An in situ, reversible fluorescent paper sensor for selective detection of ambient CO2†
Chu
Zhang
,
Yiwen
Ding
,
Min
Zhou
,
Yu
Xiang
 and
Aijun
Tong
and
Aijun
Tong
 *
*
Department of Chemistry, Beijing Key Laboratory for Microanalytical Methods and Instrumentation, Key Laboratory of Bioorganic Phosphorus Chemistry and Chemical Biology (Ministry of Education), Tsinghua University, Beijing 100084, China. E-mail: tongaj@tsinghua.edu.cn
First published on 8th December 2022
Abstract
Analyzing the components and contents of environmental gases is of great importance for industrial production, human health, and environmental safety. Carbon dioxide (CO2) has drawn great concern for its adverse effects on climate change and physical health. Simple, in situ, and selective methods for CO2 detection are thus highly demanded. This work presents a versatile and easily fabricated paper sensor for the selective detection of ambient CO2. The sensor consists of two components with a fluorescent molecule ANT-PPh3 as the sensing element and an ionic liquid [DBUH]+[Im]− for the absorption of CO2. Based on the photo-induced electron transfer (PET) between ANT-PPh3 and [DBUH]+[Im]−, the sensor exhibited fluorescence enhancement in the presence of CO2, and the response process is reversible. The sensor exhibits improved selectivity for CO2 over SO2 and NO2 compared with most reported works, with good stability and fast response to human breath. Without complex instrumentation and gas pre-collection processes, the early notification of high indoor CO2 concentrations was realized using this sensor. Taking advantage of its intuitive fluorescence changes, specific responsiveness, and convenience, we believe our designed CO2 sensor could be applied practically in monitoring and indicating high indoor CO2 concentrations in living scenarios.
1. Introduction
The components and contents of the surrounding air are related to many aspects of industrial production, human health, and environmental safety.1,2 There is a growing demand for sensors that can detect and monitor various gases in both environmental and biological systems. Their applications include monitoring air quality, detecting hazardous gases, and clinical diagnosis. The target gases vary between volatile organic compounds3 (VOCs), explosive gases,4 highly-toxic gases,5 and the other common gases,6,7 depending on different scenarios. The analytical methods behind these sensors can be primarily divided into mass spectrometry,8 electrochemical methods,9 IR absorption,10 and colorimetric/fluorescent methods.11 Generally, mass spectrometry is often the golden standard for detecting most gases, but it requires expensive instrumentation and complicated operations.12 Electrochemical gas sensors can reach an ultra-high sensitivity. However, they usually need high operating temperatures, causing energy consumption, and are unsuitable for in situ monitoring. Sensors based on IR absorption are also bothered by poor selectivity and limited sensitivity. By contrast, fluorescent gas sensors rely on highly selective reactions between target gases and sensors, which are especially appreciated for their versatility, low cost, good selectivity, and in situ sensing abilities.13,14Recently, growing concerns over global warming have generated widespread discussion.15 As one of the most important greenhouse gases, carbon dioxide (CO2) contributes immeasurably to worldwide climate change. Excessive CO2 emissions can bring significant environmental problems to our society.16 Additionally, high concentrations of CO2 can cause physical effects. When the indoor concentration of CO2 rises to 1500 ppm, long-time exposure can lead to dizziness and a lack of focus. Furthermore, several physiological dysfunctions and irreversible damage may occur after a long-time exposure when CO2 concentration is above 3000 ppm. Barely able to be perceived, people tend to ignore this potential danger.1,17,18 Thus, in situ monitoring of indoor concentrations of CO2 is essential in confined or densely populated spaces like mines or classrooms. In this perspective, fluorescent CO2 sensors become advantageous for their selective sensing and convenience.
Nevertheless, previously reported CO2 sensors based on fluorescent methods are mainly performed in solution,19–26 which is inconvenient for practical use (Table S1, ESI†). Jiang et al. developed a quantitative CO2 sensor based on AIE-active organogels.27 However, the detection setup required the presence of volatile amines, which are harmful to the human body. Meldrum et al. developed a paper sensor for CO2 based on a pH-sensitive fluorescent dye.28 The formation of carbonic acid in the presence of CO2 and water vapor would trigger ratiometric fluorescence changes. Despite the high sensitivity, the sensor lacked selectivity over SO2 and NO2, while the detection required high humidity. Overall, existing solid-state fluorescent sensors for CO2 usually require pre-collection processes or specific detecting environments. There is rare in situ solid-state fluorescent sensor that can detect ambient CO2 concentrations. Portable, selective and robust sensors for ambient CO2 detection are still highly demanded.
