRef.
- M. Thomas, T. Partridge, B. H. Harthorn and N. Pidgeon, Nat. Energy, 2017, 2, 17054 CrossRef.
- W. Zhao, S. Hu, L. Hou, T. Yang, X. Li, B. Guo and Z. Yang, Pet. Explor. Dev., 2020, 47, 1–11 CrossRef.
- S. Hu, W. Zhao, L. Hou, Z. Yang, R. Zhu, S. Wu, B. Bai and X. Jin, Pet. Explor. Dev., 2020, 47, 877–887 CrossRef.
- L. Wang, Y. Tian, X. Yu, C. Wang, B. Yao, S. Wang, P. H. Winterfeld, X. Wang, Z. Yang, Y. Wang, J. Cui and Y.-S. Wu, Fuel, 2017, 210, 425–445 CrossRef CAS.
- Z. Kang, Y. Zhao and D. Yang, Appl. Energy, 2020, 269, 115121 CrossRef CAS.
- R. C. Ryan, T. D. Fowler, G. L. Beer and V. Nair, Oil Shale: A Solution to the Liquid Fuel Dilemma, 2010, vol. 1032, pp. 161–183 Search PubMed.
- A. R. Brandt, Environ. Sci. Technol., 2008, 42, 7489–7495 CrossRef CAS PubMed.
- W. A. Symington, R. D. Kaminsky, W. P. Meurer, G. A. Otten, M. M. Thomas and J. D. Yeakel, Oil Shale: A Solution to the Liquid Fuel Dilemma, 2010, vol. 1032, pp. 185–216 Search PubMed.
- T. D. Fowler, and H. J. Vinegar, SPE Western Regional Meeting, OnePetro, San Jose, California, 2009 Search PubMed.
- C. Shen, SPE Rocky Mountain Petroleum Technology Conference, Denver, Colorado, 2009 Search PubMed.
- P. M. Crawford and J. C. Killen, Oil Shale: A Solution to the Liquid Fuel Dilemma, 2010, vol. 1032, pp. 21–60 Search PubMed.
- A. K. Burnham, R. L. Day, M. P. Hardy and P. H. Wallman, Oil Shale: A Solution to the Liquid Fuel Dilemma, 2010, vol. 1032, pp. 149–160 Search PubMed.
- Z. Kang, Y. Zhao, D. Yang, L. Tian and X. Li, J. Pet. Sci. Eng., 2020, 186, 106785 CrossRef CAS.
- Y. Lu, Z. Wang, Z. Kang, W. Li, D. Yang and Y. Zhao, RSC Adv., 2022, 12, 16329–16341 RSC.
- Y. Sun, S. Kang, S. Wang, L. He, W. Guo, Q. Li and S. Deng, Energy Fuels, 2019, 33, 2106–2114 CrossRef CAS.
- Z. Wang, S. Deng, Q. Gu, X. Cui, Y. Zhang and H. Wang, Energy Fuels, 2014, 28, 2305–2313 CrossRef CAS.
- A. Kruse and E. Dinjus, J. Supercrit. Fluids, 2007, 39, 362–380 CrossRef CAS.
- D. Bröll, C. Kaul, A. Krämer, P. Krammer, T. Richter, M. Jung, H. Vogel and P. Zehner, Angew. Chem., Int. Ed., 1999, 38, 2998–3014 CrossRef.
- H. Weingärtner and E. U. Franck, Angew. Chem., Int. Ed., 2005, 44, 2672–2692 CrossRef.
- C. Rodriguez Correa and A. Kruse, J. Supercrit. Fluids, 2018, 133, 573–590 CrossRef CAS.
- L. Guo, H. Jin and Y. Lu, J. Supercrit. Fluids, 2015, 96, 144–150 CrossRef CAS.
- M. T. Timko, A. F. Ghoniem and W. H. Green, J. Supercrit. Fluids, 2015, 96, 114–123 CrossRef CAS.
- Y. Matsumura, H. Nonaka, H. Yokura, A. Tsutsumi and K. Yoshida, Fuel, 1999, 78, 1049–1056 CrossRef CAS.
- G. V. Deshpande, G. D. Holder, A. A. Bishop, J. Gopal and I. Wender, Fuel, 1984, 63, 956–960 CrossRef CAS.
- Y. Qian, C. Zuo, J. Tan and J. He, Energy, 2007, 32, 196–202 CrossRef CAS.
- S. S. Toor, L. Rosendahl and A. Rudolf, Energy, 2011, 36, 2328–2342 CrossRef CAS.
