Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Engineering a bromophenol derivative for rapid detection of Hg2+/CH3Hg+ in both environmental and biological samples through a unique activation process†
Tapendu
Samanta
,
Narayan
Das
,
Diptendu
Patra
,
Pawan
Kumar
 and
Raja
Shunmugam
and
Raja
Shunmugam
 *
*
Polymer Research Centre, Department of Chemical Sciences, Indian Institute of Science Education and Research, Kolkata, West Bengal 741246, India. E-mail: email-sraja@iiserkol.ac.in
First published on 20th March 2023
Abstract
Reaction-based sensory systems are always preferred for detecting Hg2+ and its organic form, CH3Hg+. A simple bromophenol derivative (BDT) has been developed as a cheap, small and ultrasensitive sensor for both Hg2+ and CH3Hg+ ions with rapid detection ability. The transformation of the 1,3-dithiolane segment to the formyl group has been utilized here as a key feature for the turn “off–on” fluorescence response due to the activation of the ESIPT process during the detection of the analytes. Most of the 1,3-dithiolane systems required a long response time for CH3Hg+, but BDT has been demonstrated as an excellent system that required a low response time to detect the organic form of mercury. Having a limit of detection (LOD) value of 3.8 nM and 0.8 μM towards Hg2+ and CH3Hg+ respectively in an aqueous medium, BDT served as an excellent detection probe with environmental detection capability. It has been supported by ICP-MS analysis of commercially available vermilion. BDT has been utilized to detect Hg2+ in a biological system with excellent efficiency along with ecological and real sample analysis. Furthermore, the desulfurization process triggered by Hg2+ has been proved using 1H NMR analysis and spectroscopic fluorescence techniques. These observations encouraged us to claim BDT as an excellent cheap and easily synthesizable efficient tool for detecting inorganic and organic mercury in controlled, environmental, and biological systems.
Sustainability spotlightThe bioaccumulation of Hg(II) and methylmercury (CH3Hg+), due to their solid binding tendency to thiols of different enzymes and proteins, caused several physiological issues such as kidney damage, central nervous system damage, motion disorder, and even death. Therefore, to avoid the hazardous effect of Hg2+and CH3Hg+, an upper limit of Hg2+ has been set as 10 nM by the U.S. Environmental Protection Agency (EPA). By this virtue, the development of selective and sensitive detection strategies for mercury in environmental and living systems is an important issue to be addressed by researchers. However, traditional methods need expensive instrumentation and complicated experimental procedures despite high sensitivity and precision. In this respect, reaction-based probes are preferable due to their high selectivity and sensitivity. To the best of our knowledge, herein, we report the smallest 1,3-dithiolane-based probe BDT, where a diformyl system (BDA) has been modified using a 1,2-ethanethiol segment. BDT showed excellent selectivity and sensitivity towards Hg2+ and methylmercury ions in an aqueous environment and biological system comprehensively. |
Introduction
The toxic effect of mercury is well known for Minamata disease and poisoning in Iraq.1–3 Mercury contamination is mainly originated from the release of mercury from different chemical industries.4,5 Uncontrolled release has increased the amount of mercury in surface water and heavily affects humankind throughout the world.6 The bioaccumulation of Hg(II) and methylmercury (CH3Hg+) due to their solid binding tendency to thiols of different enzymes and proteins caused several physiological issues such as kidney damage, central nervous system damage, motion disorder, and even death.7,8 On the other hand, organic species of mercury are known to be highly toxic.9,10 Among different organic mercury species, CH3Hg+ is a commonly known toxicant that is significantly more harmful than the inorganic Hg2+ ions. It is frequently found in marine seafood. Compared to Hg2+, CH3Hg+ can easily penetrate biological membranes and the blood–brain barrier and thus affect the nervous system.11 To avoid the hazardous effect of Hg2+ and CH3Hg+, an upper limit of Hg2+ has been set as 10 nM by the U.S. Environmental Protection Agency (EPA).12 By this virtue, the development of selective and sensitive detection strategies for mercury in environmental and living systems is an important issue to be addressed by researchers. Several traditional methods such as atomic absorption spectroscopy (AAS), inductively coupled plasma mass spectrometry (ICP-MS), atomic fluorescence spectrometry (AFS), chromatographic techniques, and voltammetry studies are available for the detection of mercury with high sensitivity and accuracy.10–20 Despite high sensitivity and precision, traditional methods need expensive instrumentation and complicated experimental procedures.21–24 However, the spectroscopic fluorescence technique has emerged as a point of interest in recent times due to its comprehensive sensitivity, response, and easy sample preparation process.25–29 In the last few years, most fluorescent probes for Hg2+ were designed using heteroatom-containing ligand systems. However, these kinds of probes suffer from low selectivity due to reversible complexation and interference from different ions.30–32 In this respect, reaction-based probes are preferable due to their high selectivity and sensitivity, as an Hg-triggered reaction with a probe leads to a new fluorogenic or chromogenic substance formation with unique spectral properties.33 The deprotection of thioacetals to aldehydes or ketones, promoted by Hg2+ is one of the most utilized reactions to design a chemodosimeter with high selectivity.34–36 The formation of thioacetals can be achieved simply by reacting thiols with aldehyde or ketone segments of any molecule and can be deprotected explicitly by Hg2+ ions.37 Besides, very few reaction-based probes have been reported for methylmercury, to date and most of the probes require a high concentration of CH3Hg+ and a considerable reaction time due to the minor thiophilic nature of methylmercury.38–41Generally, three well-known fluorescence mechanisms, such as PET (Photoinduced Electron Transfer), FRET (Förster Resonance Energy Transfer), and ICT (Internal Charge Transfer), are explored vastly for the development of sensor molecules. However, excited-state intramolecular proton transfer (ESIPT) based probes have received great attention in terms of applications due to their unique photophysical properties such as large stokes shift, good photostability, and high quantum yield.42,43
By this virtue, to the best of our knowledge, herein, we report the smallest 1,3-dithiolane based probe BDT, where a diformyl system (BDA) has been modified using a 1,2-ethanethiol segment. BDT has been designed so that the Hg2+ triggered deprotection of the 1,3-dithiolane moiety to an aldehyde can turn on the ESIPT process to produce a unique emission signal. 2-(2′-Hydroxyphenyl) benzothiazole (HBT) is a well-known ESIPT active fluorophore used as the basic unit for developing different sensors throughout the years, and –OH and benzothiazole moieties are involved in the proton transfer process.44,45 But in the case of BDT, Hg2+ and CH3Hg+ promoted thioacetal deprotection and produced a basic keto–enol tautomerizing unit that activated the ESIPT process by showing intense intensity green fluorescence as output. Moreover, a single benzene ring containing BDT showed excellent selectivity and sensitivity towards Hg2+ and methylmercury ions in an aqueous environment and biological system comprehensively.
Experimental section
We have synthesized BDT in two steps starting from 4-bromophenol (Scheme 1, ESI†). Initially, 4-bromophenol has been converted to a dialdehyde system (BDA) using the Duff reaction where hexamethylenetetramine (HMTA) and TFA have been used as reagents. In the second step, BDA has been converted to BDT using 1,3-ethanedithiol. All these products have been successfully characterized using 1H NMR, 13C NMR, and ESI-MS techniques. The detailed synthetic methods and characterization of each compound have been discussed in the ESI (Fig. S1–S6†).Results and discussion
Photophysical properties of BDT
The initial photophysical study of BDT showed absorbance maxima at 306 nm in a DMSO/PBS (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v, pH 7.4) medium. However, the addition of Hg2+ resulted in the appearance of a new absorbance maximum at 350 nm (Fig. S7, ESI†). In terms of fluorescence emission, BDT showed an emission maximum of around 526 nm upon adding an Hg2+ ion, which was absent initially (Fig. S8, ESI†). The selectivity of BDT was thoroughly investigated using fluorescence spectroscopy for various metal ions such as Na+, K+, Li+, Mg2+, Ca2+, Cu2+, Zn2+, Pb2+, Co2+, Fe2+, Cd2+, Sn2+, Fe3+, Cr3+ and Hg2+ in a DMSO/PBS (4
1, v/v, pH 7.4) medium. However, the addition of Hg2+ resulted in the appearance of a new absorbance maximum at 350 nm (Fig. S7, ESI†). In terms of fluorescence emission, BDT showed an emission maximum of around 526 nm upon adding an Hg2+ ion, which was absent initially (Fig. S8, ESI†). The selectivity of BDT was thoroughly investigated using fluorescence spectroscopy for various metal ions such as Na+, K+, Li+, Mg2+, Ca2+, Cu2+, Zn2+, Pb2+, Co2+, Fe2+, Cd2+, Sn2+, Fe3+, Cr3+ and Hg2+ in a DMSO/PBS (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
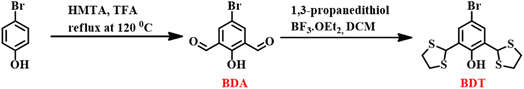
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v, pH 7.4) medium, where the 526 nm emission band was observed only for the Hg2+ ion (Fig. 1A). Selectivity studies of BDT (Fig. 1B) established the precise nature of our probe towards Hg2+ ions, which is the beneficial aspect of reaction-based systems. Due to the activation of the ESIPT phenomenon, BDT showed green emissive nature in the presence of Hg2+ but remained non-emissive for other analytes, as depicted in Fig. 1C.