Ionic liquids (ILs) are a versatile class of functional materials with various physicochemical properties.29 They can offer inherent advantages in terms of facile synthesis, low cost, and easy tunability, making them promising in the field of optical sensing.30 IL-based fluorescent sensors were reported to detect H2O2,31 SO2,32 and halogen anions.33 Combined with polymer membranes, ILs can serve as solid-state sensors for in situ, simple, and handy detection.34 More importantly, ILs have been successfully utilized as highly efficient CO2 absorbents due to their intriguing reaction performance, such as efficient binding, good stability, and rapid response between ILs and CO2.35,36 These good metrics have made ILs competitive candidates for manufacturing fluorescent CO2 sensors.
Herein, we report an in situ, reversible fluorescent paper sensor to realize ambient CO2 sensing based on a PET (photo-induced electron transfer) process using an ionic liquid ([DBUH]+[Im]−) and a functional fluorescent molecule (ANT-PPh3). Due to the interactions between triphenylphosphine cation moieties and anions like F−, ANT-PPh3 was previously reported to detect anions in an aqueous solution.37 We combined ANT-PPh3 with an amine-based ionic liquid, [DBUH]+[Im]−,38 to construct a paper-based fluorescent sensor for CO2 detection. Here, [DBUH]+[Im]− functions as a reversible CO2 absorbent. After exposure to CO2, the imidazole anion [Im]− of [DBUH]+[Im]− would transfer to carbamate species [ImCOO]−, which subsequently weakens the PET process from [Im]− to ANT-PPh3, and finally leads to fluorescence enhancement. The proposed mechanism is verified through fluorescence spectra, 31P-NMR spectra, and DFT calculations. Since [DBUH]+ [Im]− can release the adsorbed CO2 at higher temperatures, this sensor is reusable after heating at 60 °C for 30 min. The sensor shows improved selectivity over H2O, SO2, and NO2 compared to previously reported CO2 sensors, with good stability and fast response to human breath. We successfully performed in situ notification of high concentrations of indoor CO2, showing its convenience and practicality.
2. Experimental section
2.1 Synthesis and characterization of ANT-PPh3
ANT-PPh3 was synthesized following the steps shown in Scheme 1(A).37 The final product was a yellow solid, collected by filtration with a total yield of 64%. 1H-NMR (400 MHz, DMSO-d6): δ 7.97 (dd, J = 6.8, 3.2 Hz, 4H), 7.82–7.73 (m, 6H), 7.60–7.42 (m, 24H), 6.98 (dd, J = 6.9, 3.0 Hz, 4H), 6.12 (d, J = 13.8 Hz, 4H). 13C-NMR (101 MHz, DMSO-d6): δ 135.582, 134.896, 134.845, 134.795, 130.939, 130.388, 130.326, 130.265, 130.179, 125.956, 125.560, 122.348, 118.221, 117.364, 24.310, 23.819. ESI-MS (m/z): calcd for C52H42F12P4 [M-PF6]+: 873.2398, found: 873.2351.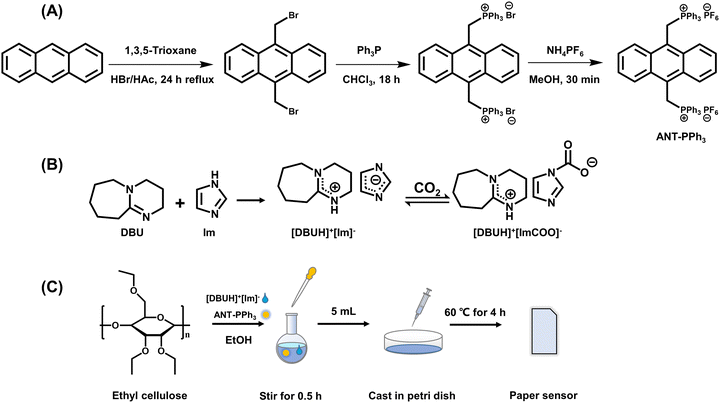 | ||
| Scheme 1 (A) Synthetic route of ANT-PPh3; (B) synthetic route of [DBUH]+[Im]− and its absorption of CO2; (C) schematic illustrations for the fabrication of the paper sensor for CO2. | ||
2.2 Synthesis and characterization of [DBUH]+[Im]−
The ionic liquid [DBUH]+[Im]− was prepared through neutralization between the super base DBU and imidazole shown in Scheme 1(B).38 Imidazole was dissolved in dichloromethane. Then equivalent DBU was added dropwise under vigorous stirring. After 12 h, the solvent was removed by a rotary evaporator. The remaining ionic liquid was dried at 60 °C for 24 h in a vacuum drying oven. 1H-NMR (400 MHz, chloroform-d): δ 7.62 (s, 1H), 7.05 (s, 2H), 3.22 (ddd, J = 20.5, 10.2, 5.1 Hz, 7H), 2.37 (s, 2H), 1.78 (p, J = 6.0 Hz, 2H), 1.59 (d, J = 29.5 Hz, 7H). 13C-NMR (101 MHz, chloroform-d): δ 162.102, 135.389, 121.924, 77.162, 52.930, 48.426, 43.658, 36.915, 29.755, 28.505, 25.969, 22.442.2.3 Preparation of CO2 paper sensor
The CO2 paper sensor was prepared as shown in Scheme 1(C). The detailed preparation process is as follows. Ethyl cellulose (EC, 5g) was dissolved in 100 mL ethanol and stirred for 3 h at 75 °C. Then 500 μL [DBUH]+[Im]− and 1.0 mg ANT-PPh3 were added into 10 mL ethyl cellulose solution. After 0.5 h stirring, the mixture was transferred into a petri dish (5 mL) and kept in a vacuum drying oven at 60 °C for 4 h to prepare the required paper sensor. The original sensor was cut into rectangles (1.3 × 2.5 cm) for the subsequent measurements.2.4 Adsorption–desorption cycles
The CO2 paper sensor was first exposed to gaseous CO2 using a balloon filled with high-purity CO2. Then, the sensor was kept in a vacuum drying oven at 60 °C for 30 min to facilitate CO2 desorption. The adsorption–desorption process was repeated, and the fluorescence spectra were recorded accordingly.2.5 CO2 detection
For measurement of the limit of detection (LOD), a CO2 gas sensing apparatus was constructed, as shown in Scheme S1A (ESI†). Argon (Ar, purity 99.999%) was used as the carrier gas, and the purity of gaseous CO2 was 99.999%. The flow rate of Ar was fixed at 400 mL min−1. The concentration of CO2 was controlled by changing the flow rates of CO2, which varied from 2 to 12 mL min−1. Mixed gas was delivered into a glass reactor. After 25 min, the sensor was taken out, and the fluorescence spectrum was recorded.For selective experiments, the sensor was placed inside a dish which was sealed by a rubber plug. Gaseous N2, Ar, O2, CO2 (>99 vol%), and SO2/NO2 (2 vol%) were continuously blown into the sealed dish through balloons. After 15 min, the fluorescence spectrum was recorded. Other experiments were performed in sealed glass bottles. 1 mL of different organic solvents or aqueous solutions were placed inside the bottle in advance. Then the sensor was placed, followed by quick sealing. After 15 min, the sensor was taken out, and the fluorescence spectrum was recorded. Lower concentrations of SO2 were prepared by adding H2SO4 aqueous solution (1.0 M) into NaHSO3 aqueous solution. Lower concentrations of NO2 were prepared by adding H2SO4 aqueous solution (1.0 M) into NaNO2 aqueous solution.39 Gaseous NH3 was prepared from concentrated ammonia (12.0 M).
For the simulation of high concentrations of indoor CO2, NaHCO3 aqueous solutions of different concentrations as the origin of gaseous CO2 were used. In a typical experiment, 30 mL (0.15 M) NaHCO3 aqueous solution was placed in a sealed glass cover. A commercial CO2 detector was applied to indicate the average CO2 concentration inside (Scheme S1B, ESI†). After 4 h, the sensor was taken out, and the fluorescence spectra were recorded.
3. Results and discussion
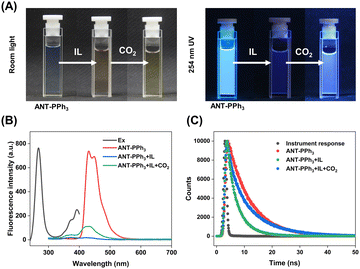
As shown in Scheme 1(A), the fluorescent functional molecule ANT-PPh3 was synthesized and characterized through 1H-NMR, 13C-NMR, and ESI-MS (Fig. S1–S3, ESI†).37 As shown in Fig. 1 and Fig. S6 (ESI†), ANT-PPh3 is strongly fluorescent with bright blue emission peaked at 430 nm and shows two absorption maxima at 268 nm and 390 nm. The ionic liquid molecule [DBUH]+[Im]− was synthesized through the neutralization reaction between DBU and imidazole40 and was characterized through 1H-NMR and 13C-NMR (Fig. S4 and S5, ESI†). As illustrated in Scheme 1(B), [DBUH]+[Im]− could reversibly absorb CO2, leading to the formation of the corresponding carbamate.38 To confirm the absorption of CO2 by [DBUH]+[Im]−, we measured its 13C-NMR and IR spectra. According to the IR spectra of [DBUH]+[Im]−, two new bands (1709 cm−1, 1281 cm−1) appeared after bubbling CO2 for 1 min (Fig. S7, ESI†), which is assigned to the stretching vibrations of C = O and the C–N bonds of the carbamate.4113C-NMR also shows a new peak of carbonyl carbon at 163.89 ppm (Fig. S8, ESI†). These facts confirmed the CO2 absorption ability of [DBUH]+[Im]−.Continuously, we investigated the optical behavior of the ANT-PPh3 + [DBUH]+[Im]− system upon CO2 stimulation. As is shown in Fig. 1(A), in the presence of [DBUH]+[Im]−, the fluorescence of ANT-PPh3 is significantly quenched. Through bubbling CO2 for 1 min, the blue emission gradually recovers. At the same time, the quantum yield of ANT-PPh3 decreased from 71.8% to 6.3% with [DBUH]+[Im]−, then recovered to 11.4% in the presence of CO2. The fluorescence lifetime shares the same trend, varying from 7.8 ns to 4.0 ns, then to 8.5 ns (Fig. 1(C) and Table S2, ESI†). These changes in quantum yields and fluorescence lifetimes indicate the occurrence of fluorescence quenching based on PET processes as expected.42
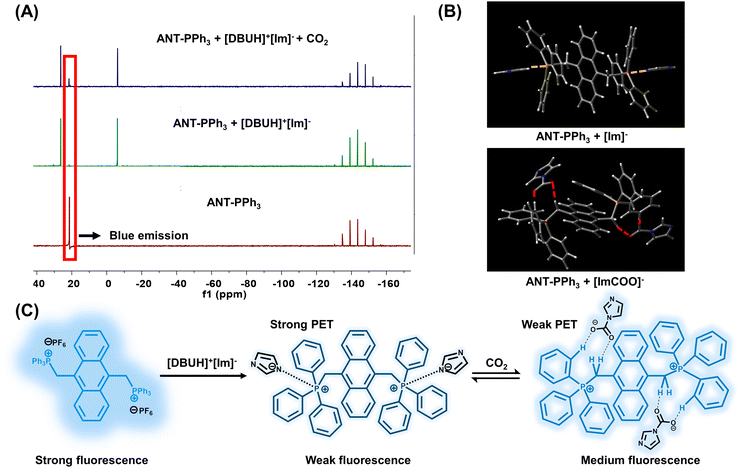
To further validate the sensing mechanism and the interactions among ANT-PPh3, [DBUH]+[Im]−, and CO2, we investigated 31P-NMR spectra. As is shown in Fig. 2(A), the original chemical shift of ANT-PPh3 lies at 21.20 ppm. Upon adding excess [DBUH]+[Im]−, this peak shifts to 26.55 ppm, and a new peak appears at −6.18 ppm. After bubbling CO2 for 1 min, the original peak regenerates at 21.20 ppm, along with the partial recovery of the blue emission. Besides, in the presence of imidazole (Im) alone, no evident chemical shift of ANT-PPh3 was observed. If imidazole was converted to its anion ([Im]−) with equivalent NaOH in advance, the 31P-NMR spectrum was nearly identical to the situation of adding [DBUH]+[Im]− (Fig. S9, ESI†). Therefore, we assumed that interactions between [Im−] and ANT-PPh3 caused these changes in 31P-NMR spectra. Next, we conducted theoretical DFT calculations with the B3LYP-d3 function and 6-31g* basis sets. The DFT-optimized structures are shown in Fig. 2(B). The yellow dashed lines show the interactions between [Im−] and the P atom of ANT-PPh3, with a distance comparable to common hydrogen bonds (2.01 Å). After bubbling CO2, the newly-formed carbamate ([ImCOO]−) only shows limited interactions with the P atom. Instead, O⋯C–H interactions are formed between [ImCOO]− and ANT-PPh3. These differences in the binding modes may lead to changes in the 31P-NMR spectra.
The frontier molecular orbital investigations of ANT-PPh3, ANT-PPh3 + [Im]−, and ANT-PPh3 + [ImCOO]− adducts were also performed, and the results are displayed in Fig. S10 (ESI†). The highest occupied molecular orbitals (HOMOs) are located on the anthracene unit in ANT-PPh3, whereas in the presence of [Im]− and [ImCOO]−, the HOMOs switch to benzene and imidazole units. The corresponding bandgaps decrease from 3.29 eV to 3.12 eV and 2.83 eV, respectively. The higher HOMOs of ANT-PPh3 + [Im]− adduct and ANT-PPh3 + [ImCOO]− adduct thus facilitate the occurrence of PET processes.43–45 The proposed mechanism is shown in Fig. 2(C). The PET process caused by [Im]− is stronger than [ImCOO]−, leading to the transformation from weak fluorescence to medium fluorescence, namely CO2-induced fluorescence enhancement of the ANT-PPh3 + [DBUH]+[Im]− system.
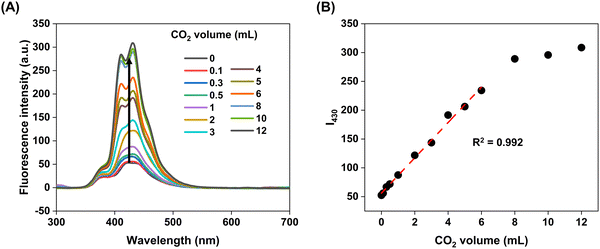
By changing the amount of [DBUH]+[Im]−, we investigated the response of the ANT-PPh3 + [DBUH]+[Im]− system toward a certain amount of CO2 in DMSO. The fluorescence enhancement ratio ((It − I0)/I0) at 430 nm of the solution firstly increases and then decreases with adding more [DBUH]+[Im]− (Fig. S11, ESI†). We determined the optimal volume of [DBUH]+[Im]− in the mixed solution. As shown in Fig. 3 and Fig. S12 (ESI†), after bubbling different volumes of high-purity gaseous CO2 through a syringe, the absorbance of the solution at 268 nm slowly decreased, while the fluorescence intensity at 430 nm dramatically increased. There is a good linear relationship (R2 = 0.992) between the fluorescence intensities and the amount of CO2 at lower levels, indicating that this system may have great potential to be a CO2 turn-on sensor.
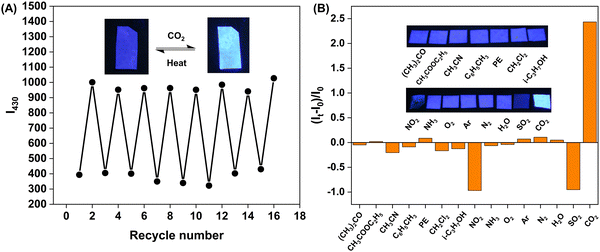
Taking advantage of the above characteristics, we fabricated paper sensors using ethyl cellulose as the substrate. For the stability test, we put the sensor in a sealed dish under ordinary room light for 7 days (Fig. S13, ESI†). The fluorescence intensities at 430 nm are nearly unchanged. In addition, photochemical stability is one of the key features determining the detecting performance of a fluorescent sensor. Under continuous excitation of white LED and 254 nm UV, the photostability of the paper sensor was investigated. As is shown in Fig. S14 (ESI†), under 254 nm UV, the fluorescence intensity quenches only 20% after 8 h. And with continuous exposure to white LED, the fluorescence intensity remains the same after 12 h. These data demonstrate that the sensor has good stability to support its daily application. After 1 min exposure to high-purity gaseous CO2, the quantum yield of this paper sensor increased from 17.0% to 33.6%, and the fluorescence lifetime increased from 6.9 ns to 10 ns (Fig. S15, ESI†). Due to the reversible reaction between [DBUH]+[Im]− and CO2, this paper sensor also shows a reversible response to CO2. As shown in Fig. 4(A), the sensor becomes strongly fluorescent by blowing high-purity CO2 into a sealed quartz dish. Then, after being heated at 60 °C for 30 min, the sensor becomes weakly fluorescent again. This reuse cycle could repeat over eight times.
The specificity of CO2 sensors has become a long-standing concern. Based on PET-quenching or pH sensing mechanisms, previously reported CO2 sensors are often affected by acidic gases like SO2 and NO2. To demonstrate the selectivity, we investigated the response of the CO2 paper sensor toward other common gases (H2O, N2, O2) and some VOCs. As is shown in Fig. 4(B), the fluorescence intensities of the sensor remain nearly unchanged after adding the interfering gases for 15 min, indicating that they exert a negligible effect on the detection of CO2. A noticeable fluorescence enhancement occurs in the presence of CO2, which can be observed by the naked eye. Especially, SO2 and NO2 cause evident fluorescence quenching, which is entirely different from CO2. To further check the fluorescence quenching caused by SO2 and NO2, excessive SO2 and NO2 gases were respectively injected into ANT-PPh3 + [DBUH]+[Im]− in DMSO, and the solutions were nearly non-fluorescent (Fig. S17, ESI†). As is shown in Fig. S18 (ESI†), after bubbling SO2 or NO2, the original chemical shift of ANT-PPh3 at around 21 ppm does not regenerate, accounting for the fluorescence quenching. As both acidic gases, SO2 and NO2 can also react with [DBUH]+[Im]−, forming addictives like [ImSOO]− and [ImNOO]−.41,46 Unlike [ImCOO]−, [ImSOO]− and [ImNOO]− species may exhibit a more intensive PET effect than [Im]−, leading to different fluorescence responses amongst CO2 and NO2/SO2.
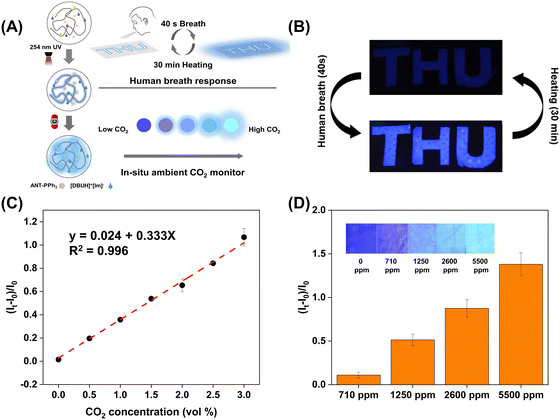
The potential competing effects of NO2 and SO2 were also investigated. After exposure to SO2 and NO2 (200 ppm) for 3 h, the fluorescence spectra of the sensor are nearly identical, and no interference is observed when detecting ambient CO2 (Fig. S19, ESI†). In living environments, the concentrations of NO2 and SO2 are minimal (<1–2 ppm).32,47 Thus, the selective detection of ambient CO2 over SO2 and NO2 can be realized. Compared with other reported sensors, this CO2 paper sensor possesses excellent analytical capability for the selective detection of CO2. Additionally, this sensor could respond to CO2 in the exhaled air (Fig. 5(B) and Fig. S20, ESI†). The paper sensor was cut into the shape of letters “T”, “H”, and “U”. After continuous exposure to human breath for 40 s, the letters became strongly emissive. This phenomenon can be restored repeatedly by heating for half an hour. Human breath consists of a mixture of CO2 (∼5%), water vapor (∼5%), N2 (∼74%), O2 (∼15%), trace gases, and various VOCs.28 These results demonstrate that this paper sensor can detect CO2 in complex atmospheres.
To find the LOD, we use the experimental setup shown in Scheme S1A (ESI†). The flow rate of high-purity Ar was fixed at 400 mL min−1. By adjusting the flow rates of CO2, we can precisely control the concentrations of CO2 in the gas mixture with the mass flow meter. We then investigated the fluorescence spectra of the paper sensor in the gas reactor after 25 min exposure to 0.5–3.0 vol% CO2. The standard deviation of the noise level was obtained by continuously blowing high-purity Ar into the same reactor. The fluorescence enhancement ratios ((It − I0)/I0) at 430 nm gradually increased with CO2 concentrations rising. An excellent linear calibration line (R2 = 0.996) was attained, and according to LOD = 3Sb × m−1, where Sb is the standard deviation of fluorescence enhancement ratios ((It − I0)/I0) for 5 blank samples, m is the slope of the linear correlation between (It − I0)/I0 and CO2 concentrations, the LOD was calculated to be 0.183 vol%.
In order to verify the potential of this paper sensor for detecting ambient CO2 in real scenarios, we used sealed glass covers and NaHCO3 aqueous solutions to simulate indoor environments with high CO2 concentrations (Scheme S1B, ESI†). We used a commercial CO2 detector to determine the CO2 concentrations inside the glass cover. As is shown in Fig. S21 (ESI†), the fluorescence intensities of CO2 paper sensors increase over time. According to the fluorescence enhancement ratios ((It − I0)/I0) at 430 nm, we can determine and monitor higher levels of indoor CO2 concentrations (Fig. 5(D)). The recommended indoor CO2 concentration should be less than 1500 ppm.48 With this paper sensor, indoor CO2 concentrations above this level can be visualized and notified. These results support the application of this sensor in living scenarios, which may be very helpful in ensuring our physical health.
4. Conclusion
In summary, we have rationally designed an in situ, reversible fluorescent paper sensor for the selective detection of ambient CO2 based on a novel fluorescent sensing system. Composed of a fluorescent molecule ANT-PPh3 and a CO2 absorbing element, the ionic liquid [DBUH]+[Im]−, the mixture of ANT-PPh3 + [DBUH]+[Im]− is fabricated into a paper sensor with ethyl cellulose as the substrate. Encountering [DBUH]+[Im]−, the fluorescence of ANT-PPh3 was quenched owing to the intense PET process. After exposure to CO2, the formation of [ImCOO]− weakened the PET process and finally caused fluorescence enhancement. This proposed sensing mechanism, confirmed by 31P-NMR spectra and DFT calculations, endows the sensor with excellent selectivity over SO2, NO2, H2O, and other VOCs. Due to the reversible reaction between [DBUH]+[Im]− and CO2, the sensor can be regenerated after heating and then reused up to eight times. We successfully conducted simulation experiments of early notification of ambient CO2 using this sensor. Compared with the previously reported CO2 sensors, this sensor exhibits improved selectivity and does not require particular detection environments or gas pre-collection processes. This PET-based strategy may promote the design of new approaches to developing more fluorescent systems with unique selectivity. We believe our designed CO2 sensor could be applied practically in monitoring high concentrations of CO2 for daily healthcare.Author contributions
Chu Zhang: investigation, conceptualization, methodology, validation, formal analysis, data curation, writing-original draft, writing-review & editing, visualization, Yiwen Ding: resources, formal analysis, Min Zhou: resources, formal analysis, Yu Xiang: supervision, methodology, Aijun Tong: supervision, funding acquisition, project administration, conceptualization, writing-review & editing. All authors discussed the results and commented on the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Key R&D Program of China (2021YFA1200901) and the National Natural Science Foundation of China (21974076, 21621003). We sincerely appreciate Mr Qingyu Meng (Department of Chemistry, Tsinghua University) for his valuable discussion on the NMR study and contributions to DFT calculations. We thank Mr Xin Tan (Department of Chemistry, Tsinghua University) for his kind help in the construction of gas detecting equipment.Notes and references
- M. Tainio, Z. Jovanovic Andersen, M. J. Nieuwenhuijsen, L. Hu, A. de Nazelle, R. An, L. M. T. Garcia, S. Goenka, B. Zapata-Diomedi, F. Bull and T. H. Sá, Environ. Int., 2021, 147, 105954 CrossRef CAS PubMed.
- M. R. Heal, P. Kumar and R. M. Harrison, Chem. Soc. Rev., 2012, 41, 6606–6630 RSC.
- C. Zhang, Y. Zheng, Y. Ding, X. Zheng, Y. Xiang and A. Tong, Talanta, 2022, 236, 122845 CrossRef CAS PubMed.
- G. Wang, M. Li, Q. Wei, Y. Xiong, J. Li, Z. Li, J. Tang, F. Wei and H. Tu, ACS Sens., 2021, 6, 1849–1856 CrossRef CAS PubMed.
- P. Zheng, W. Cao, Y. Zhang, F. Li and M. Zhang, ACS Sens., 2022, 7, 1946–1957 CrossRef CAS PubMed.
- L. Di, Z. Xia, H. Wang, Y. Xing and Z. Yang, Sens. Actuators, B, 2021, 326, 128987 CrossRef CAS.
- Y.-J. Gao, G. Romolini, H. Huang, H. Jin, R. A. Saha, B. Ghosh, M. De Ras, C. Wang, J. A. Steele, E. Debroye, J. Hofkens and M. B. J. Roeffaers, J. Mater. Chem. C, 2022, 10, 12191–12196 RSC.
- C. Wang, A. Mbi and M. Shepherd, IEEE Sens. J., 2010, 10, 54–63 Search PubMed.
- S. M. Majhi, A. Mirzaei, H. W. Kim, S. S. Kim and T. W. Kim, Nano Energy, 2021, 79, 105369 CrossRef CAS PubMed.
- L. Ciaffoni, G. Hancock, J. J. Harrison, J.-P. H. van Helden, C. E. Langley, R. Peverall, G. A. D. Ritchie and S. Wood, Anal. Chem., 2013, 85, 846–850 CrossRef CAS PubMed.
- O. Green, P. Finkelstein, M. A. Rivero-Crespo, M. D. R. Lutz, M. K. Bogdos, M. Burger, J.-C. Leroux and B. Morandi, J. Am. Chem. Soc., 2022, 8717–8724 CrossRef CAS PubMed.
- X. Zhou, S. Lee, Z. Xu and J. Yoon, Chem. Rev., 2015, 115, 7944–8000 CrossRef CAS PubMed.
- Y. Zhang, S. Yuan, G. Day, X. Wang, X. Yang and H.-C. Zhou, Coord. Chem. Rev., 2018, 354, 28–45 CrossRef CAS.
- W.-J. Yoon, S. Yang, J. Jang, M. Oh, M. Rim, H. Ko, J. Koo, S.-I. Lim, Y.-J. Choi and K.-U. Jeong, J. Mater. Chem. C, 2022, 10, 11316–11322 RSC.
- D. Leaf, H. J. H. Verolme and W. F. Hunt, Environ. Int., 2003, 29, 303–310 CrossRef PubMed.
- T. Gasser, C. Guivarch, K. Tachiiri, C. D. Jones and P. Ciais, Nat. Commun., 2015, 6, 7958 CrossRef CAS PubMed.
- U. Satish, M. J. Mendell, K. Shekhar, T. Hotchi, D. Sullivan, S. Streufert and W. J. Fisk, Environ. Health Perspect., 2012, 120, 1671–1677 CrossRef CAS PubMed.
- L. de Lary, A. Loschetter, O. Bouc, J. Rohmer and C. M. Oldenburg, Int. J. Greenhouse Gas Control, 2012, 9, 322–333 CrossRef CAS.
- Z. Guo, N. R. Song, J. H. Moon, M. Kim, E. J. Jun, J. Choi, J. Y. Lee, C. W. Bielawski, J. L. Sessler and J. Yoon, J. Am. Chem. Soc., 2012, 134, 17846–17849 CrossRef CAS PubMed.
- H. Yuan, Y. Fan, C. Xing, R. Niu, R. Chai, Y. Zhan, J. Qi, H. An and J. Xu, Anal. Chem., 2016, 88, 6593–6597 CrossRef CAS PubMed.
- H. Mardani, H. Roghani-Mamaqani, S. Shahi and M. Salami-Kalajahi, ACS Appl. Polym. Mater., 2022, 4, 1816–1825 CrossRef CAS.
- S. Kang, J. Kim, J.-H. Park, C. K. Ahn, C.-H. Rhee and M. S. Han, Dyes Pigm., 2015, 123, 125–131 CrossRef CAS.
- Y.-J. Jin, B.-C. Moon and G. Kwak, Dyes Pigm., 2016, 132, 270–273 CrossRef CAS.
- Y. Liu, Y. Tang, N. N. Barashkov, I. S. Irgibaeva, J. W. Y. Lam, R. Hu, D. Birimzhanova, Y. Yu and B. Z. Tang, J. Am. Chem. Soc., 2010, 132, 13951–13953 CrossRef CAS PubMed.
- Y. Ma and L.-Y. L. Yung, Anal. Chem., 2014, 86, 2429–2435 CrossRef CAS PubMed.
- H. Wang, D. Chen, Y. Zhang, P. Liu, J. Shi, X. Feng, B. Tong and Y. Dong, J. Mater. Chem. C, 2015, 3, 7621–7626 RSC.
- Y. Ma, M. Cametti, Z. Džolić and S. Jiang, J. Mater. Chem. C, 2018, 6, 9232–9237 RSC.
- H. Wang, S. I. Vagin, B. Rieger and A. Meldrum, ACS Appl. Mater. Interfaces, 2020, 12, 20507–20513 CrossRef CAS PubMed.
- P. Goodrich, H. Q. N. Gunaratne, J. Jacquemin, L. Jin, Y. Lei and K. R. Seddon, ACS Sustainable Chem. Eng., 2017, 5, 5635–5641 CrossRef CAS.
- S. Zeng, X. Zhang, L. Bai, X. Zhang, H. Wang, J. Wang, D. Bao, M. Li, X. Liu and S. Zhang, Chem. Rev., 2017, 117, 9625–9673 CrossRef CAS PubMed.
- Q.-H. Zhu, W.-L. Yuan, L. Zhang, G.-H. Zhang, L. He and G.-H. Tao, Anal. Chem., 2019, 91, 6593–6599 CrossRef CAS PubMed.
- S. Che, Q. Shou, Y. Fan, X. Peng, C. Zhou, H. Fu and Y. She, ACS Sustainable Chem. Eng., 2022, 10, 2784–2792 CrossRef CAS.
- T. Mizuta, K. Sueyoshi, T. Endo and H. Hisamoto, Anal. Chem., 2021, 93, 4143–4148 CrossRef CAS PubMed.
- Z. Dai, R. D. Noble, D. L. Gin, X. Zhang and L. Deng, J. Membr. Sci., 2016, 497, 1–20 CrossRef CAS.
- G. Cui, J. Wang and S. Zhang, Chem. Soc. Rev., 2016, 45, 4307–4339 RSC.
- C. Wang, X. Luo, H. Luo, D.-E. Jiang, H. Li and S. Dai, Angew. Chem., Int. Ed., 2011, 50, 4918–4922 CrossRef CAS PubMed.
- W. Huang, H. Lin and H. Lin, Sens. Actuators, B, 2011, 153, 404–408 CrossRef CAS.
- X. Zhu, M. Song and Y. Xu, ACS Sustainable Chem. Eng., 2017, 5, 8192–8198 CrossRef CAS.
- H. Yu, F. Dong, B. Li and F. S. Riehle, Sens. Actuators, B, 2019, 299, 126983 CrossRef CAS.
- F.-F. Chen, K. Huang, Y. Zhou, Z.-Q. Tian, X. Zhu, D.-J. Tao, D.-E. Jiang and S. Dai, Angew. Chem., Int. Ed., 2016, 55, 7166–7170 CrossRef CAS PubMed.
- S. F. R. Taylor, M. McClung, C. McReynolds, H. Daly, A. J. Greer, J. Jacquemin and C. Hardacre, Ind. Eng. Chem. Res., 2018, 57, 17033–17042 CrossRef CAS.
- K. Xu, J. Zhao, D. Escudero, Z. Mahmood and D. Jacquemin, J. Phys. Chem. C, 2015, 119, 23801–23812 CrossRef CAS.
- W. Sun, M. Li, J. Fan and X. Peng, Acc. Chem. Res., 2019, 52, 2818–2831 CrossRef CAS PubMed.
- D. Escudero, Acc. Chem. Res., 2016, 49, 1816–1824 CrossRef CAS PubMed.
- J. Harathi, R. Selva Kumar, S. K. Ashok Kumar, D. Saravanakumar, S. Senthilkumar and K. Thenmozhi, J. Mater. Chem. C, 2022, 10, 7949–7961 RSC.
- A. J. Greer, S. F. R. Taylor, H. Daly, M. G. Quesne, N. H. de Leeuw, C. R. A. Catlow, J. Jacquemin and C. Hardacre, ACS Sustainable Chem. Eng., 2021, 9, 7578–7586 CrossRef CAS PubMed.
- H. Long, A. Harley-Trochimczyk, T. Pham, Z. Tang, T. Shi, A. Zettl, C. Carraro, M. A. Worsley and R. Maboudian, Adv. Funct. Mater., 2016, 26, 5158–5165 CrossRef CAS.
- T. Vehviläinen, H. Lindholm, H. Rintamäki, R. Pääkkönen, A. Hirvonen, O. Niemi and J. Vinha, J. Occup. Environ. Hyg., 2016, 13, 19–29 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2tc05116h |
| This journal is © The Royal Society of Chemistry 2023 |