- A. A. Peterson, F. Vogel, R. P. Lachance, M. Fröling, J. M. J. Antal and J. W. Tester, Energy Environ. Sci., 2008, 1, 32–65 RSC.
- L. Guo and H. Jin, Int. J. Hydrogen Energy, 2013, 38, 12953–12967 CrossRef CAS.
- H. Jin, Y. Chen, Z. Ge, S. Liu, C. Ren and L. Guo, Int. J. Hydrogen Energy, 2015, 40, 16096–16103 CrossRef CAS.
- S. N. Reddy, S. Nanda, A. K. Dalai and J. A. Kozinski, Int. J. Hydrogen Energy, 2014, 39, 6912–6926 CrossRef CAS.
- J. A. Okolie, S. Nanda, A. K. Dalai, F. Berruti and J. A. Kozinski, Renewable Sustainable Energy Rev., 2020, 119, 109546 CrossRef CAS.
- A. Abourriche, M. Oumam, H. Hannache, A. Adil, R. Pailler, R. Naslain, M. Birot and J. P. Pillot, J. Supercrit. Fluids, 2009, 51, 24–28 CrossRef CAS.
- M. Morimoto, Y. Sugimoto, Y. Saotome, S. Sato and T. Takanohashi, J. Supercrit. Fluids, 2010, 55, 223–231 CrossRef CAS.
- R. Rana, S. Nanda, A. Maclennan, Y. Hu, J. A. Kozinski and A. K. Dalai, J. Energy Chem., 2019, 31, 107–118 CrossRef.
- P. Postorino, R. H. Tromp, M. A. Ricci, A. K. Soper and G. W. Neilson, Nature, 1993, 366, 668–670 CrossRef CAS.
- E. S. Fois, M. Sprik and M. Parrinello, Chem. Phys. Lett., 1994, 223, 411–415 CrossRef CAS.
- N. Akiya and P. E. Savage, Chem. Rev., 2002, 102, 2725–2750 CrossRef CAS.
- Q. Zhao, L. Guo, Z. Huang, L. Chen, H. Jin and Y. Wang, Energy Fuels, 2018, 32, 1685–1692 CrossRef CAS.
- Q. Zhao, L. Guo, Y. Wang, H. Jin, L. Chen and Z. Huang, Energy Fuels, 2020, 34, 360–367 CrossRef CAS.
- M. Watanabe, S.-n. Kato, S. Ishizeki, H. Inomata and R. L. Smith, J. Supercrit. Fluids, 2010, 53, 48–52 CrossRef CAS.
- J. Vilcáez, M. Watanabe, N. Watanabe, A. Kishita and T. Adschiri, Fuel, 2012, 102, 379–385 CrossRef.
- Q. Liu, D. Zhu, X. Tan, P. Yuan, Z. Cheng, W. Yuan and J. Yang, AIChE J., 2016, 62, 207–216 CrossRef CAS.
- R. O. Canıaz and C. Erkey, Chem. Eng. Res. Des., 2014, 92, 1845–1863 CrossRef.
- W. He, Y. Sun and X. Shan, J. Anal. Appl. Pyrolysis, 2021, 155, 105091 CrossRef CAS.
- D. Song, X. Wang, C. Wu, S. Meng, M. Zhang, H. Li, H. Jiao, X. Liu, X. Jin and J. Tuo, Energy Fuels, 2021, 35, 358–373 CrossRef CAS.
- W. Ma, L. Hou, X. Luo, S. Tao, P. Guan, J. Liu and S. Lin, Fuel, 2020, 259, 116211 CrossRef CAS.
- W. Ma, L. Hou, X. Luo, J. Liu, S. Tao, P. Guan and Y. Cai, J. Pet. Sci. Eng., 2020, 190, 107035 CrossRef CAS.
- L. Hou, W. Ma, X. Luo and J. Liu, Fuel, 2020, 279, 118497 CrossRef CAS.
- F. Yu, P. Sun, K. a. Zhao, L. Ma and X. Tian, Fuel, 2020, 261, 116412 CrossRef CAS.
- Z. Wang, S. Deng, Q. Gu, Y. Zhang, X. Cui and H. Wang, Fuel Process. Technol., 2013, 110, 103–108 CrossRef CAS.
- T. V. Le Doan, N. W. Bostrom, A. K. Burnham, R. L. Kleinberg, A. E. Pomerantz and P. Allix, Energy Fuels, 2013, 27, 6447–6459 CrossRef CAS.