1, v/v, pH 7.4) medium, where the 526 nm emission band was observed only for the Hg2+ ion (Fig. 1A). Selectivity studies of BDT (Fig. 1B) established the precise nature of our probe towards Hg2+ ions, which is the beneficial aspect of reaction-based systems. Due to the activation of the ESIPT phenomenon, BDT showed green emissive nature in the presence of Hg2+ but remained non-emissive for other analytes, as depicted in Fig. 1C.
After a successful investigation of the selectivity of BDT, we have determined the response dynamics towards Hg2+ under the same experimental conditions used in the initial photophysical experiments. A rapid response time of 1 minute, required for a complete reaction between BDT and Hg2+, was evident from the time-dependent fluorescence studies (Fig. S9, ESI†). This detection kinetics confirmed the 1 minute incubation time of Hg2+ with BDT for all future spectroscopic experiments. Reaction dynamics and selectivity studies confirmed the novelty of BDT towards detecting Hg2+ ions in terms of rapid response and high specificity. The quantum yield of BDT in the presence of Hg2+ has been evaluated using quinine sulfate as reference (eqn (1), ESI†). The value of quantum yield for BDT is calculated to be 0.11.
Sensitivity and detection parameters are essential for a probe to be an excellent candidate for sensing any analyte. Towards this, we have carried out a fluorescence titration study of BDT with varying concentrations of Hg2+ in a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
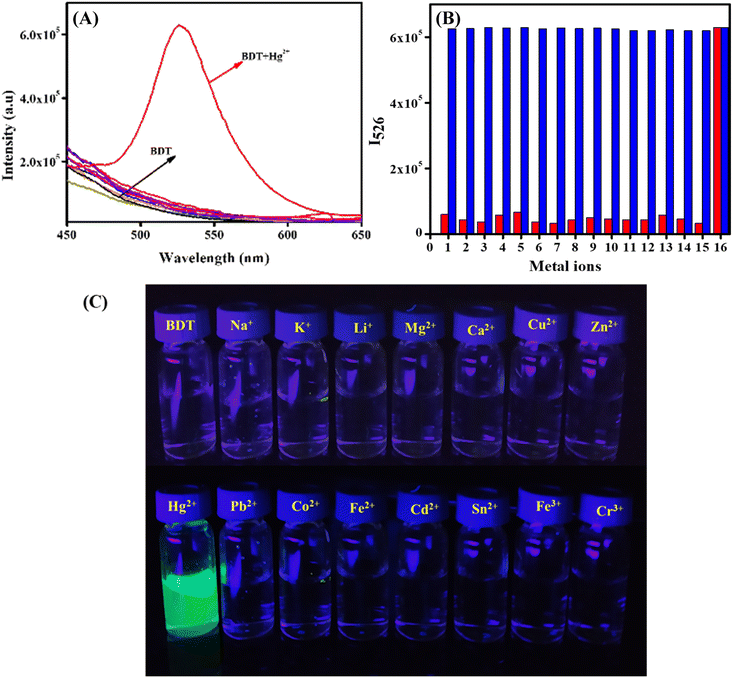
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 DMSO/PBS (v/v, pH = 7.4) medium. As expected, the emission intensity of BDT at 526 nm increased gradually with the progressive addition of Hg2+ ions (0–60 μM) (Fig. 2A), with a change in emission from colourless to green (Fig. 2A, inset). A drastic 33-fold increase in emission intensity at 526 nm till two equivalent Hg2+ addition followed by plateau formation (Fig. S10, ESI†) convincingly suggested the gradual desulfurization process. The absorption studies of BDT with changing concentrations of Hg2+ (0–100 μM) (Fig. S11, ESI†) also exhibited a similar kind of spectroscopic change which again supported our claim of the desulfurization process.
1 DMSO/PBS (v/v, pH = 7.4) medium. As expected, the emission intensity of BDT at 526 nm increased gradually with the progressive addition of Hg2+ ions (0–60 μM) (Fig. 2A), with a change in emission from colourless to green (Fig. 2A, inset). A drastic 33-fold increase in emission intensity at 526 nm till two equivalent Hg2+ addition followed by plateau formation (Fig. S10, ESI†) convincingly suggested the gradual desulfurization process. The absorption studies of BDT with changing concentrations of Hg2+ (0–100 μM) (Fig. S11, ESI†) also exhibited a similar kind of spectroscopic change which again supported our claim of the desulfurization process.
From the titration study, a calibration plot has been constructed in 0–20 μM for BDT. The linear plot of intensity at 526 nm with the concentration of Hg2+ showed excellent linearity with an adjacent R2 value of 0.99025. This linear plot has been utilized to calculate the theoretical lowest detection limit (LOD = 3σ/K) of BDT with a values of 3.8 nM for Hg2+, which is well comparable with other desulfurization based reported probes (Table S1, ESI†) and well below the permissible limit of 10 nM.
Detection of CH3Hg+ by BDT
Reaction-based probes of Hg2+ are also reactive towards CH3Hg+ in a similar mechanism. In this context, we carried out the sensing behavior studies of BDT towards the CH3Hg+ ion under identical experimental conditions. The initial time-dependent studies confirmed the rapid detection ability of BDT towards CH3Hg+ in DMSO/PBS (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v, pH = 7.4) (Fig. S12, ESI†). Furthermore, a quick response time of BDT defined the excellent factor for detecting CH3Hg+ compared to other various reported probes (Table S1, ESI†). Furthermore, a gradual increase in the I505 nm value was observed for BDT upon progressive addition of CH3Hg+ ions (Fig. 3A). Titrimetric results have been utilized to construct the calibration curve (Fig. 3B) in the concentration of 0–90 μM for methylmercury. This linear plot with an R2 value of 0.98932 has been utilized to calculate the LOD value of 0.8 μM for BDT towards CH3Hg+ in a controlled experimental medium.
1, v/v, pH = 7.4) (Fig. S12, ESI†). Furthermore, a quick response time of BDT defined the excellent factor for detecting CH3Hg+ compared to other various reported probes (Table S1, ESI†). Furthermore, a gradual increase in the I505 nm value was observed for BDT upon progressive addition of CH3Hg+ ions (Fig. 3A). Titrimetric results have been utilized to construct the calibration curve (Fig. 3B) in the concentration of 0–90 μM for methylmercury. This linear plot with an R2 value of 0.98932 has been utilized to calculate the LOD value of 0.8 μM for BDT towards CH3Hg+ in a controlled experimental medium.
The obtained results of selectivity, response time, and LOD values suggested the superiority of the probe in comparison to various reaction-based reported fluorescent probes in recent time (Table S1, ESI†). Furthermore, all these results established the highly selective and sensitive sensing ability of the chemodosimeter BDT towards both Hg2+ and CH3Hg+ in aq. media, which can be utilized to detect toxic analytes with high efficiency.
Study of the sensing mechanism
To further validate the Hg2+ triggered desulfurization process of the 1,3-dithiolane moiety, we investigated the sensing mechanism by performing the 1H NMR titration experiment (Fig. 4A) of BDT in the absence and presence of Hg2+ ions. Upon adding excess (3 equivalent) Hg2+ to BDT, the protons corresponding to the 1,3-dithiolane moiety, i.e., Hb and Hc, had disappeared entirely, and a new peak around 10.1 ppm appeared for –CHO groups. As a consequence of this desulfurization reaction, aromatic signals are shifted downfield. The new spectrum is identical to the 1H NMR spectrum of BDA (Fig. S1, ESI†), which firmly established the Hg2+ triggered desulfurization reaction of the 1,3-dithiolane moiety to form the formyl group, which activates the ESIPT process via keto–enol tautomerization (as shown in Fig. 4B). This conversion was further supported by the similar emission spectral nature of BDA and BDT + Hg2+ under identical experimental conditions (Fig. S13, ESI†). Therefore, a similar reaction process is also expected for CH3Hg+ towards BDT to show the changes in emission properties, as we proved in our previous work.46 This mechanism has been further supported by the ESI-MS study of BDT in the presence of Hg2+(Fig. S14, ESI†), where m/z = 227.195 represents the mass of BDA which confirms the desulfurization of BDT. | ||
| Fig. 4 (A)1H NMR spectra of BDT in the absence and presence of Hg2+ in DMSO-d6. (B) Probable sensing mechanism of BDT for detecting Hg2+/CH3Hg+. | ||
Practical application ability
For practical application purposes, environmental water samples were also investigated using BDT. For this, we have collected IISER Kolkata pond water to determine Hg2+ with the help of our probe BDT. However, real-life water samples contain several other competitive components, which can cause interference towards detecting Hg2+ by BDT. Therefore, we have carried out the estimation of the Hg2+ level with the help of the standard addition method.47 Probe BDT could quantify the added Hg2+ precisely with excellent recovery (Table S2, ESI†), which entirely established the applicability of our probe towards the detection of Hg2+ in real-life samples. Furthermore, to further support the relevance of our probe towards real samples, we have checked the amount of Hg2+ present in commercially available powder vermilion using BDT and verified with ICP-MS (Table S2, ESI†). The obtained result suggested the excellent applicability of BDT towards the detection of Hg2+ in real samples.Detection of Hg2+ in biological samples
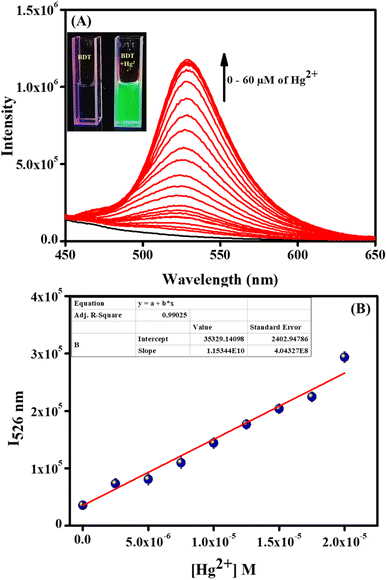
After confirming the precise and sensitive detection ability of BDT for Hg2+ and CH3Hg+, it is essential to check the detection ability of our probe at the cellular level. Towards this, BDT has been utilized to detect Hg2+ in biological systems as an incredible turn-on fluorescent probe. The initial cell viability studies of BDT revealed its low cytotoxicity effects (Fig. S14, ESI†). Due to having the less toxic effect of BDT on cells, we carried out a cellular imaging study by varying the Hg2+ ion concentration. The fluorescence intensity change of BDT in immobilized HeLa cells was investigated by changing the concentration of Hg2+ ions. From Fig. 5A–C, it could be easily observed that with the increasing concentration of Hg2+, the fluorescence intensity of BDT tagged HeLa cells increased. These results suggested that the reaction-based fluorescence probe BDT could monitor Hg2+ in cells with excellent specificity and efficiency. | ||
| Fig. 5 Confocal laser scanning microscope images of HeLa cells pre-treated with 10 μM BDT in the presence of (A) 0 μM, (B) 10 μM, and (C) 20 μM Hg2+ ions. Excitation wavelength: 405 nm. | ||
Conclusion
We designed and synthesized one of the smallest, most specific, and economic reaction-based probes (BDT) to identify and quantify Hg2+ and CH3Hg+ in environmental and biological samples through the ‘ESIPT’ activation process. BDT was demonstrated as a precise and sensitive probe for detecting inorganic and organic mercury with a theoretical detection limit of 3.8 nM and 0.8 μM, respectively. The desulfurization reaction for the formation of dialdehyde systems was proved with the help of 1H NMR spectroscopy and fluorescence spectroscopic studies. With the capability to detect the analyte at the cellular level, we believe that BDT will provide big advantages towards detecting Hg2+ in biosamples. Further improvement can be made using dialdehyde systems through increased π-conjugation for sensing purposes, and we think that BDT will initiate this progression.Author contributions
T. S and N. D have equal contribution to this work. D. P and P. K performed all in vitro biology experiments. R. S. supervised throughout this project.Conflicts of interest
There is no conflict of interest.Acknowledgements
T. S thanks IISER-K for fellowship. N. D thanks UGC for the fellowship. P. K thanks, DBT INSPIRE for the fellowship. D. P thanks IISER-K for the fellowship. R. S thanks IISER-K, DBT, and the Ministry of Textiles, Government of India, for funding. The authors thank IISER-K for infrastructure and facilities.References
- M. Altrichter, Assessing potential for community-based management of peccaries through common pool resource theory in the rural area of the Argentine Chaco, Ambio, 2008, 37, 108–113 CrossRef PubMed.
- K. P. Lisha and T. Pradeep, Towards a practical solution for removing inorganic mercury from drinking water using gold nanoparticles, Gold Bull., 2009, 42, 144–152 CrossRef CAS.
- P. A. Ariya, M. Amyot, A. Dastoor, D. Deeds, A. Feinberg, G. Kos, A. Poulain, A. Ryjkov, K. Semeniuk, M. Subir and K. Toyota, Mercury physicochemical and biogeochemical transformation in the atmosphere and at atmospheric interfaces: A review and future directions, Chem. Rev., 2015, 115, 3760–3802 CrossRef CAS PubMed.
- S. L. Pan, K. Li, L. L. Li, M. Y. Li, L. Shi, Y. H. Liu and X. Q. Yu, A reaction-based ratiometric fluorescent sensor for the detection of Hg (II) ions in both cells and bacteria, Chem. Commun., 2018, 54, 4955–4958 RSC.
- J. García-Calvo, S. Vallejos, F. C. García, J. Rojo, J. M. García and T. Torroba, A smart material for the in situ detection of mercury in fish, Chem. Commun., 2016, 52, 11915–11918 RSC.
- C. Song, W. Yang, N. Zhou, R. Qian, Y. Zhang, K. Lou, R. Wang and W. Wang, Fluorescent theranostic agents for Hg2+ detection and detoxification treatment, Chem. Commun., 2015, 51, 4443–4446 RSC.
- T. W. Clarkson, L. Magos and G. J. Myers, The toxicology of mercury—current exposures and clinical manifestations, N. Engl. J. Med., 2003, 349, 1731–1737 CrossRef CAS PubMed.
- (a) R. Von Burg, Inorganic mercury, J. Appl. Toxicol., 1995, 15, 483–493 CrossRef CAS PubMed; (b) J. Chen, J. Pan and S. Chen, A naked-eye colorimetric sensor for Hg2+ monitoring with cascade signal amplification based on target-induced conjunction of split DNAzyme fragments, Chem. Commun., 2017, 53, 10224–10227 RSC.
- W. F. Fitzgerald, C. H. Lamborg and C. R. Hammerschmidt, Marine biogeochemical cycling of mercury, Chem. Rev., 2007, 107, 641–662 CrossRef CAS PubMed.
- S. Oh, J. Jeon, J. Jeong, J. Park, E. T. Oh, H. J. Park and K. H. Lee, Fluorescent detection of methyl mercury in aqueous solution and live cells using fluorescent probe and micelle systems, Anal. Chem., 2020, 92, 4917–4925 CrossRef CAS PubMed.
- A. Chatterjee, M. Banerjee, D. G. Khandare, R. U. Gawas, S. C. Mascarenhas, A. Ganguly, R. Gupta and H. Joshi, Aggregation-induced emission-based chemodosimeter approach for selective sensing and imaging of Hg (II) and methylmercury species, Anal. Chem., 2017, 89, 12698–12704 CrossRef CAS PubMed.
- V. Iyengar and J. Woittiez, Trace elements in human clinical specimens: evaluation of literature data to identify reference values, Clin. Chem., 1988, 34, 474–481 CrossRef CAS.
- A. T. Townsend, K. A. Miller, S. McLean and S. Aldous, The determination of copper, zinc, cadmium and lead in urine by high resolution ICP-MS, J. Anal. At. Spectrom., 1998, 13, 1213–1219 RSC.
- C. B’hymer and J. A. Caruso, Arsenic and its speciation analysis using high-performance liquid chromatography and inductively coupled plasma mass spectrometry, J. Chromatography A, 2004, 1045, 1–13 CrossRef PubMed.
- K. C. Thompson and R. J. Reynolds, Atomic Absorption. Fluorescence and Flame Emission Spectroscopy, Charles Griftin, London, 2nd edn, 1978, 51, pp. 175–176 Search PubMed.
- J. L. Gómez-Ariza, D. Sánchez-Rodas, I. Giráldez and E. Morales, A comparison between ICP-MS and AFS detection for arsenic speciation in environmental samples, Talanta, 2000, 51, 257–268 CrossRef.
- T. Nakazato and H. Tao, A high-efficiency photooxidation reactor for speciation of organic arsenicals by liquid chromatography− hydride generation−ICPMS, Anal. Chem., 2006, 78, 1665–1672 CrossRef CAS PubMed.
- D. Xu, L. Tang, M. Tian, P. He and X. Yan, A benzothizole-based fluorescent probe for Hg2+ recognition utilizing ESIPT coupled AIE characteristics, Tetrahedron Lett., 2017, 58, 3654–3657 CrossRef CAS.
- Y. H. Lee, H. Liu, J. Y. Lee, S. H. Kim, S. K. Kim, J. L. Sessler, Y. Kim and J. S. Kim, Dipyrenylcalix [4] arene—a fluorescence-based chemosensor for trinitroaromatic explosives, Chem. – Eur. J., 2010, 16, 5895–5901 CrossRef CAS PubMed.
- M. E. Germain and M. J. Knapp, Optical explosives detection: from color changes to fluorescence turn-on, Chem. Soc. Rev., 2009, 38, 2543–2555 RSC.
- A. Aliberti, P. Vaiano, A. Caporale, M. Consales, M. Ruvo and A. Cusano, Fluorescent chemosensors for Hg2+ detection in aqueous environment, Sens. Actuators, B, 2017, 247, 727–735 CrossRef CAS.
- L. Zong, Y. Xie, Q. Li and Z. Li, A new red fluorescent probe for Hg2+ based on naphthalene diimide and its application in living cells, reversibility on strip papers, Sens. Actuators, B, 2017, 238, 735–743 CrossRef CAS.
- I. Kim, N. E. Lee, Y. J. Jeong, Y. H. Chung, B. K. Cho and E. Lee, Micellar and vesicular nanoassemblies of triazole-based amphiphilic probes triggered by mercury (II) ions in a 100% aqueous medium, Chem. Commun., 2014, 50, 14006–14009 RSC.
- P. Srivastava, S. S. Razi, R. Ali, R. C. Gupta, S. S. Yadav, G. Narayan and A. Misra, Selective naked-eye detection of Hg2+ through an efficient turn-on photoinduced electron transfer fluorescent probe and its real applications, Anal. Chem., 2014, 86, 8693–8699 CrossRef CAS PubMed.
- M. H. Lee, S. W. Lee, S. H. Kim, C. Kang and J. S. Kim, Nanomolar Hg (II) detection using Nile blue chemodosimeter in biological media, Org. Lett., 2009, 11, 2101–2104 CrossRef CAS PubMed.
- Z. Guo, W. Zhu, M. Zhu, X. Wu and H. Tian, Near-Infrared Cell-Permeable Hg2+-Selective ratiometric fluorescent chemodosimeters and fast indicator paper for MeHg+ based on tricarbocyanines, Chem. – Eur. J., 2010, 16, 14424–14432 CrossRef CAS PubMed.
- V. Singh, P. Srivastava, S. PrakashVerma, A. Misra, P. Das and N. Singh, A new fluorescent pyrene–pyridine dithiocarbamate probe: A chemodosimeter to detect Hg2+ in pure aqueous medium and in live cells, J. Lumin., 2014, 154, 502–510 CrossRef CAS.
- M. Y. Chae and A. W. Czarnik, Fluorometricchemodosimetry. Mercury (II) and silver (I) indication in water via enhanced fluorescence signaling, J. Am. Chem. Soc., 1992, 114, 9704–9705 CrossRef CAS.
- K. C. Song, J. S. Kim, S. M. Park, K. C. Chung, S. Ahn and S. K. Chang, Fluorogenic Hg2+ selective chemodosimeter derived from 8-hydroxyquinoline, Org. Lett., 2006, 8, 3413–3416 CrossRef CAS PubMed.
- Y. Gao, T. Ma, Z. Ou, W. Cai, G. Yang, Y. Li, M. Xu and Q. Li, Highly sensitive and selective turn-on fluorescent chemosensors for Hg2+ based on thioacetal modified pyrene, Talanta, 2018, 178, 663–669 CrossRef CAS PubMed.
- Z. Ruan, Y. Shan, Y. Gong, C. Wang, F. Ye, Y. Qiu, Z. Liang and Z. Li, Novel AIE-active ratiometric fluorescent probes for mercury (II) based on the Hg2+-promoted deprotection of thioketal, and good mechanochromic properties, J. Mater. Chem. C, 2018, 6, 773–780 RSC.
- Y. Tang, D. Lee, J. Wang, G. Li, J. Yu, W. Lin and J. Yoon, Development of fluorescent probes based on protection–deprotection of the key functional groups for biological imaging, Chem. Soc. Rev., 2015, 44, 5003–5015 RSC.
- P. CAS, J. Shanmugapriya, S. Singaravadivel, G. Sivaraman and D. Chellappa, Anthracene-Based Highly Selective and Sensitive Fluorescent “Turn-on” Chemodosimeter for Hg2+, ACS Omega, 2018, 3, 12341–12348 CrossRef PubMed.
- J. Zhao, S. Ji, Y. Chen, H. Guo and P. Yang, Excited state intramolecular proton transfer (ESIPT): from principal photophysics to the development of new chromophores and applications in fluorescent molecular probes and luminescent materials, Phys. Chem. Chem. Phys., 2012, 14, 8803–8817 RSC.
- (a) V. S. Padalkar and S. Seki, Excited-state intramolecular proton-transfer (ESIPT)-inspired solid state emitters, Chem. Soc. Rev., 2016, 45, 169–202 RSC; (b) J. E. Kwon and S. Y. Park, Adv. Mater., 2011, 23, 3615–3642 CrossRef CAS PubMed.
- J. Wu, W. Liu, J. Ge, H. Zhang and P. Wang, New sensing mechanisms for design of fluorescent chemosensors emerging in recent years, Chem. Soc. Rev., 2011, 40, 3483–3495 RSC.
- S. Sahana, G. Mishra, S. Sivakumar and P. K. Bharadwaj, A 2-(2′-hydroxyphenyl) benzothiazole (HBT)–quinoline conjugate: a highly specific fluorescent probe for Hg2+ based on ESIPT and its application in bioimaging, Dalton Trans., 2015, 44, 20139–20146 RSC.
- L. Deng, Y. Li, X. Yan, J. Xiao, C. Ma, J. Zheng, S. Liu and R. Yang, Ultrasensitive and highly selective detection of bioaccumulation of methyl-mercury in fish samples via Ag0/Hg0 amalgamation, Anal. Chem., 2015, 87, 2452–2458 CrossRef CAS PubMed.
- Y. Liu, M. Chen, T. Cao, Y. Sun, C. Li, Q. Liu, T. Yang, L. Yao, W. Feng and F. Li, A cyanine-modified nanosystem for in vivo upconversion luminescence bioimaging of methylmercury, J. Am. Chem. Soc., 2013, 135, 9869–9876 CrossRef CAS PubMed.
- (a) M. Santra, B. Roy and K. H. Ahn, A “reactive” ratiometric fluorescent probe for mercury species, Org. Lett., 2011, 13, 3422–3425 CrossRef CAS PubMed; (b) F. Song, S. Watanabe, P. E. Floreancig and K. Koide, Oxidation-resistant fluorogenic probe for mercury based on alkyne oxymercuration, J. Am. Chem. Soc., 2008, 130, 16460–16461 CrossRef CAS PubMed; (c) L. Zhang, L. Zhang, X. Zhang, P. Liu, Y. Wang, X. Han and L. Chen, Fluorescent imaging to provide visualized evidences for mercury induced hypoxia stress, J. Hazard. Mater., 2023, 444, 130374 CrossRef CAS PubMed; (d) Y. Wang, L. Zhang, X. Han, L. Zhang, X. Wang and L. Chen, Fluorescent probe for mercury ion imaging analysis: Strategies and applications, Chem. Eng. J., 2021, 406, 127166 CrossRef CAS; (e) Y. Wang, M. Gao, Q. Chen, F. Yu, G. Jiang and L. Chen, Associated Detection of Superoxide Anion and Mercury(II) under Chronic Mercury Exposure in Cells and Mice Models via a Three-Channel Fluorescent Probe, Anal.Chem., 2018, 90(16), 9769–9778 CrossRef CAS PubMed.
- Y. K. Yang, S. K. Ko, I. Shin and J. Tae, Fluorescent detection of methylmercury by desulfurization reaction of rhodaminehydrazide derivatives, Org. Biomol. Chem., 2009, 7, 4590–4593 RSC.
- T. Chen, T. Wei, Z. Zhang, Y. Chen, J. Qiang, F. Wang and X. Chen, Highly sensitive and selective ESIPT-based fluorescent probes for detection of Pd2+ with large Stocks shifts, Dyes Pigm., 2017, 140, 392–398 CrossRef CAS.
- Z. Huang, S. Ding, D. Yu, F. Huang and G. Feng, Aldehyde group assisted thiolysis of dinitrophenyl ether: a new promising approach for efficient hydrogen sulfide probes, Chem. Commun., 2014, 50, 9185–9187 RSC.
- I. J. Chang, K. S. Hwang and S. K. Chang, Selective Hg2+ signaling via dithiane to aldehyde conversion of an ESIPT fluorophore, Dyes Pigm., 2017, 137, 69–74 CrossRef CAS.
- Y. Zhou, X. He, H. Chen, Y. Wang, S. Xiao, N. Zhang, D. Li and K. Zheng, An ESIPT/ICT modulation based ratiometric fluorescent probe for sensitive and selective sensing Hg2+, Sens. Actuators, B, 2017, 247, 626–631 CrossRef CAS.
- T. Samanta, N. Das, D. Patra, P. Kumar, B. Sharmistha and R. Shunmugam, Reaction-Triggered ESIPT Active Water-Soluble Polymeric Probe for Potential Detection of Hg2+/CH3Hg+ in Both Environmental and Biological Systems, ACS Sustainable Chem. Eng., 2021, 9, 5196–5203 CrossRef CAS.
- C. C. Huang and H. T. Chang, Selective gold-nanoparticle-based “turn-on” fluorescent sensors for detection of mercury (II) in aqueous solution, Anal. Chem., 2006, 78, 8332–8338 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Materials and methods, synthetic procedures, NMR and ESI-MS spectra, UV-vis and fluorescence spectra, linear plots, time dependent studies, table for comparison among reported literature studies, and real sample analysis summary table. See DOI: https://doi.org/10.1039/d3su00012e |
| This journal is © The Royal Society of Chemistry 2023 |