Bis-imidazole ring-containing bipolar organic small molecule cathodes for high-voltage and ultrastable lithium-ion batteries†
Liping
Zheng
ab,
Jiayi
Ren
d,
Huige
Ma
bc,
Mingsheng
Yang
d,
Xiaorong
Yan
d,
Rui
Li
bc,
Qian
Zhao
d,
Jianze
Zhang
ab,
Haifeng
Fu
f,
Xiong
Pu
 abc,
Mingjun
Hu
abc,
Mingjun
Hu
 *d and
Jun
Yang
*bce
*d and
Jun
Yang
*bce
aSchool of Chemistry and Chemical Engineering, Center on Nanoenergy Research, Guangxi University, Nanning 530004, China
bBeijing Institute of Nanoenergy and Nanosystems, Chinese Academy of Sciences, Beijing 101400, China. E-mail: yangjun@binn.cas.cn
cSchool of Nanoscience and Technology, University of Chinese Academy of Sciences, Beijing 100049, China
dSchool of Materials Science and Engineering, Beihang University, Beijing 100191, China. E-mail: mingjunhu@buaa.edu.cn
eShenzhen Institute for Advanced Study, University of Electronic Science and Technology of China, Shenzhen 518000, China
fBeijing Electromechanical Research Institute Co. Ltd, Beijing 100083, China
First published on 15th November 2022
Abstract
Organic cathode materials are attractive for rechargeable lithium-ion batteries due to their advantages in sustainability and designability of the molecular structure as well as the high upper limit of theoretical capacity. However, their practical application faces the problems of a short cycle life and low working potential. To address these issues, we synthesized a novel bis-imidazole ring-containing organic small molecule compound 2,6-bis(4-(diphenylamino)phenyl)benzo[1,2-d:4,5-d′]diimidazole-4,8(1H,5H)-dione (BNBQ). It possesses bipolar charge storage characteristics with n-type C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, p-type triphenylamine groups and p-type bis-imidazole rings, endowing it with a good specific capacity and high redox potential. Attributed to the large conjugated molecular structure, strong π–π interaction and good crystallinity, it exhibits excellent electrochemical performances. As a consequence, Li-ion half-cells assembled based on the organic cathode and lithium anode deliver high average discharge voltages of about 3.64 V, salient initial specific capacity (133 mA h g−1 at 100 mA g−1), and good capacity retention (63% after 5000 cycles at 1000 mA g−1), outperforming most previously reported bipolar organic small molecular cathodes of lithium-ion batteries. This is the first report that the active bis-imidazole rings were employed for energy storage, and will further enrich the library of organic electrochemical active groups.
O, p-type triphenylamine groups and p-type bis-imidazole rings, endowing it with a good specific capacity and high redox potential. Attributed to the large conjugated molecular structure, strong π–π interaction and good crystallinity, it exhibits excellent electrochemical performances. As a consequence, Li-ion half-cells assembled based on the organic cathode and lithium anode deliver high average discharge voltages of about 3.64 V, salient initial specific capacity (133 mA h g−1 at 100 mA g−1), and good capacity retention (63% after 5000 cycles at 1000 mA g−1), outperforming most previously reported bipolar organic small molecular cathodes of lithium-ion batteries. This is the first report that the active bis-imidazole rings were employed for energy storage, and will further enrich the library of organic electrochemical active groups.
Introduction
To achieve carbon neutrality and reduce the consumption of traditional fossil energy, it is necessary to develop green, environmentally friendly, and sustainable new energy or energy carriers.1–3 As an energy storage device capable of directly converting chemical energy into electrical energy, electrochemical batteries have been intensively studied in the past few years.4,5 Among them, lithium-ion batteries (LIBs) have become the dominant energy storage devices in the commercial secondary battery market due to good discharge performance, high energy density and mature processing technology, and are widely used in electric vehicles and portable electronic products.4,6 However, the rapidly growing LIB demand poses significant challenges, because commercially available LIBs predominantly use inorganic cathode materials which generally have defects of slow kinetics, large production energy consumption, and non-renewability.7–9.Compared with traditional inorganic electrode materials, organic electrode materials have the advantages of high theoretical specific capacity, good structural designability, low environmental footprint, etc.10,11 Encouragingly, recent years have witnessed the vigorous development of a large number of organic materials as LIB cathodes involving multiple redox-active functional groups, such as carbonyl (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), pyrazine/imine (C
O), pyrazine/imine (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) groups, cyano groups (C
N) groups, cyano groups (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N), azo (N
N), azo (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N),7,12–15 and so on. Practically, in a considerable range of redox-active organic molecules, carbonyl-based compounds, like cyclohexanone,16p-benzoquinone,17 and 9,10-anthraquinone,17 have significant advantages as cathode materials for LIBs, due to their widespread presence in nature, high specific capacity, and outstanding redox activity.5 The enolation process between lithium ions and carbonyl groups nicely explains the redox mechanism of electrochemically reactive carbonyl groups.16,18 However, the high solubility of small carbonyl-based electrode materials in popular organic electrolytes usually leads to rapid capacity decay during cycling, and thus low practical application value.4,19 To solve these problems, several strategies have been carried out for the improvements, such as polymerization or chemical combination to increase the molecular weight,20 grafting,21 and compositing.22 Polymerization has emerged as one of the most effective methods to inhibit the dissolution of organic cathode materials and improve cycling performance.23–25 However, it is relatively difficult to synthesize a defect-less and highly conjugated carbonyl-based polymer due to the strong electron-withdrawing ability and weak aromaticity of quinones, resulting in poor conductivity and thus incomplete utilization of active sites.7,26,27 Distinctive from polymerization, chemical combination with a few small molecules to increase the molecular weight to a certain degree is another method to inhibit the dissolution of organic compounds, which not only possesses better flexibility of molecular structure design, but also led to less defects, better crystallinity and stronger interlaminar π–π interaction, both of which are beneficial to improving the specific capacity and rate performance beyond cycling stability.
N),7,12–15 and so on. Practically, in a considerable range of redox-active organic molecules, carbonyl-based compounds, like cyclohexanone,16p-benzoquinone,17 and 9,10-anthraquinone,17 have significant advantages as cathode materials for LIBs, due to their widespread presence in nature, high specific capacity, and outstanding redox activity.5 The enolation process between lithium ions and carbonyl groups nicely explains the redox mechanism of electrochemically reactive carbonyl groups.16,18 However, the high solubility of small carbonyl-based electrode materials in popular organic electrolytes usually leads to rapid capacity decay during cycling, and thus low practical application value.4,19 To solve these problems, several strategies have been carried out for the improvements, such as polymerization or chemical combination to increase the molecular weight,20 grafting,21 and compositing.22 Polymerization has emerged as one of the most effective methods to inhibit the dissolution of organic cathode materials and improve cycling performance.23–25 However, it is relatively difficult to synthesize a defect-less and highly conjugated carbonyl-based polymer due to the strong electron-withdrawing ability and weak aromaticity of quinones, resulting in poor conductivity and thus incomplete utilization of active sites.7,26,27 Distinctive from polymerization, chemical combination with a few small molecules to increase the molecular weight to a certain degree is another method to inhibit the dissolution of organic compounds, which not only possesses better flexibility of molecular structure design, but also led to less defects, better crystallinity and stronger interlaminar π–π interaction, both of which are beneficial to improving the specific capacity and rate performance beyond cycling stability.
In addition to the solubility problem, most organic cathode materials also encounter low reduction potential. Usually, the voltage (vs. Li/Li+) of organic cathodes is in the range of 2 to 3 V, much lower than the voltage of commercial inorganic cathode materials.28 Although previous studies have reported some p-type free radical compounds and heteroatom substituted aromatic rings that can produce stable cation intermediates by combining with electrolyte anions and show much higher redox potentials than n-type compounds,29–31 they still suffer from relatively low capacity and poor cycling life. For example, Yan et al. recently reported a p–π conjugated dioxin small-molecular cathode active material, and although the material had a high discharge voltage of 4.4 V vs. Li/Li+, the capacity decayed rapidly within 100 cycles.45 Therefore, the practical application value of p-type cathodes is greatly weakened by the low specific capacity and poor cycling stability.7,30–34 It is a necessity to further enhance the comprehensive electrochemical performance of p-type cathode materials.
Tetraamino-p-benzoquinone (TABQ) with high-density functional groups not only has a high theoretical specific capacity, but is also easily extended into larger molecules due to the presence of four side amino groups, which makes it a desirable building unit of organic electrode materials of LIBs.21,35,36 Herein, we employed TABQ as the raw material to not only contribute the low-voltage capacity but also behave as a bridging agent, and aromatic aldehydes 4-(N,N-diphenylamino)benzaldehyde (NDP) as p-type redox-active units to offer high-voltage capacity.37 Consequently, the NDP units were connected with TABQ through bis-imidazole rings, and small-molecular 2,6-bis(4-(diphenylamino)phenyl)benzo[1,2-d:4,5-d′]diimidazole-4,8(1H,5H)-dione (BNBQ), was synthesized. Electrochemical characterization studies confirm that BNBQ displays good electrochemical performance due to the introduction of active bis-imidazole rings that could enhance conjugation and capacity. This is the first report where a bis-imidazole ring-containing organic small molecule material is used as the cathode of LIBs. BNBQ shows multistage discharge voltage plateaus, and an average discharge voltage of about 3.64 V. The reversible specific capacity reaches 133 mA h g−1 at 100 mA g−1 (theoretical capacity for BNBQ is 238 mA h g−1, ESI S4†),7,38 and after 5000 cycles at 1000 mA g−1, the capacity retention is maintained at 63%. It is thought that the high electrochemical performances of BNBQ are ascribed to the rich redox active sites, large conjugation, π–π stacking, and high crystallinity, and thus high capacity, good conductivity and low solubility in electrolytes.14.
Results and discussion
Synthesis and characterization of BNBQ
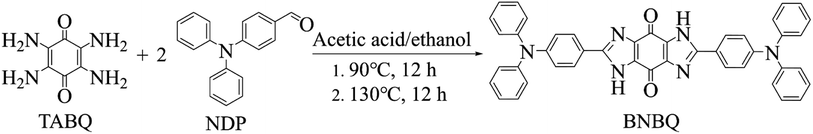
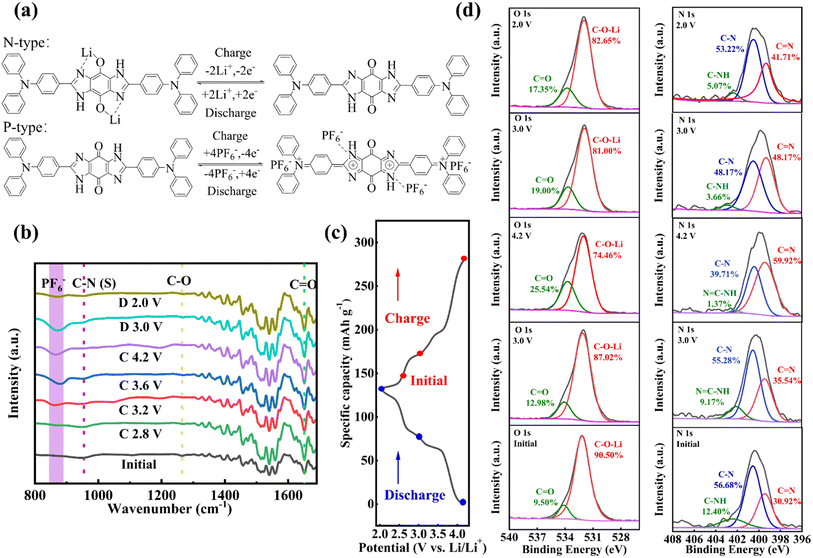
TABQ was prepared via a previously reported method.39 The synthesis of the benzoquinone-based compounds involved a Schiff-based solvothermal condensation reaction of TABQ with NDP at 90 °C for 12 h, and then at 130 °C for 12 h with 62% yield (Scheme 1).40–42 The detailed experimental process was depicted in the ESI.† The obtained tetraamino-p-benzoquinone derivative was characterized using a variety of methods.As illustrated in Fig. 1, the morphology and crystalline structure of BNBQ was investigated by powder X-ray diffraction (PXRD) and scanning electron microscopy (SEM). The PXRD patterns of BNBQ showed clear positions of characteristic peaks (Fig. 1b, Fig. S3†), indicative of a high crystallinity. SEM was used to describe the morphology of BNBQ. The typical lathlike structure is presented in Fig. 1c. The thermal gravimetric analysis demonstrated that the temperature at 10% weight loss for BNBQ was 412 °C in a nitrogen atmosphere (Fig. 1d), which illustrated that the small-molecule compound BNBQ using bis-imidazole rings to connect two active functional groups had better thermal stability compared to most organic small-molecule compounds with a decomposition temperature of about 200 °C.16,17,29,43–45 In combination with 1H nuclear magnetic resonance (NMR, Fig. S1b†), no peaks were found between the chemical shifts of 4.5–5.0 ppm, suggesting that –NH2 of TABQ (Fig. S1a†) had been fully reacted. Furthermore, the presence of a strong peak at 675.2494 in the high-resolution mass spectrum demonstrated the formation of BNBQ (Fig. S2†).
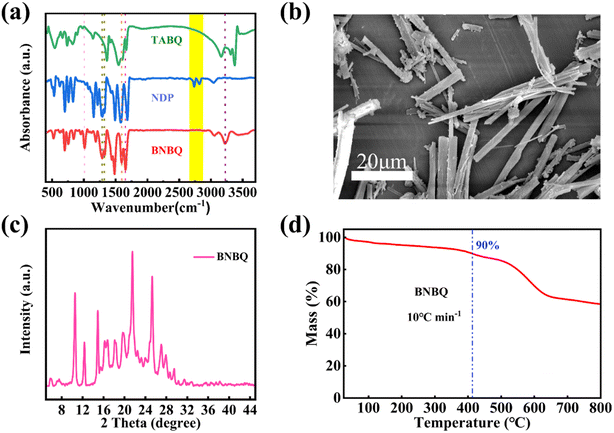 | ||
| Fig. 1 (a) FT-IR spectra of TABQ, NDP, and BNBQ; (b) SEM images of BNBQ; (c) XRD patterns of BNBQ; (d) TG curves of BNBQ. | ||
Fourier-transform infrared spectroscopy (FT-IR) and X-ray photoelectron spectroscopy (XPS) spectra were adopted to analyze the chemical structure and surface information of the resultant products. The strong absorptions at 1284 and 1319 cm−1 in the FT-IR spectrum of BNBQ were assigned to the asymmetric and symmetric vibration of the C–N group in triphenylamine rings, and the peaks of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (1654 cm−1), C
O (1654 cm−1), C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N (1591 cm−1), Ar–H (3049 cm−1) and N–H (3218 cm−1) groups of BNBQ could also be observed. The appearance of C
N (1591 cm−1), Ar–H (3049 cm−1) and N–H (3218 cm−1) groups of BNBQ could also be observed. The appearance of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and N–H indicated the formation of bis-imidazole rings, and the disappearance of O
N and N–H indicated the formation of bis-imidazole rings, and the disappearance of O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H stretching bands (2732 and 2812 cm−1) of NDP implied that the aldehyde-amine condensation reaction was complete.6,7,25,46–49 Furthermore, the XPS spectrum of BNBQ displayed the obvious signals of C, O, and N elements50 (Fig. 2a). The C 1s high-resolution spectrum of BNBQ could be fitted into four subpeaks at binding energies of 284.7, 285.7, 287.7, and 291.6 eV, corresponding to the aromatic sp2 carbons, C–N, C–O/C
C–H stretching bands (2732 and 2812 cm−1) of NDP implied that the aldehyde-amine condensation reaction was complete.6,7,25,46–49 Furthermore, the XPS spectrum of BNBQ displayed the obvious signals of C, O, and N elements50 (Fig. 2a). The C 1s high-resolution spectrum of BNBQ could be fitted into four subpeaks at binding energies of 284.7, 285.7, 287.7, and 291.6 eV, corresponding to the aromatic sp2 carbons, C–N, C–O/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, and π–π*, respectively. For the N 1s spectrum (Fig. 2c), the peak could be ascribed to three subpeaks at 397.9 eV for C
N, and π–π*, respectively. For the N 1s spectrum (Fig. 2c), the peak could be ascribed to three subpeaks at 397.9 eV for C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, 399.4 eV for C–N, and 401.8 eV for N
N, 399.4 eV for C–N, and 401.8 eV for N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–NH, implying the formation of imidazole rings. Meanwhile, the O 1s peak could be divided into two subpeaks at 534.1 eV for C
C–NH, implying the formation of imidazole rings. Meanwhile, the O 1s peak could be divided into two subpeaks at 534.1 eV for C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and 531.1 eV for C–O (Fig. 2d). All the above-mentioned results indicated that BNBQ had been successfully synthesized.
O and 531.1 eV for C–O (Fig. 2d). All the above-mentioned results indicated that BNBQ had been successfully synthesized.
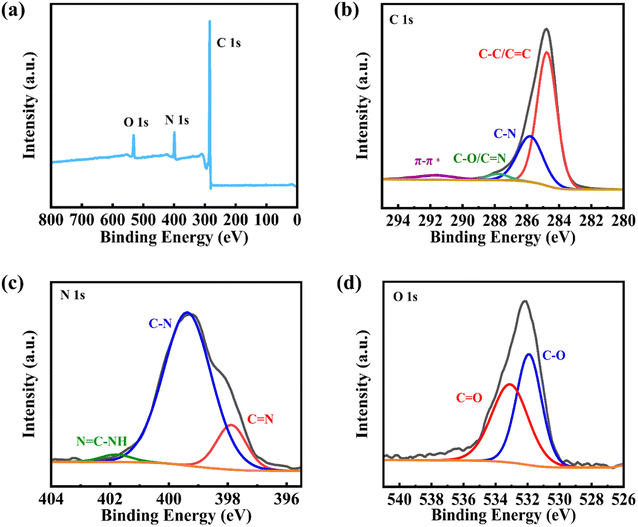 | ||
| Fig. 2 The XPS survey spectrum of BNBQ (a) and the corresponding high-resolution spectra of C1s (b), N1s (c), and O1s (d). | ||
Electrochemical characterization
The electrochemical performance of BNBQ as a cathode material was tested by assembling half-cells with lithium metal as the counter electrode and 1.0 M LiPF6 in ethylene carbonate (EC)/dimethyl carbonate (DEC) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
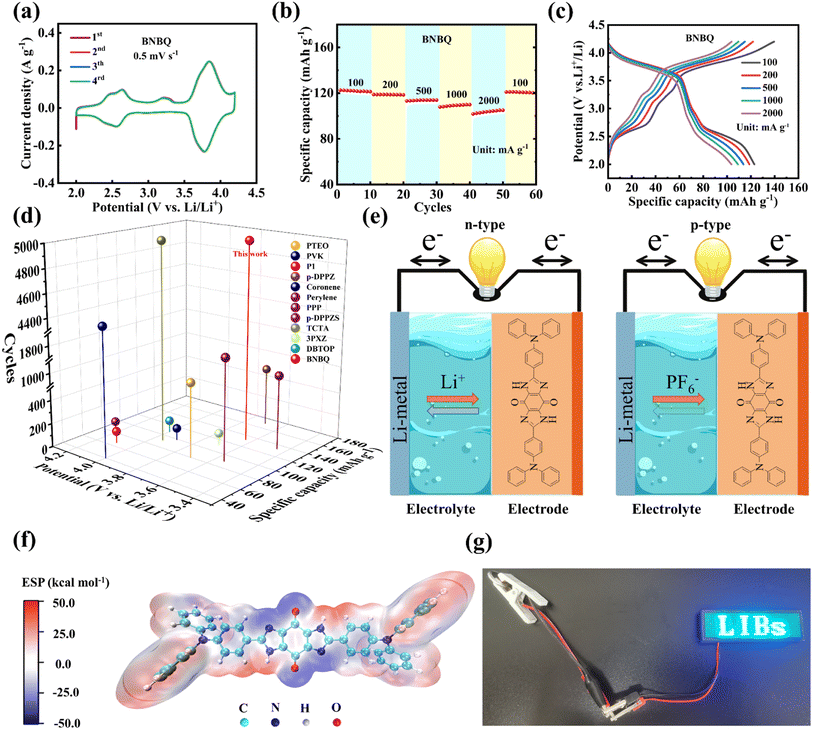
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) as the electrolyte. The cells were first studied by cyclic voltammetry (CV) measurements in the voltage range of 2.0–4.2 V (vs. Li/Li+) at a scan rate of 0.5 mV s−1 (Fig. 3a). BNBQ exhibited two pairs of distinct redox peaks at 2.63/2.56, and 3.85/3.77 V, respectively. Furthermore, there was an obvious oxidation peak at around 3.25 V, but the reduction peak intensity at 2.98 V was very small. The redox peaks at 2.63/2.56 V were attributed to the reversible lithiation/delithiation procedures of the carbonyl groups in the p-quinone unit, and the peaks of 3.85/3.77 V for BNBQ originated from the p-type doping/dedoping in the triphenylamine groups, which were consistent with that reported in previous literature studies.7,24,51,52 In addition, the peaks of 3.25/2.98 V for BNBQ should belong to the p-type doping/dedoping of the bis-imidazole groups, which could not be found in the CV curves of both NDP and TABQ (Fig. 4bS4a†). To predict redox-active sites for Li+ and PF6− storage in the BNBQ molecule, the molecular electrostatic potential (MESP) method was employed via Multiwfn. On the van der Waals surface of the BNBQ molecule, nucleophilic reactions tended to occur in the more positive MESP regions (red regions), while electrophilic reactions would occur in more negative MESP regions (blue regions). As a result, in BNBQ molecules, the O in p-benzoquinone and pyridinic N in imidazole rings with more negative MESP values should be the active sites for Li+ uptake (Fig. 3f),53 and –NH– of the imidazole ring with a positive MESP might be attractive to anions, which would be beneficial for PF6− insertion.
1 v/v) as the electrolyte. The cells were first studied by cyclic voltammetry (CV) measurements in the voltage range of 2.0–4.2 V (vs. Li/Li+) at a scan rate of 0.5 mV s−1 (Fig. 3a). BNBQ exhibited two pairs of distinct redox peaks at 2.63/2.56, and 3.85/3.77 V, respectively. Furthermore, there was an obvious oxidation peak at around 3.25 V, but the reduction peak intensity at 2.98 V was very small. The redox peaks at 2.63/2.56 V were attributed to the reversible lithiation/delithiation procedures of the carbonyl groups in the p-quinone unit, and the peaks of 3.85/3.77 V for BNBQ originated from the p-type doping/dedoping in the triphenylamine groups, which were consistent with that reported in previous literature studies.7,24,51,52 In addition, the peaks of 3.25/2.98 V for BNBQ should belong to the p-type doping/dedoping of the bis-imidazole groups, which could not be found in the CV curves of both NDP and TABQ (Fig. 4bS4a†). To predict redox-active sites for Li+ and PF6− storage in the BNBQ molecule, the molecular electrostatic potential (MESP) method was employed via Multiwfn. On the van der Waals surface of the BNBQ molecule, nucleophilic reactions tended to occur in the more positive MESP regions (red regions), while electrophilic reactions would occur in more negative MESP regions (blue regions). As a result, in BNBQ molecules, the O in p-benzoquinone and pyridinic N in imidazole rings with more negative MESP values should be the active sites for Li+ uptake (Fig. 3f),53 and –NH– of the imidazole ring with a positive MESP might be attractive to anions, which would be beneficial for PF6− insertion.
As presented in Fig. 3c, the BNBQ cathodes had two distinct discharge plateaus (with an average value of 3.64 V, Fig. S6†), attributed to the deinsertion of PF6− combined with positive triphenylamine groups at high voltage and the lithiation processes of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, respectively (Fig. 3e). Additionally, BNBQ also exhibited narrow charge–discharge plateaus at about 3.25 V and 2.98 V attributed to the doping/dedoping of bis-imidazole rings, which were in good agreement with the CV results. The specific capacity of BNBQ was mainly contributed by the high-voltage capacity, and the percentage of capacity contribution from different functional groups was 29.33% for the p-quinone unit, 9.76% for bis-imidazole groups, and 60.91% for triphenylamine groups, respectively. Compared with the 40% utilization rate of active triphenylamine groups in NDP (Fig. S4c†), the utilization of triphenylamine groups was significantly increased to 91.8% due to the presence of bisimidazole rings in BNBQ (Fig. S7b†). Furthermore, the peak voltage differences of the two redox pairs of BNBQ were 0.07 and 0.08 V, respectively, which was relatively small and indicated that the cathode material had low polarization and rapid redox dynamics. This should be attributed to the large conjugated structure and good conductivity of the as-prepared organic molecule that contains triphenylamine groups. Fig. 3b shows the rate capability of the cathode material at different ampere densities (100–2000 mA g−1), and the reversible discharge capacities were 123.1, 118.8, 113.9, 109.9, and 105.0 mA h g−1 at the current densities of 100, 200, 500, 1000, and 2000 mA g−1, respectively. When the current density returned to 100 mA g−1, the specific capacity of BNBQ could be increased to 121.1 again, very close to the initial values.
O, respectively (Fig. 3e). Additionally, BNBQ also exhibited narrow charge–discharge plateaus at about 3.25 V and 2.98 V attributed to the doping/dedoping of bis-imidazole rings, which were in good agreement with the CV results. The specific capacity of BNBQ was mainly contributed by the high-voltage capacity, and the percentage of capacity contribution from different functional groups was 29.33% for the p-quinone unit, 9.76% for bis-imidazole groups, and 60.91% for triphenylamine groups, respectively. Compared with the 40% utilization rate of active triphenylamine groups in NDP (Fig. S4c†), the utilization of triphenylamine groups was significantly increased to 91.8% due to the presence of bisimidazole rings in BNBQ (Fig. S7b†). Furthermore, the peak voltage differences of the two redox pairs of BNBQ were 0.07 and 0.08 V, respectively, which was relatively small and indicated that the cathode material had low polarization and rapid redox dynamics. This should be attributed to the large conjugated structure and good conductivity of the as-prepared organic molecule that contains triphenylamine groups. Fig. 3b shows the rate capability of the cathode material at different ampere densities (100–2000 mA g−1), and the reversible discharge capacities were 123.1, 118.8, 113.9, 109.9, and 105.0 mA h g−1 at the current densities of 100, 200, 500, 1000, and 2000 mA g−1, respectively. When the current density returned to 100 mA g−1, the specific capacity of BNBQ could be increased to 121.1 again, very close to the initial values.
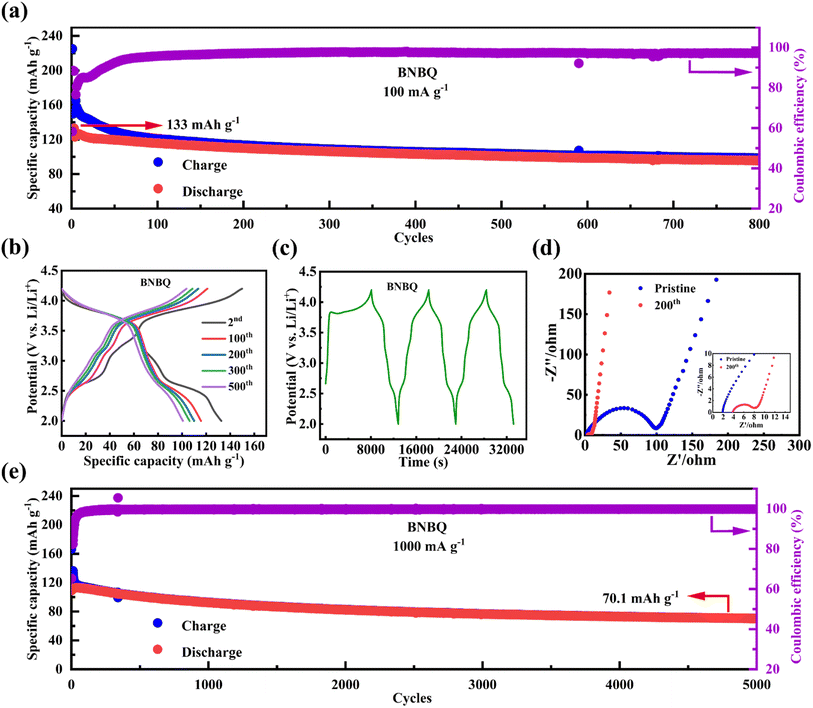
Fig. 4a shows that the BNBQ cathode had a high initial discharge specific capacity of 133 mA h g−1 in a long cycling test at 100 mA g−1. In terms of the high average discharge voltage, the energy density of the BNBQ electrode can reach 437 W h kg−1 at 100 mA g−1 (Fig. S8†). However, it displayed a rather low coulombic efficiency in the first few cycles, due to the formation of a solid electrolyte interface (SEI) and the redox-shuttle effect of the partially dissolved active materials (Fig. 4c).34,54 After a few activation cycles, the coulombic efficiency rapidly increased and reached 98%, implying reversible lithiation/delithiation procedures in BNBQ electrodes. After the 200th cycle, the oxidation peak at 2.57 V decreased, but the peak at 3.20 V, 3.83/3.82 was still obvious, illustrating a good p-type charge/discharge stability (Fig. S9†).
The reversible specific capacity and long-term cycling stability of BNBQ were compared with those of previously reported p-type and bipolar organic cathode materials in Li-organic batteries, including DBTOP,45 3PXZ,34 and so on,7,31,55,56 and the results are shown in Table S2.† It could be seen that BNBQ delivered the best cycling stability in these cathode materials, and only the polymer p-DPPZ had a higher reversible specific capacity than BNBQ (Fig. 3d). These results indicated that the as-obtained bipolar quinone-based organic molecule with extended conjugated structures was an excellent cathode candidate material of LIBs, and BNBQ with the bis-imidazole rings as the linker had outstanding electrochemical performance, indicating the importance of suitable molecular structure design.57 Meanwhile, the electrochemical performance of BNBQ as a cathode material of sodium-ion batteries was also evaluated, and a high discharge voltage (∼3.2 V vs. Na+/Na) and good cycling stability could also be obtained (Fig. S10 and 11†).11.
Electrochemical impedance spectroscopy (EIS) was applied to probe Li-ion diffusion kinetics. The Nyquist plots of the organic cathode material exhibited a semicircle at high frequencies and a sloping line at low frequencies, assigned to the charge-transfer resistance (Rct) and Warburg impedance (Fig. 4d).6 The Rct value of the BNBQ electrode was about 100 Ω before cycling, and decreased to 8 Ω after the 200th cycle at 100 mA g−1, which may be attributed to the gradual activation of cathode materials during the charge/discharge process due to the infiltration of electrolytes. Likewise, attributed to the decreased charge transfer resistance, the battery also showed the palmary rate performance. To further explore the durability of electrode materials, cyclability was tested at a higher current density of 1000 mA g−1 (Fig. 4e). After 5000 cycles, the capacity retention was 63% (70.1 mA h g−1). The excellent cycle stability at 1000 mA g−1 should be ascribed to the significant structural stability of BNBQ during the charge and discharge process. In addition, the BNBQ electrode showed nearly 100% coulombic efficiency during long-cycle testing, implying few side reactions and high redox reversibility. The morphologies of the samples after long cycles were also investigated using SEM (Fig. S13†), and it was found that BNBQ was not fragmented completely even after 5000 cycles, suggesting a fair structure stability as a result of the bis-imidazone linker.
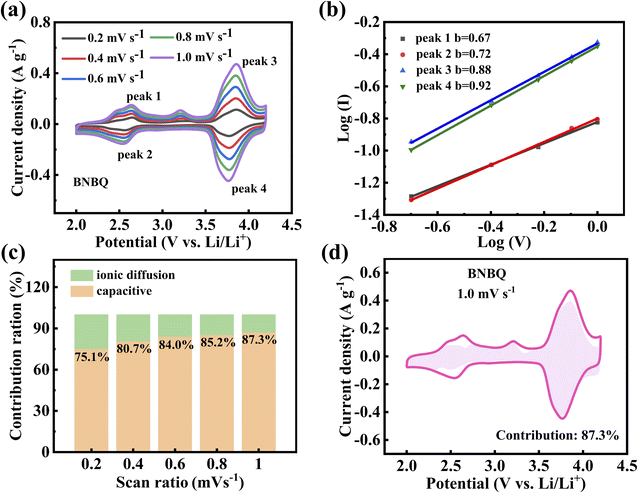
The electrochemical kinetics of the bipolar organic cathode was further evaluated based on the CV curves of BNBQ measured at different scan rates from 0.2 to 1.0 mV s−1. The cyclic voltammetry (CV) curves of the BNBQ electrode at different scan rates are presented in Fig. 5a. The relationship between the peak current (i, A) and scan rate (ν, mV s−1) could be expressed by using equation: i = ανb,58 where i was the current value associated with a certain voltage, and ν was the scan rate. The b-value could be obtained by calculating the slope by plotting the log(i) versus log(ν) curves, as displayed in Fig. 5b. When b approached 0.5, it meant a diffusion-controlled kinetic process, and a b value close to 1.0 suggested the domination of a capacitive process.36 The b values obtained from the peaks 1 to 4 were 0.67, 0.72, 0.88, and 0.92, respectively, demonstrating that the kinetics process of BNBQ was predominantly controlled by the non-diffusion process. The contribution of a capacitive process and a diffusion-limited redox process in overall capacity could be quantified through the equation i = k1ν + k2ν1/2,58 where k1 and k2 were constants which could be confirmed by plotting ν1/2versus i/ν1/2 at specific potentials. As shown in Fig. 5c–d, with the increase in scan rates, the capacitive contribution gradually increased and corresponded to 75.1%, 80.7%, 84.0%, 85.2%, and 87.3% at 0.2 mV s−1, 0.4 mV s−1, 0.6 mV s−1, 0.8 mV s−1, and 1 mV s−1, respectively. These results suggested that charge storage in BNBQ was primarily a fast surface-controlled pseudocapacitive process, guaranteeing fast reaction kinetics.
The redox mechanism of the BNBQ electrode was determined by ex situ X-ray photoelectron spectroscopy (XPS) and FT-IR spectroscopy of the electrodes at different charge/discharge stages. Energy dispersive spectroscopy (EDS) displayed a uniform distribution of C, N, O, and F elements in the BNBQ electrode (Fig. S14a and 14b†). The possible charge and discharge mechanisms of the BNBQ electrode are shown in Fig. 6a. It was shown that the organic small molecule compound could be inserted by anions and deinserted by cations in the charge process and reverse in the discharge process due to the presence of p-type triphenylamine and n-type carbonyl groups and showed bipolar characteristics. The ex situ FT-IR spectra in Fig. 6b show that the vibrational peak of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond at 1654 cm−1 gradually increased during the charging process and then gradually decreased during the discharging process, corresponding to the delithiation and lithiation procedures, respectively, indicating the reversible and stable electrochemical reactions during the charge and discharge process, but its real utilization rate was not very high. In addition, the peak at 858 cm−1 could be assigned to the PF6− anion. After charging to 4.2 V, the peak of the PF6− anions gradually increased, and when discharging to 2.0 V, it gradually disappeared, indicating that the anion could be well extracted from the BNBQ cathode, demonstrating high reversibility of insertion and deinsertion of PF6−. In the meantime, XPS was used to gain further insights into the lithiation/delithiation mechanism of the BNBQ electrode. The high-resolution ex situ XPS spectra of O 1s and N 1s are shown in Fig. 6d. The O 1s spectrum of the initial BNBQ electrode could be fitted into two peaks assigned to C
O bond at 1654 cm−1 gradually increased during the charging process and then gradually decreased during the discharging process, corresponding to the delithiation and lithiation procedures, respectively, indicating the reversible and stable electrochemical reactions during the charge and discharge process, but its real utilization rate was not very high. In addition, the peak at 858 cm−1 could be assigned to the PF6− anion. After charging to 4.2 V, the peak of the PF6− anions gradually increased, and when discharging to 2.0 V, it gradually disappeared, indicating that the anion could be well extracted from the BNBQ cathode, demonstrating high reversibility of insertion and deinsertion of PF6−. In the meantime, XPS was used to gain further insights into the lithiation/delithiation mechanism of the BNBQ electrode. The high-resolution ex situ XPS spectra of O 1s and N 1s are shown in Fig. 6d. The O 1s spectrum of the initial BNBQ electrode could be fitted into two peaks assigned to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (534.3 eV) and C–O (532.1 eV), respectively.6,35,59 In the fully charged state, the peak intensity of C
O (534.3 eV) and C–O (532.1 eV), respectively.6,35,59 In the fully charged state, the peak intensity of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O was slightly enhanced while the peak intensity of C–O was slightly weakened, implying a delithiation process. However, after again discharging to 2.0 V, the C–O–Li peak was enhanced once again, suggesting a reversible reaction of BNBQ with lithium. Furthermore, similar reversible changes could be found in the N 1s XPS spectrum at different redox states of the electrode. The peaks at 399.5 eV and 402.5 eV were assigned to C
O was slightly enhanced while the peak intensity of C–O was slightly weakened, implying a delithiation process. However, after again discharging to 2.0 V, the C–O–Li peak was enhanced once again, suggesting a reversible reaction of BNBQ with lithium. Furthermore, similar reversible changes could be found in the N 1s XPS spectrum at different redox states of the electrode. The peaks at 399.5 eV and 402.5 eV were assigned to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and C–NH, respectively. In the charged state, the peak for C–NH decreases remarkably, accompanied by the enhanced intensity of C
N and C–NH, respectively. In the charged state, the peak for C–NH decreases remarkably, accompanied by the enhanced intensity of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N. After discharging, the peak intensity of C–NH was recovered and that of C
N. After discharging, the peak intensity of C–NH was recovered and that of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds were decreased, indicating that triphenylamine groups and bis-imidazole rings also take part in energy storage.7,58 The above results demonstrated the transformation between C–O and C
N bonds were decreased, indicating that triphenylamine groups and bis-imidazole rings also take part in energy storage.7,58 The above results demonstrated the transformation between C–O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O as well as between p-type doped N and normal N during discharge–charge cycles, showing bipolar features for charge storage.
O as well as between p-type doped N and normal N during discharge–charge cycles, showing bipolar features for charge storage.
Conclusion
The novel bipolar quinone-based compound BNBQ is synthesized by a Schiff-based solvothermal condensation reaction of TABQ with NDP, and the relationship between its structure and electrochemical performance as the cathode of LIBs is also explored. The original material has relatively low solubility in common organic electrolytes and good conductivity, and thus demonstrates excellent electrochemical performance. The small-molecular BNBQ with bis-imidazole rings to connect p-benzoquinone and triphenylamine groups, possesses a high specific capacity (133 mA h g−1 at 100 mA g−1), a good rate capability (105 mA h g−1 at 2000 mA g−1), a high average discharge voltage of about 3.64 V, and excellent cycle stability (70.1 mA h g−1 after 5000 cycles at 1000 mA g−1), which is considered outstanding for the organic small molecule cathode of LIBs, and can be attributed to abundant active sites and the highly stable and conjugated molecular structure of BNBQ, endowing it with nice conductivity and low solubility in common solvents. The ex situ FT-IR and XPS spectra analyses identify CO, the triphenylamine groups and bis-imidazole rings of BNBQ as the redox-active centers and reveal the reversible Li+ and PF6− storage mechanism. The introduction of bis-imidazole rings can not only contribute to the specific capacity by its own redox reaction, but can also enhance the utilization rate of other redox active sites, such as triphenylamine groups (from the original 40% to 91.8%), as well as the cycling stability due to hydrogen bonds and enhanced conjugation. Obviously, the bipolar quinone-based organic cathode linked via bis-imidazole rings displays special advantages in contrast to other bipolar small molecular electrodes. This is the first report that an active bis-imidazole ring-containing small molecule is used as a cathode material of LIBs, and can provide an alternative option for the design and development of an organic small molecule cathode with high voltage, high capacity and super stability.Author contributions
Conceptualization, M. J. H. and J. Y.; experiments, L. P. Z., J. Y. R., H. G. M., M. S. Y., X. R. Y., R. L., Q. Z., J. Z. Z., and X. P.; theoretical calculations, L. P. Z. and J. Z. Z.; review and editing, M. J. H., H. F. F., and J. Y.; all authors carried out a final check.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge the financial support by the National Natural Science Foundation of China (21771017, 51702009 and 51971157), “Hundred Talents Program” of the Chinese Academy of Sciences, Fundamental Research Funds for the Central Universities, and Shenzhen Science and Technology Program (JCYJ20210324115412035 and ZDSYS20210813095534001).Notes and references
- S. Ding, M. J. Hülsey, J. Pérez-Ramírez and N. Yan, Joule, 2019, 3, 2897–2929 CrossRef CAS.
- G. Zhou, X. An, C. Zhou, Y. Wu, Y.-E. Miao and T. Liu, Compos. Commun., 2020, 22, 100519 CrossRef.
- H. Yang, J. Lee, J. Y. Cheong, Y. Wang, G. Duan, H. Hou, S. Jiang and I.-D. Kim, Energy Environ. Sci., 2021, 14, 4228–4267 RSC.
- Y. Lu and J. Chen, Nat. Rev. Chem., 2020, 4, 127–142 CrossRef CAS.
- P. Poizot, J. Gaubicher, S. Renault, L. Dubois, Y. Liang and Y. Yao, Chem. Rev., 2020, 120, 6490–6557 CrossRef CAS PubMed.
- G. Zhao, H. Li, Z. Gao, L. Xu, Z. Mei, S. Cai, T. Liu, X. Yang, H. Guo and X. Sun, Adv. Funct. Mater., 2021, 31, 2101019 CrossRef CAS.
- C. Zhao, Z. Chen, W. Wang, P. Xiong, B. Li, M. Li, J. Yang and Y. Xu, Angew. Chem., Int. Ed. Engl., 2020, 59, 11992–11998 CrossRef CAS PubMed.
- S. Zheng, D. Shi, D. Yan, Q. Wang, T. Sun, T. Ma, L. Li, D. He, Z. Tao and J. Chen, Angew. Chem., Int. Ed. Engl., 2022, 61, e202117511 CAS.
- M. Xiong, W. Tang, B. Cao, C. Yang and C. Fan, J. Mater. Chem. A, 2019, 7, 20127–20131 RSC.
- Z. Song, Y. Qian, X. Liu, T. Zhang, Y. Zhu, H. Yu, M. Otani and H. Zhou, Energy Environ. Sci., 2014, 7, 4077–4086 RSC.
- S. Wu, W. Wang, M. Li, L. Cao, F. Lyu, M. Yang, Z. Wang, Y. Shi, B. Nan, S. Yu, Z. Sun, Y. Liu and Z. Lu, Nat. Commun., 2016, 7, 13318 CrossRef CAS PubMed.
- Y. Shi, J. Yang, J. Yang, Z. Wang, Z. Chen and Y. Xu, Adv. Funct. Mater., 2022, 2111307, DOI:10.1002/adfm.202111307.
- X. Yang, L. Gong, X. Liu, P. Zhang, B. Li, D. Qi, K. Wang, F. He and J. Jiang, Angew. Chem., Int. Ed. Engl., 2022, 61, e202207043 CAS.
- J. Yang, P. Xiong, Y. Shi, P. Sun, Z. Wang, Z. Chen and Y. Xu, Adv. Funct. Mater., 2020, 30, 1909597 CrossRef CAS.
- S. Gu, S. Wu, L. Cao, M. Li, N. Qin, J. Zhu, Z. Wang, Y. Li, Z. Li, J. Chen and Z. Lu, J. Am. Chem. Soc., 2019, 141, 9623–9628 CrossRef CAS PubMed.
- Y. Lu, X. Hou, L. Miao, L. Li, R. Shi, L. Liu and J. Chen, Angew. Chem., Int. Ed. Engl., 2019, 58, 7020–7024 CrossRef CAS PubMed.
- Y. Wu, R. Zeng, J. Nan, D. Shu, Y. Qiu and S.-L. Chou, Adv. Energy Mater., 2017, 7, 1700278 CrossRef.
- M. E. Bhosale, S. Chae, J. M. Kim and J.-Y. Choi, J. Mater. Chem. A, 2018, 6, 19885–19911 RSC.
- Y. Lu, Q. Zhang, L. Li, Z. Niu and J. Chen, Chem, 2018, 4, 2786–2813 CAS.
- M. Ruby Raj, R. V. Mangalaraja, D. Contreras, K. Varaprasad, M. V. Reddy and S. Adams, ACS Appl. Energy Mater., 2019, 3, 240–252 CrossRef.
- Z. Luo, L. Liu, J. Ning, K. Lei, Y. Lu, F. Li and J. Chen, Angew. Chem., Int. Ed. Engl., 2018, 57, 9443–9446 CrossRef CAS PubMed.
- X. Gong, J. Zheng, Y. Zheng, S. Cao, H. Wen, B. Lin and Y. Sun, Electrochim. Acta, 2020, 356, 136858 CrossRef CAS.
- J. Lopez, D. G. Mackanic, Y. Cui and Z. Bao, Nat. Rev. Mater., 2019, 4, 312–330 CrossRef CAS.
- H. Li, T. Wu, Y. Chen, Y. Liu, Z. Jiang, X. Zhang, G. Dai and Y. Zhao, Compos. Commun., 2021, 28, 100947 CrossRef.
- S. Gu, X. Ma, J. Chen, R. Hao, Z. Wang, N. Qin, W. Zheng, Q. Gan, W. Luo, M. Li, Z. Li, K. Liao, H. Guo, G. Liu, K. Zhang and Z. Lu, J. Energy Chem., 2022, 69, 428–433 CrossRef CAS.
- Z. Song, Y. Qian, T. Zhang, M. Otani and H. Zhou, Adv. Sci., 2015, 2, 1500124 CrossRef PubMed.
- J. Xie, Z. Wang, P. Gu, Y. Zhao, Z. J. Xu and Q. Zhang, Sci. China Mater., 2016, 59, 6–11 CrossRef CAS.
- C. Zhang, S. Chen, G. Zhou, Q. Hou, S. Luo, Y. Wang and G. Shi, J. Electrochem. Soc., 2021, 168, 050548 CrossRef CAS.
- É. Deunf, P. Moreau, É. Quarez, D. Guyomard, F. Dolhem and P. Poizot, J. Mater. Chem. A, 2016, 4, 6131–6139 RSC.
- M. Yao, H. Sano, H. Ando and T. Kiyobayashi, Sci. Rep., 2015, 5, 10962 CrossRef CAS PubMed.
- M. E. Speer, M. Kolek, J. J. Jassoy, J. Heine, M. Winter, P. M. Bieker and B. Esser, Chem. Commun., 2015, 51, 15261–15264 RSC.
- M. Lee, J. Hong, B. Lee, K. Ku, S. Lee, C. B. Park and K. Kang, Green Chem., 2017, 19, 2980–2985 RSC.
- J.-K. Kim, F. Thébault, M.-Y. Heo, D.-S. Kim, Ö. Hansson, J.-H. Ahn, P. Johansson, L. Öhrström, A. Matic and P. Jacobsson, Electrochem. Commun., 2012, 21, 50–53 CrossRef CAS.
- K. Lee, I. E. Serdiuk, G. Kwon, D. J. Min, K. Kang, S. Y. Park and J. E. Kwon, Energy Environ. Sci., 2020, 13, 4142–4156 RSC.
- Z. Tian, V. S. Kale, Y. Wang, S. Kandambeth, J. Czaban-Jozwiak, O. Shekhah, M. Eddaoudi and H. N. Alshareef, J. Am. Chem. Soc., 2021, 143, 19178–19186 CrossRef CAS PubMed.
- Z. Chen, J. Wang, T. Cai, Z. Hu, J. Chu, F. Wang, X. Gan and Z. Song, ACS Appl. Mater. Interfaces, 2022, 14, 27994–28003 CrossRef CAS PubMed.
- W. Ni, J. Cheng, X. Li, G. Gu, L. Huang, Q. Guan, D. Yuan and B. Wang, RSC Adv., 2015, 5, 9221–9227 RSC.
- J. Lee, H. Kim and M. J. Park, Chem. Mater., 2016, 28, 2408–2416 CrossRef CAS.
- S. Stang, A. Lebkücher, P. Walter, E. Kaifer and H.-J. Himmel, Eur. J. Inorg. Chem., 2012, 2012, 4833–4845 CrossRef CAS.
- R. Manivannan, A. Satheshkumar and K. P. Elango, New J. Chem., 2013, 37, 3152 RSC.
- P. R. Lakshmi, P. Jayasudha and K. P. Elango, Spectrochim. Acta, Part A, 2019, 213, 318–323 CrossRef CAS PubMed.
- J. L. Segura, M. J. Mancheno and F. Zamora, Chem. Soc. Rev., 2016, 45, 5635–5671 RSC.
- X. Liu and Z. Ye, Adv. Energy Mater., 2020, 11, 2003281 CrossRef.
- Z. Lin, H. Y. Shi, L. Lin, X. Yang, W. Wu and X. Sun, Nat. Commun., 2021, 12, 4424 CrossRef CAS PubMed.
- Y. Zheng, H. Ji, J. Liu, Z. Wang, J. Zhou, T. Qian and C. Yan, Nano Lett., 2022, 22, 3473–3479 CrossRef CAS PubMed.
- Y. Zhao, M. Wu, H. Chen, J. Zhu, J. Liu, Z. Ye, Y. Zhang, H. Zhang, Y. Ma, C. Li and Y. Chen, Nano Energy, 2021, 86, 106055 CrossRef CAS.
- Y. Zhao, M. Wu, H. Zhang, Z. Ge, C. Li, Y. Ma and Y. Chen, Energy Storage Mater., 2022, 47, 141–148 CrossRef.
- Y. Gao, G. Li, F. Wang, J. Chu, P. Yu, B. Wang, H. Zhan and Z. Song, Energy Storage Mater., 2021, 40, 31–40 CrossRef.
- Z. Xiong, B. Sun, H. Zou, R. Wang, Q. Fang, Z. Zhang and S. Qiu, J. Am. Chem. Soc., 2022, 144, 6583–6593 CrossRef CAS PubMed.
- C. Ye, Y. Qiu, X. Luo, M. Chen, Y. Zhong, B. Wu, L. Xing, Y. Liao and W. Li, Chem. Eng. J., 2021, 403, 126366 CrossRef CAS.
- M. Wu, Y. Zhao, B. Sun, Z. Sun, C. Li, Y. Han, L. Xu, Z. Ge, Y. Ren, M. Zhang, Q. Zhang, Y. Lu, W. Wang, Y. Ma and Y. Chen, Nano Energy, 2020, 70, 104498 CrossRef CAS.
- W. Wang, C. Zhao, J. Yang, P. Xiong, H. Su and Y. Xu, Sci. China Mater., 2021, 64, 2938–2948 CrossRef CAS.
- Z. Tie, S. Deng, H. Cao, M. Yao, Z. Niu and J. Chen, Angew. Chem., Int. Ed. Engl., 2022, 61, e202115180 CrossRef CAS PubMed.
- S. Lee, K. Lee, K. Ku, J. Hong, S. Y. Park, J. E. Kwon and K. Kang, Adv. Energy Mater., 2020, 10, 2001635 CrossRef CAS.
- L. M. Zhu, A. W. Lei, Y. L. Cao, X. P. Ai and H. X. Yang, Chem. Commun., 2013, 49, 567–569 RSC.
- K. Oyaizu, T. Kawamoto, T. Suga and H. Nishide, Macromolecules, 2010, 43, 10382–10389 CrossRef CAS.
- Z. Li, Q. Jia, Y. Chen, K. Fan, C. Zhang, G. Zhang, M. Xu, M. Mao, J. Ma, W. Hu and C. Wang, Angew. Chem., Int. Ed. Engl., 2022, e202207221 CAS.
- X. Peng, Y. Xie, A. Baktash, J. Tang, T. Lin, X. Huang, Y. Hu, Z. Jia, D. J. Searles, Y. Yamauchi, L. Wang and B. Luo, Angew. Chem., Int. Ed. Engl., 2022, 61, e202203646 CAS.
- X. X. Luo, W. H. Li, H. J. Liang, H. X. Zhang, K. D. Du, X. T. Wang, X. F. Liu, J. P. Zhang and X. L. Wu, Angew. Chem., Int. Ed. Engl., 2022, 61, e202117661 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ta07199a |
| This journal is © The Royal Society of Chemistry 2023 |