- Y. Ma, T. Cao, L. Snowdon, M. Qian, Q. Jiang, M. Li, N. Mahlstedt and B. Horsfield, Energy Fuels, 2017, 31, 10378–10392 CrossRef CAS.
- S. Li and C. Yue, Fuel, 2003, 82, 337–342 CrossRef CAS.
- D. Song, J. Tuo, M. Zhang, C. Wu, L. Su, J. Li, Y. Zhang and D. Zhang, J. Nat. Gas Sci. Eng., 2019, 62, 171–183 CrossRef CAS.
- H. Guo, J. Lin, Y. Yang and Y. Liu, Fuel, 2014, 118, 186–193 CrossRef CAS.
- L. Zhang, M. Sun, Q. Lv, C. F. Ukaomah, Q. Hu, B. Yu, J. Zhang, X. Liang, G. Wang and S. Jiang, Energy Fuels, 2021, 35, 4345–4357 CrossRef CAS.
- J. Chen and X. Xiao, Fuel, 2014, 129, 173–181 CrossRef CAS.
- Z. Chen, Y. Tian, D. Lai, J.-h. Zhan, Z. Han, G. Xu and S. Gao, Energy Convers. Manage., 2020, 224, 113358 CrossRef.
- B. Chen, M. Yuan, Y. You, S. Wang, J. Shen, X. Han, X. Jiang and Y. Guo, Energy Convers. Manage., 2021, 243, 114405 CrossRef CAS.
- Q. Wang, Y. Ma, S. Li, C. Yue and L. He, Energy Convers. Manage., 2019, 187, 29–40 CrossRef.
- L. Wang, D. Yang, X. Li, J. Zhao, G. Wang and Y. Zhao, Energies, 2018, 11, 2297 CrossRef.
- A. Y. Bychkov, G. A. Kalmykov, I. A. Bugaev, A. G. Kalmykov and E. V. Kozlova, Moscow Univ. Geol. Bull., 2015, 70, 299–304 CrossRef.
- C. Fang, S. Li, G. Ma, H. Wang and Z. Huang, Pet. Sci., 2012, 9, 532–534 CrossRef CAS.
- I. Johannes, H. Luik, J. A. Bojesen- Koefoed, L. Tiikma, N. Vink and L. Luik, Oil Shale, 2012, 29, 206–221 CrossRef CAS.
- Y. Sun, L. He, S. Kang, W. Guo, Q. Li and S. Deng, Energies, 2018, 11, 842 CrossRef.
- E. Popov, A. Kalmykov, A. Cheremisin, A. Bychkov, T. Bondarenko, N. Morozov and I. Karpov, J. Pet. Sci. Eng., 2017, 156, 852–857 CrossRef CAS.
- S. Deng, Z. Wang, Y. Gao, Q. Gu, X. Cui and H. Wang, J. Anal. Appl. Pyrolysis, 2012, 98, 151–158 CrossRef CAS.
- S. Deng, Z. Wang, Q. Gu, F. Meng, J. Li and H. Wang, Fuel Process. Technol., 2011, 92, 1062–1067 CrossRef CAS.
- O. M. Ogunsola and N. Berkowitz, Fuel Process. Technol., 1995, 45, 95–107 CrossRef CAS.
- A. K. Abourriche, M. Oumam, H. Hannache, M. Birot, Y. Abouliatim, A. Benhammou, Y. El Hafiane, A. M. Abourriche, R. Pailler and R. Naslain, J. Supercrit. Fluids, 2013, 84, 98–104 CrossRef CAS.
- Z. Shuai, L. Xiaoshu, L. Qiang and S. Youhong, Chem. Eng. J., 2020, 394, 125037 CrossRef CAS.
- P. J. Jarboe, P. A. Candela, W. Zhu and A. J. Kaufman, Energy Fuels, 2015, 29, 7897–7909 CrossRef CAS.
- M. Allawzi, A. Al-Otoom, H. Allaboun, A. Ajlouni and F. Al Nseirat, Fuel Process. Technol., 2011, 92, 2016–2023 CrossRef CAS.
- M. Koel, S. Ljovin, K. Hollis and J. Rubin, Pure Appl. Chem., 2001, 73, 153–159 CrossRef CAS.
- L. Tiikma, I. Johannes, H. Luik, A. Zaidentsal and N. Vink, J. Anal. Appl. Pyrolysis, 2009, 85, 502–507 CrossRef CAS.
- J. D. Tucker, B. Masri and S. Lee, Energy Sources, 2000, 22, 453–463 CrossRef CAS.
- N. Olukcu, J. Yanik, M. Saglam, M. Yuksel and M. Karaduman, Energy Fuels, 1999, 13, 895–902 CrossRef CAS.
