Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Sequential extraction of high-value added molecules from grape pomaces using supercritical fluids with water as a co-solvent†
Gayane
Hayrapetyan
 ab,
Karen
Trchounian
b,
Laurine
Buon
c,
Laurence
Noret
a,
Benoît
Pinel
d,
Jeremy
Lagrue
d and
Ali
Assifaoui
*a
ab,
Karen
Trchounian
b,
Laurine
Buon
c,
Laurence
Noret
a,
Benoît
Pinel
d,
Jeremy
Lagrue
d and
Ali
Assifaoui
*a
aUMR PAM, Equipe PCAV, Université de Bourgogne/Institut Agro, 1 Esp. Erasme, Agrosup, 21000 Dijon, France. E-mail: ali.assifaoui@u-bourgogne.fr
bDepartment of Biochemistry, Microbiology and Biotechnology, Yerevan State University, 1 Alex Manoogian St, Yerevan 0025, Armenia
cCERMAV (Centre de Recherches sur les Macromolécules Végétales), 38041 Grenoble, France
dSFE Process, 107 Bd Tolstoï, 54510 Tomblaine, France
First published on 4th September 2023
Abstract
Inspired by the concept of organic waste valorisation and heading towards a sustainable economy, a green chemistry extraction technique involving supercritical carbon dioxide (SC-CO2) along with water as a co-solvent was employed for the main winery by-product (grape pomaces). The objective was to selectively extract high-value added molecules, in particular phenolic compounds and polysaccharides. The experimental design involved applying three distinct temperature conditions (40, 60, and 80 °C), and a constant pressure of 400 bar. Phenolic compounds and high-molecular weight isolates (449–478 kDa) containing low methoxyl (% DE = 23) pectic substances were detected in the SC-CO2 extracts accompanied by water as a co-solvent. The phenolic acids, namely, gallic (GA), protocatechuic (PCA), coumaric (CouA), and caftaric (CTA), and flavonoids, namely, procyanidin B1 (PRC B1), procyanidin B2 (+) (PRC B2), (+) catechin (CT), and (−) epicatechin (ECT) were found in all the extracts under the tested experimental conditions. The following study underscores the potential of the pressurized CO2/H2O medium as an effective solvent with minimal environmental impacts for the comprehensive valorisation of the main winery by-product, specifically targeting polysaccharides and phenolic compounds.
Sustainability spotlightThis study focuses on the extraction of valuable molecules such as polyphenols and fibers from grape pomace, a by-product of wine production, using a green chemistry technique called supercritical fluid extraction. These extracted molecules have potential applications as antioxidants in wine production. The research highlights the significance of specific United Nations sustainable development goals, including affordable and clean energy (SDG 7), industry, innovation, and infrastructure (SDG 9), and climate action (SDG 13). |
1. Introduction
In the past two decades, serious attention has been paid to the possible applications of “truly green chemistry” methods such as supercritical fluid extraction (SFE) and subcritical water extraction (SWE). These are based on primarily non-toxic, non-flammable and widely available molecules such as water and carbon dioxide (CO2). The methods were proved to be efficient for the total valorisation of high-value added molecules such as antioxidants, polyphenols and grape seed oil from various industrial wastes including grape, apple and hazelnut pomaces.1–4This work focuses on the SC-CO2 extraction, which is based on the application of carbon dioxide (CO2) as a solvent. The CO2 solvent under its supercritical condition (T = 31.2 °C, P = 74 bar) shows increased affinity towards non-polar substances. Thus, research on SC-CO2 recently reported in the literature primarily focuses on the extraction of oils.5–8 Moreover, the added values of the SC-CO2 process are its high recyclability rate compared to the conventional techniques and the overall reduced energy cost.9 However, CO2 alone is not a good solvent; to increase its solvating power and also attract hydrophilic molecules, the process is often associated with the implementation of additional co-solvents (ethanol/methanol).10,11
The valorization of by-products is often a challenge due to their complex nature. The grape pomace is the main winery by-product that makes up to 20–25% of total freshly pressed grape fruit.12 It is a potential source of high-value added products such as dietary fibres, oils, phenolic compounds and anthocyanins with various proven health benefits.13,14 It consists of skins (42.4%), seeds (22.5%), stalks (24.9%) and other minor components. The grape skin is rich in polysaccharides such as cellulose, hemicellulose, xyloglucan, arabinan, xylan, and mannan that constitute 30–35%, among which ∼20% are moderately methyl-esterified (62%) pectic substances, some structural proteins (<5%) and phenolic compounds.15
Despite its considerable socio-economic importance, contributing to a global wine production of approximately 260 million hectoliters in 2022,16 winemaking produces a substantial quantity of winery by-products. Although the pomace is a natural and completely biodegradable source, its huge amounts in vineyards generated within a short period of time are often associated with various environmental hazards such as soil (change in pH) and water pollution, unpleasant odors, insect attraction and spread of diseases.17 While in some countries the pomace is directly disposed in vineyards as a fertilizer, the European Union (EU) regulations request to send the winery by-products into the distillery sites for alcohol, spirits and piquette production only (Commission Delegated Regulation (Eu) 2018/273 of 11 December 2017, Article 14).
In the grape skin, phenolic compounds are cross-linked to pectic polysaccharides via hydrophobic interactions and hydrogen bonds and are later released upon fruit maturation.15,18 The phenolic compounds are secondary metabolites of plant-based organisms; these molecules are considered as natural alternatives to synthetic antioxidants and have been extensively studied using traditional and green chemistry extraction techniques over the years.10,18–20 Furthermore, water as a co-solvent in the SC-CO2 process is not applied commonly, and only some studies showed its potential application for the extraction of phenolic compounds. Da Porto et al.2 targeted mainly proanthocyanidins and phenols from the grape pomace by applying both water and/or ethanol 15% (w/w) as co-solvents in the SC-CO2 process under 40, 50, and 60 °C and 100 or 200 bar pressure experimental conditions.
Polysaccharides such as pectin in grapes are responsible for various physiological processes such as immune response to abiotic and biotic stresses.21 It has quite important economic value and is extensively used in food, cosmetic and pharmaceutical industries such as gelling, emulsifying and stabilizing agents. Some alternative extraction methods such as ultrasound22 and microwave-assisted (from citrus)23–25 were applied for pectin extraction in order to find better alternatives to the outdated conventional acid extraction. However, the alternative methods continue the use of harmful chemicals, and hence, they could not be considered “fully green”. In the context of finding sustainable solutions with the least environmental impact, the SC-CO2 together with water as a co-solvent is a promising medium and could potentially target polysaccharides and phenolic compounds. Furthermore, in contrast to SWE, which involves significantly higher temperatures (T = 100–374 °C), SC-CO2 operates at lower temperatures, thus safeguarding extracts from heat-induced degradation. There is indeed a huge gap in the research dedicated to the pectin extraction by fully green chemistry methods, especially by SC-CO2. To the best of our knowledge, only a study by the time of writing reported the extraction of bioactive compounds including pectin by SC-CO2 alone.26 The aim of this work is to valorise the main winery by-product (grape pomaces) by SC-CO2 along with water as a co-solvent. The extracted molecules were further characterized by various physical and biochemical methods.
2. Materials and methods
2.1. Materials
The grape pomace was obtained from a white grape variety (Marsannay Aligoté) from Côte de Nuits vineyard, France and stored at −20 °C in the plastic thermo bags. The wet grape pomace had a moisture content of 73.2%.The standards of white wine-related phenolic compounds such as gallic, protocatechuic, hydroxybenzoic, caftaric, gentisic, caffeic, coumaric, and chlorogenic acids and hydroxytyrosol, tyrosol, procyanidin B1, B2, (+) catechin and (−) epicatechin were purchased from Sigma-Aldrich (Steinheim, Germany). The monosaccharide standards (mannitol, L-fucose, L-rhamnose, L-arabinose, D-galactose, D-glucose, D-mannose, D-xylose, D-galacturonic and glucuronic acids) were purchased from Acros Organics and Sigma-Aldrich. Sodium hydroxide (NaOH) solution 46/48% (Fisher Bioblock), sodium acetate (NaOAc) (Sigma-Aldrish), trifluoroacetic acid (TFA) (Merck) and all the above-mentioned chemicals were of analytical grade.
2.2. Sample pre-treatment
Prior to extraction, the grape pomace was subjected to pre-treatment steps involving oven-drying at 50 °C followed by grinding using an industrial grinder (SK 100, RETSCH®). The powder was passed through 1 mm mesh. The exact particle size distribution within the powder was determined using a laboratory sieve shaker and the size variation of particles was 50% of 200 μm, 40% under 400–500 μm and the remaining 10% less <1 mm which included larger particles mainly from the grape seeds. The powder was stored in thermo bags at −20 °C until further use.2.3. Supercritical CO2 extraction on grape pomaces
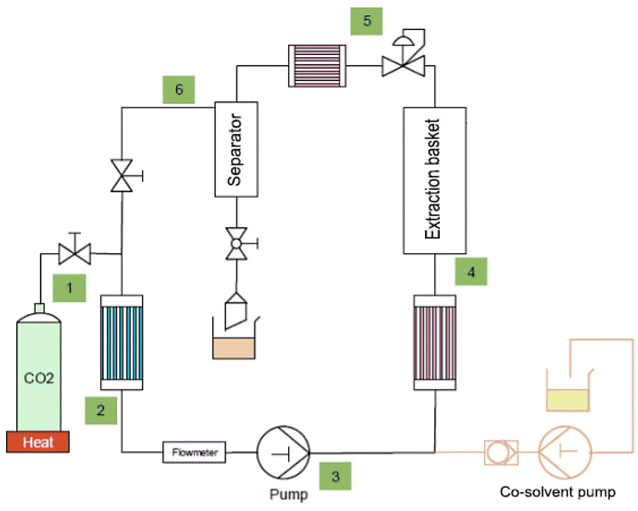
The supercritical SC-CO2 machine (SFE process, Nancy, France) coupled with two pumps, main CO2 and co-solvent, with a maximum pressure capacity of 1000 bar was used in this study. The instrument is equipped with an extraction basket (stainless steel) with a capacity of 100 mL and an autoclave cylinder, which directs the extracts to the back pressure regulator (BPR) (Fig. 1). The cooling system was filled with glycerol and the CO2 flow was recycled in the system. The observation of the extracts was possible thanks to the transparent sapphire separator. Various temperature sensors were placed in the system monitoring important temperature changes within the system.The experimental plan was designed based on Moller's diagram and the experimental conditions were chosen to fall within the supercritical region of CO2, accompanied by the highest density values. An exact mass (26 g) of dry grape pomace powder was introduced into the extraction basket, the experiments were conducted twice for each condition and the extracts were collected in the corresponding containers. A sequential extraction process was applied to the matrix at a constant pressure of 400 bar, which was chosen to be efficient according to the preliminary experimental data. Moving forward the extracts will be labelled: SC-40, SC-60 and SC-80 which correspond to the applied temperatures 40, 60, and 80 °C and a constant pressure of 400 bar accordingly.
The supercritical condition of CO2 can be reached at different temperature and pressure values, where the minimal conditions are T = 31.2 °C and P = 74 bar. At the beginning of the process, CO2 exists in a mixed gas/liquid form, and then it undergoes decompression under high pressures. As it advances towards the pre-heating step and just before entering the extraction basket, it transitions into a liquid state. This marks the point of extraction. The process is further followed by the decompression step after the back pressure regulation. Eventually CO2 leaves the system by liberating into the atmosphere. It should be noted that Moller's diagram (enthalpy–entropy chart) is applicable when pure CO2 is applied and it changes depending on the co-solvents introduced.
The pomace was initially defatted using a continuous flow of CO2 (30 g min−1) for an hour under each tested experimental condition. Upon the termination of oil extraction, ethanol as a co-solvent was introduced into the system to collect the remaining oily substances. The characterization of the extracted oil will not be presented in this article.
Following the de-fatting process, water as a co-solvent (flow rate = 10 g min−1) was introduced into the system. The use of water as a co-solvent requires precise management of the temperature in the sapphire separator, which is a crucial factor for extract collection. For instance, higher temperatures (>30 °C) in the sapphire separator lead to the condensation of water and may initiate the formulation of CO2 ice blocks in the condenser, thus affecting the extract collection. Hence, it is important to maintain the separator temperature within the range of 20 to 30 °C, along with a pressure of 50 bar, to ensure that CO2 remains in liquid form for its uninterrupted circulation.
The extraction stopped manually following the color change in the transparent sapphire separator, when the extracts from deep burgundy tuned into pale pink. All the extracts were collected in the corresponding glass containers and stored at −4 °C until future characterizations. These liquid fractions were first characterized in terms of phenolic compounds by UHPLC and then alcohol insoluble fractions (AIF) were separated by alcoholic precipitation to target mainly pectic polysaccharides. The isolates were later characterized by FT-IR (Fourier-transform infrared) spectroscopy, HPAEC-PAD (high-performance anion-exchange chromatography/pulsed amperometric detection) and HPSEC (high-performance size-exclusion chromatography) for monosaccharide composition and molecular weight determination.
2.4. Isolation of alcohol insoluble fractions (AIFs)
SC-CO2 and water as co-solvent liquid extracts further undergo an isolation process, where the extracts were precipitated by 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v of ethanol (96%) and kept under stirring at room temperature for one hour. The precipitated alcohol insoluble fractions (AIFs) were separated by centrifugation (1750 g, 30 minutes). The pellet was separated from the supernatant by filtration and washed against 70, 96 and 99% ethanol to eliminate impurities and small sugar components.22 Later the substances were dissolved in 10 mM NaCl solution and the pH was adjusted to 7 with NaOH. The isolates were subjected to thorough dialysis (Mw cut-off: 3.5 kDa) against deionized water, with liquid changes carried out every two hours throughout the day and kept overnight. Finally, the isolates were freeze-dried (−57 °C) (Heto PowerDry PL 6000, Thermo Electron Corporation) at a pressure lower than 1 hPa. The isolates were stored under dry conditions until further analysis.
1 v/v of ethanol (96%) and kept under stirring at room temperature for one hour. The precipitated alcohol insoluble fractions (AIFs) were separated by centrifugation (1750 g, 30 minutes). The pellet was separated from the supernatant by filtration and washed against 70, 96 and 99% ethanol to eliminate impurities and small sugar components.22 Later the substances were dissolved in 10 mM NaCl solution and the pH was adjusted to 7 with NaOH. The isolates were subjected to thorough dialysis (Mw cut-off: 3.5 kDa) against deionized water, with liquid changes carried out every two hours throughout the day and kept overnight. Finally, the isolates were freeze-dried (−57 °C) (Heto PowerDry PL 6000, Thermo Electron Corporation) at a pressure lower than 1 hPa. The isolates were stored under dry conditions until further analysis.
2.5. UHPLC-DAD/FLD phenolic compound analysis
The phenolic profile of SC-CO2 and water as co-solvent liquid extracts under the tested temperature conditions was determined by ultra-high-performance liquid chromatography (UHPLC). The method was adapted from Nicolantonaki et al.27 with slight modifications. UPLC H-Class (Waters, Milford, MA, USA) equipped with a quaternary pump (QSM), an autosampler (SM-FTN), and fluorimetric (FLR Acquity) and diode array (Acquity PDA) detectors were used for the analysis. White wine-related phenolic compounds such as gallic, protocatechuic, hydroxybenzoic, caftaric, gentisic, caffeic, coumaric, and chlorogenic acids and hydroxytyrosol, tyrosol, procyanidin B1, B2, (+) catechin and (−) epicatechin were used to detect their presence in the extracts.The SC-CO2 extracts prior injections were filtered using a PTFE filter (0.45 μm PTFE, Restek, Lisses, France) and 2 μL of each filtrate was loaded into a C18 acid-resistant column (Raptor ARS-18, 150 × 2.1 mm, 1.8 μm, 1000 bar, Restek, Lisses, France). Reverse-phase chromatography has been used. The stationary phase was composed of non-polar (hydrophobic) silica-grafted C18 alkyl chains. A binary solvent was run at a flow rate of 0.30 mL min−1 employing a mobile phase (A) H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol
methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) trifluoroacetic acid (TFA) (95
trifluoroacetic acid (TFA) (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.28%) and (B) methanol (100%). The optimized elution system consisted of a stepwise gradient as follows: 3% of B for 5 min, 3–5% B in 3 min, 5% of B for 9 min, 5–9% B for 2 min, 9% B for 6 min, 9–14.3% for 10 min, 14.3–23% of B in 1 min and 23–100% B for 9 min. The column temperature was set at 35 ± 2 °C and the autosampler at 12 °C. Acquisitions, solvent delivery and detectors were controlled using the Empower 2 program.
0.28%) and (B) methanol (100%). The optimized elution system consisted of a stepwise gradient as follows: 3% of B for 5 min, 3–5% B in 3 min, 5% of B for 9 min, 5–9% B for 2 min, 9% B for 6 min, 9–14.3% for 10 min, 14.3–23% of B in 1 min and 23–100% B for 9 min. The column temperature was set at 35 ± 2 °C and the autosampler at 12 °C. Acquisitions, solvent delivery and detectors were controlled using the Empower 2 program.
The diode array detector was set at 320 nm for trans-caftaric, gentisic, trans-caffeic, chlorogenic, trans-coutaric, 2-S-glutathionylcaftaric (GRP), and trans-ferulic acids at 305 nm for salicylic, trans-coumaric, at 280 nm for gallic and at 260 nm for protocatechuic and hydroxybenzoic acids. The fluorescence detector was set at λex = 270 nm and λem = 322 nm for hydroxytyrosol, tyrosol, catechin, epicatechin, proanthocyanidin B1, and proanthocyanidin B2.
2.6. Molecular weight determination
The average molecular weight (Mw) of polysaccharides was commonly determined by size exclusion chromatography (SEC) coupled with multi-angle light scattering (MALS) and refractive index detection. After separation in a SEC column by hydrodynamic volume, the MALS detector measures the intensity of the light scattered by polysaccharides in the solution. This measure is directly proportional to molar mass and to the concentration of polysaccharides, which was determined using a refractive index detector.In this work, an HPSEC system (High Performance Size-Exclusion Chromatography – Shimadzu) coupled with a 3-angles light scattering detector (Mini Dawn Treos – WYATT) associated with a refractive index detector (Optilab – WYATT) and a UV detector (Shimadzu) was used.
The SEC columns used for the separation were two Shodex SB 806M HQ in series associated with a pre-column SB-G 6B thermostated at 30 °C. The solvent used was 0.1 M sodium nitrate (NaNO3) including 0.3 g L−1 sodium azide (NaN3) (pH: 6) and the flow rate was 0.5 mL min−1 with an elution time of 70 minutes.
The value of the refractive index increment dn/dc used for this analysis is 0.146 mL g−1, which is a value commonly used for polysaccharide detection (pectin). The lyophilized isolates were dissolved under stirring during 24 h at a concentration of 1 mg mL−1 in an eluent composed of 0.1 M sodium nitrate + 0.03% sodium azide (NaN3). Afterwards, they were filtered through a 0.2 μm filter and 100 μL of each was injected. Softer package Astra 6 (Wyatt Technology Co., USA) has been used for the data treatment.
2.7. Monosaccharide composition
HPAEC-PAD (high-performance anion-exchange chromatography/pulsed amperometric detection) was used for the determination of the monosaccharide profile in the isolates. The freeze-dried isolates undergo hydrolysis prior to injection into the chromatograph. Around 4 mg of samples were solubilized in 1 mL of deionized water and stirred for around 45 minutes at room temperature. Then, 1 mL of 4 N TFA was added to the samples and they were placed into a dry bath for 4 hours at 110 °C. After cooling, the samples were filtered through a filter (0.2 μm) and then diluted six times in ultrapure water before injection into the HPAEC-PAD system, equipped with a pre-column and CarboPac PA1 column (250 × 2 mm). The compounds were detected using a DIONEX ICS-6000 ED electrochemical detector (ThermoFisher) equipped with gold on PTFE disposable working electrodes and an Ag/AgCl reference electrode.The column was thermostated beforehand at 28 °C and the volume injected was 5 μL. Pulse potentials (E, volts) and durations (t, ms) were E1 = 0.1, t1 = 0, E2 = 0.1, t2 = 200, E3 = 0.1, t3 = 400, E4 = −2.0, t4 = 410, E5 = −2.0, t5 = 420, E5 = 0.6, t5 = 430, E6 = −0.1, t6 = 440, and E7 = −0.1, t7 = 500.
The elution was carried out at 0.18 mL min−1 under the following conditions: −t = 0 to t = 27 min: NaOH 0.016 M, t = 27 to t = 29.1 min: NaOH 0.016 M + linear gradient in NaOAc from 0 to 0.02 M, t = 29.1 to t = 37 min: linear gradient in NaOH from 0.016 M to 0.1 M and linear gradient in NaOAc from 0.02 M to 0.5 M, t = 37 to t = 43.1 min: a linear gradient in NaOH from 0.1 M to 0.2 M + NaOAc 0.5 M. A calibration curve of the following monosaccharides was carried out with respect to the following elution order: mannitol, fucose, rhamnose, arabinose, galactose, glucose, mannose, xylose, galacturonic acid and glucuronic acid.
2.8. Fourier transform infrared (FT-IR) spectroscopy
The degree of esterification (DM) was determined by Fourier transform infrared (FT-IR) spectroscopy (PerkinElmer, Spectrum 65, The United States). The spectrum was recorded by applying 32 scans within a wavelength ranging from 600 to 4000 cm−1 with a resolution of 4 cm−1. Low methoxyl pectin (Cargill) was used as a reference. The peak area of 1744 cm−1 (COOH and COOCH3) over the sum of the peak areas of 1744 cm−1 and 1613 cm−1 (COO−) was used to determine the degree of methyl esterification.28 The calculations were performed using the Origin Pro 2021 software package.2.9. Statistical analysis
One-way ANOVA was used to compare group means and Bonferroni's and Tukey's tests were used to compare the statistical differences between each mean group under tested temperature conditions. All the data presented are the average of minimum three repetitions under each testing condition. The statistical tests were performed using the Origin Pro 2021 software package.3. Results and discussion
3.1. SC-CO2 + H2O liquid extracts
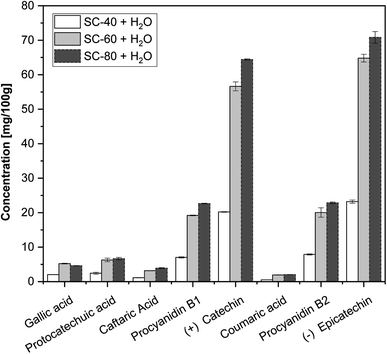
SC-CO2 and water as co-solvent liquid extracts were of a burgundy colour, which is associated with grape's skin-related water-soluble pigments such as anthocyanins. It is a common practice to use gallic acid equivalents (GAE) by the Folin Ciocalteau reagent for the determination of the overall yield of phenolics; however, this work mainly focuses on the identification and nature of molecules extracted with water as a co-solvent. The composition of phenolic compounds in the wine pomace depends on several parameters such as grape variety, soil composition, terrain and environmental factors. The existence of various phenolic compounds was confirmed with the UV and fluorescence spectra; however, only those with standards were able to be quantified. Phenolic acids, namely, gallic (GA), protocatechuic (PCA), coumaric (CouA) and caftaric (CTA) acids, and flavonoids, namely, procyanidin B1 (PRC B1), procyanidin B2 (+) (PRC B2), (+) catechin (CT) and (−) epicatechin (ECT) were detected in all the extracts (Fig. 2, S.1, Table S.1).† The total yields of water-soluble phenolic compounds under tested temperature conditions with water as a co-solvent were 64.4, 177.2 and 198 mg/100 g gallic acid equivalent (GAE/100 g grape pomace powder) for the SC-40, SC-60 and SC-80 + H2O liquid extracts accordingly.High-molecular weight polyphenols such as procyanidines (B1 and B2) were also detected in the liquid extracts, which reached their highest value at 80 °C under 400 bar.
Solubility itself is a complex process, which depends on various factors such as the nature of molecules targeted and the extraction parameters. A number of studies in the related literature mainly focused on the experimental part of SC-CO2 with ethanol or methanol as a co-solvent.10,31,32 In an earlier work, (+) catechin and (−) epicatechin were extracted from grape skin by SC-CO2 with 20% ethanol as the co-solvent under the optimal extraction condition at 60 °C and 250 bar and the values ranged from 53.6 to 89.7 and 8.94 to 233.6 mg/100 g depending on the grape skin origin.10 Arce et al. reported even lower values of total polyphenol content from different grape pomaces measured by the Folin-Ciocalteu reagents, which varied between 13 and 30 mg/100 g under the conditions of 5% MeOH, 300 bar, and 50 °C (ref. 32) while much higher recovery was observed in this study using water as a co-solvent. In the present study, when water was used as a co-solvent, the affinity of carbon dioxide towards hydrophilic molecules was significantly enhanced. This enhancement facilitated the extraction of predominantly hydrophilic phenolic compounds. Similar patterns were also reported by other authors working on the SC-CO2 process.2,33
The interactions of carbon dioxide with water molecules under higher pressure conditions lack theoretical understanding. According to the model proposed by Galanakis et al.,34 phenols are generally better solubilized in polar protic media such as ethanol and methanol or hydroalcoholic mixtures; however, some of them such as gallic, cinnamic, and coumaric acids have preference over water instead.
Several thermodynamic properties such as tunable density, high diffusivity and low viscosity are typical to compressible solvents such as carbon dioxide and may have an impact on interfacial properties. Rocha et al.35 Investigated water and CO2 interactions at two pressure (200 and 280 bar) and two temperature values (45 and 65 °C) via molecular dynamics computer simulations. They have shown similarities between CO2 in water (C/W) interface and other conventional oil-in-water (O/W) interfaces, which can be adapted to extract amphiphilic molecules. It must be considered the non-negligible solubility of CO2 in water (24 molecules of CO2 in 1000 water molecules under 200 bar and at 45 °C). The increasing attraction of CO2 towards hydrophilic compounds (SC-80) at higher temperatures observed in this work is in alliance with the theoretical approach discussed.
3.2. Polysaccharides in SC-CO2 and water as co-solvent isolates
The extracts obtained using SC-CO2 with water as a co-solvent under all temperature conditions (40, 60, and 80 °C) underwent alcohol precipitation and purification steps, as described previously. The goal was to isolate components primarily composed of pectic polysaccharides.The SC-40 + H2O liquid extract in this work after alcohol precipitation and separation steps, exhibited no isolates. This outcome is not surprising, considering that 40 °C is still relatively low for the extraction of targeted pectic polysaccharides. The isolates appeared at higher temperatures SC-60 and SC-80 + H2O; therefore, for polysaccharide extraction, the temperature here is a key factor. The extraction with SC-CO2 manually stopped when the burgundy extract became paler, indicating that CO2 under this condition reached its limits of extraction. The extraction yield exhibited an inverse correlation with the extraction time, displaying higher yields during the initial stages of extraction. Subsequently, the yield reached a plateau as the matrix became depleted. This observation aligns with findings from the existing literature.31
The yield of freeze-dried isolates after purification steps was estimated to be 1.4 g/100 g (on dry weight) and 3.2 g/100 g (on dry weight) for the samples SC-60 and SC-80, respectively. The CO2 amount used during the essays overall varied from 1870 g for SC-40, 2690 g for SC-60 and 3540 g for SC-80 respectively. This information could be important for scaling up the SC-CO2 process. The experimental design included three temperature conditions 40, 60, 80 °C at a constant pressure of 400 bar, which corresponds to the density of supercritical fluid of 1000, 900 and 800 kg m−3 respectively when only CO2 is applied. However, once modifiers such as ethanol or water are applied, the density of pure CO2 changes; therefore, for the reproducibility of the results, it is misleading to consider the density values.
It is well known that at higher temperatures, the density of CO2 is low, thus its solubility decreases, while the contrary effect was observed with water as the co-solvent. Furthermore, it should be noted that when CO2 is mixed with water, it is no longer under a supercritical condition. This change is not necessarily a disadvantage, especially when polysaccharides are the target. In addition, CO2 coupled with water as the co-solvent also changes the pH of the medium,36 consequently high pressure and lower pH conditions are naturally favorable for breaking down the cell-wall components and liberating polysaccharides and phenolic compounds.
3.3. Monosaccharide composition
High-performance anion-exchange chromatography/pulsed amperometric detection (HPAEC-PAD) of freeze-dried samples showed that SC-CO2 + H2O isolates contained the following monosaccharides: rhamnose, arabinose, galactose, glucose, mannose, xylose, galacturonic and glucuronic acids (Table 1 and Fig. 3). The total sugar content was estimated to be 23.84 ± 0.65 and 16.86 ± 0.19 (on dry weight) for the SC-60 and SC-80 + H2O isolates accordingly. Valiente et al.37 reported a similar total sugar content of 22.4% (on dry weight) in white grape variety.| Temp. [°C] | Rha | Ara | Gal | Glc | Man | Xyl | GalA | GlcA | % Total |
|---|---|---|---|---|---|---|---|---|---|
| a Fucose: not included-traces. | |||||||||
| 60 | 2.24 ± 0.13 | 3.26 ± 0.08 | 3.51 ± 0.09 | 1.55 ± 0.03 | 3.51 ± 0.14 | 1.00 ± 0.02 | 6.75 ± 0.13 | 2.02 ± 0.03 | 23.84 ± 0.65 |
| 80 | 1.18 ± 0.02 | 2.04 ± 0.02 | 2.08 ± 0.04 | 2.04 ± 0.02 | 2.35 ± 0.02 | 0.82 ± 0.02 | 5.31 ± 0.03 | 1.04 ± 0.02 | 16.86 ± 0.19 |
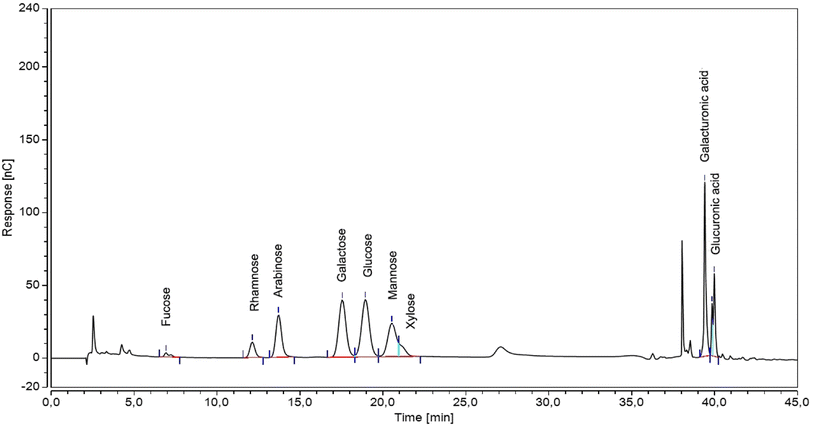 | ||
| Fig. 3 HPAEC-PAD chromatogram obtained for the SC-CO2 + H2O isolate from the grape pomace. Waveform used: gold, carbo, quad = carbohydrates, gold on PTFE disposable working electrodes. | ||
The GalA content is highly dependent on various factors such as source quality, extraction and hydrolysis methods. The pectin backbone is composed of repeating sugar units, primarily D-galacturonic acid, linked together by α-1,4-glycosidic bonds. Therefore, the GalA content is an important indicator of pectin in the isolates. The data provided in the Table 1 show that the GalA content decreased by increasing the temperature from 6.75 ± 0.13 to 5.31 ± 0.03 for SC-60 and SC-80 + H2O respectively.
Low values of uronic acid from the grape pomace indicate the presence of small amounts of pectic substances in the samples.37 It is also possible that CO2 may have too much acidified the medium and had degraded pectin's backbone.
The existence of pectic substances in the SC-CO2 + H2O isolates could be explained by the dissolution of CO2 in water under high-pressure conditions. While conventional pectin extraction methods rely on hot acid treatment in the pH range of 1–3, SC-CO2 + H2O under high pressures is a naturally acidified medium, which effectively targets polysaccharides by breaking down the cell wall components. Moreover, the acidified medium does not pose an environmental concern, as liberated CO2 by the end of the process returns to the atmosphere, elevating the medium's pH.36
3.4. Degree of esterification and molecular weight
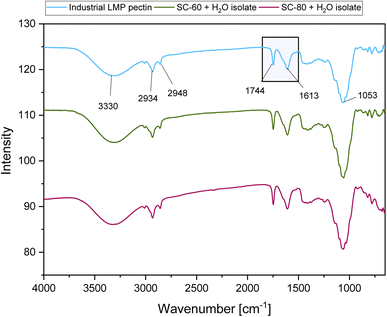
The degree of esterification of the extracts was characterized by FT-IR spectroscopy (Fig. 4). Carbohydrates usually show high absorbance between 1200 and 950 cm−1 wavenumbers, which is also known as a ‘‘fingerprint’’ region for the polysaccharide determination. The absorbance at 3000–3740 cm−1 is associated with the O–H stretching vibrations, while –CH, –CH2, and –CH3 groups are found at 2934–2948 cm−1. The most important region for the determination of the degree of methyl esterification lies between 1800 cm−1 and 1500 cm−1. The band at 1613 cm−1 is associated with non-methyl-esterified carboxyl group vibrations (O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O) and at 1744 cm−1 with methyl-esterified carboxylic groups (C
C–O) and at 1744 cm−1 with methyl-esterified carboxylic groups (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) accordingly.22,28
O) accordingly.22,28
The pectic substances in the isolates were of lower degrees of methylation: 23.8 ± 0.5% for SC-60 and 22.8 ± 1.0% for SC-80 + H2O isolates accordingly. Similar results were found in the work of Colodel et al.,12 on acid-extracted low methoxyl pectin (DE = 18%, Mw 154 kDa) from white grape variety (Chardonnay) pomaces. We can suppose here that the SC-CO2 + H2O medium activated some natural enzymes present in the grape pomace.
Commercial pectin extraction often yields high-methoxyl pectin (HMP), and low-methoxyl pectin (LMP) needs to further undergo chemical transformations for the groups to be hydrolysed (de-esterification). This is usually achieved by acidic, alkaline or enzymatic treatments (methylesterase). The process augments the production cost and is harmful to the environment. Despite the low GalA content, pectic substances in the isolates obtained in this work are of lower degrees of methylation (LMP) without additional chemical modification.
3.5. Molecular weight of SC-CO2 and water isolates
The preliminary studies in this work showed that 350–400 bar is an optimal pressure window for the extraction of phenolic compounds and higher molecular weight compounds. The molecular weight analysis of SC-CO2 and water as co-solvent isolates showed that they were indeed of a complex nature. The HPSEC chromatograms obtained from the extracts using a refractive index detector showed the presence of several populations of macromolecules (Fig. 5 and 6). When associated with the signal of light scattering and the UV detector at 280 nm, two populations of molecules were observed. The first population was eluted between 27 and 32 min with an average molecular weight (Mw) of ∼950 and 650 kDa for isolates SC-60 and SC-80 + H2O respectively. These fractions represent 4 wt% followed by the second population eluted between 32 min and 38 min, with an average Mw ∼ 300 and 200 kDa for the isolates SC-60 and SC-80 representing 10 wt%. These Mw values were calculated by considering that these two populations of low elution time and maximum light scattering signal are from polysaccharide molecules.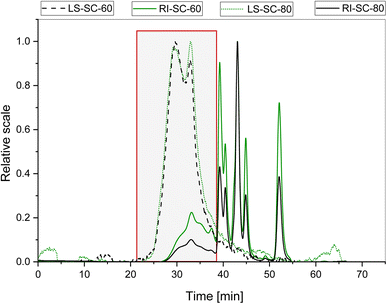 | ||
| Fig. 5 HPSEC-MALS-RI elution profile of isolates from the extracts SC-60 and SC-80 + H2O. Light scattering (LS-90° – dashed line) and refractive index (RI – solid line) detectors. | ||
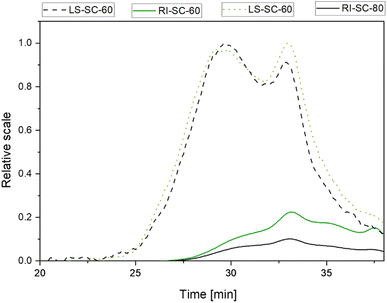 | ||
| Fig. 6 HPSEC-MALS-RI elution profile of isolates from the extracts SC-60 and SC-80 + H2O. Light scattering (LS-90° – dashed line) and refractive index (RI – solid line) detectors. | ||
The absence of a signal in light scattering after 40 minutes of elution time indicates the existence of very small molecules with the molecular weight out of the detection capacity.
The combined average molecular weights (Mw) of pectic substances were estimated to be 478 ± 6 kDa and 449 ± 3 kDa for the isolates from SC-60 and SC-80 + H2O, respectively. Moderately higher temperatures accelerated the thermal motion of molecules enabling better penetration of the solvent into the plant matrix and initiated the extraction of high-molecular weight components. A slightly lower molecular weight observed at 80 °C could indicate the beginning of an early degradation of the polymer chain. Pectin's molecular weight could range significantly based on the source material and extraction method applied (50 to 2000 kDa).38 Commonly used hot acid pectin extraction (HCl) results in lower to medium molecular weight of pectins. In the literature, several values have been reported for pectins isolated from sugar beet (70–355 kDa), banana (87 to 248 kDa) and orange, lemon and bigarade peels (88, 90 and 80 kDa) respectively.39–41
The average Mw of pectin extracted from the grape pomace by ultrasound-assisted methods ranged from 111 to 205 kDa (ref. 22) accordingly. Relatively low-molecular weight pectic substances were estimated by the subcritical water extraction (SWE) technique for citrus peel (CPP) to be 56 to 69 kDa and (APP) apple pomace pectin (APP) to be 28 to 65 kDa.42 The quality of the pectin is associated with high molecular weight. The lower values presented from the authors by SWE may be due to the hydrolysis of pectin's chain at high temperatures (120–130 °C). The results of this work highlight the potential application of the SC-CO2 process with water as the co-solvent not only for the successful extraction of various phenolic compounds but also high-molecular weight polysaccharides. Relatively low temperatures applied by this process put the extracts under lower risk to undergo heat-related degradations.
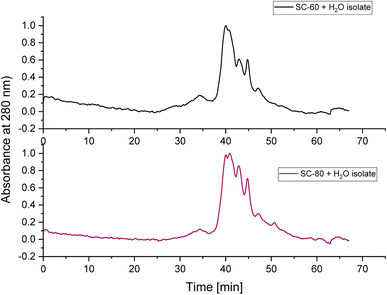
The UV absorption at 280 nm is characteristic of aromatic compounds such as proteins and polyphenol complexes in the isolates (Fig. 7). The supplementary DUMAS nitrogen analysis showed the nitrogen amount in the isolates to be ranging from 8.2 to 9.5% (on dry weight) while applying a pea protein factor of 5.4 to the SC-CO2 + H2O isolates. Fig. 7 illustrates the UV absorbance at 280, which confirms the existence of residual protein/polyphenol complexes in the isolates.
4. Conclusions
The potential of novel technologies such as SC-CO2 process towards sustainable waste management with minimum environmental impact was the scope of this study. The present work demonstrated that the grape pomace could be fully valorized for the extraction of phenolic compounds and fibers including pectic substances by the SC-CO2 process, applying water as a co-solvent. The isolates contained pectic substances, proteins (8–9.5% on dry weight) and other natural fibres. Moreover, the pectin has low methoxyl content (LMP, ∼23%) without any additional chemical modifications, which is of great industrial interest. The estimated molecular weight of the isolates was around 478 ± 6 kDa and 449 ± 3 kDa for SC-60 and SC-80 + H2O respectively. The pectin extraction method (SC-CO2 + H2O) employed in this study is entirely eco-friendly and avoids the use of harmful chemicals, which can result in acidic wastewater and significant environmental issues, as is the case with conventional pectin extraction methods. The quite efficiency of the extraction is due to the acidification of the medium through specific interactions between CO2 and water under supercritical conditions. This work clearly illustrates the SC-CO2 process and water as the co-solvent is a powerful medium, which targets not only smaller phenolic compounds but larger sized polysaccharides such as pectin. These extracted molecules could be reused in the wine-making process to enhance its quality and play a role in promoting a circular economy in this sector. Although the method proved to be promising for the extraction of valuable molecules, separation and isolation pose a challenge. Further investigations are needed to fully understand the potential of this technique for the possible valorization of other by-products from different sources.Conflicts of interest
The authors have no conflicts to disclose.Acknowledgements
This work is a part of the project (Valvigne 2021 and Probio+ 2019), supported by the Conseil Régional de Bourgogne Franche-Comte and the European Union through the PO FEDER-FSE Bourgogne 2014/2020 programs. The authors also thank the DIVVA technological platform for the access to various techniques. The authors would like to sincerely thank Pr Camille Loupiac and Ms Bernadette Rollin for protein Dumas analysis and specially Dr Adrien Lerbret for the technical assistance while working on the SFE machine.References
- L. Casas, C. Mantell, M. Rodríguez, E. J. M. de la Ossa, A. Roldán, I. D. Ory, I. Caro and A. Blandino, J. Food Eng., 2010, 96, 304–308 CrossRef CAS.
- C. Da Porto, D. Decorti and A. Natolino, J. Supercrit. Fluids, 2014, 87, 1–8 CrossRef CAS.
- G. Ferrentino, K. Morozova, O. K. Mosibo, M. Ramezani and M. Scampicchio, J. Cleaner Prod., 2018, 186, 253–261 CrossRef CAS.
- L. Manna, C. A. Bugnone and M. Banchero, J. Supercrit. Fluids, 2015, 104, 204–211 CrossRef CAS.
- X. Cao and Y. Ito, J. Chromatogr. A, 2003, 1021, 117–124 CrossRef CAS PubMed.
- M. L. Martínez, M. A. Mattea and D. M. Maestri, J. Food Eng., 2008, 88, 399–404 CrossRef.
- C. P. Passos, R. M. Silva, F. A. Da Silva, M. A. Coimbra and C. M. Silva, J. Supercrit. Fluids, 2009, 48, 225–229 CrossRef CAS.
- B. Pavlić, S. Vidović, J. Vladić, R. Radosavljević and Z. Zeković, J. Supercrit. Fluids., 2015, 23–28 CrossRef.
- V. Hessel, N. N. Tran, M. R. Asrami, Q. D. Tran, N. V. D. Long, M. Escribà-Gelonch, J. O. Tejada, S. Linke and K. Sundmacher, Green Chem., 2022, 24, 410–437 RSC.
- A. Chafer, M. C. Pascual-Martí, A. Salvador and A. Berna, J. Sep. Sci., 2005, 28, 2050–2056 CrossRef CAS PubMed.
- T. Vatai, M. Škerget and Ž. Knez, J. Food Eng., 2009, 90, 246–254 CrossRef CAS.
- C. Colodel, L. C. Vriesmann, R. F. Teófilo and C. L. de Oliveira Petkowicz, Int. J. Biol. Macromol., 2020, 161, 204–213 CrossRef CAS PubMed.
- L. Luo, Y. Cui, S. Zhang, L. Li, H. Suo and B. Sun, J. Chromatogr. B, 2017, 1068–1069, 201–209 CrossRef CAS PubMed.
- C. Negro, L. Tommasi and A. Miceli, Bioresour. Technol., 2003, 87, 41–44 CrossRef CAS PubMed.
- M. Lecas and J.-M. Brillouet, Phytochemistry, 1994, 35, 1241–1243 CrossRef CAS.
- P. Roca, Oiv-international Organisation of Vine and Wine-State of the World Vine and Wine Sector, 2023 Search PubMed.
- M. A. Bustamante, R. Moral, C. Paredes, A. Pérez-Espinosa, J. Moreno-Caselles and M. D. Pérez-Murcia, Waste Manag., 2008, 28, 372–380 CrossRef CAS PubMed.
- M. Pinelo, M. Rubilar, M. Jerez, J. Sineiro and M. J. Núñez, J. Agric. Food Chem., 2005, 53, 2111–2117 CrossRef CAS PubMed.
- N. Paixao, R. Perestrelo, J. Marques and J. Camara, Food Chem., 2007, 105, 204–214 CrossRef CAS.
- G. J. Soleas, J. Dam, M. Carey and D. M. Goldberg, J. Agric. Food Chem., 1997, 45, 3871–3880 CrossRef CAS.
- L. N. Gerschenson, E. N. Fissore, A. M. Rojas, A. M. Idrovo Encalada, E. F. Zukowski and R. A. Higuera Coelho, Food Hydrocolloids, 2021, 118, 106799 CrossRef CAS.
- R. Minjares-Fuentes, A. Femenia, M. C. Garau, J. A. Meza-Velázquez, S. Simal and C. Rosselló, Carbohydr. Polym., 2014, 106, 179–189 CrossRef CAS PubMed.
- M. L. Fishman, H. K. Chau, P. D. Hoagland and A. T. Hotchkiss, Food Hydrocolloids, 2006, 20, 1170–1177 CrossRef CAS.
- S. S. Hosseini, F. Khodaiyan and M. S. Yarmand, Carbohydr. Polym., 2016, 140, 59–65 CrossRef CAS PubMed.
- J. Prakash Maran, V. Sivakumar, K. Thirugnanasambandham and R. Sridhar, Carbohydr. Polym., 2014, 101, 786–791 CrossRef CAS PubMed.
- M. Á. Rivas, R. Casquete, M. de Guía Córdoba, M. J. Benito, A. Hernández, S. Ruiz-Moyano and A. Martín, LWT--Food Sci. Technol., 2021, 145, 111305 CrossRef CAS.
- M. Nikolantonaki, C. Coelho, L. Noret, M. Zerbib, B. Vileno, D. Champion and R. D. Gougeon, Food Chem., 2019, 270, 156–161 CrossRef CAS PubMed.
- G. D. Manrique and F. M. Lajolo, Postharvest Biol. Technol., 2002, 25, 99–107 CrossRef CAS.
- E. E. Yilmaz, E. B. Özvural and H. Vural, J. Supercrit. Fluids, 2011, 55, 924–928 CrossRef CAS.
- K. Dwyer, F. Hosseinian and M. R. Mark Rod, J. Food Res., 2014, 91–106 CrossRef.
- L. Fiori, D. de Faveri, A. A. Casazza and P. Perego, CyTA--J. Food, 2009, 7, 163–171 CrossRef CAS.
- L. Arce, A. G. Lista, A. Ríos and M. Valcárcel, Anal. Lett., 2001, 34, 1461–1476 CrossRef CAS.
- M. Pinelo, A. Ruiz-Rodríguez, J. Sineiro, F. J. Señoráns, G. Reglero and M. J. Núñez, Eur. Food Res. Technol., 2007, 226, 199–205 CrossRef CAS.
- C. M. Galanakis, V. Goulas, S. Tsakona, G. A. Manganaris and V. Gekas, Int. J. Food Prop., 2013, 382–396 CrossRef CAS.
- S. R. P. da Rocha, K. P. Johnston, R. E. Westacott and P. J. Rossky, J. Phys. Chem. B, 2001, 105, 12092–12104 CrossRef CAS.
- A. R. C. Morais, A. M. Da Costa Lopes and R. Bogel-Łukasik, Chem. Rev., 2015, 115, 3–27 CrossRef CAS PubMed.
- C. Valiente, E. Arrigoni, R. M. Esteban and R. Amado, J. Food Sci., 1995, 60, 818–820 CrossRef CAS.
- O. Kurita, T. Fujiwara and E. Yamazaki, Carbohydr. Polym., 2008, 74, 725–730 CrossRef CAS.
- T. Happi Emaga, S. N. Ronkart, C. Robert, B. Wathelet and M. Paquot, Food Chem., 2008, 108, 463–471 CrossRef CAS PubMed.
- N. El Fihry, K. El Mabrouk, M. Eeckhout, H. A. Schols, Y. Filali-Zegzouti and H. Hajjaj, LWT--Food Sci. Technol., 2022, 162, 113508 CrossRef CAS.
- S. Levigne, M.-C. Ralet and J.-F. Thibault, Carbohydr. Polym., 2002, 49, 145–153 CrossRef CAS.
- X. Wang, Q. Chen and X. Lü, Food Hydrocolloids, 2014, 38, 129–137 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3su00183k |
| This journal is © The Royal Society of Chemistry 2023 |