Polydopamine-modified decellularized intestinal scaffolds loaded with adipose-derived stem cells promote intestinal regeneration†
Jian
Wan
 *abc,
Tianqi
Wu
*a,
Keyi
Wang
d,
Kai
Xia
a,
Lu
Yin‡
a and
Chunqiu
Chen‡
a
*abc,
Tianqi
Wu
*a,
Keyi
Wang
d,
Kai
Xia
a,
Lu
Yin‡
a and
Chunqiu
Chen‡
a
aCenter for Difficult and Complicated Abdominal Surgery, Shanghai Tenth People's Hospital, Tongji University School of Medicine, Shanghai, 200072, China. E-mail: justwanjian@126.com; wtqttkk@126.com; xkky1029@163.com; 1400819@tongji.edu.cn; chenchunqiu6@126.com
bDepartment of Hepatobiliary and Pancreatic Surgery, Affiliated Hospital of Nantong University, Nantong, 226000, China
cResearch Center of Clinical Medicine, Affiliated Hospital of Nantong University, Nantong, 226000, China
dDepartment of Urology, Shanghai Tenth People's Hospital, Tongji University School of Medicine, Shanghai, 200072, China. E-mail: wangkeyi0910@163.com
First published on 28th November 2022
Abstract
Regeneration of gastrointestinal tissues remains a great challenge due to their unique microenvironment. Functional composite decellularized scaffolds have shown great potential in gastrointestinal repair and inducing gastrointestinal tissue-specific proliferation. In this study, polydopamine (PDA)-mediated surface modification of decellularized intestinal scaffolds (DIS), combined with adipose tissue-derived stem cells (ADSC), was used to promote intestinal wound healing while avoiding intestinal resection. The results showed that DIS had good biocompatibility and could maintain the growth and proliferation of ADSC. Moreover, PDA-coated DIS not only had anti-infection ability but could also further promote the secretory activity for the paracrine effects of ADSC. ADSC cultured on PDA-DIS produced significantly higher levels of anti-inflammatory and proangiogenic cytokines than those cultured on plastic plates or DIS. In vivo, ADSC-PDA-DIS significantly promoted intestinal wound closure in rat intestinal defect models. Moreover, ADSC-PDA-DIS was able to induce more neovascularization at 4 weeks postoperatively and promoted macrophage recruitment to accelerate wound healing. Taken together, the results showed that PDA-modified DIS could significantly improve the efficacy of stem cell therapy, and ADSC-PDA-DIS could improve the wound healing process with anti-infection effects, enhancing neovascularization and immunoregulation, which may be of great clinical significance for gastrointestinal regeneration.
Introduction
In recent years, the development of whole-organ acellular technology has provided new ideas for regenerative medicine. Both the decellularized scaffolds themselves and various biological scaffolds derived from them are utilized for the reconstruction and regeneration of organs and tissues.1 To date, relatively simple organ grafts, such as tissue-engineered vascular grafts, segments of upper airways and urethras, have been used with great success in humans.2,3 However, for complex organs, including the esophagus, stomach and small intestine, there is more to explore due to their unique physiological microenvironment.In the field of regenerative medicine and tissue engineering, the small intestinal submucosa (SIS) is undoubtedly a great success of acellular material development and is widely used to repair damaged tissues and organs, including those of the cardiovascular system,4 digestive system5 and urinary system.6 However, the poor mechanical properties of SIS limit its wider application.7 With the rapid development of decellularization technology,8 decellularized scaffolds of the stomach and small intestine with full-layer structures can be prepared,9,10 which greatly improves the possibility of repairing full-layer defects of the digestive tract.11 The preservation of the whole structure of the small intestine provides a good platform for promoting intestinal regeneration after transplantation. However, previous studies have confirmed varying degrees of extracellular matrix (ECM) and cytokine loss during the decellularization process, suggesting the need for further research on ECM materials. To overcome this problem, SIS-based biomaterials or surface modifications of SIS have been developed. In recent years, mussel-inspired adhesive proteins have attracted much attention because of their strong adhesion to various substances in moist environments. Polydopamine (PDA), a mussel-inspired molecule, has been widely employed as an adhesive layer on various biomedical substrates.12 First, the synthesis of PDA is simple, efficient and cost-saving, which can promote the large-scale application of PDA. Second, PDA not only has good biocompatibility but also has high stability and is widely used to regulate the reactions between cells and biomaterials. PDA modification can enhance the adhesion and proliferation of engrafted cells on scaffolds and improve the biocompatibility of grafted biomaterials in vivo.13,14 In addition, studies have shown that PDA can reduce cell oxidative stress damage induced by reactive oxygen species (ROS) and improve cell viability.15–17 Moreover, surface modification of PDA allows the material surface to have good secondary reactivity, leading to continued bridging of the required biomolecules, such as antibacterial molecules, to provide the material with specific antibacterial properties.18,19
In recent years, mesenchymal stem cells (MSC) have shown promising prospects in the treatment of various diseases due to their excellent therapeutic effects. The introduction of adipose tissue-derived stem cells (ADSC), a type of MSC, as a key tool in stem cell-based therapies in wound healing has been successful, such as for treating full-thickness skin defects and spinal cord injury.20,21 Studies have confirmed that ADSC can not only differentiate into a variety of cells, such as fibrocytes, vascular endothelial cells and epithelial cells but also secrete a variety of growth factors, such as bFGF, HGF and VEGF, which can promote collagen synthesis and accelerate the migration of fibroblasts, ultimately promoting wound healing.22 However, the harsh wound environment may weaken the therapeutic potential of transplanted cells. Biomaterial-based cell therapy, which can be used to transplant different types of cells into damaged areas, is often considered a good option. Moreover, cotransplantation of biomaterials can be used fill the lesion cavity and mediate directed growth of cells. Some researchers have used biomaterials recellularized with MSC for tissue repair in vivo, and the results showed that the composite scaffolds could not only accelerate tissue healing but also promote tissue regeneration.23–25
In this study, decellularized intestinal scaffolds (DIS) were prepared by immersion and agitation in detergents, and PDA was used for surface modification. Subsequently, ADSC were recellularized in PDA-DIS, and the composite scaffolds were used to reconstruct intestinal defects in rats to explore the feasibility of decellularized scaffolds for repairing intestinal defects. Finally, this study provides an experimental and theoretical basis for tissue engineering to realize intestinal regeneration.
Materials and methods
Preparation of DIS
The decellularization procedure for the DIS was performed based on a previously reported method.26 Porcine intestines were harvested from adult animals (6–8 months at the time of slaughter) at a local slaughterhouse. After thorough lavage, the intestines were cut into 1–2 cm pieces. Then, the samples were decellularized in 4% sodium deoxycholate (Sigma-Aldrich, Cat# D6750) for at least 12 h (3 consecutive 4 h washes) and 200 μg mL−1 DNase-I (Sigma-Aldrich, Cat# 11284932001) for 12 h, ultimately alternating with washing steps in phosphate buffered saline (PBS) for 48 h. Finally, the DIS was sterilized by incubation for 2 h with 0.1% PAA/4% EtOH and rinsed six times with sterile PBS for 10 min each time.Decellularization assessment
The native tissues and DIS were fixed with 4% paraformaldehyde overnight and embedded in paraffin. Sections were stained with hematoxylin and eosin (H&E), Masson trichrome, and sirius red (SR) to confirm the removal of cells and retention of ECM after decellularization. Scanning electron microscopy (SEM) was adopted to confirm the ultrastructure of the ECM. Selected crucial ECM components, including collagen I, collagen IV and fibronectin, in native and decellularized samples were further detected by immunofluorescence (IF). For quantification of the DNA, collagen and glycosaminoglycans (GAGs), the samples (n = 3) were analyzed by a DNA Extraction Kit (Solarbio, Cat# D1700), Hydroxyproline Assay Kit (Nanjing Jiancheng Bioengineering Institute, Cat# A030-1-1) and Blyscan Assay Kit (Biocolor, Cat# B1000) following the manufacturer's instructions. Mechanical analysis of the DIS and native intestine was conducted in a tensiometer rack (NK500N; HLD, China) (Fig. S1, ESI†). Tensile stress was the load force that the samples (n = 3) were subjected to when it broke in the test.Isolation and culture of ADSC
Adipose tissues were obtained from the mesentery of patients undergoing partial intestinal resection and isolated based on collagenase digestion.27 This study was approved by the Ethics Committee of the Shanghai Tenth People's Hospital, affiliated with Tongji University School of Medicine, and conducted in accordance with the tenets of the Declaration of Helsinki. The mesenteric adipose tissues were minced and digested with 0.1% collagenase I (Sigma-Aldrich, Cat# C0130) at 37 °C on a shaking table for 1 h. Then, the tissues were filtered through a cell strainer (100 μm), and the remaining cells were washed with Dulbecco's modified Eagle's medium (DMEM; Thermo Fisher Scientific, Cat# C11875500BT) containing 10% fetal bovine serum (FBS; Biological Industries, Cat# 1925624) and 1% penicillin/streptomycin (P/S; Thermo Fisher Scientific, Cat# 15140122) 3 times. Finally, the cells were resuspended in complete DMEM and cultured under standard conditions (37 °C, 5% CO2). All the cells used in the experiments were obtained from passages 3 to 6. For adipogenic induction, cells were cultured in adipogenic differentiation medium (Cyagen, Cat# HUXMD-90031) for 2 weeks and stained with an Oil Red O staining kit (Solarbio, Cat# G1262). For osteogenic induction, cells were cultured in osteogenic differentiation medium (Cyagen, Cat# HUXMD-90021) for 3 weeks and stained with Alizarin Red S solution (Solarbio, Cat# G1452). Cell surface protein expression of ADSC was analyzed by flow cytometry and IF. For flow cytometry, 5 × 105 ADSC were resuspended in 100 μL staining solution and stained with 2.5 μL PE anti-human CD29 antibody (BioLegend, Cat# 303003), PE anti-human CD34 (BioLegend, Cat# 343505), FITC anti-human CD44 (BioLegend, Cat# 338803), PE anti-human CD45 (BioLegend, Cat# 304007) and APC anti-human CD90 (BioLegend, Cat# 328113). After incubation for 30 min at 4 °C in the dark and washing with FACS fluid solution, the labeled ADSC were measured using a flow cytometer (BD Biosciences, Germany). All the experiments included negative controls with corresponding isotype controls.Synthesis of PDA and cytocompatibility studies
PDA was prepared via a previously reported method.28 Two milliliters of NH4OH, 40 mL of ethanol and 90 mL of water were mixed and stirred at room temperature for 0.5 h. Then, 0.5 g of dopamine hydrochloride (Sigma-Aldrich, Cat# H8502) in 10 mL of ddH2O was added to the above mixture for a 24 h reaction. After centrifugation, washing with water and drying, the PDA was stored at 4 °C until further use. For cytocompatibility studies, ADSC were evenly plated at a density of 1 × 106 cells per well in 6-well culture plates (Corning, Cat# 3516). After reaching 80% confluence, the cells were incubated with different concentrations of PDA for another 24 h. The cellular viability of ADSC was assessed using CCK8 (Meilunbio, Cat# MA0218) and calcein AM and PI (Meilunbio, Cat# MA0361) following the manufacturer's instructions. For flow cytometry, at least 1 × 106 mL−1 ADSC in each specimen were resuspended in binding buffer. Then, ADSC were stained with the desired Annexin V-FITC and PI (Meilunbio, Cat# MA0220) and incubated for 30 min at room temperature in the dark. The labeled ADSC were measured using a flow cytometer (BD Biosciences, Germany). The apoptosis rate was calculated as late apoptotic cells (Q2) + early apoptotic cells (Q3).Fabrication of PDA-DIS and histocompatibility assay
PDA was dissolved in PBS and dispersed evenly by ultrasonic oscillation. For the fabrication of PDA-DIS, DIS were immersed in different concentrations of PDA solution (25 μg mL−1, 50 μg mL−1, 100 μg mL−1, 200 μg mL−1 and 400 μg mL−1) for 24 h at room temperature. The surface microstructure of the samples was observed by SEM. For the histocompatibility assay, the DIS and PDA-DIS (n = 3) were sterilized and sectioned into 5 × 5 mm2 sections and implanted into the dorsal region of Sprague–Dawley (SD) rats weighing 200–220 g. At selected time points (1 w and 4 w), the samples were harvested for H&E staining. The in vitro degradation rates of DIS and PDA-DIS were determined by collagenase digestion. In brief, samples were immersed in 0.1% collagenase solution, removed and weighed at 24 and 48 h.Antibacterial property
The antibacterial properties of the DIS and PDA-DIS were evaluated using E. coli and S. aureus suspension. The specimens were placed in Eppendorf (EP) tubes and mixed with 1 mL of E. coli or S. aureus suspension at a concentration of 5 × 104 cfu mL−1. Bacteria cultured without specimens were used as a negative control. After culturing for 3 h, an aliquot of 100 μL of E. coli or S. aureus suspension from each specimen was mixed with resazurin for 1 h in 96-well plates, and terminated promptly with 3% SDS. Plates were read in a microplate reader (Thermo Fisher Scientific, USA) at 570 nm, with a reference wavelength of 600 nm. For colony formation, the above incubated E. coli or S. aureus suspension was diluted 5000 times, and 200 μL was coated on the LB agar plate at 37 °C for another 24 h.ADSC cultivated in DIS and PDA-DIS
For ADSC culture in DIS and PDA-DIS, 2 × 105 cells in 200 μL of DMEM were engrafted into the DIS and fixed with tailored metal rings (Fig. 3E). All 3D cell cultures, as with 2D cultures, were performed under standard conditions (37 °C, 5% CO2). To evaluate the cell engraftment rate on the scaffolds, the supernatant was collected, and unseeded cells were counted after 4 h of incubation. After an adhesion time of 4 h, 800 μL of medium was added to the plates, and the cells were cultured for 3 days.Collection of conditioned medium
To obtain the conditioned medium (CM), 2 × 105 ADSC were evenly plated on 24-well plastic plates, DIS and PDA-DIS in complete DMEM for 24 h and then supplemented with FBS-free DMEM for another 24 h. The supernatant was collected and centrifuged at 5000 rpm to remove the dead cells and cell debris. The concentrations of the paracrine factors prostaglandin E2 (PGE2), transforming growth factor-β1 (TGF-β1) and vascular endothelial growth factor (VEGF) in CM were determined by a Human PGE2 ELISA Kit (Elabscience, Cat# E-EL-0034c), Human VEGF ELISA Kit (Multi sciences, Cat# EK183), and Human TGF-β1 ELISA Kit (Multi sciences, Cat# EK981) according to the manufacturer's instructions.HUVEC wound healing, migration and tube-formation assay
Wound healing assay
HUVECs were purchased from Cyagen Biosciences and cultured in ECM medium (Cyagen, Cat# 90011). Cells from passages 3 to 10 were used for subsequent experiments. HUVECs were evenly plated in 6-well plates and incubated until 80% confluence. The cells were then wounded with sterile 200 μL tips, and the floating cells were washed away with PBS. For the treatment with CM, serum-free DMEM, ADSC-CM, ADSC-DIS-CM and ADSC-PDA-DIS-CM were added to the culture plates. The wound scars were captured at 0 and 24 h from the same position using an inverted microscope.HUVEC migration assays
The migration properties of different CMs on HUVECs were examined using Transwell chambers with an 8 μm pore size (Corning, Cat# 3422). A total of 1000 cells in 100 μL of FBS-free DMEM were added to the upper chamber of the Transwells, and the lower chamber was filled with 800 μL serum-free DMEM, ADSC-CM, ADSC-DIS-CM and ADSC-PDA-DIS-CM. HUVECs treated with FBS-free DMEM were used as negative controls. After incubating at 37 °C for 12 h, the noninvading cells on the upper surface of the chambers were wiped off. The migrated cells were fixed and stained with 0.5% crystal violet solution (Solarbio, G1065). For quantification, the cells were counted by a microscope at 200 × magnification in three random fields.Tube formation assay
For tube formation assays, HUVECs were seeded on Matrigel (Corning, Cat# 354234) (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ×
× ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 104 cells per well) in 48-well plates (Corning, Cat# 3548) and cultured with ECM medium containing 50% (v/v) CM. After 6–8 h of culture, tube formation was assessed with a microscope at 100 × magnification, and the length and size of the tube were quantified by ImageJ angiogenesis analyzer software based on three repeat experiments.
104 cells per well) in 48-well plates (Corning, Cat# 3548) and cultured with ECM medium containing 50% (v/v) CM. After 6–8 h of culture, tube formation was assessed with a microscope at 100 × magnification, and the length and size of the tube were quantified by ImageJ angiogenesis analyzer software based on three repeat experiments.
Macrophage polarization assay
Mouse RAW264.7 macrophages were seeded in 24-well plates (Corning, Cat# 3524) and incubated until 70% confluence. For phenotype stimulation, cells were incubated with different CMs containing LPS (100 ng mL−1; Sigma-Aldrich, Cat# L2630) for 24 h. Samples supplemented with LPS alone served as positive controls. The samples and supernatants were collected for IF, western blotting and qRT–PCR.In vivo characterization
Male SD rats (200–220 g, 10–12 weeks old) used in the experiment were purchased from SLAC Laboratory Animal Co., Ltd. (Shanghai, China). All animals were kept under ambient conditions with a 12 h light per dark cycle and free access to water and food. All animal work was approved by the Ethics Committee of the Shanghai Tenth People's Hospital, affiliated with Tongji University School of Medicine. The abdomens of the rats were disinfected with iodophor three times, and incisions were made in the middle abdomen. A 0.5 × 0.5 cm2 surgical defect involving all the layers was made in the cecum. Then, the defect area was mounted and repaired with sterile DIS, ADSC-DIS and ADSC-PDA-DIS (n = 6) by applying 4–0 nonabsorbable sutures in an uninterrupted manner. The repaired areas were harvested at 1 w and 4 w postoperation, and histological staining was performed. For quantitative analysis of neovascularization, collagen expression and distribution of macrophages, images were captured at 200 × magnification in random fields in each group and analyzed by ImageJ software. Each experiment was repeated three times. To test the long-term toxicity of PDA, blood and main organs were collected at 4 w postoperation for biochemical detection and histological staining.IF
First, the sample sections were retrieved by antigen retrieval buffers (Meilunbio, Cat# MA0180). After permeabilization with 0.5% Triton X-100 and blocking with 5% bovine serum albumin (BSA; Sigma, Cat# A1933), the sections were incubated with primary antibodies at 4 °C overnight. The next morning, the primary antibodies were removed, and the sections were incubated with secondary antibodies and DAPI (Meilunbio, Cat# MA0128) at room temperature. Finally, the sections were visualized using an Olympus fluorescence microscope. The primary and secondary antibodies used in this study included rabbit anti-collagen I, rabbit anti-collagen IV, rabbit anti-fibronectin, mouse anti-CD29, rabbit anti-CD44, rabbit anti-CD90, rabbit anti-alpha smooth muscle actin (α-SMA), mouse anti-vimentin, rabbit anti-iNOS, rat anti-F4/80, mouse anti-CD31, mouse anti-CD206, Alexa Fluor 488 and Cy3 (Table S2, ESI†).qRT–PCR
Total RNA from cultured cells was isolated using a Total RNA Kit (Vazyme, Cat# R701) according to the manufacturer's instructions. To generate cDNA, 1 μg of total RNA was used as a template with a First Strand cDNA Synthesis Kit (Vazyme, Cat# R323). Real-time qPCR was performed with SYBR Green Master Mix (Vazyme, Cat# Q711) and detected using an ABI 7500 Real-Time PCR System (Applied Biosystems, USA). Gene expression levels were quantified using the 2−ΔΔCt method. Gene-specific primers are listed in Additional file (Table S1, ESI†).Western blotting
Total protein from the cells was extracted using RIPA buffer (Beyotime, Cat# P0013B), and the concentrations were determined using a BCA Protein Assay Kit (Beyotime, Cat# P0010) according to the manufacturer's instructions. Equal amounts of the protein were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE; Beyotime, Cat# P0012AC) and transferred onto polyvinylidene fluoride (PVDF) membranes (Beyotime, Cat# FFP39). After blocking, the protein was incubated with primary antibodies at 4 °C overnight and then with the secondary antibody for 1 h. The membranes were visualized using a gel imaging system (Bio-Rad, USA). The following specific antibodies were used: rabbit anti-αTubulin, rabbit anti-liver arginase (Arg1), rabbit anti-TNFα, rabbit anti-IL6 and goat anti-rabbit IgG (H + L) (Table S2, ESI†).Statistical analysis
All data analyses were conducted using SPSS 22.0. Data are expressed as the mean ± standard deviation of at least three independent experiments. All data were tested for normal distribution, followed by Student's t test and one-way ANOVA for comparison between groups. Differences were considered significant at P < 0.05.Results
Characterization of DIS
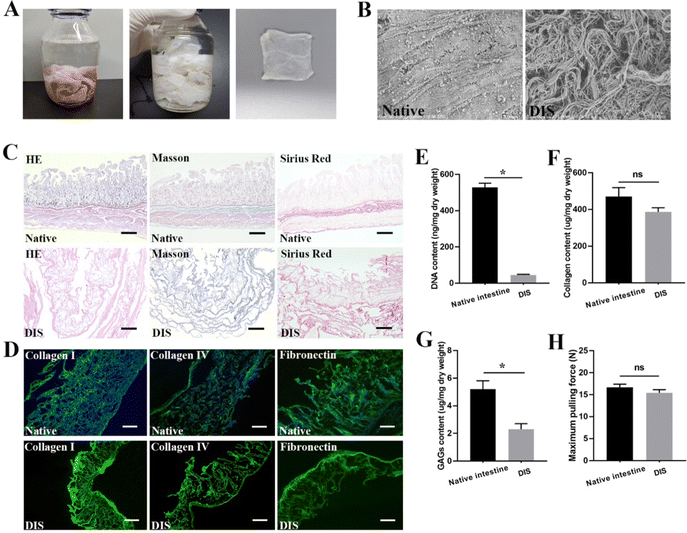
After infusion of 4% sodium deoxycholate for 24 h, the small intestine lost its natural color gradually until it became semitransparent at the macroscopic level (Fig. 1A). H&E staining and SEM analysis confirmed the almost complete removal of cells and ultrastructure maintenance after the decellularization process (Fig. 1B and C). Quantification of the residual DNA demonstrated that the DNA content of DIS was 45.77 ± 2.08 ng mg−1 dry weight, in contrast to 528.9 ± 12.97 ng mg−1 for the native intestine (Fig. 1E) (P < 0.05). According to the results of Masson trichrome and SR staining, collagen was well preserved, and no significant differences were observed between the native tissues and DIS (Fig. 1C). The collagen content in the DIS was 386.8 ± 13.07 ng mg−1 dry weight, compared to 470.9 ± 27.55 ng mg−1 dry weight in the native intestine, as determined through indirect quantification of the amino acid hydroxyproline (Fig. 1F) (P > 0.05). Based on the IF results, the distribution and expression of key structural ECM proteins, including collagen I, collagen IV and fibronectin, were well maintained in DIS after decellularization (Fig. 1D). Previous studies have shown that GAGs are easily degraded in the process of decellularization. In this study, the total content of GAGs in the DIS and native tissue was 2.3 ± 0.23 and 5.2 ± 0.35 μg mg−1 dry tissue weight, respectively (P < 0.05) (Fig. 1G). Although GAGs were significantly reduced, 44.2% were retained in DIS. According to the tensile test, the maximum pulling force of DIS was 15.4 ± 0.42 N, in contrast to 16.7 ± 0.42 N for the native intestine (Fig. 1H) (P > 0.05).Culture of ADSC
Details regarding the identification of ADSC are provided in the experimental section and Fig. S2 (ESI†). After the third passage, ADSC presented typical spindle-shaped and fibroblast-like morphology (Fig. S2A, ESI†). Following 2–3 weeks of osteogenic and adipogenic induction, mineralized nodules and lipid droplets were observed by Alizarin Red and Oil Red O staining (Fig. S2B and C, ESI†). By flow cytometry and IF, ADSC showed high expression of the classic MSC surface markers CD29, CD44, and CD90 and the absence of CD34 and CD45 (Fig. S2D and E, ESI†).Biocompatibility of PDA and PDA-DIS
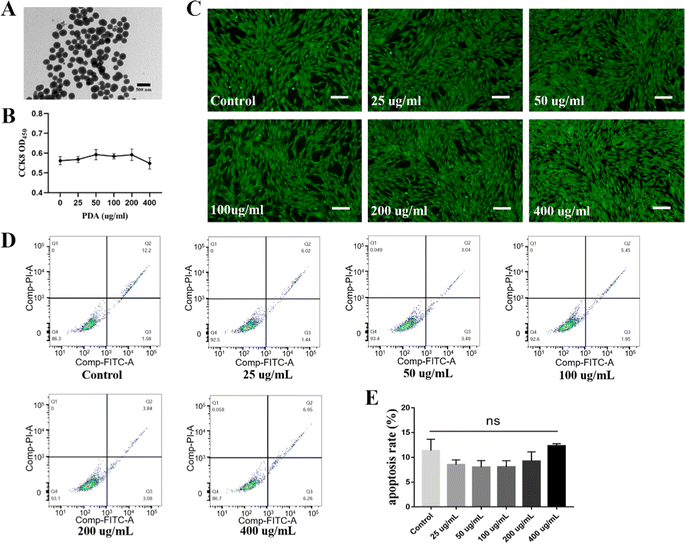
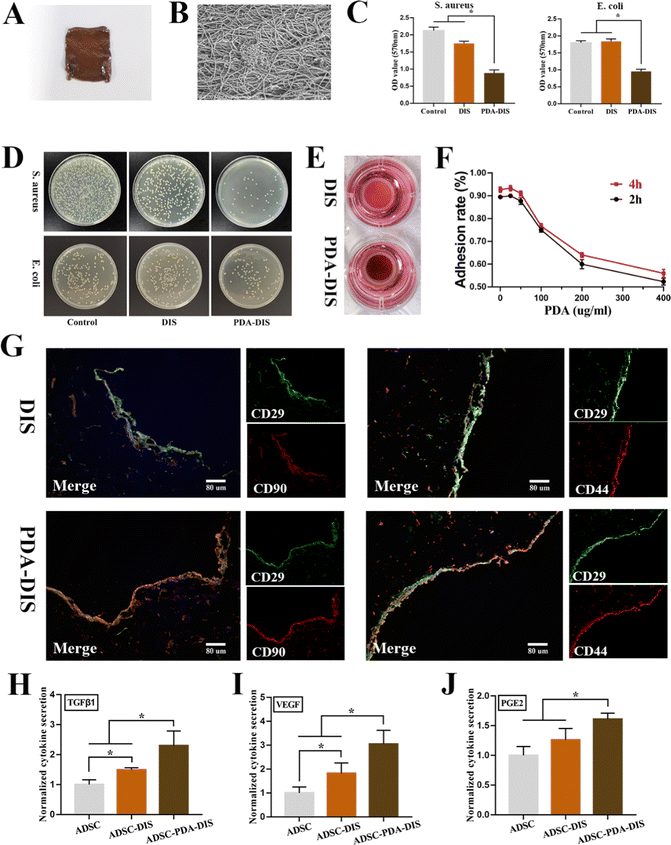
Transmission electron microscopy (TEM) showed that the average diameter of the PDA nanoparticles (NPs) was 200 nm, with a monodispersed spherical structure (Fig. 2A). ADSC were incubated with different concentrations of PDA, and no significant cytotoxicity was observed in any group. The CCK-8 assay showed that the proliferation ability of ADSC did not change significantly at a PDA concentration lower than 400 μg mL−1 (Fig. 2B). Additionally, no obvious dead cells were found by calcein AM and PI staining (Fig. 2C). Moreover, FITC-Annexin V and PI staining followed by flow cytometry analysis revealed that the apoptosis rate in the PDA group was not significantly higher than that in the control group (Fig. 2D and E). Together, these results indicated that PDA had good cytocompatibility. To prepare PDA-DIS, the DIS was immersed in PDA solution for 24 h, and the color of the scaffold changed to brown (Fig. 3A). SEM characterization of the microstructures revealed a uniform nanolayer on the surface of the PDA-DIS (Fig. 3B). To evaluate the histocompatibility of the scaffolds, the DIS and PDA-DIS samples were removed from the rats and evaluated by histological analysis. It was demonstrated that both scaffolds were histocompatible. H&E staining showed that both scaffolds triggered angiogenesis after implantation (Fig. S3, ESI†). After immersed the scaffolds in 0.1% collagenase for 24 h and 48 h. It showed that the degradation rate of PDA-DIS was significantly lower than that of DIS, indicating that PDA could significantly prolong the implantation time of DIS (Fig. S4, ESI†). E. coli and S. aureus were selected as the model bacterium in this experiment. After incubation with scaffolds for 3 h, the bacterial activity in the PDA-DIS was weakened (Fig. 3C). To further elucidate the antibacterial effect, the bacterial suspensions were cultured on LB agar plates, and fewer bacterial colonies were formed in the PDA-DIS group than in the control and DIS groups (Fig. 3D). These results together indicated that the PDA coating conferred an antibacterial property.Repopulation of ADSC in the scaffolds
Considering that excessive PDA coating would affect the adhesion of ADSC, we calculated the adhesion rate of ADSC in PDA-DIS at different concentrations. The results showed that the cell adhesion rate decreased significantly when the PDA concentration reached 100 μg mL−1 (Fig. 3F). Therefore, PDA solutions with a concentration of 50 μg mL−1 were selected for DIS coating in subsequent experiments. After 3 days of culture, the repopulated scaffolds were harvested for histological analysis. ADSC could not only be engrafted into the scaffolds but also showed MSC surface markers (CD29, CD44, and CD90), indicating that the cytoactivity of ADSC was not affected in the DIS or PDA-DIS (Fig. 3G). The cytokine secretion levels of ADSC seeded on plastic plates, DIS and PDA-DIS were characterized with ELISA. In general, the secretion of TGFβ1, VEGF and PGE2 in PDA-DIS was promoted to varying degrees compared to that in the control and DIS groups (Fig. 3H–J). These results indicated that scaffolds coated with polydopamine strongly modulated the secretion of cytokines relevant to inflammation and angiogenesis in ADSC.In vitro proangiogenic and immunoregulatory function of ADSC paracrine products
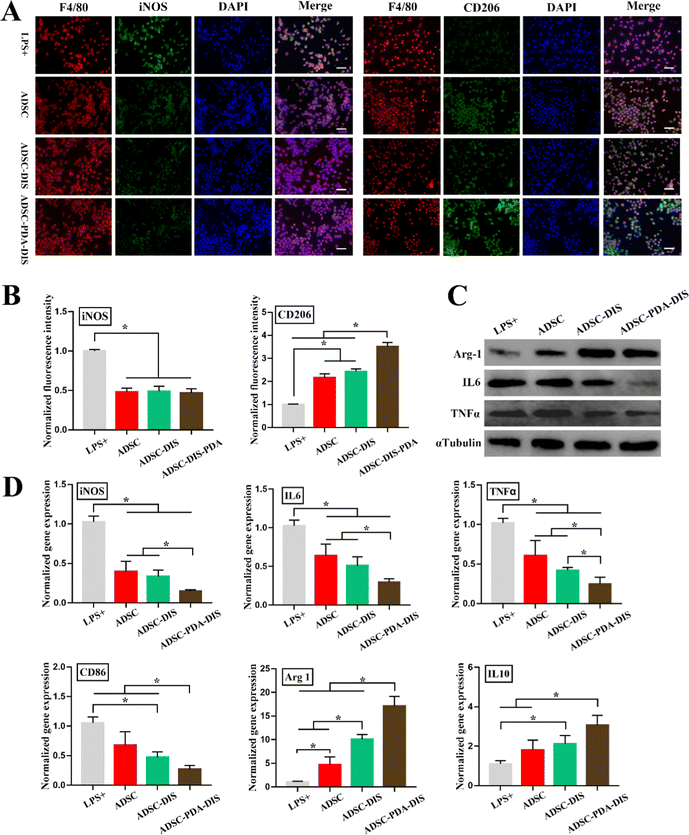
HUVEC wound healing, migration and tube formation assays were conducted to analyze the proangiogenic function of the CM of ADSC. The wound closure percentages were 30.56 ± 3.2%, 47.35 ± 0.77%, 50.44 ± 0.81% and 66.75 ± 1.17% in the blank control, ADSC, ADSC-DIS and ADSC-PDA-DIS groups, respectively. The migration ability of HUVECs stimulated with ADSC-PDA-DIS CM was significantly enhanced compared to that of HUVECs in the other three groups (P < 0.05) (Fig. 4A and D). Meanwhile, transwell migration assays demonstrated that the numbers of migrated HUVECs in the blank control group, ADSC group, ADSC-DIS group and ADSC-PDA-DIS group were 44 ± 7.7, 77 ± 4.8, 98 ± 5.9 and 140 ± 7.9, respectively (200 × magnification). Similarly, ADSC-PDA-DIS CM showed an enhanced migration capability compared to the CM from the ADSC and ADSC-DIS groups (Fig. 4B and E). Moreover, tube formation assays showed that the total branching length of the tubes formed in the ADSC-PDA-DIS CM group was statistically higher than those from the blank control, ADSC and ADSC-DIS groups (Fig. 4C and F).Regarding immunoregulation function, Raw264.7 cells were immunostained with F4/80 (pan-macrophage marker), iNOS (M1 macrophage marker) and CD206 (M2 macrophage marker). IF demonstrated that the expression of iNOS decreased after CM stimulation compared with that in the LPS+ group. In contrast, as a marker of M2 polarization, CD206 was most significantly expressed in the ADSC-PDA-DIS group, followed by the ADSC and ADSC-DIS groups, and was lowest in the LPS+ group (Fig. 5A and B). Meanwhile, the mRNA expression levels related to anti-inflammatory factors (Arg-1 and IL-10) were significantly upregulated in the ADSC, ADSC-DIS and ADSC-PDA-DIS groups, whereas the mRNA levels of proinflammatory factors (iNOS, IL6, TNFα and CD86) showed the reverse trend (Fig. 5D). Notably, the ADSC-PDA-DIS group showed the most potent anti-inflammatory effects. Subsequently, the protein expression levels of anti-inflammatory and proinflammatory factors from different CMs were confirmed by western blotting. Similarly, the anti-inflammatory effects of the ADSC-PDA-DIS group were significantly enhanced (Fig. 5C).
PDA-coated DIS accelerated the healing of intestinal defects
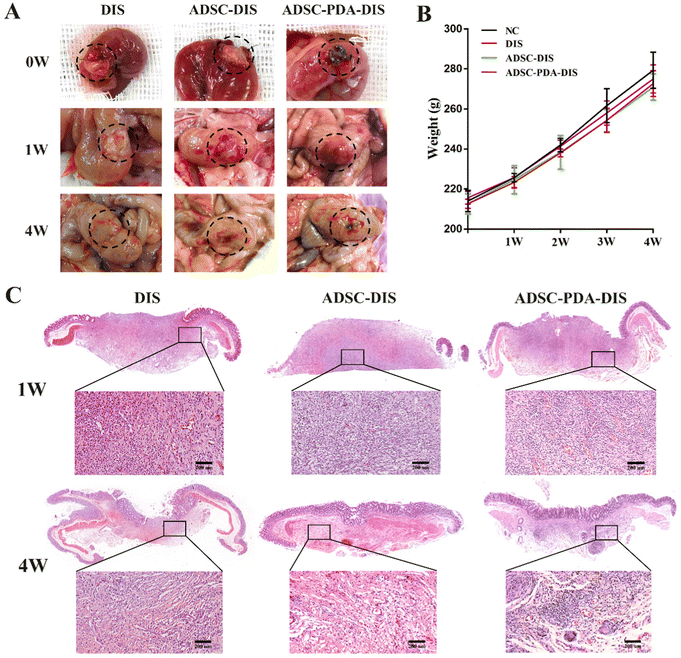
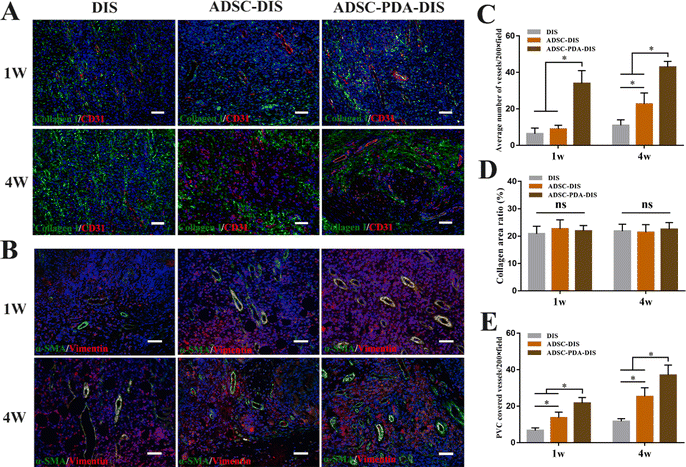
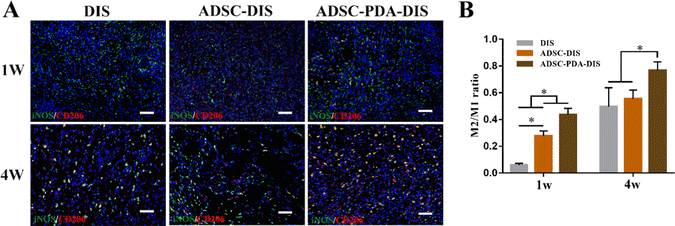
The transplanted scaffolds were analyzed in the 1st and 4th weeks after intestine repair. At the macroscopic level, DIS, ADSC-DIS and ADSC-PDA-DIS could repair intestinal defects without intestinal fistula. In addition, intestinal inflammation seemed to be weaker in the ADSC-DIS and ADSC-PDA-DIS groups in the first week (Fig. 6A). We determined the weight of rats for 4 consecutive weeks, and the weight of rats in the 3 groups did not decrease significantly compared to unoperated rats, indicating that the repair surgery did not cause nutritional disorders in the rats (Fig. 6B). Histological staining of the scaffolds in the repaired area showed that the ADSC-PDA-DIS group showed more neovascularization at both 1 w and 4 w postoperation, while little neovascularization was observed in the DIS groups (Fig. 6C). IF analysis further confirmed higher expression levels of CD31 (labeling ECs) and αSMA (labeling perivascular cells, PVCs) in the ADSC-PDA-DIS group (Fig. 7A and B). Further quantitative analysis showed that the expression of CD31 and αSMA in the ADSC-PDA-DIS group was significantly higher than that in the DIS and ADSC-DIS groups (P < 0.05) (Fig. 7C and E). On the other hand, large populations of vimentin+ cells were found in the scaffolds, indicating infiltration of host fibroblasts into the scaffolds. Notably, the expression level of collagen was not significantly different among the three groups. This was because the scaffolds were inherently rich in collagen (Fig. 7D). The IF assay showed the phenotype distribution of macrophages at 1 w and 4 w after transplantation. The iNOS level of M1 macrophages in the DIS group was higher than that in the other two groups, and CD206 expression in M2 macrophages in the ADSC-PDA-DIS group was stronger (Fig. 8A). Quantitative analysis showed that the M2/M1 ratio in the ADSC-PDA-DIS group was markedly higher than that in the DIS and ADSC-DIS groups at 1 w and 4 w after transplantation (Fig. 8B). Ultimately, biosafety evaluation was carried out, and blood analysis showed negligible variations in biochemical indexes among the DIS, ADSC-DIS and ADSC-PDA-DIS groups (Fig. S5A, ESI†). Additionally, examination of H&E-stained histological sections of major organs showed no prominent injuries (Fig. S5B, ESI†).Discussion
Decellularized scaffolds have shown promising prospects in the repair of organ defects or replacement of malfunctioning parts of the gastrointestinal tract and have attracted great interest from researchers in the field of gastroenterology.29,30 In this study, DIS were prepared, and surface modification was carried out by incubation in PDA. Then, ADSC were implanted on PDA-DIS to form composite scaffolds for the repair of intestinal defects in rats. ADSC-PDA-DIS was prepared in this study to enhance neovascularization after repair in vivo, improve anti-inflammatory effects, explore the feasibility of using decellularized scaffolds to repair intestinal defects, and provide innovative ideas for using tissue engineering for intestinal repair and regeneration.Given the extensive development of decellularization technology, decellularized scaffolds have been successfully fabricated for various important organs with complex structures, such as the heart, lung, kidney, liver and bladder.31,32 Decellularized scaffolds possess characteristics that synthetic materials find difficult to mimic, such as natural ECM structures, high bioactivity, and low immunogenicity.33,34 Especially in gastrointestinal tissue engineering, the decellularized intestine not only preserves the basic intestinal structure such as the mucosal layer, submucosa and muscular layer, but also intact crypt-villus, which could provide a reasonable microtopography for intestinal graft reconstruction.35 This is the biggest advantage of DIS compared with other synthetic biomaterials. As described in this study, the main structures of the ECM in DIS, such as collagen I, collagen IV and fibronectin, were well preserved compared to the native tissues. Of the GAGs, which are indicative of tissue remodeling, 44.2% were retained in DIS. Well-designed decellularization procedures can produce a nontoxic and biocompatible matrix that ultimately triggers cell infiltration and angiogenesis after engraftment.36 Moreover, it is well known that the retention of growth factors in decellularized matrices can induce the adhesion and migration of various cells, such as endothelial cells, epithelial cells and smooth muscle cells,37 which play a critical role in tissue regeneration and remodeling.
Given that the intestinal tract is a bacterium-rich environment, an important challenge for using intestinal grafts is the prevention of infection. The anti-infection ability of biomaterials has important clinical significance for repairing tissue defects.38 PDA has been proven to have antibacterial and antifungal effects against a wide range of microorganisms. Moreover, additional biomolecules can be anchored to the PDA coating via Schiff-base and Michael addition reactions, providing a greater variety of surface properties to the biomaterials.39 Our results showed that DIS with PDA modification had antibacterial properties. What is noteworthy is that as there are gastrointestinal microbiota in the intestine, which is important for the normal function of the intestine other major organ system.40 At present, relevant studies have confirmed that PDA has good compatibility with microbial and mammalian cells, and has little effect on their biological activities.41,42 In future studies, 16S ribosomal RNA gene sequencing or metagenomic sequencing will help us to further evaluate the effects of the composite scaffold on the intestinal microbiota and improve the therapeutic efficacy of the scaffold.
Although the DIS coating with a higher PDA concentration did not affect the histocompatibility of the scaffold,43 it did affect the cytocompatibility. At present, many studies focus on the chemical and biological factors of PDA affecting cell activity, but physical factors cannot be ignored. In our experiment, PDA concentrations in the range of 0–400 μg mL−1 had no obvious toxicity toward ADSC. However, it was found that when the concentration of PDA-modified DIS was greater than 100 μg mL−1, the cell adhesion decreased significantly, possibly because an excessively dense PDA coating could hinder the adhesion and migration of ADSC. Therefore, we chose 50 μg mL−1 PDA for the coating of DIS in subsequent experiments.
An important concern regarding surface modification is whether it affects the function of transplanted cells. Materials for surface modification should have good cytocompatibility, which can not only promote the adhesion and migration of engrafted cells but also induce targeted differentiation of cells.44,45 In our study, IF staining was performed on ADSC planted in DIS and PDA-DIS, and the results showed that the surface markers of ADSC were positive for CD29, CD44 and CD90. The results showed that the cell characteristics of ADSC in scaffolds did not change, even in the case of PDA coating, which did not affect the cytoactivity of ADSC. Many studies have shown that MSC promote cell proliferation, tissue repair and regeneration through autocrine and paracrine pathways.13,46 The growth factors secreted by MSC, including HGF, VEGF, TGF-β and bFGF, could enhance the survival or proliferation of stem cells in a hostile microenvironment.47 Furthermore, these factors are essential for maintaining the stemness of MSC.48 In this study, TGFβ1, PGE2 and VEGF were detected in ADSC-derived CM, and the CM derived from ADSC-DIS and ADSC-PDA-DIS was enhanced, which is consistent with previous studies;49 that is, 3D scaffolds could provide a unique microenvironment to enhance ADSC secretion. At the same time, paracrine effects were also enhanced in 3D scaffolds, and there is increasing evidence that the immune regulation and angiogenesis of MSC are largely attributable to paracrine factors.50 Several studies have investigated the therapeutic effects of MSC-derived paracrine factors on different disorders, including brain injury,51 myocardial infarction,47 bronchopulmonary dysplasia52 and ovarian insufficiency.53 In this study, HUVEC wound healing, migration and tube formation assays confirmed that ADSC-derived CM could promote the migration and proliferation of endothelial cells and that DIS, and PDA modification enhanced the paracrine effect of ADSC. MSC suspensions can be directly injected into or around the wound to promote wound healing. However, the harsh wound environment may hinder the implantation of MSC, resulting in a limited number of MSC with therapeutic potential. The transmission modes of engrafted cells combined with scaffolds have been proven to significantly improve the survival rate and retention rate of MSC at the wound site and improve wound healing.54,55 For example, in the field of skin repair, many researchers prefer hydrogel systems loaded with MSC to maximize the therapeutic effects of MSC.56,57 Properly designed scaffolds will serve as templates to guide cell adhesion, proliferation and differentiation and ultimately promote tissue repair and regeneration. Our study showed that after intestinal defect repair in rats, no intestinal fistula or other complications occurred, and the DIS was compatible with the tissue in vivo. Moreover, vascular networks in the graft area were examined at weeks 1 and 4 posttransplantation. According to CD31 and αSMA staining, which labels ECs and PVCs, the degree of vascularization in ADSC-PDA-DIS was higher, which highlighted the necessity of improving the histocompatibility of scaffolds.
Another major challenge for organ repair is functional tissue regeneration. Several previous studies have demonstrated that the microenvironments generated by MSC can promote an improved environment for wound healing.58,59 Factors such as VEGF and TGFβ1 can enhance neovascularization and wound re-epithelialization, significantly improve collagen matrix deposition, and ultimately reduce excessive ECM accumulation and scar formation.60,61 For some scaffolds without collagen fibers, the regeneration of collagen fibers after transplantation is crucial. Only by avoiding disorderly collagen fibers can excessive hyperplasia of scars be prevented. According to our results, there was no significant difference in collagen staining among the three groups after scaffold transplantation, which may be because DIS itself contains a large amount of collagen. It is precisely because of this advantage that the mechanical properties of DIS scaffolds with tensile strength can be guaranteed, ultimately ensuring that transplantation failure will not occur due to DIS rupture in the early stage of transplantation. So as to realize the great clinical transformation value of this biomaterial.
The M2 phenotype of macrophages, another key cause of tissue remodeling, is also receiving increasing attention.62 Multiple studies have shown that a higher M2/M1 ratio was associated with more organizational remodeling and vascularization.63 In our study, ADSC-PDA-DIS CM significantly promoted the expression of M2-related polarization factors (Arg-1, IL-10, and CD206) in RAW264.7 cells while inhibiting the expression of M1-related polarization factors (TNF-α, iNOS, CD86 and IL-6). The IF results showed decreased levels of the M1 polarization marker iNOS and increased levels of the M2 polarization marker CD206 in the ADSC-PDA-DIS CM group compared with the other groups. These findings confirmed that the PDA-DIS induced paracrine secretion of ADSC modulated the immune microenvironment, promoted vascularization, and promoted tissue regeneration. Finally, we conducted a biosafety assessment. There was no significant difference in body weight or biochemical parameters of blood among the three groups. In addition, H&E staining of major organs revealed no obvious lesions. These results reflect the absence of significant toxicity of PDA in vivo.
In previous similar studies, researchers used untreated mice with intestinal mucosal damage as a control group, so the mice could survive even with this mild damage.64 However, the model we established was a full-thickness intestinal defect. It was almost impossible for rats to survive in this condition without any treatment. The sham operation group or the sutured group were not taken as the control group because the regeneration of native intestinal cells was not observed during the research cycle of this experiment. In this case, comparing the patch area to normal intestinal structure does not seem to make sense. The main objective of this study is to explore the use of biological scaffolds as a template for intestinal regeneration. In the future, this scaffold can be used to reconstruct an artificial intestine in vitro and perform intestine transplantation for patients with intestinal failure. So we selected the DIS group as the “control group” in the study. For gastrointestinal tissue engineering, considering its special structure and function, various attempts have only partially simulated gastrointestinal characteristics at present, and there is still a long way to go before it is applied to the clinic. Taking our study as an example, although the ADSC-PDA-DIS composite scaffold was established in this study to promote the rapid vascularization and immune cell infiltration of intestinal wounds after in vivo repair. However, no regeneration of native intestinal cells and structures has been observed in scaffolds. To restore the function of digesting food and absorbing nutrients, the reconstruction of mature intestinal epithelial lineages, crypt-villus structures and the enteric nervous system is essential. Some studies have observed the regeneration of native intestinal cells after the transplantation of DIS-derived ECM combined with organoids in vivo, which undoubtedly brings new hope for intestinal regeneration. For this non-full-thickness defect, ECM hydrogel may be a good choice, but for the need to achieve the regeneration of the whole intestinal structure, the full-thickness structure of the scaffold seems to have more advantages in clinical translation. It does restore intestinal continuity in a short time and provided a promising template for intestinal regeneration. In the future, on the basis of this study, the cotransplantation of multiple multilineage cells such as endothelial cells, small intestinal stem cells, intestinal organoids and neural crest cells will greatly expand its application.35,65
Conclusions
This study shows that PDA-DIS not only has good biocompatibility and can support the growth of ADSC but also provides a unique microenvironment to regulate the paracrine function of ADSC. Most importantly, in vivo transplantation for repairing intestinal defects can quickly establish vascularization and promote intestinal healing and regeneration. The composite scaffold prepared in this experiment provides a new direction for tissue engineering for the treatment of intestinal diseases, highlighting innovations in and the importance of surface modification.Data availability statements
All data included in this study are available for readers from the corresponding author upon reasonable request.Conflicts of interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.References
- D. A. Taylor, L. C. Sampaio, Z. Ferdous, A. S. Gobin and L. J. Taite, Acta Biomater., 2018, 74, 74–89 CrossRef CAS PubMed.
- S. Pashneh-Tala, S. MacNeil and F. Claeyssens, Tissue Eng., Part B, 2016, 22, 68–100 CrossRef CAS PubMed.
- A. Singh, T. J. Bivalacqua and N. Sopko, Sex. Med. Rev., 2018, 6, 35–44 CrossRef.
- L. C. Haney, H. F. Ahmed, A. Dani, P. Chin, K. Thangappan, N. Madsen, F. Zafar, J. S. Tweddell and D. L. S. Morales, Ann. Thorac. Surg., 2021, 114, 1475–1483 CrossRef PubMed.
- M. Wang, Y. Q. Li, J. Cao, M. Gong, Y. Zhang, X. Chen, M. X. Tian and H. Q. Xie, J. Mater. Chem. B, 2017, 5, 7059–7071 RSC.
- X. Z. Zhang, Y. L. Jiang, J. G. Hu, L. M. Zhao, Q. Z. Chen, Y. Liang, Y. Zhang, X. X. Lei, R. Wang, Y. Lei, Q. Y. Zhang, J. Li-Ling and H. Q. Xie, Bioact. Mater., 2021, 6, 1827–1838 CrossRef CAS PubMed.
- G. Cao, Y. Huang, K. Li, Y. Fan, H. Xie and X. Li, J. Mater. Chem. B, 2019, 7, 5038–5055 RSC.
- A. E. Mayorca-Guiliani, O. Willacy, C. D. Madsen, M. Rafaeva, S. Elisabeth Heumüller, F. Bock, G. Sengle, M. Koch, T. Imhof, F. Zaucke, R. Wagener, T. Sasaki, J. T. Erler and R. Reuten, Nat. Protoc., 2019, 14, 3395–3425 CrossRef CAS PubMed.
- P. Giuffrida, M. Curti, W. Al-Akkad, C. Biel, C. Crowley, L. Frenguelli, A. Telese, A. Hall, D. Tamburrino, G. Spoletini, G. Fusai, F. P. Tinozzi, A. Pietrabissa, G. R. Corazza, P. De Coppi, M. Pinzani, A. Di Sabatino, K. Rombouts and G. Mazza, Inflammatory Bowel Dis., 2019, 25, 1740–1750 CrossRef.
- A. J. Boys, S. L. Barron, D. Tilev and R. M. Owens, Front. Bioeng. Biotechnol., 2020, 8, 589960 CrossRef.
- L. Feng, Y. L. Hu, P. Ma, Y. Feng, Y. B. Guo, H. Huang, P. Li, Q. S. Mao and W. J. Xue, J. Biomed. Mater. Res., Part B, 2021, 109, 451–462 CrossRef CAS.
- B. D. James, K. M. Kimmins, M. T. Nguyen, A. J. Lausch and E. D. Sone, Sci. Rep., 2021, 11, 23998 CrossRef CAS PubMed.
- T. Li, H. Ma, H. Ma, Z. Ma, L. Qiang, Z. Yang, X. Yang, X. Zhou, K. Dai and J. Wang, ACS Appl. Mater. Interfaces, 2019, 11, 17134–17146 CrossRef CAS.
- T. Su, M. Zhang, Q. Zeng, W. Pan, Y. Huang, Y. Qian, W. Dong, X. Qi and J. Shen, Bioact. Mater., 2021, 6, 579–588 CrossRef CAS PubMed.
- Q. Wang, R. Zhang, M. Lu, G. You, Y. Wang, G. Chen, C. Zhao, Z. Wang, X. Song, Y. Wu, L. Zhao and H. Zhou, Biomacromolecules, 2017, 18, 1333–1341 CrossRef CAS.
- Z. Deng, W. Wang, X. Xu, Y. Nie, Y. Liu, O. E. C. Gould, N. Ma and A. Lendlein, ACS Appl. Mater. Interfaces, 2021, 13, 10748–10759 CrossRef CAS PubMed.
- S. Zhang, Q. Ou, P. Xin, Q. Yuan, Y. Wang and J. Wu, Biomater. Sci., 2019, 7, 4230–4236 RSC.
- Y. Cong, T. Xia, M. Zou, Z. Li, B. Peng, D. Guo and Z. Deng, J. Mater. Chem. B, 2014, 2, 3450–3461 RSC.
- J. Park, T. F. Brust, H. J. Lee, S. C. Lee, V. J. Watts and Y. Yeo, ACS Nano, 2014, 8, 3347–3356 CrossRef CAS PubMed.
- F. Cofano, M. Boido, M. Monticelli, F. Zenga, A. Ducati, A. Vercelli and D. Garbossa, Int. J. Mol. Sci., 2019, 20, 2698 CrossRef CAS PubMed.
- L. Mazini, L. Rochette, B. Admou, S. Amal and G. Malka, Int. J. Mol. Sci., 2020, 21, 1306 CrossRef CAS PubMed.
- L. Liu, P. W. Chiu, P. K. Lam, C. C. Poon, C. C. Lam, E. K. Ng and P. B. Lai, Br. J. Surg., 2015, 102, e158–168 CrossRef CAS.
- J. Chu, P. Shi, X. Deng, Y. Jin, H. Liu, M. Chen, X. Han and H. Liu, J. Biophotonics, 2018, 11, e201700336 CrossRef PubMed.
- Q. Wang, Y. Jin, X. Deng, H. Liu, H. Pang, P. Shi and Z. Zhan, Biomaterials, 2015, 53, 659–668 CrossRef CAS.
- X. Li, Y. Wang, R. Ma, X. Liu, B. Song, Y. Duan, J. Guo, G. Feng, T. Cui, L. Wang, J. Hao, H. Wang and Q. Gu, Biomed. Mater., 2021, 16, 035023 CrossRef CAS.
- G. G. Giobbe, C. Crowley, C. Luni, S. Campinoti, M. Khedr, K. Kretzschmar, M. M. De Santis, E. Zambaiti, F. Michielin, L. Meran, Q. Hu, G. van Son, L. Urbani, A. Manfredi, M. Giomo, S. Eaton, D. Cacchiarelli, V. S. W. Li, H. Clevers, P. Bonfanti, N. Elvassore and P. De Coppi, Nat. Commun., 2019, 10, 5658 CrossRef.
- X. Huang, G. Q. Fei, W. J. Liu, J. Ding, Y. Wang, H. Wang, J. L. Ji and X. Wang, Acta Pharmacol. Sin., 2020, 41, 612–619 CrossRef CAS.
- X. Bao, J. Zhao, J. Sun, M. Hu and X. Yang, ACS Nano, 2018, 12, 8882–8892 CrossRef CAS.
- A. L. Speer, X. Ren, E. P. McNeill, J. M. Aziz, S. M. Muir, D. I. Marino, P. Dadhich, K. Sawant, R. Ciccocioppo, A. Asthana, K. N. Bitar and G. Orlando, Cytotherapy, 2021, 23, 381–389 CrossRef CAS PubMed.
- K. Kanetaka and S. Eguchi, Regener. Ther., 2020, 15, 129–137 CrossRef.
- J. Liao, B. Xu, R. Zhang, Y. Fan, H. Xie and X. Li, J. Mater. Chem. B, 2020, 8, 10023–10049 RSC.
- D. Choudhury, M. Yee, Z. L. J. Sheng, A. Amirul and M. W. Naing, Acta Biomater., 2020, 115, 51–59 CrossRef.
- X. Zhang, X. Chen, H. Hong, R. Hu, J. Liu and C. Liu, Bioact. Mater., 2022, 10, 15–31 CrossRef CAS.
- M. S. Massaro, R. Pálek, J. Rosendorf, L. Červenková, V. Liška and V. Moulisová, Mater. Sci. Eng., C, 2021, 127, 112203 CrossRef CAS PubMed.
- L. Meran, I. Massie, S. Campinoti, A. E. Weston, R. Gaifulina, L. Tullie, P. Faull, M. Orford, A. Kucharska, A. Baulies, L. Novellasdemunt, N. Angelis, E. Hirst, J. König, A. M. Tedeschi, A. F. Pellegata, S. Eli, A. P. Snijders, L. Collinson, N. Thapar, G. M. H. Thomas, S. Eaton, P. Bonfanti, P. De Coppi and V. S. W. Li, Nat. Med., 2020, 26, 1593–1601 CrossRef CAS.
- A. K. Nowocin, A. Southgate, S. M. Gabe and T. Ansari, J. Tissue Eng. Regener. Med., 2016, 10, E23–33 CrossRef CAS.
- F. E. Uhl, F. Zhang, R. A. Pouliot, J. J. Uriarte, S. Rolandsson Enes, X. Han, Y. Ouyang, K. Xia, G. Westergren-Thorsson, A. Malmström, O. Hallgren, R. J. Linhardt and D. J. Weiss, Acta Biomater., 2020, 102, 231–246 CrossRef CAS PubMed.
- Y. Tao, X. B. Cheng, Z. J. Wang, R. W. Tan, X. Q. Yu, Z. W. Zhai and J. G. Han, Mater. Sci. Eng., C, 2021, 119, 111645 CrossRef CAS PubMed.
- I. Singh, G. Dhawan, S. Gupta and P. Kumar, Front. Microbiol., 2020, 11, 607099 CrossRef.
- H. Wozniak, T. S. Beckmann, L. Fröhlich, T. Soccorsi, C. Le Terrier, A. de Watteville, J. Schrenzel and C. P. Heidegger, Crit. Care, 2022, 26, 250 CrossRef.
- C. Pan, J. Li, W. Hou, S. Lin, L. Wang, Y. Pang, Y. Wang and J. Liu, Adv. Mater., 2021, 33, e2007379 CrossRef.
- L. Xie, Q. Li, Y. Liao, Z. Huang, Y. Liu, C. Liu, L. Shi, Q. Li and M. Yuan, Molecules, 2022, 27, 6461 CrossRef CAS PubMed.
- L. Y. Li, L. Y. Cui, R. C. Zeng, S. Q. Li, X. B. Chen, Y. Zheng and M. B. Kannan, Acta Biomater., 2018, 79, 23–36 CrossRef CAS PubMed.
- R. Xue, Y. Qian, L. Li, G. Yao, L. Yang and Y. Sun, Stem Cell Res. Ther., 2017, 8, 148 CrossRef PubMed.
- J. C. Silva, M. S. Carvalho, R. N. Udangawa, C. S. Moura, J. M. S. Cabral, C. L. da Silva, F. C. Ferreira, D. Vashishth and R. J. Linhardt, J. Biomed. Mater. Res., Part B, 2020, 108, 2153–2166 CrossRef CAS.
- Z. Li, H. Wang, B. Yang, Y. Sun and R. Huo, Mater. Sci. Eng., C, 2015, 57, 181–188 CrossRef CAS PubMed.
- C. Sid-Otmane, L. P. Perrault and H. Q. Ly, J. Transl. Med., 2020, 18, 336 CrossRef PubMed.
- Y. W. Eom, J. E. Oh, J. I. Lee, S. K. Baik, K. J. Rhee, H. C. Shin, Y. M. Kim, C. M. Ahn, J. H. Kong, H. S. Kim and K. Y. Shim, Biochem. Biophys. Res. Commun., 2014, 445, 16–22 CrossRef CAS PubMed.
- N. Su, P. L. Gao, K. Wang, J. Y. Wang, Y. Zhong and Y. Luo, Biomaterials, 2017, 141, 74–85 CrossRef CAS.
- S. Maacha, H. Sidahmed, S. Jacob, G. Gentilcore, R. Calzone, J. C. Grivel and C. Cugno, Stem Cells Int., 2020, 2020, 4356359 Search PubMed.
- Z. Wang, Y. Wang, Z. Wang, J. S. Gutkind, Z. Wang, F. Wang, J. Lu, G. Niu, G. Teng and X. Chen, Stem Cells, 2015, 33, 456–467 CrossRef CAS.
- J. Obendorf, C. Fabian, U. H. Thome and M. Laube, Stem Cell Res. Ther., 2020, 11, 525 CrossRef CAS PubMed.
- L. Ling, X. Feng, T. Wei, Y. Wang, Y. Wang, Z. Wang, D. Tang, Y. Luo and Z. Xiong, Stem Cell Res. Ther., 2019, 10, 46 CrossRef CAS PubMed.
- L. Moussa, G. Pattappa, B. Doix, S. L. Benselama, C. Demarquay, M. Benderitter, A. Sémont, R. Tamarat, J. Guicheux, P. Weiss, G. Réthoré and N. Mathieu, Biomaterials, 2017, 115, 40–52 CrossRef CAS PubMed.
- J. Koh, D. R. Griffin, M. M. Archang, A. C. Feng, T. Horn, M. Margolis, D. Zalazar, T. Segura, P. O. Scumpia and D. Di Carlo, Small, 2019, 15, e1903147 CrossRef.
- K. C. Murphy, J. Whitehead, D. Zhou, S. S. Ho and J. K. Leach, Acta Biomater., 2017, 64, 176–186 CrossRef CAS PubMed.
- D. Sivaraj, K. Chen, A. Chattopadhyay, D. Henn, W. Wu, C. Noishiki, N. J. Magbual, S. Mittal, A. M. Mermin-Bunnell, C. A. Bonham, A. A. Trotsyuk, J. A. Barrera, J. Padmanabhan, M. Januszyk and G. C. Gurtner, Front. Bioeng. Biotechnol., 2021, 9, 660145 CrossRef PubMed.
- X. Zheng, Z. Ding, W. Cheng, Q. Lu, X. Kong, X. Zhou, G. Lu and D. L. Kaplan, Adv. Healthcare Mater., 2020, 9, e2000041 CrossRef.
- X. R. Zhang, Y. Z. Huang, H. W. Gao, Y. L. Jiang, J. G. Hu, J. K. Pi, A. J. Chen, Y. Zhang, L. Zhou and H. Q. Xie, Stem Cell Res. Ther., 2020, 11, 150 CrossRef CAS.
- X. Zhang, X. Kang, L. Jin, J. Bai, W. Liu and Z. Wang, Int. J. Nanomed., 2018, 13, 3897–3906 CrossRef CAS.
- T. Sun, Z. Huang, W. C. Liang, J. Yin, W. Y. Lin, J. Wu, J. M. Vernes, J. Lutman, P. Caplazi, S. Jeet, T. Wong, M. Wong, D. J. DePianto, K. B. Morshead, K. H. Sun, Z. Modrusan, J. A. Vander Heiden, A. R. Abbas, H. Zhang, M. Xu, E. N. N’Diaye, M. Roose-Girma, P. J. Wolters, R. Yadav, S. Sukumaran, N. Ghilardi, R. Corpuz, C. Emson, Y. G. Meng, T. R. Ramalingam, P. Lupardus, H. D. Brightbill, D. Seshasayee, Y. Wu and J. R. Arron, Sci. Transl. Med., 2021, 13, eabe0407 CrossRef CAS.
- K. E. Martin and A. J. García, Acta Biomater., 2021, 133, 4–16 CrossRef CAS PubMed.
- Y. Liu and T. Segura, Front. Bioeng. Biotechnol., 2020, 8, 609297 CrossRef.
- R. Cruz-Acuña, M. Quirós, A. E. Farkas, P. H. Dedhia, S. Huang, D. Siuda, V. García-Hernández, A. J. Miller, J. R. Spence, A. Nusrat and A. J. García, Nat. Cell Biol., 2017, 19, 1326–1335 CrossRef PubMed.
- M. J. Workman, M. M. Mahe, S. Trisno, H. M. Poling, C. L. Watson, N. Sundaram, C. F. Chang, J. Schiesser, P. Aubert, E. G. Stanley, A. G. Elefanty, Y. Miyaoka, M. A. Mandegar, B. R. Conklin, M. Neunlist, S. A. Brugmann, M. A. Helmrath and J. M. Wells, Nat. Med., 2017, 23, 49–59 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2tb01389d |
| ‡ Jian Wan and Tianqi Wu contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2023 |