- Y. Jiang, Y. Luo, Y. Lu, C. Qin and H. Liu, Energy, 2016, 97, 173–181 CrossRef CAS.
- M. W. Amer, J. S. A. Alhesan, M. Marshall, Y. Fei, W. R. Jackson and A. L. Chaffee, Energy Convers. Manage., 2022, 252, 115108 CrossRef CAS.
- Z. R. Nasyrova, G. P. Kayukova, Y. V. Onishchenko, V. P. Morozov and A. V. Vakhin, Energy Fuels, 2020, 34, 1329–1336 CrossRef CAS.
- O. N. Fedyaeva, V. R. Antipenko, D. Y. Dubov, T. V. Kruglyakova and A. A. Vostrikov, J. Supercrit. Fluids, 2016, 109, 157–165 CrossRef CAS.
- H. Hu, J. Zhang, S. Guo and G. Chen, Fuel, 1999, 78, 645–651 CrossRef CAS.
- K. El harfi, C. Bennouna, A. Mokhlisse, M. Ben chanâa, L. Lemée, J. Joffre and A. Amblès, J. Anal. Appl. Pyrolysis, 1999, 50, 163–174 CrossRef CAS.
- X. Liang, Q. Zhao, Y. Dong, L. Guo, Z. Jin and Q. Liu, Energy Fuels, 2021, 35, 7657–7665 CrossRef CAS.
- W. Zhao, S. Hu and L. Hou, Pet. Explor. Dev., 2018, 45, 563–572 CrossRef.
- H. Jin, L. Guo, J. Guo, Z. Ge, C. Cao and Y. Lu, Int. J. Hydrogen Energy, 2015, 40, 7523–7529 CrossRef CAS.
- Z. Ge, S. Guo, L. Guo, C. Cao, X. Su and H. Jin, Int. J. Hydrogen Energy, 2013, 38, 12786–12794 CrossRef CAS.
- L. Hou, W. Ma, X. Luo, S. Tao, P. Guan and J. Liu, Mar. Pet. Geol., 2020, 116, 104348 CrossRef CAS.
- Y. Wang, Y. Wang, X. Meng, J. Su and Q. Long, Oil Gas Geol., 2019, 40, 678–684 Search PubMed.
- F. Zhao, B. Li, D. Che and S. Liu, J. Mol. Struct., 2022, 1261, 132878 CrossRef CAS.
- L. Ballice, Ind. Eng. Chem. Res., 2006, 45, 906–912 CrossRef CAS.
- M. Al-Harahsheh, O. Al-Ayed, J. Robinson, S. Kingman, A. Al-Harahsheh, K. Tarawneh, A. Saeid and R. Barranco, Fuel Process. Technol., 2011, 92, 1805–1811 CrossRef CAS.
- L. Ballice, Fuel Process. Technol., 2005, 86, 673–690 CrossRef CAS.
- S. A. Sitnov, A. V. Vakhin, I. I. Mukhamatdinov, Y. V. Onishchenko and D. A. Feoktistov, Pet. Sci. Technol., 2019, 37, 687–693 CrossRef CAS.
- G. P. Kayukova, A. N. Mikhailova, I. P. Kosachev, D. A. Feoktistov and A. V. Vakhin, Energy Fuels, 2018, 32, 6488–6497 CrossRef CAS.
- J. Rouquerol, G. Baron, R. Denoyel, H. Giesche, J. Groen, P. Klobes, P. Levitz, A. V. Neimark, S. Rigby, R. Skudas, K. Sing, M. Thommes and K. Unger, Pure Appl. Chem., 2011, 84, 107–136 CrossRef.
- T. Cao, Z. Song, G. Liu, Q. Yin and H. Luo, Pet. Geol. Recovery Effic., 2016, 23, 1–8 Search PubMed.
- J. Chang, X. Fan, Z. Jiang, X. Wang, L. Chen, J. Li, L. Zhu, C. Wan and Z. Chen, Appl. Clay Sci., 2022, 216, 106334 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Lists of detailed experimental data in Section 3.1. Hydrocarbon generation characteristics in sub- and supercritical water and experiment of shales pyrolysis in N2 atmosphere are shown in Tables S1–S5 and Fig. S1. Detailed experimental data in Section 3.2. Effect of inorganic minerals on oil and gas generation from kerogen are shown in the Tables S6–S8. See DOI: https://doi.org/10.1039/d2se01361d |
| This journal is © The Royal Society of Chemistry 2023 |

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
: